Background: The mechanisms eliciting metabolic activation in satellite cells are unclear.
Results: Noncanonical Sonic Hedgehog is activated following muscle injury, which activates AMPKα1 to induce Warburg-like glycolysis and promote satellite cell activation and proliferation.
Conclusion: AMPKα1 is required for Warburg-like glycolysis in satellite cells, which promotes satellite cell activation and muscle regeneration.
Significance: AMPK promotes satellite cell activation during muscle regeneration.
Keywords: AMP-activated kinase (AMPK), myogenesis, regeneration, skeletal muscle, Sonic Hedgehog (SHH), satellite cell
Abstract
Satellite cells are the major myogenic stem cells residing inside skeletal muscle and are indispensable for muscle regeneration. Satellite cells remain largely quiescent but are rapidly activated in response to muscle injury, and the derived myogenic cells then fuse to repair damaged muscle fibers or form new muscle fibers. However, mechanisms eliciting metabolic activation, an inseparable step for satellite cell activation following muscle injury, have not been defined. We found that a noncanonical Sonic Hedgehog (Shh) pathway is rapidly activated in response to muscle injury, which activates AMPK and induces a Warburg-like glycolysis in satellite cells. AMPKα1 is the dominant AMPKα isoform expressed in satellite cells, and AMPKα1 deficiency in satellite cells impairs their activation and myogenic differentiation during muscle regeneration. Drugs activating noncanonical Shh promote proliferation of satellite cells, which is abolished because of satellite cell-specific AMPKα1 knock-out. Taken together, AMPKα1 is a critical mediator linking noncanonical Shh pathway to Warburg-like glycolysis in satellite cells, which is required for satellite activation and muscle regeneration.
Introduction
Skeletal muscle is the main component in animal locomotion system. It is also the major tissue sustaining respiration and the primary peripheral tissue utilizing glucose and fatty acids, important in preventing obesity and type 2 diabetes (1–3). Skeletal muscle fibers are frequently damaged during exercise and because of physical trauma or diseases such as Duchenne muscular dystrophy (4, 5). Efficient regeneration following muscle injury is critical for maintaining the normal physiological function of skeletal muscle. On the other hand, insufficient muscle regeneration replaces muscle fibers with fibrotic tissue and weakens the contractile function of muscle, which is a key etiological factor leading to progressive muscle weakness associated with aging and muscle dystrophic diseases (6–8).
Despite the presence of multiple types of myogenic cells in skeletal muscle, satellite cells are the major postnatal myogenic cells indispensable for muscle regeneration (9). Satellite cells maintain in a quiescent stage and become activated when muscle regeneration process is triggered (10, 11). Activated satellite cells proliferate to expand their population and undergo further myogenic differentiation orchestrated by sequential expression of myogenic regulatory factors, Myf5, MyoD, myogenin, and MRF4 (12).
Recent studies show that stem cells including satellite cells rely on glycolysis to provide energy (13, 14), likely because of their limited access to oxygen because of deep location in the tissue and the need to prevent the damage from reactive oxygen species (15–17). Satellite cells have relatively small cytoplasm and few mitochondria (10, 18), resulting in low metabolic rates. However, metabolism of stem cells rapidly elevates during wakening from their quiescent state, providing energy needed for stem cell proliferation and further differentiation (10). Warburg glycolysis is a primarily source of energy for certain cancer cells, which allows their fast proliferation (19, 20). Cancer cells and stem cells share metabolic similarity, and recently, Warburg-like glycolysis was identified in induced stem cells (21). Moreover, a Warburg-like glycolysis was discovered in differentiated C2C12 myotubes and brown fat (22).
AMP-activated kinase (AMPK)2 is a master regulator of metabolism that has an α catalytic subunit with two isoforms, α1 and α2 (23). Here, we report that AMPKα1 is the dominant isoform in satellite cells, and AMPK α1 mediates Warburg-like glycolysis needed for satellite cell activation following muscle injury. Consequently, satellite cell-specific AMPKα1 KO impairs muscle regeneration, characterized by reduced satellite cell activation and muscle structure restoration.
Experimental Procedures
Mice
All animals were handled in accordance with protocols approved by the Animal Use and Care Committees of Washington State University. Wild-type C57BL/6 mice, B6.129S-Pax7tm1(cre/ERT2)Gaka/J mice (catalog no. 017763) in which tamoxifen-inducible Cre recombinase is driven by the endogenous mouse Pax7 promoter, B6(Cg)-Prkaa1tm1.1Sjm/J mice (catalog no. 014141) in which AMPKα1 exon 3 was flanked by two loxP sites, and Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice (catalog no. 007576) in which a membrane-targeted tdTomato is floxed and EGFP is expressed when cross-bred to Cre recombinase-expressing mice, were obtained from Jackson Laboratory (Bar Harbor, ME). B6.129S-Pax7tm1(cre/ERT2)Gaka/J mice were cross-bred with Prkaa1tm1.1Sjm/J mice to generate tamoxifen-inducible satellite cell-specific AMPKα1 KO mouse strain (Pax7cre/AMPKα1fl/fl). Pax7cre/AMPKα1fl/fl mice were cross-bred with Gt(ROSA)26Sortm4(ACTB-tdTomato,-EGFP)Luo/J mice to generate tamoxifen-inducible satellite cell-specific AMPKα1 KO mouse strain with tamoxifen-inducible satellite cell-specific EGFP expression (Pax7Cre/AMPKα1fl/fl/tdtomato,EGFP).
Antibodies and Chemicals
Antibodies against AMPKα (antibody 2532), and phospho-AMPKα at Thr-172 (antibody 2535), rabbit anti-Ki67 Alexa Fluor 488 (antibody 11882), rabbit anti-PFKFB3 (antibody 13123), rabbit anti-phospho-AMPK substrate motif (antibody 5759), goat anti-mouse Alexa Fluor 555 (antibody 4409), and goat anti-rat Alexa Fluor 488 (antibody 4416) were purchased from Cell Signaling (Danvers, MA). Anti-embryonic myosin heavy chain (F1.652), anti-Pax7 (PAX7), anti-MHC (MF20), and anti-β-tubulin (E7) mouse monoclonal antibodies were obtained from the Developmental Studies Hybridoma Bank (Iowa City, IA). Rabbit anti-AMPKα1 antibody (ABIN737886) and rabbit anti-AMPKα2 (ABIN680458) antibody were obtained from Antibodies-Online Inc. (Atlanta, GA). Mouse anti-MyoD antibody (sc32758), smoothened agonist (SAG), and cyclopamine were purchased from Santa Cruz (Dallas, TX). Rat anti-laminin antibody (4H8-2) was purchased from Enzo (Faringdale, NY). Goat anti-mouse IgG1 Alexa Fluor 555 (A-21127) was purchased from Life Technologies Inc. IRDye 800CW goat anti-rabbit secondary antibody and IRDye 680 goat anti-mouse secondary antibody were purchased from LI-COR Biosciences (Lincoln, NE). Cardiotoxin, tamoxifen and Oil-Red O were purchased from Sigma. Basic FGF2 (233-FB-025) and anti-mouse integrin α7 APC was purchased from R&D Systems (Minneapolis, MN). Anti-mouse CD45 PE-Cy7, anti-mouse CD16/CD32, and flow cytometry buffer were purchased from eBioscience (San Diego, CA). Anti-mouse TER-119 PE-Cy7, anti-mouse Sca-1 APC-Cy7, and anti-mouse CD31 PE-Cy7 antibodies were purchased from BioLegend (San Diego, CA). Gill's hematoxylin (catalog no. 26030-10) and Eosin Y-Phloxine B (catalog no. 26051-21) were purchased from Electron Microscopy Sciences (Hatfield, PA). AICAR was purchased from Toronto Research Chemicals (Toronto, Canada).
Fluorescence-activated Cell Sorting
Tibialis anterior (TA) muscle was digested in DMEM with collagenase D and dispase II as previously described (24). Cells were blocked in anti-mouse CD16/CD32 antibody and then stained with anti-mouse CD45 PE-Cy7, anti-mouse TER119 PE-Cy7, anti-mouse CD31 PE-Cy7, anti-mouse Sca-1 APC-Cy7, and anti-mouse integrin α7 APC antibodies. Stained cells were sorted on FACSaria (BD Biosciences, San Jose, CA) and analyzed by FlowJo (Treestar, Inc., San Carlos, CA). Gates were made based on fluorescence minus one control.
Cell Culture
Satellite cells were resuspended in F-10 medium with 20% FBS, 1% antibiotic mixture and 5 ng/ml FGF2, and seeded on collagen-coated plates. Myogenic differentiation of satellite cells was induced by switching medium to DMEM supplemented with 2% horse serum and 1% antibiotic mixture. Nonmyogenic cells were cultured in DMEM with 10% FBS and 1% antibiotic mixture.
Satellite Cell Proliferation Essay
Satellite cells were isolated as previously described (25) with modifications. Muscle was removed from the hind limbs of 3–4-month-old mice. Muscle was cut into small pieces and digested in digestion buffer containing collagenase D and dispase II for about 30 min. Muscle slurry was passed through a 100-μm cell strainer. Filtrate was centrifuged for 5 min at 350 × g. Cell pellet was resuspended and cultured in F-10 medium with 20% FBS, 5 ng/ml FGF2, and 1% antibiotic mixture on collagen-coated plates. Satellite cells were enriched by preplating. Fast attaching nonmyogenic cells were also collected. Five thousand satellite cells were seeded in each well of 12 well plates. Cells were then trypsinized and counted at 1, 2, and 3 days after to determine the cell proliferation rate.
Single Muscle Fiber Culture
Single muscle fibers were isolated as previously described (26) with modification. The extensor digitorum longus muscle was removed from 1-month-old Pax7Cre/AMPKα1fl/fl/tdtomato,EGFP and Pax7Cre/tdtomato,EGFP mice that had been treated with tamoxifen. Extensor digitorum longus muscle was digested in digestion buffer containing collagenase D. Extensor digitorum longus muscle was then carefully flushed to release single muscle fibers. Intact single muscle fibers were then transferred to 24-well plates with one muscle fiber in each well and cultured in high glucose DMEM with 20% FBS, 5 ng/ml FGF2, 110 mg/ml sodium pyruvate, and 1% antibiotic mixture.
Glucose Uptake Test
Glucose uptake test was performed using glucose uptake cell base assay kit from Cayman (Ann Arbor, MI) following the manufacturer's protocol. The cells were seeded onto 96-well plates at a density of 1 × 104 cells/well. Cells were cultured with fluorescently labeled deoxyglucose analog, and fluorescence was detected using Synergy H1 hybrid reader (BioTek, Winooski, VT).
Real Time Quantitative PCR
Total RNA was extracted using TRIzol (Sigma) followed by DNase (New England BioLabs Inc., Ipswich, MA) treatment, and cDNA was synthesized using a reverse transcription kit (Bio-Rad). Real time PCR was carried out using CFX real time PCR detection system (Bio-Rad) with a SYBR Green real time PCR kit from Bio-Rad. After amplification, a melting curve (0.01 °C/s) was used to confirm product purity, and agarose gel electrophoresis was performed to confirm that only a single product of the right size was amplified. Relative mRNA content was normalized to 18S rRNA content (24). Primer sequences and their respective PCR fragment lengths are listed below. 18S rRNA (110 bp), forward 5′-TGCTGTCCCTGTATGCCTCT-3′ and reverse 5′-TGTAGCCACGCTCGGTCA-3′; Pax7 (115 bp), forward 5′-TTGGGGAACACTCCGCTGTGC-3′ and reverse 5′-CAGGGCTTGGGAAGGGTTGGC-3′; MyoD (100 bp), forward 5′-TCTGGAGCCCTCCTGGCACC-3′ and reverse 5′-CGGGAAGGGGGAGAGTGGGG-3′; Myf5 (125 bp), forward 5′-AAACTCCGGGAGCTCCGCCT-3′ and reverse 5′-GGCAGCCGTCCGTCATGTCC-3′; Myogenin (97 bp), forward 5′-GAGATCCTGCGCAGCGCCAT-3′ and reverse 5′-CCCCGCCTCTGTAGCGGAGA-3′; Smo (121 bp) forward 5′-GGCCTGACTTTCTGCGTTGCACACC-3′ and reverse 5′-GGGTTGTCTGTTCGCACCAAGG-3′; Shh (182 bp) forward 5′-CAGCGGCAGATATGAAGGGAAGA-3′ and reverse 5′-CAGGCCACTGGTTCATCACAGA-3′; Gli1 (188 bp) forward 5′-AGGTCTGCGTGGTAGAGGGAA-3′ and reverse 5′-GTTGGCTTGGTGGCAAAAGGG-3′; Ptch1 (121 bp) forward 5′-GCAAGTTTTTGGTTGTGGGTCTCC-3′ and reverse 5′-TCTCGACTCACTCGTCCACCAA-3′; AMPKα1 (246 bp) forward 5′-TGTCTCTGGAGGAGAGCTATTTGA-3′ and reverse 5′-GGTGAGCCACAGCTTGTTCTT-3′; and AMPKα2 (150 bp) forward 5′-CAGAAGATTCGCAGTTTAGATGTTGT-3′ and reverse 5′-ACCTCCAGACACATATTCCATTACC-3′.
Immunoblotting Analyses
Immunoblotting analysis was performed as previously described using an Odyssey Infrared Imaging System (LI-COR Biosciences) (27). Band density was normalized to β-tubulin content.
Immunocytochemical Staining
Cells grown on multiple well plates were fixed in cold methanol for 10 min, permeabilized with 0.1% Triton X-100 for 5 min, blocked with 1% BSA, and incubated with primary antibodies at 4 °C overnight. Cells were then stained with corresponding secondary antibodies (1:1,000) for 1 h. Images were taken using a EVOS microscope.
Immunohistochemical Staining
TA muscle was fixed in cold 4% paraformaldehyde and frozen in isopentane cooled in liquid nitrogen. Frozen tissue was sectioned (5–10 μm thick). Sections were heated in citrate buffer for 20 min, blocked in 5% goat serum in TBS containing 0.3% Triton X-100, and stained with primary antibodies and corresponding fluorescent secondary antibodies. Sections were then mounted in a mounting medium containing DAPI (Vector Laboratories, Burlingame, CA).
Quantification of Satellite Cells and EMH+ Muscle Fibers
Pax7+ cells with nuclei identified by DAPI staining were classified as satellite cells. For each TA muscle sample, the number of satellite cells and EMH+ muscle fibers on four randomly picked microscopic fields of each of three sections at different depths of the muscle were counted (four fields/section, three sections/muscle). Average numbers obtained from the three examined sections of each muscle sample were used as a biological replicate for comparative analysis.
Hemotoxylin Staining
TA muscle frozen sections were rinsed in PBS, stained with Gill's hemotoxylin, and counterstained with eosin Y following the manufacturer's protocol.
l-Lactate Assay
Ten thousand cells were seeded in each well of 96-well plates. 24 h after seeding, cell culture medium was collected and tested for lactate content using an l-lactate assay kit from Eton Bioscience, Inc. (San Diego, CA) following the manufacturer's instruction.
Oxygen Consumption Assay
200,000 cells were seeded in each well of 6-well plates. One day after seeding, cell culture medium was changed with fresh medium. Oxygen content in medium was measured after 30 min of incubation with Orion 3-Star Pus Dissolved Oxygen Meter (Thermo Scientific, Waltham, MA). Oxygen consumption was calculated from the difference between the oxygen content in medium after 30 min of incubation and the oxygen content in fresh medium.
Statistics
For all studies, at least three independent experiments were conducted. All data are expressed as means ± S.D. The data were analyzed using the general linear model of SAS (SAS Inst. Inc., Cary, NC), and t test or Tukey range test was used to determine significance of differences among means. p < 0.05 was considered significant.
Results
Expression of AMPKα1 and AMPKα2 during Skeletal Muscle Regeneration
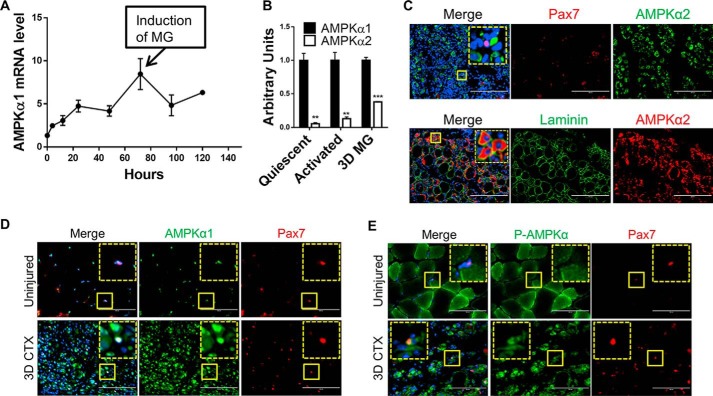
To test the role of AMPKα1 in muscle regeneration, we first measured the expression of AMPKα1 during the proliferation and differentiation of isolated satellite cells. AMPKα1 expression profoundly increased (∼6-fold) during the activation and proliferation of satellite cells. After induction of myogenesis, the expression of AMPKα1 dropped first, followed by a slight increase (Fig. 1A), which is consistent with a previous report showing AMPKα1 activity in muscle increases during muscle regeneration, whereas the activity of AMPKα2 remain unchanged (24, 28). Moreover, AMPKα1 expression was consistently and significantly higher than AMPKα2 in quiescent, activated, and differentiating satellite cells (Fig. 1B). These data support our hypothesis that AMPKα1 is important in activating satellite cell proliferation.
FIGURE 1.
Dominant expression of AMPKα1 in satellite cells. A, AMPKα1 mRNA expression during WT satellite cell proliferation and differentiation by real time PCR. B, AMPKα1 and AMPKα2 mRNA expression in WT quiescent satellite cell isolated from uninjured muscle, WT activated satellite cells isolated from injured muscle, and isolated WT satellite cells at 3 days of myogenic differentiation. C, IHC staining detecting AMPKα2 in regenerating TA muscle from WT mice at 7 days after CTX injection combined with laminin or Pax7 IHC staining. Scale bars, 200 μm. D, IHC staining detecting AMPKα1 in uninjured TA muscle and regenerating TA muscle from WT mice at 3 days after CTX injection combined with Pax7 staining. Scale bars, 100 μm. E, IHC staining detecting p-AMPKα in uninjured TA muscle and regenerating TA muscle from WT mice at 3 days after CTX injection combined with Pax7 staining. Scale bars, 100 μm. MG, myogenic differentiation. The magnified images of areas marked by solid yellow lines are shown in the corners of corresponding pictures outlined by dotted sline. **, p < 0.01; ***, p < 0.0001 versus control; means ± S.D.; n ≥ 3.
To test whether AMPKα1 is critical for muscle regeneration following injury, we analyzed the expression of AMPKα1 and AMPKα2 in TA muscle injured by cardiotoxin (CTX) injection using immunohistochemical (IHC) staining after injury. AMPKα2 was found to be expressed primarily in the cytoplasm of well differentiated myogenic cells and regenerating muscle fibers (Fig. 1C). In contrast, AMPKα1 was found mainly in mononucleated cells including satellite cells (Fig. 1D). Moreover, we found that AMPKα1 was also expressed in satellite cells in uninjured muscle (Fig. 1D). However, p-AMPKα was only detected in satellite cells after injury, indicating AMPKα1 activation in satellite cells during muscle regeneration, which might be involved in satellite cell activation and proliferation (Fig. 1E). These data further suggest that AMPKα1 has a regulatory role in satellite cell activation, whereas AMPKα2 plays a major role in regulating metabolism in differentiated muscle fibers. Therefore, we focused further studies on the role of AMPKα1 in satellite activation and proliferation.
AMPKα1 Knock-out Reduces Satellite Cell Proliferation
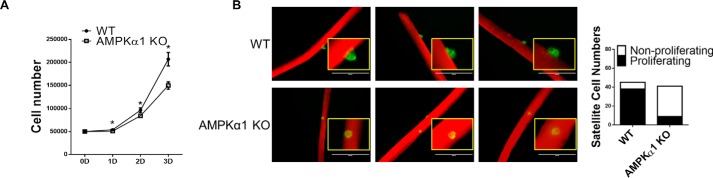
To better understand the influence of AMPKα1 KO on satellite cell activation and proliferation, we then tested the proliferation of satellite cells isolated from tamoxifen-treated Pax7cre/AMPKfl/fl mice in which AMPKα1 gene is specifically deleted in satellite cells. Satellite cell-specific AMPKα1 KO was verified by different assays (Fig. 2, A–E). Indeed, AMPKα1 KO satellite cells proliferated slower than WT myoblasts (Fig. 3A). Because isolated satellite cells lack the niche environment, we further prepared single muscle fibers from tamoxifen-treated Pax7cre/tdomato,EGFP mice and Pax7cre/AMPKα1fl/fl/tdomato,EGFP mice, in which satellite cells express membrane-located EGFP upon tamoxifen treatment accompanied with AMPKα1 KO specifically in satellite cells. 48 h after muscle fiber isolation, satellite cells on muscle fibers were compared. Although 38 of 45 observed WT satellite cells had activated and started to proliferate, only 9 of 41 observed AMPKα1 KO satellite cells showed sign of proliferation, which suggested less efficient satellite cell activation and proliferation because of AMPKα1 KO (Fig. 3B).
FIGURE 2.
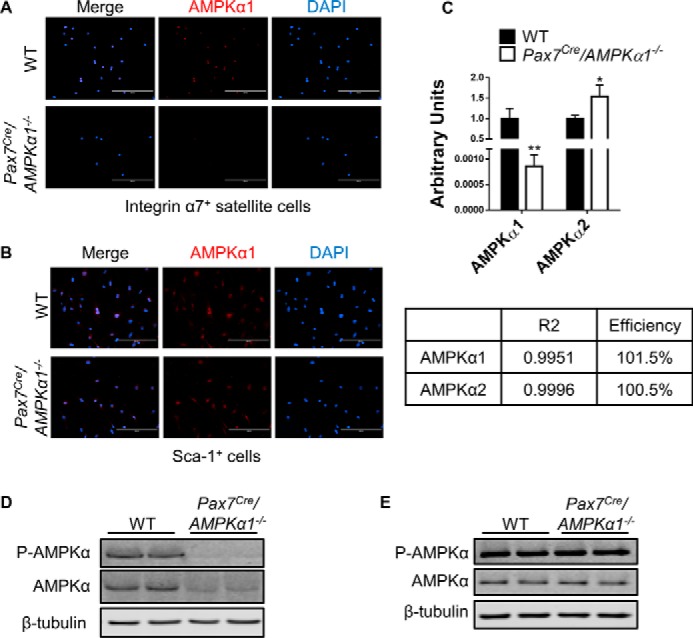
Satellite cell-specific KO of AMPKα1. A and B, ICC staining detecting AMPKα1 in Lin−/Sca-1−/integrin α7+ satellite cells (A) and Lin−/Sca-1+ cells (B) isolated from tamoxifen-treated AMPKα1fl/fl (WT) mice and Pax7cre/AMPKα1fl/fl (Pax7Cre/AMPKα1−/−) mice. C, real time PCR detecting the expression of AMPKα1 and AMPKα2 in satellite cells isolated from WT mice and Pax7Cre/AMPKα1−/− mice. D and E, Western blot detecting the expression of AMPKα1 in nonmyogenic cells (D) and satellite cells (E) isolated from muscle of WT mice and Pax7Cre/AMPKα1−/− mice. *, p < 0.05; **, p < 0.01 versus control; means ± S.D.; n ≥ 3. Scale bars, 200 μm.
FIGURE 3.
AMPKα1 KO satellite cells proliferated slower than WT cells in vitro. A, proliferation of satellite cells isolated from tamoxifen-treated AMPKα1fl/fl (WT) mice and tamoxifen-treated Pax7cre/AMPKα1fl/fl mice (AMPKα1 KO) mice. B, proliferation of satellite cell on single muscle fibers isolated from tamoxifen-treated Pax7Cre/tdtomato,EGFP (WT) and tamoxifen-treated Pax7Cre/AMPKα1fl/fl/tdtomato,EGFP (AMPKα1 KO) mice at 48 h after isolation. *, p < 0.05 versus control; means ± S.D.; n ≥ 3. Scale bars, 200 μm.
Smoothened Agonist Promotes Satellite Proliferation through Activating AMPK
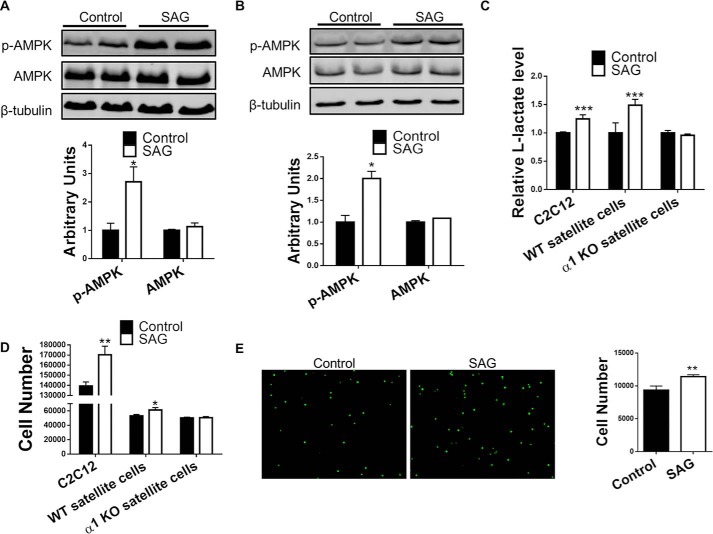
It has been recently reported that noncanonical Sonic Hedgehog (Shh) signaling promotes a Warburg-like glycolysis in differentiated C2C12 myotubes (22). Therefore, we questioned whether this pathway is also present in undifferentiated myoblasts and satellite cells and whether AMPKα1 has a mediatory role in eliciting Warburg-like glycolysis. We first tested the effect of Smoothened (Smo) agonist (SAG), an activator of Shh pathway, on AMPK activity in C2C12 myoblasts and WT satellite cells. C2C12 myoblasts and WT satellite cells were treated with 200 nm SAG for only 10 min to avoid the activation of canonical Shh signaling (22, 29). In both C2C12 cells and WT satellite cells, 10 min of SAG treatment activated AMPK (Fig. 4, A and B). l-Lactate assay revealed increased glycolysis rates in C2C12 cells and WT satellite cells in response to SAG treatment, which was absent in AMPKα1 KO satellite cells (Fig. 4C).
FIGURE 4.
SAG treatment promotes satellite cell activation and proliferation through AMPKα1. A, representative Western blot of experiments testing phosphorylated AMPK (p-AMPK) and total AMPK protein levels in C2C12 with or without 10 min of SAG (200 nm) treatment. B, representative Western blot of experiments testing phosphorylated AMPK (p-AMPK) and total AMPK protein levels in WT satellite cells with or without 10 min of SAG (200 nm) treatment. C, l-lactate assay measure l-lactate in culture medium of C2C12 cells, WT satellite cells, and AMPKα1 (α1) KO satellite cells with or without 24 h of SAG (200 nm) treatment. D, proliferation of C2C12 cells, WT satellite cells, and AMPKα1 (α1) KO satellite cells during 24 h of culture with or without 24 h of SAG (200 nm) treatment. E, quantification of GFP+ satellite cells in SAG (200 nm) treated and untreated total muscle-resident cells from tamoxifen-treated Pax7Cre/tdtomato,EGFP mice 48 h after cell isolation. *, p < 0.05; **, p < 0.01; ***, p < 0.0001 versus control; means ± S.D.; n ≥ 3. Scale bars, 200 μm.
In addition, SAG promoted the proliferation of both C2C12 cells and purified WT satellite cells but failed to promote proliferation of purified AMPKα1 KO satellite cells (Fig. 4D), suggesting that the proliferative effects of SAG treatment on satellite cells require AMPKα1. Skeletal muscle contains multiple cell types that interact with satellite cells and participate in muscle regeneration (30). To better understand the potential effect of SAG treatment on satellite cell activation and proliferation in the presence of other cell types, muscle tissue slurry from tamoxifen-treated Pax7cre/tdomato,EGFP mice expressing EGFP in Pax7+ cells was obtained by enzymatic digestion of muscle tissue and plated without sorting. 48 h later, all cells were harvested, and EGFP-positive satellite cells were quantified. We found that SAG treatment increased the number of EGFP+ satellite cells, further supporting the promotive effect of Shh signaling on satellite activation and proliferation (Fig. 4E).
Selective Activation of noncanonical Shh Promotes Satellite Cell Activation and Proliferation
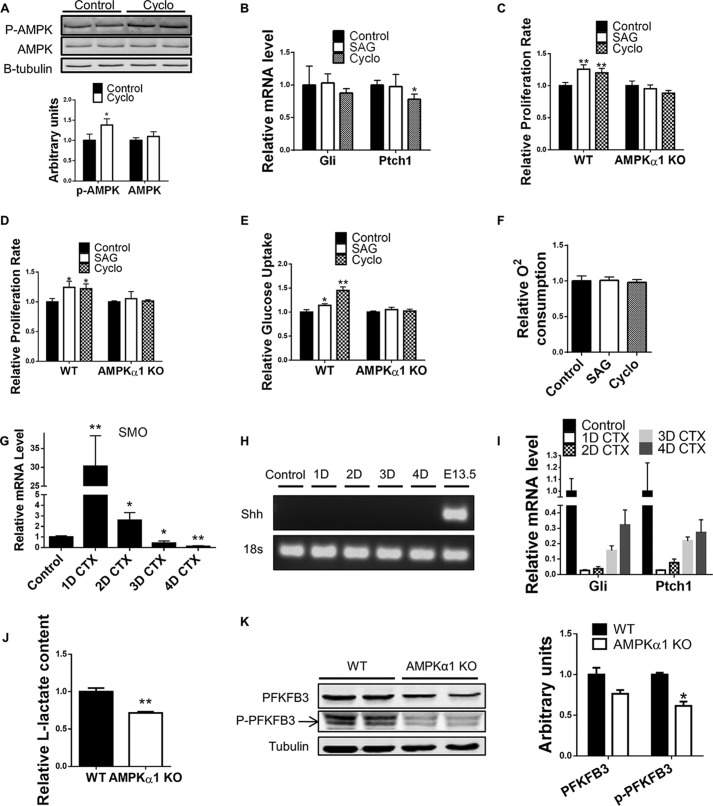
To further test whether the observed effects of SAG on satellite cells was through noncanonical Shh signaling, WT primary myoblasts were treated with cyclopamine, a noncanonical Shh specific activator, which is also known to inhibit canonical Shh (22). 10 min of cyclopamine treatment successfully activated AMPK (Fig. 5A). Considering that noncanonical Shh signaling has relatively rapid response, we chose to only treat cells for 1 h with low doses of cyclopamine or SAG so that the impact of canonical Shh signaling on cell proliferation was minimized. Indeed, 1 h of SAG treatment did not change the expression of the two canonical Shh targets, Gli1 and Ptch1 (31, 32), in primary myoblasts 24 h after treatment (Fig. 5B). Similarly, 1 h of cyclopamine treatment did not change the expression of Gli1 and only caused a slight reduction in Ptch1 expression 24 h after treatment (Fig. 5B). Therefore, fresh muscle tissue slurry from tamoxifen-treated Pax7cre/tdomato,EGFP mice and Pax7cre/AMPKα1fl/fl/tdomato,EGFP mice with satellite cell-specific AMPKα1 KO was treated with SAG and cyclopamine for 1 h in culture medium. Tissue slurry was then collected and cultured in SAG and cyclopamine-free medium. SAG and cycolopamine treatments were able to increase the number of EGFP+ satellite cells from Pax7cre/tdomato,EGFP mice at 48 h after treatment but failed to do so to EGFP+ satellite cells without AMPKα1 (isolated from tamoxifen-treated Pax7cre/AMPKα1fl/fl/tdomato,EGFP mice), indicating an AMPKα1-dependent stimulatory effect of noncanonical Shh on satellite cell activation and proliferation (Fig. 5C). The same treatments were applied to purified satellite cells. We found that 1-h treatment of SAG and cyclopamine increased the number of purified WT myoblasts but not that of AMPKα1 KO myoblasts, indicating a similar AMPKα1-dependent stimulatory effect of noncanonical Shh on satellite cell proliferation (Fig. 5D). Moreover, glucose uptake of WT myoblasts was increased after 20 min of SAG or cyclopamine treatment, but no effect was observed for AMPKα1 KO myoblasts (Fig. 5E). In addition, oxygen consumption was not changed in satellite cells treated with SAG or cyclopamine for 30 min, indicating a specific activation of glycolysis (Fig. 5F).
FIGURE 5.
Noncanonical Shh promotes satellite cell activation and proliferation through AMPKα1. A, representative Western blot of experiments testing phosphorylated AMPK (p-AMPK) and total AMPK protein levels in WT satellite cells with or without 10 min of cyclopamine (200 nm) treatment. B, real time PCR showing Gli mRNA expression and Ptch1 mRNA expression 24 h after 1 h of SAG treatment (200 nm) and cyclopamine treatment (200 nm). C, quantification of GFP+ satellite cells in SAG-treated (200 nm, 1 h), cyclopamine-treated (Cyclo, 200 nm, 1 h), and untreated muscle-resident cells from muscle of tamoxifen-treated Pax7Cre/tdtomato,EGFP (WT) mice and tamoxifen-treated Pax7Cre/AMPKα1fl/fl/tdtomato,EGFP (AMPKα1 KO) mice 48 h after treatment. D, proliferation of purified WT satellite cells, and AMPKα1 KO satellite cells during 24 h culture following 1 h of 200 nm SAG, 1 h of 200 nm cyclopamine, or vehicle only control treatments. E, relative glucose uptake of WT and AMPKα1 KO satellite cells during 20 min of 200 nm SAG treatment, 20 min of 200 nm cyclopamine treatment, or control treatment. F, oxygen consumption of WT satellite cells during 30 min of 200 nm SAG treatment, 30 min of 200 nm cyclopamine treatment, or control treatment. G, real time PCR showing Smo mRNA expression at 1, 2, 3, and 4 days after injury. H, representative image of electrophoresis following real time PCR showing undetected Shh expression in undamaged muscle and in muscle at 1, 2, 3, and 4 days after injury. Day 13.5 mouse embryo was used as a positive control. I, real time PCR showing Gli mRNA expression and Ptch1 mRNA expression in muscle before injury and at 1, 2, 3, and 4 days after injury. J, l-lactate assay measured l-lactate content in culture medium of WT and AMPKα1 KO satellite cells. K, representative Western blot of experiments testing PFKFB3 and phospho-PFKFB3 protein levels in WT and AMPKα1 KO satellite cells. *, p < 0.05; **, p < 0.01 versus control; means ± S.D.; n ≥ 3.
To assess the presence of canonical Shh and noncanonical Shh signaling during muscle regeneration, expression of Shh and Smo during muscle regeneration was analyzed. Surprisingly, a 30-fold increase in Smo expression was seen during the first day after muscle injury, showing profound activation of Shh signaling in the initiation of muscle regeneration, even though the expression gradually decreased in the following days (Fig. 5G). In contrast, no Shh expression was detected, which was not due to technical problems, because a high level of Shh expression was detected in day 13.5 embryo tissue, a positive control (Fig. 5H). In addition, the expression of two canonical Shh targets, Gli1 and Ptch1, dropped dramatically after injury and remained considerably lower afterward, indicating that noncanonical but not canonical Shh signaling was active in the initiation stage of muscle regeneration (Fig. 5I). The decreased Smo expression after 2 days post injury likely marked a completion of satellite cell activation and metabolic switch from glycolysis to oxidative phosphorylation (OXPHOS) during myogenic differentiation; myogenic differentiation requires elevated OXPHOS and noncanonical Shh reduces OXPHOS (22, 33).
Consistently, a reduced glycolytic rate was observed in AMPKα1 KO satellite cells compared with WT satellite cells (Fig. 5J). In addition, we found that 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3 (PFKFB3), an important enzyme in glycolysis which is activated by AMPK through phosphorylation at Ser-461, was also down-regulated in AMPKα1 KO myoblasts (Fig. 5K) (34). PFKFB3 has been reported to regulate satellite cell proliferation (35). Therefore down-regulated PFKFB3 might be another hurdle caused by disrupted Smo-AMPK axis, which hampered glycolysis and proliferation of AMPKα1 KO satellite cells.
Direct Activation of AMPK by AICAR Promoted Satellite Cell Activation
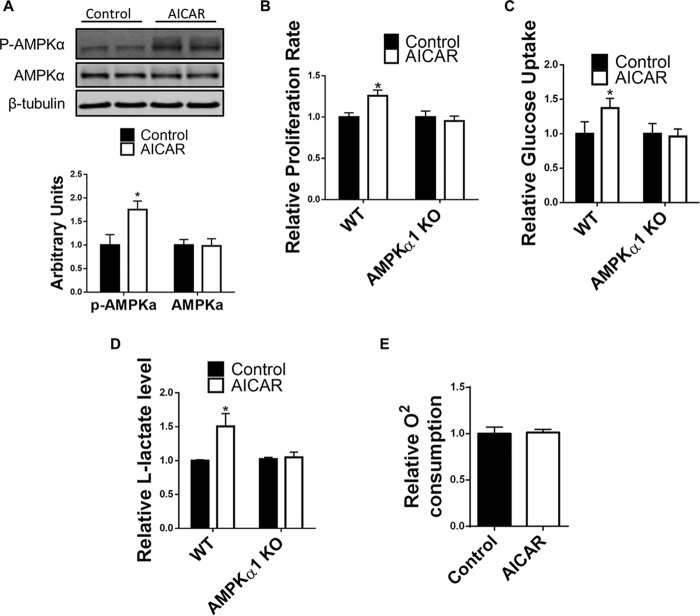
AICAR, a direct activator of AMPK, was used to test the effect of AMPK on satellite activation. 1 h of 250 μm AICAR treatment successfully increased AMPK activity in isolated satellite cells (Fig. 6A). Cells were then isolated from whole muscle tissue of tamoxifen-treated Pax7cre/tdomato,EGFP mice without sorting. 1 h of 250 μm AICAR treatment after cell isolation also increased the number of GFP+ satellite 48 h after AICAR treatment (Fig. 6B). However, the same treatment failed to increase the number of GFP+ satellite cells isolated from tamoxifen-treated Pax7cre/AMPKα1fl/fl/tdomato,EGFP mice, which indicates that direct activation of AMPKα1 promotes satellite cell activation. Moreover, 20 min of AICAR treatment enhanced glucose uptake in WT satellite cells but not in AMPKα1 satellite cells (Fig. 6C). Lactate content in culture medium of WT satellite cells increased by 24-h AICAR treatment, but no difference was found in AMPKα1 KO satellite cells treated with AICAR, indicating an enhanced glycolysis in WT satellite cells mediated by enhanced AMPKα1 activity (Fig. 6D). In addition, oxygen consumption was not altered in satellite cells treated with AICAR, clearly showing a specific effect of AICAR treatment on glycolysis (Fig. 6E).
FIGURE 6.
Activation of AMPKα1 by AICAR promotes satellite cell activation. A, representative Western blot of experiments testing phosphorylated AMPK (p-AMPK) and total AMPK protein levels in WT satellite cells with or without 1 h of AICAR (250 μm) treatment. B, quantification of GFP+ satellite cells in AICAR-treated (250 μm, 1 h) and untreated total muscle-resident cells from muscle of tamoxifen-treated Pax7Cre/tdtomato,EGFP (WT) mice and tamoxifen-treated Pax7Cre/AMPKα1fl/fl/tdtomato,EGFP (AMPKα1 KO) mice 48 h after treatment. C, relative glucose uptake of WT and AMPKα1 KO satellite cells during 20 of min 250 μm AICAR treatment or control treatment. D, l-lactate assay measure l-lactate in the culture medium of WT satellite cells and AMPKα1 KO satellite cells treated with AICAR (250 μm) or PBS for 24 h. E, oxygen consumption of WT satellite cells during 30 min AICAR (250 μm) and control treatments. *, p < 0.05 versus control; means ± S.D.; n ≥ 3.
Satellite Cell-specific KO of AMPKα1 Attenuates Muscle Regeneration
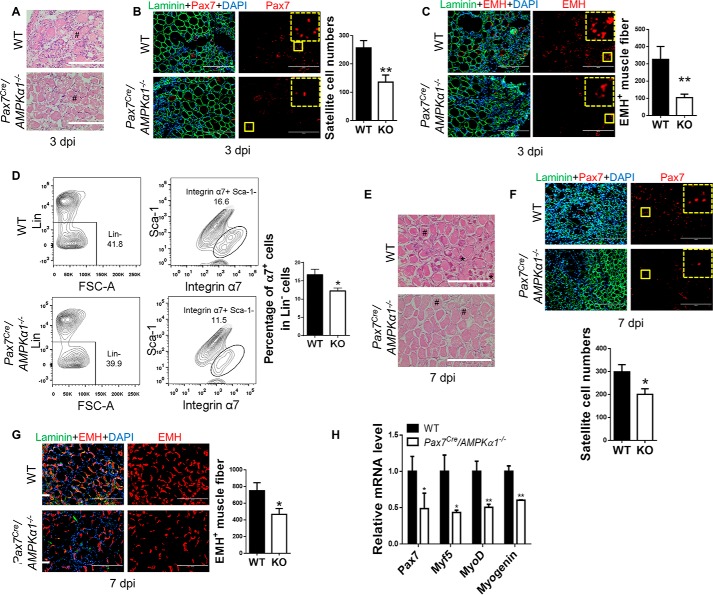
To better understand the role of AMPKα1 in the activation and proliferation of satellite cells during muscle regeneration while avoiding possible confounding effects of AMPKα1 KO on cells other than satellite cells in muscle regeneration, we then employed a tamoxifen-inducible satellite cell-specific AMPKα1 KO mouse model (Pax7cre/AMPKα1fl/fl). CTX was injected to the TA muscle of tamoxifen-treated AMPKα1fl/fl mice and Pax7cre/AMPKα1fl/fl (Pax7Cre/AMPKα1−/−) mice, which showed similar degree of initial muscle damage (Fig. 7A). However, the number of Pax7+ satellite cells and EMH+ muscle fibers was reduced in muscle from Pax7Cre/AMPKα1−/− mice compared with AMPKα1fl/fl mice at 3 days post injury (Fig. 7, B and C). Because there was no difference in satellite cell density before injury, the reduced number of satellite cells and regenerating muscle fibers suggested attenuation of satellite cell activation and proliferation.
FIGURE 7.
Satellite cell-specific AMPKα1 KO attenuated muscle regeneration. Satellite cell-specific AMPKα1 KO was achieved by tamoxifen-injection of Pax7cre/AMPKα1fl/fl mice. TA muscle of tamoxifen-treated AMPKα1fl/fl (WT) mice and Pax7cre/AMPKα1fl/fl (Pax7cre/AMPKα1−/−) mice were injured by CTX injection. A, hemotoxylin staining of TA muscle at 3 days post injury showing a large quantity of necrotic muscle fibers (#). B and C, Pax7+ satellite cells (B) and EMH+ muscle fibers (C) in TA muscle at 3 days post injury and quantification analyzed by IHC staining. D, Lin−/Sca-1−/integrin α7+ satellite cells in the TA muscle at 7 days post injury analyzed by FACS. E, hemotoxylin staining of TA muscle at 7 days post injury showing necrotic muscle fibers (#) and regenerating muscle fibers (*). F and G, Pax7+ satellite cells (F) and EMH+ muscle fibers (G) in TA muscle at 7 days post injury and quantification analyzed by IHC staining. H, Pax7, Myf5, MyoD, and myogenin mRNA levels in muscle at 7 days post injury. dpi, days post injury; KO, Pax7cre/AMPKα1−/−. Magnified images of areas marked by solid yellow lines are shown in the corners of corresponding pictures outlined by dotted lines. *, p < 0.05; **, p < 0.01 versus control; means ± S.D.; n ≥ 3. Scale bars, 200 μm.
In agreement, in FACS sorting, fewer CD45−/TER119−/CD31−(Lin−)Sca-1−/integrin α7+ satellite cells were present in muscle from Pax7Cre/AMPKα1−/− mice at 7 days post injury (11, 36) (Fig. 7D). Similar differences were observed in IHC staining (Fig. 7F). Although many newly formed muscle fibers were present in muscle from AMPKα1fl/fl mice, a smaller number of newly formed muscle fibers were observed in muscle from Pax7Cre/AMPKα1−/− mice (Fig. 7, E and G). Consistently, Pax7, Myf5, MyoD, and myogenin were all expressed lower in muscle from Pax7Cre/AMPKα1−/− mice at 7 days post injury compared with AMPKα1fl/fl mice, indicating a less active muscle regeneration (Fig. 7H).
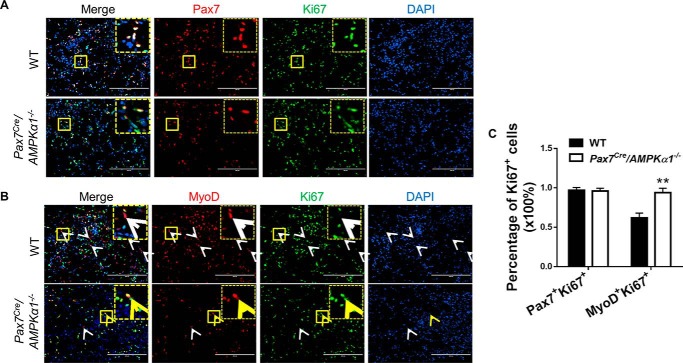
Moreover, at 3 days post injury, Pax7+ satellite cells in both AMPKα1fl/fl mice and Pax7Cre/AMPKα1−/− mice were proliferating as indicated by Ki67 expression (Fig. 8, A and C). However, although some MyoD+ myoblasts in AMPKα1fl/fl mice lost Ki67 expression indicating cell cycle arrest and initiation of myogenic differentiation, most MyoD+ myoblasts in Pax7Cre/AMPKα1−/− mice remained positive for Ki67, indicating a delayed myogenic differentiation and a prolonged duration for proliferation (Fig. 8, B and C). Such a change was likely needed for compensating the impaired capacity of AMPKα1 KO myoblasts to proliferate. These data clearly demonstrate that AMPKα1 KO negatively affects satellite cell proliferation.
FIGURE 8.
Reduced myogenic differentiation in satellite cells with AMPKα1 KO during muscle regeneration. A, IHC co-stained Pax7+ and Ki67+ cells in muscle of WT and Pax7cre/AMPKα1−/− mice at 3 days after injury. B, IHC co-stained MyoD+ and Ki67+ cells in muscle of WT and Pax7cre/AMPKα1−/− mice at 3 days after injury. C, quantification of Pax7+/Ki67+ cells and MyoD+/Ki67+ cells in the muscle of WT and Pax7Cre/AMPKα1−/− mice at 3 days after injury. White arrowhead, MyoD+Ki67− cells; yellow arrowhead, MyoD+Ki67+ cells. Magnified images of area marked by solid yellow lines are shown in the corners of corresponding pictures outlined by dotted lines. **, p < 0.01 versus control; means ± S.D.; n ≥ 3. Scale bars, 200 μm.
Discussion
Recent studies suggest the importance of metabolic transition in regulating the quiescence, activation, proliferation, and differentiation of stem cells (14, 15, 37). OXPHOS and glycolysis are two major metabolic pathways generating energy in mammalian cells (38). OXPHOS is more efficient in generating energy than glycolysis. However, OXPHOS requires ample oxygen supply and functional mitochondria to be accomplished, whereas glycolysis does not require oxygen and mainly occurs in cytoplasm (39). Many stem cells reside deep in tissue where access to oxygen is very limited (16, 17). Moreover, most quiescent stem cells are relatively small with few immature mitochondria, indicating that glycolysis is a suitable metabolic pathway to supply energy (40–42). In addition, glycolysis also provides intermediates needed for the synthesis of cellular components for stem cell self-renewal and proliferation such as nucleotides and lipids (43). On the other hand, overactive OXPHOS exposes stem cells to reactive oxygen species and in consequence increases the risk of DNA damage, inducing mutagenesis and impairing their stemness (15). Because of these advantages of glycolysis, stem cells mainly relay on glycolysis for energy generation (13, 14). However, the relatively inefficient energy generation of glycolysis poses a challenge for stem cells to sustain multiple energy demanding processes associated with stem cell activation and proliferation.
Warburg glycolysis was first discovered in cancer cells (19, 20). It is characterized by enhanced glycolysis even when oxygen is available. Because of the inefficient energy generation of glycolysis, Warburg glycolysis is associated with dramatically increased glucose uptake, which provides enough fuel to glycolysis to generate ample energy required for rapid and uncontrolled cell proliferation of cancer cells (44, 45). Cancer cells and stem cells share similar characteristics including the ability to rapidly proliferate. Recently, a few studies reported that Warburg-like glycolysis is present in different types of stem cells and proliferating nontumor cells to support their energy demand during proliferation (21, 46, 47).
Satellite cells are adult stem cells residing in skeletal muscle (48, 49). They stay in a quiescent state with low metabolic rate under normal conditions (10). However, their activation is rapidly triggered by muscle injury, followed by their proliferation and differentiation to mediate muscle regeneration (50). During the initial stage of muscle regeneration, satellite cells involve many rounds of cell proliferation before differentiation (50). Similar to other stem cells, quiescent satellite cells have immature mitochondria and a very low metabolic rate, and glycolysis supplies most energy for satellite cells (10, 18). Robust mitochondria biogenesis and OXPHOS only start when myogenic differentiation is initiated (10, 51). Therefore, there is a significant demand of energy to support the large biomass formation during satellite cell proliferation before myogenic differentiation. It was recently reported that activation and proliferation of satellite cells are associated with increased glycolytic metabolism (52). However, it remained unknown how satellite cells regulate their metabolism to support these processes. Although it has been shown that AMPK promotes Warburg-like glycolysis in C2C12 myotubes, AMPK was shown to suppress Warburg-like glycolysis in certain cancer cells, suggesting the roles of AMPK in regulating metabolism might vary depending on cell types (22, 53). Canonical Shh signaling is a well known signaling pathway promoting myogenic cell proliferation during prenatal myogenesis (54–56). Our study revealed the critical role of a distinct noncanonical Shh signaling in muscle regeneration where it promotes satellite cell activation and proliferation during the initiation of muscle regeneration through enhancing glycolysis in satellite cells, a process mediated by AMPKα1.
In summary, for the first time, to our knowledge, we showed that AMPKα1 is important in muscle regeneration through mediating noncanonical Shh-triggered Warburg-like glycolysis in satellite cells, which is required for satellite activation and proliferation during muscle regeneration (Fig. 9). Because AMPK activity is attenuated because of a number of pathophysiological conditions such as obesity and type 2 diabetes, and drugs targeting AMPK are widely available as anti-diabetic drugs, our data suggest the possibility of applying these drugs to activate AMPK to facilitate muscle regeneration.
FIGURE 9.
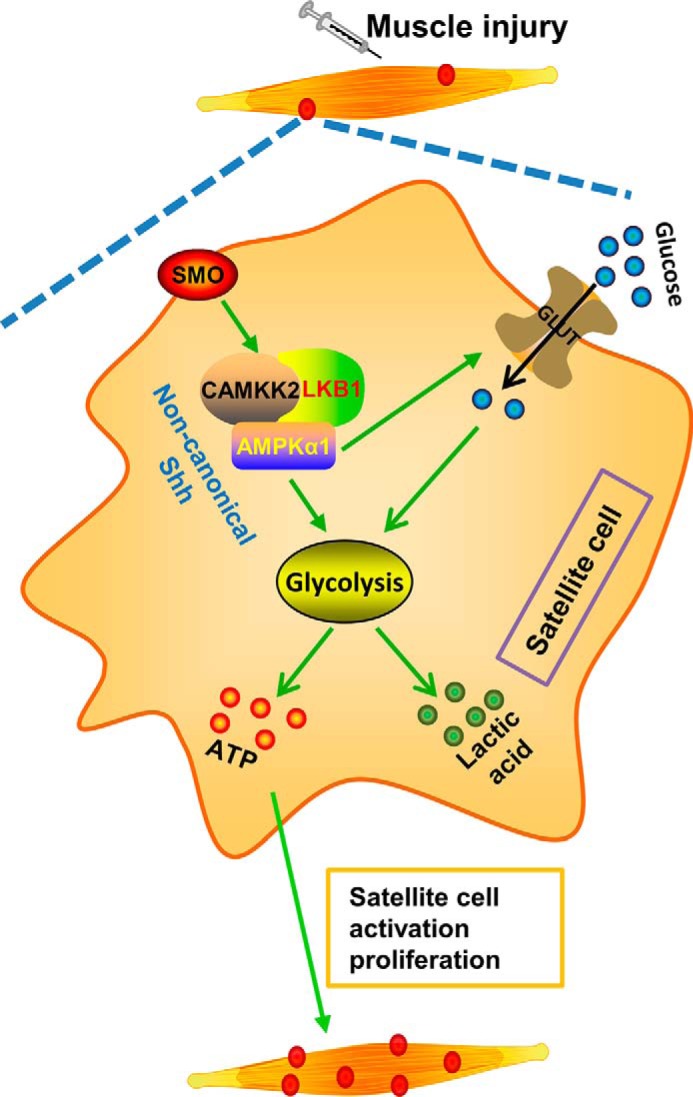
Schematic diagram shows the proposed mechanism for enhanced satellite cell activation and proliferation by noncanonical Shh via AMPKα1 during muscle regeneration. Muscle injury triggers noncanonical Shh pathway in satellite cells, which activates AMPKα1 and sequentially promotes a Warburg-like glycolysis supporting satellite cell activation and proliferation. When AMPKα1 is absent in satellite cells, the proposed pathway is disrupted, resulting in reduced satellite cell activation and proliferation and the attenuated muscle regeneration.
Author Contributions
X. F. and M. D. designed experiments. X. F. performed experiments and analyzed data. X. F. and M. D. interpreted the results of experiments. X. F. prepared the figures. X. F. and M. D. drafted the manuscript. M. Z. and M. V. D. provided critical tools for experiments. All authors reviewed the results and approved the final version of the manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant 1R01HD067449. The authors declare that they have no conflicts of interest with the contents of this article.
- AMPK
- AMP-activated protein kinase
- OXPHOS
- oxidative phosphorylation
- PFKFB3
- 6-phosphofructo-2-kinase/fructose-2,6-biphosphatase 3
- SAG
- smoothened agonist
- TA
- tibialis anterior
- CTX
- cardiotoxin
- IHC
- immunohistochemical
- AICAR
- 5-aminoimidazole-4-carboxamide ribonucleotide.
References
- 1.Schenk S., and Horowitz J. F. (2007) Acute exercise increases triglyceride synthesis in skeletal muscle and prevents fatty acid-induced insulin resistance. J. Clin. Investig. 117, 1690–1698 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Youn J. Y., and Cai H. (2015) Fueling up skeletal muscle to reduce obesity: a TrkB story. Chem. Biol. 22, 311–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kennedy J. W., Hirshman M. F., Gervino E. V., Ocel J. V., Forse R. A., Hoenig S. J., Aronson D., Goodyear L. J., and Horton E. S. (1999) Acute exercise induces GLUT4 translocation in skeletal muscle of normal human subjects and subjects with type 2 diabetes. Diabetes 48, 1192–1197 [DOI] [PubMed] [Google Scholar]
- 4.Irintchev A., and Wernig A. (1987) Muscle damage and repair in voluntarily running mice: strain and muscle differences. Cell Tissue Res. 249, 509–521 [DOI] [PubMed] [Google Scholar]
- 5.Webster C., Silberstein L., Hays A. P., and Blau H. M. (1988) Fast muscle fibers are preferentially affected in Duchenne muscular dystrophy. Cell 52, 503–513 [DOI] [PubMed] [Google Scholar]
- 6.Uezumi A., Fukada S., Yamamoto N., Takeda S., and Tsuchida K. (2010) Mesenchymal progenitors distinct from satellite cells contribute to ectopic fat cell formation in skeletal muscle. Nat. Cell Biol. 12, 143–152 [DOI] [PubMed] [Google Scholar]
- 7.Bernasconi P., Torchiana E., Confalonieri P., Brugnoni R., Barresi R., Mora M., Cornelio F., Morandi L., and Mantegazza R. (1995) Expression of transforming growth factor-β1 in dystrophic patient muscles correlates with fibrosis: pathogenetic role of a fibrogenic cytokine. J. Clin. Invest. 96, 1137–1144 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Li H., Malhotra S., and Kumar A. (2008) Nuclear factor-κB signaling in skeletal muscle atrophy. J. Mol. Med. 86, 1113–1126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sambasivan R., Yao R., Kissenpfennig A., Van Wittenberghe L., Paldi A., Gayraud-Morel B., Guenou H., Malissen B., Tajbakhsh S., and Galy A. (2011) Pax7-expressing satellite cells are indispensable for adult skeletal muscle regeneration. Development 138, 3647–3656 [DOI] [PubMed] [Google Scholar]
- 10.Montarras D., L'Honoré A., and Buckingham M. (2013) Lying low but ready for action: the quiescent muscle satellite cell. FEBS J. 280, 4036–4050 [DOI] [PubMed] [Google Scholar]
- 11.Kuang S., Kuroda K., Le Grand F., and Rudnicki M. A. (2007) Asymmetric self-renewal and commitment of satellite stem cells in muscle. Cell 129, 999–1010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sabourin L. A., and Rudnicki M. A. (2000) The molecular regulation of myogenesis. Clin. Genet. 57, 16–25 [DOI] [PubMed] [Google Scholar]
- 13.Kondoh H., Lleonart M. E., Nakashima Y., Yokode M., Tanaka M., Bernard D., Gil J., and Beach D. (2007) A high glycolytic flux supports the proliferative potential of murine embryonic stem cells. Antioxid. Redox Signal. 9, 293–299 [DOI] [PubMed] [Google Scholar]
- 14.Folmes C. D., Dzeja P. P., Nelson T. J., and Terzic A. (2012) Metabolic plasticity in stem cell homeostasis and differentiation. Cell Stem Cell 11, 596–606 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Ochocki J. D., and Simon M. C. (2013) Nutrient-sensing pathways and metabolic regulation in stem cells. J. Cell Biol. 203, 23–33 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Eliasson P., and Jönsson J. I. (2010) The hematopoietic stem cell niche: low in oxygen but a nice place to be. J. Cell Physiol. 222, 17–22 [DOI] [PubMed] [Google Scholar]
- 17.Clarke L., and van der Kooy D. (2009) Low oxygen enhances primitive and definitive neural stem cell colony formation by inhibiting distinct cell death pathways. Stem Cells 27, 1879–1886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Bracha A. L., Ramanathan A., Huang S., Ingber D. E., and Schreiber S. L. (2010) Carbon metabolism-mediated myogenic differentiation. Nat. Chem. Biol. 6, 202–204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Warburg O., Posener K., and Negelein E. (1924) Metabolism of carcinoma cells. Biochemische Zeitschrift 152, 319–344 [Google Scholar]
- 20.Warburg O. (1956) On the origin of cancer cells. Science 123, 309–314 [DOI] [PubMed] [Google Scholar]
- 21.Vazquez-Martin A., Corominas-Faja B., Cufi S., Vellon L., Oliveras-Ferraros C., Menendez O. J., Joven J., Lupu R., and Menendez J. A. (2013) The mitochondrial H+-ATP synthase and the lipogenic switch: new core components of metabolic reprogramming in induced pluripotent stem (iPS) cells. Cell Cycle 12, 207–218 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Teperino R., Amann S., Bayer M., McGee S. L., Loipetzberger A., Connor T., Jaeger C., Kammerer B., Winter L., Wiche G., Dalgaard K., Selvaraj M., Gaster M., Lee-Young R. S., Febbraio M. A., Knauf C., Cani P. D., Aberger F., Penninger J. M., Pospisilik J. A., and Esterbauer H. (2012) Hedgehog partial agonism drives Warburg-like metabolism in muscle and brown fat. Cell 151, 414–426 [DOI] [PubMed] [Google Scholar]
- 23.Zhang B. B., Zhou G., and Li C. (2009) AMPK: an emerging drug target for diabetes and the metabolic syndrome. Cell Metab. 9, 407–416 [DOI] [PubMed] [Google Scholar]
- 24.Fu X., Zhao J. X., Zhu M. J., Foretz M., Viollet B., Dodson M. V., and Du M. (2013) AMP-activated protein kinase alpha1 but not alpha2 catalytic subunit potentiates myogenin expression and myogenesis. Mol. Cell Biol. 33, 4517–4525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rando T. A., and Blau H. M. (1994) Primary mouse myoblast purification, characterization, and transplantation for cell-mediated gene therapy. J. Cell Biol. 125, 1275–1287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Pasut A., Jones A. E., and Rudnicki M. A. (2013) Isolation and culture of individual myofibers and their satellite cells from adult skeletal muscle. J. Vis. Exp. 73, e50074. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Zhao J. X., Yue W. F., Zhu M. J., and Du M. (2011) AMP-activated protein kinase regulates β-catenin transcription via histone deacetylase 5. J. Biol. Chem. 286, 16426–16434 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mounier R., Théret M., Arnold L., Cuvellier S., Bultot L., Göransson O., Sanz N., Ferry A., Sakamoto K., Foretz M., Viollet B., and Chazaud B. (2013) AMPKα1 regulates macrophage skewing at the time of resolution of inflammation during skeletal muscle regeneration. Cell Metab. 18, 251–264 [DOI] [PubMed] [Google Scholar]
- 29.Chen J. K., Taipale J., Young K. E., Maiti T., and Beachy P. A. (2002) Small molecule modulation of Smoothened activity. Proc. Natl. Acad. Sci. U.S.A. 99, 14071–14076 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Joe A. W., Yi L., Natarajan A., Le Grand F., So L., Wang J., Rudnicki M. A., and Rossi F. M. (2010) Muscle injury activates resident fibro/adipogenic progenitors that facilitate myogenesis. Nat. Cell Biol. 12, 153–163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Ingham P. W., and McMahon A. P. (2001) Hedgehog signaling in animal development: paradigms and principles. Genes Dev. 15, 3059–3087 [DOI] [PubMed] [Google Scholar]
- 32.Nybakken K., and Perrimon N. (2002) Hedgehog signal transduction: recent findings. Curr. Opin. Genet. Dev. 12, 503–511 [DOI] [PubMed] [Google Scholar]
- 33.Ryall J. G. (2013) Metabolic reprogramming as a novel regulator of skeletal muscle development and regeneration. FEBS J 280, 4004–4013 [DOI] [PubMed] [Google Scholar]
- 34.Marsin A. S., Bouzin C., Bertrand L., and Hue L. (2002) The stimulation of glycolysis by hypoxia in activated monocytes is mediated by AMP-activated protein kinase and inducible 6-phosphofructo-2-kinase. J. Biol. Chem. 277, 30778–30783 [DOI] [PubMed] [Google Scholar]
- 35.Repele A., Lupi R., Eaton S., Urbani L., De Coppi P., and Campanella M. (2013) Cell metabolism sets the differences between subpopulations of satellite cells (SCs). BMC Cell Biol. 14, 24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blanco-Bose W. E., Yao C.-C., Kramer R. H., and Blau H. M. (2001) Purification of mouse primary myoblasts based on α7 integrin expression. Exp. Cell Res. 265, 212–220 [DOI] [PubMed] [Google Scholar]
- 37.Varum S., Rodrigues A. S., Moura M. B., Momcilovic O., Easley C. A. 4th, Ramalho-Santos J., Van Houten B., and Schatten G. (2011) Energy metabolism in human pluripotent stem cells and their differentiated counterparts. PLoS One 6, e20914. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Wu M., Neilson A., Swift A. L., Moran R., Tamagnine J., Parslow D., Armistead S., Lemire K., Orrell J., Teich J., Chomicz S., and Ferrick D. A. (2007) Multiparameter metabolic analysis reveals a close link between attenuated mitochondrial bioenergetic function and enhanced glycolysis dependency in human tumor cells. Am. J. Physiol. Cell Physiol. 292, C125–C136 [DOI] [PubMed] [Google Scholar]
- 39.Jose C., Bellance N., and Rossignol R. (2011) Choosing between glycolysis and oxidative phosphorylation: a tumor's dilemma? Biochim. Biophys. Acta 1807, 552–561 [DOI] [PubMed] [Google Scholar]
- 40.Chen C. T., Shih Y. R., Kuo T. K., Lee O. K., and Wei Y. H. (2008) Coordinated changes of mitochondrial biogenesis and antioxidant enzymes during osteogenic differentiation of human mesenchymal stem cells. Stem Cells 26, 960–968 [DOI] [PubMed] [Google Scholar]
- 41.Chung S., Dzeja P. P., Faustino R. S., Perez-Terzic C., Behfar A., and Terzic A. (2007) Mitochondrial oxidative metabolism is required for the cardiac differentiation of stem cells. Nat. Clin. Pract. Cardiovasc. Med. 4, S60–S67 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Armstrong L., Tilgner K., Saretzki G., Atkinson S. P., Stojkovic M., Moreno R., Przyborski S., and Lako M. (2010) Human induced pluripotent stem cell lines show stress defense mechanisms and mitochondrial regulation similar to those of human embryonic stem cells. Stem Cells 28, 661–673 [DOI] [PubMed] [Google Scholar]
- 43.Lunt S. Y., and Vander Heiden M. G. (2011) Aerobic glycolysis: meeting the metabolic requirements of cell proliferation. Annu. Rev. Cell Dev. Biol. 27, 441–464 [DOI] [PubMed] [Google Scholar]
- 44.Semenza G. L. (2007) HIF-1 mediates the Warburg effect in clear cell renal carcinoma. J. Bioenerg. Biomembr. 39, 231–234 [DOI] [PubMed] [Google Scholar]
- 45.Macheda M. L., Rogers S., and Best J. D. (2005) Molecular and cellular regulation of glucose transporter (GLUT) proteins in cancer. J. Cell Physiol. 202, 654–662 [DOI] [PubMed] [Google Scholar]
- 46.Wang T., Marquardt C., and Foker J. (1976) Aerobic glycolysis during lymphocyte proliferation. Nature 261, 702–705 [DOI] [PubMed] [Google Scholar]
- 47.Brand K. A., and Hermfisse U. (1997) Aerobic glycolysis by proliferating cells: a protective strategy against reactive oxygen species. FASEB J. 11, 388–395 [DOI] [PubMed] [Google Scholar]
- 48.Mauro A. (1961) Satellite cell of skeletal muscle fibers. J. Biophys. Biochem. Cytol. 9, 493–495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Katz B. (1961) The terminations of the afferent nerve fibre in the muscle spindle of the frog. Philos. Trans. R. Soc. London B Biol. Sci. 243, 221–240 [Google Scholar]
- 50.Collins C. A., Olsen I., Zammit P. S., Heslop L., Petrie A., Partridge T. A., and Morgan J. E. (2005) Stem cell function, self-renewal, and behavioral heterogeneity of cells from the adult muscle satellite cell niche. Cell 122, 289–301 [DOI] [PubMed] [Google Scholar]
- 51.Wagatsuma A., and Sakuma K. (2013) Mitochondria as a potential regulator of myogenesis. ScientificWorldJournal 2013, 593267. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ryall J. G., Dell'Orso S., Derfoul A., Juan A., Zare H., Feng X., Clermont D., Koulnis M., Gutierrez-Cruz G., Fulco M., and Sartorelli V. (2015) The NAD+-dependent SIRT1 deacetylase translates a metabolic switch into regulatory epigenetics in skeletal muscle stem cells. Cell Stem Cell 16, 171–183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Faubert B., Boily G., Izreig S., Griss T., Samborska B., Dong Z., Dupuy F., Chambers C., Fuerth B. J., Viollet B., Mamer O. A., Avizonis D., DeBerardinis R. J., Siegel P. M., and Jones R. G. (2013) AMPK is a negative regulator of the Warburg effect and suppresses tumor growth in vivo. Cell Metab. 17, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Duprez D., Fournier-Thibault C., and Le Douarin N. (1998) Sonic Hedgehog induces proliferation of committed skeletal muscle cells in the chick limb. Development 125, 495–505 [DOI] [PubMed] [Google Scholar]
- 55.Marcelle C., Ahlgren S., and Bronner-Fraser M. (1999) In vivo regulation of somite differentiation and proliferation by Sonic Hedgehog. Dev. Biol. 214, 277–287 [DOI] [PubMed] [Google Scholar]
- 56.Krüger M., Mennerich D., Fees S., Schäfer R., Mundlos S., and Braun T. (2001) Sonic Hedgehog is a survival factor for hypaxial muscles during mouse development. Development 128, 743–752 [DOI] [PubMed] [Google Scholar]