Background: ThiJ/DJ-1/PfpI protein family members possess glutathione-independent glyoxalase activity.
Results: S. cerevisiae Hsp31, an efficient glyoxalase among all paralogs is essential for cytoprotection under oxidative stress conditions.
Conclusion: The robust glyoxalase activity of Hsp31 aids in the maintenance of glutathione levels, and its mitochondrial localization provides protection against oxidative stress.
Significance: This is the first demonstration of the mechanisms of ThiJ/DJ-1/PfpI family in combating oxidative stress in yeast.
Keywords: heat shock protein (HSP), mitochondria, mitochondrial transport, molecular chaperone, oxidative stress, Parkinson disease, protein folding, yeast genetics
Abstract
Methylglyoxal (MG) is a reactive metabolic intermediate generated during various cellular biochemical reactions, including glycolysis. The accumulation of MG indiscriminately modifies proteins, including important cellular antioxidant machinery, leading to severe oxidative stress, which is implicated in multiple neurodegenerative disorders, aging, and cardiac disorders. Although cells possess efficient glyoxalase systems for detoxification, their functions are largely dependent on the glutathione cofactor, the availability of which is self-limiting under oxidative stress. Thus, higher organisms require alternate modes of reducing the MG-mediated toxicity and maintaining redox balance. In this report, we demonstrate that Hsp31 protein, a member of the ThiJ/DJ-1/PfpI family in Saccharomyces cerevisiae, plays an indispensable role in regulating redox homeostasis. Our results show that Hsp31 possesses robust glutathione-independent methylglyoxalase activity and suppresses MG-mediated toxicity and ROS levels as compared with another paralog, Hsp34. On the other hand, glyoxalase-defective mutants of Hsp31 were found highly compromised in regulating the ROS levels. Additionally, Hsp31 maintains cellular glutathione and NADPH levels, thus conferring protection against oxidative stress, and Hsp31 relocalizes to mitochondria to provide cytoprotection to the organelle under oxidative stress conditions. Importantly, human DJ-1, which is implicated in the familial form of Parkinson disease, complements the function of Hsp31 by suppressing methylglyoxal and oxidative stress, thus signifying the importance of these proteins in the maintenance of ROS homeostasis across phylogeny.
Introduction
The key metabolic pathways are highly conserved among all eukaryotic organisms that utilize both glycolytic and mitochondrial pathways for normal cellular functions (1). Although these metabolic pathways are known to be tightly regulated, there is increasing evidence that many metabolites have detrimental effects upon accumulation within the cell (2, 3). Among those, glyoxal and methylglyoxal (MG)4 are potent α-ketoaldehydes that are primarily formed as by-products of glucose metabolism or “glycolysis” in all living organisms (4–6). The other sources of glyoxal generation are from the enzymatic conversion of intermediates during fatty acid and amino acid metabolism (7), collectively referred to as reactive carbonyl species. The reactive carbonyl groups of these glyoxals react indiscriminately with the arginine and lysine residues of proteins, leading to the formation of advanced glycation end-products (8–10). As a result of nucleophilic addition, glyoxals irreversibly modify proteins, including various cellular antioxidant enzymes such as glutathione reductase, glutathione peroxidase, and catalase, leading to significant risks of oxidative stress (11). The toxicity of these reactive aldehydes is limited not only to the proteome but also extends to lipids and nucleic acids (12, 13). Organisms possess appropriately evolved detoxifying pathways to neutralize the toxic effects of these harmful metabolites. The detoxification of glyoxals is achieved by two independent mechanisms: first by methylglyoxal reductase enzyme, where MG is converted into d-lactic acid (14); and second, by the glyoxalase systems, comprising two glutathione-dependent enzymes (glyoxalase I and II) that efficiently convert MG into d-lactic acid (15).
The accumulation of advanced glycation end-products and higher levels of glyoxals generates an elevation in the reactive oxygen species (ROS) leading to acute oxidative stress conditions. Although ROS is known to be harmful to cellular constituents, low levels of ROS act as signaling molecules in various pathways, including cell development and division (16, 17). Mitochondria are important centers of ROS generation through the complexes of the respiratory electron transport chain (18). In addition, other pro-oxidants such as NADPH oxidase, xanthine oxidase, glucose oxidase, and lipoxygenase are involved in redox reactions contributing to the elevation of ROS in the cell (18–20). In a healthy cellular milieu, the ROS levels are stringently regulated by the action of various enzymatic or non-enzymatic antioxidant systems, including catalase, superoxide dismutase, glutathione, and thioredoxins (21–23). Imbalance in the ROS homeostasis generates oxidative stress resulting in damage to cellular macromolecules like proteins, lipids, and nucleic acids, which cause aberrations in cellular functions (16, 17, 24). Additionally, acute oxidative stress also causes organellar damage leading to organellar dysfunction (25, 26). Importantly, mitochondrial dysfunction is one of the prominent cellular etiologies associated with premature aging, cardiovascular and retinal disorders, atherosclerosis, and several neurodegenerative diseases such as Alzheimer, Parkinson, and Huntington diseases (27–30).
Parkinson disease (PD), the second most prevalent age-related neurodegenerative disorder, is characterized by progressive loss of dopaminergic neurons in the substantia nigra pars compacta (31). Although PD is predominantly sporadic in nature, mutations in at least five gene loci are implicated in the development of familial PD (32, 33). These include SNCA (encoding α-synuclein), PARK7 (encoding DJ-1), PARK2 (encoding parkin), PINK1 (encoding Pink1), and LRRK2 (encoding leucine-rich repeat kinase 2) (31, 32, 34–36). Among these genes, DJ-1 belongs to the ThiJ/DJ-1/PfpI superfamily of proteins involved in a plethora of functions such as transcriptional regulation, mitochondrial complex stabilization, and RNA binding (37–41). Additionally, DJ-1 has been shown recently to function as an oxidative stress sensor, and its absence leads to increased risk of oxidative stress in dopaminergic neurons of the substantia nigra (42). The homologs of DJ-1 are reported across other species, including Drosophila and Caenorhabditis elegans (43, 44). In contrast to humans, Drosophila has two homologs, namely DJ-1α and DJ-1β (44). Mutations in both, or in DJ-1β alone, cause a Parkinsonian-like syndrome in Drosophila, characterized by increased sensitivity to oxidative stress and decreased mobility (45, 46). The members of ThiJ/DJ-1/PfpI superfamily are also reported in lower eukaryotes, including bacterial species. In Escherichia coli, it is referred to as Hsp31 and is shown to perform multiple functions, including holdase chaperone activity, acid resistance, and aminopeptidase activity of broad specificity (47–49). On the other hand, in yeast, there are four homologs (Hsp31, Hsp32, Hsp33, and Hsp34) belonging to the ThiJ/DJ-1/PfpI family, also classified as the Hsp31 mini-family, for which the functional diversity is poorly understood. Some recent studies indicate that yeast Hsp31 proteins are required against extraneous stress and during the diauxic shift for survival under stationary phase conditions (50–52).
Although human DJ-1 is the most extensively studied protein in this superfamily in connection with PD pathology, the functional significance of Hsp31 and its paralogs in yeast is still elusive. In the current study, we have demonstrated the importance of Hsp31 mini-family proteins in protecting cells against oxidative stress induced by MG. Hsp31 exhibits robust GSH-independent glyoxalase activity, and it is critical for glyoxal detoxification as well as suppression of ROS levels. Importantly, human DJ-1 complements the growth of yeast under oxidative and glyoxal stress conditions, signifying its functional conservation across species. Mechanistically, our findings show that Hsp31 maintains GSH and NADPH homeostasis thereby protecting mitochondrial integrity. In summary, our report highlights the specific importance of yeast Hsp31 mini-family proteins in the maintenance of redox homeostasis by modulating cellular antioxidant levels.
Experimental Procedures
Plasmid Construction, Yeast Strains, and Genetic Analysis
The haploid yeast strain BY4741 (MATa his3Δ1 leu2Δ0 met15Δ0 ura3Δ0) used in this study was obtained from Open Biosystems. Haploid Δhsp31 strain was generated by homologous recombination of the corresponding PCR product containing a kanMX4 cassette and flanking sequences of the HSP31 loci. The amplification was performed using pFA6-KanMX4 vector as a template and P1 and P2 primers, respectively (Table 1). To select for positive colonies, transformants were plated on YPD (yeast extract-peptone-dextrose) medium containing G418 sulfate (400 μg/ml), and the deletion was further verified by PCR using specific primers (P3 and P4). Δhsp32 and Δhsp33 strains were generated by homologous recombination of the corresponding PCR products consisting of the hphNT1 cassette and flanking regions of the HSP32 and HSP33 loci. Amplification was performed by using the pYM16 vector as template and P5 and P6 as primers. To screen for positive colonies, transformants were plated on YPD medium containing hygromycin (250 μg/ml). Because the upstream and downstream sequences of the two genes were identical, we confirmed the knockouts of Δhsp32 and Δhsp33 by amplifying the downstream sequences with the P7 and P9 primers and subjected the products for BsgI digestion. Because the downstream sequence of HSP32, but not HSP33, has the BsgI restriction site, the digestion profile is different for both knockouts. On the basis of the digestion profiles, we confirmed the Δhsp32 and Δhsp33 single knockouts. The knockouts were further confirmed by amplification with another set of primers, P8 and P9, and subjected to digestion with BsgI. The Δhsp34 single deletion and Δhsp31Δhsp34 double deletions were generated in WT and Δhsp31 backgrounds using the URA3 coding sequence as the disruption cassette and P10 and P11 primers, respectively (Table 1). To isolate positive candidates, yeast transformants were plated on a selective synthetic uracil dropout medium. The colonies for hsp34 knockouts were verified by PCR with confirmation primers P12 and P13.
TABLE 1.
List of primers used in this study
| Primer No. | Primer name | Primer sequence 5′ → 3′ |
|---|---|---|
| P1 | Hsp31 KO Fwd | CTTCCCACTGGCTAATTACACAGATAAAACTCAAACAAATTTATACGTACGCTGCAGGTCGAC |
| P2 | Hsp31 KO Rev | TCAGTTTTTTAAAGCGTCGATGGATCTTACGGCAGTGGAGTGCGCATCGATGAATTCGAGCTCG |
| P3 | Hsp31 KO confirmation kanMX4 Fwd | CTGCCCAGATGCGAAGTTAAGTGC |
| P4 | Hsp31 KO confirmation Rev | GCAAATAACCATTAGATAACTG |
| P5 | Hsp32/33KO Fwd | CTAGAACACTTTTCTCCTTCATTCAAAAAGAAAACTGGCCTTGCACGTACGCTGCAGGTCGAC |
| P6 | Hsp32/33KO Rev | CAAGCCAAAAAAAAGAAAAAAAAAGGAAAAAAAAGAAAACACAGCATCGATGAATTCGAGCTCG |
| P7 | Hsp32/33 KO confirmation hphNT1 Fwd | CGTGTACGCATGTAACATTATAC |
| P8 | Hsp32/33 KO confirmation gene Fwd | GAAACAAAAGACAAGGGAATTGTTG |
| P9 | Hsp32/33 KO confirmation Rev | AAGATATTGGTTGGCAGAAATGTTTAATAAAAC |
| P10 | Hsp34 KO Fwd | ATGACTCCAAAAAGAGCGCTAATATCTCTTACTTCATACCATGTCGAAAGCTACATATA |
| P11 | Hsp34 KO Rev | TAAGAACGAGAGGTTAGGATTTATTTTGTCGCCCAAGTCCTTAGTTTTGCTGGCCGCAT |
| P12 | Hsp34 KO confirmation URA Fwd | CCGTGGATGATGTGGTCTCTAC |
| P13 | Hsp34 KO confirmation Rev | GTGTAACATGCTGCTTCCTAGCA |
| P14 | Hsp31 ORF Fwd | CGCGGATCCAATGGCCCCAAAAAAAGTTTTACTCG |
| P15 | Hsp31 ORF Rev | ACGCGTCGACTCAGTTTTTTAAAGCGTCG |
| P16 | DJ-1 ORF Fwd | GGCGGATCCATGGCTTCCAAAAGAGCTCTGGT |
| P17 | DJ-1 ORF FLAG Rev | ACGCGTCGACCTACTTATCGTCGTCATCCTTGTAATCGTCTTTAAGAACAAGTGG |
| P18 | Hsp31-HA genomic tag Fwd | AGGTGTGAATCCTGCTTCTGCGCACTCCACTGCCGTAAGATCCATCGACGCTTTAAAAAACCGTACGCTGCAGGTCGAC |
| P19 | Hsp31-HA genomic tag Rev | TAAAGTTCCTTACATCTATATAGTAGTACAAAGGAAATTCTAATTATCAACCTTTGGCTCAATCGATGAATTCGAGCTCG |
| P20 | GSH1-HA genomic tag Fwd | TTTAGGAGCTGAAATTGCAGAATATGTAAAAAAAAATAAGCCTTCAATAGAAAGCAAATGTCGTACGCTGCAGGTCGAC |
| P21 | GSH1-HA genomic tag Rev | CAATCACCGTGTCACCCAAATCGATAATGTCAACTTTCTTTCACAACCGAAGTAAAAGGAGTATCGATGAATTCGAGCTCG |
| P22 | GSH2-HA genomic tag Fwd | TACTTCAAATGAAGGTGGAGTGGCGGCAGGATTCGGATGTTTGGACAGTATTATTCTTTACCGTACGCTGCAGGTCGAC |
| P23 | GSH2-HA genomic tag Rev | TAATTGTTAATCAAGTTCTAGCATCATCTTCCTAGCATCTATGTGTATAGTACATGTACACATCGATGAATTCGAGCTCG |
| P24 | GLR1-HA genomic tag Fwd | GATTTCGATAATTGTGTTGCTATTCATCCGACTAGCGCAGAAGAATTGGTTACTATGAGACGTACGCTGCAGGTCGAC |
| P25 | GLR1-HA genomic tag Rev | GTTTACAAGAGTACAAAAGTCCAGTATAGCAACAGCAGATTGGACGTCTAGTTTCGTTGCTATCGATGAATTCGAGCTCG |
| P26 | Hsp31- C-FLAG- Rev | ACGCGTCGACTCACTTATCGTCGTCATCCTTGTAATCGTTTTTTAAAGCGTCG |
For complementation analysis, the ORF corresponding to HSP31 was amplified from yeast genomic DNA using primers P14 and P15. Human DJ-1 was amplified along with FLAG tag using P16 and P17 primers and the DJ-1-pcDNA3.1/gs construct as a template. Both the HSP31 and DJ-1 products were subsequently cloned in pRS415 vector using BamHI and SalI restriction sites. The mutations in HSP31 and human DJ-1 were generated by PCR-based site-directed mutagenesis using the QuikChange method (Stratagene) and suitable primer sequences (Table 1). For protein expression and purification, the ORFs corresponding to HSP31, HSP34, and DJ-1 were amplified and cloned into pRSF-Duet vector using BamHI and SalI restriction sites.
For genomic tagging of HSP31, GSH1, GSH2, and GLR1 with a C-terminal HA tag, the coding sequence corresponding to an HA-kanMX4 cassette was amplified from pYM14 vector by cassette-specific primers containing flanking sequences of the C termini of the respective ORFs (53). Primers used for amplifying the cassettes with respective ORF-specific flanking regions were HSP31 (P18 and P19), GSH1 (P20 and P21), GSH2 (P22 and P23), and GLR1 (P24 and P25) (Table 1). The positive recombinants were selected by plating transformants on YPD-G418 sulfate (400 μg/ml) medium or YPD-hygromycin (250 μg/ml) medium.
Growth Analyses
For spot analysis, the respective yeast strains were grown overnight in liquid YPD or synthetic dropout media. Total yeast cells equivalent to A600 = 1.0 were harvested at mid-log phase. The cells were resuspended in sterile water and subjected to treatment with either 1 mm H2O2 or 7 mm MG followed by incubation at 30 °C for 2 or 5 h, respectively. Subsequently, the cells were washed in sterile water and subjected to 10-fold serial dilutions. Each dilution was spotted on YPD or synthetic leucine dropout media followed by incubation at 30 °C for 36 h. To obtain growth curves, the cells were grown overnight, and equivalent amounts of cells were diluted in 25-ml flasks and grown at 30 °C. 1 mm H2O2 was added to the flasks once the cultures reached A600 = 0.2 followed by further incubation at 30 °C. Absorbance was measured at 600 nm at the indicated time intervals using a UV-visible spectrophotometer, and the data were fitted with GraphPad Prism 5.0 software.
Protein Purification
For purification of N-terminal His6-tagged yeast proteins (WT Hsp31, WT Hsp34, and their mutants), expression was carried out in E. coli BL-21(DE3) strain at 30 °C. Once growth reached an A600 value of 0.6, the cells were subjected to induction by 0.5 mm isopropyl 1-thio-β-d-galactopyranoside for 8 h. Cells were harvested by centrifugation and lysed in lysis buffer (20 mm HEPES, pH 7.5, 100 mm KCl, 20 mm imidazole, 1 mm PMSF, and 10% glycerol) containing 0.2 mg/ml lysozyme followed by incubation at 4 °C for 1 h. The samples were treated gently with 0.5% deoxycholate, and further lysed by sonicating six times (20 s each) at 25% amplitude in ice with 2-min intervals using an Ultrasonic processor. The cell lysates were clarified by centrifugation at 18,000 × g for 30 min, and the supernatant was incubated with Ni2+-NTA-Sepharose (GE Healthcare) for 2 h at 4 °C. Unbound proteins and nonspecific contaminants were removed by multiple washes in lysis buffer followed by sequential single washes with high salt buffer (20 mm HEPES, pH 7.5, 1 m KCl, 20 mm imidazole, and 10% glycerol) and imidazole buffer (20 mm HEPES, pH 7.5, 150 mm KCl, 40 mm imidazole, and 10% glycerol). Finally, the bound proteins were eluted using elution buffer (20 mm HEPES, pH 7.5, 100 mm KCl, 250 mm imidazole, and 10% glycerol) and dialyzed against appropriate assay buffers to remove imidazole. Proteins obtained by this method were ∼95% pure.
Glyoxalase Activity Assays
For estimating the glyoxalase activity of the purified proteins, WT Hsp31 or mutants (1.0 μg) and WT Hsp34 or mutants (40 μg) were incubated with 0.4 mm MG in a reaction buffer containing 20 mm HEPES sodium salt, pH 7.5, 80 mm KCl, and 10% glycerol for 30 min at 30 °C. As a negative control, MG alone was incubated with 1.0 μg of BSA. After 30 min, the reaction was terminated using 0.1% 2,4-dinitrophenylhydrozine and incubated at 42 °C for 10 min. Subsequently, 10% NaOH was added, and the reaction was further incubated at 42 °C for another 10 min to develop the color. The decrease in absorbance at 530 nm due to the reduction in methylglyoxal levels was used to calculate the activity of the protein.
For determining the in vivo glyoxalase activity of Hsp31, the strains were grown up to mid-exponential phase at 30 °C followed by treatment with 0.5% allyl alcohol (to inhibit other glyoxalases in the cell such as glyoxalase I and II) for 1 h (54). Lysates were prepared in buffer (1.2 m sorbitol and 20 mm KH2PO4, pH 7.5) containing 0.2 mg/ml zymolyase using a glass bead treatment method, and the proteins were quantified using the Bradford assay (Bio-Rad). Lysate amounts corresponding to 50 μg of protein were incubated with 0.3 mm methylglyoxal for 30 min at 30 °C, and the activity was measured similarly as described for purified proteins.
Measurement of Methylglyoxal Levels by 1H-NMR Spectroscopy
Cell lysates were prepared in a buffer (1.2 m sorbitol and 20 mm KH2PO4, pH 7.5) containing 0.2 mg/ml zymolyase using glass bead treatment. In each case, 1 mg of lysate was incubated with 0.3 mm methylglyoxal for 30 min at 30 °C and then mixed with 40 μl of D2O for the estimation of methylglyoxal by 1H-NMR spectroscopy (Bruker 800-MHz NMR). The integral values of individual methylglyoxal peaks were analyzed using TopSpin 3.2 software, and the normalized values were plotted using GraphPad Prism 5.0.
Measurement of ROS Levels
Yeast cells were harvested from early exponential growth phase (A600 ∼ 0.6) by centrifugation at room temperature and treated with 1 mm H2O2 (1 h) or 7 mm MG (5 h) at 30 °C. Subsequently, the cells were treated with 100 μm H2DCFDA (peroxide-specific) or 5 μm MitoSOX Red (mitochondrial superoxide-specific) for 20 min followed by a single wash with 1× PBS. For measurement of ROS levels by flow cytometry, the treated cells were analyzed using a BD FACSCanto II flow cytometer. The MFI values from 2000 events were analyzed using WinMDI 2.0 software. For imaging, the cells were mounted with Antifade (Invitrogen) and subjected to fluorescence microscopy (Delta Vision Elite fluorescence microscope).
Measurement of Glutathione Levels
Yeast cells grown in liquid medium to early log phase (A600 ∼ 0.6) at permissible temperature were harvested and treated with 1× PBS containing 30 μm monochlorobimane dye for 30 min at 30 °C allowing the formation of a GSH-specific adduct (55, 56). Fluorescence due to adduct formation (350/460 nm, excitation/emission) was measured by fluorescence microscopy (Delta Vision Elite fluorescence microscope) and flow cytometry (BD FACSVerse) using a Tecan Infinite 200 PRO microplate reader. GSH/GSSG ratios were quantified using the GSH/GSSG-Glo assay kit (Promega).
Measurement of Total NADPH Levels
Yeast cells grown to early log phase (A600 ∼ 0.6) at 30 °C were harvested and partially digested with zymolase. The spheroplast was subjected to NADPH measurement using a NADP/NADPH-Glo assay kit (Promega). Luminance was measured using a Tecan Infinite 200 PRO microplate reader. The values were normalized and were represented using GraphPad Prism 5.0.
Mitochondrial Morphology Analysis by Fluorescence Microscopy
For visualization of mitochondrial morphology, WT, Δhsp31, and Δhsp31/Hsp31WT cells expressing mitochondrial targeting sequence (MTS)-mCherry were harvested at exponential phase (A600 ∼ 0.6) by centrifugation. MTS-mCherry specifically decorates mitochondria allowing us to visualize the mitochondrial morphology. Fixed amount of cells (∼0.2 O.D.) from all the strains were treated with 1 mm H2O2 at room temperature for 1 h. The cells were washed once with 1× PBS, mounted with Antifade (Invitrogen) on agarose pads, and observed under a Delta Vision Elite fluorescence microscope (GE Healthcare) using an ×100 objective lens. The λex/λem for mCherry was 587 nm/610 nm.
Statistical Analysis
All Statistical analyses were performed using GraphPad Prism 5.0 software. Error bars represent S.E. and are derived from three replicates. For significance testing by one-way analysis of variance, Dunnett's multiple comparison post test and Tukey's multiple comparison tests were used to compare against WT values and between columns, respectively. Asterisks used in Fig. 1, C and E; Fig. 2, B, C, and E; Fig. 3, B–D, F; Fig. 5, B, D, and F; Fig. 6, B, C, E, F, and H; Fig. 7H; and Fig. 8, F and G represent the following significance values: *, p ≤ 0.05; **, p ≤ 0.01; ***, p ≤ 0.001.
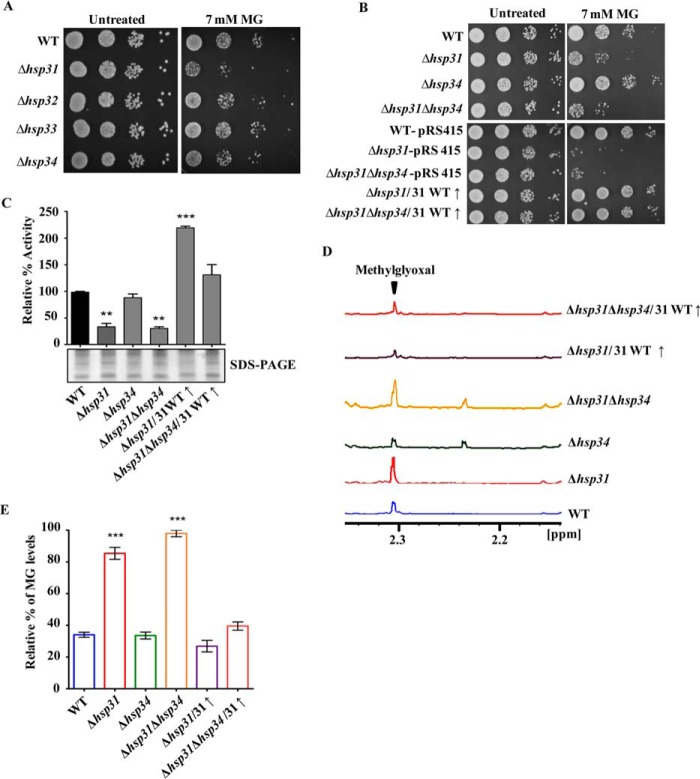
FIGURE 1.
Analysis of growth sensitivity to methylglyoxal and in vivo glyoxalase activity of Hsp31. A, growth phenotype analysis. Yeast cells equivalent to A600 = 1.0 from each strain were treated with 7 mm methylglyoxal and subjected to spot analysis on YPD medium. Images were captured after 36 h of growth at 30 °C. B complementation of Δhsp31 and Δhsp31Δhsp34 by WT Hsp31 was similarly tested by spotting on synthetic leucine dropout plates (SD Leu− medium). Images were captured after 46 h of growth. C, in vivo glyoxalase activity assay. Glyoxalase activities of cell lysates are represented from respective strains normalized vis-à-vis the WT. The SDS-PAGE image is indicated as a quantitative measure of the equal amounts of lysates used for the assay. D and E, measurement of glyoxal levels by 1H-NMR spectroscopy. Cell lysates from different strains were incubated with 3 mm methylglyoxal at 30 °C, and its levels were measured after 30 min of incubation. The peak indicates the relative methylglyoxal level. E, comparison of integral values of methylglyoxal peaks from different strains.
FIGURE 2.
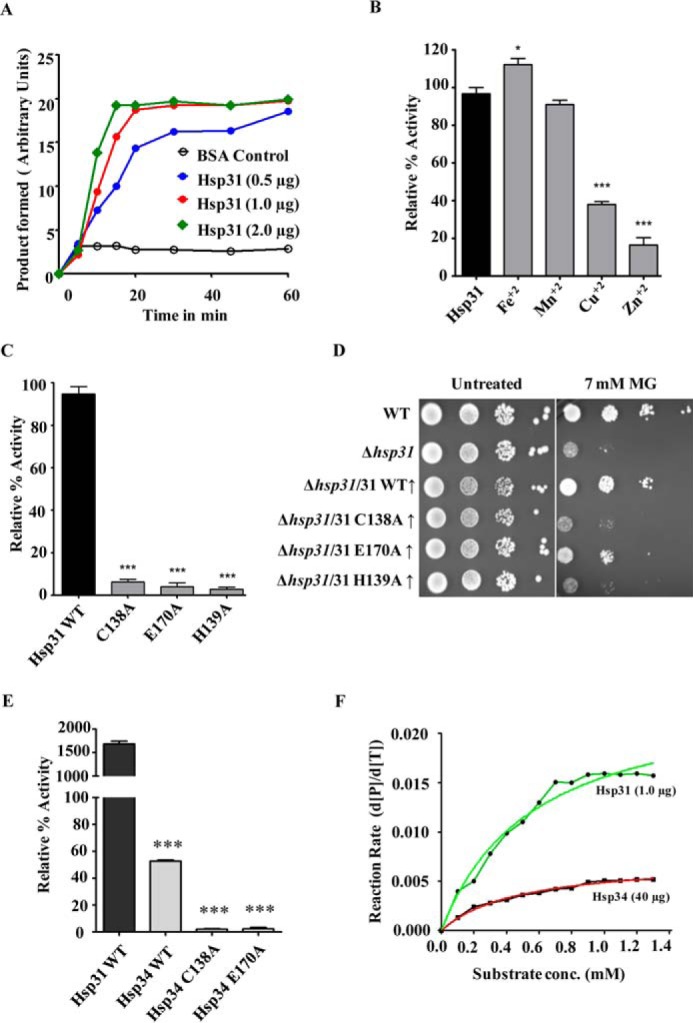
Estimation of glyoxalase activity in vitro. A, measurement of glyoxalase activities of the purified proteins. Increasing concentrations of purified yeast Hsp31 were incubated with 0.4 mm methylglyoxal, and absorbance changes at 530 nm were monitored against time. BSA was used as a negative control. B, metal ion specificity for glyoxalase activity of Hsp31. The purified protein was incubated with 100 mm concentration of various divalent metal ions and subjected for estimation of methylglyoxalase activity. The activity values are normalized with respect to purified Hsp31 protein without metal ion. C, glyoxalase activity of Hsp31 mutants compared with that of WT Hsp31. Purified wild type and Hsp31 mutants were subjected to an estimation of relative glyoxalase activities. D, growth phenotype of Hsp31 catalytic triad mutants. Wild type and mutant yeast strains pretreated with 7 mm methylglyoxal were subjected to spot analysis on SD Leu− medium. Images were captured after 36 h of growth at 30 °C. E, comparison of glyoxalase activities of WT Hsp34 and its mutants. The purified WT Hsp31, WT Hsp34, and Hsp34 mutant proteins (40 μg) were subjected to glyoxalase activity estimation. F, comparison of initial reaction rates of Hsp31 (1 μg) and Hsp34 (40 μg).
FIGURE 3.
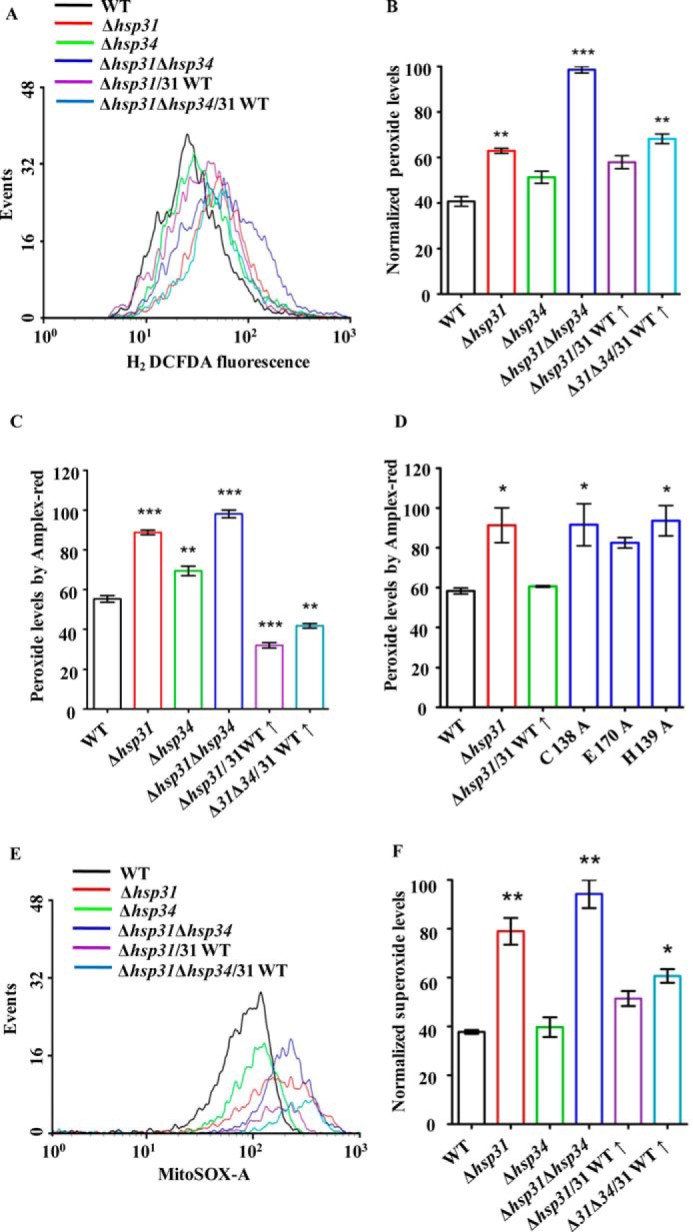
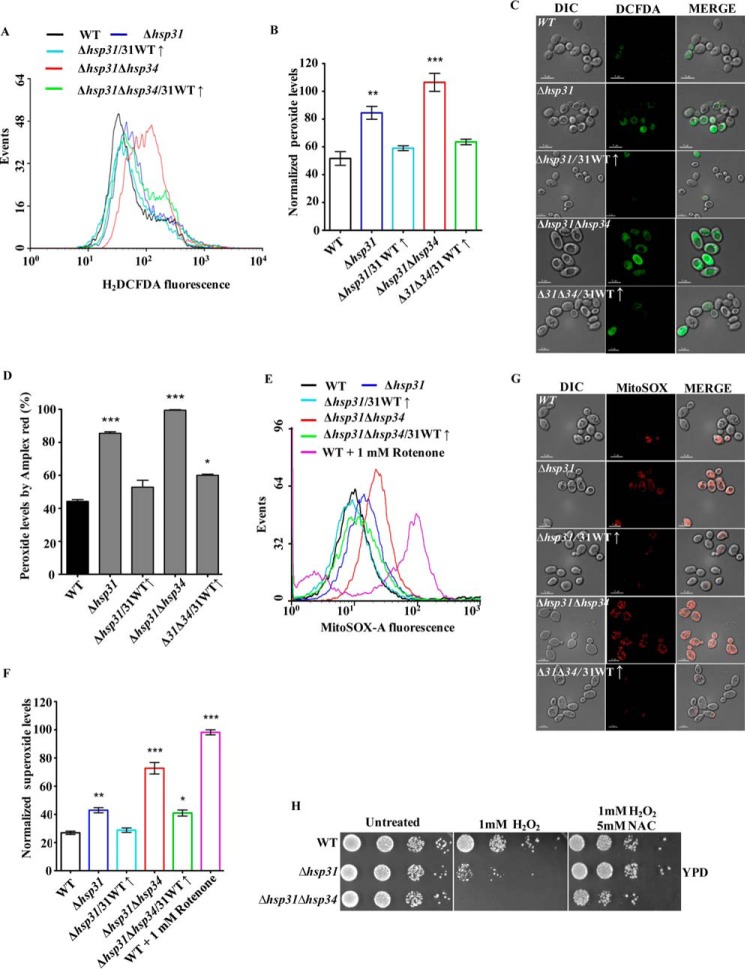
Assessment of cellular ROS levels following methylglyoxal treatment. A and B, estimation of cytosolic ROS levels by H2DCFDA staining after MG treatment. Equivalent amounts of yeast cells from each strain were subjected to 7 mm methylglyoxal treatment followed by staining with H2DCFDA dye and analysis by flow cytometry (A). The total peroxide levels were quantified by normalization of data obtained from flow cytometry (B). C, estimation of total cellular ROS levels by Amplex Red. The total cellular ROS was measured in each indicated strain using an alternate ROS-specific indicator, Amplex Red, at 570/585 nm (excitation/emission) in a spectrofluorometer. D, measurement of peroxide levels in Hsp31 mutants. Δhsp31 cells expressing Hsp31 mutants were subjected to methylglyoxal treatment, and peroxide levels were estimated by Amplex Red staining. E and F, measurement of mitochondrial superoxide levels using MitoSOX Red. Cells from the indicated strains were subjected to staining by a specific mitochondrial superoxide indicator, MitoSOX Red, after treatment with methylglyoxal and then analyzed by flow cytometry (E). The total superoxide levels quantified from the data were obtained by flow cytometry (F).
FIGURE 5.
Regulation of cellular ROS levels by Hsp31 protein. A–C, measurement of total ROS levels by H2DCFDA dye after H2O2 treatment. Equivalent amounts of cells from each strain were subjected to 1 mm H2O2 treatment followed by staining with H2DCFDA and analysis by flow cytometry (A). The total peroxide level for each strain was quantified by normalization of data obtained from flow cytometry (B). Fluorescence imaging is shown of H2DCFDA-stained yeast cells from WT, Δhsp31, Δhsp31/31(↑), Δhsp31Δhsp34, and Δhsp31Δhsp34/31(↑) strains after treatment with 1 mm H2O2 by Delta Vision Elite microscope using a ×100 objective lens; the cells in all the panels were imaged at identical exposures to compare the fluorescence intensity levels (C). D, assessment of cellular peroxide levels. WT, Δhsp31, Δhsp31/31(↑), Δhsp31Δhsp34, and Δhsp31Δhsp34/31(↑) strains were treated with Amplex Red after pretreatment with 1 mm H2O2 and subjected to spectrofluorometry. E–G, estimation of mitochondrial superoxide levels using MitoSOX Red. Equivalent amounts of cells from each strain were subjected to 1 mm H2O2 treatment followed by staining with MitoSOX Red and analysis by flow cytometry. WT cells were treated with 1 mm rotenone for 15 min and used as a positive control for MitoSOX Red staining (E). The total superoxide levels in each strain were quantified from the normalized mean fluorescent intensity values obtained by flow cytometry (F). Fluorescence imaging of relative superoxide levels in WT, Δhsp31, Δhsp31/31(↑), Δhsp31Δhsp34 and Δhsp31Δhsp34/31(↑) strains by MitoSOX Red staining following 1 mm H2O2 treatment. The cells in all the panels were imaged at identical exposures to compare the fluorescence intensity levels (G). H, suppression of oxidative stress by Hsp31 overexpression and NAC treatment. Yeast cells from wild type and deletion strains were treated with 1 mm H2O2 in the presence or absence of 5 mm NAC for 1 h followed by spot test analysis in YPD medium. The images were acquired after 36 h of incubation at 30 °C.
FIGURE 6.
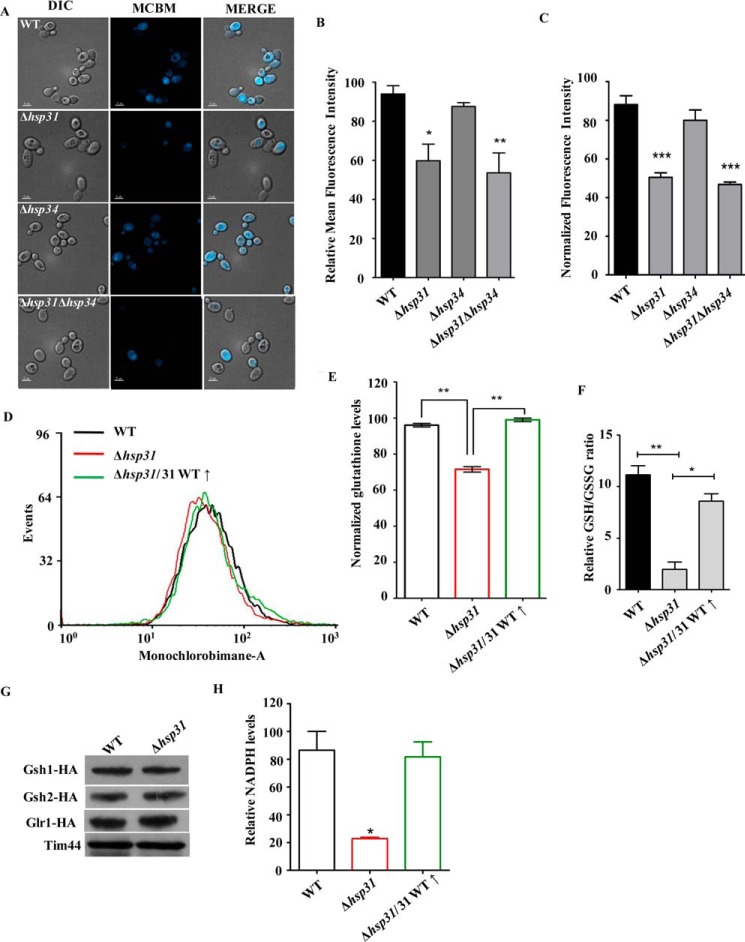
Modulation of cellular glutathione levels by Hsp31. A–C, estimation of cellular glutathione levels. Equivalent amounts of cells from deletion strains (Δhsp31, Δhsp34, and Δhsp31Δhsp34) were treated with a glutathione-specific dye, monochlorobimane, and subjected to fluorescence imaging analysis by Delta Vision Elite microscope using a ×100 objective lens. The cells in all the panels were imaged at identical exposures to compare the fluorescence intensity levels (A). The mean fluorescence intensity obtained from imaging analysis (n = 13) was quantified using ImageJ software (B). Cells from the indicated strains were treated with 30 μm monochlorobimane and subjected to fluorescence intensity measurement using a Tecan Infinite 200 PRO microplate reader. The relative normalized glutathione levels in comparison with WT are represented (C). D and E, measurement of glutathione levels by flow cytometry. The total glutathione levels from the indicated yeast strains (WT, Δhsp31, and Δhsp31/WT (↑)) were measured by treating them with monochlorobimane dye followed by flow cytometry (D). The total glutathione levels were quantitated by normalization of data obtained from mean fluorescent intensity values using flow cytometric analysis (E). F, estimation of reduced (GSH) and oxidized glutathione (GSSG) ratio. The relative GSH/GSSG ratios were estimated to indicate strains by using a GSH/GSSG-Glo assay kit. G, estimation of protein levels. The expression levels of enzymes involved in GSH biosynthesis (Gsh1, Gsh2, and Glr1) in the indicated strains (WT and Δhsp31) were quantified using HA-specific antibodies. Tim44 protein was used as a loading control. H, estimation of total NADPH levels. The total cellular NADPH level was measured in the indicated strains (WT, Δhsp31, and Δhsp31/WT (↑)) using an NADP/NADPH-Glo assay kit from Promega.
FIGURE 7.
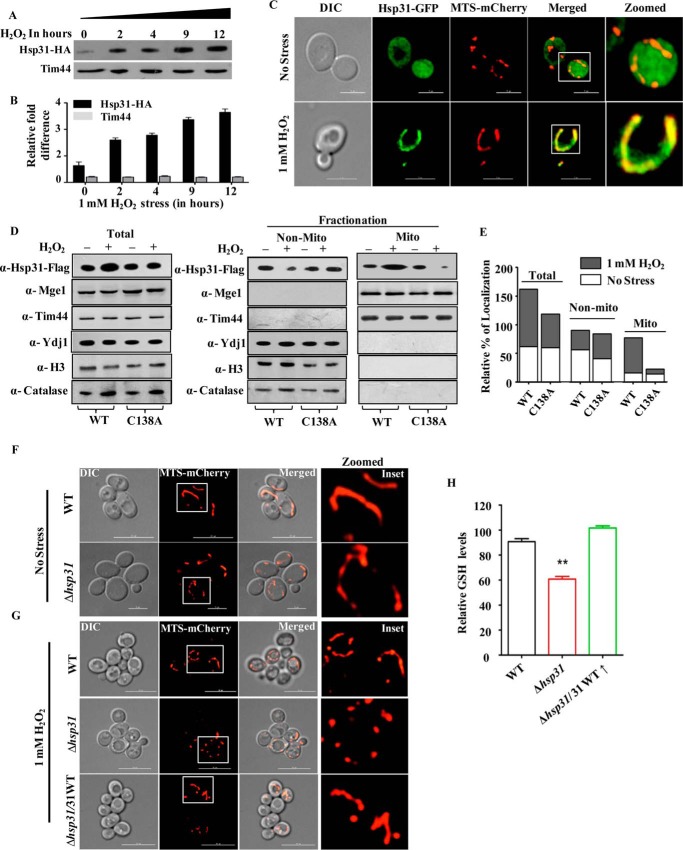
Mitochondrial localization of Hsp31 under oxidative stress. A and B, steady-state levels of Hsp31 in response to oxidative stress. Wild type yeast cells were subjected to 1 mm H2O2 treatment to induce oxidative stress followed by evaluation of steady-state levels of protein in the whole-cell lysates at the indicated time intervals by immunoblotting using anti-HA antibody. The Tim44 protein level served as a loading control (A). The steady-state level of Hsp31 protein was estimated by densitometric analysis of the immunoblots obtained in A using Fujifilm MultiGauge software (B). C, mitochondrial localization of Hsp31 under oxidative stress. The oxidative stress-induced colocalization of Hsp31-GFP with the mitochondria-expressing marker protein, MTS-mCherry, was visualized by imaging analysis using a Delta Vision Elite fluorescence microscope with a ×100 objective lens. D and E, biochemical analysis for Hsp31 localization. The localization of Hsp31 was tested in lysates prepared from total, purified non-mitochondria and mitochondria isolated from yeast strains Δhsp31/Hsp31WT-FLAG and Δhsp31/Hsp31C138A-FLAG by immunoblotting with anti-FLAG-specific antibody. Mitochondrial Tim44 protein was used as the loading control (D). The purity of the mitochondria was tested using various organelle-specific antibodies as indicated (D). The immunoblot was quantitated by densitometry to estimate the relative -fold enrichment of wild type and Hsp31C138A protein in total, non-mitochondria and mitochondria under oxidative stress (E). F and G, assessment of mitochondrial morphology. The mitochondrial morphology of WT and Δhsp31 cells transformed with the MTS-mCherry construct was assessed by fluorescence microscopy prior to H2O2 treatment (F). Similarly, yeast strains (WT, Δhsp31, and Δhsp31/Hsp31WT(↑)) harboring MTS-mCherry-pRS413 were treated with 1 mm H2O2 and observed under a Delta Vision Elite fluorescence microscope with ×100 objective lens for changes in mitochondrial morphology (G). H, estimation of mitochondrial GSH levels. The total glutathione levels were quantified in purified mitochondria isolated from the indicated strains using the GSH/GSSG-Glo assay kit.
FIGURE 8.
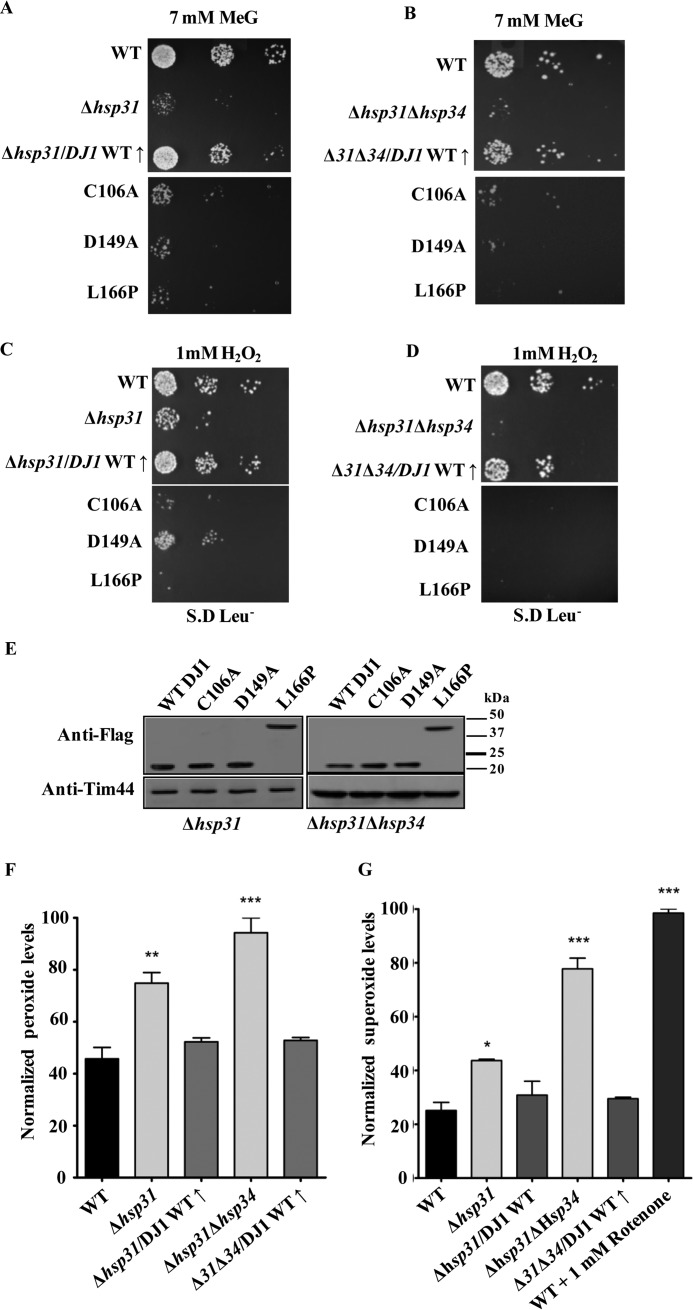
Functional complementation of Δhsp31 by human DJ-1 (hDJ-1). A–D, growth phenotype analyses. Yeast strains (Δhsp31 and Δhsp31Δhsp34) transformed with WT hDJ-1 and its PD variants were treated with either 7 mm methylglyoxal (A and B) or 1 mm H2O2 (C and D) and subjected to spot analysis on synthetic leucine dropout medium. Images shown were captured after 42 h of incubation at 30 °C. E, quantification of levels overexpressed in hDJ-1 PD variants in Δhsp31 and Δhsp31Δhsp34 strains by immunoblotting with anti-HA antibody (upper panel). Tim44 served as the loading control (bottom panel). F and G, suppression of ROS levels by WT hDJ-1. The total ROS levels of Δhsp31 and Δhsp31Δhsp34 strains overexpressing WT hDJ-1 were estimated by H2DCFDA staining following pretreatment with 1 mm H2O2 and analyzed by flow cytometry. The data represented are in the post-normalization stage (F). Similarly, the superoxide levels were estimated by MitoSOX Red staining through flow cytometric analysis after pretreatment with 1 mm H2O2. Total superoxide levels were quantified by normalization of data obtained from flow cytometry (G). WT cells treated with 1 mm rotenone served as a positive control.
Miscellaneous
Mitochondrion isolation was performed using standard protocols (57). The antibody against Tim44 was gifted by Prof. Elizabeth A. Craig, and Hsp31 antibody was raised by immunization of rabbits using purified Hsp31 protein. The antibodies against the FLAG and HA epitopes were obtained from Sigma-Aldrich. All fine chemicals used were also from Sigma-Aldrich, unless otherwise specified. Delta Vision Elite microscope (GE Healthcare) was used for fluorescence imaging, and analytical flow cytometry was performed using BD FACSCanto II unless otherwise specified.
Results
YDR533Cp (Hsp31) Possesses Robust Methylglyoxalase Activity and Protects Cells from Glyoxal Toxicity
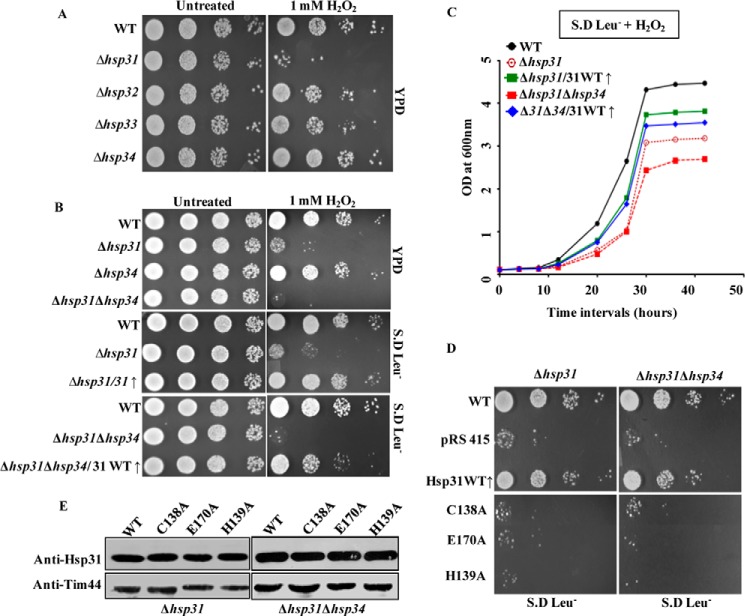
To investigate the role of the Hsp31 class of proteins (Hsp31, Hsp32, Hsp33, and Hsp34) in combating toxicity due to the metabolic intermediates glyoxals, we first generated single Δhsp31, Δhsp32, Δhsp33, and Δhsp34 knock-out yeast strains in BY4741 background by homologous recombination (see “Experimental Procedures”). The strains were subjected to phenotypic analysis after treatment with MG. We observed that the Δhsp31 mutant was highly sensitive to extraneous MG stress as compared with the wild type (Fig. 1A). On the other hand, the Δhsp32, Δhsp33, and Δhsp34 paralogs did not exhibit any significant growth defects under MG stress (Fig. 1A). At the amino acid sequence level, Hsp31 is distinct from the other three paralogs (Hsp32, Hsp33, and Hsp34). In contrast, Hsp32, -33, and -34 proteins are highly identical except for differences in a few amino acids, thus leading to redundancy in their respective functions. To determine whether the deletion of another paralog would lead to enhanced stress sensitivity of Δhsp31, we generated the Δhsp31Δhsp34 double mutant. Intriguingly, the Δhsp31Δhsp34 mutant showed a subtle additive effect on its growth phenotype compared with the Δhsp31 mutant alone, indicating a minor role in protecting cells against MG stress (Fig. 1B, upper panel). To ascertain the involvement of Hsp31 in protecting cells against MG stress, we overexpressed WT Hsp31 in Δhsp31 and Δhsp31Δhsp34 mutant strains and analyzed for growth sensitivity. Indeed, the mutant yeast strains exhibited a reversion in MG stress sensitivity, behaving similar to the wild type strain (Fig. 1B, lower panel).
To analyze the role of Hsp31 in neutralizing cellular MG toxicity, we first measured the in vivo glyoxalase activity using cell lysates. Upon incubating 50 μg of wild type cell lysate with 0.3 mm MG, robust glyoxalase activity was observed (Fig. 1C). On the other hand, the lysates from Δhsp31 and Δhsp31Δhsp34 mutants showed significant reduction in glyoxalase activity in comparison with WT (Fig. 1C). At the same time, the Δhsp34 mutant exhibited a minor reduction in activity consistent with its growth phenotype in the presence of MG. Strikingly, the lysates of the Δhsp31 and Δhsp31Δhsp34 yeast strains overexpressed with WT Hsp31 showed a remarkable enhancement in glyoxalase activity, correlating with the reversion in their growth phenotypes under MG stress (Fig. 1C). To confirm the reduction in MG levels in the lysates, we measured the changes in MG levels using 1H-NMR spectroscopy. The MG peak was traced to a chemical shift of 2.33 ppm (Fig. 1D, arrow), similar to a previous report (47). The integral values obtained for the MG peak were quantitated to measure the total levels of MG in the lysates. In agreement with the glyoxalase activity assay, a significant level of MG was accumulated in Δhsp31 and Δhsp31Δhsp34 strains in comparison with the WT (Fig. 1E). At the same time, Δhsp31 and Δhsp31Δhsp34 yeast mutants containing overexpressed WT Hsp31 showed significantly reduced levels of MG in comparison with the parent strains alone (Fig. 1E).
To establish direct evidence in favor of glyoxalase activity, we purified the Hsp31 class of proteins to homogeneity by introducing a histidine tag at the N terminus and using Ni2+-NTA affinity chromatography. To measure in vitro glyoxalase activity, increasing amounts of purified protein (0.5, 1.0, and 2.0 μg) were incubated with MG in a reaction mixture. Interestingly, the Hsp31 protein showed a robust glyoxalase activity as compared with buffer alone blank and BSA, which were incubated with the substrate to serve as negative controls (Fig. 2A). This observation was consistent with the previous observations in Hsp3101 of Schizosaccharomyces pombe and Glx3 of Candida albicans (58, 59). The protein exhibited typical Michaelis-Menten enzyme kinetics with an estimated Km of 3.854 × 10−4 m and Vmax of 3.073 × 10−2 m. Interestingly, the catalytic constant (Kcat) of yeast Hsp31 was found to be 150 min−1, which was 2-fold faster than human DJ-1 (60). The crystal structure of Hsp31 protein revealed the significance of catalytic histidine residue in binding divalent metal ions such as Zn2+, thereby modulating its activity (61). Therefore, to investigate the effect of metals on Hsp31 activity, we performed the glyoxalase assay in the presence of various divalent metal ions including Zn2+. The activity of Hsp31 was differentially modulated by the addition of different metal ions as cofactors. Importantly, Zn2+ and Cu2+ were found to inhibit activity, whereas, Fe2+ ions showed an enhancement of activity (Fig. 2B). The crystal structure of Hsp31 predicted residues Cys-138, His-139, and Glu-170 as possibly forming a putative catalytic triad (59, 61). We generated mutants by replacing the catalytic triad residues with alanine at their respective positions. The mutants were purified by Ni2+-NTA affinity chromatography and subjected to glyoxalase activity measurements under similar conditions. Interestingly, all the mutants showed marked reductions in their glyoxalase activity (Fig. 2C). In addition, unlike WT, the mutants failed to rescue the in vivo growth sensitivity toward MG treatment (Fig. 2D), thus further highlighting the importance of the predicted catalytic residues for Hsp31 function.
The sequence and structural similarities between Hsp31 and Hsp34 paralogs suggest that the putative catalytic triad residues are highly conserved in both paralogs. To analyze for functional overlap among these paralogs, we tested for glyoxalase activity of purified Hsp34 protein in vitro. Hsp34 exhibited a very weak glyoxalase activity, even at the higher concentration of protein used as compared with Hsp31 under similar conditions (Fig. 2E). To compare the kinetics of Hsp34 with Hsp31, we measured the initial rates of reaction for Hsp31 (1 μg) and Hsp34 (40 μg). As indicated in Fig. 2F, the initial rate of reaction for Hsp34 was much lower than that of Hsp31. This observation further substantiated our earlier findings that with Hsp34 being a weak glyoxalase, its deletion contributes to a subtle difference in the growth phenotype under MG stress. To demonstrate the specificity of Hsp34 glyoxalase activity, we also generated two similar catalytic triad mutants, C138A and E170A in Hsp34. Both mutants showed a significant reduction in glyoxalase activity as compared with the wild type (Fig. 2E). Taken together, our analyses reveal the importance of the glyoxalase activity of Hsp31 class proteins in reducing cellular glyoxal toxicity in Saccharomyces cerevisiae.
Hsp31 Represses Methylglyoxal-induced Oxidative Stress in S. cerevisiae
Previous reports have revealed that methylglyoxal induces oxidative stress in osteoblastic MC3T3-E1 cells (62). In addition, elevated MG levels induce apoptosis through the generation of reactive oxygen species in nerve-derived Schwann cells (12, 63). Therefore, we hypothesized that MG treatment could induce oxidative stress leading to loss of growth. To delineate the effect of MG in the cellular pathways leading to the loss of viability of yeast cells, we assessed the levels of ROS. First, we measured the cytosolic ROS levels in different backgrounds, including deletion and overexpression strains (WT, Δhsp31, Δhsp34, Δhsp31Δhsp34, Δhsp31/WT31(↑), and Δhsp31Δhsp34/WT31(↑)) after treatment with MG using H2DCFDA dye. The dye produces a green fluorescence signal upon modification by peroxide species and enables the measurement of the total cellular ROS levels either by flow cytometry (FACS) or by fluorescence microscopy. The flow cytometric analyses revealed that both Δhsp31 and Δhsp34 strains showed an enhancement in the ROS levels in comparison with the WT (Fig. 3, A and B). At the same time, the double deletion strain (Δhsp31Δhsp34) showed a synergistic robust increment in the total ROS levels (Fig. 3, A and B). On the other hand, overexpression of WT Hsp31 in Δhsp31 and Δhsp31Δhsp34 strains significantly reduced the ROS levels, consistent with the observed reversion in the growth phenotype (Fig. 3, A and B). To further support our findings, the overall cellular ROS levels were measured by using an alternative cytosolic peroxide-specific dye, Amplex Red (64, 65). In concordance with our observations with H2DCFDA, Amplex Red staining after treating yeast cells with MG showed an elevation in cellular ROS levels in the Δhsp31 and Δhsp31Δhsp34 strains as compared with the WT (Fig. 3C). Additionally, the deletion mutant strains overexpressed with WT Hsp31 exhibited a restoration of ROS to basal levels (Fig. 3C). Notably however, the expression of Hsp31 catalytic triad mutants, which failed to rescue the growth defective phenotype under MG stress, was also unable to reduce the peroxide levels in the Δhsp31 deletion background (Fig. 3D).
Mitochondria are a major source of ROS production, due to incomplete reduction of molecular oxygen during the respiration process. Therefore, we tested for increment in the mitochondrial superoxide levels by using MitoSOX Red dye. The dye is selectively targeted to the mitochondrial compartment and gets oxidized by superoxide ions, thus exhibiting a red fluorescence signal upon DNA binding (66, 67). In consistency with H2DCFDA data, a similar pattern of increment in the red fluorescence was observed for the Δhsp31 and Δhsp31Δhsp34 deletion strains (Fig. 3, E and F). Additionally, the superoxide content was restored back to basal levels when the deletion strains were complemented with WT Hsp31(↑) (Fig. 3, E and F). In summary, our studies show that the Hsp31 class of proteins in S. cerevisiae plays a critical role in maintaining the levels of the metabolic intermediate glyoxal, thus regulating redox balance within the cell.
Hsp31 Functions as a ROS-regulating Protein in S.cerevisiae
Previous reports suggest that Hsp31 is an oxidative stress-responsive protein in S. cerevisiae (51). However, the cellular regulatory mechanisms involving the Hsp31 class of proteins in ROS sensing are still elusive. To elucidate this phenomenon, we first subjected single deletion yeast strains (Δhsp31, Δhsp32, Δhsp33, and Δhsp34) in BY4741 background to extraneous oxidative stress. As compared with untreated controls (Fig. 4A, left panel), Δhsp31 strain was found highly sensitive to 1 mm H2O2 treatment over the other paralogs, (Fig. 4A right panel) which is consistent with the previous observations (50, 51). On the contrary, Δhsp32, Δhsp33, Δhsp34 paralogs did not exhibit significant growth defects due to extraneous stress, similar to methylglyoxal stress (Fig. 4A right panel). However, the double deletion strain (Δhsp31Δhsp34) exhibited an enhancement in sensitivity to H2O2 treatment as compared with Δhsp31 alone (Fig. 4, B and C). To ascertain the specificity of the observed phenotype, both the Δhsp31 and Δhsp31Δhsp34 strains were complemented with WT Hsp31 overexpressed under the TEF promoter and subjected to growth analyses. Indeed, overexpression of WT Hsp31 was able to rescue the oxidative stress-sensitive growth phenotype in both solid and liquid culture mediums (Fig. 4, B and C). Interestingly, the catalytic triad mutants of Hsp31 did not support the growth of either Δhsp31 or Δhsp31Δhsp34 strains when subjected to a similar extraneous oxidative stress (Fig. 4D, left and right panels). Under these growth conditions, the overexpressed levels of the mutant proteins were comparable with the WT in both single and double deletion strains, indicating that the loss of function cannot be attributed to decreased availability of the mutants (Fig. 4E).
FIGURE 4.
Suppression of peroxide stress by Hsp31 paralogs. A, growth phenotype analysis. Yeast cells from wild type and each Hsp31 paralog deleted strains were subjected to spot analysis in the absence (left panel) and presence of 1 mm H2O2 treatment (right panel) on YPD medium. B and C, rescue of Δhsp31 by exogenous expression of Hsp31. Equivalent amounts of cells from each strain, kept untreated (left panel) or treated with 1 mm H2O2 (right panel) for 2 h, were subjected to spot analysis on leucine dropout medium. The growth rescue was monitored on SD Leu− medium up to 42 h (B). Similarly, the growth of Δhsp31, Δhsp31Δhsp34, Δhsp31/31(↑), and Δhsp31Δhsp34/31(↑) strains were assessed in liquid SD Leu− medium up to 42 h in the presence of 1 mm H2O2 (C). D, suppression of peroxide stress by Hsp31 mutants. Δhsp31 and Δhsp31Δhsp34 strains overexpressed with WT Hsp31 and its mutants were subjected to 1 mm H2O2 and spotted on SD Leu− medium. Images were taken after 40 h of incubation at 30 °C. E, Western blot showing the expression of WT Hsp31 and mutants in both Δhsp31and Δhsp31Δhsp34 (upper panels). Tim44 served as the internal loading control (lower panels).
To establish a functional correlation between the growth sensitivity of the deletion strains with the extraneous oxidative stress conditions, we first assessed the accumulation of total ROS levels under H2O2-treated and untreated conditions by using H2DCFDA dye-based flow cytometric analysis. Both the Δhsp31 and Δhsp31Δhsp34 strains showed a robust increment in the cellular ROS levels as compared with WT (Fig. 5, A and B). Retrospectively, overexpression of WT Hsp31 suppressed the accumulation of overall ROS levels (Fig. 5, A and B). These data were consistent with the ROS measurements performed with another specific dye, Amplex Red (Fig. 5D). The flow cytometric findings were further confirmed by fluorescence microscopic imaging analysis of the yeast cells, which were subjected to similar oxidative stress and monitored for ROS levels using H2DCFDA dye. As indicated in Fig. 5C, both the Δhsp31 and Δhsp31Δhsp34 strains exhibited enhanced green florescence indicative of elevated peroxide species as compared with treated WT control (Fig. 5C, compare panels 1, 2, and 4 (from top to bottom)). On the other hand, the overexpression of WT Hsp31 restores the normal basal ROS levels (Fig. 5C, compare panels 1–5 (from top to bottom)). Similarly, to measure the changes in the mitochondrial superoxide levels arising from extraneous oxidative stress, the deletion strains were analyzed using MitoSOX Red dye. Flow cytometric analysis revealed that both the Δhsp31 and Δhsp31Δhsp34 strains exhibited enhancement in the superoxide levels (Fig. 5, E and F). The observed -fold increment for the double deletion strain was significant and comparable with that of the positive control treated with rotenone, which is known to cause elevation in the superoxide levels by decoupling electron transport chain complex (Fig. 5, E and F). The elevated mitochondrial superoxide levels in the Δhsp31 and Δhsp31Δhsp34 strains were further confirmed by fluorescence microscopic imaging analysis. Similar to the FACS results, the single and double deletions strains showed a significant accumulation in mitochondrial superoxide levels compared with WT (Fig. 5G, compare panels 1, 2, and 4 (top to bottom)). Moreover, overexpression of WT Hsp31 in deletion backgrounds restored the normal superoxide levels (Fig. 5G, compare panels 1–5 (top to bottom)).
Previously, N-acetylcysteine (NAC) has been widely utilized to scavenge free radicals and protect cells from ROS-mediated oxidative damage (68). To compare whether the rescue of the growth phenotype by Hsp31 is similar to NAC, a growth assay was performed after H2O2 treatment in the presence or absence of NAC. Interestingly, the growth recovery observed for Δhsp31 and Δhsp31Δhsp34 strains after NAC treatment was comparable with the rescue by overexpression of WT Hsp31 under the TEF promoter (Figs. 5H and 4B). These results provide compelling evidence suggesting that Hsp31 can function as an oxidative stress-relieving protein by regulating the intracellular ROS levels in S. cerevisiae.
Hsp31 Provides Cytoprotection against Oxidative Stress by Modulating Cellular GSH and NADPH Levels
Glutathione is a major and important intracellular low molecular weight, thiol-containing, antioxidant molecule that plays a critical role in the cellular defense against oxidative stress (69). The ratio of reduced and oxidized glutathione is a measure of the degree of oxidative stress encountered by the cell. Because Δhsp31 mutant cells showed enhancement in ROS levels under metabolic stress conditions, we hypothesized that Hsp31 protein is probably involved in regulating cellular antioxidant levels in response to oxidative stress. To delineate the mechanism of how Hsp31 class proteins provide cytoprotection, we first measured the total basal cellular glutathione level, which is a potent reducing agent inside the cell. We utilized cell-permeable monochlorobimane, a dye that reacts with cellular GSH and gives blue fluorescence when excited at 380 nm (55). Additionally, we measured the basal levels of GSH in WT and hsp31 mutant cells using microscopic imaging analysis. As compared with the WT, Δhsp31 and Δhsp31Δhsp34 mutant cells showed a reduction in the total GSH levels (Fig. 6A). Upon quantitation, about a 30–35% reduction of basal GSH levels was observed in Δhsp31 and Δhsp31Δhsp34 strains (Fig. 6B). At the same time, deletion of Hsp34 led to a marginal (∼5%) reduction in GSH levels, suggesting that Hsp31 plays an important role in maintaining the cellular GSH levels (Fig. 6, A and B). These findings were further validated by direct fluorescence measurements using spectrofluorometric and flow cytometric methods. As indicated in Fig. 6, C–E, about a 25–30% reduction in the basal cellular GSH levels was observed in both Δhsp31 and Δhsp31Δhsp34 strains, consistent with the imaging analysis (Fig. 6, C–E). At the same time, the levels of oxidized forms of GSH increased in the deletion Δhsp31 background (Fig. 6F). Furthermore, the reduction in the GSH levels was restored upon overexpressing Hsp31 in the deletion background (Fig. 6F).
To gain further insights into how Hsp31 class of proteins enhances the cellular GSH pool, we measured the levels of enzymes involved in its biosynthetic pathway. For this purpose, we first chromosomally tagged glutamate-cysteine ligases GSH1 and GSH2, enzymes that regulate the glutathione biosynthesis (70). Interestingly, Δhsp31 cells did not exhibit any difference in the expression levels of GSH1 and GSH2 as compared with the WT (Fig. 6G). Similarly, we also analyzed glutathione reductase enzyme (GLR1) levels, which play a critical role in the maintenance of the GSH pool by reducing oxidized glutathione disulfide forms (GSSG) in the glutathione peroxidase cycle. As indicated in Fig. 6G, the levels of glutathione reductase in Δhsp31 cells were also comparable with those of the WT, suggesting that GSH levels are regulated by downstream events. Glutathione reductase functions as a dimer utilizing NADPH as a cofactor to donate electrons for the reduction of glutathione disulfide into GSH (71). As reported previously, the NADPH supply from the pentose phosphate pathway is crucial for maintaining the redox cycles of glutathione in the cells (72). Therefore, we measured the total NADPH levels in Δhsp31 cells. Interestingly, we observed a 60% depletion in the NADPH level in Δhsp31 in comparison with WT (Fig. 6H). At the same time, overexpression of Hsp31 exhibited restoration of NADPH levels in Δhsp31 (Fig. 6H). Collectively, these findings suggest that Hsp31 modulates the NADPH levels and thereby maintains reduced glutathione levels to protect cells from oxidative stress.
Mitochondrial Localization of Hsp31 Protects the Organellar Integrity by Suppressing Oxidative Stress via Redistribution of GSH Levels
Although the Hsp31 class of proteins localizes predominantly in the cytosol for ROS regulation, its mitochondrial oxidative stress suppressive function is quite intriguing (Fig. 5, D–F). Therefore, we tested for the partitioning of Hsp31 into mitochondria during oxidative stress. The up-regulation of Hsp31 upon exposure to oxidative stress was monitored by HA-tagging the protein at the C terminus using the genomic integration method. Upon immunoblotting using HA-specific antibodies, a time-dependent increment in the steady-state levels of Hsp31 was observed up to a maximum of 4-fold in response to 12 h of exposure to 1 mm H2O2 stress (Fig. 7, A and B). To determine the localization pattern under oxidative stress conditions, GFP was fused to the C terminus of Hsp31 and subjected to fluorescence imaging. To track the organellar localization, mCherry protein was fused with MTS from Neurospora crassa mitochondrial complex subunit 9 to the N terminus, which exclusively decorated the mitochondrial compartment (73, 74). The construct was then transformed into cells and subjected to imaging before and after H2O2 treatment. Importantly, as indicated in Fig. 7C, under normal conditions Hsp31-GFP was localized predominantly to the cytosol. Strikingly, under stress conditions, Hsp31was found to localize to the mitochondrial compartment, as highlighted by the merged image of Hsp31-GFP with the mCherry (Fig. 7C). These observations were further supported by subcellular fractionation analysis using total cell lysate and purified non-mitochondrial and mitochondrial fractions isolated from Δhsp31/Hsp31WT-FLAG yeast cells treated with 1 mm H2O2 or untreated controls. Upon immunoblotting with FLAG-specific antibodies, it was evident that more than 50% of the FLAG-tagged Hsp31 protein was associated with the mitochondrial fraction, similar to Tim44 and Mge1 positive protein controls (Fig. 7D). At the same time, the absence of marker proteins such as histone H3 (nuclear), catalase (peroxisomes), and Ydj1 (cytosol) confirmed the authenticity of the mitochondrial purity. To understand the mechanism of how Hsp31 translocates to the mitochondrial compartment, we generated Hsp31C138A mutant, as it was reported previously that the cysteine residue in ThiJ/DJ-1/PfpI proteins known to function as a redox sensor (75). Interestingly, the mitochondrial localization of the Hsp31C138A mutant in response to oxidative stress is significantly impaired as compared with the WT, thus revealing its importance in redox-mediated organellar translocation (Fig. 7, D and E).
To evaluate the significance of localization of Hsp31 into mitochondrial compartment, we tested for organellar integrity under stress conditions. To decorate the mitochondria, MTS-mCherry was targeted into both WT and Δhsp31cells and subjected to morphology analysis after treatment with 1 mm H2O2. The WT and Δhsp31cells maintained a normal tubular mitochondrial morphology in the absence of oxidative stress (Fig. 7F). However, unlike WT cells, fragmented mitochondria were observed in Δhsp31cells upon exposure to oxidative stress (Fig. 7G). On the other hand, the overexpression of Hsp31 restored mitochondrial morphology in Δhsp31 cells, thus highlighting the critical function of Hsp31 in suppressing mitochondrial oxidative stress and thereby preserving its integrity (Fig. 7G). To unravel how Hsp31 reduces mitochondrial oxidative stress, we measured the distribution of GSH levels in the organelle upon exposure to 1 mm H2O2 stress. GSH levels were significantly reduced in the Δhsp31 mitochondria in comparison with the WT (Fig. 7H). Remarkably, the overexpression of Hsp31 restores the organellar GSH levels in Δhsp31 cells (Fig. 7H). In summary, our findings revealed the importance of Hsp31 class proteins in providing organellar cytoprotection under oxidative stress by elevating the production of GSH as well as its redistribution to the mitochondrial compartment.
Human Homolog DJ-1 Complements the ROS Regulatory Function of Hsp31 in S. cerevisiae
Human DJ-1 exhibits glyoxalase activity and provides protection to neurons against oxidative stress (60). Based on sequence homology, Hsp31 is predicted to be the closest ortholog among the yeast paralogs. Because DJ-1 family proteins exhibit ROS scavenging function across species, we tested for a possible growth complementation of human DJ-1 in Δhsp31 and Δhsp31Δhsp34 cells. The strains were subjected to spot analysis in the presence of 7 mm methylglyoxal. Human DJ-1 rescued the growth of both single and double deletions (Fig. 8, A and B). Most notably, the mutants DJ-1C106A (catalytic triad and implicated in familial PD), DJ-1L166P, and DJ-1D149A (familial PD) were defective in rescuing the growth of Δhsp31 and Δhsp31Δhsp34 cells in the presence of methylglyoxal. This observation therefore reveals the central importance of glyoxalase activity across kingdoms for growth under stress conditions (Fig. 8, A and B). Similarly, human DJ-1 rescued the oxidative stress sensitivity of Δhsp31 and Δhsp31Δhsp34 cells when exposed to 1 mm H2O2 (Fig. 8, C and D). At the same time, DJ-1 PD mutants were also unable to complement the growth defects of both single and double deletion strains in the presence of oxidative stress (Fig. 8, C and D), consistent with yeast Hsp31 glyoxalase mutants. To eliminate the possibility of these differences arising due to altered protein expression, lysates from mutants were subjected to Western blotting followed by immunodetection with FLAG-specific antibodies. As indicated in Fig. 8E, all of the DJ-1 mutants were expressed comparably with WT levels (Fig. 8E). However, DJ-1L166P mutant showed a mobility shift compared with other PD mutants, probably due to polyubiquitination, which is known to occur in the mammalian system (Fig. 8E). However, we did not observe faster clearance of the L166P mutant, possibly due to the attachment of short ubiquitin chains with less than four subunits, which are known to remain stable for longer times as are other reported proteins (76).
To test the suppression of oxidative stress by human DJ-1, we assessed the accumulation of total ROS levels by H2DCFDA dye using flow cytometry. Overexpression of DJ-1 in Δhsp31 and Δhsp31Δhsp34 strains suppressed the elevated ROS to levels comparable with WT (Fig. 8F). In addition, the overexpression of DJ-1 also neutralized the superoxide levels as indicated by MitoSOX Red dye staining in Δhsp31 and Δhsp31Δhsp34 strains (Fig. 8G). These findings provide direct evidence in favor of the conserved functions of the ThiJ/DJ-1/PfpI family proteins in combating oxidative stress across species boundaries.
Discussion
Our present study has uncovered the ROS regulatory role of yeast Hsp31 class of proteins in suppressing the oxidative stress induced by accumulation of toxic metabolic intermediates such as MG. Using yeast genetic and biochemical analyses, we have revealed the vital mechanistic and molecular details of Hsp31 in combating oxidative stress generated by elevation in the levels of glyoxals and reactive oxygen species.
Robust Methylglyoxalase Activity of Hsp31 Protects against Cellular Damages by Glyoxals and Oxidative Stress in S. cerevisiae
Although organisms comprise multiple types of glyoxalases for the efficient detoxification of MG accumulated during normal metabolic processes, their functions are largely dependent on the availability of GSH as cofactors. Moreover, the availability of GSH is rate-limiting under oxidative stress conditions, and thus cells demand alternate modes of detoxification of MG. In this report, we have highlighted the significance of the yeast Hsp31 class of proteins, members of the ThiJ/DJ-1/PfpI family possessing robust glyoxalase activity for the suppression of MG-mediated toxicity. Interestingly, the glyoxalase activity of Hsp31-class proteins is GSH-independent, hence providing a unique advantage for cells to combat glyoxal stress (Fig. 9 model).
FIGURE 9.
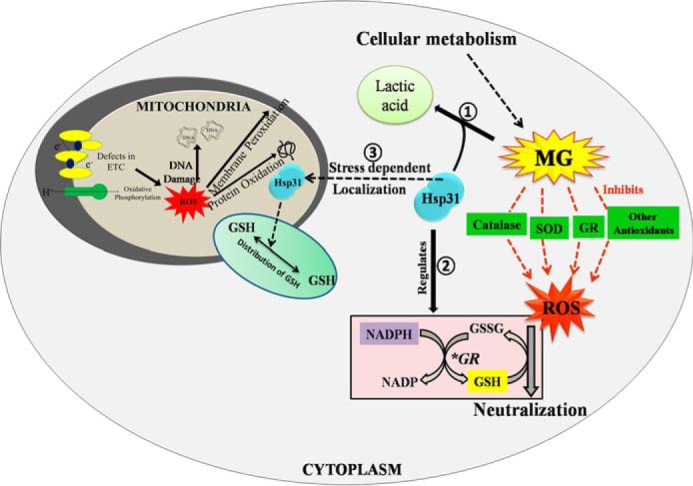
Model depicting mechanistic details of S. cerevisiae Hsp31 protein in regulating cellular oxidative stress. Step 1, the robust glyoxalase activity of Hsp31 ensures the elimination of toxic methylglyoxal species that arise as a by-product of various metabolic pathways. Step 2, Hsp31 is involved in regulating the GSH/GSSG ratio and NADPH homeostasis, which enables the maintenance of redox homeostasis. Step 3, Hsp31 translocates to the mitochondria and also protects the mitochondrial functions by redistributing GSH levels.
Previous findings reveal that Hsp31 from E. coli exhibits GSH-independent glyoxalase activity, which efficiently converts MG to lactic acid (47). Contrastingly, however, E. coli Hsp31 plays a minor role in combating oxidative stress but provides acid resistance during starvation conditions in E. coli (49). On the other hand, human DJ-1 protein, a member of the ThiJ/DJ-1/PfpI family implicated in familial PD, displays redox-based chaperone activity as well as methylglyoxalase activity (38, 60). Because of the conservation of these family proteins across species, we have demonstrated here that YDR533Cp (Hsp31) of S. cerevisiae, a member of the novel ThiJ/DJ-1/PfpI family, possesses robust glyoxalase activity and efficiently suppresses glyoxal toxicity. Besides, the yeast genome encodes for three additional paralogs, namely Hsp32, Hsp33, and Hsp34, which are highly identical in nature but possess a ∼40-fold lower glyoxalase activity as compared with Hsp31 and are hence unable to suppress glyoxal toxicity. Mammalian DJ-1 is known to regulate oxidative stress and reduce ROS levels in dopaminergic neurons. Our results show that deletion of Hsp31 leads to enhancement of the overall cellular ROS and mitochondrial superoxide levels. On the contrary, overexpression of WT Hsp31 restores the ROS to basal levels and suppresses mitochondrial oxidative stress, thus playing an important role in redox homeostasis. Moreover, our results provide the first experimental evidence revealing that human DJ-1 possesses canonical functions and could suppress glyoxal toxicity as well as the oxidative stress phenotype of Hsp31 mutants, thereby signifying the functional conservation of the ThiJ/DJ-1/PfpI family across phylogenetic boundaries. The predicted catalytic triad amino acids, namely Cys-138, His-139, and Glu-170, were found to be very critical for the glyoxalase and ROS regulative functions of Hsp31. On the other hand, the catalytic mutants of human DJ-1, C106A and L166P, which are implicated in familial PD, failed to restore the ROS regulatory effect in Hsp31 deletion cells, suggesting that glyoxalase activity is indispensable for its function across species. Based on our findings, we therefore hypothesized that, unlike E. coli, the ThiJ/DJ-1/PfpI family proteins play a major role in combating oxidative stress and maintaining ROS homeostasis in eukaryotes (Fig. 9 model).
Hsp31 Maintains Glutathione and NADPH Homeostasis to Combat Oxidative Stress
The maintenance of steady-state levels of reduced glutathione is critical for cells to survive against oxidative stress-mediated damages. In yeast, the biosynthesis of reduced glutathione is a regulated process requiring two important energy-utilizing enzymes, namely γ-glutamyl-cysteine synthase (Gsh1) and glutathione synthetase (Gsh2) (70). At the same time, oxidized glutathione (GSSG) formed during ROS scavenging is recycled to its reduced state by glutathione reductase enzyme (Glr1), which requires NADPH as a cofactor (71). Under stress conditions, the activity of key biosynthetic enzymes and the levels of protective antioxidants such as GSH are regulated by several transcription factors, including Nrf2, the activity of which is modulated by ThiJ/DJ-1/PfpI family proteins (77, 78).
Our findings provide evidence showing that yeast Hsp31 plays an important role in glutathione homeostasis. The deletion of Hsp31 leads to a decrease in the GSH levels, and overexpression restores intracellular glutathione levels. As a result, the presence of Hsp31 provides cytoprotection to cells by robustly suppressing the cytosolic and mitochondrial ROS levels under both MG and H2O2 stress conditions. Human DJ-1 was shown to up-regulate intracellular glutathione levels by increasing glutamate-cysteine ligase mRNA levels during oxidative stress (79). Intriguingly, our results show that levels of glutathione biosynthetic enzymes Gsh1 and Gsh2 and recycling enzyme Glr1 are relatively unaffected by Hsp31expression in yeast. On the other hand, during oxidative stress, Glr1-dependent recycling of GSSG to GSH is a key rate-determining event to counter excessive ROS, which is largely dependent on NADPH availability (71) (Fig. 9 model). Intriguingly, glyoxals are known to inhibit various NAD(P)H-generating enzymes, leading to NADPH depletion, a major cause of increased oxidative stress upon glyoxal accumulation (80). In line with this observation, our results suggest that the deletion of Hsp31 led to a significant reduction in total NADPH levels. At the same time, NADPH levels were significantly restored upon Hsp31 overexpression to the WT. On the basis of our findings, it is reasonable to conclude that the robust glyoxalase activity of Hsp31 reduces glyoxal stress, thereby maintaining the intracellular NADPH and glutathione levels required to combat oxidative stress (Fig. 9 model).
Hsp31 Protects Mitochondrial Integrity by Its Relocalization and Redistribution of GSH Levels under Oxidative Stress Conditions
Based on our observations, it is evident that Hsp31 plays two significant roles in preserving mitochondrial function by maintaining its integrity. Foremost, the level of Hsp31 is up-regulated upon oxidative stress to suppress mitochondrial superoxide levels. Enhanced mitochondrial oxidative stress results in its fragmentation via alteration in the mitochondrial dynamics (81). In agreement with this hypothesis, our studies demonstrate that deletion of Hsp31 leads to fragmentation of mitochondria under oxidative stress. Strikingly, the mitochondrial morphology was restored upon overexpression of Hsp31 in the deletion background, thus signifying its critical role in maintaining organelle integrity. Because Hsp31 regulates total cellular GSH availability, it therefore aids in the redistribution into mitochondria to suppress ROS-mediated damages to the organelle. On the other hand, mitochondrial isoform of Glr1 enzyme may play a crucial role in the recycling of oxidized glutathione into its reduced form, thereby maintaining GSH homeostasis (82). Secondly, Hsp31 is predominantly a cytosolic protein; under oxidative stress, it relocalizes into mitochondria to protect its integrity and function. In humans, DJ-1 is known to stabilize complex I as well as interact with mtHsp70; these are the key components regulating mitochondrial oxidative stress (83). Importantly, our study demonstrates that the Cys-138 residue is critical for the mitochondrial translocation property. These results are in agreement with reports on mammalian DJ-1, which is known to translocate to mitochondria upon oxidation of cysteine residue (75). Recent reports show that MG also is involved in the glycation of mitochondrial proteins leading to inhibition of respiration, which is implicated in several pathological conditions in humans (84–86). Intriguingly, DJ-1 family members from E. coli and humans also possess protein deglycase activity, which repairs and reactivates proteins from glycation (87, 88). Although we have not measured the protein deglycase activity of yeast Hsp31, we speculate that the localization of Hsp31 to mitochondria under oxidative stress conditions may contribute to repairing the mitochondrial proteins from glycation, thus protecting organellar integrity. Collectively, our findings underscore the importance of yeast Hsp31 family proteins in the maintenance of the mitochondrial life cycle, which is a key factor in several pathological conditions such as familial PD, where human DJ-1 is known to play a significant role in disease progression.
In summary, our reports for the first time highlight the significance of ThiJ/DJ-1/PfpI family proteins in protecting the cells against glyoxal stress, which in turn maintains ROS homeostasis in S. cerevisiae by three different mechanisms, as represented in the “model” figure (Fig. 9). First, the robust glyoxalase activity of Hsp31 reduces glyoxal toxicity by converting MG to a less harmful intermediate (lactic acid), thereby alleviating oxidative stress that arises due to the glycation of enzymatic antioxidants. Second, Hsp31 maintains glutathione and NADPH homeostasis, which facilitates the recycling of reduced GSH for the neutralization of excess ROS. Third, enhanced levels of Hsp31 aids in the translocation and redistribution of GSH into mitochondria to suppress elevated levels of ROS and maintain mitochondrial integrity, thereby providing overall cytoprotection.
Author Contributions
P. D., K. B., A. V. G., and M. S. designed the study, analyzed the data, and wrote the paper. K. B., S. S., and S. S. A. performed the experiments. K. B. prepared all the figures. All authors reviewed the results and approved the final version of the manuscript.
Acknowledgments
Yeast Tim44-specific antibody was a kind gift from Prof. Elizabeth A Craig, University of Wisconsin-Madison. We thank Dr. Mark R. Cookson, NIA, National Institutes of Health, for providing the DJ-1 construct. We also thank Dr. H. S. Athreya and Dr. Garima Jaipuria for their help with NMR spectroscopic studies, and we thank the Indian Institute of Science for use of the flow cytometry facility.
This work was supported by a Swarnajayanthi fellowship from the Department of Science and Technology (Grant ID: DST/SJF/LSA-01/2011–2012) and the Department of Biotechnology, India (to P. D.) and by a senior research fellowship from the Council of Scientific and Industrial Research, India (to K. B., A. V. G., and M. S.). The authors declare that they have no conflicts of interest with the contents of this article.
- MG
- methylglyoxal
- ROS
- reactive oxygen species
- PD
- Parkinson disease
- Ni2+-NTA
- nickel-nitrilotriacetic acid
- NAC
- N-acetylcysteine
- MTS
- mitochondrial targeting sequence
- YPD
- yeast extract-peptone-dextrose.
References
- 1.Wellen K. E., and Thompson C. B. (2010) Cellular metabolic stress: considering how cells respond to nutrient excess. Mol. Cell 40, 323–332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Treichel J. L., Henry M. M., Skumatz C. M., Eells J. T., and Burke J. M. (2003) Formate, the toxic metabolite of methanol, in cultured ocular cells. Neurotoxicology 24, 825–834 [DOI] [PubMed] [Google Scholar]
- 3.Sharpe J. A., Hostovsky M., Bilbao J. M., and Rewcastle N. B. (1982) Methanol optic neuropathy: a histopathological study. Neurology 32, 1093–1100 [DOI] [PubMed] [Google Scholar]
- 4.Thornalley P. J., Langborg A., and Minhas H. S. (1999) Formation of glyoxal, methylglyoxal and 3-deoxyglucosone in the glycation of proteins by glucose. Biochem. J. 344, 109–116 [PMC free article] [PubMed] [Google Scholar]
- 5.Mlakar A., Batna A., Dudda A., and Spiteller G. (1996) Iron (II) ions induced oxidation of ascorbic acid and glucose. Free Radic. Res. 25, 525–539 [DOI] [PubMed] [Google Scholar]
- 6.Wells-Knecht K. J., Zyzak D. V., Litchfield J. E., Thorpe S. R., and Baynes J. W. (1995) Mechanism of autoxidative glycosylation: identification of glyoxal and arabinose as intermediates in the autoxidative modification of proteins by glucose. Biochemistry 34, 3702–3709 [DOI] [PubMed] [Google Scholar]
- 7.Lee K. W., Simpson G., and Ortwerth B. (1999) A systematic approach to evaluate the modification of lens proteins by glycation-induced crosslinking. Biochim. Biophys. Acta 1453, 141–151 [DOI] [PubMed] [Google Scholar]
- 8.Ahmed M. U., Brinkmann Frye E., Degenhardt T. P., Thorpe S. R., and Baynes J. W. (1997) N-Epsilon-(carboxyethyl)lysine, a product of the chemical modification of proteins by methylglyoxal, increases with age in human lens proteins. Biochem. J. 324, 565–570 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Ahmed N., Argirov O. K., Minhas H. S., Cordeiro C. A., and Thornalley P. J. (2002) Assay of advanced glycation endproducts (AGEs): surveying AGEs by chromatographic assay with derivatization by 6-aminoquinolyl-N-hydroxysuccinimidyl-carbamate and application to N-epsilon-carboxymethyl-lysine- and N-epsilon-(1-carboxyethyl)lysine-modified albumin. Biochem. J. 364, 1–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Abordo E. A., Minhas H. S., and Thornalley P. J. (1999) Accumulation of α-oxoaldehydes during oxidative stress: a role in cytotoxicity. Biochem. Pharmacol. 58, 641–648 [DOI] [PubMed] [Google Scholar]
- 11.Shangari N., and O'Brien P. J. (2004) The cytotoxic mechanism of glyoxal involves oxidative stress. Biochem. Pharmacol. 68, 1433–1442 [DOI] [PubMed] [Google Scholar]
- 12.Thornalley P. J. (1996) Pharmacology of methylglyoxal: formation, modification of proteins and nucleic acids, and enzymatic detoxification: a role in pathogenesis and antiproliferative chemotherapy. Gen. Pharmacol. 27, 565–573 [DOI] [PubMed] [Google Scholar]
- 13.Kasai H., Iwamoto-Tanaka N., and Fukada S. (1998) DNA modifications by the mutagen glyoxal: adduction to G and C, deamination of C and GC, and GA cross-linking. Carcinogenesis 19, 1459–1465 [DOI] [PubMed] [Google Scholar]
- 14.Murata K., Fukuda Y., Simosaka M., Watanabe K., Saikusa T., and Kimura A. (1985) Metabolism of 2-oxoaldehyde in yeasts: purification and characterization of NADPH-dependent methylglyoxal-reducing enzyme from Saccharomyces cerevisiae. Eur. J. Biochem. 151, 631–636 [DOI] [PubMed] [Google Scholar]
- 15.Thornalley P. J. (1990) The glyoxalase system: new developments towards functional characterization of a metabolic pathway fundamental to biological life. Biochem. J. 269, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Fleury C., Mignotte B., and Vayssiere J. L. (2002) Mitochondrial reactive oxygen species in cell death signaling. Biochimie 84, 131–141 [DOI] [PubMed] [Google Scholar]
- 17.Thannickal V. J., and Fanburg B. L. (2000) Reactive oxygen species in cell signaling. Am. J. Physiol. Lung Cell. Mol. Physiol. 279, L1005–L1028 [DOI] [PubMed] [Google Scholar]
- 18.Adam-Vizi V., and Chinopoulos C. (2006) Bioenergetics and the formation of mitochondrial reactive oxygen species. Trends Pharmacol. Sci. 27, 639–645 [DOI] [PubMed] [Google Scholar]
- 19.Edens W. A., Sharling L., Cheng G., Shapira R., Kinkade J. M., Lee T., Edens H. A., Tang X., Sullards C., Flaherty D. B., Benian G. M., and Lambeth J. D. (2001) Tyrosine cross-linking of extracellular matrix is catalyzed by Duox, a multidomain oxidase/peroxidase with homology to the phagocyte oxidase subunit gp91phox. J. Cell Biol. 154, 879–891 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Smeitink J., van den Heuvel L., and DiMauro S. (2001) The genetics and pathology of oxidative phosphorylation. Nat. Rev. Genet. 2, 342–352 [DOI] [PubMed] [Google Scholar]
- 21.Chelikani P., Fita I., and Loewen P. C. (2004) Diversity of structures and properties among catalases. Cell. Mol. Life Sci. 61, 192–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.McCord J. M., and Fridovich I. (1969) Superoxide dismutase: an enzymic function for erythrocuprein (hemocuprein). J. Biol. Chem. 244, 6049–6055 [PubMed] [Google Scholar]
- 23.Pompella A., Visvikis A., Paolicchi A., De Tata V., and Casini A. F. (2003) The changing faces of glutathione, a cellular protagonist. Biochem. Pharmacol. 66, 1499–1503 [DOI] [PubMed] [Google Scholar]
- 24.Storz G., Christman M. F., Sies H., and Ames B. N. (1987) Spontaneous mutagenesis and oxidative damage to DNA in Salmonella typhimurium. Proc. Natl. Acad. Sci. U.S.A. 84, 8917–8921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rose S., Frye R. E., Slattery J., Wynne R., Tippett M., Pavliv O., Melnyk S., and James S. J. (2014) Oxidative stress induces mitochondrial dysfunction in a subset of autism lymphoblastoid cell lines in a well-matched case control cohort. PLoS ONE 9, e85436. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Li N., Brun T., Cnop M., Cunha D. A., Eizirik D. L., and Maechler P. (2009) Transient oxidative stress damages mitochondrial machinery inducing persistent beta-cell dysfunction. J. Biol. Chem. 284, 23602–23612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lin M. T., and Beal M. F. (2006) Mitochondrial dysfunction and oxidative stress in neurodegenerative diseases. Nature 443, 787–795 [DOI] [PubMed] [Google Scholar]
- 28.Dai D. F., Chiao Y. A., Marcinek D. J., Szeto H. H., and Rabinovitch P. S. (2014) Mitochondrial oxidative stress in aging and healthspan. Longev. Healthspan 3, 6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Damiano M., Galvan L., Déglon N., and Brouillet E. (2010) Mitochondria in Huntington's disease. Biochim. Biophys. Acta 1802, 52–61 [DOI] [PubMed] [Google Scholar]
- 30.Walker F. O. (2007) Huntington's disease. Lancet 369, 218–228 [DOI] [PubMed] [Google Scholar]
- 31.Davie C. A. (2008) A review of Parkinson's disease. Br. Med. Bull. 86, 109–127 [DOI] [PubMed] [Google Scholar]
- 32.Cookson M. R., and Bandmann O. (2010) Parkinson's disease: insights from pathways. Hum. Mol. Genet. 19, R21–R27 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Satake W., Nakabayashi Y., Mizuta I., Hirota Y., Ito C., Kubo M., Kawaguchi T., Tsunoda T., Watanabe M., Takeda A., Tomiyama H., Nakashima K., Hasegawa K., Obata F., Yoshikawa T., Kawakami H., Sakoda S., Yamamoto M., Hattori N., Murata M., Nakamura Y., and Toda T. (2009) Genome-wide association study identifies common variants at four loci as genetic risk factors for Parkinson's disease. Nat. Genet. 41, 1303–1307 [DOI] [PubMed] [Google Scholar]
- 34.Goswami A. V., Samaddar M., Sinha D., Purushotham J., and D'Silva P. (2012) Enhanced J-protein interaction and compromised protein stability of mtHsp70 variants lead to mitochondrial dysfunction in Parkinson's disease. Hum. Mol. Genet. 21, 3317–3332 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Dauer W., and Przedborski S. (2003) Parkinson's disease: mechanisms and models. Neuron 39, 889–909 [DOI] [PubMed] [Google Scholar]
- 36.Sulzer D. (2007) Multiple hit hypotheses for dopamine neuron loss in Parkinson's disease. Trends Neurosci. 30, 244–250 [DOI] [PubMed] [Google Scholar]
- 37.Chen J., Li L., and Chin L. S. (2010) Parkinson disease protein DJ-1 converts from a zymogen to a protease by carboxyl-terminal cleavage. Hum. Mol. Genet. 19, 2395–2408 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Shendelman S., Jonason A., Martinat C., Leete T., and Abeliovich A. (2004) DJ-1 is a redox-dependent molecular chaperone that inhibits α-synuclein aggregate formation. PLos Biol. 2, e362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Zhou W., Zhu M., Wilson M. A., Petsko G. A., and Fink A. L. (2006) The oxidation state of DJ-1 regulates its chaperone activity toward α-synuclein. J. Mol. Biol. 356, 1036–1048 [DOI] [PubMed] [Google Scholar]
- 40.Xu J., Zhong N., Wang H., Elias J. E., Kim C. Y., Woldman I., Pifl C., Gygi S. P., Geula C., and Yankner B. A. (2005) The Parkinson's disease-associated DJ-1 protein is a transcriptional co-activator that protects against neuronal apoptosis. Hum. Mol. Genet. 14, 1231–1241 [DOI] [PubMed] [Google Scholar]
- 41.van der Brug M. P., Blackinton J., Chandran J., Hao L. Y., Lal A., Mazan-Mamczarz K., Martindale J., Xie C., Ahmad R., Thomas K. J., Beilina A., Gibbs J. R., Ding J., Myers A. J., Zhan M., Cai H., Bonini N. M., Gorospe M., and Cookson M. R. (2008) RNA binding activity of the recessive parkinsonism protein DJ-1 supports involvement in multiple cellular pathways. Proc. Natl. Acad. Sci. U.S.A. 105, 10244–10249 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Kim R. H., Smith P. D., Aleyasin H., Hayley S., Mount M. P., Pownall S., Wakeham A., You-Ten A. J., Kalia S. K., Horne P., Westaway D., Lozano A. M., Anisman H., Park D. S., and Mak T. W. (2005) Hypersensitivity of DJ-1-deficient mice to 1-methyl-4-phenyl-1,2,3,6-tetrahydropyrindine (MPTP) and oxidative stress. Proc. Natl. Acad. Sci. U.S.A. 102, 5215–5220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Chen P., DeWitt M. R., Bornhorst J., Soares F. A., Mukhopadhyay S., Bowman A. B., and Aschner M. (2015) Age- and manganese-dependent modulation of dopaminergic phenotypes in a C. elegans DJ-1 genetic model of Parkinson's disease. Metallomics 7, 289–298 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Menzies F. M., Yenisetti S. C., and Min K. T. (2005) Roles of Drosophila DJ-1 in survival of dopaminergic neurons and oxidative stress. Curr. Biol. 15, 1578–1582 [DOI] [PubMed] [Google Scholar]
- 45.Meulener M., Whitworth A. J., Armstrong-Gold C. E., Rizzu P., Heutink P., Wes P. D., Pallanck L. J., and Bonini N. M. (2005) Drosophila DJ-1 mutants are selectively sensitive to environmental toxins associated with Parkinson's disease. Curr. Biol. 15, 1572–1577 [DOI] [PubMed] [Google Scholar]
- 46.Park J., Kim S. Y., Cha G. H., Lee S. B., Kim S., and Chung J. (2005) Drosophila DJ-1 mutants show oxidative stress-sensitive locomotive dysfunction. Gene 361, 133–139 [DOI] [PubMed] [Google Scholar]
- 47.Subedi K. P., Choi D., Kim I., Min B., and Park C. (2011) Hsp31 of Escherichia coli K-12 is glyoxalase III. Mol. Microbiol. 81, 926–936 [DOI] [PubMed] [Google Scholar]
- 48.Mujacic M., Bader M. W., and Baneyx F. (2004) Escherichia coli Hsp31 functions as a holding chaperone that cooperates with the DnaK-DnaJ-GrpE system in the management of protein misfolding under severe stress conditions. Mol. Microbiol. 51, 849–859 [DOI] [PubMed] [Google Scholar]
- 49.Mujacic M., and Baneyx F. (2007) Chaperone Hsp31 contributes to acid resistance in stationary-phase Escherichia coli. Appl. Environ. Microbiol. 73, 1014–1018 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Miller-Fleming L., Antas P., Pais T. F., Smalley J. L., Giorgini F., and Outeiro T. F. (2014) Yeast DJ-1 superfamily members are required for diauxic-shift reprogramming and cell survival in stationary phase. Proc. Natl. Acad. Sci. U.S.A. 111, 7012–7017 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Skoneczna A., Miciałkiewicz A., and Skoneczny M. (2007) Saccharomyces cerevisiae Hsp31p, a stress response protein conferring protection against reactive oxygen species. Free Radic. Biol. Med. 42, 1409–1420 [DOI] [PubMed] [Google Scholar]
- 52.Wilson M. A. (2014) Metabolic role for yeast DJ-1 superfamily proteins. Proc. Natl. Acad. Sci. U.S.A. 111, 6858–6859 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Janke C., Magiera M. M., Rathfelder N., Taxis C., Reber S., Maekawa H., Moreno-Borchart A., Doenges G., Schwob E., Schiebel E., and Knop M. (2004) A versatile toolbox for PCR-based tagging of yeast genes: new fluorescent proteins, more markers and promoter substitution cassettes. Yeast 21, 947–962 [DOI] [PubMed] [Google Scholar]
- 54.Kwolek-Mirek M., Bednarska S., Bartosz G., and Biliński T. (2009) Acrolein toxicity involves oxidative stress caused by glutathione depletion in the yeast Saccharomyces cerevisiae. Cell Biol. Toxicol. 25, 363–378 [DOI] [PubMed] [Google Scholar]
- 55.Nair S., Singh S. V., and Krishan A. (1991) Flow cytometric monitoring of glutathione content and anthracycline retention in tumor cells. Cytometry 12, 336–342 [DOI] [PubMed] [Google Scholar]
- 56.Apostolova N., Gomez-Sucerquia L. J., Moran A., Alvarez A., Blas-Garcia A., and Esplugues J. V. (2010) Enhanced oxidative stress and increased mitochondrial mass during efavirenz-induced apoptosis in human hepatic cells. Br. J. Pharmacol. 160, 2069–2084 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sinha D., Joshi N., Chittoor B., Samji P., and D'Silva P. (2010) Role of Magmas in protein transport and human mitochondria biogenesis. Hum. Mol. Genet. 19, 1248–1262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhao Q., Su Y., Wang Z., Chen C., Wu T., and Huang Y. (2014) Identification of glutathione (GSH)-independent glyoxalase III from Schizosaccharomyces pombe. BMC Evol. Biol. 14, 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 59.Hasim S., Hussin N. A., Alomar F., Bidasee K. R., Nickerson K. W., and Wilson M. A. (2014) A glutathione-independent glyoxalase of the DJ-1 superfamily plays an important role in managing metabolically generated methylglyoxal in Candida albicans. J. Biol. Chem. 289, 1662–1674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Lee J. Y., Song J., Kwon K., Jang S., Kim C., Baek K., Kim J., and Park C. (2012) Human DJ-1 and its homologs are novel glyoxalases. Hum. Mol. Genet. 21, 3215–3225 [DOI] [PubMed] [Google Scholar]
- 61.Wilson M. A., St Amour C. V., Collins J. L., Ringe D., and Petsko G. A. (2004) The 1.8-A resolution crystal structure of YDR533Cp from Saccharomyces cerevisiae: a member of the DJ-1/ThiJ/PfpI superfamily. Proc. Natl. Acad. Sci. U.S.A. 101, 1531–1536 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Suh K. S., Choi E. M., Rhee S. Y., and Kim Y. S. (2014) Methylglyoxal induces oxidative stress and mitochondrial dysfunction in osteoblastic MC3T3-E1 cells. Free Radic. Res. 48, 206–217 [DOI] [PubMed] [Google Scholar]
- 63.Du J., Suzuki H., Nagase F., Akhand A. A., Yokoyama T., Miyata T., Kurokawa K., and Nakashima I. (2000) Methylglyoxal induces apoptosis in Jurkat leukemia T cells by activating c-Jun N-terminal kinase. J. Cell. Biochem. 77, 333–344 [DOI] [PubMed] [Google Scholar]
- 64.Srivastava S., Sinha D., Saha P. P., Marthala H., and D'Silva P. (2014) Magmas functions as a ROS regulator and provides cytoprotection against oxidative stress-mediated damages. Cell Death Dis. 5, e1394. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Vernekar A. A., Sinha D., Srivastava S., Paramasivam P. U., D'Silva P., and Mugesh G. (2014) An antioxidant nanozyme that uncovers the cytoprotective potential of vanadia nanowires. Nat. Commun. 5, 5301. [DOI] [PubMed] [Google Scholar]
- 66.Mukhopadhyay P., Rajesh M., Yoshihiro K., Haskó G., and Pacher P. (2007) Simple quantitative detection of mitochondrial superoxide production in live cells. Biochem. Biophys. Res. Commun. 358, 203–208 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Saha P. P., Kumar S. K., Srivastava S., Sinha D., Pareek G., and D'Silva P. (2014) The presence of multiple cellular defects associated with a novel G50E iron-sulfur cluster scaffold protein (ISCU) mutation leads to development of mitochondrial myopathy. J. Biol. Chem. 289, 10359–10377 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Zafarullah M., Li W. Q., Sylvester J., and Ahmad M. (2003) Molecular mechanisms of N-acetylcysteine actions. Cell. Mol. Life Sci. 60, 6–20 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Grant C. M., MacIver F. H., and Dawes I. W. (1996) Glutathione is an essential metabolite required for resistance to oxidative stress in the yeast Saccharomyces cerevisiae. Curr. Genet. 29, 511–515 [DOI] [PubMed] [Google Scholar]
- 70.Penninckx M. J. (2002) An overview on glutathione in Saccharomyces versus non-conventional yeasts. FEMS Yeast Res. 2, 295–305 [DOI] [PubMed] [Google Scholar]
- 71.Grant C. M., Collinson L. P., Roe J. H., and Dawes I. W. (1996) Yeast glutathione reductase is required for protection against oxidative stress and is a target gene for yAP-1 transcriptional regulation. Mol. Microbiol. 21, 171–179 [DOI] [PubMed] [Google Scholar]
- 72.Williams A. C., and Ford W. C. (2004) Functional significance of the pentose phosphate pathway and glutathione reductase in the antioxidant defenses of human sperm. Biol. Reprod. 71, 1309–1316 [DOI] [PubMed] [Google Scholar]
- 73.Knox C., Sass E., Neupert W., and Pines O. (1998) Import into mitochondria, folding and retrograde movement of fumarase in yeast. J. Biol. Chem. 273, 25587–25593 [DOI] [PubMed] [Google Scholar]
- 74.John G. B., Anjum R., Khar A., and Nagaraj R. (2002) Subcellular localization and physiological consequences of introducing a mitochondrial matrix targeting signal sequence in Bax and its mutants. Exp. Cell Res. 278, 198–208 [DOI] [PubMed] [Google Scholar]
- 75.Canet-Avilés R. M., Wilson M. A., Miller D. W., Ahmad R., McLendon C., Bandyopadhyay S., Baptista M. J., Ringe D., Petsko G. A., and Cookson M. R. (2004) The Parkinson's disease protein DJ-1 is neuroprotective due to cysteine-sulfinic acid-driven mitochondrial localization. Proc. Natl. Acad. Sci. U.S.A. 101, 9103–9108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Hicke L. (2001) Protein regulation by monoubiquitin. Nat. Rev. Mol. Cell Biol. 2, 195–201 [DOI] [PubMed] [Google Scholar]
- 77.Clements C. M., McNally R. S., Conti B. J., Mak T. W., and Ting J. P. (2006) DJ-1, a cancer- and Parkinson's disease-associated protein, stabilizes the antioxidant transcriptional master regulator Nrf2. Proc. Natl. Acad. Sci. U.S.A. 103, 15091–15096 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Im J. Y., Lee K. W., Woo J. M., Junn E., and Mouradian M. M. (2012) DJ-1 induces thioredoxin 1 expression through the Nrf2 pathway. Hum. Mol. Genet. 21, 3013–3024 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Zhou W., and Freed C. R. (2005) DJ-1 up-regulates glutathione synthesis during oxidative stress and inhibits A53T α-synuclein toxicity. J. Biol. Chem. 280, 43150–43158 [DOI] [PubMed] [Google Scholar]
- 80.Morgan P. E., Sheahan P. J., and Davies M. J. (2014) Perturbation of human coronary artery endothelial cell redox state and NADPH generation by methylglyoxal. PLoS ONE 9, e86564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Wu S., Zhou F., Zhang Z., and Xing D. (2011) Mitochondrial oxidative stress causes mitochondrial fragmentation via differential modulation of mitochondrial fission-fusion proteins. FEBS J 278, 941–954 [DOI] [PubMed] [Google Scholar]
- 82.Outten C. E., and Culotta V. C. (2004) Alternative start sites in the Saccharomyces cerevisiae GLR1 gene are responsible for mitochondrial and cytosolic isoforms of glutathione reductase. J. Biol. Chem. 279, 7785–7791 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Hayashi T., Ishimori C., Takahashi-Niki K., Taira T., Kim Y. C., Maita H., Maita C., Ariga H., and Iguchi-Ariga S. M. (2009) DJ-1 binds to mitochondrial complex I and maintains its activity. Biochem. Biophys. Res. Commun. 390, 667–672 [DOI] [PubMed] [Google Scholar]
- 84.Ray S., Dutta S., Halder J., and Ray M. (1994) Inhibition of electron flow-through complex I of the mitochondrial respiratory chain of Ehrlich ascites carcinoma cells by methylglyoxal. Biochem. J. 303, 69–72 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Biswas S., Ray M., Misra S., Dutta D. P., and Ray S. (1997) Selective inhibition of mitochondrial respiration and glycolysis in human leukaemic leucocytes by methylglyoxal. Biochem. J. 323, 343–348 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Rosca M. G., Monnier V. M., Szweda L. I., and Weiss M. F. (2002) Alterations in renal mitochondrial respiration in response to the reactive oxoaldehyde methylglyoxal. Am. J. Physiol. Renal Physiol. 283, F52–F59 [DOI] [PubMed] [Google Scholar]
- 87.Mihoub M., Abdallah J., Gontero B., Dairou J., and Richarme G. (2015) The DJ-1 superfamily member Hsp31 repairs proteins from glycation by methylglyoxal and glyoxal. Biochem. Biophys. Res. Commun. 463, 1305–1310 [DOI] [PubMed] [Google Scholar]
- 88.Richarme G., Mihoub M., Dairou J., Bui L. C., Leger T., and Lamouri A. (2015) Parkinsonism-associated protein DJ-1/Park7 is a major protein deglycase that repairs methylglyoxal- and glyoxal-glycated cysteine, arginine, and lysine residues. J. Biol. Chem. 290, 1885–1897 [DOI] [PMC free article] [PubMed] [Google Scholar]