Abstract
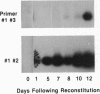
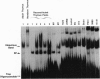
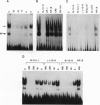
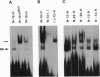
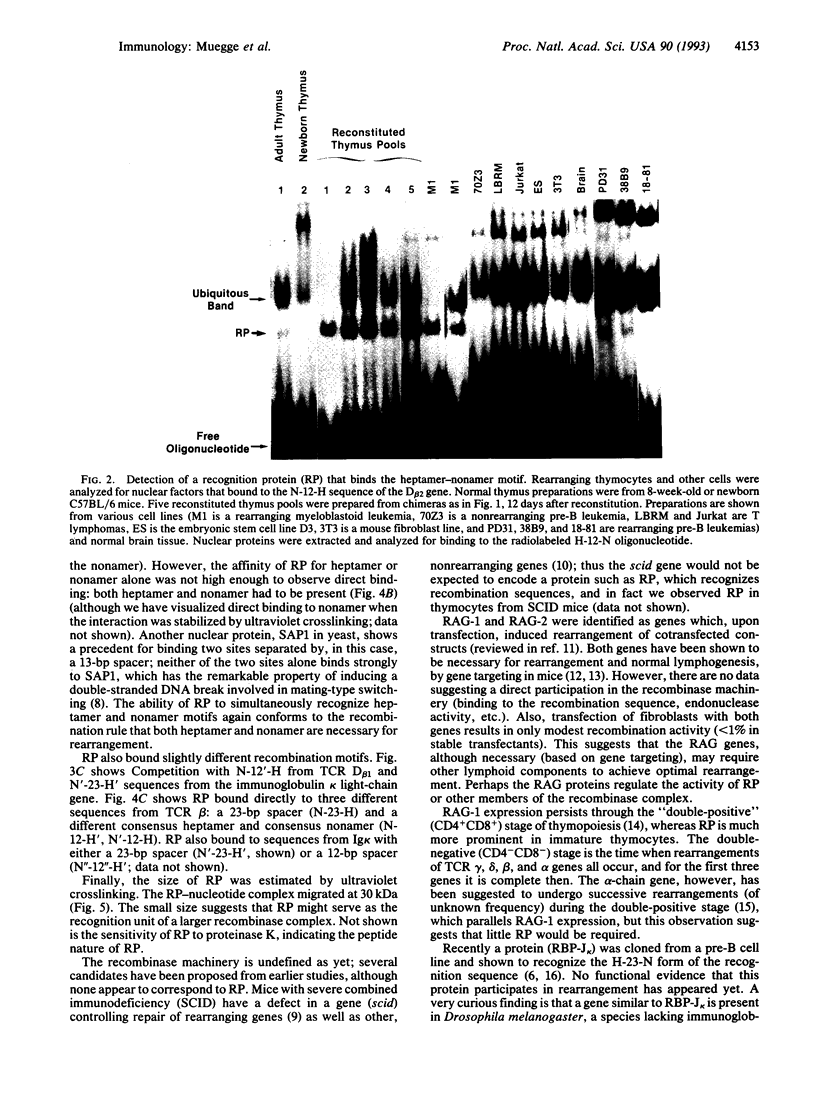
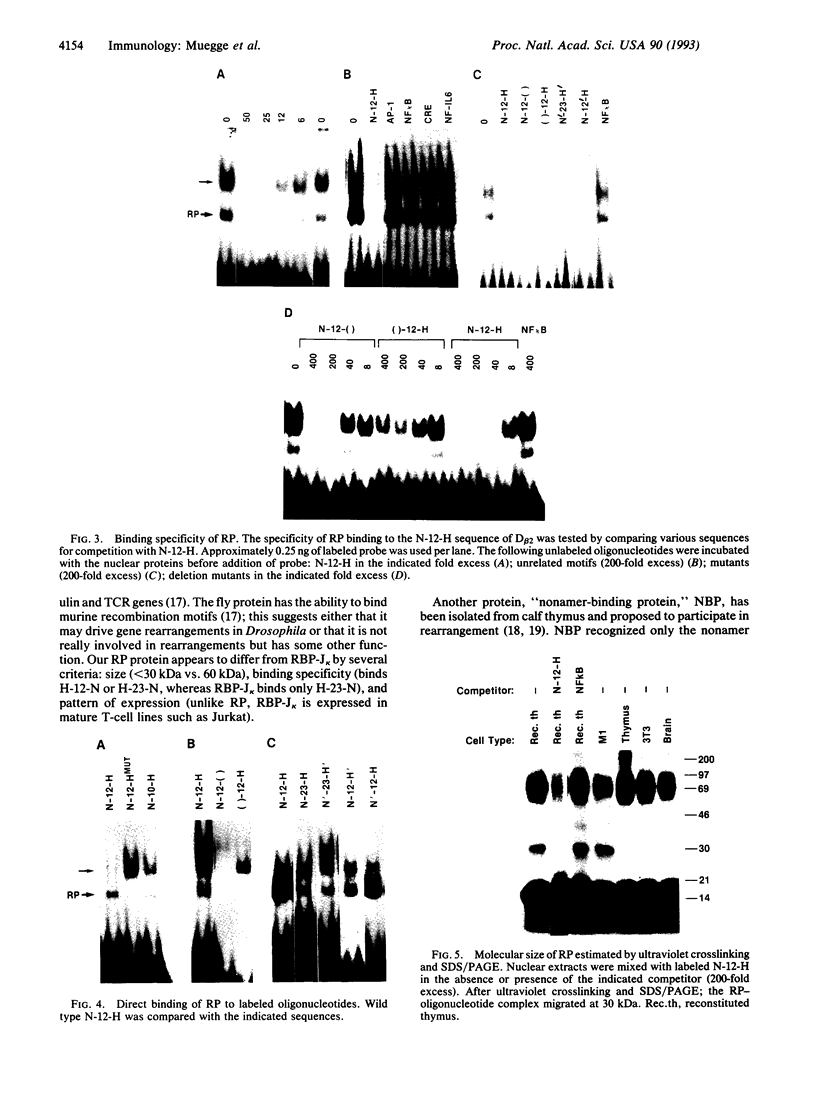
Rearrangement of T-cell antigen receptor and immunoglobulin genes occurs in immature lymphoid cells by an unknown mechanism. To identify components of the rearrangement machinery, we isolated a population of murine thymocytes enriched for rearranging pre-T cells. In the nuclear fraction of these cells, we detected a protein that specifically bound the recombination sequences that flank T-cell receptor and immunoglobulin genes and are required for their rearrangement. This protein recognized both heptamer and nonamer motifs of the recombination sequence, separated by either 12 or 23 bp. The protein complexed with the recombination sequence oligonucleotide had an apparent molecular mass of 30 kDa. The binding characteristics of the protein and its presence in rearranging thymocytes and cell lines suggest that it could serve as the recognition unit of a recombinase complex.
Full text
PDF


Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Akira S., Okazaki K., Sakano H. Two pairs of recombination signals are sufficient to cause immunoglobulin V-(D)-J joining. Science. 1987 Nov 20;238(4830):1134–1138. doi: 10.1126/science.3120312. [DOI] [PubMed] [Google Scholar]
- Arcangioli B., Klar A. J. A novel switch-activating site (SAS1) and its cognate binding factor (SAP1) required for efficient mat1 switching in Schizosaccharomyces pombe. EMBO J. 1991 Oct;10(10):3025–3032. doi: 10.1002/j.1460-2075.1991.tb07853.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borgulya P., Kishi H., Uematsu Y., von Boehmer H. Exclusion and inclusion of alpha and beta T cell receptor alleles. Cell. 1992 May 1;69(3):529–537. doi: 10.1016/0092-8674(92)90453-j. [DOI] [PubMed] [Google Scholar]
- Bosma M. J., Carroll A. M. The SCID mouse mutant: definition, characterization, and potential uses. Annu Rev Immunol. 1991;9:323–350. doi: 10.1146/annurev.iy.09.040191.001543. [DOI] [PubMed] [Google Scholar]
- Fulop G. M., Phillips R. A. The scid mutation in mice causes a general defect in DNA repair. Nature. 1990 Oct 4;347(6292):479–482. doi: 10.1038/347479a0. [DOI] [PubMed] [Google Scholar]
- Furukawa T., Kawaichi M., Matsunami N., Ryo H., Nishida Y., Honjo T. The Drosophila RBP-J kappa gene encodes the binding protein for the immunoglobulin J kappa recombination signal sequence. J Biol Chem. 1991 Dec 5;266(34):23334–23340. [PubMed] [Google Scholar]
- Guy-Grand D., Vanden Broecke C., Briottet C., Malassis-Seris M., Selz F., Vassalli P. Different expression of the recombination activity gene RAG-1 in various populations of thymocytes, peripheral T cells and gut thymus-independent intraepithelial lymphocytes suggests two pathways of T cell receptor rearrangement. Eur J Immunol. 1992 Feb;22(2):505–510. doi: 10.1002/eji.1830220232. [DOI] [PubMed] [Google Scholar]
- Halligan B. D., Desiderio S. V. Identification of a DNA binding protein that recognizes the nonamer recombinational signal sequence of immunoglobulin genes. Proc Natl Acad Sci U S A. 1987 Oct;84(20):7019–7023. doi: 10.1073/pnas.84.20.7019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hesse J. E., Lieber M. R., Mizuuchi K., Gellert M. V(D)J recombination: a functional definition of the joining signals. Genes Dev. 1989 Jul;3(7):1053–1061. doi: 10.1101/gad.3.7.1053. [DOI] [PubMed] [Google Scholar]
- Lewis S., Gellert M. The mechanism of antigen receptor gene assembly. Cell. 1989 Nov 17;59(4):585–588. doi: 10.1016/0092-8674(89)90002-0. [DOI] [PubMed] [Google Scholar]
- Li M., Morzycka-Wroblewska E., Desiderio S. V. NBP, a protein that specifically binds an enhancer of immunoglobulin gene rearrangement: purification and characterization. Genes Dev. 1989 Nov;3(11):1801–1813. doi: 10.1101/gad.3.11.1801. [DOI] [PubMed] [Google Scholar]
- Lieber M. R., Hesse J. E., Mizuuchi K., Gellert M. Developmental stage specificity of the lymphoid V(D)J recombination activity. Genes Dev. 1987 Oct;1(8):751–761. doi: 10.1101/gad.1.8.751. [DOI] [PubMed] [Google Scholar]
- Matsunami N., Hamaguchi Y., Yamamoto Y., Kuze K., Kangawa K., Matsuo H., Kawaichi M., Honjo T. A protein binding to the J kappa recombination sequence of immunoglobulin genes contains a sequence related to the integrase motif. Nature. 1989 Dec 21;342(6252):934–937. doi: 10.1038/342934a0. [DOI] [PubMed] [Google Scholar]
- Miyake S., Sugiyama H., Tani Y., Fukuda T., Kishimoto S. Identification of a recombinational signal sequence-specific DNA-binding protein(s) of Mr 115,000 in the nuclear extracts from immature lymphoid cell lines. J Immunogenet. 1990 Feb-Apr;17(1-2):67–75. doi: 10.1111/j.1744-313x.1990.tb00860.x. [DOI] [PubMed] [Google Scholar]
- Mombaerts P., Iacomini J., Johnson R. S., Herrup K., Tonegawa S., Papaioannou V. E. RAG-1-deficient mice have no mature B and T lymphocytes. Cell. 1992 Mar 6;68(5):869–877. doi: 10.1016/0092-8674(92)90030-g. [DOI] [PubMed] [Google Scholar]
- Muegge K., Williams T. M., Kant J., Karin M., Chiu R., Schmidt A., Siebenlist U., Young H. A., Durum S. K. Interleukin-1 costimulatory activity on the interleukin-2 promoter via AP-1. Science. 1989 Oct 13;246(4927):249–251. doi: 10.1126/science.2799385. [DOI] [PubMed] [Google Scholar]
- Oettinger M. A., Schatz D. G., Gorka C., Baltimore D. RAG-1 and RAG-2, adjacent genes that synergistically activate V(D)J recombination. Science. 1990 Jun 22;248(4962):1517–1523. doi: 10.1126/science.2360047. [DOI] [PubMed] [Google Scholar]
- Parker C. S., Topol J. A Drosophila RNA polymerase II transcription factor contains a promoter-region-specific DNA-binding activity. Cell. 1984 Feb;36(2):357–369. doi: 10.1016/0092-8674(84)90229-0. [DOI] [PubMed] [Google Scholar]
- Shinkai Y., Rathbun G., Lam K. P., Oltz E. M., Stewart V., Mendelsohn M., Charron J., Datta M., Young F., Stall A. M. RAG-2-deficient mice lack mature lymphocytes owing to inability to initiate V(D)J rearrangement. Cell. 1992 Mar 6;68(5):855–867. doi: 10.1016/0092-8674(92)90029-c. [DOI] [PubMed] [Google Scholar]
- Tycko B., Sklar J. Chromosomal translocations in lymphoid neoplasia: a reappraisal of the recombinase model. Cancer Cells. 1990 Jan;2(1):1–8. [PubMed] [Google Scholar]