Abstract
For a long time, protein transport into the extracellular space was believed to strictly depend on signal peptide-mediated translocation into the lumen of the endoplasmic reticulum. More recently, this view has been challenged, and the molecular mechanisms of unconventional secretory processes are beginning to emerge. Here, we focus on unconventional secretion of fibroblast growth factor 2 (FGF2), a secretory mechanism that is based upon direct protein translocation across plasma membranes. Through a combination of genome-wide RNAi screening approaches and biochemical reconstitution experiments, the basic machinery of FGF2 secretion was identified and validated. This includes the integral membrane protein ATP1A1, the phosphoinositide phosphatidylinositol-4,5-bisphosphate (PI(4,5)P2), and Tec kinase, as well as membrane-proximal heparan sulfate proteoglycans on cell surfaces. Hallmarks of unconventional secretion of FGF2 are: (i) sequential molecular interactions with the inner leaflet along with Tec kinase-dependent tyrosine phosphorylation of FGF2, (ii) PI(4,5)P2-dependent oligomerization and membrane pore formation, and (iii) extracellular trapping of FGF2 mediated by heparan sulfate proteoglycans on cell surfaces. Here, we discuss new developments regarding this process including the mechanism of FGF2 oligomerization during membrane pore formation, the functional role of ATP1A1 in FGF2 secretion, and the possibility that other proteins secreted by unconventional means make use of a similar mechanism to reach the extracellular space. Furthermore, given the prominent role of extracellular FGF2 in tumor-induced angiogenesis, we will discuss possibilities to develop highly specific inhibitors of FGF2 secretion, a novel approach that may yield lead compounds with a high potential to develop into anti-cancer drugs.
Keywords: fibroblast growth factor (FGF), heparan sulfate, membrane transport, plasma membrane, tumor cell biology, Tec kinase, phosphoinositides, protein translocation across membranes, unconventional protein secretion
ER/Golgi-dependent versus Unconventional Protein Secretion
For many years, it has been a dogma in molecular cell biology that, in mammalian cells, transport of proteins into the extracellular space depends on signal peptide-mediated translocation into the endoplasmic reticulum (ER)2 (1). Then, transport of secretory proteins occurs through vesicular intermediates that travel via the Golgi apparatus to the cell surface. Upon membrane fusion of post-Golgi transport vesicles with the plasma membrane, secretory proteins are dispatched into the extracellular space (2, 3). However, with the identification of extracellular growth factors and cytokines that lack signal peptides, such as fibroblast growth factor 2 (FGF2) and interleukin 1β (IL-1β), it became clear that protein secretion from mammalian cells is mechanistically more diverse than previously assumed (4–9). In terms of molecular mechanisms, two major types of ER/Golgi-independent secretion of soluble cargoes have been identified: (i) secretion by direct translocation across the plasma membrane (with FGF2 being a classical example (8, 10, 11)) and (ii) secretion through vesicular intermediates such as autophagosomes or exosomes derived from multivesicular bodies (with IL-1β being an example (4, 7, 9)). In this review, we will focus on the pathway of unconventional secretion of FGF2. This secretory mechanism is based upon direct protein translocation across plasma membranes. It appears to represent an ancient mechanism that ensures secretion of proteins whose functionality could not be maintained when passing through the ER/Golgi system, for example by inactivation through O-glycosylation (12). Both cellular components and cis elements within FGF2 required for unconventional secretion have been identified, shedding light on the molecular mechanism by which FGF2 is secreted from mammalian cells.
The Unconventional Secretory Pathway of FGF2
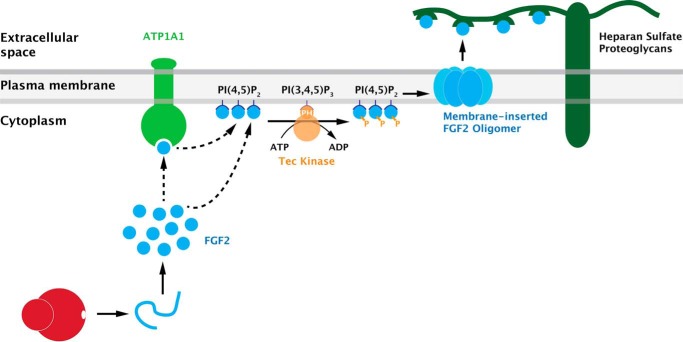
As illustrated in Fig. 1, unconventional secretion of FGF2 from cells is based upon direct translocation across plasma membranes (11, 13, 14). However, as opposed to other mechanisms of protein translocation across membranes (15, 16), FGF2 translocation into the extracellular space does not rely on a conventional protein-conducting channel translocating cargoes in an unfolded state. Rather, FGF2 membrane translocation is based upon the ability of FGF2 oligomers to form membrane pores in the plasma membrane (11, 17). This process is initiated by FGF2 recruitment to the inner leaflet mediated by the phosphoinositide PI(4,5)P2 (13, 18, 19). As a result, FGF2 undergoes oligomerization that, in turn, causes membrane insertion and the formation of membrane pores (11, 17). Recently, two cysteine residues on the molecular surface of FGF2 have been demonstrated to be critical for PI(4,5)P2-dependent oligomerization of FGF2 at membrane surfaces (20). They are required for the formation of intermolecular disulfide bridges that drive FGF2 oligomerization and membrane pore formation. Intriguingly, these cysteine residues are uniquely present in FGF2, i.e. they are absent from all FGF family members carrying signal peptides for ER/Golgi-dependent secretion. This observation suggests a specific role in unconventional secretion. Indeed, FGF2 variants lacking these surface cysteines are not secreted from cells with a phenotype being even stronger than what has previously been observed for FGF2 mutants that are deficient in binding to PI(4,5)P2 (18, 20).
FIGURE 1.
A current view of the molecular mechanism of unconventional secretion of FGF2. FGF2 secretion is mediated by direct translocation across the plasma membrane. This process involves sequential interactions of FGF2 with components at the inner leaflet, including ATP1A1 (the α subunit of the Na/K ATPase), the phosphoinositide PI(4,5)P2, and Tec kinase. As a result, membrane-inserted oligomers form in a PI(4,5)P2-dependent manner, structures that have been interpreted as intermediates in FGF2 membrane translocation. Membrane-proximal heparan sulfate proteoglycans are required to trap FGF2 on cell surfaces, the final step in the overall process of FGF2 secretion. Modified from research originally published in the Journal of Biological Chemistry. Zacherl, S., La Venuta, G., Müller, H. M., Wegehingel, S., Dimou, E., Sehr, P., Lewis, J. D., Erfle, H., Pepperkok, R., and Nickel, W. A direct role for ATP1A1 in unconventional secretion of fibroblast growth factor 2. J. Biol. Chem. 2015; 290: 3654–3665. © the American Society for Biochemistry and Molecular Biology.
As discussed above, the direct consequence of PI(4,5)P2-induced oligomerization of FGF2 is the formation of lipidic pores in plasma membranes, the key event in unconventional secretion of FGF2 (11, 13, 17, 20). The structure of FGF2-induced membrane pores has been proposed to be characterized by a toroidal architecture with the FGF2 oligomer in the center and the PI(4,5)P2 binding sites being localized in the periphery (11, 17). This conclusion was drawn from the observation that, upon FGF2 oligomerization and membrane pore formation, both membrane passage of small fluorescent tracer molecules and transbilayer diffusion of membrane lipids could be observed (11, 17). In support of this, diacylglycerol, a cone-shaped lipid that interferes with membrane curvature stabilized by PI(4,5)P2, was found to inhibit membrane pore formation by FGF2 oligomers (11, 17). Based upon these findings, the role of PI(4,5)P2 in unconventional secretion of FGF2 is likely to be three-fold: (i) recruitment of FGF2 at the plasma membrane, (ii) orienting FGF2 molecules at the inner leaflet favoring oligomerization, and (iii) stabilizing local curvature to allow for the formation of a membrane pore with a toroidal structure.
What Is the Precise Role of Membrane-inserted FGF2 Oligomers in FGF2 Membrane Translocation and How Do Cell Surface Heparan Sulfates Function in FGF2 Secretion?
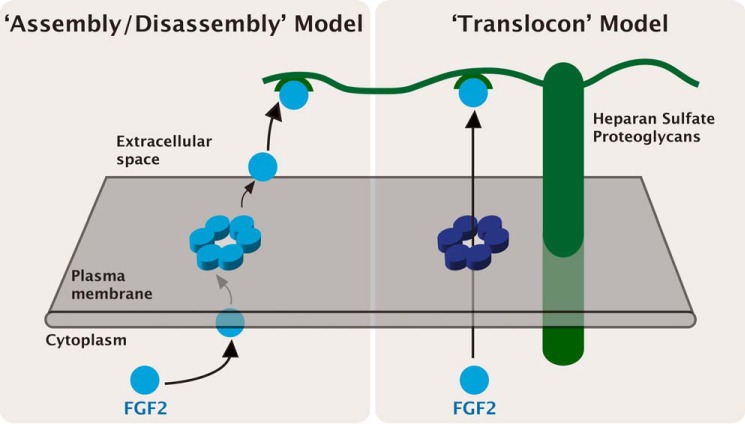
There are two principal possibilities of how membrane-inserted FGF2 oligomers could promote FGF2 membrane translocation (Fig. 2). One view is that FGF2 oligomers may insert into membranes only transiently followed by disassembly and release to the extracellular side (Fig. 2; “assembly/disassembly” model). This model has been discussed previously (11) and proposes membrane-inserted oligomers of FGF2 to be intermediate forms of the monomeric FGF2 cargo that is found on cell surfaces. Alternatively, FGF2 oligomers may form stable but highly dynamic membrane pores that can occur in open and closed states (Fig. 2; “translocon” model). Here, the role of the oligomer would be to act as a highly specific translocon mediating physical passage of FGF2 monomers across plasma membranes. Although not fully conclusive at this point, this model is supported by the recently observed tight association of FGF2 oligomers with membranes (21). The “translocon” model also eliminates the need of conversion of disulfide-linked FGF2 oligomers into monomers on the surface of plasma membranes, a process that would be difficult to explain considering the oxidative environment of the extracellular space. However, both models are consistent with the previously established role of membrane-proximal heparan sulfate proteoglycans on cell surfaces that form an extracellular trap required for FGF2 translocation (5, 6, 11, 13, 22). FGF2 firmly binds to heparan sulfates and, therefore, it is not released into cellular supernatants but rather remains associated with cell surfaces (23, 24). However, FGF2 undergoes intercellular spreading by direct cell-cell contacts (25). Thus, during the lifetime of a FGF2 molecule, the role of heparan sulfate proteoglycans is three-fold: (i) mediating the final step of FGF2 secretion (22, 25), (ii) protection of FGF2 on cell surfaces against degradation (26), and (iii) mediating FGF2 signaling as part of a ternary complex containing FGF2, heparan sulfates, and FGF high affinity receptors (27, 28). Based upon sequential interactions of FGF2 with PI(4,5)P2 at the inner leaflet and heparan sulfates on cell surfaces, the proposed mechanisms of FGF2 membrane translocation (Fig. 2) both offer a molecular basis for directionality of FGF2 transport into the extracellular space. Furthermore, both models are consistent with previous studies demonstrating FGF2 membrane translocation in a fully folded state (29, 30) as they require the formation of defined oligomers during membrane pore formation. In addition, molecular interactions of FGF2 with both PI(4,5)P2 and heparan sulfates depend on proper folding of FGF2 (30). Because FGF2 membrane translocation occurs at the level of plasma membranes, these findings suggest an intrinsic quality control mechanism that limits unconventional secretion to fully folded and therefore functional forms of FGF2 (30).
FIGURE 2.
Alternative models for FGF2 membrane translocation: the “assembly/disassembly” model versus the “translocon” model. The exact role of membrane-inserted FGF2 oligomers as intermediates in FGF2 membrane translocation is not fully understood. Two principal mechanisms have been postulated, the “assembly/disassembly” model and the “translocon” model.
The Role of Tec Kinase in Unconventional Secretion of FGF2
The role of membrane pores is further emphasized by the observation that Tec kinase, a non-receptor tyrosine kinase that has previously been described in the context of immune cell development and activation (31), is a regulatory factor of FGF2 secretion (Fig. 1). The role of Tec kinase in FGF2 secretion was initially recognized through a genome-wide RNAi screen designed to identify factors involved in this process (10, 32). Tec kinase was shown to phosphorylate FGF2, a process that facilitates the formation of membrane pores by FGF2 oligomers (13, 17). The role of Tec kinase-mediated tyrosine phosphorylation in FGF2 secretion from cells was further corroborated by demonstrating Tec kinase-independent secretion of phosphomimetic variant forms of FGF2 (32). Tec kinase contains a pleckstrin homology domain (PH domain) that mediates PI(3,4,5)P3-dependent membrane recruitment. Following activation of various kinds of receptors, PI(3,4,5)P3 levels increase, which, in turn, causes recruitment of Tec kinase to the inner leaflet. Tec kinase then becomes phosphorylated by plasma membrane-resident Src kinases or by autophosphorylation within its activation loop resulting in enzymatic activation (33). In its activated state, Tec kinase phosphorylates target proteins (34). Therefore, FGF2 phosphorylation is likely to occur at the inner leaflet of the plasma membrane (13). As FGF2 is a key signaling molecule in the context of many cancers, Tec kinase-regulated secretion of FGF2 represents an interesting link to the up-regulation of PI 3-kinases in many tumor cells (35). PI 3-kinases catalyze the formation of PI(3,4,5)P3, and high cellular levels of this phosphoinositide are likely to support efficient secretion of FGF2, which, in turn, promotes tumor cell proliferation.
ATP1A1, a New Component of the Unconventional Machinery Mediating FGF2 Secretion from Cells
As discussed above, a key approach in the identification of molecular components involved in FGF2 secretion has been a genome-wide RNAi screen that led to the identification of Tec kinase as a regulatory component of FGF2 secretion (13, 32). In addition to Tec kinase, this screen also revealed ATP1A1 as a gene product whose down-regulation causes a substantial drop in FGF2 secretion efficiency (36). ATP1A1 is the α1-chain of the Na/K-ATPase that is composed of a hetero-oligomer made from two α-chains and two β-chains. Both α-chains and β-chains come in four isoforms (ATP1A1–4; ATP1B1–4) that are differentially expressed in different types of cells and tissues (37). The α2/β2 hetero-oligomeric configuration is essential to form a functional Na/K-ATPase required for generating the electrochemical potential associated with the plasma membrane of mammalian cells (37). Intriguingly, down-regulation of ATP1B1 and ATP1B3 chains (the principal forms of β-chains expressed in HeLa cells) did not cause inhibition of FGF2 secretion, suggesting that it is not the fully assembled form of the Na/K-ATPase that is required for FGF2 secretion (36). In support of this idea, unassembled forms of ATP1A1 have indeed been proposed to exist in the plasma membrane based upon calculations of distinct turnover times of α-chains versus β-chains (38). The described findings are further consistent with earlier observations from other laboratories reporting inhibition of FGF2 secretion in the presence of ouabain, an inhibitor of the Na/K-ATPase (23, 39, 40). Intriguingly, these studies revealed that reagents compromising the membrane potential generated by the Na/K-ATPase do not inhibit FGF2 secretion (40). This suggests that the classical function of the fully assembled Na/K-ATPase is not required and, therefore, these studies support the idea of a role of ATP1A1 in FGF2 secretion based on its unassembled form.
Initial insight into the mechanism by which ATP1A1 participates in unconventional secretion of FGF2 was obtained, demonstrating a direct interaction between the cytoplasmic domain of ATP1A1 and FGF2 (36). Using purified recombinant components, a specific interaction with sub-micromolar affinity was determined. In addition, direct evidence for an interaction between endogenous ATP1A1 and FGF2 in cells was provided, employing the Duo-Link in situ proximity assay (36). Intriguingly, we recently found that in-cell interactions between the cytoplasmic domain of ATP1A1 and FGF2 are inhibited in the presence of ouabain.3 These findings provide a straightforward explanation for the inhibition of FGF2 secretion both in the presence of ouabain (23, 39, 40) and after down-regulation of ATP1A1 (36), suggesting a requirement for an interaction between ATP1A1 and FGF2 at the inner leaflet of plasma membranes. Thus, as illustrated in Fig. 1, a number of components of the molecular machinery required for FGF2 secretion have been identified, all of which are either permanently or transiently (Tec kinase following cellular activation) in physical association with the plasma membrane. At the inner leaflet, FGF2 interacts with at least three components, ATP1A1, PI(4,5)P2, and Tec kinase. As indicated by dashed arrows in Fig. 1, the sequence of these interactions is not fully established, especially with regard to the point of action of ATP1A1. However, based upon our current knowledge, the scheme shown in Fig. 1 is plausible with ATP1A1 acting as an initial contact at the inner leaflet. Following handover to PI(4,5)P2 along with Tec kinase-mediated phosphorylation of FGF2, membrane pore formation is initiated (Fig. 1). Irrespective of the precise sequence of events, the combined findings discussed here establish the plasma membrane as the subcellular site of FGF2 membrane translocation.
Are There Other Unconventionally Secreted Proteins That Form PI(4,5)P2-dependent Membrane Pores?
Recently, evidence was obtained suggesting that the molecular mechanism by which FGF2 is secreted from cells is also used by other proteins. Like FGF2, the HIV-1 transactivator of transcription (HIV-Tat) is an unconventionally secreted protein lacking a signal peptide (41). HIV-Tat is a small protein of 86–101 amino acids that is synthesized in very early steps of viral infection. HIV-Tat is a regulatory protein that is involved in viral gene expression by enhancing transcriptional rates and, therefore, is crucial for viral viability (42, 43). In addition to these classical functions, HIV-Tat is secreted from infected cells followed by uptake by non-infected cells (41, 44–46). An extracellular localization of HIV-Tat is also evident from studies demonstrating substantial levels of HIV-Tat in the serum of patients diseased with AIDS (47, 48). Despite the lack of a signal peptide, secretion of HIV-Tat was shown to occur independent of cell damage and, therefore, HIV-Tat was classified as a protein secreted by unconventional means (5, 8, 11, 41, 49–51). Extracellular HIV-Tat is supposed to be essential for viral spread and plays an important role in AIDS pathogenesis by modulating the immune response (49–51). Recent studies indicate that vaccination with anti-Tat antibodies slows down AIDS progression and may restore immune functions (52–54). Intriguingly, HIV-Tat has previously been demonstrated to exit infected T cells in a PI(4,5)P2-dependent manner (41, 55, 56). Using reconstitution experiments, it has now been shown that HIV-Tat has properties similar to FGF2. These properties include PI(4,5)P2-dependent membrane binding, oligomerization, and membrane pore formation (21). Also, HIV-Tat has been demonstrated to bind to heparan sulfate proteoglycans (44) that may play a similar role in HIV-Tat secretion when compared with what is known for FGF2 (22, 25). These findings point at a general mechanism by which at least one sub-group of proteins lacking signal peptides are secreted from mammalian cells based upon PI(4,5)P2-dependent pore formation in the plasma membrane.
Outlook
As outlined above, the molecular mechanism explaining how proteins without signal peptides such as FGF2 can be secreted from cells is beginning to emerge. Beyond the intriguing capability of FGF2 to physically traverse plasma membranes utilizing a previously unknown mechanism of protein translocation across membranes, these insights lead to new strategies in drug development for cancer therapy. With attempts to use inhibitors of FGF receptor tyrosine kinases in cancer therapy turning out to be challenging (57), it appears highly attractive to develop inhibitors that block FGF2 secretion from tumor cells. This approach is likely to yield highly specific lead compounds as transport of the vast majority of secretory proteins does rely on a different mechanism, the ER/Golgi-dependent pathway. Based upon the schematic shown in Fig. 1, obvious targets for lead compound development are interactions of FGF2 with machinery components at the inner leaflet, i.e. PI(4,5)P2, Tec kinase, and ATP1A1. Furthermore, it appears promising to develop inhibitors that prevent PI(4,5)P2-dependent oligomerization of FGF2. Such compounds would have the potential to block the formation of FGF2-induced membrane pores, the key intermediates in unconventional secretion of FGF2. With such inhibitors at hand, a new class of drugs targeting tumor-induced angiogenesis may become available. In addition, such drugs might have the potential to target the role of FGF2 as a survival factor of tumor cells, a process that depends on an autocrine FGF2 secretion/signaling loop and causes a block of tumor cell apoptosis (58).
This work was supported by the German Research Council (DFG-SFB 638, DFG-SFB/TRR 83, DFG Ni 423/6-1 and DFG-GRK1188) and the AID-NET program of the Federal Ministry for Education and Research of Germany, as well as the DFG Cluster of Excellence, CellNetworks. The authors declare that they have no conflicts of interest with the contents of this article.
C. Legrand and W. Nickel, unpublished results.
- ER
- endoplasmic reticulum
- HIV-Tat
- human immunodeficiency virus transactivator of transcription
- PI(4,5)P2
- phosphatidylinositol 4,5-bisphosphate
- PI(3,4,5)P3
- phosphatidylinositol 3,4,5-triphosphate.
References
- 1.Rapoport T. A. (2007) Protein translocation across the eukaryotic endoplasmic reticulum and bacterial plasma membranes. Nature 450, 663–669 [DOI] [PubMed] [Google Scholar]
- 2.Rothman J. E. (1994) Mechanisms of intracellular protein transport. Nature 372, 55–63 [DOI] [PubMed] [Google Scholar]
- 3.Rothman J. E., and Wieland F. T. (1996) Protein sorting by transport vesicles. Science 272, 227–234 [DOI] [PubMed] [Google Scholar]
- 4.Malhotra V. (2013) Unconventional protein secretion: an evolving mechanism. EMBO J. 32, 1660–1664 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Nickel W., and Rabouille C. (2009) Mechanisms of regulated unconventional protein secretion. Nat. Rev. Mol. Cell Biol. 10, 148–155 [DOI] [PubMed] [Google Scholar]
- 6.Nickel W., and Seedorf M. (2008) Unconventional mechanisms of protein transport to the cell surface of eukaryotic cells. Annu. Rev. Cell Dev. Biol. 24, 287–308 [DOI] [PubMed] [Google Scholar]
- 7.Piccioli P., and Rubartelli A. (2013) The secretion of IL-1β and options for release. Semin. Immunol. 25, 425–429 [DOI] [PubMed] [Google Scholar]
- 8.Rabouille C., Malhotra V., and Nickel W. (2012) Diversity in unconventional protein secretion. J. Cell Sci. 125, 5251–5255 [DOI] [PubMed] [Google Scholar]
- 9.Zhang M., and Schekman R. (2013) Cell biology: unconventional secretion, unconventional solutions. Science 340, 559–561 [DOI] [PubMed] [Google Scholar]
- 10.Nickel W. (2010) Pathways of unconventional protein secretion. Curr. Opin. Biotechnol. 21, 621–626 [DOI] [PubMed] [Google Scholar]
- 11.Steringer J. P., Müller H. M., and Nickel W. (2015) Unconventional secretion of fibroblast growth factor 2: a novel type of protein translocation across membranes? J. Mol. Biol. 427, 1202–1210 [DOI] [PubMed] [Google Scholar]
- 12.Wegehingel S., Zehe C., and Nickel W. (2008) Rerouting of fibroblast growth factor 2 to the classical secretory pathway results in post-translational modifications that block binding to heparan sulfate proteoglycans. FEBS Lett. 582, 2387–2392 [DOI] [PubMed] [Google Scholar]
- 13.Nickel W. (2011) The unconventional secretory machinery of fibroblast growth factor 2. Traffic 12, 799–805 [DOI] [PubMed] [Google Scholar]
- 14.Schäfer T., Zentgraf H., Zehe C., Brügger B., Bernhagen J., and Nickel W. (2004) Unconventional secretion of fibroblast growth factor 2 is mediated by direct translocation across the plasma membrane of mammalian cells. J. Biol. Chem. 279, 6244–6251 [DOI] [PubMed] [Google Scholar]
- 15.Neupert W., and Herrmann J. M. (2007) Translocation of proteins into mitochondria. Annu. Rev. Biochem. 76, 723–749 [DOI] [PubMed] [Google Scholar]
- 16.Osborne A. R., Rapoport T. A., and van den Berg B. (2005) Protein translocation by the Sec61/SecY channel. Annu. Rev. Cell Dev. Biol. 21, 529–550 [DOI] [PubMed] [Google Scholar]
- 17.Steringer J. P., Bleicken S., Andreas H., Zacherl S., Laussmann M., Temmerman K., Contreras F. X., Bharat T. A., Lechner J., Müller H. M., Briggs J. A., García-Sáez A. J., and Nickel W. (2012) Phosphatidylinositol 4,5-bisphosphate (PI(4,5)P2)-dependent oligomerization of fibroblast growth factor 2 (FGF2) triggers the formation of a lipidic membrane pore implicated in unconventional secretion. J. Biol. Chem. 287, 27659–27669 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Temmerman K., Ebert A. D., Müller H. M., Sinning I., Tews I., and Nickel W. (2008) A direct role for phosphatidylinositol-4,5-bisphosphate in unconventional secretion of fibroblast growth factor 2. Traffic 9, 1204–1217 [DOI] [PubMed] [Google Scholar]
- 19.Temmerman K., and Nickel W. (2009) A novel flow cytometric assay to quantify interactions between proteins and membrane lipids. J. Lipid Res. 50, 1245–1254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Müller H. M., Steringer J. P., Wegehingel S., Bleicken S., Münster M., Dimou E., Unger S., Weidmann G., Andreas H., García-Sáez A. J., Wild K., Sinning I., and Nickel W. (2015) Formation of disulfide bridges drives oligomerization, membrane pore formation and translocation of fibroblast growth factor 2 to cell surfaces. J. Biol. Chem. 290, 8925–8937 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Zeitler M., Steringer J. P., Müller H. M., Mayer M. P., and Nickel W. (2015) HIV-Tat forms phosphoinositide-dependent membrane pores implicated in unconventional protein secretion. J. Biol. Chem. 290, 21976–21984 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Nickel W. (2007) Unconventional secretion: an extracellular trap for export of fibroblast growth factor 2. J. Cell Sci. 120, 2295–2299 [DOI] [PubMed] [Google Scholar]
- 23.Engling A., Backhaus R., Stegmayer C., Zehe C., Seelenmeyer C., Kehlenbach A., Schwappach B., Wegehingel S., and Nickel W. (2002) Biosynthetic FGF-2 is targeted to non-lipid raft microdomains following translocation to the extracellular surface of CHO cells. J. Cell Sci. 115, 3619–3631 [DOI] [PubMed] [Google Scholar]
- 24.Trudel C., Faure-Desire V., Florkiewicz R. Z., and Baird A. (2000) Translocation of FGF2 to the cell surface without release into conditioned media. J. Cell Physiol. 185, 260–268 [DOI] [PubMed] [Google Scholar]
- 25.Zehe C., Engling A., Wegehingel S., Schäfer T., and Nickel W. (2006) Cell-surface heparan sulfate proteoglycans are essential components of the unconventional export machinery of FGF-2. Proc. Natl. Acad. Sci. U.S.A. 103, 15479–15484 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Nugent M. A., and Iozzo R. V. (2000) Fibroblast growth factor-2. Int. J. Biochem. Cell Biol. 32, 115–120 [DOI] [PubMed] [Google Scholar]
- 27.Presta M., Dell'Era P., Mitola S., Moroni E., Ronca R., and Rusnati M. (2005) Fibroblast growth factor/fibroblast growth factor receptor system in angiogenesis. Cytokine Growth Factor Rev. 16, 159–178 [DOI] [PubMed] [Google Scholar]
- 28.Ribatti D., Vacca A., Rusnati M., and Presta M. (2007) The discovery of basic fibroblast growth factor/fibroblast growth factor-2 and its role in haematological malignancies. Cytokine Growth Factor Rev. 18, 327–334 [DOI] [PubMed] [Google Scholar]
- 29.Backhaus R., Zehe C., Wegehingel S., Kehlenbach A., Schwappach B., and Nickel W. (2004) Unconventional protein secretion: membrane translocation of FGF-2 does not require protein unfolding. J. Cell Sci. 117, 1727–1736 [DOI] [PubMed] [Google Scholar]
- 30.Torrado L. C., Temmerman K., Müller H. M., Mayer M. P., Seelenmeyer C., Backhaus R., and Nickel W. (2009) An intrinsic quality-control mechanism ensures unconventional secretion of fibroblast growth factor 2 in a folded conformation. J. Cell Sci. 122, 3322–3329 [DOI] [PubMed] [Google Scholar]
- 31.Yang W. C., Collette Y., Nunès J. A., and Olive D. (2000) Tec kinases: a family with multiple roles in immunity. Immunity 12, 373–382 [DOI] [PubMed] [Google Scholar]
- 32.Ebert A. D., Laussmann M., Wegehingel S., Kaderali L., Erfle H., Reichert J., Lechner J., Beer H. D., Pepperkok R., and Nickel W. (2010) Tec-kinase-mediated phosphorylation of fibroblast growth factor 2 is essential for unconventional secretion. Traffic 11, 813–826 [DOI] [PubMed] [Google Scholar]
- 33.Bradshaw J. M. (2010) The Src, Syk, and Tec family kinases: distinct types of molecular switches. Cell. Signal. 22, 1175–1184 [DOI] [PubMed] [Google Scholar]
- 34.Lewis C. M., Broussard C., Czar M. J., and Schwartzberg P. L. (2001) Tec kinases: modulators of lymphocyte signaling and development. Curr. Opin. Immunol. 13, 317–325 [DOI] [PubMed] [Google Scholar]
- 35.Liu P., Cheng H., Roberts T. M., and Zhao J. J. (2009) Targeting the phosphoinositide 3-kinase pathway in cancer. Nat. Rev. Drug Discov. 8, 627–644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Zacherl S., La Venuta G., Müller H. M., Wegehingel S., Dimou E., Sehr P., Lewis J. D., Erfle H., Pepperkok R., and Nickel W. (2015) A direct role for ATP1A1 in unconventional secretion of fibroblast growth factor 2. J. Biol. Chem. 290, 3654–3665 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Kaplan J. H. (2002) Biochemistry of Na,K-ATPase. Annu. Rev. Biochem. 71, 511–535 [DOI] [PubMed] [Google Scholar]
- 38.Yoshimura S. H., Iwasaka S., Schwarz W., and Takeyasu K. (2008) Fast degradation of the auxiliary subunit of Na+/K+-ATPase in the plasma membrane of HeLa cells. J. Cell Sci. 121, 2159–2168 [DOI] [PubMed] [Google Scholar]
- 39.Dahl J. P., Binda A., Canfield V. A., and Levenson R. (2000) Participation of Na,K-ATPase in FGF-2 secretion: rescue of ouabain-inhibitable FGF-2 secretion by ouabain-resistant Na,K-ATPase α subunits. Biochemistry 39, 14877–14883 [DOI] [PubMed] [Google Scholar]
- 40.Florkiewicz R. Z., Anchin J., and Baird A. (1998) The inhibition of fibroblast growth factor-2 export by cardenolides implies a novel function for the catalytic subunit of Na+,K+-ATPase. J. Biol. Chem. 273, 544–551 [DOI] [PubMed] [Google Scholar]
- 41.Debaisieux S., Rayne F., Yezid H., and Beaumelle B. (2012) The ins and outs of HIV-1 Tat. Traffic 13, 355–363 [DOI] [PubMed] [Google Scholar]
- 42.Campbell G. R., and Loret E. P. (2009) What does the structure-function relationship of the HIV-1 Tat protein teach us about developing an AIDS vaccine? Retrovirology 6, 50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Ruben S., Perkins A., Purcell R., Joung K., Sia R., Burghoff R., Haseltine W. A., and Rosen C. A. (1989) Structural and functional characterization of human immunodeficiency virus tat protein. J. Virol. 63, 1–8 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Chang H. C., Samaniego F., Nair B. C., Buonaguro L., and Ensoli B. (1997) HIV-1 Tat protein exits from cells via a leaderless secretory pathway and binds to extracellular matrix-associated heparan sulfate proteoglycans through its basic region. Aids 11, 1421–1431 [DOI] [PubMed] [Google Scholar]
- 45.Frankel A. D., and Pabo C. O. (1988) Cellular uptake of the tat protein from human immunodeficiency virus. Cell 55, 1189–1193 [DOI] [PubMed] [Google Scholar]
- 46.Tyagi M., Rusnati M., Presta M., and Giacca M. (2001) Internalization of HIV-1 tat requires cell surface heparan sulfate proteoglycans. J. Biol. Chem. 276, 3254–3261 [DOI] [PubMed] [Google Scholar]
- 47.Shi B., Raina J., Lorenzo A., Busciglio J., and Gabuzda D. (1998) Neuronal apoptosis induced by HIV-1 Tat protein and TNF-α: potentiation of neurotoxicity mediated by oxidative stress and implications for HIV-1 dementia. J. Neurovirol. 4, 281–290 [DOI] [PubMed] [Google Scholar]
- 48.Xiao H., Neuveut C., Tiffany H. L., Benkirane M., Rich E. A., Murphy P. M., and Jeang K. T. (2000) Selective CXCR4 antagonism by Tat: implications for in vivo expansion of coreceptor use by HIV-1. Proc. Natl. Acad. Sci. U.S.A. 97, 11466–11471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Ensoli B., Barillari G., Salahuddin S. Z., Gallo R. C., and Wong-Staal F. (1990) Tat protein of HIV-1 stimulates growth of cells derived from Kaposi's sarcoma lesions of AIDS patients. Nature 345, 84–86 [DOI] [PubMed] [Google Scholar]
- 50.Ensoli B., Buonaguro L., Barillari G., Fiorelli V., Gendelman R., Morgan R. A., Wingfield P., and Gallo R. C. (1993) Release, uptake, and effects of extracellular human immunodeficiency virus type 1 Tat protein on cell growth and viral transactivation. J. Virol. 67, 277–287 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Rubartelli A., Poggi A., Sitia R., and Zocchi M. R. (1998) HIV-I Tat: a polypeptide for all seasons. Immunol. Today 19, 543–545 [DOI] [PubMed] [Google Scholar]
- 52.Bellino S., Tripiciano A., Picconi O., Francavilla V., Longo O., Sgadari C., Paniccia G., Arancio A., Angarano G., Ladisa N., Lazzarin A., Tambussi G., Nozza S., Torti C., Focà E., Palamara G., Latini A., Sighinolfi L., Mazzotta F., Di Pietro M., Di Perri G., Bonora S., Mercurio V. S., Mussini C., Gori A., Galli M., Monini P., Cafaro A., Ensoli F., and Ensoli B. (2014) The presence of anti-Tat antibodies in HIV-infected individuals is associated with containment of CD4+ T-cell decay and viral load, and with delay of disease progression: results of a 3-year cohort study. Retrovirology 11, 49. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Ensoli B., Fiorelli V., Ensoli F., Cafaro A., Titti F., Buttò S., Monini P., Magnani M., Caputo A., and Garaci E. (2006) Candidate HIV-1 Tat vaccine development: from basic science to clinical trials. AIDS 20, 2245–2261 [DOI] [PubMed] [Google Scholar]
- 54.Ensoli F., Cafaro A., Casabianca A., Tripiciano A., Bellino S., Longo O., Francavilla V., Picconi O., Sgadari C., Moretti S., Cossut M. R., Arancio A., Orlandi C., Sernicola L., Maggiorella M. T., Paniccia G., Mussini C., Lazzarin A., Sighinolfi L., Palamara G., Gori A., Angarano G., Di Pietro M., Galli M., Mercurio V. S., Castelli F., Di Perri G., Monini P., Magnani M., Garaci E., and Ensoli B. (2015) HIV-1 Tat immunization restores immune homeostasis and attacks the HAART-resistant blood HIV DNA: results of a randomized phase II exploratory clinical trial. Retrovirology 12, 33. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Rayne F., Debaisieux S., Bonhoure A., and Beaumelle B. (2010) HIV-1 Tat is unconventionally secreted through the plasma membrane. Cell Biol. Int. 34, 409–413 [DOI] [PubMed] [Google Scholar]
- 56.Rayne F., Debaisieux S., Yezid H., Lin Y. L., Mettling C., Konate K., Chazal N., Arold S. T., Pugnière M., Sanchez F., Bonhoure A., Briant L., Loret E., Roy C., and Beaumelle B. (2010) Phosphatidylinositol-(4,5)-bisphosphate enables efficient secretion of HIV-1 Tat by infected T-cells. EMBO J. 29, 1348–1362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Touat M., Ileana E., Postel-Vinay S., André F., and Soria J. C. (2015) Targeting FGFR signaling in cancer. Clin. Cancer Res. 21, 2684–2694 [DOI] [PubMed] [Google Scholar]
- 58.Pardo O. E., Wellbrock C., Khanzada U. K., Aubert M., Arozarena I., Davidson S., Bowen F., Parker P. J., Filonenko V. V., Gout I. T., Sebire N., Marais R., Downward J., and Seckl M. J. (2006) FGF-2 protects small cell lung cancer cells from apoptosis through a complex involving PKCϵ, B-Raf and S6K2. EMBO J. 25, 3078–3088 [DOI] [PMC free article] [PubMed] [Google Scholar]