Background: Mechanisms that preferentially deliver B cell receptor-antigen complexes to receptive MHC class II molecules are poorly defined.
Results: Internalized B cell receptor-antigen complexes associate with class II molecules.
Conclusion: In B cells, B cell receptor-antigen complexes associate with peptide-loaded class II molecules, potentially defining sites of class II ligand acquisition.
Significance: Antigen-specific B cells control the loading of cognate antigen-derived peptide onto class II molecules.
Keywords: antigen presentation, antigen processing, FRET, imaging, lymphocyte, MHC, B cell receptor (BCR), antigen-presenting cell (APC)
Abstract
Antigen processing and MHC class II-restricted antigen presentation by antigen-presenting cells such as dendritic cells and B cells allows the activation of naïve CD4+ T cells and cognate interactions between B cells and effector CD4+ T cells, respectively. B cells are unique among class II-restricted antigen-presenting cells in that they have a clonally restricted antigen-specific receptor, the B cell receptor (BCR), which allows the cell to recognize and respond to trace amounts of foreign antigen present in a sea of self-antigens. Moreover, engagement of peptide-class II complexes formed via BCR-mediated processing of cognate antigen has been shown to result in a unique pattern of B cell activation. Using a combined biochemical and imaging/FRET approach, we establish that internalized antigen-BCR complexes associate with intracellular class II molecules. We demonstrate that the M1-paired MHC class II conformer, shown previously to be critical for CD4 T cell activation, is incorporated selectively into these complexes and loaded selectively with peptide derived from BCR-internalized cognate antigen. These results demonstrate that, in B cells, internalized antigen-BCR complexes associate with intracellular MHC class II molecules, potentially defining a site of class II peptide acquisition, and reveal a selective role for the M1-paired class II conformer in the presentation of cognate antigen. These findings provide key insights into the molecular mechanisms used by B cells to control the source of peptides charged onto class II molecules, allowing the immune system to mount an antibody response focused on BCR-reactive cognate antigen.
Introduction
T lymphocyte activation is driven by the recognition of complexes of antigen-derived peptide and MHC molecules on the surface of an antigen-presenting cell (APC).2 Cytotoxic CD8-positive T cells recognize peptide-MHC class I complexes and generally kill the APC, whereas helper CD4-positive T cells recognize peptide-MHC class II complexes and help/activate the APC. Although the mechanism of formation of MHC class I-peptide complexes is understood in considerable molecular detail (1), the mechanisms of formation of MHC class II-peptide complexes are less well understood.
There are three major types of MHC class II-restricted APCs: dendritic cells, B cells, and macrophages. B cells are unique among this group in that they express a clonally restricted antigen-specific receptor, the B cell receptor (BCR). Antigen engagement of the BCR drives clonal selection of antigen-specific B cells. Moreover, BCR-mediated antigen processing and presentation are 100- to 1000-fold more efficient than non-BCR-mediated antigen processing (2–4). In addition, peptide-class II complexes formed via BCR-mediated antigen processing have unique B cell-activating properties (5). Therefore, B cells are an excellent model system for an in-depth analysis of the mechanisms that might control the source of peptides loaded onto MHC class II molecules.
MHC class I molecules acquire peptide in the endoplasmic reticulum (ER) within a class I peptide-loading complex that contains multiple components. The foundation of the class I complex is the transporter associated with antigen processing, which transports antigen-derived peptides from the cytosol to the ER lumen. Peptide-receptive MHC class I molecules (i.e. class I heavy chain and β2-microglobulin) associate with the transporter associated with antigen processing via the adaptor/chaperone tapasin, which facilitates class I peptide loading/editing. Other chaperones, such as ERp57 (which binds tapasin) and calreticulin, associate with peptide-receptive class I molecules to stabilize the molecule and facilitate peptide loading. Currently it is unclear whether any proteases, such as ER aminopeptidase associated with antigen processing (which mediates peptide trimming), are part of this class I complex. Nevertheless, the complex functions to ensure efficient loading of antigen-derived peptides onto MHC class I molecules.
MHC class II molecules are αβ dimers that assemble in the ER under guidance of the chaperone CD74 (also known as invariant chain (Ii)) (6). Ii facilitates initial class II assembly, and a portion of the Ii molecule called class II-associated Ii peptide (CLIP) occupies the class II peptide-binding groove. Ii then directs class II molecules to MHC class II-enriched compartments within the endocytic pathway, where Ii is degraded and class II is loaded with antigen-derived peptide under the “guidance” of the chaperone DM (7).
Therefore, both MHC class I and class II molecules interact with multiple chaperones that mediate MHC peptide loading (i.e. tapasin, calreticulin, and ERp57 for class I and Ii and DM for class II). However, although proximity to the transporter associated with antigen processing controls which peptides are loaded onto class I molecules, it is unclear whether and how peptides loaded onto MHC class II are controlled. In this report, we establish that, in B lymphocytes, antigen (Ag)-BCR complexes associate with intracellular MHC class II molecules. In addition, we establish the M1-paired MHC class II conformer (shown previously shown to have high T cell activation potential (8)) as the class II molecule that preferentially associates with intracellular Ag-BCR complexes. Finally, we show that the M1-paired class II conformer associates with the CD79 signaling subunit of the BCR and is loaded selectively with peptide derived from the processing of BCR-internalized antigen.
Materials and Methods
Cells
A20μWT cells expressing a transfected phosphorylcholine-specific human IgM BCR were grown and used as reported previously (9). K46μ cells (and derivatives) were grown in RPMI 1640 medium, 10% FBS, 50 μm 2-mercaptoethanol, 1× sodium pyruvate, and 1× non-essential amino acids. K46μ cells were transfected by electroporation with I-AKα and I-AKβ in the expression vectors pcDNA3.1 and pcDNA3.1/hygro, respectively (Invitrogen), grown in medium containing 650 μg/ml G418 (Corning Cellgro, catalog no. 61-234-RG) and 1 mg/ml hygromycin B (Corning Cellgro, catalog no. 30-240-CR), and cloned by limiting dilution. Clone 1D6 A10 (expressing I-Ak levels similar to B10.Br splenic B cells) was used for the study. K46μ cells were also transfected by electroporation with the I-AKα, I-AKβ-CFP (pcDNA3.1/hygro) and CD79a-YFP (pcDNA3.1/zeo) expression vectors, grown in media containing 650 μg/ml G418 (Corning Cellgro, catalog no. 61-234-RG), 1 mg/ml hygromycin B, and 500 μg/ml Zeocin (Invitrogen, catalog no. R25001), and cloned by limiting dilution (cells transfected with only CD79A-YFP were also made and selected only with Zeocin). Clone 2C1 (expressing I-Ak levels similar to B10.Br splenic B cells) was used for the study.
B cells were purified from whole spleen by negative selection with a MACS B cell isolation kit (Miltenyi Biotec, catalog no. 130-090-862) on MACS separation columns (Miltenyi Biotec, catalog no. 130-042-401) according to the instructions of the manufacturer. Fractionated B cells were >85% B220+ by flow cytometry.
Mice
MD4.B10.Br mice were bred at Taconic Farms Inc. B10.BR/SgSnJ (B10.Br) mice for breeding were purchased from The Jackson Laboratory. Mice were housed in the Albany Medical College Animal Resource Facility under specific pathogen-free conditions. The Albany Medical College Institutional Animal Care and Use Committee approved all reported protocols.
Biochemical Analysis of Ag-BCR—MHC Class II Association
Cells were pulsed with biotin-labeled BCR ligand (A20μWT (PC-BSA-biotin or biotin anti-human IgM, Jackson ImmunoResearch Laboratories, catalog no. 109-006-129), B10.Br splenic B cells (biotin anti-IgMb AF6-78, BD Biosciences, catalog no. 553519, or biotin anti-IgM R6–60.2, BD Biosciences, catalog no. 553406), 1D6 A10 (biotin anti-IgM RMM1, BioLegend, catalog no. 406504)) at 37 °C in Hanks' balanced salt solution and 0.1% BSA for the indicated time. Cells were then washed and lysed on ice for 30 min in 10 mm Tris, 150 mm NaCl, and 5 mm EDTA (pH 7.5) (TNE) and 0.05% Brij-58 (containing protease inhibitors). Insoluble material was removed by centrifugation for 15 min at 16,000 × g. Btn-ligand-BCR—class II complexes were recovered by pulldown with 100 μl of a 10% suspension of streptavidin-agarose (Thermo Scientific, catalog no. 20353) overnight at 4 °C with constant inversion. Each pulldown was washed three times with 1.5 ml of TNE and 0.05% Brij-58 and then resuspended in SDS-PAGE sample buffer. Samples were analyzed by SDS-PAGE/Western blot as reported previously (10), using the following antibodies: class II (1630a rabbit anti-mouse class II β chain cytoplasmic tail + donkey anti-rabbit IgG-HRP (BioLegend, catalog no. 406401)), IgM (rabbit anti-mouse IgM (Jackson ImmunoResearch Laboratories, catalog no. 315-005-020) + donkey anti-rabbit IgG-HRP), Ii (In-1 (BD Biosciences, catalog no. 555317) + goat anti-rat IgG2b-HRP (Thermo Scientific, catalog no. PA1–84179)), transferrin receptor (rabbit anti-mouse transferrin receptor (Abcam, catalog no. ab84036) + donkey anti-rabbit IgG-HRP), GAPDH (6C5 (Ambion, catalog no. AM4300) + goat anti-mouse IgG-HRP (Calbiochem, catalog no. 401253)), and btn-labeled ligand (streptavidin-HRP (Pierce, catalog no. 21124)) and Pierce Dura or Femto ECL substrate (Pierce, catalog nos. 34076 and 34096, respectively).
Confocal Microscopy
Images of 2C1 cells (expressing I-Ak-CFP and CD79a-YFP) were acquired on a Zeiss LSM510 META-NLO laser-scanning microscope system equipped with a Plan-Apochromat ×63/1.4 numerical aperture oil objective lens, an argon laser (at 458 and 514 nm) for excitation of CFP and YFP, respectively, and standard emission filters for CFP and YFP.
Confocal FRET Microscopy of Ag-BCR—MHC Class II Association
Images were acquired on a Zeiss LSM510 META-NLO laser-scanning microscope system as above. Optimal acquisition settings were determined using a double-labeled sample to avoid saturation. In Zen software (Zeiss USA, Thornwood, NY), the image display was configured for three channels: a donor excitation/donor emission (donor channel), an acceptor excitation/acceptor emission (acceptor channel), and a donor excitation/acceptor emission (FRET channel). Images were collected sequentially at 512 × 512 pixels, 8-bit depth, a mean of two images, and ×1 zoom. Photomultiplier tube gain and black level settings, laser power, and pinhole were set at identical levels and remained unchanged for all subsequent collections. Double-labeled single-label acceptor and single-label donor images were collected with identical settings. Double-labeled cells expressed CFP and YFP fusion constructs, whereas single-labeled cells expressed only CFP or YFP fusion constructs. Single-labeled images were used to correct for FRET signal contamination such as spectral bleedthrough in double-labeled images.
Precision FRET Analysis
To calculate the FRET efficiency (E%), we used a quantitative analysis algorithm called PFRET that removes the donor and acceptor spectral bleedthrough and corrects the variation in fluorophore expression level associated with FRET imaging (11–13). Nine images were collected for background correction, spectral bleedthrough correction, and FRET analysis using the donor, acceptor, and FRET channels described above: three single-label donor reference images, three single-label acceptor reference images, and three double-label images. The three double-labeled images were named as follows: quenched donor (qD), i.e. donor excitation/donor emission (donor channel); acceptor (A), i.e. acceptor excitation/acceptor emission (acceptor channel); and uncorrected FRET, i.e. donor excitation/acceptor emission (FRET channel). Then images were background-subtracted and processed by the PFRET software to generate the corrected FRET images (PFRET images) that represent the actual energy transfer levels (PFRET levels) and were used for quantitative analyses. A custom-written ImageJ macro was used to select above threshold regions of interest (ROIs) (10 × 10 pixels) of the 8-bit grayscale fluorescence intensities of uncorrected FRET, A, and qD images, excluding zero and saturated pixels (12). Under our imaging conditions, there were less than 5% saturated pixels. These ROIs were subsequently applied to the other images to extract the different grayscale fluorescence intensity values for the different parameters tested, including PFRET (actual energy transfer levels according to the PFRET spectral bleedthrough correction algorithm), uncorrected FRET, qD, and A levels. These values were transferred to an Excel spreadsheet (Microsoft, Redmond, WA) for calculation of the additional parameters E%, unquenched D (D = PFRET + qD), and D/A ratios. Energy transfer efficiency, E%, is calculated as a percentage of energy transfer in relation to the unquenched donor, i.e. D = qD + γ × PFRET, as described in the equation E% = 100 × (γ × PFRET / D) or E% = 100 × [1 − (qD / D)]. γ Value (γ) is a function of the quantum yield of the fluorophores and of the detection setup. Because all of our imaging conditions remain constant, the γ value does not affect the interpretation of the relative E% data when E% is calculated assuming γ = 1 (13, 14).
Discrimination between a Clustered and a Random Protein Distribution
When acceptor and donor pairs are restricted to membrane surfaces and expressed in high concentrations, it is crucial to determine that the FRET results from specific protein-protein interactions. It is important to exclude molecular crowding because of protein overexpression. Mathematical models have been used to discriminate clustered from random membrane protein organizations on the basis of the relationship between E% and A levels at specific ranges of D/A ratios, which are determined experimentally using quantitative FRET analysis (15, 16). In random situations, the likelihood of an acceptor colocalizing with a given donor is positively correlated with acceptor levels and leads to an increase in E%. Conversely, in a clustered situation where molecules, by definition, are in nanometer range proximity, E% is largely independent of A levels, and it does not decrease to zero when A trends to zero.
Class II SDS Stability Assay
Ag-BCR—class II complexes were isolated as detailed above. Samples in SDS-PAGE sample buffer were incubated either at ∼22 °C (room temperature) or 100 °C for 2 min before analysis by SDS-PAGE and Western blotting.
Ia.2 Status of Ag-BCR-associated MHC Class II Molecules
Ag-BCR—class II complexes were isolated as detailed above. MHC class II molecules were released from Ag-BCR complexes by incubation in TNE and 1.0% Triton X-100 for 2–4 h at room temperature. Released I-Ak class II molecules were then immunoprecipitated with either 11-5.2 (BioLegend, catalog no. 110002, which selectively binds M1-paired I-Ak class II molecules) or 10-3.6 (BioLegend, catalog no. 109902, which binds all I-Ak class II molecules), as reported previously (10).
Analysis of Peptide Loading onto MHC Class II Conformers
Splenic B cells from an MD4.B10.Br mouse (expressing a transgenic HEL-specific IgM BCR and I-Ak class II molecules (5)) were pulsed overnight with 100 nm HEL to allow BCR-mediated antigen processing and formation of derivative HEL peptide-I-Ak class II complexes. The cells were then washed, lysed in TNE, 0.5% deoxycholate, and 1% Nonidet P-40 containing protease inhibitors, and a sequential immunoprecipitation approach (10) was used to determine the Ia.2 status of the I-Ak class II molecules loaded with HEL46–61 peptide.
Class II CD79 Association
Cells (B10.Br splenocytes, 1D6 A10 cells, or 1D6 A10 cells expressing CD79A-YFP) were lysed in 0.05% Brij-58 lysis buffer. Total and Ia.2+ I-Ak MHC class II molecules were immunoprecipitated from WCL with either the 10-3.6 or 11-5.2 mAb and PGS. Samples were probed for class II β chain and either CD79A (rabbit anti-CD79A, a gift from Dr. Lei Jin, AMC) or CD79A-YFP (rabbit anti-YFP, Abcam, catalog no. ab6556) by Western blot analysis (Pierce, Dura ECL substrate).
Results
Formation of Intracellular Antigen-BCR·MHC Class II Complexes
Within the ER, MHC class I molecules associate with the transporter associated with antigen processing, which is a source of class I peptides. To determine whether MHC class II molecules associate with any potential sources of class II peptide, we focused on B lymphocytes, which have an Ag-specific receptor (the BCR), and determined whether internalized Ag-BCR complexes associate with intracellular MHC class II molecules (Fig. 1A).
FIGURE 1.
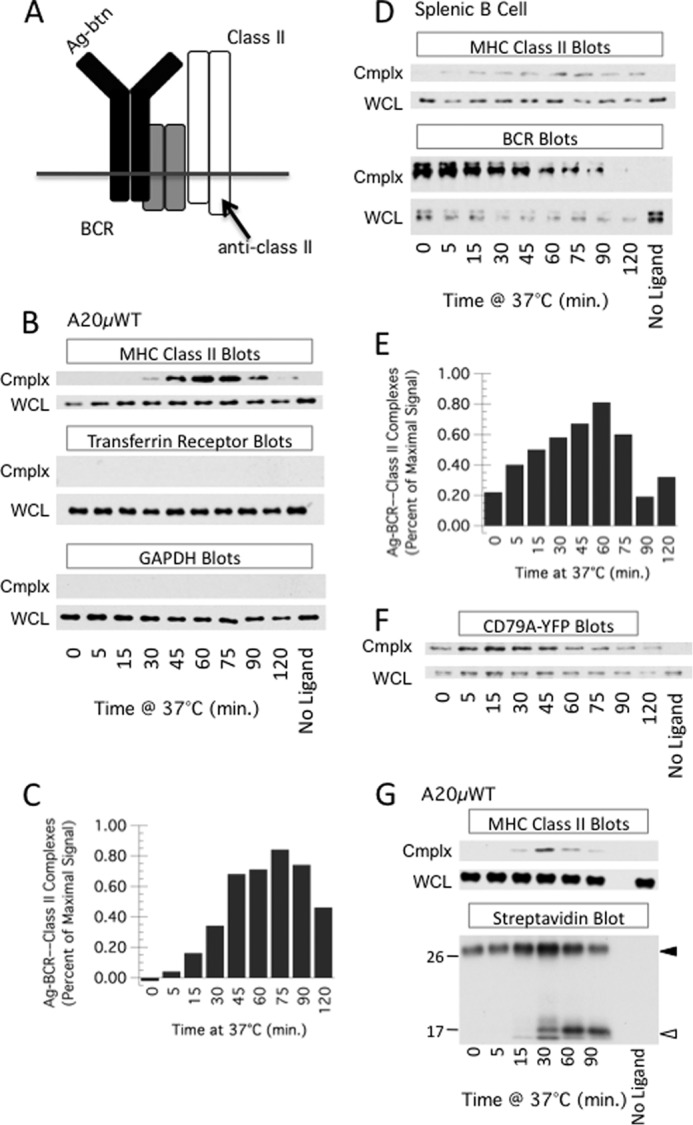
Time-dependent association of antigen-B cell receptor complexes with MHC class II molecules. A, diagram of the approach. B cells are pulsed with biotin-labeled BCR ligand (antigen or anti-BCR mAb). Cells are lysed, and biotin-labeled BCR ligand (and associated proteins) are recovered by pulldown with streptavidin beads. Pulldowns are probed for MHC class II by Western blot analysis for class II β chain cytoplasmic tail (anti-class II). B, A20μWT B cells were pulsed with PC-BSA-btn for the indicated times and then analyzed for PC-BSA-btn—BCR—class II complexes (cmplx) as diagrammed in A. WCL was also analyzed for class II. Shown are representative results from one of six independent experiments. Isolated complexes and WCL were also probed for transferrin receptor and GAPDH. Shown are representative results from one of three independent experiments. In all experiments, both cmplx and WCL blots for each marker (e.g. class II) were developed with the same ECL reagent and exposed for the same time. C, quantitative analysis of average level of PC-BSA-btn—BCR—class II complexes across six independent experiments. D and E, splenic B cells were pulsed with anti-IgM-btn and ligand-BCR-class II complexes detected as in B and C (quantitation of results from three independent experiments). Isolated complexes and WCL were also probed for the IgM heavy chain of the BCR (BCR Blots). The difference in signal between “No Ligand” and T = 0 in the WCL is likely due to ligation-induced association of some BCR molecules with detergent-insoluble cellular elements such as the cytoskeleton. F, 1D6 A10 CD79A-YFP cells were pulsed with anti-IgM-btn for the indicated time, lysed in Brig-58 lysis buffer, and BCR—anti-IgM-btn was pulled down with streptavidin beads. The pulldown (Cmplx) and WCL were then analyzed by Western blotting with anti-YFP antibody. Shown are representative results from one of two independent experiments. G, A20μWT B cells were cultured in the continued presence of low levels of biotin-labeled F(ab')2 fragments of goat anti-human IgM antibody for the indicated times. The cells were analyzed for ligand-BCR-class II complexes as in B. Parallel streptavidin pulldown samples were also analyzed for anti-BCR-btn by streptavidin-HRP blotting. Closed and open arrowheads indicate the position of the anti-BCR-btn antibody (heavy chain F(ab')2 fragment and/or intact light chain) and proteolytic breakdown fragments, respectively. The position of the molecular weight markers (in kilodaltons) is shown. The increase in signal for the intact heavy chain and CD79-YFP (F) from 0–30 min is due to ligand binding to intracellular receptors that are recruited to the cell surface upon exposure to BCR ligand (data not shown). Shown are representative results from one of three independent experiments.
To accomplish this, A20μWT B cells (expressing a PC-specific human IgM BCR (9)) were pulsed with antigen (PC-BSA-biotin) for various times. The cells were then lysed in a mild detergent (0.05% Brij-58, to preserve non-covalent BCR-class II associations), and biotin-Ag-BCR complexes (and associated proteins) were isolated using streptavidin-coated beads. The streptavidin pulldown (cmplx) as well as WCL were probed for MHC class II molecules by Western blot analysis (Fig. 1, B and C). Under these conditions, there is a time- and ligand-dependent coisolation of MHC class II molecules with the captured Ag-BCR complexes, with maximal coisolation occurring at about 1 h after antigen exposure. A similar approach was used with splenic B cells, using biotin-labeled anti-IgM antibody as a BCR ligand (Fig. 1, D and E), and there is a similar time- and ligand-dependent coisolation of MHC class II molecules with the captured ligand-BCR complexes.
To assess the selectivity of the pulldown protocol, both Ag-BCR pulldowns and WCL from antigen-pulsed A20μWT B cells were probed for GAPDH (a cytosolic protein) as well as transferrin receptor (Fig. 1B), neither of which would be expected to associate with the BCR or MHC class II molecules. Neither GAPDH nor the transferrin receptor was found to associate with internalized Ag-BCR complexes, illustrating the selectivity of the Ag-BCR—MHC class II pulldown. To determine whether the pulldown also contains the CD79 subunit of the BCR, pulldowns from B cells expressing YFP-tagged CD79A (Fig. 2) were probed with anti-YFP (Fig. 1F). The results demonstrate that CD79 is also present in the isolated complexes (nuanced changes in the relative levels of IgH and CD79 in the pulldowns over time may be due to ligand-induced changes in the strength of IgH/L-CD79 association (17) or dynamic BCR association with detergent-insoluble aspects of the cytoskeleton). Together, the results in Fig. 1 reveal that Ag-BCR complexes associate with MHC class II molecules in a time- and antigen-dependent manner.
FIGURE 2.
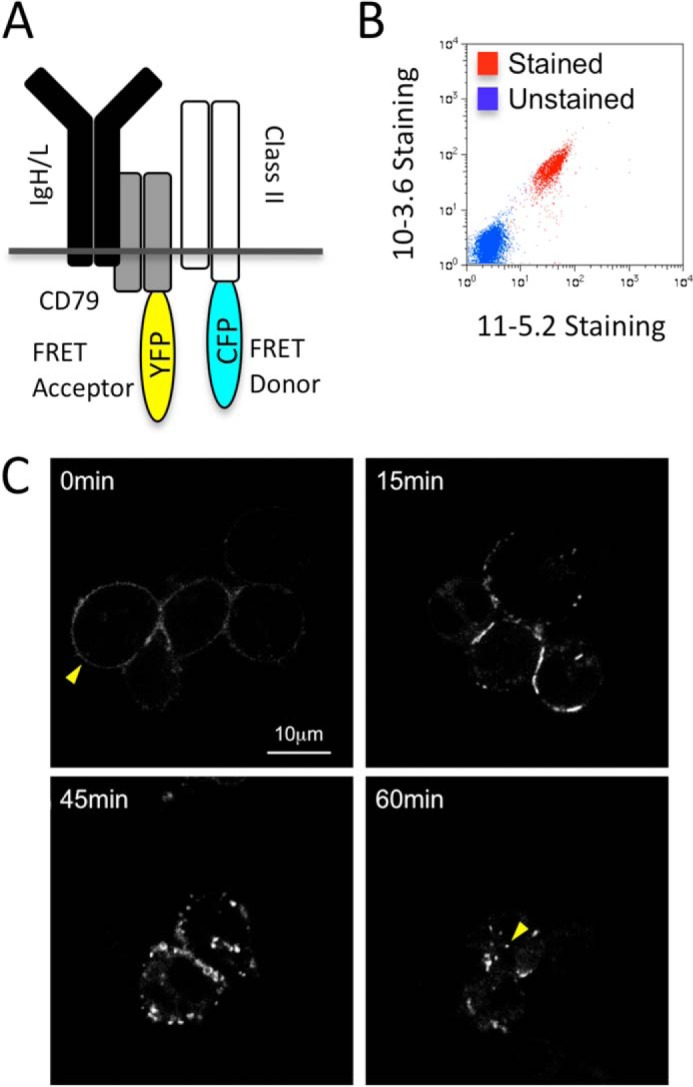
Characterization of FRET B cells. A, diagram of fluorescent protein-labeled molecules. K46μ B cells were sequentially transfected with expression vectors encoding expression of CD79a-YFP (FRET acceptor) and MHC class II-CFP (FRET donor, I-Ak β chain-CFP + unlabeled α chain). B, non-permeabilized stable CD79a-YFP/class II-CFP transfectants (2C1 FRET B cells) were stained with anti-I-Ak mAbs (10-3.6 anti-total I-Ak and 11-5.2 anti-Ia.2+ I-Ak) and analyzed by flow cytometry. Unlabeled cells were also analyzed. Shown are representative results from one of three independent experiments. C, 2C1 cells were pulsed with anti-BCR antibody on ice or for up to 60 min at 37 °C. The cells were then imaged for CD79a-YFP by confocal microscopy. Mid-plane slices of representative images are shown. In the T = 0 image, the yellow arrow indicates cell surface CD79a-YFP (cell surface BCR). In the T = 60 image, the yellow arrow indicates one example of intracellular CD79a-YFP (intracellular BCR). Shown are representative results from one of three independent experiments.
Next, A20μWT B cells were pulsed with a biotin-labeled anti-IgM antibody (which is susceptible to degradation by endosomal proteases), and BCR-class II associations were analyzed as above (Fig. 1G). Under these conditions, there is, again, a time- and ligand-dependent coisolation of MHC class II molecules with captured ligand-BCR complexes. In addition, analysis of the pulldowns by streptavidin-HRP Western blot (to detect the anti-IgM-btn) revealed that the peak of Ag-BCR—MHC class II association (∼30 min) is coincident with initiation of detectable proteolytic degradation of the BCR-bound anti-IgM-btn (Fig. 1G, closed and open arrowheads indicate the intact heavy chain of the anti-BCR antibody and proteolytic breakdown fragments, respectively). Furthermore, the streptavidin pulldowns and WCL from splenic B cells were analyzed for BCR IgM heavy chain (Fig. 1D). There is a decrease in BCR IgM heavy chain in the pulldown samples with time, which is likely due to proteolytic degradation of the BCR heavy chain itself and/or degradation of the biotin-labeled ligand (which would result in a decrease in the level of isolated ligand-BCR complexes). These results demonstrate that internalized Ag-BCR complexes associate with MHC class II molecules at a time corresponding to the initiation of proteolytic processing of the BCR/BCR-bound antigen.
Although the results from the coprecipitation studies establish an association between Ag-BCR complexes with MHC class II molecules, it is possible that the observed BCR-class II complexes may have either formed at the cell surface or come together aberrantly as a result of detergent solubilization. To investigate these possibilities, we took an alternative live-cell imaging approach. K46μ B cells were transfected with expression vectors encoding CFP-tagged I-Ak class II molecules (i.e. CFP-labeled Aβk β chain and unlabeled Aαk α chain) as well as YFP-tagged CD79a (which will associate with endogenous CD79b and the rest of the BCR, Fig. 2A). The resulting B cells express cell surface I-Ak MHC class II molecules (Fig. 2B), establishing that the CFP-tagged class II molecules can traffic through the biosynthetic pathway and arrive at the cell surface. In addition, exposure of the cells to anti-BCR antibody (to engage cell surface BCR molecules and induce BCR internalization) results in the redistribution of YFP-labeled BCR molecules from the cell surface into intracellular compartments (Fig. 2C), establishing the endocytic capacity of the YFP-labeled BCR molecules. These FRET-compatible B cells were then used for live-cell FRET imaging using confocal laser-scanning microscopy to determine whether ligand-induced BCR internalization drives formation of intracellular BCR-YFP—class II-CFP complexes.
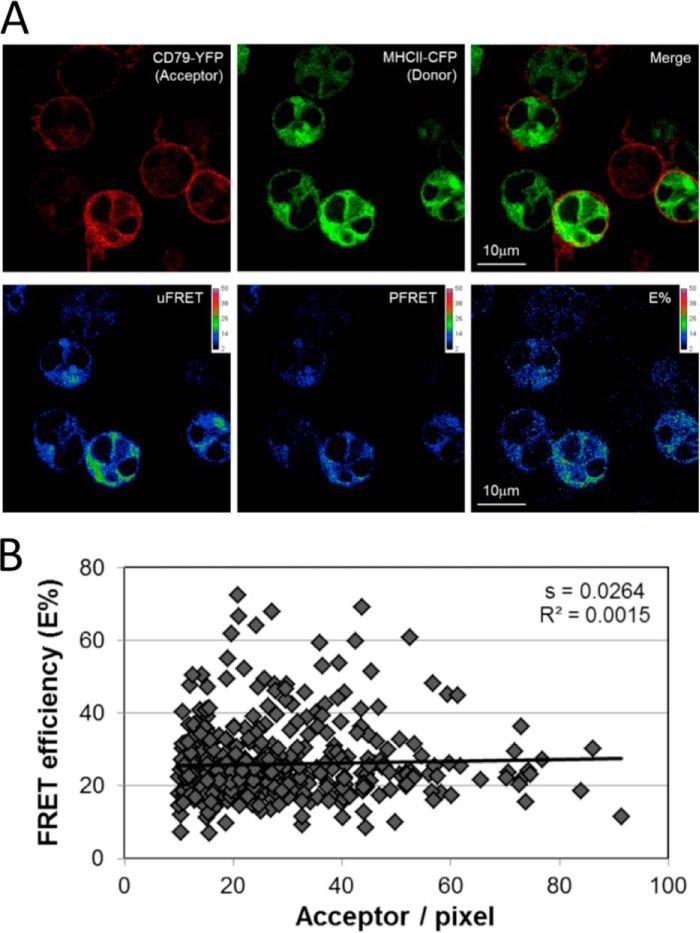
The FRET-compatible B cells were first imaged in the absence of BCR engagement to establish the relative distribution of the BCR and class II and determine whether there was any “baseline” FRET. As shown in Fig. 3A, although there is some heterogeneity in the observed patterns, YFP-tagged BCR (i.e. CD79a-YFP) is found predominantly at the cell surface (and within intracellular compartments in some cells). In contrast, CFP-tagged class II is found at higher levels within intracellular compartments (possibly the ER/Golgi or MHC class II-enriched compartments). Under these resting conditions, there is a low level of FRET that is localized to intracellular compartments, possibly representing complexes of constitutively internalized BCR (18) and intracellular class II. Importantly, when FRET efficiency was compared with the level of FRET acceptor (YFP) across multiple events (Fig. 3B), it becomes apparent that FRET efficiency is independent of acceptor level (the slope of the resulting best fit line is ≤0.03, which we have shown previously to be the threshold for detecting effects of molecular crowding (15, 16)), meaning that molecular crowding (simple colocalization of the BCR and class II to the same subcellular compartment) is not driving FRET in this system.
FIGURE 3.
Distribution of BCR-YFP and class II-CFP in non-BCR-engaged B cells. A, 2C1 B cells were imaged by confocal laser-scanning microscopy and analyzed for FRET between BCR-YFP (i.e. CD79a-YFP) and class II-CFP (i.e. Aβk-CFP). The top row shows the distribution of each protein alone, followed by a two-color overlay. The bottom row shows uncorrected FRET (uFRET), corrected FRET (PFRET) and FRET corrected for varying donor levels (E%). See “Experimental Procedures” for details. B, multiple images were analyzed on a pixel-by-pixel basis for both FRET efficiency (E%) and acceptor level. The resulting analysis reveals that the slope of the linear curve fit is <0.03, which we have shown previously to be the threshold for detecting effects of molecular crowding (15, 16). Therefore, molecular crowding is not driving FRET in this system. This relationship did not change in samples stimulated with BCR ligand (data not shown).
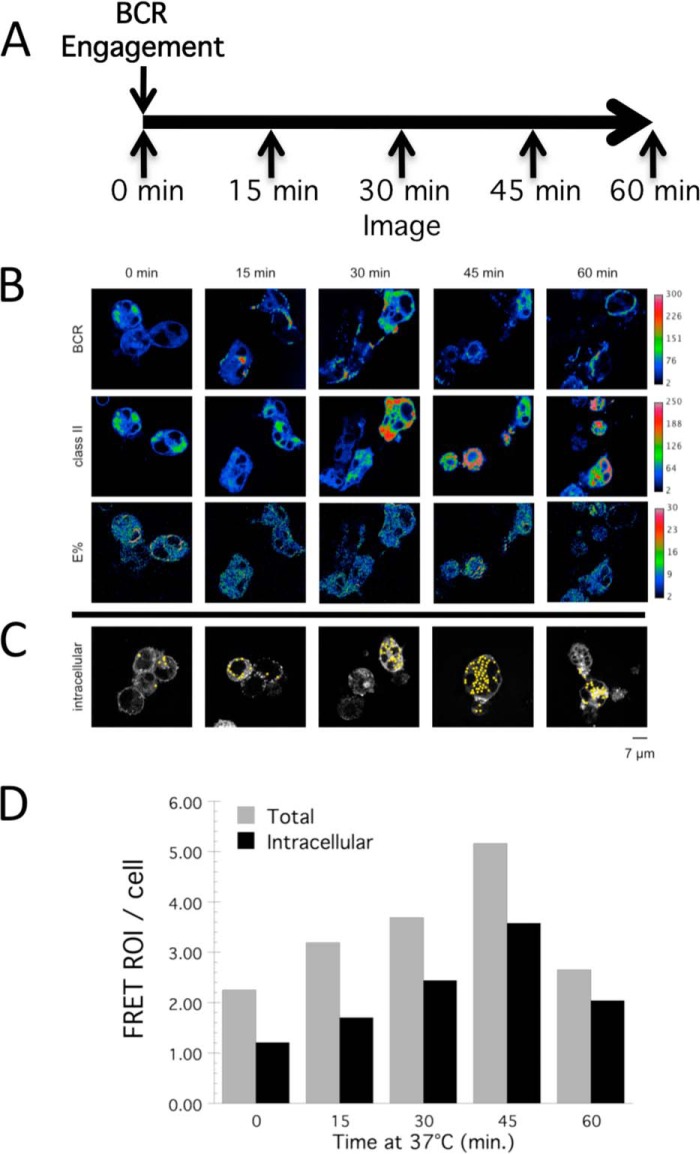
FRET-compatible B cells were then pulsed with unlabeled anti-IgM antibody (to drive BCR-YFP internalization) and were subjected to live-cell confocal FRET imaging at multiple time points between 0 and 60 min. (Fig. 4A). FRET events were identified after image acquisition by computer algorithm using our previously reported custom ImageJ FRET ROI selection macro (12). We then manually assigned a membrane or intracellular location to each identified FRET ROI. Using this approach (Fig. 4, B–D), it is apparent that BCR cross-linking drives a marked increase in the number of FRET ROI per cell, peaking 45 min after initiation of BCR cross-linking (Fig. 4D). This increase was evident whether we considered all cell-associated FRET ROIs or only counted obviously intracellular ROIs. Commensurate with the results shown in Fig. 3, there is a low number of FRET ROIs present in unstimulated cells. However, upon BCR engagement at 37 °C, there was a marked 3- to 4-fold increase in the number of subcellular compartments bearing detectable levels of BCR-YFP—class II-CFP complexes (i.e. FRET) over a time frame similar to that shown by a biochemical approach (Fig. 1). Overall, these results confirm and extend the biochemical analysis presented in Fig. 1 and establish that internalized Ag-BCR complexes associate with intracellular MHC class II molecules. It is currently unclear whether this is a direct association or an indirect association mediated by a still to be defined linker molecule.
FIGURE 4.
Association of BCR and MHC class II molecules within intracellular compartments. A, time line of the experimental protocol. B, representative images of anti-BCR-pulsed FRET cells from each time point. Rows from top to bottom: CD79a-YFP (BCR), I-Ak-CFP (class II), corrected FRET between CD79a-YFP, and class II-CFP (E%). C, representative images with identified intracellular FRET events. D, quantitation of the number of FRET events/cell at each time point across all samples analyzed. Both total FRET events/cell (Total) and morphologically identifiable intracellular FRET events/cell (Intracellular) are reported.
The Functional State of BCR-associated MHC Class II Molecules
During their lifetime, MHC class II molecules associate first with Ii, which is a chaperone that assists in both class II folding and delivery to antigen-processing compartments, and with antigen-derived peptide, which is ultimately presented to CD4 T cells. To investigate the potential function of the identified Ag-BCR—class II complexes, we determined the state of the BCR-associated MHC class II molecules.
Ag-BCR—class II complexes isolated from antigen-pulsed A20μWT cells (Fig. 1) were analyzed for Ii by Western blotting (Fig. 5A). Although Ii can easily be detected in WCL derived from ∼104 A20μWT cells, Ii is not detectable in Ag-BCR—class II complexes isolated from ∼2 × 106 (∼200-fold more) cells. This suggests that the class II molecules within the Ag-BCR—class II complexes are either devoid of Ii or that the lysis conditions used do not retain Ii in the isolated complexes. Studies with the 30-2 mAb, which binds I-Ab—CLIP complexes (19), and BCR-class II complexes isolated from I-Ab expressing C57Bl/6 splenocytes also failed to reveal class II-CLIP within the complexes (data not shown). These results suggest that intracellular association of MHC class II with Ag-BCR complexes occurs concurrent with or subsequent to class II dissociation from Ii/CLIP.
FIGURE 5.
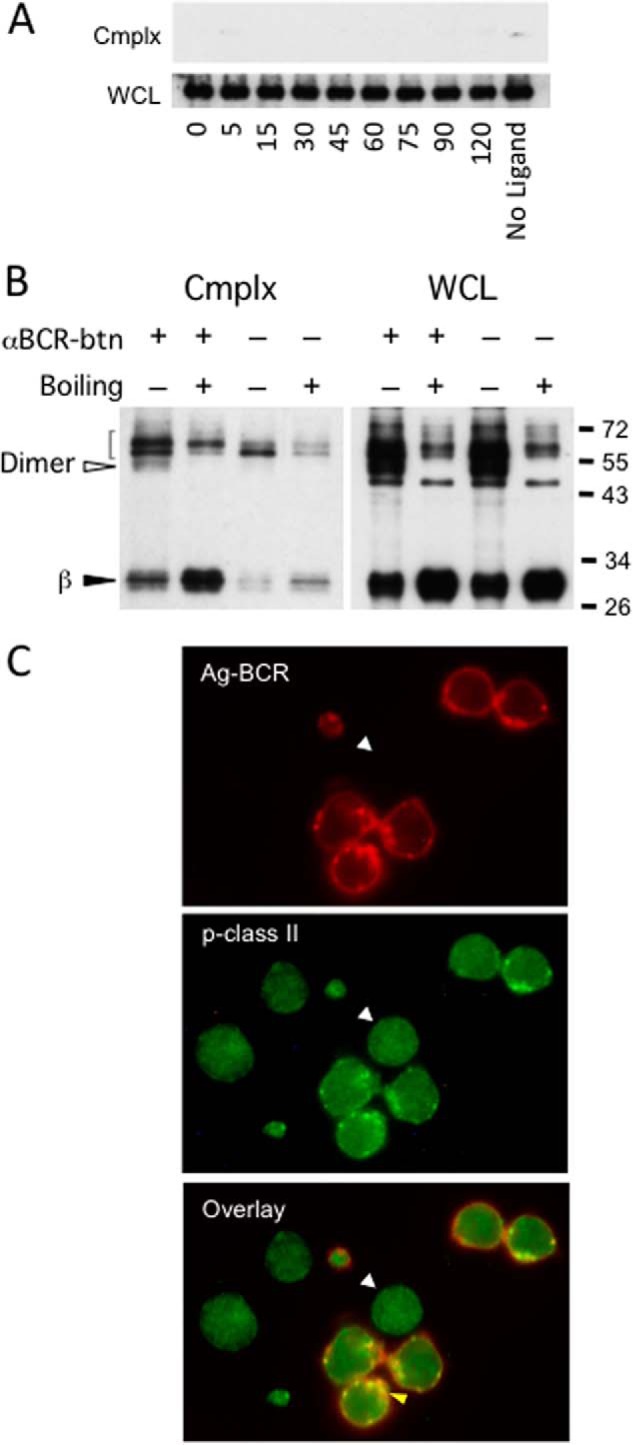
The presence of peptide-class II complexes within antigen-BCR-class II complexes. A, A20μWT cells were pulsed with PC-BSA-btn, lysed, and analyzed for ligand-BCR complexes as in Fig. 1, but pulldowns and WCL were probed for Ii (CD74) by Western blot analysis. Shown are representative results from one of three independent experiments. B, 1D6 A10 I-Ak-expressing K46μ B cells were pulsed for 1 h with anti-IgM-btn and anti-IgM-btn—BCR—class II complexes isolated by streptavidin pulldown. The isolated complexes were released with SDS-PAGE sample buffer either at room temperature or at 100 °C (boiling). The released class II molecules were analyzed by SDS-PAGE and Western blotting for MHC class II β chain. The open arrowhead indicates the position of SDS-stable class II αβ dimers. The closed arrowhead indicates free β chain. The bracket indicates background bands. Shown are representative results from one of three independent experiments. C, MD4.B10.Br B cells (expressing an HEL-specific BCR and I-Ak class II molecules) were pulsed with 100 nm HEL for 4 h. The cells were then fixed, stained for BCR-bound HEL (2D1 anti-HEL, red) and derivative HEL46–61—I-Ak peptide-class II complexes (C4H3, green), and imaged by confocal microscopy. The lack of staining of trace-contaminating non-B cells (arrowheads) for Ag-BCR complexes or derivative peptide-class II complexes (these cells exhibit no puncta of FITC staining) confirms the specificity of staining. Shown are representative results from one of three independent experiments.
Because complex-associated class II appears to be at a post-Ii phase, it may be associated with some form of peptide antigen. One readout for class II-peptide complexes is the formation of SDS-stable class II αβ dimers (i.e. class II αβ dimers resistant to dissociation by SDS at room temperature but sensitive to SDS-induced dissociation at 100 °C) (20). Therefore, we determined the SDS stability of the class II molecules within isolated Ag-BCR—class II complexes. Ag-BCR—class II complexes isolated from antigen-pulsed I-Ak-expressing 1D6 A10 B cells were dissociated with SDS-PAGE sample buffer either at room temperature or at 100 °C and then analyzed for SDS-stable MHC class II molecules by Western blotting (Fig. 5B). As shown in Fig. 5B, under these conditions, unboiled WCL as well as isolated Ag-BCR—class II complexes (Cmplx) both possess SDS-stable MHC class II αβ dimers (open arrowhead) that convert to free β chain (and undetected α chain) upon boiling (closed arrowhead). Although the lower level of class II in Ag-BCR—class II complexes isolated from splenocytes (Fig. 1D) hindered direct detection of SDS-stable class II in these cells, a single pilot experiment did reveal a detectable increase in free β chain upon heating of complexes isolated from these cells (data not shown). These results are consistent with the notion that at least a portion of the class II molecules in the Ag-BCR—class II complexes have bound antigen-derived peptide.
To confirm and extend the biochemical analysis of class II peptide loading to an intact untransformed B cell, we used immunofluorescence microscopy to determine the subcellular distribution of both Ag-BCR complexes and derivative peptide-class II complexes in splenic B cells. B cells from an MD4.B10.Br mouse (expressing both a transgenic HEL-specific BCR and I-Ak MHC class II molecules) were pulsed with 10 nm HEL for 4 h at 37 °C, and the distribution of both HEL-BCR and HEL46–61—I-Ak complexes was determined (Fig. 5C). As expected, most Ag-BCR complexes were present within intracellular vesicles, consistent with their rapid internalization. Staining for derivative HEL46–61—I-Ak peptide class II complexes 4 h after the antigen pulse revealed that most peptide-class II complexes are present within the same intracellular compartments as internalized Ag-BCR complexes. Analysis of multiple 4-h images resulted in an average Pearson coefficient of colocalization of 0.63 (range, 0.58–0.69), confirming a significant level of colocalization of internalized Ag-BCR complexes and derivative peptide-MHC class II complexes.
These findings are consistent with the biochemical and FRET-based studies establishing the intracellular association of Ag-BCR complexes and MHC class II molecules and, along with the results of the SDS stability study, suggest that at least a fraction of the class II molecules within these intracellular assemblies is bound to antigen-derived peptide. Therefore, association of MHC class II molecules with internalized Ag-BCR complexes appears to represent a phase in the life of a class II molecule somewhere between synthesis/assembly in the ER and cell surface expression as a peptide-class II complex.
Previous studies have established that accelerated conversion of Ag-BCR complexes to derivative peptide-class II complexes is promoted by the signaling-dependent ubiquitination of lipid raft-resident Ag-BCR complexes (21–23). To determine whether signaling-dependent BCR ubiquitination is necessary for association of internalized Ag-BCR complexes with class II molecules, the impact of blocking src family kinase-dependent BCR signaling (the first step in the BCR signaling and ubiquitination pathways) on the kinetics of Ag-BCR—class II complex formation was determined (Fig. 6). At doses of src kinase inhibitor known to block BCR signaling and signaling-dependent BCR ubiquitination (21, 22), we failed to observe a difference in the kinetics of Ag-BCR—class II complex formation. This finding suggests that the association of internalized Ag-BCR complexes and class II molecules is not dependent on signaling-dependent BCR ubiquitination.
FIGURE 6.
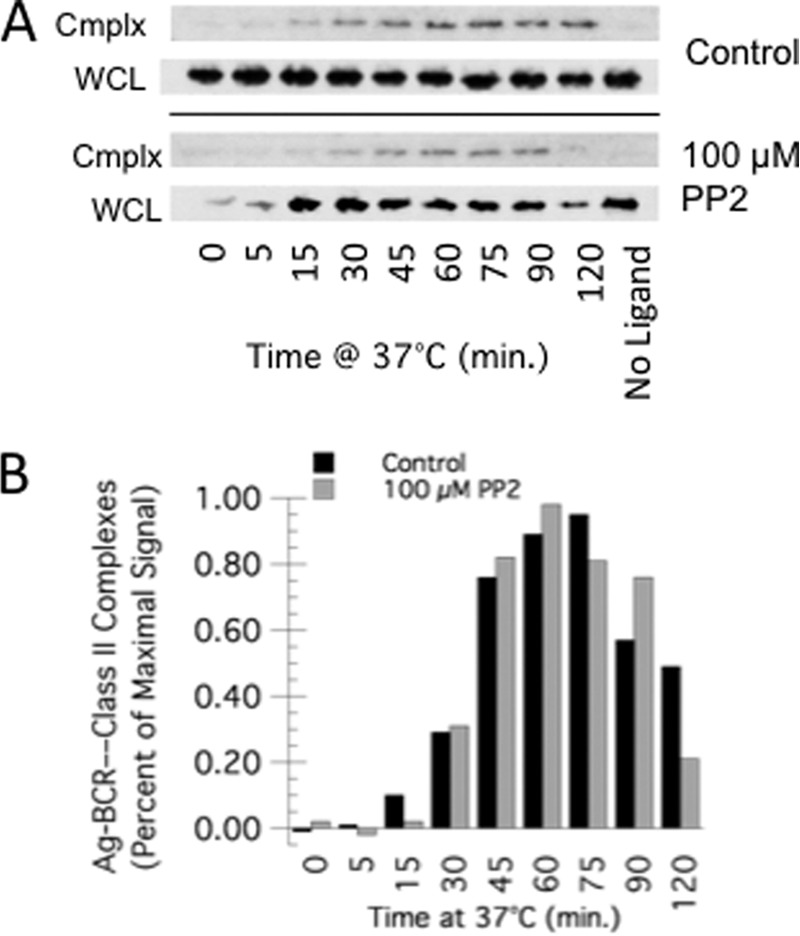
BCR signaling is not required for Ag-BCR association with MHC class II molecules. A, A20μWT B cells were pretreated with 100 μm PP2 (an Src kinase inhibitor) for 20 min at 37 °C or left untreated. The cells were then pulsed with PC-BSA-btn for the indicated time, and PC-BSA-btn—BCR—class II complexes (cmplx) were isolated and analyzed as in Fig. 1. Shown are representative results from one of three independent experiments (the ECL exposure for the cmplx blots of control and PP2-treated cells was identical). B, quantitative analysis of the average level of PC-BSA-btn-BCR—class II complex levels in control and PP2-treated cells across three independent experiments.
A Unique Role for M1-paired MHC Class II in BCR-mediated Antigen Processing and Presentation
Recently, we established that MHC class II molecules exist in two conformational states on the basis of alternative pairing of transmembrane domain GXXXG dimerization motifs (8, 10, 24) (Fig. 7A). Most (∼90%) cell surface class II molecules exist as an M2-paired conformer that resides outside of lipid rafts and lacks the ability to drive intracellular calcium signaling in B cells. In contrast, ∼10% of class II molecules exist as an M1-paired conformer that is marked by the presence of the Ia.2 epitope recognized by the 11-5.2 anti-I-Ak mAb. This Ia.2+ M1-paired I-Ak class II conformer is highly effective at driving T cell stimulation, is enriched in lipid rafts, and has the ability to drive intracellular calcium signaling in B cells (8, 10).
FIGURE 7.
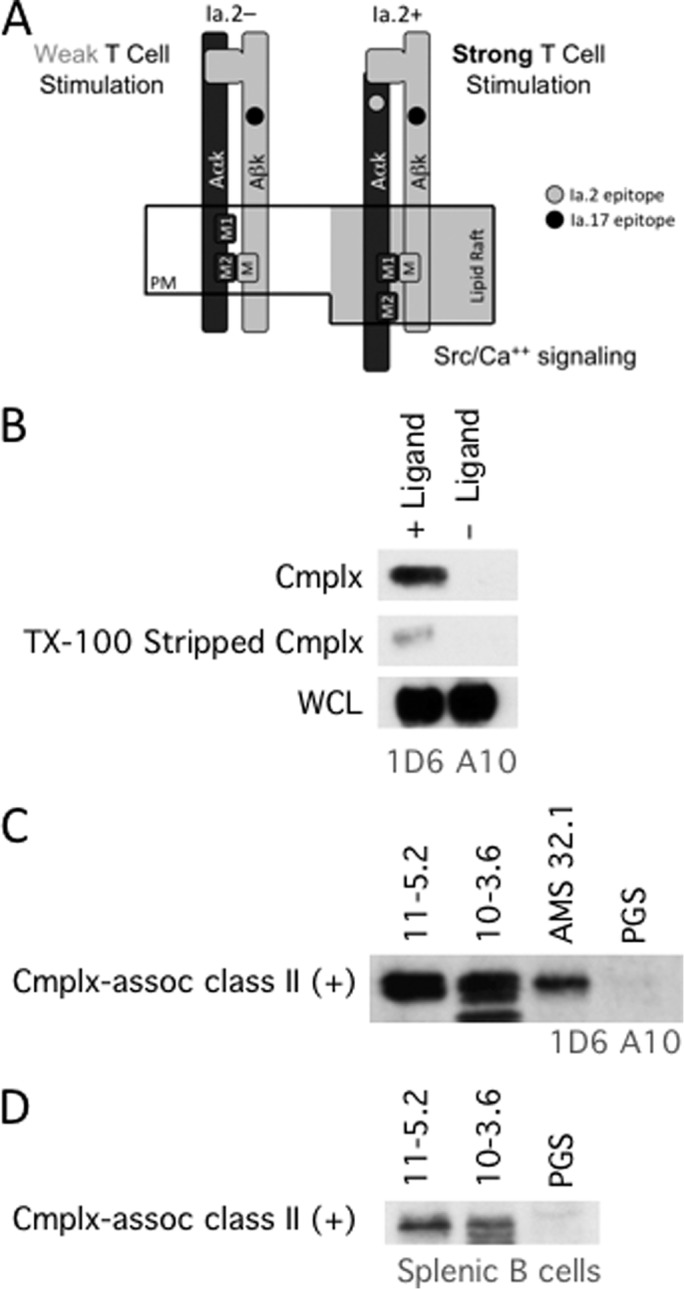
Selective association of M1-paired class II with internalized Ag-BCR complexes. A, diagrammatic summary of Ia.2+ M1-paired I-Ak class II molecules and Ia.2− M2-paired I-Ak class II molecules. See Refs. 8, 10 for details. PM, plasma membrane. B, anti-IgM-btn—BCR—class II complexes were isolated from 1D6 A10 B cells by streptavidin pulldown as in Fig. 1 (+ Ligand, anti-IgM-btn-pulsed cells; − Ligand, no anti-IgM-btn (negative control)). A fraction of the pulldown was analyzed for MHC class II β chain (Cmplx). The remainder of the pulldown was treated with 1% Triton X-100 at room temperature to release non-covalently associated class II molecules. The Triton X-100-stripped beads were then analyzed for remaining unreleased class II. Shown are representative results from one of three independent experiments. C, the Triton X-100 pulldown supernatant (containing released MHC class II molecules) was divided into four equal samples and immunoprecipitated with either PGS alone (negative control) or PGS plus 11-5.2 (anti-Ia.2+ I-Ak class II), 10-3.6 (anti-Ia.2- I-Ak class II), or AMS-32.1 (anti-I-Ad class II). Immunoprecipitates were analyzed for MHC class II β chain by Western blotting. Across three independent experiments, the band density for the 11-5.2 IP was 1.36 times that of 10-3.6 (± 0.24 S.D.). D, Ag-BCR—class II complexes isolated from splenic B cells pulsed with anti-IgM-btn were analyzed for Ia.2+ I-Ak class II as in C. Across three independent experiments, the band density for the 11-5.2 IP was 1.16 times that of 10-3.6 (± 0.21 S.D.). B–D show representative results from one of three independent experiments. The lower molecular weight band present in the 10-3.6 IP in C and D is a nonspecific background band because it is also seen in IPs from lysis buffer.
To further investigate the role of these two distinct class II conformers in MHC class II immunobiology, we determined the Ia.2 status of the Ag-BCR-associated I-Ak class II molecules. Ligand-BCR—I-Ak complexes were isolated from either 1D6 A10 or splenic B cells that had been pulsed with anti-IgM-btn. I-Ak class II was then released from the complexes by treatment with 1% Triton X-100 (a harsh detergent that disrupts the non-covalent association between the BCR and class II but preserves the class II αβ dimer, Fig. 7B). The Ia.2 status of the released I-Ak class II molecules (i.e. M1 versus M2 paired) was then determined by immunoprecipitation of the released I-Ak class II with either the 11-5.2 mAb (which only binds Ia.2+ M1-paired I-Ak class II) or the 10-3.6 mAb (which binds all I-Ak class II, Fig. 7, C and D). If all Ag-BCR-associated class II molecules are M1-paired, then both mAb should bring down the same amount of class II. However, if there is a significant level of Ia.2− M2-paired class II in the complexes, the pan-reactive 10-3.6 mAb should precipitate significantly more class II than the M1-specific 11-5.2 mAb. The results demonstrate that both antibodies precipitated similar amounts of complex-released I-Ak class II molecules, meaning that most class II molecules in the Ag-BCR—class II complex are in the Ia.2+ M1-paired conformation.
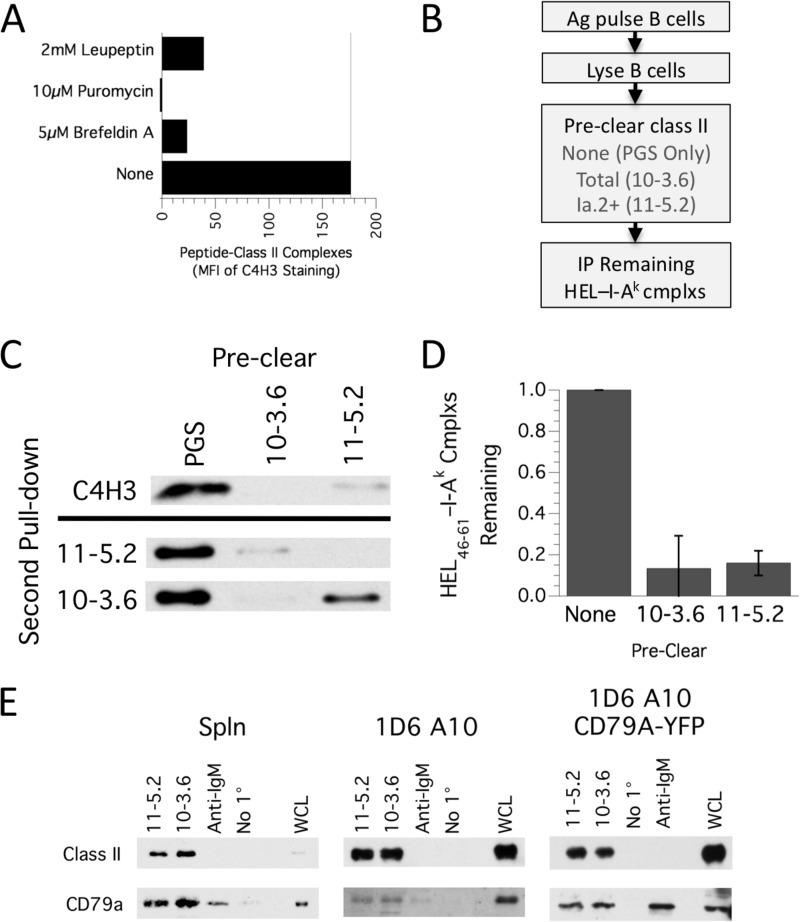
To further test the idea that the majority of Ag-BCR-associated I-Ak class II molecules are in the Ia.2+ M1-paired conformation, we took a biochemical approach and investigated the Ia.2 status of I-Ak class II molecules loaded selectively with a peptide derived from BCR-internalized antigen. Splenic B cells from MD4.B10.Br mice were pulsed overnight with 100 nm HEL (which only allows BCR-mediated antigen uptake and processing) to allow formation and cell surface expression of derivative HEL46–61—I-Ak complexes. Under these conditions, HEL-derived peptides are selectively loaded onto newly synthesized I-Ak class II molecules because pharmacological agents that block the synthesis (puromycin), intracellular trafficking (Brefeldin A), or proteolytic maturation (leupeptin) of MHC class II molecules block the expression of HEL46–61—I-Ak complexes (Fig. 8A).
FIGURE 8.
Selective loading of antigen-BCR-derived peptide onto M1-paired MHC class II molecules. A, MD4.B10.Br B cells were pulsed overnight with 100 nm HEL in the presence of the listed pharmacological agents (leupeptin, a protease inhibitor, blocks Ii degradation; puromycin, a protein synthesis inhibitor, or Brefeldin A disrupt ER/Golgi vesicle-mediated trafficking). The level of cell surface HEL46–61—I-Ak peptide-class II complexes was determined by staining with C4H3 mAb and analysis by flow cytometry. Plotted is the MFI of HEL-induced C4H3 staining (MFI of C4H3 staining of HEL-pulsed cells minus MFI of C4H3 staining of non-HEL-pulsed cells). Shown are representative results from one of three independent experiments. MFI, mean fluorescent intensity. B, diagram of the experimental flow generating the results presented in C and D. IP, immunoprecipitation. C, detergent lysates of HEL-pulsed MD4.B10.Br B cells were precleared with PGS only (negative control) or PGS plus 10-3.6 (anti-total I-Ak class II) or PGS plus 11-5.2 (anti-Ia.2+ I-Ak class II). HEL46–61—I-Ak peptide-class II complexes remaining in the precleared supernatants were immunoprecipitated with C4H3 and PGS. Immunoprecipitates were analyzed for MHC class II β chain by Western blotting. Shown are representative results from one of three independent experiments. As a control, the precleared SN was also analyzed by IP with either the 10-3.6 or 11-5.2 anti-I-Ak mAbs, as reported previously (10) (blots below the black line). D, quantitative analysis of HEL46–61—I-Ak peptide-class II complexes remaining in the precleared supernatants. In each experiment, the level of complexes in the PGS precleared sample was set to 1.00, and the level of complexes in the other samples was reported as a fraction of this value. Results are mean ± S.E. across three independent experiments. E, the indicated cell type was lysed in 0.05% Brij-58 and either Ia.2+ or total I-Ak class II molecules immunoprecipitated with 11-5.2 or 10-3.6 mAb, respectively. As controls, samples were also immunoprecipitated with anti-IgM (which brings down associated CD79 but no class II) or just protein G-Sepharose (No 1°, which brings down neither class II nor CD79). Each sample, along with some WCL, was then probed for class II β chain and CD79A/CD79A-YFP by Western blotting. For each cell type, shown are representative results from one of three independent experiments.
To determine the Ia.2 status of the peptide-class II complexes generated by antigen-specific B cells, the ability of either the Ia.2-specific 11-5.2 mAb or pan-reactive 10-3.6 anti-I-Ak mAb to “preclear” HEL46–61—I-Ak peptide-class II complexes (recognized by the C4H3 mAb) was determined (Fig. 8B). Consistent with the observation that M1-paired class II molecules selectively associate with intracellular Ag-BCR complexes (Fig. 7), essentially all HEL46–61—I-Ak peptide-class II complexes formed by HEL-pulsed MD4.B10.Br B cells were precleared by both the 11-5.2 and 10-3.6 mAbs (Fig. 8, C and D), meaning that they are predominantly Ia.2+ M1-paired peptide-class II complexes. Importantly, this finding is consistent with our reported observation that the M1 conformer-specific 11-5.2 mAb can completely block formation of cell-cell conjugates between HEL-specific CD4 T cells and HEL-pulsed MD4 B cells (10).
Finally, because M1-paired class II molecules selectively associate with Ag-BCR complexes (Fig. 7) and appear to be the primary recipients of peptides derived from the processing of BCR-associated antigen (Fig. 8), we sought to determine whether these class II molecules also associate with CD79, the BCR signaling subunit shown previously to also associate with MHC class II molecules (25, 26). Accordingly, both M1-paired and total I-Ak class II molecules were immunoprecipitated from either splenic B cells or two related B cell lines. The level of CD79 associated with the isolated class II was determined by Western blot analysis (Fig. 8E). The analysis revealed a similar level of CD79A associated with both total and Ia.2+ M1-paired class II, indicating that CD79 primarily associates with M1-paired MHC class II molecules.
Discussion
The results presented here establish that internalized Ag-BCR complexes associate with intracellular MHC class II molecules at a point subsequent to dissociation from Ii/CLIP but before surface expression of peptide-class II complexes and that the class II molecules that enter these complexes are predominantly in the M1-paired conformation. These findings represent a significant step forward in our understanding of exogenous antigen processing/presentation by antigen-specific B cells and suggest that future study of other class II APCs, such as dendritic cells and macrophages, may reveal an ability for other antigen receptors, such as Fc receptors and DEC-205, to associate with intracellular class II in these cells.
Attempts to detect the class II chaperone DM (which facilitates CLIP release/class II peptide loading) within the isolated Ag-BCR—class II complexes have not been fruitful (data not shown). However, Macmillan et al. (27) have reported recently that DM interacts with the Fab region of internalized Ag-BCR complexes to catalyze the release of BCR-bound antigen. In this report, we isolate Ag-BCR—MHC class II complexes by pulldown of biotin-labeled antigen. Therefore, any complexes possessing significant levels of DM molecules may not be recovered because the DM would have catalyzed the release of the biotin-labeled antigen from the complex. Taken together, our work and the work of Macmillan et al. (27) suggest that internalized Ag-BCR complexes may interact with intracellular class II or class II-DM complexes, where DM catalyzes Ag-BCR dissociation as a potential prelude to proteolytic antigen processing and class II association. This hypothesis will require further investigation.
Prior work by Mitchell et al. (28) established that peptides derived from simultaneous processing of two different BCR-bound antigens (in the same cell) compete with each other for binding to limiting numbers of MHC class II molecules, whereas peptides stemming from simultaneous processing of an antigen internalized via fluid-phase endocytosis do not compete with peptides derived from Ag-BCR complexes. The authors interpreted these results to mean that there is a limiting step in the intracellular processing of Ag-BCR complexes. In light of our current findings, it seems possible that this limiting step could be an association of internalized Ag-BCR complexes with intracellular class II or class II-DM complexes.
BCR-mediated antigen processing is known to result in the formation of peptide-class II complexes with unique properties. For example, in vitro engagement of peptide-class II complexes formed via fluid-phase processing of non-cognate antigen results in B cell death, whereas engagement of peptide-class II formed via BCR-mediated processing of cognate antigen results in B cell survival and expansion (5). Moreover, in vivo B cell-T cell interactions in non-immunized mice (presumably mediated by T cell recognition of class II molecules loaded with peptides from self-antigens internalized by fluid-phase endocytosis) are transient in nature, lasting less than 10 min. In contrast, B-T conjugates in immunized mice formed subsequent to BCR-mediated processing of cognate antigen are long-lasting (in some cases lasting over an hour) and are highly motile, with B cells leading T cells over long migratory paths (29). The results presented in this report suggest that the unique properties of these BCR-generated peptide-class II complexes may be the result of an association of internalized Ag-BCR complexes with M1-paired MHC class II molecules. In contrast, bypassing the BCR (e.g. fluid-phase uptake of high-dose antigen) may result in antigen access to both M1- and M2-paired class II and development of B cells expressing peptide-class II complexes with different signaling properties.
Although MHC class II molecules can signal via multiple mechanisms (25), class II-driven intracellular calcium signaling in B cells appears to occur primarily by class II-associated CD79 molecules (25). Moreover, the 11-5.2 mAb, which selectively binds M1-paired I-Ak class II molecules, is highly effective at driving class II calcium signaling in B cells whereas pan-reactive mAb are not (30). This suggests that one consequence of association of internalized Ag-BCR complexes with M1-paired class II could be the transfer of BCR-derived CD79 signaling modules to M1-paired class II molecules, providing them with their unique signaling properties.
In summary, we established that internalized Ag-BCR complexes associate with intracellular MHC class II molecules and that the class II molecules that enter this complex are predominantly in the M1-paired conformation. These findings represent a significant step forward in our understanding of MHC class II immunobiology in antigen-specific B cells and provide a potential explanation for some of the unique signaling properties of BCR-generated peptide-class II complexes.
Author Contributions
M. B. and L. D. designed the study; acquired, analyzed, and interpreted data; and helped write the report. H. T. and K. N. designed the study and acquired, analyzed, and interpreted data. J. R. D. conceived the project; designed the study; acquired, analyzed, and interpreted data; and helped write the report.
Acknowledgments
We thank the Albany Medical College Imaging Core Facility, Center for Immunology and Microbial Disease Immunology Core Facility, and Albany Medical College Animal Resources Facility. We also thank Drs. Jon Harton and Joe Mazurkiewicz for discussions and Dr. Michelle Lennartz for discussions and critical reading of the manuscript.
This work was supported by National Institutes of Health Grants AI-097673 (to J. R. D.) and CA-161782 (to M. B.). The authors declare that they have no conflicts of interest with the contents of this article.
- APC
- antigen-presenting cell
- BCR
- B cell receptor
- ER
- endoplasmic reticulum
- Ii
- invariant chain (CD74)
- CLIP
- class II-associated invariant chain peptide
- Ag
- antigen
- PC
- phosphorylcholine
- qD
- quenched donor
- A
- acceptor
- ROI
- region of interest
- HEL
- hen egg lysozyme
- WCL
- whole cell lysate
- PGS
- protein G-Sepharose
- cmplx
- complex
- BSA
- bovine serum albumin
- btn
- biotin
- CFP
- cyan fluorescent protein
- YFP
- yellow fluorescent protein.
References
- 1.Hulpke S., and Tampé R. (2013) The MHC I loading complex: a multitasking machinery in adaptive immunity. Trends Biochem. Sci. 38, 412–420 [DOI] [PubMed] [Google Scholar]
- 2.Chesnut R. W., and Grey H. M. (1985) Antigen presenting cells and mechanisms of antigen presentation. Crit. Rev. Immunol. 5, 263–316 [PubMed] [Google Scholar]
- 3.Chesnut R. W., and Grey H. M. (1986) Antigen presentation by B cells and its significance in T-B interactions. Adv. Immunol. 39, 51–94 [DOI] [PubMed] [Google Scholar]
- 4.Lanzavecchia A. (1987) Antigen uptake and accumulation in antigen-specific B cells. Immunol. Rev. 99, 39–51 [DOI] [PubMed] [Google Scholar]
- 5.Nashar T. O., and Drake J. R. (2005) The pathway of antigen uptake and processing dictates MHC class II-mediated B cell survival and activation. J. Immunol. 174, 1306–1316 [DOI] [PubMed] [Google Scholar]
- 6.Cresswell P. (1996) Invariant chain structure and MHC class II function. Cell 84, 505–507 [DOI] [PubMed] [Google Scholar]
- 7.Mellins E. D., and Stern L. J. (2014) HLA-DM and HLA-DO, key regulators of MHC-II processing and presentation. Curr. Opin. Immunol. 26, 115–122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Dixon A. M., Drake L., Hughes K. T., Sargent E., Hunt D., Harton J. A., and Drake J. R. (2014) Differential transmembrane domain GXXXG motif pairing impacts major histocompatibility complex (MHC) class II structure. J. Biol. Chem. 289, 11695–11703 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Drake J. R., Webster P., Cambier J. C., and Mellman I. (1997) Delivery of B cell receptor-internalized antigen to endosomes and class II vesicles. J. Exp. Med. 186, 1299–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Busman-Sahay K., Sargent E., Harton J. A., and Drake J. R. (2011) The Ia.2 epitope defines a subset of lipid raft-resident MHC class II molecules crucial to effective antigen presentation. J. Immunol. 186, 6710–6717 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Elangovan M., Wallrabe H., Chen Y., Day R. N., Barroso M., and Periasamy A. (2003) Characterization of one- and two-photon excitation fluorescence resonance energy transfer microscopy. Methods 29, 58–73 [DOI] [PubMed] [Google Scholar]
- 12.Talati R., Vanderpoel A., Eladdadi A., Anderson K., Abe K., and Barroso M. (2014) Automated selection of regions of interest for intensity-based FRET analysis of transferrin endocytic trafficking in normal vs. cancer cells. Methods 66, 139–152 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Wallrabe H., Bonamy G., Periasamy A., and Barroso M. (2007) Receptor complexes cotransported via polarized endocytic pathways form clusters with distinct organizations. Mol. Biol. Cell 18, 2226–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Wallrabe H., Elangovan M., Burchard A., Periasamy A., and Barroso M. (2003) Confocal FRET microscopy to measure clustering of ligand-receptor complexes in endocytic membranes. Biophys. J. 85, 559–571 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kenworthy A. K., and Edidin M. (1998) Distribution of a glycosylphosphatidylinositol-anchored protein at the apical surface of MDCK cells examined at a resolution of <100 A using imaging fluorescence resonance energy transfer. J. Cell Biol. 142, 69–84 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Kenworthy A. K., Petranova N., and Edidin M. (2000) High-resolution FRET microscopy of cholera toxin B-subunit and GPI-anchored proteins in cell plasma membranes. Mol. Biol. Cell 11, 1645–1655 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Vilen B. J., Nakamura T., and Cambier J. C. (1999) Antigen-stimulated dissociation of BCR mIg from Ig-α/Ig-β: implications for receptor desensitization. Immunity 10, 239–248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cassard S., Salamero J., Hanau D., Spehner D., Davoust J., Fridman W. H., and Bonnerot C. (1998) A tyrosine-based signal present in Ig α mediates B cell receptor constitutive internalization. J. Immunol. 160, 1767–1773 [PubMed] [Google Scholar]
- 19.Eastman S., Deftos M., DeRoos P. C., Hsu D. H., Teyton L., Braunstein N. S., Hackett C. J., and Rudensky A. (1996) A study of complexes of class II invariant chain peptide: major histocompatibility complex class II molecules using a new complex-specific monoclonal antibody. Eur. J. Immunol. 26, 385–393 [DOI] [PubMed] [Google Scholar]
- 20.Sadegh-Nasseri S., Stern L. J., Wiley D. C., and Germain R. N. (1994) MHC class II function preserved by low-affinity peptide interactions preceding stable binding. Nature 370, 647–650 [DOI] [PubMed] [Google Scholar]
- 21.Katkere B., Rosa S., Caballero A., Repasky E. A., and Drake J. R. (2010) Physiological-range temperature changes modulate cognate antigen processing and presentation mediated by lipid raft-restricted ubiquitinated B cell receptor molecules. J. Immunol. 185, 5032–5039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Katkere B., Rosa S., and Drake J. R. (2012) The Syk-binding ubiquitin ligase c-Cbl mediates signaling-dependent B cell receptor ubiquitination and B cell receptor-mediated antigen processing and presentation. J. Biol. Chem. 287, 16636–16644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Zhang M., Veselits M., O'Neill S., Hou P., Reddi A. L., Berlin I., Ikeda M., Nash P. D., Longnecker R., Band H., and Clark M. R. (2007) Ubiquitinylation of Ig β dictates the endocytic fate of the B cell antigen receptor. J. Immunol. 179, 4435–4443 [DOI] [PubMed] [Google Scholar]
- 24.King G., and Dixon A. M. (2010) Evidence for role of transmembrane helix-helix interactions in the assembly of the Class II major histocompatibility complex. Mol. Biosyst. 6, 1650–1661 [DOI] [PubMed] [Google Scholar]
- 25.Jin L., Stolpa J. C., Young R. M., Pugh-Bernard A. E., Refaeli Y., and Cambier J. C. (2008) MHC class II structural requirements for the association with Igα/β, and signaling of calcium mobilization and cell death. Immunol. Lett. 116, 184–194 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Lang P., Stolpa J. C., Freiberg B. A., Crawford F., Kappler J., Kupfer A., and Cambier J. C. (2001) TCR-induced transmembrane signaling by peptide/MHC class II via associated Ig-α/β dimers. Science 291, 1537–1540 [DOI] [PubMed] [Google Scholar]
- 27.Macmillan H., Strohman M. J., Ayyangar S., Jiang W., Rajasekaran N., Spura A., Hessell A. J., Madec A. M., and Mellins E. D. (2014) The MHC class II cofactor HLA-DM interacts with Ig in B cells. J. Immunol. 193, 2641–2650 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Mitchell R. N., Barnes K. A., Grupp S. A., Sanchez M., Misulovin Z., Nussenzweig M. C., and Abbas A. K. (1995) Intracellular targeting of antigens internalized by membrane immunoglobulin in B lymphocytes. J. Exp. Med. 181, 1705–1714 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Okada T., Miller M. J., Parker I., Krummel M. F., Neighbors M., Hartley S. B., O'Garra A., Cahalan M. D., and Cyster J. G. (2005) Antigen-engaged B cells undergo chemotaxis toward the T zone and form motile conjugates with helper T cells. PLoS Biol. 3, e150. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Nashar T. O., and Drake J. R. (2006) Dynamics of MHC class II-activating signals in murine resting B cells. J. Immunol. 176, 827–838 [DOI] [PubMed] [Google Scholar]