Abstract
Aims
To test the hypothesis that chocolate consumption is associated with a lower risk of heart failure (HF).
Methods and Results
We prospectively studied 20,278 men from the Physicians’ Health Study. Chocolate consumption was assessed between 1999 and 2002 via a self-administered food frequency questionnaire and HF was ascertained through annual follow-up questionnaires with validation in a subsample. We used Cox regression to estimate multivariable adjusted relative risk of HF. During a mean follow-up of 9.3 years, there were 876 new cases of HF. The mean age at baseline was 66.4 ± 9.2 years. Hazard ratios (95% CI) for HF were 1.0 (ref), 0.86 (0.72–1.03), 0.80 (0.66–0.98), 0.92 (0.74–1.13), and 0.82 (0.63–1.07), for chocolate consumption of less than 1/month, 1–3/week, 2–4/week, and 5+/week, respectively, after adjusting for age, body mass index (BMI), smoking, alcohol, exercise, energy intake, and history of atrial fibrillation (p for quadratic trend = 0.62). In a secondary analysis, chocolate consumption was inversely associated with risk of HF in men whose BMI was <25 kg/m2 (HR (95% CI) = 0.59 (0.37–0.94) for consumption of 5+ servings/week, p for linear trend = 0.03) but not in those with BMI of 25+ kg/m2 (HR (95% CI) = 1.01 (0.73–1.39), p for linear trend = 0.42, p for interaction=0.17).
Conclusions
Our data suggest that moderate consumption of chocolate might be associated with a lower risk of HF in male physicians..
Keywords: epidemiology, heart failure, risk factors, nutrition
Introduction
Heart failure (HF) is a major comorbidity in older adults and remains a public health burden in the United States since it is associated with high healthcare expenditures.1,2 At 40 years of age, the lifetime risk of HF is 1 in 5, and by age 65, HF incidence approaches 10 per 1000 individuals.3,4 HF contributes to 1 in 9 deaths, and the costs of HF are estimated to be $31 billion annually and anticipated to reach $70 billion by 2030.4 Thus it is important to improve primary prevention of HF. Currently, lifestyle factors, such as regular exercise, and diet, including moderate alcohol intake, and consumption of fruits, vegetables, breakfast cereals, and fish high in omega-3 fatty acids, have been shown to be related to lower risk of HF.5,6
Prior research has suggested that dark chocolate and cocoa intake are associated with reduced risk of cardiovascular disease and cardiovascular mortality.7–10 The benefit of chocolate consumption on risk of CVD may be due to favorable effects of cocoa products on blood pressure, which is a major risk factor for HF.10–13
One prior study assessed the impact of chocolate intake on the incidence of HF in the Swedish Mammography Cohort.14 In 9 years of follow-up, for women who consumed 1–3 servings/month, there was a 26% lower risk of HF compared to no regular intake (95% CI = 5%–42%), and a 32% lower risk of HF (95% CI=7%–50%) with consumption of 1–3 servings/week, and there was no association with 3 or more servings per week.14 Such data could have been confounded by overall healthy diet among chocolate consumers. Since no prior research has been published on the relation of chocolate intake with HF in healthy men we sought to assess whether moderate chocolate consumption is associated with lower risk of HF in US male physicians. In a secondary aim, we examined whether the relation between chocolate intake and risk of HF differs in lean versus overweight and obese individuals.
Methods
Study population
The Physicians’ Health Study (PHS) I is a completed, randomized, double-blind, placebo-controlled trial designed to study low-dose aspirin and β-carotene for the primary prevention of cardiovascular disease and cancer among US male physicians. In 1997, the PHS II enrolled 7,641 physicians from the PHS I and 7,000 new physicians to study the effects of β-carotene, vitamin C, E, and a multivitamin on cardiovascular disease and cancer. Detailed descriptions of the PHS I and II have previously been published.15,16 Of the 29,071 total participants in the PHS, 21,075 completed a food frequency questionnaire (FFQ) between 1999 and 2002 and were alive at baseline. We excluded individuals with prevalent HF at the time of the FFQ (n=470) and missing data for chocolate consumption (n=327), for a final sample of 20,278 used in the present analysis. Each participant gave written informed consent, and the institution review board at Brigham and Women’s Hospital approved the study protocol.
Ascertainment of HF in the PHS
HF outcomes in the PHS were determined with the use of annual follow-up questionnaires mailed to each participant to obtain information on compliance with the intervention and the occurrence of new medical diagnoses. Cases of HF were identified by self-reported diagnosis. HF diagnoses in the PHS were previously validated by reviewing medical records in a subsample with a high positive predictive value (91%) in the PHS.17,18
Characterization of chocolate consumption
Information on chocolate consumption was obtained by using a FFQ. Participants were asked to report their average consumption of 1 oz of chocolate during the past year. Possible answers were never or less than once per month; 1–3/month; 1/week; 2–4/week; 5–6/week; 1/day; 2–3/day; 4–5/day; and 6+/day. For current analyses, we collapsed adjacent categories to obtain stable estimate (never or <1/month; 1–3/month; 1/week, 2–4/week, and 5+/week) because only 5% of subjects indicated consumption of chocolate of 1 or more times a day. The validity and reproducibility of FFQs have been previously published.19,20
Other variables
Information on demographic variables, body mass index (BMI), cigarette smoking, exercise, alcohol consumption, and history of diabetes, hypertension, and MI were collected at baseline. Information on incident co-morbidities, such as diabetes, hypertension, and MI, were collected through annual follow-up questionnaires as previously described.
Statistical Analysis
We used Cox regression analysis to calculated hazard ratios (HRs) and corresponding 95% confidence intervals (CIs). Person time was calculated from date of food frequency questionnaire to first date of HF, date of death, or date of last available follow-up. Proportional hazards assumption was assessed by including an interaction with logarithmic person time in the model. We used generalized additive models to assess whether fitting continuous variables as such or as categorical variables improved the fit of the model. We assessed confounding by age (<=55, >55–65, >65–75, >75), BMI (continuous), race (white/non-white), smoking (never, past, current), alcohol consumption (never, 1–3/month, 1–6/week, and 1+/day), exercise (<1/day, 1–2 days/week, 3–4 days/week, 5–7 days/week), caloric intake (tertiles), sodium intake (tertiles), fruit and vegetable intake (tertiles), red meat intake (tertiles), and history of atrial fibrillation (yes/no). A variable was considered a confounder if its adjustment led to a ≥ 10% change in the regression β coefficient for any chocolate category. A p value for linear trend was obtained by creating a new variable that was assigned median chocolate intake in each category, and p for quadratic trend was obtained by fitting a quadratic term for median chocolate consumption in a hierarchical model.
The initial model was adjusted for age. The parsimonious model accounted for age, BMI, smoking, alcohol consumption, exercise, caloric intake, and history of atrial fibrillation. We then considered hypertension (yes/no) and diabetes (yes/no) as potential mediators and did not adjust for them in the multivariable model. For each categorical variable we created indicator variables for missing observations.
In secondary analysis, we stratified BMI into <25 kg/m2 (n=8,907) and ≥25 kg/m2 (n=11,368). Analysis was completed using SAS version 9.3 (SAS Institute, Cary, North Carolina). All p values were 2-tailed, and the significance level was set at an alpha of 0.05.
Results
The characteristics of the 20,278 US male physicians are presented in Table 1 according to chocolate consumption. The mean age of study participants at baseline was 66.4 ± 9.2 (range 50.2–97.6). Median chocolate consumption was 1–3 time per month. Compared to consumption of <1/month, higher chocolate consumption was associated with being white, lower alcohol consumption, a higher proportion of never smokers, a lower prevalence of baseline diabetes, hypertension, MI, and atrial fibrillation, and higher energy intake.
Table 1.
Baseline characteristics of 20,278 participants in the Physicians’ Health Study according to chocolate intake
| Chocolate intake (servings/week) | ||||||
|---|---|---|---|---|---|---|
| Characteristics1 | 0 | <1 | 1 | 2–4 | 5+ | P for trend |
| n | 5359 | 5907 | 3919 | 3234 | 1859 | |
| Age (y) | 67.0 ± 8.8 | 66.0 ± 8.9 | 66.2 ± 9.2 | 66.2 ± 9.6 | 66.7 ± 10.1 | 0.39 |
| BMI (kg/m2) | 25.6 ± 3.3 | 25.9 ± 3.4 | 25.8 ± 3.2 | 25.8 ± 3.3 | 25.6 ±3.3 | 0.24 |
| White | 85.4 | 91.3 | 94.2 | 95.6 | 95.7 | <0.01 |
| Smoking status | ||||||
| Never | 50.0 | 54.0 | 56.9 | 57.8 | 56.3 | <0.01 |
| Past | 46.0 | 42.9 | 39.9 | 38.8 | 40.0 | <0.01 |
| Current | 3.8 | 3.1 | 3.2 | 3.3 | 3.6 | 0.47 |
| Alcohol consumption | ||||||
| Rarely/Never | 17.0 | 15.4 | 16.8 | 18.6 | 20.3 | <0.01 |
| 1–3×/month | 6.6 | 7.3 | 8.0 | 8.8 | 8.7 | <0.01 |
| 1–6×/week | 35.6 | 38.7 | 39.4 | 39.7 | 37.7 | <0.01 |
| 1 or more/day | 40.3 | 38.0 | 35.1 | 32.3 | 32.8 | <0.01 |
| Exercise (days per week) | ||||||
| <1 | 38.0 | 37.3 | 35.8 | 36.5 | 37.6 | 0.20 |
| 1–2 | 15.3 | 16.7 | 17.3 | 16.3 | 15.3 | 0.56 |
| 3–4 | 28.1 | 29.2 | 30.2 | 30.9 | 29.1 | 0.03 |
| 5–7 | 16.7 | 15.3 | 15.1 | 14.4 | 16.3 | 0.08 |
| Prevalent diabetes | 11.4 | 6.5 | 5.2 | 5.0 | 4.8 | <0.01 |
| Prevalent hypertension | 49.6 | 46.2 | 44.4 | 42.5 | 43.3 | <0.01 |
| Prevalent MI | 4.8 | 3.9 | 3.0 | 3.0 | 3.7 | <0.01 |
| Prevalent atrial fibrillation | 9.1 | 7.9 | 7.9 | 8.6 | 8.6 | 0.50 |
| Calories (kcal) | 1534.9 ± 481.1 | 1626.2 ± 485.5 | 1705.6 ± 499.8 | 1795.8 ± 509.5 | 2033.6 ± 579.9 | <0.01 |
| Sodium (mg) | 1356.6 ± 528.2 | 1455.4 ± 532.5 | 1527.1 ± 546.3 | 1576.0 ± 541.3 | 1690.7 ± 607.4 | <0.01 |
| Fruits and Vegetables (s/d) | 4.8 ± 2.9 | 4.8 ± 2.6 | 4.9 ± 2.6 | 4.9 ± 2.5 | 5.2 ± 3.0 | <0.01 |
| Red Meat (s/d) | 0.6 ± 0.6 | 0.7 ± 0.6 | 0.7 ± 0.6 | 0.8 ± 0.6 | 0.9 ± 0.7 | <0.01 |
Data are presented as means ± SD, median (IQR), or percentages. Few participants had missing data: BMI (n=3), race (n=52), smoking (n=14), alcohol (n=117), exercise (n=349), calories (n=894), sodium (n=894), fruit and vegetable intake (n=1), red meat intake (n=21)
In a Cox regression adjusted for age, HRs for HF were 1.00 (reference), 0.86 (0.72–1.02), 0.79 (0.65–0.96), 0.91 (0.74–1.11), and 0.82 (0.64–1.06) for chocolate consumption of <1/month, 1–3/month, 1/week, and 2–4/week, and 5+/week respectively (p for linear and quadratic trend = 0.35 and 0.51, respectively). In a parsimonious model adjusted for age, BMI, smoking, alcohol, exercise, energy intake, and prevalent atrial fibrillation, compared to consumption of <1/month, consumption of 1/week, was associated with a 20% lower risk of HF (95% CI: 2% to 34%), Table 2. As expected, additional adjustment for hypertension and diabetes slightly attenuated this relationship: HRs for HF were 1.00 (reference), 0.90 (0.76–1.08), 0.86 (0.70–1.05), 0.98 (0.80–1.21), and 0.89 (0.68–1.16) across categories of chocolate consumption.
Table 2.
Hazard ratios (95% CI) for heart failure according to chocolate consumption in the Physicians’ Health Study
| Hazard ratios (95% CI) |
||||
|---|---|---|---|---|
| Frequency of chocolate intake | Cases/Person- time |
Crude Incidence Rate (per 1000 person-years) |
Age Adjusted | Model 11 |
| Never or <1/month | 259/48,669 | 5.32 | 1.00 | 1.00 |
| 1–3/month | 243/55,622 | 4.37 | 0.86 (0.72–1.02) | 0.86 (0.72–1.03) |
| 1/week | 153/37,119 | 4.12 | 0.79 (0.65–0.96) | 0.80 (0.66–0.98) |
| 2–4/week | 144/30,585 | 4.71 | 0.91 (0.74–1.11) | 0.92 (0.74–1.13) |
| 5+/week | 77/17,128 | 4.50 | 0.82 (0.64–1.06) | 0.82 (0.63–1.07) |
| p for linear trend | 0.35 | 0.41 | ||
| p for quadratic trend | 0.51 | 0.62 | ||
Adjusted for age (≤55, 55–≤65, 65–≤75, 75+), BMI (continuous), alcohol (never, 1–3/month, 1–6/week, and 1+/day), smoking (never, past, current), exercise (<1/day, 1–2 days/week, 3–4 days/week, 5–7 days/week), caloric intake (tertiles), and prevalent atrial fibrillation (yes/no)
In secondary analyses, we examined the relation of chocolate with risk of HF stratified by BMI, and showed a significant linear relation in individuals with a BMI <25 kg/m2 (HRs for HF were 1.00 (reference), 0.86 (0.65–1.15), 0.70 (0.50–0.99), 0.72 (0.50–1.04), 0.59 (0.37–0.94), across consecutive categories of chocolate consumption (p for linear trend=0.03). For individuals with a BMI ≥25 kg/m2, there was no significant association with HF (HRs and 95% CIs: 1.00 (reference), 0.87 (0.69–1.09), 0.88 (0.68–1.13), 1.06 (0.82–1.37), 1.01 (0.73–1.39) for chocolate consumption of <1/month 1–3/month, 1/week, and 2–4/week, and 5+/week respectively, p for linear trend=0.42, p for interaction (BMI*chocolate)=0.17, Figure 1.
Figure 1.
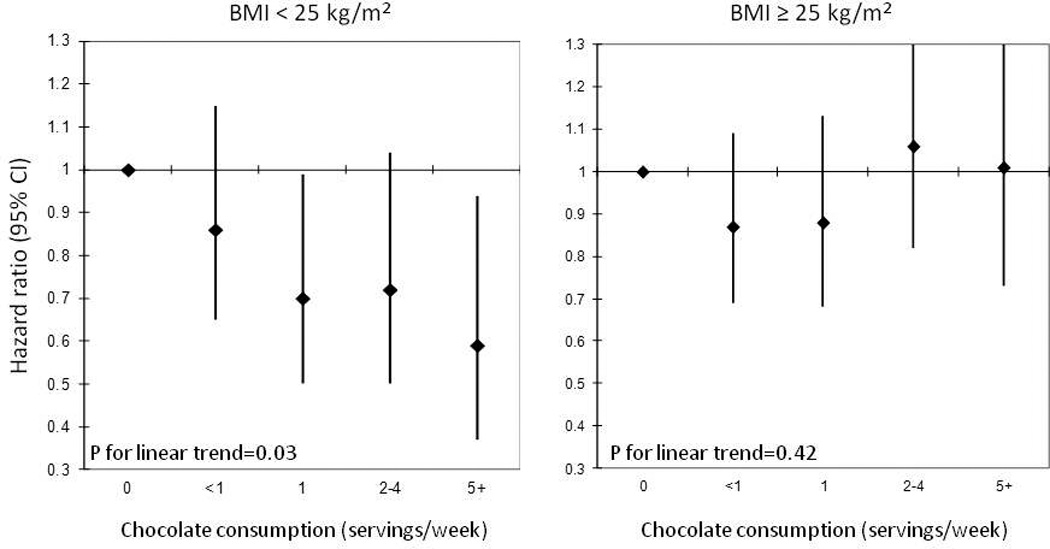
Hazard ratios (95% CI) for heart failure according to chocolate consumption stratified by body mass index1,2
1. Adjusted for age (≤55, 55–≤65, 65–≤75, 75+), BMI (continuous), alcohol (never, 1–3/month, 1–6/week, and 1+/day), smoking (never, past, current), exercise (<1/day, 1–2 days/week, 3–4 days/week, 5–7 days/week), caloric intake (tertiles), and prevalent atrial fibrillation (yes/no)
2. P for interaction = 0.17
Discussion
In this prospective cohort study, we found that moderate chocolate consumption was associated with a lower risk of HF in male physicians after adjustment for important confounders. In a secondary analysis, this inverse association was slightly stronger and stepwise in lean individuals.
Chocolate intake has been previously shown to be associated with lower blood pressure.10–13 In short term trials, cocoa intake resulted in a significant reduction in mean systolic blood pressure by 2.77 mmHg (95% CI = −4.72, −0.82), and a reduction in diastolic blood pressure by 2.20 mmHg (95% CI = −3.46, −0.93).11 Elevated blood pressure is a major risk factor for HF.21–24 Our findings could be partly attributable to a decrease in blood pressure since we found that including hypertension in the model slightly attenuated our results.
Previous randomized control trials have suggested that flavonoids in chocolate may be responsible for the beneficial effect of chocolate on blood pressure, along with other risk factors for HF, including increasing HDL cholesterol, improving endothelial function, and reducing inflammation.25,26 Cocoa products are rich is plant phytochemicals, especially flavonoids, with strong antioxidant properties.27 Experiments with endothelial cell cultures have shown that flavonoids reduce the down-regulation of nitric oxide synthase, thereby promoting the production of nitric oxide and help maintain normal cardiac function.28,29 By increasing nitric oxide production and decreasing oxidative stress, flavonoids could be playing a role to lower the risk of HF. In patients with HF, a randomized control trial found that feeding flavanol-rich chocolate significantly improved endothelial function suggesting an improvement in vascular function.30
To our knowledge, only one prior study has prospectively tested whether chocolate consumption is related to risk of HF in healthy individuals.14 In the Swedish Mammography Cohort of 31,823 women, compared to no chocolate consumption, multivariable adjusted HFs for HF were 0.74 (0.58–0.95), 0.68 (0.50–0.93), 1.09 (0.74–1.62), and 1.23 (0.73–2.08) for chocolate intake of 1–3/month, 1–2/week, 3–6/week, and ≥ 1 serving/day, which is similar, albeit a stronger association, when compared to our current study. In individuals with a prior myocardial infarction in the Stockholm Heart Epidemiology Program, between 1/month and ≤1/week of chocolate consumption was associated with a 32% lower risk of HF (95% CI = 3%-53%) compared to no consumption.31 This suggests that chocolate consumption may be beneficial even in patients with prior coronary heart disease. The authors did not stratify by BMI for risk of HF, however, moderate chocolate consumption was associated with risk of cardiac mortality in patients with a BMI < 30 kg/m2, but not in those with a higher BMI.31
In a secondary analysis, we found the association between chocolate consumption and heart failure in lean subjects to be stronger, although statistically not significantly different from overweight/obese males. This finding is worth pursuing in an independent cohort. It is possible that the benefits from flavonoid consumption in overweight individuals could be outweighed by the overall harm from metabolic syndrome. Alternatively, low risk of HF in people with normal BMI makes it easier to detect a small effect size compared to overweight and obese individuals.
Our study had a few limitations. Due to the observational design of the current study, we cannot exclude residual or unmeasured confounding as an alternate explanation of our results. Chocolate consumption could have been misclassified due to self-reports, because chocolate was only assessed at baseline, and because we didn’t have data on amounts of chocolate consumed. Additionally, our study did not have data on type of chocolate consumed, so it is unclear whether we would have obtained stronger results had we been able to restrict analysis to dark chocolate. We did not collect data to distinguish HF with and without preserved left ventricular function. Finally, our population was predominately Caucasian male physicians who may have had different behaviors than the general population; hence, our results may not be generalizable to women or other ethnic groups.
Strengths of our study include the prospective design of the PHS, a large sample size, the collection of a large number of key confounding variables, and a high positive predictive value (91%) of self-reported HF in male physicians.5
In conclusion, our data showed an inverse but non-linear relation between chocolate intake and risk of HF in male physicians.
Acknowledgements
Funding: This work was supported by the National Heart, Lung, and Blood Institute [R21 HL088081 to LD]. The Physicians’ Health Study is supported by the National Cancer Institute [CA-34944, CA-40360, and CA-097193], and from the National Heart, Lung, and Blood Institute [HL-26490 and HL-34595].
We are indebted to the participants in the PHS for their outstanding commitment and cooperation and to the entire PHS staff for their expert and unfailing assistance.
Dr. Djousse has received in the past ad hoc travel support to an international scientific meeting from The Hershey Company scientific meeting from the Hershey, Inc.
Abbreviations
- HF
heart failure
- PHS
Physicians’ Health Study
- FFQ
food frequency questionnaire
- BMI
body mass index
Footnotes
Conflict of interest: All other authors confirm no potential conflict of interest.
References
- 1.Goldberg RJ, Spencer FA, Farmer C, Meyer TE, Pezzella S. Incidence and hospital death rates associated with heart failure: A community-wide perspective. Am J Med. 2005;118:728–734. doi: 10.1016/j.amjmed.2005.04.013. [DOI] [PubMed] [Google Scholar]
- 2.Goldberg R, Glatfelter K, Burbank-Schmidt E, Farmer C, Spencer F, Meyer T. Trends in mortality attributed to heart failure in worcester, massachusetts, 1992 to 2001. Am J Cardiol. 2005;95:1324–1328. doi: 10.1016/j.amjcard.2005.01.076. [DOI] [PubMed] [Google Scholar]
- 3.LloydJones DMS, Larson MG, Leip EP, Beiser A, D'Agostino RB, Kannel WB, Murabito JMS, Vasan RS, Benjamin EJS, Levy D. Lifetime risk for developing congestive heart failure: The framingham heart study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 4.Go AS, Mozaffarian D, Roger VL, Benjamin EJ, Berry JD, Blaha MJ, Dai S, Ford ES, Fox CS, Franco S, Fullerton HJ, Gillespie C, Hailpern SM, Heit JA, Howard VJ, Huffman MD, Judd SE, Kissela BM, Kittner SJ, Lackland DT, Lichtman JH, Lisabeth LD, Mackey RH, Magid DJ, Marcus GM, Marelli A, Matchar DB, McGuire DK, Mohler ER, 3rd, Moy CS, Mussolino ME, Neumar RW, Nichol G, Pandey DK, Paynter NP, Reeves MJ, Sorlie PD, Stein J, Towfighi A, Turan TN, Virani SS, Wong ND, Woo D, Turner MB American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics--2014 update: A report from the american heart association. Circulation. 2014;129:e28–e292. doi: 10.1161/01.cir.0000441139.02102.80. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Djousse L, Driver JA, Gaziano MJ. Relation between modifiable lifestyle factors and lifetime risk of heart failure. JAMA. 2009;302:394–400. doi: 10.1001/jama.2009.1062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Mozaffarian D, Bryson CL, Lemaitre RN, Burke GL, Siscovick DS. Fish intake and risk of incident heart failure. J Am Coll Cardiol. 2005;45:2015–2021. doi: 10.1016/j.jacc.2005.03.038. [DOI] [PubMed] [Google Scholar]
- 7.Zhang Z, Xu G, Liu X. Chocolate intake reduces risk of cardiovascular disease: Evidence from 10 observational studies. Int J Cardiol. 2013 doi: 10.1016/j.ijcard.2012.12.036. [DOI] [PubMed] [Google Scholar]
- 8.Buitrago-Lopez A, Sanderson J, Johnson L, Warnakula S, Wood A, Di Angelantonio E, Franco OH. Chocolate consumption and cardiometabolic disorders: Systematic review and meta-analysis. BMJ: British Medical Journal. 2011;343:d4488. doi: 10.1136/bmj.d4488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Khawaja O, Gaziano JM, Djoussé L. Chocolate and coronary heart disease: A systematic review. Curr Atheroscler Rep. 2011;13:447–452. doi: 10.1007/s11883-011-0203-2. [DOI] [PubMed] [Google Scholar]
- 10.Buijsse B, Feskens EJ, Kok FJ, Kromhout D. Cocoa intake, blood pressure, and cardiovascular mortality: The zutphen elderly study. Arch Intern Med. 2006;166:411. doi: 10.1001/archinte.166.4.411. [DOI] [PubMed] [Google Scholar]
- 11.Ried K, Sullivan TR, Fakler P, Frank OR, Stocks NP. Effect of cocoa on blood pressure. The Cochrane Library. 2012 doi: 10.1002/14651858.CD008893.pub2. Published online ahead of print 15 August 2012. [DOI] [PubMed] [Google Scholar]
- 12.Grassi D, Necozione S, Lippi C, Croce G, Valeri L, Pasqualetti P, Desideri G, Blumberg JB, Ferri C. Cocoa reduces blood pressure and insulin resistance and improves endothelium-dependent vasodilation in hypertensives. Hypertension. 2005;46:398–405. doi: 10.1161/01.HYP.0000174990.46027.70. [DOI] [PubMed] [Google Scholar]
- 13.Desch S, Schmidt J, Kobler D, Sonnabend M, Eitel I, Sareban M, Rahimi K, Schuler G, Thiele H. Effect of cocoa products on blood pressure: Systematic review and meta-analysis. Am J Hypertens. 2010;23:97–103. doi: 10.1038/ajh.2009.213. [DOI] [PubMed] [Google Scholar]
- 14.Mostofsky E, Levitan EB, Wolk A, Mittleman MA. Chocolate intake and incidence of heart failure a population-based prospective study of middle-aged and elderly women. Circulation: Heart Failure. 2010;3:612–616. doi: 10.1161/CIRCHEARTFAILURE.110.944025. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Final report on the aspirin component of the ongoing physicians' health study. steering committee of the physicians' health study research group. N Engl J Med. 1989;321:129–135. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 16.Christen WG, Gaziano JM, Hennekens CH. Design of physicians' health study II—a randomized trial of beta-carotene, vitamins E and C, and multivitamins, in prevention of cancer, cardiovascular disease, and eye disease, and review of results of completed trials. Ann Epidemiol. 2000;10:125–134. doi: 10.1016/s1047-2797(99)00042-3. [DOI] [PubMed] [Google Scholar]
- 17.HO KK L, Anderson KM, Kannel WB, Grossman WL, Daniel Survival after the onset of congestive heart failure in framingham heart study subjects. Circulation. 1993;88:107–115. doi: 10.1161/01.cir.88.1.107. [DOI] [PubMed] [Google Scholar]
- 18.Djousse L, D.Sc, Gaziano JM. Alcohol consumption and risk of heart failure in the physicians' health study I. Circulation. 2007;115:34–39. doi: 10.1161/CIRCULATIONAHA.106.661868. [DOI] [PubMed] [Google Scholar]
- 19.Willett WC, Sampson L, Stampfer MJ, Rosner B, Bain C, Witschi J, Hennekens CH, SPEIZER FE. Reproducibility and validity of a semiquantitative food frequency questionnaire. Am J Epidemiol. 1985;122:51–65. doi: 10.1093/oxfordjournals.aje.a114086. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Stampfer MJ, Colditz GA, Litin LB, Willett WC. Reproducibility and validity of an expanded self-administered semiquantitative food frequency questionnaire among male health professionals. Am J Epidemiol. 1992;135:1114–1126. doi: 10.1093/oxfordjournals.aje.a116211. [DOI] [PubMed] [Google Scholar]
- 21.Wilhelmsen L, Rosengren A, Eriksson H, Lappas G. Heart failure in the general population of men--morbidity, risk factors and prognosis. J Intern Med. 2001;249:253–261. doi: 10.1046/j.1365-2796.2001.00801.x. [DOI] [PubMed] [Google Scholar]
- 22.Levy D, Larson MG, Vasan RS, Kannel WB, Ho KKL. The progression from hypertension to congestive heart failure. JAMA. 1996;275:1557–1562. [PubMed] [Google Scholar]
- 23.Kannan A, Janardhanan R. Hypertension as a risk factor for heart failure. Curr Hypertens Rep. 2014;16:1–8. doi: 10.1007/s11906-014-0447-7. [DOI] [PubMed] [Google Scholar]
- 24.He J, Ogden LG, Bazzano LA, Vupputuri S, Loria C, Whelton PK. Risk factors for congestive heart failure in US men and women: NHANES I epidemiologic follow-up study. Arch Intern Med. 2001;161:996–002. doi: 10.1001/archinte.161.7.996. [DOI] [PubMed] [Google Scholar]
- 25.Hooper L, Kay C, Abdelhamid A, Kroon PA, Cohn JS, Rimm EB, Cassidy A. Effects of chocolate, cocoa, and flavan-3-ols on cardiovascular health: A systematic review and meta-analysis of randomized trials. Am J Clin Nutr. 2012;95:740–751. doi: 10.3945/ajcn.111.023457. [DOI] [PubMed] [Google Scholar]
- 26.Shrime MG, Bauer SR, McDonald AC, Chowdhury NH, Coltart CEM, Ding EL. Flavonoid-rich cocoa consumption affects multiple cardiovascular risk factors in a meta-analysis of short-term studies. J Nutr. 2011;141:1982–1988. doi: 10.3945/jn.111.145482. [DOI] [PubMed] [Google Scholar]
- 27.Lee KW, Kim YJ, Lee HJ, Lee CY. Cocoa has more phenolic phytochemicals and a higher antioxidant capacity than teas and red wine. J Agric Food Chem. 2003;51:7292–7295. doi: 10.1021/jf0344385. [DOI] [PubMed] [Google Scholar]
- 28.Fernández-Murga L, Tarín J, García-Perez M, Cano A. The impact of chocolate on cardiovascular health. Maturitas. 2011;69:312–321. doi: 10.1016/j.maturitas.2011.05.011. [DOI] [PubMed] [Google Scholar]
- 29.Ramirez-Sanchez I, Maya L, Ceballos G, Villarreal F. (−)-Epicatechin activation of endothelial cell endothelial nitric oxide synthase, nitric oxide, and related signaling pathways. Hypertension. 2010;55:1398–1405. doi: 10.1161/HYPERTENSIONAHA.109.147892. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Flammer AJ, Sudano I, Wolfrum M, Thomas R, Enseleit F, Périat D, Kaiser P, Hirt A, Hermann M, Serafini M. Cardiovascular effects of flavanol-rich chocolate in patients with heart failure. Eur Heart J. 2012;33:2172–2180. doi: 10.1093/eurheartj/ehr448. [DOI] [PubMed] [Google Scholar]
- 31.Janszky I, Mukamal KJ, Ljung R, Ahnve S, Ahlbom A, Hallqvist J. Chocolate consumption and mortality following a first acute myocardial infarction: The stockholm heart epidemiology program. J Intern Med. 2009;266:248–257. doi: 10.1111/j.1365-2796.2009.02088.x. [DOI] [PubMed] [Google Scholar]


