Abstract
Background
Progestin-only contraceptives (POCs) are appropriate for many women who cannot or should not take estrogen. Many POCs are long-acting, cost-effective methods of preventing pregnancy. However, concern about weight gain can deter the initiation of contraceptives and cause early discontinuation among users.
Objectives
The primary objective was to evaluate the association between progestin-only contraceptive use and changes in body weight.
Search strategy
We searched MEDLINE, CENTRAL, POPLINE, EMBASE, LILACS, ClinicalTrials.gov, and ICTRP, and contacted investigators to identify other trials.
Selection criteria
All comparative studies were eligible that examined a POC versus another method or no contraceptive. The primary outcome was mean change in body weight or body composition.
Data collection and analysis
Two authors extracted the data. We computed the mean difference with 95% confidence interval (CI) for continuous variables and odds ratio with 95% CI for dichotomous variables.
Main results
We did not conduct meta-analysis due to the various contraceptive methods and weight change measures. Fifteen studies examined progestin-only pills (N=1), Norplant (N=4), and depot medroxyprogesterone acetate (DMPA) (N=10). Comparison groups were similar for weight change in 11 studies. Four studies showed differences in weight or body composition change for POCs compared to no hormonal method. Adolescents using DMPA had a greater increase in body fat (%) versus a group using no hormonal method (mean difference 11.00; 95% CI 2.64 to 19.36). The DMPA group also had a greater decrease in lean body mass (%) (mean difference −4.00; 95% CI −6.93 to −1.07). In another study, weight gain (kg) was greater for the DMPA group than an IUD group (mean difference 2.28, 2.71, 3.17, respectively). The differences were notable within the normal weight and overweight subgroups. One study showed the Norplant (six-capsule) group had greater weight gain (kg) than a non-hormonal IUD group (mean difference 0.47 (95% CI 0.29 to 0.65) and a group using non-hormonal or no method (mean difference 0.74; 95% CI 0.52 to 0.96). Another study also showed a Norplant group also had greater weight gain (kg) than an IUD group (mean difference 1.10; 95% CI 0.36 to 1.84).
Authors’ conclusions
We found little evidence of weight gain when using POCs. Mean gain was less than 2 kg for most studies up to 12 months, and usually similar for the comparison group using another contraceptive. Appropriate counseling about typical weight gain may help reduce discontinuation of contraceptives due to perceptions of weight gain.
PLAIN LANGUAGE SUMMARY
Effects of progestin-only birth control on weight
Progestin-only contraceptives (POCs) can be used by women who cannot or should not take the hormone estrogen. Many POCs are long acting, cost less than some other methods, and work well to prevent pregnancy. Some people worry that weight gain is a side effect of these birth control methods. Concern about weight gain can keep women from using these methods, or cause women to stop using them early, which can lead to unplanned pregnancy. We looked at studies of progestin-only birth control and changes in body weight.
We did computer searches for studies of progestin-only birth control compared to another birth control method or no contraceptive. We also wrote to researchers to find other trials. The main focus was on change in body weight.
Of 15 studies, 4 showed the groups differed in weight gain or body mass. Differences were noted when a POC was compared to no hormonal birth control. In one study, the group using the injectable ‘depo’ had a greater increase in body fat at six months than a group with no hormonal birth control. The depo group also had a greater decrease in lean body mass than the ‘no hormonal’ group. In another study, the depo group gained more weight by one, two, and three years compared to an IUD group. The difference was noted in the subgroups of normal weight or overweight (but not obese) women. Two studies showed a Norplant (implant) group had more weight gain at six months and one year than an IUD group. A Norplant group also gained more weight by six months than a group using another non-hormonal method or no birth control.
We found little evidence of weight gain when using progestin-only birth control. Mean weight gain was less than 2 kg for most studies up to 12 months. The groups using other birth control methods had about the same weight gain. Good counseling about typical weight gain may help women avoid stopping birth control early due to worries about weight gain.
BACKGROUND
Description of the condition
Weight gain is often considered a side effect of using hormonal contraceptives (Goldberg 2007; Raymond 2007). This perception may be based on self-report of side effects rather than actual weight changes (Paul 1997; Berenson 2008). A Cochrane review revealed no clear evidence of weight gain with the use of combined hormonal contraceptives (Gallo 2008). Many clinicians and women also believe that progestin-only contraceptives cause weight gain (FWHC 2010; WebMD 2010).
Concern about weight gain can deter the initiation of contraceptives and cause early discontinuation among users. In a United States (US) study of bone mineral density, weight gain was reported more often by women using depot medroxyprogesterone acetate (DMPA) than those using a low-dose oral contraceptive (Berenson 2008). A national study in New Zealand found that weight gain was the most common side effect reported with DMPA use after menstrual disturbances (Paul 1997). In the US, weight gain has been reported to be a major reason for discontinuing DMPA use (Bonny 2004). Some evidence suggests that DMPA is a concern for adolescents who are already obese (Curtis 2009). Levonorgestrel implants have also been implicated for weight gain (Sivin 2003). The gain may be greater among women in the US than among those in China, and may be partly attributable to differences in dietary habits. In US studies, half of all implant discontinuations were attributed to the side effects of headache, weight, and mood changes (Sivin 2003).
Description of the intervention
Progestin-only contraceptives (POCs) do not contain estrogen, unlike combined hormonal contraceptives that have both progestin and estrogen. Therefore, POCs are appropriate for women who cannot or should not take estrogen(ACOG2006). The World Health Organization classes POCs as category 1 for women who are obese (body mass index >= 30 kg/m2). Category 1 is a condition with no restriction for use of the contraceptive method. For obese adolescents, DMPA is category 2 due to possible effects on bone mineral density (WHO 2010). For category 2, method advantages generally outweigh the theoretical or proven risks. POCs are also category 1 for breastfeeding women who are at least six weeks postpartum (WHO 2010), while combined hormonal contraceptives are category 3 for such women.
Oral contraceptives are the most commonly used reversible method in more developed areas of the world at 16.5%(UN2007), and include combined oral contraceptives (COCs) as well as progestin-only pills (POPs) (Grimes 2010). The introduction of a new progestin-only oral contraceptive in Europe (Faculty of Family Planning 2003) has renewed interest in this class of oral contraceptives. Intrauterine devices (IUDs) lead in less developed areas at 16.5%, but hormonal IUDs are not widely used. Next in usage are injectables and implants; the combined rate is 3.4% worldwide (UN 2007). Some injectable contraceptives are combined, i.e., contain estrogen and progestin, while others like DMPA are progestin-only.
Injectables and implants are long-acting, thus freeing women from daily action to prevent unintended pregnancy. Long-acting methods are among the most cost-effective contraceptives in many areas. Studies of long-acting contraceptives are often of longer duration than those for combined oral contraceptives (COCs), making study of weight change over time more feasible.
How the intervention might work
In general, weight gain is due to an increase in one or more factors: fluid retention, muscle mass, and fat deposition. An early mechanistic study of DMPA and weight gain found no significant changes influid compartmental size, creatinine excretion rate, or nitrogen metabolism (Amatayakul 1980). The researchers reported an association between skinfold thickness and weight gain, indicating the gain was related to increased body fat. A study of risk factors for weight gain among adolescent DMPA users showed that appetite decreased while on DMPA (Bonny 2004). However, a brief randomized controlled trial (RCT) of DMPA versus placebo examined energy intake and expenditure as well as weight gain (Pelkman 2001). Meals were provided along with a range of snacks. Menstrual phase (pre-treatment) was associated with differences in energy intake and expenditure, but DMPA did not affect intake, expenditure, or weight gain.
The possible causal association between contraceptives and weight gain is difficult to study. During adolescence, some weight gain is developmentally normal and appropriate. Also, people tend to gain weight over time (Flegal 2000). No consensus exists regarding what is excessive weight gain. Ideally, studies would define a clinically important weight gain a priori, but weight change is rarely a primary outcome in contraceptive studies.
Examining contraceptive use and weight gain can be complicated by the initial weight of the users. We know little about how overweight women metabolize hormonal contraceptives, since many studies exclude overweight women (Edelman 2009; Lopez 2009). Further, concern about contraceptive effectiveness among overweight women (Grimes 2005; Trussell 2009) may lead to different usage by women of greater body weight. Some contraceptive methods that require a medical procedure have been associated with body weight. Among obese women in a US survey of contraceptive use (Schraudenbach 2009), tubal ligation was similar to pill use at nearly 26%. Among lower BMI groups, the figures were 13.5% and 19.5%, respectively. Implant use was relatively low overall, but higher among overweight (0.9%) and obese women (1.2%) than among normal or underweight women (0.4%). IUD use ranged from 4.1% to 4.6% across the BMI groups. In contrast, within an RCT focused on postpartum weight reduction, women with a BMI of 35 or higher were less likely to use ‘effective’ contraceptive than those with a BMI less than 30 (Chin 2009). Effective contraception referred to hormonal methods, IUDs, or sterilization.
Why it is important to do this review
Currently no comprehensive systematic review existson progestin only contraceptives and weight change. Concern about weight gain might deter women from using these effective contraceptives and health care providers from recommending them. We are not examining effectiveness nor focusing on overweight women. Many reviews have examined effectiveness of specific progestin only contraceptives, such as progestin-only pills (Grimes 2010) and IUDs (Grimes 2007). Further, a Cochrane review examined effectiveness of hormonal contraceptives for overweight women versus women who were not overweight (Lopez 2010).
Progestin-only contraceptives are an attractive option for many women. The cost can be less than that of COCs in some areas, and many postpartum women can use them. Further, POCs are appropriate for many obese women, an important factor given the worldwide epidemic of obesity (Ogden 2007; Prentice 2006).
OBJECTIVES
The primary objective was to evaluate the potential association between progestin-only contraceptive use and changes in body weight.
METHODS
Criteria for considering studies for this review
Types of studies
We included studies of progestin-only contraceptives for contraception and the effect on weight change. Reports had to contain information on the specific contraceptive method(s) examined. We did not anticipate finding many randomized controlled trials addressing weight change. Therefore, we searched for studies with comparative data on a progestin-only contraceptive versus another contraceptive (differing in formulation, dose, or regimen) or no contraceptive. This includes comparisons of a progestin-only contraceptive with a combination contraceptive as well as comparisons of two different types of progestin-only contraceptives. We eliminated studies focused on women with specific health problems, such as diabetes or HIV. We also excluded studies of contraceptives as treatment for specific disorders, e.g., acne, hirsutism, or polycystic ovary syndrome.
Types of participants
Participants were the women inthe studies who usedthe progestin-only contraceptive for contraception or who had the comparison intervention or placebo.
Types of interventions
Any progestin-only contraceptive could have been examined, such as an oral contraceptive, an injectable, an implant, or a hormonal IUD. Treatment duration must have been at least three cycles. Progestin method of interest must have been specified and not combined in a group with another method (e.g., a group that could have used either DMPA or norethisterone enanthate (NET-EN)). The comparison could have been another progestin-only contraceptive or a group of contraceptives, such as COCs. We did not include comparison groups identified only as OC users, since the oral contraceptives could have been POPs or COCs.
Types of outcome measures
The primary outcome was the continuous outcome of mean change in body weight, BMI, or body composition (e.g., percent body fat) over time with the use of progestin-only contraceptives. If mean change in body weight or BMI was not available per study arm, we examined the dichotomous outcome of loss or gain of a specified amount of weight in each study arm. Measured weight was used (not self-reported weight).
Search methods for identification of studies
Electronic searches
We searched MEDLINE, Cochrane Central Register of Controlled Trials (CENTRAL), POPLINE, EMBASE, and LILACS. We also searched for trials via ClinicalTrials.gov and the search portal of the International Clinical Trials Registry Platform (ICTRP). The search strategies are shown below.
MEDLINE via PubMed
(contraceptive agents, female [Mesh] OR contraceptive devices, female [Mesh] OR contracept*) AND (progest* OR “progestin only” OR “progestin only” contracept* OR “progestin only pill” OR progestin* OR progesteron* OR progestational, hormones, synthetic OR progestogen* OR progesterone OR gestagen OR “progestogen only”) AND (body weight changes OR weight gain OR weight loss OR body mass index OR BMI OR weight) NOT (cancer [ti] OR polycystic [ti] OR exercise [ti] OR physical activity [ti] OR postmenopaus*[ti])
limited to human, female.
POPLINE
(progestin only contracept*/contraceptive agents, progestin/low-dose progestins) & (weight/weight gain/weight loss/body weight/BMI/body mass index/weight change)
CENTRAL
weight OR body mass index OR BMI in Abstract and contraception OR contraceptive in Title, Abstract or Keywords.
NOT premenstrual OR dysmenor* OR endometr * OR *androgen* OR HIV OR polycystic OR PCOS OR cancer OR exercise OR anorexia OR bulimic in Record Title.
NOT postmenopausal OR post-menopausal OR hormone therapy OR male hormonal OR male contracept* OR testosterone in Record Title.
EMBASE
(contraceptive agent, progestin –side effects
or
((contraceptive device or contraceptives or contracept*) and (gestagen! or progest? or progestin? or progesterone? or progestational, hormones, synthetic or progestogen?or progestin()only or progestin()only()contracept or progestin()only()pill or progestogen()only)))
and
(body weight! or weight gain or weight reduction or weight()loss or body()mass()index)
not (cancer or polycystic or exercise or physical() activity or postmenopaus? or oral contraceptives, combined)
and body weight/de
limited to human
LILACS
contraceptive agents or Agentes Anticonceptivos Femeninos or Anticoncepcionais Femininos or contraceptive devices, female or Dispositivos Anticonceptivos Femeninos or Dispositivos Anticoncepcionais Femininos or contraceptives or Anticonceptivos or Anticoncepcionais [Words]
and weight or body weight or Peso Corporal or weight gain or Aumento de Peso or Ganho de Peso or weight reduction or weight loss or Pérdida de Peso or Perda de Peso or body weight changes or Cambios en el Peso Corporal or Alterações do Peso Corporal or body mass index or BMI [Words]
ClinicalTrials.gov
Search terms: overweight OR obese OR obesity OR weight OR body mass index OR BMI
Condition: NOT (HIV OR polycystic OR PCOS OR cancer OR anorexia OR pulmonary OR metabolic OR amenorrhea)
Intervention: contraceptive OR contraception
Study type: interventional studies
Gender: studies with female participants
ICTRP
Intervention: contraceptive OR contraception
Condition: contraceptive OR contraception
Intervention: progestin OR progestin-only OR IUD OR implant OR medroxyprogesterone OR norethisterone
Searching other resources
We examined reference lists of relevant articles and contacted investigators in the field to seek additional unpublished trials or published trials.
Data collection and analysis
Selection of studies
We assessed for inclusion all titles and abstracts identified during the literature searches. One author reviewed the search results and identified reports for inclusion or exclusion. A second author also examined the reports identified for appropriate categorization according to the criteria above.
All comparative study designs were considered. For example, studies could have been randomized controlled trials (RCTs), other prospective studies (provided intervention; assignment not random), observational studies of existing contraceptive users, case control studies, or retrospective chart reviews. Post hoc analysis from any of these types of studies was also considered. However, the studies had to meet the other Criteria for considering studies for this review.
We excluded studies that only reported mean weight pre- and post-treatment. Even RCTs could not be used with mean weights only, since the comparisons in our review were not necessarily those on which the randomization was based.
Data extraction and management
One author abstracted the data and entered the information into RevMan. Another author conducted a second data abstraction and verified correct data entry. Any discrepancies were resolved by discussion or with a third author if necessary.
Assessment of risk of bias in included studies
The randomized controlled trials were examined for methodological quality, according to recommended principles (Higgins 2009). Methodology considered included randomization method, allocation concealment, blinding, and losses to follow up and early discontinuation. Adequate methods for allocation concealment include a centralized telephone system and the use of sequentially-numbered, opaque, sealed envelopes (Schulz 2002). In addition, high losses to follow up threaten validity (Strauss 2005).
To assess the non-randomized data, we used the principles outlined in section 13.5 of Higgins 2009 as well as the STROBE statement for reports of observational studies (Von Elm 2007). We assessed whether the analysis included adjustment for potential confounding related to weight change. The study groups could differ in ways related to the outcome, such as initial weight or BMI or previous use of hormonal contraceptives.
For all types of studies, limitations in design were presented in Risk of bias in included studies, and were considered when interpreting the results.
After conducting the review, we assessed the quality of evidence using the GRADE approach (Higgins 2009). Grades could be high, moderate, low, or very low. The initial grade was based on study design: RCTs were high; prospective, not RCTs were moderate; and retrospective studies were low. The initial rating was downgraded a level for each of the following: a) losses to follow up of 25% or more; b) inappropriate exclusions after randomization.
Measures of treatment effect
Outcomes listed in Characteristics of included studies are focused on those relevant to this review. For weight gain measure with follow up less than one year, we selected the last date. If multiple time points were reported up to one year, we used the midpoint and one year data. If data were available for more than three years, we used one year as well as the midpoint and last measure.
Dealing with missing data
We excluded studies with insufficient data on weight or BMI for analysis in this review. Reports sometimes provided results in figures without specific numbers; others presented means without any variance estimate. Many studies were more than 10 years old, making it difficult to obtain additional data from the researchers.
Assessment of heterogeneity
We expected study populations, designs, and interventions to be heterogeneous. We described the clinical and methodological diversity (or heterogeneity) of the studies. We did not pool data from studies that had different contraceptive methods (e.g., DMPA or implants), different doses of the same method, or different criteria for reporting weight change. Therefore, we were not able to conduct meta-analysis due to the range of contraceptive methods examined and different reporting for weight change. Heterogeneity is not an issue when a comparison has a single study.
Data synthesis
We examined results by the contraceptive method studied, e.g., injectable or implant, as well as by formulation, dose, or regimen as appropriate. The main comparisons for this review were between users of progestin-only contraceptives and users of another contraceptive (differing in formulation, dose, or regimen) or no hormonal contraceptive. For continuous variables, the mean difference was computed with 95% confidence interval (CI) using a fixed effect model. RevMan uses the inverse variance approach (Higgins 2009). Continuous measures include mean change in weight or BMI or percentage body fat. For dichotomous outcomes, the Mantel-Haenszel odds ratio (OR) with 95% CI was calculated using a fixed-effect model. An example is the proportion of women who gained a specified amount of weight. Fixed and random effects give the same result if no heterogeneity exists, as when a comparison includes only one study. The Peto OR was used when a study arm had no events; the Peto OR does not require correction for zero events (Higgins 2009).
Data were not available to examine weight change in relation to age. A certain amount of weight gain is part of normal development for adolescents. Studies that targeted adolescents are identified. Studies that included both adolescents and adult women did not provide outcome data for age subgroups.
When data were available, we also examined weight change in relation to initial body weight or body mass index (BMI) [weight (kg)/height (m)2]. Weight change may differ for women who were initially overweight or obese versus those who were not overweight or obese. We preferred BMI over weight alone, as a higher BMI generally reflects more body fat (CDC 2010). However, the measures and cutoffs depended on those used in the included studies. Frequently used BMI categories are 25 to 29.9 (kg/m2) for overweight and 30 or higher for obesity (CDC 2010). We also considered studies that used older US cutoffs derived from the National Health and Nutrition Examination Survey II (NHANES II) conducted from 1976 to 1980 (Najjar 1987). From NHANES II, the BMI cutoffs for women are 27.3 for overweight and 32 for obesity.
Sensitivity analysis
We conducted sensitivity analysis by removing the retrospective studies, i.e., chart reviews. The remaining prospective studies included RCTs, other prospective designs, and post hoc analysis of data from prospective studies. Further, we examined results after removing studies that had losses or discontinuations totaling 25% or more of the sample.
RESULTS
Description of studies
See: Characteristics of included studies; Characteristics of excluded studies.
Fifteen studies met our inclusion criteria. The nine prospective studies included five RCTs (WHO 1983; Salem 1988; Ball 1991; Sivin 1998; Westhoff 2007) and four with other prospective designs (Tankeyoon 1976; Castle 1978; Salem 1984; Bonny 2009). Six studies used retrospective data (Moore 1995; Taneepanichskul 1998; Espey 2000; Sule 2005; Tuchman 2005; Pantoja 2010).
The progestin-only contraceptives studied were as follows:
Oral contraceptives (OCs) containing norethisterone 350 μg or levonorgestrel 30 μg
Injectable: depot medroxyprogesterone acetate (DMPA) 150 mg/mL or 450 mg/mL (intramuscular) or 104 mg/0.65mL (subcutaneous)
Injectable: norethisterone enanthate (NET-EN) 200 mg
Implants (levonorgestrel): 6 capsules; 2 rods
Comparison groups included no hormonal contraceptive, a different regimen of the same progestin-only contraceptive, another progestin-only contraceptive, or a contraceptive or supplement containing estrogen. Where IUD users were a comparison group, the IUDs were non-hormonal.
Studies were conducted in the USA, South America, Europe, Africa, Asia, and on multiple continents. Duration of prospective follow up or retrospective data collection was 6 months to 2 years for 10 studies, while 5 studies lasted 3 years or more.
Three studies included adolescents and young women. Bonny 2009 analyzed data from a larger study of hormonal contraceptives and bone mineral density. Moore 1995 and Tuchman 2005 were retrospective chart reviews.
Risk of bias in included studies
Three of the five RCTs did not have any information on randomization method or allocation concealment (WHO 1983; Ball 1991; Westhoff 2007). The other two RCTs reported the method of randomization and allocation concealment (Salem 1988; Sivin 1998). Two RCTs had information on blinding. Ball 1991 was reportedly “single-blind.” For the trials used in Westhoff 2007, the evaluators were blinded.
Incomplete outcome data
Five of 15 studies had losses of 25% or more in at least one group. Three were prospective studies (RCTs or non-randomized) For Tankeyoon 1976, the COC group was reduced by 25%. In Castle 1978, losses to follow up were 39% (DMPA 150) versus 23% (DMPA 450). Westhoff 2007 had study discontinuation rates of 75% (DMPA-SC 104) and 79% (DMPA-IM 150). The retrospective studies generally had complete outcome data due to the inclusion criteria for cases. However, two of the six were missing substantial data from the later years. For Espey 2000 at two years, weight measures were not available for 70% of interval group and 49% of the postpartum group. In Sule 2005, the sample was reduced to 54% at three years.
Selective reporting
Taneepanichskul 1998 excluded those who developed a chronic disease or disorder during method use, which may have biased the results. Weight gain is associated with development of some diseases and disorders.
Other potential sources of bias
Two non-randomized studies conducted analysis that adjusted for potential confounders (Moore 1995; Bonny 2009). However, we did not include those analyses because at least one comparison group did not meet our inclusion criteria. Details are in Characteristics of included studies. Consequently, none of the analyses in this review were adjusted for potential confounders of weight gain.
Effects of interventions
See: Summary of findings for the main comparison All included studies; Summary of findings 2 Sensitivity analysis; Summary of findings 3 Quality of evidence by contraceptive method
Results are grouped according to three types of progestin-only contraceptive studied. One trial examined progestin-only pills, 10 studies addressed DMPA, and 4 examined Norplant (one of which also included DMPA). We have subdivided the sections into studies comparing POCs with combination contraceptives, with no hormonal contraceptive, and with other POCs.
Progestin-only oral contraceptives
In the RCT of Ball 1991, weight change at six months was similar for the norethisterone 350 μg and the levonorgestrel 30 μg groups. Mean changes were small.
Depot medroxyprogesterone acetate
POC versus a combination contraceptive
Depot medroxyprogesterone acetate 150 mg/mL (DMPA) was compared to a contraceptive or supplement that also contained estrogen in three studies. Bonny 2009 compared mean percentage changes in total body fat and lean body mass at six months for DMPA 150 plus placebo injection versus DMPA plus estradiol cypionate 5 mg (E2C). The study targeted adolescents, ages 12 to 18 years. The DMPA group was similar to the DMPA plus E2C group for mean change in total body fat (%) and in lean body mass (%). However, when compared to a group using no hormonal method, the DMPA group had a greater change in total body fat (%) (mean difference 11.00; 95% CI 2.64 to 19.36) (Analysis 3.1) and a greater change in lean body mass (%) (mean difference −4.00; 95% CI −6.93 to −1.07) (Analysis 3.2). The retrospective study of Tuchman 2005 focused on adolescents and young women, ages 12 to 21 years. At 6 and 12 months, the DMPA group was similar to the group using medroxyprogesterone acetate (MPA) plus E2C and to the COC users for mean weight change and mean percentage weight change (Tuchman 2005). In the small study of Tankeyoon 1976, the DMPA and COC groups were similar in the proportions that gained at least 1 kg by months 6 and 12. For weight loss of 1 kg or more, the groups were slightly different at six months but the confidence interval was very wide.
POC versus another POC
DMPA 150 mg/mL was compared with other DMPA formulations or regimens in three studies. In Castle 1978, the mean changes in weight at six months were small and similar for the DMPA 150 and DMPA 450 groups. The retrospective study of Espey 2000 did not show a significant difference in weight gain at one or two years for those who initiated DMPA at 20 weeks or more after pregnancy (interval group) versus initiation at 5 to 8 weeks after pregnancy (postpartum group). In the RCT analyzed in Westhoff 2007, weight change was comparable for the DMPA 150 group and the group with subcutaneous DMPA 104.
Two studies examined DMPA 150 mg/mL versus NET-EN 200 mg. In WHO 1983, mean weight changes at 12 and 24 months did not differ significantly for the DMPA group versus the group administered NET-EN at 60-day intervals. Weight change was also similar for two NET-EN regimens of 60 day-intervals versus 84-day intervals. For the RCT of Salem 1988, the units for weight were not reported (lbs or kg). However, mean changes in weight at one year were similar for the DMPA and the NET-EN groups.
POC versus no hormonal contraceptive
DMPA 150 mg/mL was compared to a non-hormonal IUD in two retrospective studies. Taneepanichskul 1998 did not show a difference between the DMPA and IUD groups at 120 months. For Pantoja 2010, mean weight gain (kg) was greater for the DMPA group versus the IUD group at one, two, and three years: mean difference 2.28 (Analysis 11.2), 2.71 (Analysis 11.3), and 3.17 (Analysis 11.4), respectively. For each year, the difference between contraceptive groups was notable within the normal to lower weight group (BMI < 25) and within the overweight group (BMI 25 to 29.9), but not within the obese group (BMI >= 30).
Norplant
Norplant (with 6 capsules) was compared to other hormonal contraceptives and to no hormonal contraceptive use.
For the retrospective study of Sule 2005, the Norplant group had a significantly greater weight increase (kg) than the IUD group at one year (mean difference 1.10; 95% CI 0.36 to 1.84) (Analysis 13.2), but not at three years. The Norplant group was similar in weight change to the COC group.
The retrospective study of Moore 1995 targeted adolescents and young women, ages 15 to 30 years. The Norplant and DMPA groups were similar in mean weight change at one year. Salem 1984 showed a greater weight change (kg) at six months for the Norplant group versus the IUD group(mean difference 0.47; 95% CI 0.29 to 0.65) (Analysis 13.1). The Norplant group also had a greater weight change (kg) than the group that used barrier, ‘local’, or no contraceptive method (mean difference 0.74; 95% CI 0.52 to 0.96) (Analysis 14.1). In the RCT of Sivin 1998, mean weight change was similar for the Norplant group versus the two-rod LNG group at one, three, and five years.
Sensitivity analysis
All study results were summarized in Summary of findings for the main comparison. For the planned sensitivity analysis, we removed retrospective studies as well as studies with high losses to follow up or method discontinuation (>= 25%) (Summary of findings 2). Two studies showed differences in weight gain or body composition between the progestin-only group and a comparison group; both lasted only six months. Bonny 2009 was a small study of adolescents from a larger trial. The DMPA group had a greater increase in body fat percentage, and a greater decrease in lean body mass, compared to the group using no hormonal contraceptive. In Salem 1984, Norplant users showed a greater weight gain than the IUD users and the group that used barrier, ‘local’, or no contraceptive method.
DISCUSSION
Summary of main results
We were not able to conduct meta-analysis due to the range of contraceptive methods examined and different reporting for weight change. Four of the 15 studies showed a significant difference between groups in weight change or body composition change (Salem 1984; Sule 2005; Bonny 2009; Pantoja 2010). Two of the four studies examined DMPA and two focused on Norplant (six capsules). The differences were noted in the comparisons with groups using no hormonal contraceptives. Of these four studies, two remained in the sensitivity analysis. Bonny 2009 examined DMPA, focused on adolescents, and appropriately measured change in body fat (%) and lean body mass (%) rather than weight change alone. The DMPA group differed from the group using no hormonal contraceptive at six months. Salem 1984 assessed Norplant and provided mean weight change (kg) at six months. The Norplant group differed from the IUD group and from a group using other non-hormonal or no contraceptive method.
Actual mean weight gain was limited at6 or 12 months (Summary of findings for the main comparison), i.e., less than 2 kg for most studies. The five studies with multi-year data showed that mean weight change was approximately twice as much at two or three years than at one year, but was generally similar for both study groups (Summary of findings for the main comparison). A total of seven studies had data from two years or more of contraceptive use:
Three RCTs compared two POCs (WHO 1983; Sivin 1998; Westhoff 2007). The DMPA or implant groups gained similar amounts of weight over one to three years.
Three retrospective studies compared a POC to a nonhormonal IUD (Taneepanichskul 1998; Sule 2005; Pantoja 2010). Only Pantoja 2010 showed more weight gain for the DMPA group at one, two, and three years, but interestingly not within the obese group (BMI >=30).
The retrospective study of Espey 2000 showed two DMPA groups to be similar in weight gain at one and two years.
Overall completeness and applicability of evidence
Of the 15 studies, 11 had data from a year or more of contraceptive use, of which 7 had data from two years or more. Weight gain (or the perception of weight gain) is frequently cited as a reason for discontinuing a contraceptive method. If a method is associated with weight gain, a year is long enough to detect some change, though the amount may not be significant. Of the seven studies with data from two years or more of contraceptive use, most showed the study groups to be similar for weight gain, regardless of whether the comparison group used POCs, combination contraceptives, or no hormonal methods.
Studies of progestin-only contraceptives and weight change were limited in type and number. Only one study of progestin-only pills was found that met our inclusion criteria. For implants, we found the most weight change data for Norplant (with six capsules). However, Norplant is no longer marketed, while single-rod and two-rod implants are currently available. However, most studies of currently marketed implants did not meet our inclusion criteria, mainly due to the lack of comparative weight change data. The one exception was a study in which Norplant was compared to a two-rod implant. Consequently, we do not have much evidence regarding weight change with currently marketed implants. Further, we did not find any studies of hormonal IUDs that met our inclusion criteria.
Quality of the evidence
After conducting the review, we assessed the quality of evidence using the GRADE approach (Higgins 2009). The level was based primarily on study design, which we downgraded one level if losses to follow up were 25% or more or if cases were inappropriately excluded after randomization. The results are shown by contraceptive method of focus and quality level (Summary of findings 3). Seven studies were rated as high or moderate quality. Progestin-only pills had one study of high quality that showed no significant difference in weight change. DMPA had two high quality and two moderate quality studies, one of which showed adolescents using DMPA had increased body fat percentage and decreased lean body mass. The six-capsule Norplant had one high quality and one moderate quality study; the latter showed a greater weight increase for Norplant users.
Sample sizes varied. Three studies had 32 to 51 participants, and were unlikely to have had sufficient power to detect differences in weight gain. Seven studies had 100 to 400 participants, and five ranged from 534 to 3172. A few had comparison groups that were not used in this review, because the group did not meet our inclusion criteria.
Most studies did not adjust for potential confounding factors of weight change. Some had adjusted analyses that we did not use since a comparison group did not meet our inclusion criteria.
Potential biases in the review process
We selected studies that had data on mean weight change, mean change in body composition, or proportion that gained or lost a specified amount of weight. Several studies were excluded due to not reporting the data we needed. Many studies were older, which limited our ability to obtain additional information from the researchers.
Agreements and disagreements with other studies or reviews
Many of the concerns about weight gain with POC use are based on perceptions and discontinuation reasons rather than measures of actual weight change (Paul 1997; Bonny 2004; Berenson 2008). We found limited evidence of significant change for POC users versus those who did not use hormonal contraceptives. Actual weight gain was relatively low up to one year. Similarly, a Cochrane review showed no clear evidence of weight gain with the use of combined hormonal contraceptives (Gallo 2008). People may gain weight over time, regardless of contraceptive use.
However, DMPA may be a concern for adolescents who are already obese (Curtis 2009). One study that focused on adolescents (Bonny 2009) showed an increase in total body fat and a decrease in lean body mass for the DMPA group versus those with no hormonal contraceptive use. Two retrospective studies included adolescents (Moore 1995 (15 to 30 years old); Tuchman 2005(12 to 21 years old). However, they did not show a significant difference between groups nor much weight gain within the DMPA or Nor-plant groups.
AUTHORS’ CONCLUSIONS
Implications for practice
We found little evidence of weight gain when using progestin-only contraceptives. Some differences were noted when a POC was compared to no hormonal contraceptive. Actual mean weight gain was low for 6 to 12 months, i.e., less than 2 kg for most studies. More weight gain was noted at two and three years, but was generally similar for both comparison groups. People may gain weight over time regardless of contraceptive use. Appropriate and accurate counseling about typical weight gain may help reduce discontinuation of contraceptives due to perceptions of weight gain.
Implications for research
The five RCTs each compared similar progestin-only contraceptives, which might account for the groups being similar for weight change. Two other prospective studies (not RCTs) and two retrospective studies showed some differences for POCs versus no hormonal contraceptives; one showed a difference at more than a year of contraceptive use. Weight gain is rarely the focus of prospective contraceptive studies. Well-designed RCTs assessing weight change over time would better address this issue. However, careful counseling and follow up are needed to avoid the high rates for losses to follow up and discontinuation found in many contraceptive studies.
SUMMARY OF FINDINGS FOR THE MAIN COMPARISON [Explanation]
| Study | Type of study | Sample size | Comparison groups | Groups that differed | Time frame | Mean changea |
|---|---|---|---|---|---|---|
| Ball 1991 | RCT | 51 | OC NET 350 μg vs LNG 30 μg | — | 6 months | 0 vs 0.6 |
| Bonny 2009 | Prospective, not randomized | 33 | DMPA + placebo vs DMPA + E2C | — | 6 months | 10.3% vs 2.8%; −3.4% vs −1.2%b |
| DMPA + placebo vs no hormonal | X | 6 months | 10.3% vs −0.1%; −3.4% vs 0.6%b |
|||
| Castle 1978 | Prospective, not randomized | 1000 | DMPA 150 vs DMPA 450 | — | 6 months | 0.33 vs 0.32 |
| Espey 2000 | Retrospective | 172 | DMPA: interval vs postpartum | — | 1 year 2 years |
4.2 vs 3.2; 7.2 vs 6.5 |
| Moore 1995 | Retrospective | 100 | Norplant vs DMPA | — | 1 year | −0.81 vs 0.06 |
| Pantoja 2010 | Retrospective | 758 | DMPA vs IUD | X (all 3 years) | 1 year 2 years 3 years |
1.76 vs −0.42; 3.1 vs 0.4; 3.9 vs 0.8 |
| Salem 1984 | Prospective, not randomized | 150 | Norplant vs IUD | X | 6 months | 1.39 vs 0.92 |
| Norplant vs other non-hormonal | X | 6 months | 1.39 vs 0.65 | |||
| Salem 1988 | RCT | 400 | DMPA vs NET-EN | — | 1 year | 3.5 vs 2.7a |
| Sivin 1998 | RCT | 1200 | Norplant vs 2-rod LNG | — | 1 year 3 years 5 years |
0.99 vs 0.90; 3.12 vs 3.12; 4.14 vs 3.54 |
| Sule 2005 | Retrospective | 516 | Norplant vs IUD | X (at 1 year) | 1 year 3 years |
2.5 vs 1.4 4.8 vs 3.9; |
| Norplant vs COC | — | 1 year 3 years |
2.5 vs 1.4; 4.8 vs 0.0 |
|||
| Taneepanichskul 1998 | Retrospective | 100 | DMPA vs IUD | — | 10 years | 10.9 vs 11.2 |
| Tankeyoon 1976 | Prospective, not randomized | 32 | DMPA vs COC | — | 1 year | 1.8 vs 3.1 (estimated) |
| Tuchman 2005 | Retrospective | 222 | DMPA vs MPA/E2C | — | 6 months; 12 months |
0.6 vs 1.2; 1.7 vs 3.0 |
| DMPA vs COC | — | 6 months; 12 months |
0.6 vs 1.1; 1.7vs 1.0 |
|||
| Westhoff 2007 | RCT | 534 | DMPA-IM150 vs DMPA-SC 104 | — | 3 years | 5.8 vs 4.5 |
| WHO 1983 | RCT | 3172 | DMPA vs NET-EN (60 days) | — | 1 year 2 years |
1.9 vs 1.7; 3.3 vs 3.3 |
| NET-EN: 60 days vs 84 days | — | 1 year 2 years |
1.7 vs 1.7; 3.3 vs 3.4 |
Weight change in kg unless otherwise specified. Salem 1988 did not report units for weight change (lbs or kg).
Mean change in total body fat or lean body mass, respectively
ADDITIONAL SUMMARY OF FINDINGS [Explanation]
| Studya | Type of study | Comparison groups | Groups differed significantly | Mean difference (95% CI) |
|---|---|---|---|---|
| Ball 1991 | RCT | OC NET 350 μg vs LNG μg 30 | no | — |
| Bonny 2009 | Prospective, not randomized | DMPA + placebo vs DMPA + E2C or no hormonal | DMPA + placebo vs no hormonal | 11.00 (2.64 to 19.36); −4.00 (−6.93 to −1.07)b |
| Salem 1984 | Prospective, not randomized | Norplant vs IUD or no hormonal | Norplant vs IUD or vs other non-hormonal | 0.47 (0.29 to 0.65); 0.74 (0.52 to 0.96)c |
| Sivin 1998 | RCT | Norplant vs 2-rod LNG | no | — |
Excludes retrospective chart reviews and studies with loss to follow up or discontinuation >= 25%.
Total body fat (%) and lean body mass (%), respectively
Weight change (kg)
| Study | Comparison groups | Mean difference (95% CI)* | Quality of evidence** |
|---|---|---|---|
| Progestin-only pills | |||
| Ball 1991 | OC NET 350 μg vs LNG 30 μg | — | High |
| DMPA | |||
| WHO 1983 | DMPA vs NET-EN (60 days) | — | High |
| NET-EN: 60 days vs 84 days | — | ||
| Salem 1988 | DMPA vs NET-EN | — | High |
| Bonny 2009 | DMPA + placebo vs DMPA + E2C | — | Moderate |
| DMPA + placebo vs no hormonal | 11.00 (2.64 to 19.36); −4.00 (−6.93 to −1.07)a |
||
| Westhoff 2007 | DMPA-IM 150 vs DMPA-SC 104 | — | Moderate |
| Castle 1978 | DMPA 150 vs DMPA 450 | — | Low |
| Pantoja 2010 | DMPA vs IUD | 3.17(2.51 to 3.83)b | Low |
| Tankeyoon 1976 | DMPA vs COC | — | Low |
| Taneepanichskul 1998 | DMPA vs IUD | — | Very low |
| Tuchman 2005 | DMPA vs MPA/E2C | — | Very low |
| DMPA vs COC | — | ||
| Espey 2000 | DMPA: interval vs postpartum | — | Very low |
| Norplant | |||
| Sivin 1998 | Norplant vs 2-rod LNG | — | High |
| Salem 1984 | Norplant vs IUD | 0.47 (0.29 to 0.65)b | Moderate |
| Norplant vs other non-hormonal | 0.74 (0.52 to 0.96)b | ||
| Moore 1995 | Norplant vs DMPA | — | Low |
| Sule 2005 | Norplant vs IUD | 1.10 (0.36 to 1.84)b | Very low |
| Norplant vs COC | — |
Data shown if groups differed significantly
Grades were initially based on study design (RCT = high; prospective, not RCT = moderate; retrospective = low). Studies were downgraded a level if losses >=25% and if cases inappropriately excluded.
Total body fat (%) and lean body mass (%), respectively
Weight (kg), except Salem 1988, which did not report units (lbs or kg).
Characteristics of included studies [ordered by study ID]
| Ball 1991 | ||
| Methods | Randomized controlled trial, likely conducted in Oxford, England. No information on method for randomization, except stratified according to prior OC use; “single-blind” (unspecified). Main study examined effects of progestin-only OC on lipoprotein levels, glucose tolerance, coagulation factors, and blood pressure. | |
| Participants | 51 women, 17 to 41 years, requesting oral contraceptives (OC). New OC users had not used an OC or hormone therapy for 3 months; switchers were changing from low-dose combined OC. Exclusion criteria: hypertension (diastolic blood pressure (BP) > 100 mm Hg, systolic BP > 140 mm Hg), smoking > 20 cigarettes/day, or diabetes. | |
| Interventions | Progestin-only pills: norethisterone (NET) 350 μg (N=23) versus levonorgestrel (LNG) 30 μg (N=23); 6 treatment cycles. | |
| Outcomes | Mean change in weight from baseline to month 6 | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | No information |
| Incomplete outcome data addressed? All outcomes | Yes | Five women did not return for follow up, and were excluded from the analysis (groups not specified). Nine withdrew after 3 months (1 NET; 8 LNG). Analysis for weight included 39 women at 6 months; loss 12/51 = 24%. |
| Bonny 2009 | ||
| Methods | Prospective study in 4 urban adolescent health clinics in large metropolitan area (USA); enrollment 2002 to 2003; part of a larger 2-year study that examined hormonal contraception and bone mineral density | |
| Participants | Postmenarchal girls 12 to 18 years of age. Inclusion criteria: requesting contraception and selecting DMPA or OC; those who did not want hormonal contraception were eligible for control group. Exclusion criteria: pregnancy or DMPA use in past 6 months; OC use in past 3 months; alcohol or drug dependence; medical condition (e.g., renal disease) or medication use (e.g., corticosteroids) associated with outcomes of interest; contraindication to estrogen use; weight > 250 lbs (upper limit for dual energy x-ray absorptiometry [DEXA] scanner); and need for confidential contraceptive care. | |
| Interventions | Choice of: 1) Depot medroxyprogesterone acetate (DMPA) (N=15): randomized to additional monthly injections of placebo (N=8) or estradiol cypionate 5 mg (E2C) (N=7) 2) Control (no hormonal contraception) (N=18) |
|
| Outcomes | Change in total body fat (%) and in lean body mass (%) from baseline to 6 months. | |
| Notes | Researchers also developed multivariate models for change in total body fat and change in lean body mass; models adjusted for potential confounders. However, the analysis also included a third group of those who chose OCs (N=18), which we did not include in our review. Type of OC was not specified and might have included progestin-only OCs as well as combination OCs, so the OC group was excluded here. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | No information |
| Incomplete outcome data addressed? All outcomes | Unclear | To remain in study, participants had to adhere to DMPA by appointment. No information on controls. |
| Castle 1978 | ||
| Methods | Prospective study in family planning center in Salisbury, Rhodesia; enrollment Jun to Dec 1976 | |
| Participants | 1000 Black women seeking contraception. Ages ranged from ‘under 20’ to 40 years or older. | |
| Interventions | 1) DMPA 150 mg every 3 months (N=500) 2) DMPA 450 mg every 6 months (N=500) |
|
| Outcomes | Mean increase in weight at 6 months | |
| Notes | Weight measured at each visit while participant wore only a gown | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | No | Allocation by patient request |
| Incomplete outcome data addressed? All outcomes | Yes | Withdrawals from study were 21 for DMPA 150 mg and 17 for DMPA 450 mg. Losses to follow-up: 39% DMPA 150 mg and 23% DMPA 450 mg. |
| Espey 2000 | ||
| Methods | Retrospective chart review at 3 Indian Health Service facilities in southwestern USA | |
| Participants | Female members of Navajo tribe. Inclusion criteria: 18 to 40 years old, completed at least 5 consecutive injections of DMPA at 10- to 14-week intervals, and had weights recorded at 1 - or 2-year intervals. Exclusion criteria: history of diabetes or thyroid disease; women in postpartum group who had pre-eclampsia or multiple gestations within index pregnancy. | |
| Interventions | DMPA initiation a) Interval (N=1 15): first injection at least 20 weeks past pregnancy of 20 weeks or more gestation b) Postpartum (N=57): first injection at 5 to 8 weeks after delivery of singleton pregnancy of 20 weeks or more gestation |
|
| Outcomes | Mean weight gain (lbs) for DMPA at 1 and 2 years by initiation group | |
| Notes | For another group, not included in this review, method of contraception was reportedly extracted from charts but specifics were not provided. Discussion noted that group “more frequently used” IUD or tubal ligation and also included COC users. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | Not applicable (NA) |
| Incomplete outcome data addressed? All outcomes | Yes | Women with incomplete records were excluded from this retrospective review. However, at 2 years, weight data were not available for 70% of the interval group and 49% of the postpartum group. |
| Moore 1995 | ||
| Methods | Retrospective chart review at rural obstetrics and gynecology clinic in Arizona (USA) | |
| Participants | Women who used the contraceptive method and were 15 to 30 years old. Exclusion criteria: prior hormonal contraceptive therapy, height < 62 inches (152.4 cm) or > 70 inches (177.8 cm), weight < 100 lbs (45.5 kg) or > 180 lbs (81.8 kg), presence of diabetes, history of thyroid disease, < 12 months postpartum. | |
| Interventions | 1) Norplant (N=50) 2) DMPA 150 mg (M=50) |
|
| Outcomes | Weight gain (kg) at 1 year by treatment group. | |
| Notes | Researchers also analyzed weight gain in model adjusted for age, height, weight, and parity. That analysis included a third group of OC users, which we excluded from this review. Type of OC was not specified and may have included progestin-only OCs as well as combination OCs. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | NA |
| Incomplete outcome data addressed? All outcomes | Yes | NA |
| Pantoja 2010 | ||
| Methods | Retrospective chart review at a university department of obstetrics and gynecology in Campina, Brazil. Chart data spanned Jan 1991 to Dec 2000. | |
| Participants | Women who chose DMPA and used the method continuously for 3 years or more and women who used TCu380A IUD for similar time period. Exclusion criteria: diabetes mellitus, hyper- or hypothyroidism, chronic renal failure, rheumatic diseases requiring chronic use of corticoids, and any type of organ transplant. Mean age was 33.5 years in both groups. | |
| Interventions | 1) DMPA (N=379) 2) TCu380A IUD (N=379) |
|
| Outcomes | Change in weight (kg) at 1, 2, and 3 years by contraceptive group and baseline BMI (kg/m2) group (BMI < 25; 25 to 29.9; >= 30) | |
| Notes | After pairing for age and baseline BMI, the samples were 379 for each contraceptive group. Weight and height were measured at baseline (time of method initiation) and annually thereafter. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | NA |
| Incomplete outcome data addressed? All outcomes | Yes | NA; charts were selected for those with 3 years of continuous use. |
| Salem 1984 | ||
| Methods | Prospective study in postpartum clinic of university hospital in Assiut, Egypt. Study focused on effect of Norplant use on lactating women and on lactation performance and infant growth. | |
| Participants | 150 lactating women. Inclusion criteria: normal delivery of normal living baby and exclusively breastfeeding, one month after delivery, and infant weight at least 3500 gm. Mean age was 29 years for each group. | |
| Interventions | Acceptors (50 in each group): 1) Norplant 2) Barrier, “local,” or no contraceptive method 3) CuT380A IUD |
|
| Outcomes | Weight gain at 6 months by study group | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | NA |
| Incomplete outcome data addressed? All outcomes | Yes | Excluded data: 1 in Norplant group who lost her baby and wanted to get pregnant; 2 who got pregnant in group with barrier, local, or no contraceptive. |
| Salem 1988 | ||
| Methods | Randomized controlled trial of DMPA and NET-EN in family planning center in Assiut, Egypt. Random numbers table was prepared by WHO. Study examined performance of 2 injectables regarding side effects, continuation, and termination. | |
| Participants | 400 women attending family planning clinic. Inclusion criteria: 18 to 40 years old, proven fertility and frequent risk of pregnancy, regular menstrual cycles, willing to rely on one method. Exclusion criteria: breast-feeding; cardiovascular disease; liver disease; known or suspected breast malignancy, genital malignancy, uterine fibroids; undiagnosed vaginal bleeding; suspected pregnancy. | |
| Interventions | 200 in each group: 1) DMPA 150 mg every 3 months 2) Norethisterone enanthate (NET-EN) 200 mg every 2 months Study duration was 1 year |
|
| Outcomes | Mean change in weight at 1 year by contraceptive group. Units were not specified (kg or lbs). | |
| Notes | Report had mean change for those who had an increase, decrease, or no change in weight. Review authors calculated combined weight change means and standard deviations. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Sealed envelopes contained assignments |
| Incomplete outcome data addressed? All outcomes | Yes | Losses to follow up were reportedly 19% for DMPA and 13.3% for NET-EN. One-year method continuation rates were 68.8% DMPA and 57.1% NET-EN. Those who finished the study were 54% and 47%, respectively. |
| Sivin 1998 | ||
| Methods | Randomized controlled trial at 7 centers, including USA and Finland. Study focused on effectiveness of reformulated 2-rod LNG implant versus 6-rod implant. Randomization by “linear congruential method”; blocks of 50 per clinic. Enrollment from 1990 to 1994. | |
| Participants | 1200 healthy women, 18 to 40 years old, who sought implant contraception. Inclusion criteria: no contraindications to implant use, willing to undergo study procedures. Exclusion criteria: cancer, severe cardiovascular problem, hyperlipidemia, diabetes mellitus, mental illness, epilepsy, severe or frequent headaches, undiagnosed genital bleeding, hyperprolactinaemia or bloody breast discharge, pelvic inflammatory disease since last pregnancy or ectopic pregnancy. | |
| Interventions | Levonorgestrel implants 1) Norplant: 6 capsules, containing levonorgestrel 216 mg (total) 2) LNG rod (Jadelle): 2 rods containing levonorgestrel 150 mg (total); different elastomer in core than earlier implant Follow up: 1, 3, 6 months, and then semi-annually up to 5 years |
|
| Outcomes | Mean weight change per year by implant group. Weight change was also presented for the 10th, 50th, and 90th percentiles of body weight (at admission). |
|
| Notes | Weighing method not specified | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Yes | Implants were in sealed opaque envelopes that were numbered sequentially, according to randomization lists. |
| Incomplete outcome data addressed? All outcomes | Yes | Two sets of Norplant were contaminated and not used (1198 analyzed) |
| Sule 2005 | ||
| Methods | Retrospective chart review at family planning clinic at a university hospital in Zaria, Nigeria. Study examined hormonal contraceptives and weight changes. | |
| Participants | All new clients registered between 01 Jan 1993 and 31 Dec 1995 and followed for at least 1 year (N=516). Included were those who used hormonal contraceptives (COC, DMPA, NET-EN, or Norplant); IUD users were considered to be controls. | |
| Interventions | Users of 1) Norplant (N=188) 2) Combined oral contraceptives (COC) (N=38) 3) IUD (N=136) (non-hormonal) |
|
| Outcomes | Mean weight gain or loss by contraceptive group at 1 year and 3 years | |
| Notes | Report had mean change for those who had an increase, decrease, or no change in weight. Review authors calculated combined weight change means and standard deviations. Injectable users were not used in this review, as DMPA and NET-EN had been grouped for analysis. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | NA |
| Incomplete outcome data addressed? All outcomes | Yes | Researchers selected charts with at least one year of data. Only 236 were followed for 3 years, producing an overall loss of 54%: Norplant 31 %, COC 95%, and IUD 56%. |
| Taneepanichskul 1998 | ||
| Methods | Retrospective study in family planning clinic at a hospital in Bangkok, Thailand. Study examined weight change in long-term users of DMPA versus IUD. | |
| Participants | 100 women, aged 37 to 50 years, attending family planning clinic. Inclusion criteria: used DMPA or IUD for 120 months, followed “regularly,” no history of smoking or alcohol intake. IUD users had not used any hormonal contraceptive. Exclusion criteria: developed chronic disease or metabolic disorder during DMPA or IUD use. | |
| Interventions | Chosen: 1) DMPA (N=50) 2) Copper T380A IUD (N=50) |
|
| Outcomes | Mean change in body weight by 120 months | |
| Notes | Weight was measured in standard manner at 120 months; methods used previously were not specified. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | NA |
| Incomplete outcome data addressed? All outcomes | Yes | Excluding those who developed a chronic disease or disorder during method use may have biased the results. Weight gain is associated with development of some diseases and disorders. |
| Tankeyoon 1976 | ||
| Methods | Prospective metabolic study in Bangkok, Thailand. Study focused on metabolic effects of the contraceptive methods. | |
| Participants | Two groups of 16 healthy women attending the family planning clinic. Inclusion criteria: > 6 weeks postpartum and no other steroid use for past 3 months. Age range was 18 to 38 years. | |
| Interventions | 1) DMPA 150 mg (3-month intervals) (N=16) 2) COC: d-norgestrel 50 μg + EE 50 μg (N=16) Follow up at 1, 2, 3, 6, 9, 12 months |
|
| Outcomes | Percent of cases with >= 1 kg increase or decrease in body weight by contraceptive method | |
| Notes | ||
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | No information |
| Incomplete outcome data addressed? All outcomes | Unclear | Losses by 12 months: DMPA 2/16 (13%); COC 4/16 (25%) Reasons for missing data not specified. |
| Tuchman 2005 | ||
| Methods | Retrospective chart review at urban, hospital-based, teen health center (USA) | |
| Participants | 222 females, aged 12 to 21 years, attending health center for contraception between 01 Jan 2001 and 31 Dec 2001. Inclusion criteria: first-time use of oral or injectable contraceptive. ‘New start’ was defined as no OC in past 3 months or DMPA in past 6 months prior to new method initiation. | |
| Interventions | Choice of: 1) DMPA every 3 months 2) Medroxyprogesterone acetate + estradiol cypionate 5 mg (MPA/E2C) monthly 2) COC |
|
| Outcomes | Mean weight change (kg) and mean percent weight change at 3, 6, 9, 12 months by contraceptive method | |
| Notes | Standardized weight and height measures described. Measures closest to baseline and each follow up were used. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | NA |
| Incomplete outcome data addressed? All outcomes | Yes | Numbers were provided for those who continued method use at each follow-up time. At 12 months, discontinuation rates were 54% to 58%. |
| Westhoff 2007 | ||
| Methods | Randomized controlled trial at sites in North and South America. Trial was evaluator-blinded. | |
| Participants | Women, 18 to 49 years old, sexually active and wanting long-term contraception. Inclusion criteria: no OC use for past 2 months, regular menstruation in past 3 months, willing to rely on DMPA for a year. Exclusion criteria: used OCs, implants, or hormonal IUD in past 2 months or DMPA-IM in past 10 months, pregnant or infertile, abnormal Pap, undiagnosed genital bleeding, other contraindications to hormonal contraceptives. | |
| Interventions | DMPA-SC 104 mg (N=266) or DMPA-IM 150 mg (N=268) every 3 months for 3 years | |
| Outcomes | Weight change at 36 months | |
| Notes | Weight assessed as safety endpoint. Researchers also analyzed weight change by BMI group: <= 25, 25 to 30, > 30 kg/m2. Report notes that no consistent differences were found by BMI groups. | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | No information |
| Incomplete outcome data addressed? All outcomes | Unclear | By 3 years, continuation in the study was 25% for DMPA-SC 104 and 21% for DMPA-IM 150. Reasons for discontinuation were unclear. |
| WHO 1983 | ||
| Methods | Phase III randomized controlled trial in 13 centers in Africa, Asia, Central and South America, and Europe. No information on method for randomization and whether any blinding was done (e.g., assessors). | |
| Participants | Non-breastfeeding women who chose injectable contraception. Exclusion criteria: contraindication for long-acting contraceptive methods. Mean age was 27.4 years. | |
| Interventions | 1) DMPA 150 mg at 90-day intervals (N=1587) 2) NET-EN 200 mg at 60-day intervals (N=789) 3) NET-EN 200 mg at 60-day intervals for 6 months then 84-day intervals (N=796) |
|
| Outcomes | Mean weight change at 12 and 24 months by contraceptive group | |
| Notes | Method for measuring weight not mentioned | |
| Risk of bias | ||
| Item | Authors’ judgement | Description |
| Allocation concealment? | Unclear | No information |
| Incomplete outcome data addressed? All outcomes | Yes | Losses to follow up were reportedly 10.7% DMPA, 8.9% NET-EN 60 days, and 9.8% NET-EN 84 days. Life-table rates for total discontinuation were 71 to 74. |
Characteristics of excluded studies [ordered by study ID]
| Study | Reason for exclusion |
|---|---|
| Agoestina 1978 | Insufficient weight change data: presented in figure without any specific numbers, other than mean gain for DMPA group in text. |
| Barsivala 1974 | Insufficient data: study duration not reported (our criteria was >= 3 months); also, authors did not specify whether the variance reported is standard deviation or standard error. |
| Beksinska 2010 | Analysis combined users of DMPA, NET-EN, or both. Authors noted the subgroups were too small to analyze separately and that differences in weight gain were not significant. |
| Berenson 1997 | Insufficient weight change data: means reported without any variance measure |
| Berenson 2009 | Insufficient weight change data: mean change reported without any variance. Of 240 who chose DMPA, 182 (76%) discontinued the method. Of the 182, 68 remained in study <= 2 more years (44 began the COC used in the study and 24 chose non-hormonal methods). Reportedly, DMPA users who had > 5% increase in weight at 6-month visit were more likely to be lost to follow up at the next visit than those who had not gained weight. |
| Bonny 2006 | Analysis combined groups that received DMPA or DMPA with estradiol supplement; researchers reported the two DMPA groups did not differ in weight gain. Method discontinuation for DMPA was 37% at 18 months. |
| Clark 2005 | Insufficient weight change data: means (not mean change) presented in a figure. Text mentions mean change for DMPA at 30 months (no variance measure) and that the control group was basically unchanged. Due to discontinuations of DMPA and initiation of hormonal contraception among controls, the samples sizes were 17% (DMPA) and 19% (controls) of baseline by the last visit. |
| Dahlberg 1982 | Insufficient weight change data: means reported without any variance measure |
| El Mahgoub 1980 | Insufficient weight change data: mean change reported without any variance measure. Also, percent that lost or gained weight was reported, but no specific amount of weight was provided. |
| Hall 1980 | Insufficient weight change data: mean change in ‘ideal body weight’ shown in figure, except for mean change for progestin-only group reported in text. |
| Havranek 1972 | Insufficient weight data: mean change for one group reported without any variance measure |
| Mangan 2002 | Comparison groups were DMPA users and OC users. Types of OC were not specified and might have included progestin-only as well as combination OC. |
| Olsson 1988 | Insufficient data for analysis: no N per group for analysis. First-year continuation rate was 59% for Norplant and 77% for Norplant-2; methods suggest these were life-table rates. |
| Ortayli 2001 | Insufficient data: report does not provide sample sizes used for analysis. Outcome data are from a pilot conducted in 1995 and the main study conducted from 1996 to 1998. |
| Risser 1999 | Comparison groups were DMPA users and OC users. Types of OC were not specified and might have included progestin-only as well as combination OC. |
| WHO 1978 | Insufficient weight data: mean gains reported without any variance measure |
| Yela 2006 | Insufficient weight change data: tables show weight and BMI means (not change) by year; text mentions mean change per group over 5 years without any variance measure. Study began in 1998. |
| Zheng 1999 | Insufficient weight change data: means reported without any variance measure |
Acknowledgments
Carol Manion of FHI developed the search strategies for several databases. David Grimes of FHI assisted with the second data abstraction.
SOURCES OF SUPPORT
Internal sources
No sources of support supplied
External sources
National Institute of Child Health and Human Development, USA.
US Agency for International Development, USA.
DATA AND ANALYSES
Comparison 1. Norethisterone 350 μg versus levonorgestrel 30 μg
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 39 | Mean Difference (IV, Fixed, 95% CI) | −0.6 [−1.76, 0.56] |
Comparison 2. DMPA 150 mg/mL + placebo versus DMPA 150 mg/mL + E2C
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total body fat (%) at 6 months | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | 7.50 [−0.47, 15.47] |
| 2 Mean change in lean body mass (%) at 6 months | 1 | 15 | Mean Difference (IV, Fixed, 95% CI) | −2.2 [−3.00, 0.60] |
Comparison 3. DMPA 150 mg/mL versus control (no hormonal method)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean change in total body fat (%) at 6 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | 11.0 [2.64, 19.36] |
| 2 Mean change in lean body mass (%) at 6 months | 1 | 26 | Mean Difference (IV, Fixed, 95% CI) | −4.0 [−6.93, −1.07] |
Comparison 4. DMPA 150 mg/mL versus MPA + E2C
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | −0.6 [−3.05, 1.85] |
| 2 Mean weight change (kg) at 12 months | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | −1.3 [−6.37, 3.77] |
| 3 Mean percentage weight change at 6 months | 1 | 70 | Mean Difference (IV, Fixed, 95% CI) | −0.60 [−4.04, 2.84] |
| 4 Mean percentage weight change at 12 months | 1 | 46 | Mean Difference (IV, Fixed, 95% CI) | −0.70 [−7.58, 6.18] |
Comparison 5. DMPA 150 mg/mL versus COC
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | −0.50 [−2.26, 1.26] |
| 2 Mean weight change (kg) at 12 months | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | 0.7 [−1.92, 3.32] |
| 3 Mean percentage weight change at 6 months | 1 | 142 | Mean Difference (IV, Fixed, 95% CI) | −0.7 [−3.10, 1.70] |
| 4 Mean percentage weight change at 12 months | 1 | 81 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 5 Weight gain >= 1 kg at month 6 | 1 | 31 | Odds Ratio (M-H, Fixed, 95% CI) | 0.39 [0.09, 1.67] |
| 6 Weight gain >= 1 kg at month 12 | 1 | 26 | Odds Ratio (M-H, Fixed, 95% CI) | 0.44 [0.08, 2.39] |
| 7 Weight loss >= 1 kg at month 6 | 1 | 31 | Peto Odds Ratio (Peto, Fixed, 95% CI) | 9.15 [0.88, 95.40] |
| 8 Weight loss >= 1 kg at month 12 | 1 | 26 | Odds Ratio (M-H, Fixed, 95% CI) | 1.83 [0.15, 23.15] |
Comparison 6. DMPA 150 mg/mL versus DMPA 450 mg/mL
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 651 | Mean Difference (IV, Fixed, 95% CI) | 0.01 [−0.48, 0.50] |
Comparison 7. DMPA 150 mg/mL initiation after pregnancy: interval (>=20 weeks) versus postpartum (5 to 8 weeks)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight gain (lbs) at 1 year | 1 | 172 | Mean Difference (IV, Fixed, 95% CI) | 2.30 [−0.94, 5.54] |
| 2 Mean weight gain (lbs) at 2 years | 1 | 64 | Mean Difference (IV, Fixed, 95% CI) | 1.60 [−4.79, 7.99] |
Comparison 8. DMPA-IM 150 mg versus DMPA-SC 104 mg
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 36 months | 1 | 121 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [−1.78, 4.38] |
Comparison 9. DMPA 150 mg/mL versus NET-EN 200 mg (60-day intervals)
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 12 months | 1 | 1162 | Mean Difference (IV, Fixed, 95% CI) | 0.20 [−0.63, 1.03] |
| 2 Mean weight change (kg) at 24 months | 1 | 604 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3 Mean weight change at 1 year | 1 | 201 | Mean Difference (IV, Fixed, 95% CI) | 0.80 [−0.10, 1.70] |
Comparison 10. NET-EN 200 mg: 60-day intervals versus 3 intervals of 60 days then 84-day intervals
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 12 months | 1 | 822 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 2 Mean weight change (kg) at 24 months | 1 | 453 | Mean Difference (IV, Fixed, 95% CI) | −0.10 [−1.35, 1.15] |
Comparison 11. DMPA 150 mg/mL versus IUD
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 120 months | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | −0.30 [−0.83, 0.23] |
| 2 Mean weight change (kg) at 1 year by baseline BMI | 1 | 758 | Mean Difference (IV, Fixed, 95% CI) | 2.28 [1.79, 2.77] |
| 2.1 BMI < 25 kg/m2 | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | 2.5 [1.90, 3.10] |
| 2.2 BMI 25 to 29.9 kg/m2 | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 2.10 [1.16, 3.04] |
| 2.3 BMI >= 30 kg/m2 | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 0.7 [−1.26, 2.66] |
| 3 Mean weight change (kg) at 2 years by baseline BMI | 1 | 758 | Mean Difference (IV, Fixed, 95% CI) | 2.71 [2.12, 3.30] |
| 3.1 BMI < 25 kg/m2 | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | 2.7 [2.02, 3.38] |
| 3.2 BMI 25 to 29.9 kg/m2 | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 3.0 [1.73, 4.27] |
| 3.3 BMI >= 30 kg/m2 | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 1.5 [−1.33, 4.33] |
| 4 Mean weight change (kg) at 3 years by baseline BMI | 1 | 758 | Mean Difference (IV, Fixed, 95% CI) | 3.17 [2.51, 3.83] |
| 4.1 BMI < 25 kg/m2 | 1 | 452 | Mean Difference (IV, Fixed, 95% CI) | 3.3 [2.52, 4.08] |
| 4.2 BMI 25 to 29.9 kg/m2 | 1 | 218 | Mean Difference (IV, Fixed, 95% CI) | 3.20 [1.82, 4.58] |
| 4.3 BMI >= 30 kg/m2 | 1 | 88 | Mean Difference (IV, Fixed, 95% CI) | 1.30 [−1.56, 4.16] |
Comparison 12. Norplant versus DMPA 150 mg/mL
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 1 year | 1 | 100 | Mean Difference (IV, Fixed, 95% CI) | −0.87 [−1.86, 0.12] |
Comparison 13. Norplant versus IUD
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 99 | Mean Difference (IV, Fixed, 95% CI) | 0.47 [0.29, 0.65] |
| 2 Mean weight change (kg) at 1 year | 1 | 324 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [0.36, 1.84] |
| 3 Mean weight change (kg) at 3 years | 1 | 190 | Mean Difference (IV, Fixed, 95% CI) | 0.90 [−0.39, 2.19] |
Comparison 14. Norplant versus barrier, ‘local’, or no contraceptive method
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 6 months | 1 | 97 | Mean Difference (IV, Fixed, 95% CI) | 0.74 [0.52, 0.96] |
Comparison 15. Norplant versus 2-rod LNG
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 1 year | 1 | 1196 | Mean Difference (IV, Fixed, 95% CI) | 0.09 [−0.33, 0.51] |
| 2 Mean weight change (kg) at 3 years | 1 | 922 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
| 3 Mean weight change (kg) at 5 years | 1 | 614 | Mean Difference (IV, Fixed, 95% CI) | 0.60 [−0.13, 1.33] |
Comparison 16. Norplant versus COC
| Outcome or subgroup title | No. of studies | No. of participants | Statistical method | Effect size |
|---|---|---|---|---|
| 1 Mean weight change (kg) at 1 year | 1 | 226 | Mean Difference (IV, Fixed, 95% CI) | 1.1 [−0.13, 2.33] |
| 2 Mean weight change (kg) at 3 years | 1 | 132 | Mean Difference (IV, Fixed, 95% CI) | Not estimable |
Analysis 1.1.
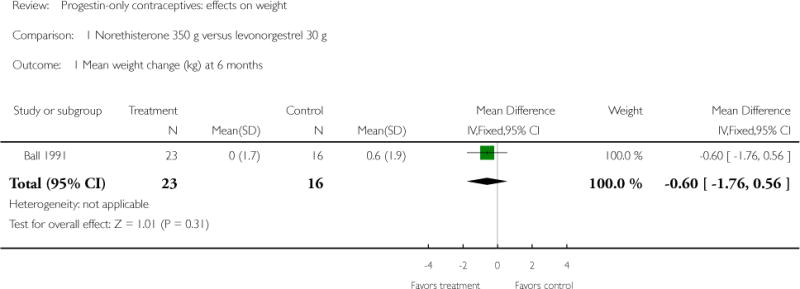
Comparison 1 Norethisterone 350 μg versus levonorgestrel 30 μg, Outcome 1 Mean weight change (kg) at 6 months.
Analysis 2.1.
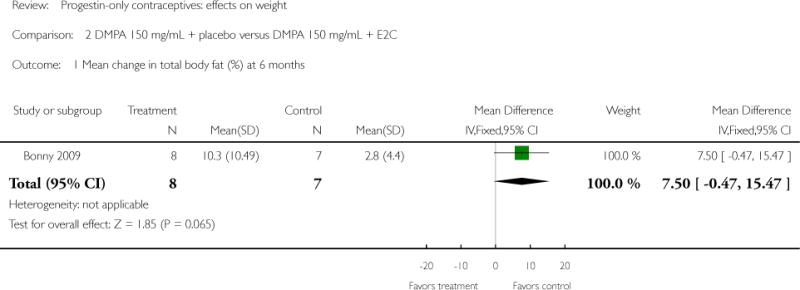
Comparison 2 DMPA 150 mg/mL + placebo versus DMPA 150 mg/mL + E2C, Outcome 1 Mean change in total body fat (%) at 6 months.
Analysis 2.2.
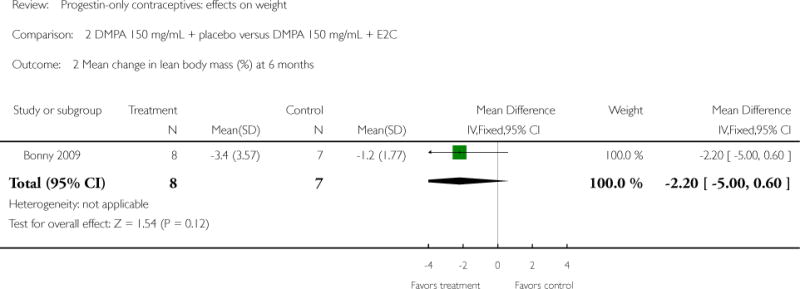
Comparison 2 DMPA 150 mg/mL + placebo versus DMPA 150 mg/mL + E2C, Outcome 2 Mean change in lean body mass (%) at 6 months.
Analysis 3.1.
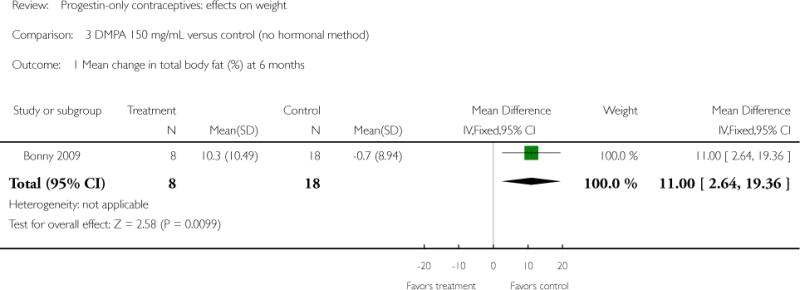
Comparison 3 DMPA 150 mg/mL versus control (no hormonal method), Outcome 1 Mean change in total body fat (%) at 6 months.
Analysis 3.2.
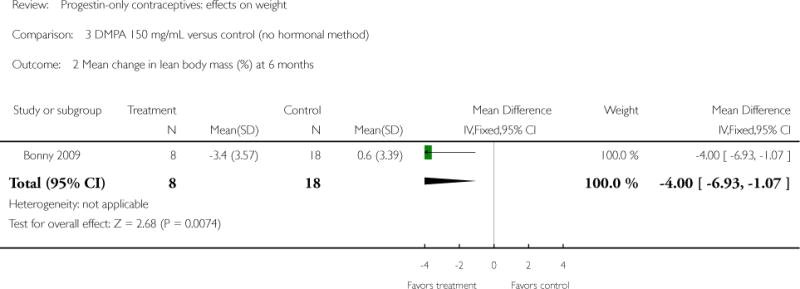
Comparison 3 DMPA 150 mg/mL versus control (no hormonal method), Outcome 2 Mean change in lean body mass (%) at 6 months.
Analysis 4.1.
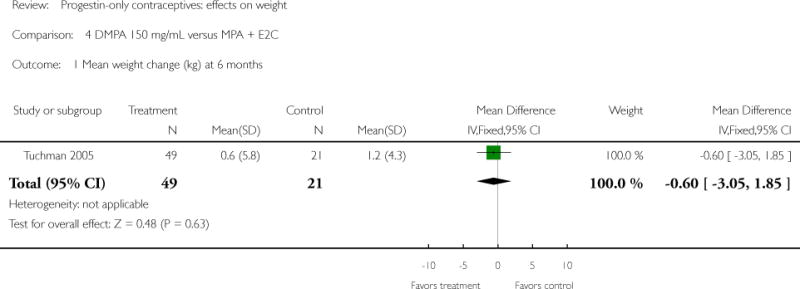
Comparison 4 DMPA 150 mg/mL versus MPA + E2C, Outcome 1 Mean weight change (kg) at 6 months.
Analysis 4.2.
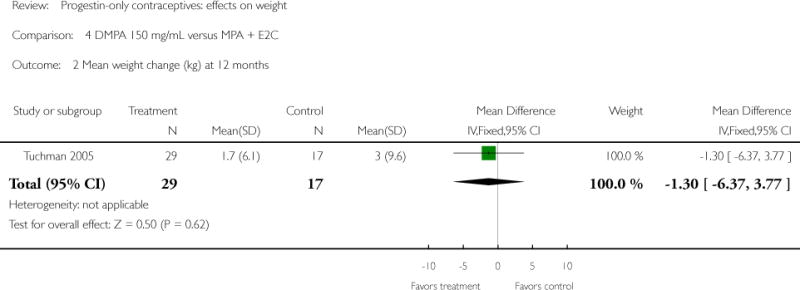
Comparison 4 DMPA 150 mg/mL versus MPA + E2C, Outcome 2 Mean weight change (kg) at 12 months.
Analysis 4.3.
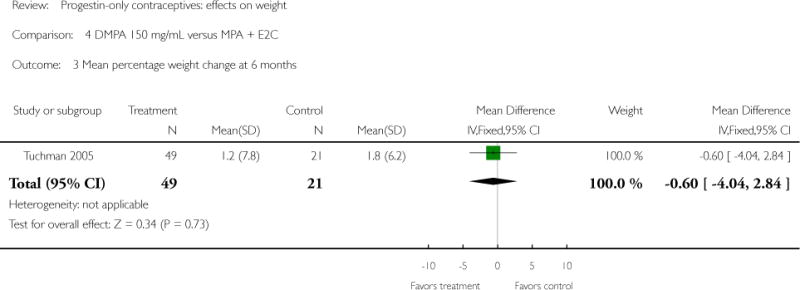
Comparison 4 DMPA 150 mg/mL versus MPA + E2C, Outcome 3 Mean percentage weight change at 6 months.
Analysis 4.4.
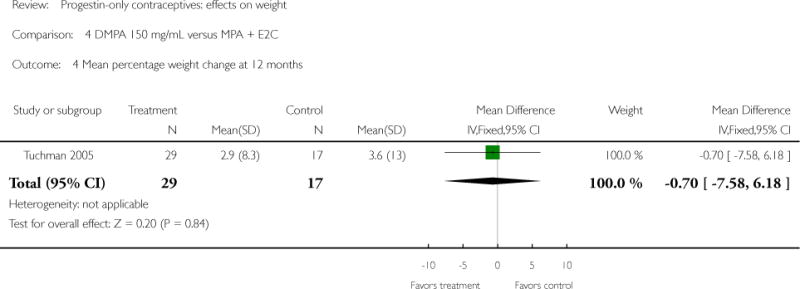
Comparison 4 DMPA 150 mg/mL versus MPA + E2C, Outcome 4 Mean percentage weight change at 12 months.
Analysis 5.1.
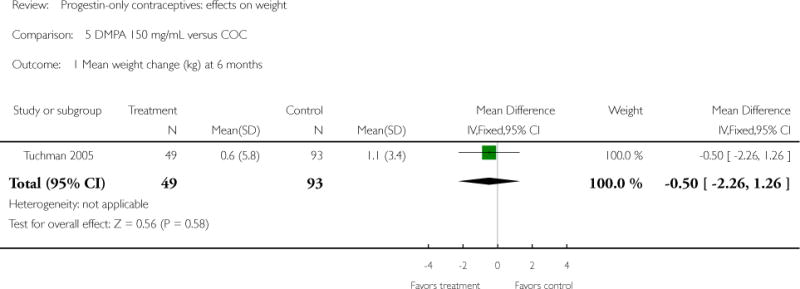
Comparison 5 DMPA 150 mg/mL versus COC, Outcome 1 Mean weight change (kg) at 6 months.
Analysis 5.2.
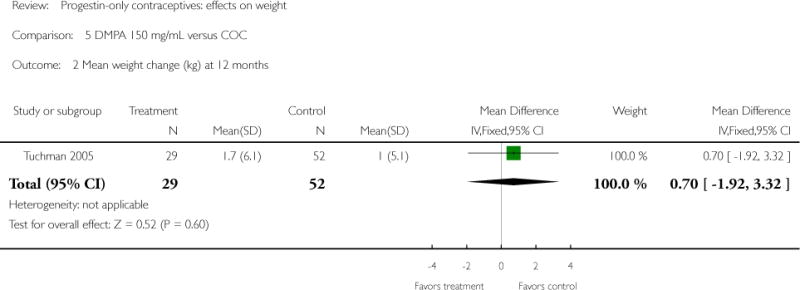
Comparison 5 DMPA 150 mg/mL versus COC, Outcome 2 Mean weight change (kg) at 12 months.
Analysis 5.3.
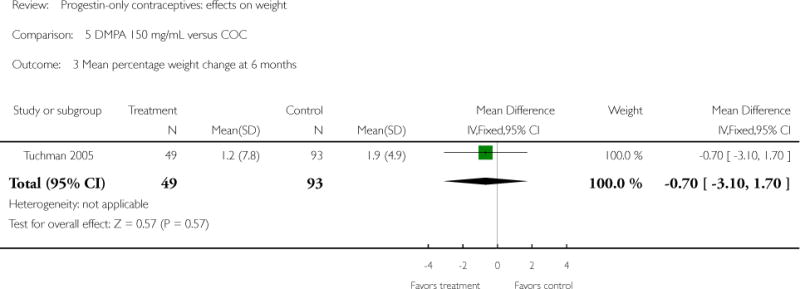
Comparison 5 DMPA 150 mg/mL versus COC, Outcome 3 Mean percentage weight change at 6 months.
Analysis 5.4.
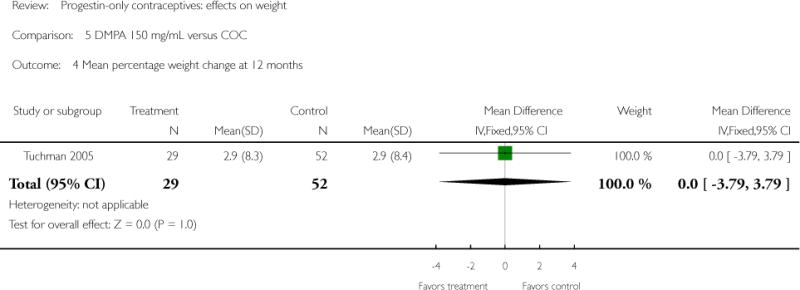
Comparison 5 DMPA 150 mg/mL versus COC, Outcome 4 Mean percentage weight change at 12 months.
Analysis 5.5.
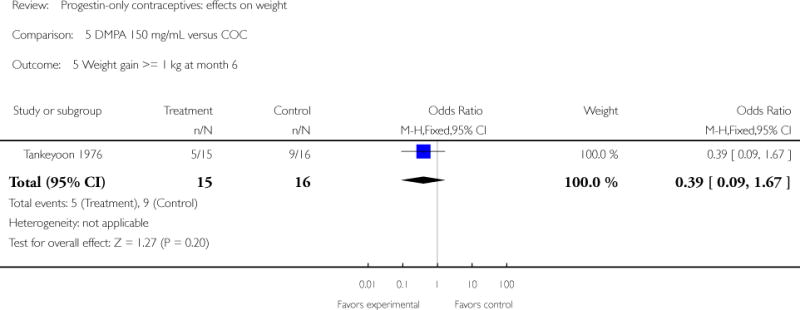
Comparison 5 DMPA 150 mg/mL versus COC, Outcome 5 Weight gain >= 1 kg at month 6.
Analysis 5.6.
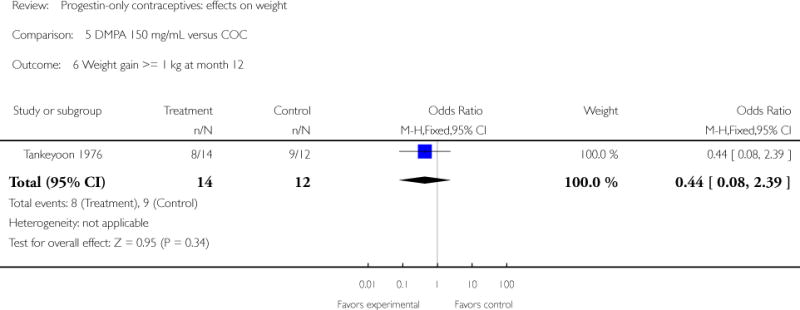
Comparison 5 DMPA 150 mg/mL versus COC, Outcome 6 Weight gain >= 1 kg at month 12.
Analysis 5.7.
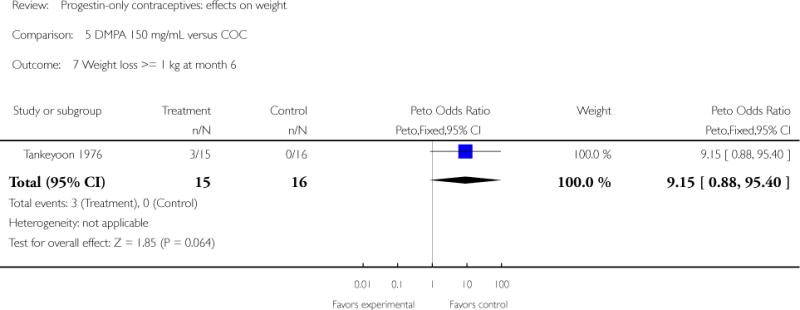
Comparison 5 DMPA 150 mg/mL versus COC, Outcome 7 Weight loss >= 1 kg at month 6.
Analysis 5.8.
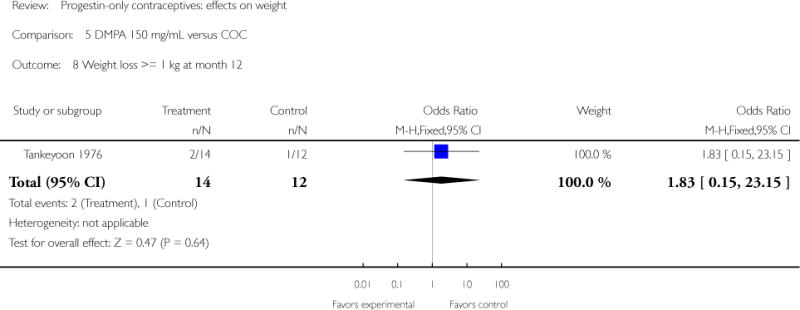
Comparison 5 DMPA 150 mg/mL versus COC, Outcome 8 Weight loss >= 1 kg at month 12.
Analysis 6.1.
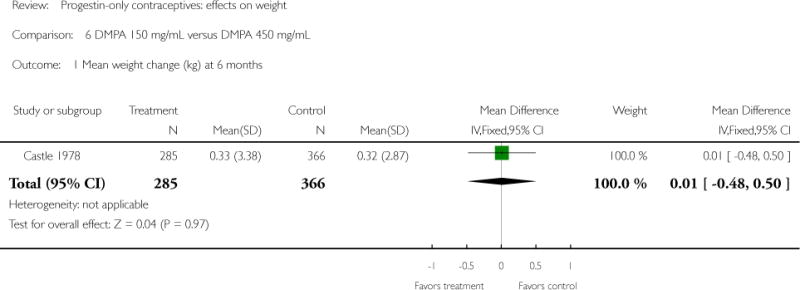
Comparison 6 DMPA 150 mg/mL versus DMPA 450 mg/mL, Outcome 1 Mean weight change (kg) at 6 months.
Analysis 7.1.
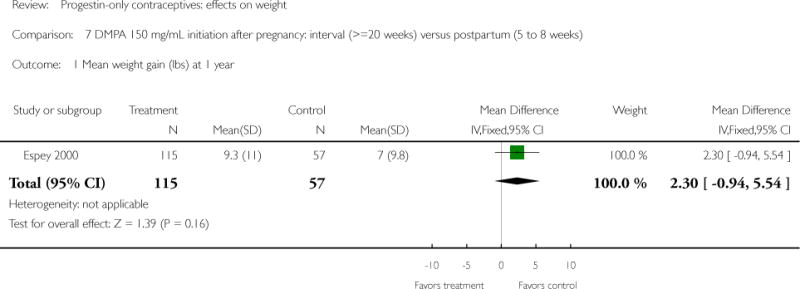
Comparison 7 DMPA 150 mg/mL initiation after pregnancy: interval (>=20 weeks) versus postpartum (5 to 8 weeks), Outcome 1 Mean weight gain (lbs) at 1 year.
Analysis 7.2.
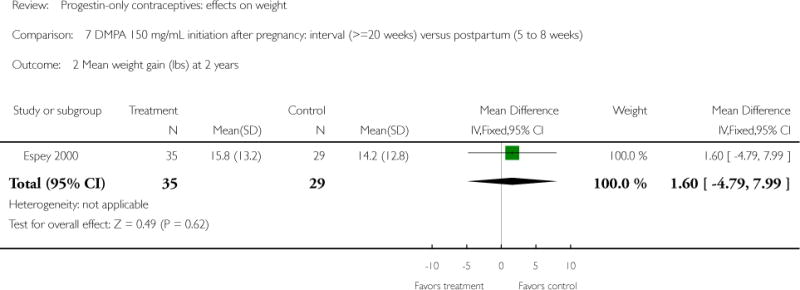
Comparison 7 DMPA 150 mg/mL initiation after pregnancy: interval (>=20 weeks) versus postpartum (5 to 8 weeks), Outcome 2 Mean weight gain (lbs) at 2 years.
Analysis 8.1.
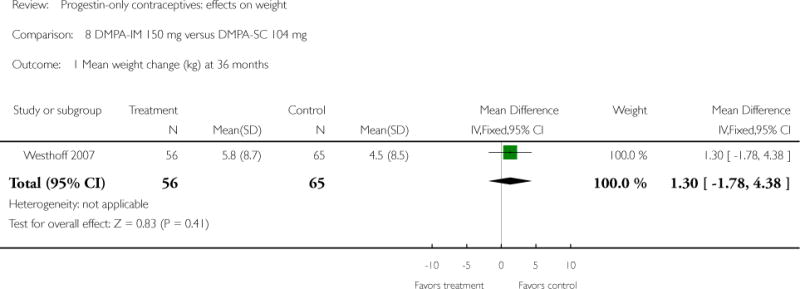
Comparison 8 DMPA-IM 150 mg versus DMPA-SC 104 mg, Outcome 1 Mean weight change (kg) at 36 months.
Analysis 9.1.
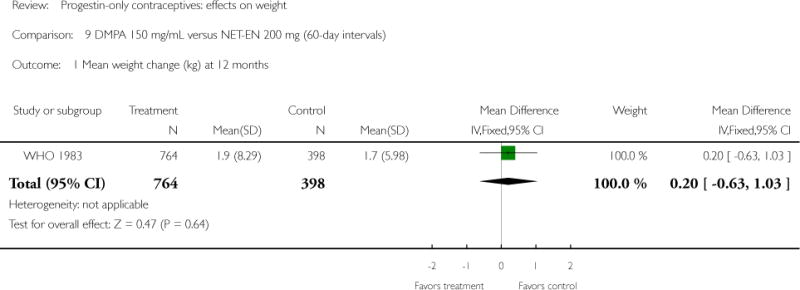
Comparison 9 DMPA 150 mg/mL versus NET-EN 200 mg (60-day intervals), Outcome 1 Mean weight change (kg) at 12 months.
Analysis 9.2.
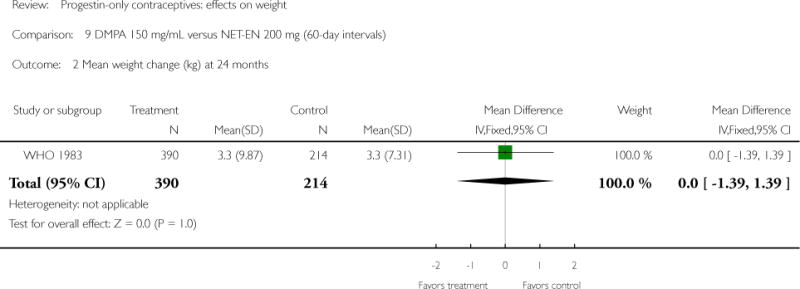
Comparison 9 DMPA 150 mg/mL versus NET-EN 200 mg (60-day intervals), Outcome 2 Mean weight change (kg) at 24 months.
Analysis 9.3.
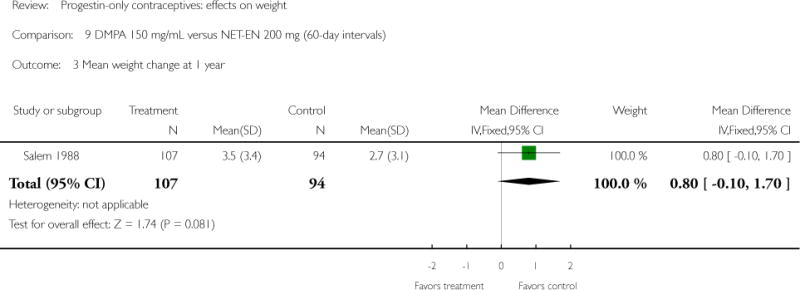
Comparison 9 DMPA 150 mg/mL versus NET-EN 200 mg (60-day intervals), Outcome 3 Mean weight change at 1 year.
Analysis 10.1.
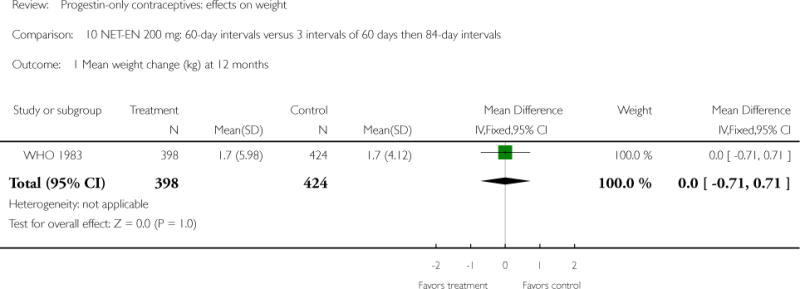
Comparison 10 NET-EN 200 mg: 60-day intervals versus 3 intervals of 60 days then 84-day intervals, Outcome 1 Mean weight change (kg) at 12 months.
Analysis 10.2.
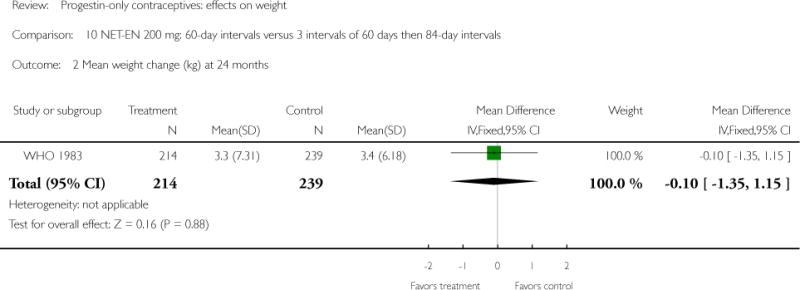
Comparison 10 NET-EN 200 mg: 60-day intervals versus 3 intervals of 60 days then 84-day intervals, Outcome 2 Mean weight change (kg) at 24 months.
Analysis 11.1.
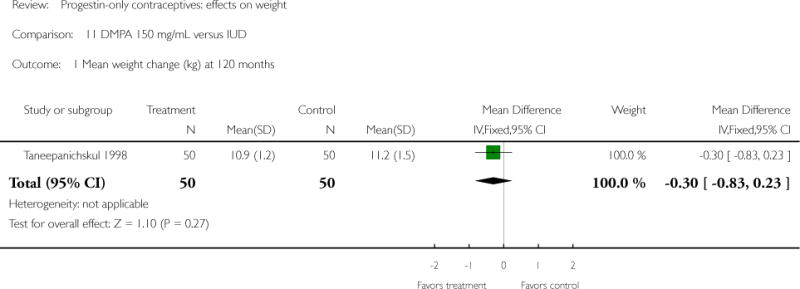
Comparison 11 DMPA 150 mg/mL versus IUD, Outcome 1 Mean weight change (kg) at 120 months.
Analysis 11.2.
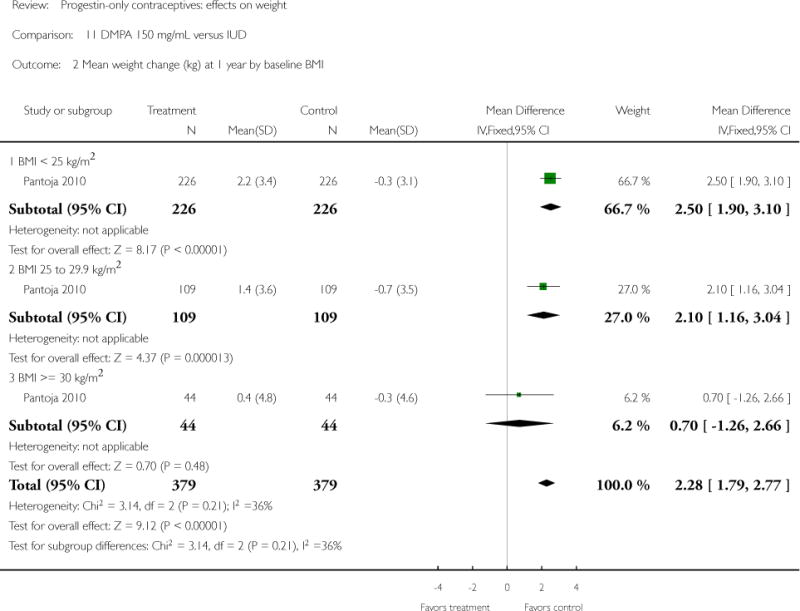
Comparison 11 DMPA 150 mg/mL versus IUD, Outcome 2 Mean weight change (kg) at 1 year by baseline BMI.
Analysis 11.3.
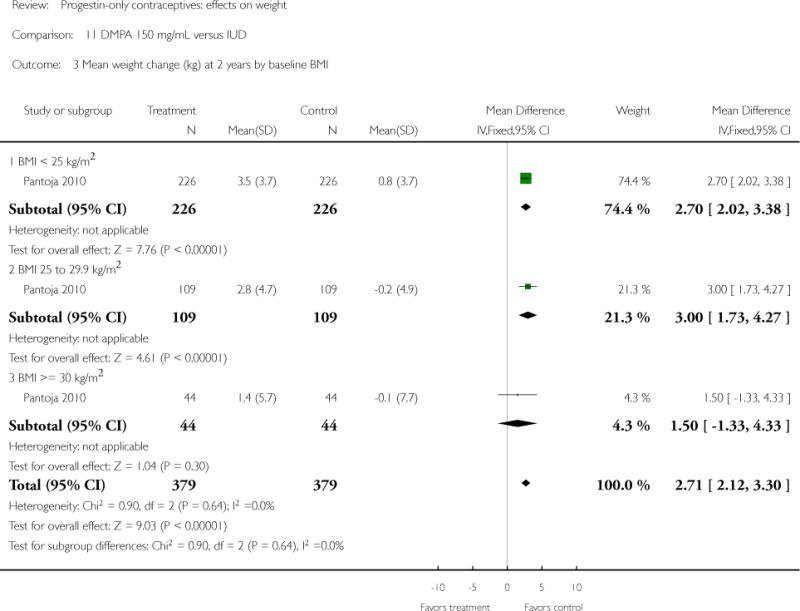
Comparison 11 DMPA 150 mg/mL versus IUD, Outcome 3 Mean weight change (kg) at 2 years by baseline BMI.
Analysis 11.4.
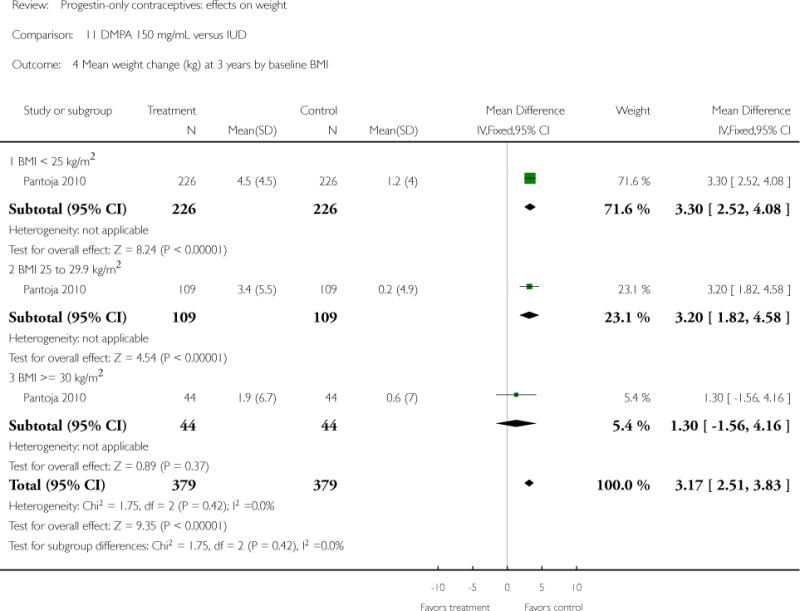
Comparison 11 DMPA 150 mg/mL versus IUD, Outcome 4 Mean weight change (kg) at 3 years by baseline BMI.
Analysis 12.1.
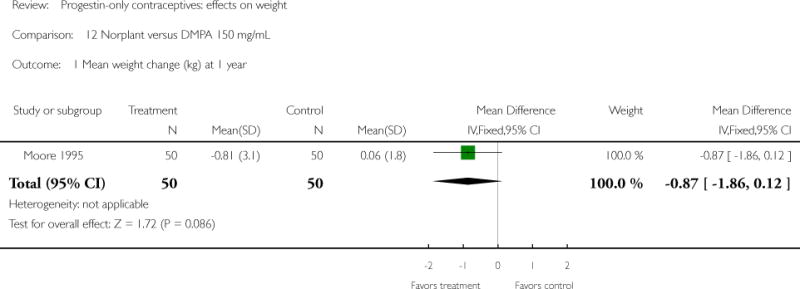
Comparison 12 Norplant versus DMPA 150 mg/mL, Outcome 1 Mean weight change (kg) at 1 year.
Analysis 13.1.
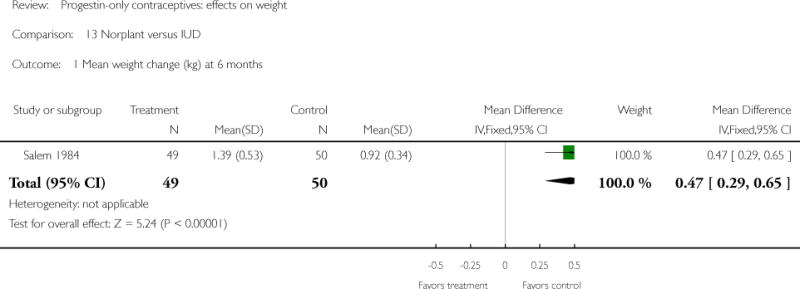
Comparison 13 Norplant versus IUD, Outcome 1 Mean weight change (kg) at 6 months.
Analysis 13.2.
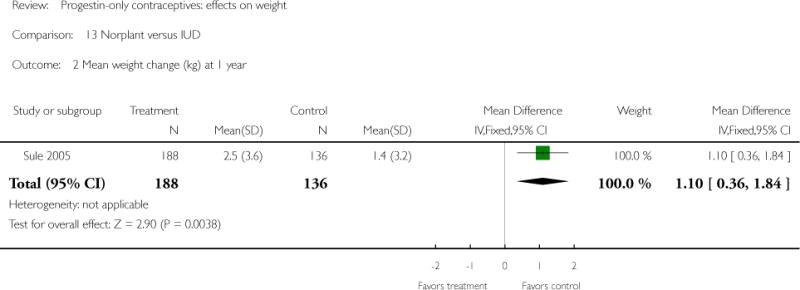
Comparison 13 Norplant versus IUD, Outcome 2 Mean weight change (kg) at 1 year.
Analysis 13.3.
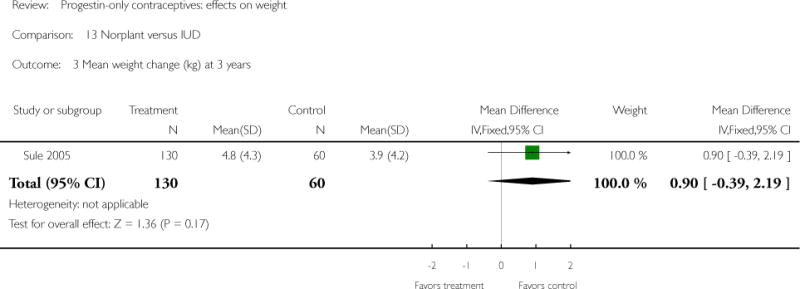
Comparison 13 Norplant versus IUD, Outcome 3 Mean weight change (kg) at 3 years.
Analysis 14.1.
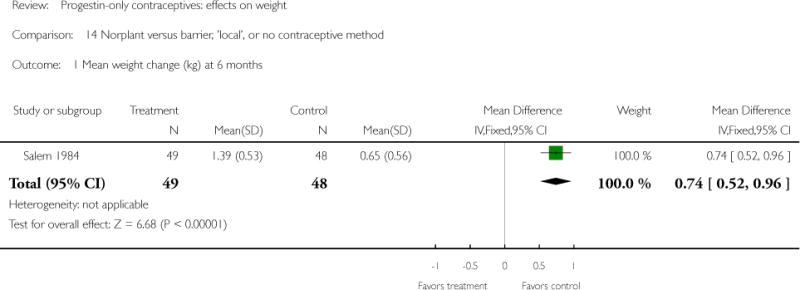
Comparison 14 Norplant versus barrier, ‘local’, or no contraceptive method, Outcome 1 Mean weight change (kg) at 6 months.
Analysis 15.1.
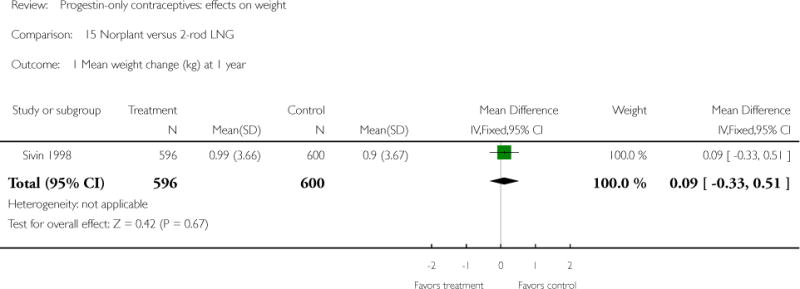
Comparison 15 Norplant versus 2-rod LNG, Outcome 1 Mean weight change (kg) at 1 year.
Analysis 15.2.
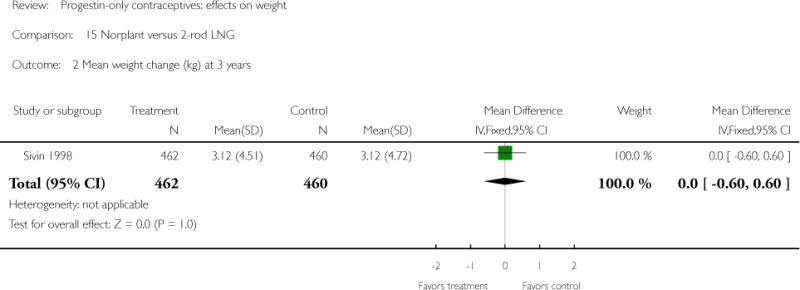
Comparison 15 Norplant versus 2-rod LNG, Outcome 2 Mean weight change (kg) at 3 years.
Analysis 15.3.
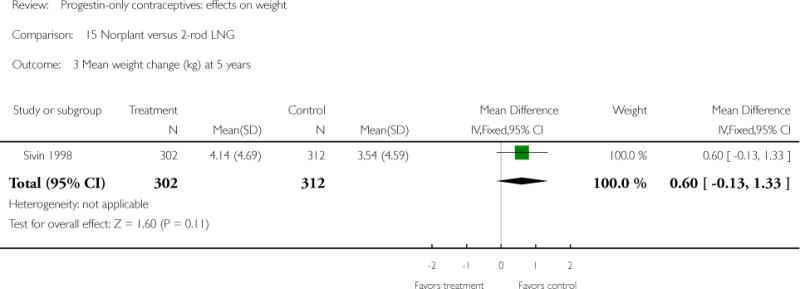
Comparison 15 Norplant versus 2-rod LNG, Outcome 3 Mean weight change (kg) at 5 years.
Analysis 16.1.
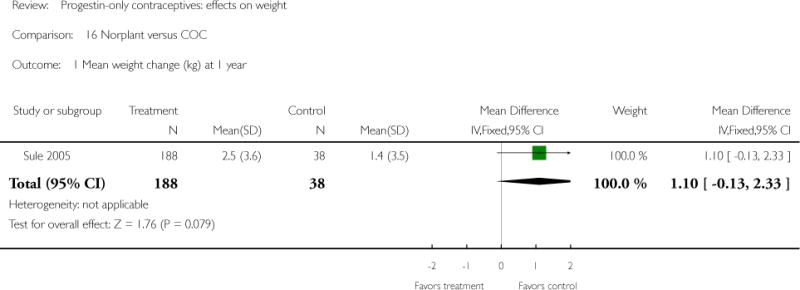
Comparison 16 Norplant versus COC, Outcome 1 Mean weight change (kg) at 1 year.
Analysis 16.2.
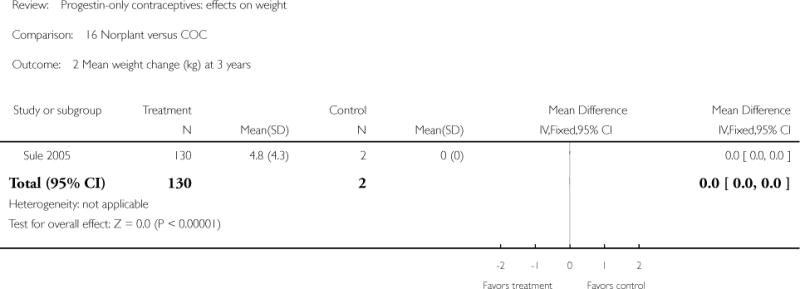
Comparison 16 Norplant versus COC, Outcome 2 Mean weight change (kg) at 3 years.
Footnotes
CONTRIBUTIONS OF AUTHORS
L Lopez reviewed the search results, extracted and entered the data, and drafted the review. A Edelman and F Helmerhorst participated in the second extraction. M Chen-Mok provided guidance on study design, data analysis, and interpretation of results. All authors reviewed and commented on the document.
DECLARATIONS OF INTEREST
The authors do not have any conflicts of interest to declare regarding this review.
References to studies included in this review
* Indicates the major publication for the study
- Ball 1991 {published data only}.Ball MJ, Ashwell E, Gillmer MD. Progestagen-only oral contraceptives: comparison of the metabolic effects of levonorgestrel and norethisterone. Contraception. 1991;44(3):223–33. doi: 10.1016/0010-7824(91)90014-7. [DOI] [PubMed] [Google Scholar]
- Bonny 2009 {published data only}.Bonny AE, Secic M, Cromer BA. A longitudinal comparison of body composition changes in adolescent girls receiving hormonal contraception. Journal of Adolescent Health. 2009;45(4):423–5. doi: 10.1016/j.jadohealth.2009.04.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Castle 1978 {published data only}.Castle WM, Sapire KE, Howard KA. Efficacy and acceptability of injectable medroxyprogesterone: a comparison of 3-monthly and 6-monthly regimens. South African Medical Journal. 1978;53(21):842–5. [PubMed] [Google Scholar]
- Espey 2000 {published data only}.Espey E, Steinhart J, Ogburn T, Qualls C. Depo-provera associated with weight gain in Navajo women. Contraception. 622:55–8. doi: 10.1016/s0010-7824(00)00144-x. 2000/12/05 2000. [DOI] [PubMed] [Google Scholar]
- Moore 1995 {published data only}.Moore LL, Valuck R, McDougall C, Fink W. A comparative study of one-year weight gain among users of medroxyprogesterone acetate, levonorgestrel implants, and oral contraceptives. Contraception. 1995;52(4):215–9. doi: 10.1016/0010-7824(95)00189-h. 1995/10/01. [DOI] [PubMed] [Google Scholar]
- Pantoja 2010 {published data only}.Pantoja M, Medeiros T, Baccarin MC, Morais S, Fernandes AM. Variation of weight among users of the contraceptive with depot-medroxyprogesterone acetate according to body mass index in a six-year follow-up. Revista Brasileira de Ginecologia e Obstetricia. 2009;31(8):380–4. doi: 10.1590/s0100-72032009000800002. [DOI] [PubMed] [Google Scholar]
- Pantoja M, Medeiros T, Baccarin MC, Morais SS, Bahamondes L, Fernandes AM. Variations in body mass index of users of depot-medroxyprogesterone acetate as a contraceptive. Contraception. 2010;81(2):107–11. doi: 10.1016/j.contraception.2009.07.008. [DOI] [PubMed] [Google Scholar]
- Salem 1984 {published data only}.Salem HT, Abdullah KA, Shaaban MM. Proceedings of the Symposium on Longterm Subdermal Contraceptive Implants. Assuit, Egypt: Assiut University; 1984. 1984. Norplant and lactation. The Norplant subdermal contraceptive system; pp. 139–49. February 23–24. [Google Scholar]
- Salem 1988 {published data only}.Salem HT, Salah M, Aly MY, Thabet AI, Shaaban MM, Fathalla MF. Acceptability of injectable contraceptives. 6. Vol. 38. Assiut, Egypt: Contraception; 1988. pp. 697–710. 1988/12/01. [DOI] [PubMed] [Google Scholar]
- Sivin 1998 {published data only}.Sivin I, Campodonico I, Kiriwat O, Holma P, Diaz S, Wan L, et al. The performance of levonorgestrel rod and Norplant contraceptive implants: a 5 year randomized study. Human Reproduction. 1998;13(12):3371–8. doi: 10.1093/humrep/13.12.3371. 1999/01/14. [DOI] [PubMed] [Google Scholar]
- Sivin I, Viegas O, Campodonico I, Diaz S, Pavez M, Wan L, et al. Clinical performance of a new two-rod levonorgestrel contraceptive implant: a three-year randomized study with Norplant implants as controls. Contraception. 1997;55(2):73–80. doi: 10.1016/s0010-7824(96)00275-2. [DOI] [PubMed] [Google Scholar]
- Sule 2005 {published data only}.Sule S, Shittu O. Weight changes in clients on hormonal contraceptives in Saria, Nigeria. African Journal of Reproductive Health. 2005;9(2):92–100. [PubMed] [Google Scholar]
- Taneepanichskul 1998 {published data only}.Taneepanichskul S, Reinprayoon D, Jaisamrarn U. Effects of DMPA on weight and blood pressure in long term acceptors. Contraception. 1999;59(5):301–3. doi: 10.1016/s0010-7824(99)00037-2. [DOI] [PubMed] [Google Scholar]
- Taneepanichskul S, Reinprayoon D, Khaosaad P. Comparative study of weight change between long-term DMPA and IUD acceptors. Contraception. 1998;58(3):149–51. [Google Scholar]
- Tankeyoon 1976 {published data only}.Tankeyoon M, Dusitsin N, Poshyachinda V, Larsson-Cohn U. A study of glucose tolerance, serum transaminase and lipids in women using depot-medroxyprogesterone acetate and a combination-type oral contraceptive. Contraception. 1976;14(2):199–214. doi: 10.1016/0010-7824(76)90088-3. [DOI] [PubMed] [Google Scholar]
- Tuchman 2005 {published data only}.Tuchman LK, Huppert JS, Huang B, Slap GB. Adolescent use of the monthly contraceptive injection. Journal of Pediatric and Adolescent Gynecology. 2005;18(4):255–60. doi: 10.1016/j.jpag.2005.05.004. [DOI] [PubMed] [Google Scholar]
- Westhoff 2007 {published data only}.Jain J, Jakimiuk AJ, Bode FR, Ross D, Kaunitz AM. Contraceptive efficacy and safety of DMPA-SC. Contraception. 2004;70(4):269–75. doi: 10.1016/j.contraception.2004.06.011. [DOI] [PubMed] [Google Scholar]
- Westhoff C, Jain JK, Milsom I, Ray A. Changes in weight with depot medroxyprogesterone acetate subcutaneous injection 104 mg/0.65 mL. Contraception. 2007;75(4):261–7. doi: 10.1016/j.contraception.2006.12.009. [DOI] [PubMed] [Google Scholar]
- WHO 1983 {published data only}.World Health Organization. Multinational comparative clinical trial of long-acting injectable contraceptives: norethisterone enanthate given in two dosage regimens and depot-medroxyprogesterone acetate. A preliminary report. Contraception. 1982;25(1):1–11. doi: 10.1016/s0010-7824(83)80002-x. 1982/01/01. [DOI] [PubMed] [Google Scholar]
- World Health Organization. Multinational comparative clinical trial of long-acting injectable contraceptives: norethisterone enanthate given in two dosage regimens and depot-medroxyprogesterone acetate. Final report. Contraception. 1983;28(1):1–20. doi: 10.1016/s0010-7824(83)80002-x. 1983/07/01. [DOI] [PubMed] [Google Scholar]
References to studies excluded from this review
- Agoestina 1978 {published data only}.Agoestina T, Biben A. Use of Depo Provera in immediate post partum and lactating period at Dr Hasan Sadikin Hospital. 1978 Report on file 10. [Google Scholar]
- Barsivala 1974 {published data only}.Barsivala V, Virkar K, Kulkarni RD. Thyroid functions of women taking oral contraceptives. Contraception. 1974;9(3):305–14. doi: 10.1016/0010-7824(74)90021-3. [DOI] [PubMed] [Google Scholar]
- Beksinska 2010 {published data only}.Beksinska ME, Smit JA, Kleinschmidt I, Milford C, Farley TM. Prospective study of weight change in new adolescent users of DMPA, NET-EN, COCs, nonusers and discontinuers of hormonal contraception. Contraception. 2010;81(1):30–4. doi: 10.1016/j.contraception.2009.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berenson 1997 {published data only}.Berenson AB, Wiemann CM, Rickerr VI, McCombs SL. Contraceptive outcomes among adolescents prescribed Norplant implants versus oral contraceptives after one year of use. American Journal of Obstetrics and Gynecology. 1997;176(3):586–92. doi: 10.1016/s0002-9378(97)70552-0. [DOI] [PubMed] [Google Scholar]
- Berenson 2009 {published data only}.Berenson AB, Rahman M. Changes in weight, total fat, percent body fat, and central-to-peripheral fat ratio associated with injectable and oral contraceptive use. American Journal of Obstetrics and Gynecology. 2009;200(3:329):e1–8. doi: 10.1016/j.ajog.2008.12.052. 2009/03/04. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonny 2006 {published data only}.Bonny A, Camlin K, Harvey R, Islam KM, Debanne S. Prospective analysis of weight changes in adolescent females initiating depot medroxyprogesterone acetate (DMPA), oral contraceptive pills (OC), or no hormonal contraceptive method. Journal of Adolescent Health. 2003;32(2):134. [Google Scholar]
- Bonny AE, Ziegler J, Harvey R, Debanne SM, Secic M, Cromer BA. Weight gain in obese and nonobese adolescent girls initiating depot medroxyprogesterone, oral contraceptive pills, or no hormonal contraceptive method. Archives of Pediatrics & Adolescent Medicine. 2006;160(1):40–5. doi: 10.1001/archpedi.160.1.40. 2006/01/04. [DOI] [PubMed] [Google Scholar]
- Clark 2005 {published data only}.Clark MK, Dillon JS, Sowers M, Nichols S. Weight, fat mass, and central distribution of fat increase when women use depot-medroxyprogesterone acetate for contraception. International Journal of Obesity. 2005;29(10):1252–8. doi: 10.1038/sj.ijo.0803023. [DOI] [PubMed] [Google Scholar]
- Clark MK, Sowers MR, Nichols S, Levy B. Bone mineral density changes over two years in first-time users of depot medroxyprogesterone acetate. Fertility and Sterility. 2004;82(6):1580–6. doi: 10.1016/j.fertnstert.2004.04.064. 2004/12/14. [DOI] [PubMed] [Google Scholar]
- Dahlberg 1982 {published data only}.Dahlberg K. Some effects of depo-medroxyprogesterone acetate (DMPA): observations in the nursing infant and in the long-term user. International Journal of Gynaecology and Obstetrics. 1982;20(1):43–8. doi: 10.1016/0020-7292(82)90044-3. [DOI] [PubMed] [Google Scholar]
- El Mahgoub 1980 {published data only}.El Mahgoub S. Body weight and cycle control of injectable contraceptives. Journal of Reproductive Medicine. 1980;24(3):119–26. [PubMed] [Google Scholar]
- Hall 1980 {published data only}.Hall WD, Douglas MB, Blumenstein BA, Hatcher RA. Blood pressure and oral progestational agents. A prospective study of 119 black women. American Journal of Obstetrics and Gynecology. 1980;136(3):344–8. doi: 10.1016/0002-9378(80)90860-1. [DOI] [PubMed] [Google Scholar]
- Havranek 1972 {published data only}.Havranek F, Tichy M, Divila F, Hejda V. Experience with the contraceptive effect of Depo-Provera, 300mg and 450mg administered at 6 month intervals [Zkusenosti s antikoncepcni ucinnosti Depo–Provery v davce 300mg a 450mg podanych v intervalech 6 mesicu] Ceskoslovenska Gynekologie. 1972;37(2):95–6. [PubMed] [Google Scholar]
- Mangan 2002 {published data only}.Mangan SA, Larsen PG, Hudson S. Overweight teens at increased risk for weight gain while using depot medroxyprogesterone acetate. Journal of Pediatric and Adolescent Gynecology. 2002;15(2):79–82. doi: 10.1016/s1083-3188(01)00147-4. [DOI] [PubMed] [Google Scholar]
- Olsson 1988 {published data only}.Olsson SE, Odlind V, Johansson ED, Sivin I. Contraception with NORPLANT implants and NORPLANT-2 implants (two covered rods). Results from a comparative clinical study in Sweden. Contraception. 1988;37(1):61–73. doi: 10.1016/0010-7824(88)90149-7. 1988/01/01. [DOI] [PubMed] [Google Scholar]
- Ortayli 2001 {published data only}.Ortayli N, Bulut A, Sahin T, Sivin I. Immediate postabortal contraception with the levonorgestrel intrauterine device, Norplant, and traditional methods. Contraception. 2001;63(6):309–14. doi: 10.1016/s0010-7824(01)00212-8. [DOI] [PubMed] [Google Scholar]
- Risser 1999 {published data only}.Risser WL, Gefter LR, Barratt MS, Risser JM. Weight change in adolescents who used hormonal contraception. Journal of Adolescent Health. 1999;24(6):433–6. doi: 10.1016/s1054-139x(98)00151-7. 1999/07/13. [DOI] [PubMed] [Google Scholar]
- WHO 1978 {published data only}.World Health Organization. Multinational comparative clinical evaluation of two long-acting injectable contraceptive steroids: noresthisterone oenanthate and medroxyprogesterone acetate. 2. Bleeding patterns and side effects. Contraception. 1978;17(5):395–406. 1978/05/01. [PubMed] [Google Scholar]
- Yela 2006 {published data only}.Yela DA, Monteiro IM, Bahamondes LG, Castillo SD, Bahamondes MV. Weight variation in users of the levonorgestrel-releasing intrauterine system, of the copper IUD and of medroxyprogesterone acetate in Brazil. Revista da Associacao Medica Brasileira. 2006;52(1):32–6. doi: 10.1590/s0104-42302006000100019. [DOI] [PubMed] [Google Scholar]
- Zheng 1999 {published data only}.Zheng SR, Zheng HM, Qian SZ, Sang GW, Kaper RF. A randomized multicenter study comparing the efficacy and bleeding pattern of a single-rod (Implanon) and a six-capsule (Norplant) hormonal contraceptive implant. Contraception. 1999;60(1):1–8. doi: 10.1016/s0010-7824(99)00053-0. 1999/11/05. [DOI] [PubMed] [Google Scholar]
Additional references
- ACOG 2006.ACOG technical bulletin Use of hormonal contraception in women with coexisting medical conditions. Washington, D.C: American College of Obstetricians and Gynecologists; 2006. American College of Obstetricians and Gynecologists. [Google Scholar]
- Amatayakul 1980.Amatayakul K, Sivasomboon B, Thanangkul O. A study of the mechanism of weight gain in medroxyprogesterone acetate users. Contraception. 1980;22(6):605–22. doi: 10.1016/0010-7824(80)90087-6. [DOI] [PubMed] [Google Scholar]
- Berenson 2008.Berenson AB, Odom SD, Breitkopf CR, Rahman M. Physiologic and psychologic symptoms associated with use of injectable contraception and 20 μg oral contraceptive pills. American Journal of Obstetrics and Gynecology. 2008;199(4)(351e):1–12. doi: 10.1016/j.ajog.2008.04.048. 2008/07/05. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bonny 2004.Bonny AE, Britto MT, Huang B, Succop P, Slap GB. Weight gain, adiposity, and eating behaviors among adolescent females on depot medroxyprogesterone acetate (DMPA) Journal of Pediatric and Adolescent Gynecolog y. 2004;17(2):109–15. doi: 10.1016/j.jpag.2004.01.006. [DOI] [PubMed] [Google Scholar]
- CDC 2010.Centers for Disease Control and Prevention. Overweight and Obesity. http://www.cdc.gov/obesity/index.html (accessed 15 June 2010)
- Chin 2009.Chin JR, Swamy GK, Ostbye T, Bastian LA. Contraceptive use by obese women 1 year postpartum. Contraception. 2009;80(5):463–8. doi: 10.1016/j.contraception.2009.03.017. 2009/10/20. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curtis 2009.Curtis KM, Ravi A, Gaffield ML. Progestogen-only contraceptive use in obese women. Contraception. 2009;80(4):346–54. doi: 10.1016/j.contraception.2009.04.006. 2009/09/16. [DOI] [PubMed] [Google Scholar]
- Edelman 2009.Edelman AB. Contraceptive considerations in obese women. Contraception. 2009;80(6):583–90. doi: 10.1016/j.contraception.2009.08.001. [DOI] [PubMed] [Google Scholar]
- Faculty of Family Planning 2003 Faculty of Family Planning and Reproductive Health Care Clinical Effectiveness Unit. New product review (April 2003) desogestrel-only pill (Cerazette) Journal of Family Planning and Reproductive Health Care. 2003;29(3):162–4. doi: 10.1783/147118903101197593. [DOI] [PubMed] [Google Scholar]
- Flegal 2000.Flegal KM, Troiano RP. Changes in the distribution of body mass index of adults and children in the US population. International Journal of Obesity and Related Metabolic Disorders. 2000;24(7):807–18. doi: 10.1038/sj.ijo.0801232. [DOI] [PubMed] [Google Scholar]
- FWHC 2010.Feminist Women’s Health Center. Mini-pills (progesterone-only oral contraceptives) http://www.fwhc.org/birth-control/minipill.htm (accessed 15 Jun 2010)
- Gallo 2008.Gallo MF, Lopez LM, Grimes DA, Schulz KF, Helmerhorst FM. Combination contraceptives: effects on weight. Cochrane Database of Systematic Reviews. 2008;(4) doi: 10.1002/14651858.CD003987.pub3. [DOI] [PubMed] [Google Scholar]
- Goldberg 2007.Goldberg AB, Grimes DA. Injectable contraceptives. In: Hatcher RA, Trussell J, Nelson AL, Cates W, Stewart F, Kowal D, editors. Contraceptive Technology. 19th. New York: Ardent Media, Inc; 2007. pp. 157–79. [Google Scholar]
- Grimes 2005.Grimes DA, Shields WC. Family planning for obese women: challenges and opportunities. Contraception. 2005;72(1):1–4. doi: 10.1016/j.contraception.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Grimes 2007.Grimes DA, Lopez LM, Manion C, Schulz KF. Cochrane systematic reviews of IUD trials: lessons learned. Contraception. 2007;75(6 Suppl):S55–9. doi: 10.1016/j.contraception.2006.12.004. [DOI] [PubMed] [Google Scholar]
- Grimes 2010.Grimes DA, Lopez LM, O’Brien PA, Raymond EG. Progestin-only pills for contraception. Cochrane Database of Systematic Reviews. 2010;(1) doi: 10.1002/14651858.CD007541. [DOI] [PubMed] [Google Scholar]
- Higgins 2009.Higgins JPT, Green S, editors. The Cochrane Collaboration. John Wiley & Sons, Ltd; 2008. Cochrane Handbook for Systematic Reviews of Interventions 5.0.2 [updated September 2009] Available from www.cochrane-handbook.org. (accessed 04 Aug 2010) [Google Scholar]
- Lopez 2009.Lopez LM, Grimes DA, Schulz KF. Steroidal contraceptives: effect on carbohydrate metabolism in women without diabetes mellitus. Cochrane Database of Systematic Reviews. 2009;(4) doi: 10.1002/14651858.CD006133.pub3. [DOI] [PubMed] [Google Scholar]
- Lopez 2010.Lopez LM, Grimes DA, Chen-Mok M, Westhoff C, Edelman A, Helmerhorst FM. Hormonal contraceptives for contraception in overweight or obese women. Cochrane Database of Systematic Reviews. 2010 doi: 10.1002/14651858.CD008452. Issue submitted. [DOI] [PubMed] [Google Scholar]
- Najjar 1987.Najjar MF, Rowland M. Anthropometric reference data and prevalence of overweight, United States, 1976–80. Vital Health Stat 11. 1987;238:1–73. [PubMed] [Google Scholar]
- Ogden 2007.Ogden CL, Carroll MD, McDowell MA, Flegal KM. NCHS data brief no 1. Hyattsville (MD): National Center for Health Statistics; 2007. Obesity among adults in the United States- no change since 2003–2004. [PubMed] [Google Scholar]
- Paul 1997.Paul C, Skegg DC, Williams S. Depot medroxyprogesterone acetate. Patterns of use and reasons for discontinuation. Contraception. 1997;56(4):209–14. doi: 10.1016/s0010-7824(97)00140-6. 1997/12/31. [DOI] [PubMed] [Google Scholar]
- Pelkman 2001.Pelkman CL, Chow M, Heinback RA, Rolls BJ. Short-term effects of a progestional contraceptive drug on food intake, resting energy expenditure, and body weight in young women. American Journal of Clinical Nutrition. 2001;73(1):19–26. doi: 10.1093/ajcn/73.1.19. [DOI] [PubMed] [Google Scholar]
- Prentice 2006.Prentice AM. The emerging epidemic of obesity in developing countries. International Journal of Epidemiology. 2006;35(1):93–9. doi: 10.1093/ije/dyi272. [DOI] [PubMed] [Google Scholar]
- Raymond 2007.Hatcher RA, Trussell J, Nelson AL, Cates W Jr, Stewart F, Kowal D, editors. Contraceptive technology. 19th. New York: Ardent Media, Inc; 2007. Raymond EG Progestin-only pills; pp. 181–91. [Google Scholar]
- Schraudenbach 2009.Schraudenbach A, McFall S. Contraceptive use and contraception type in women by body mass index category. Womens Health Issues. 2009;19(6):381–9. doi: 10.1016/j.whi.2009.08.002. 2009/11/03. [DOI] [PubMed] [Google Scholar]
- Schulz 2002.Schulz KF, Grimes DA. Allocation concealment in randomised trials: defending against deciphering. Lancet. 2002;359(9306):614–8. doi: 10.1016/S0140-6736(02)07750-4. [DOI] [PubMed] [Google Scholar]
- Sivin 2003.Sivin I. Risks and benefits, advantages and disadvantages of levonorgestrel-releasing contraceptive implants. Drug Safety. 2003;26(5):303–35. doi: 10.2165/00002018-200326050-00002. 2003/03/26. [DOI] [PubMed] [Google Scholar]
- Strauss 2005.Strauss SE, Richardson WS, Glasziou P, Haynes RB. Evidence-based Medicine: How to Practice and Teach EBM. Third. New York: Churchill Livingstone; 2005. [Google Scholar]
- Trussell 2009.Trussell J, Schwarz EB, Guthrie K. Obesity and oral contraceptive pill failure. Contraception. 2009;79(5):334–8. doi: 10.1016/j.contraception.2008.11.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- UN 2007.United Nations Department of Economic and Social Affairs. World Contraceptive Use - 2007. http://www.un.org/esa/population/publications/contraceptive2007/WallChart_WCU2007_Data.xls (accessed 16 Oct 2009)
- Von Elm 2007.Von Elm E, Altman DG, Egger M, Pocock SJ, Gøtzsche PC, Vandebroucke JP, for the STROBE Initiative The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) Statement: Guidelines for reporting observational studies. Lancet. 2007;370(9596):1453–7. doi: 10.1016/S0140-6736(07)61602-X. [DOI] [PubMed] [Google Scholar]
- WebMD 2010.WebMD. Progestin-only hormonal methods (mini-pills, implants, and shots) http://www.webmd.com/sex/birth-control/progestin-only-hormonal-methods-mini-pills-shots (accessed 15 Jun 2010)
- WHO 2010.World Health Organization. Medical Eligibility Criteria for Contraceptive Use. (Fourth) http://www.who.int/reproductivehealth/publications/family_planning/9789241563888/en/index.html (accessed 16 Feb 2011) [PubMed]


