Abstract
Background
Immunohistochemical markers to assist in the diagnosis and classification of hyperplastic endometrial epithelial proliferations would be of diagnostic use.
Methods
To examine the possible utility of PAX2 as a marker of hyperplastic endometrium, cases of normal endometrium, simple and complex hyperplasia without atypia, atypical hyperplasia and FIGO grade 1 endometrioid carcinomas were stained for PAX2.
Results
206 endometrial samples were available for interpretation of PAX2 staining. The percent of cases with complete PAX2 loss (0% of cells staining) increased with increasing severity of hyperplasia: 0% of normal proliferative and secretory endometrium (n=28), 17.4% of simple hyperplasia (n=23), 59.0% of complex hyperplasia (n=83), 74.1% of atypical hyperplasia (n=54) and 73.3% of FIGO grade 1 endometrioid cancers (n=15). Partial loss of PAX2 expression did occur in normal endometrium (17.9%) but occurred in smaller proportions of tissue and was less frequent than in simple hyperplasia (47.8% with partial loss), complex hyperplasia (32.5%), atypical hyperplasia (22.2%) and FIGO grade 1 carcinomas (20.0%). Uniform PAX2 expression was rare in complex (8.4%) and atypical hyperplasia (3.7%) and carcinoma (6.7%). When evaluating loss of PAX2 in histologically normal endometrium adjacent to lesional endometrium in a given case, statistically significant differences in staining were observed for simple hyperplasia (p=0.011), complex hyperplasia (p< 0.001), atypical hyperplasia (p<0.001) and FIGO grade 1 endometrioid cancer (p=0.003).
Conclusion
In summary, PAX2 loss appears to occur early in the development of endometrial pre-cancers and may prove useful in some settings as a diagnostic marker in determining normal endometrium from complex and atypical hyperplasia and low grade carcinomas. However, it is not useful in distinguishing between these diagnostic categories.
Keywords: Endometrial hyperplasia, PAX2, immunohistochemistry, endometrial neoplasia, biomarkers, endometrium
Introduction
The appropriate classification of endometrial proliferations is an area of both diagnostic challenge and academic debate. 1-10,8, 11-14 The World Health Organization (WHO) classification categorizes lesions into hyperplasia without atypia (simple or complex) and hyperplasia with atypia (simple or complex) based on the degree of architectural crowding and complexity and the presence of cytologic atypia. 15 Atypical hyperplasia has the highest risk of progression to or concurrent endometrial carcinoma but unfortunately is also the category with the highest diagnostic disagreement. 6, 16-18
Because of the variability in diagnosing pre-cancerous endometrial lesions, new markers are needed to support the most appropriate diagnostic classification. PAX2 belongs to a family of pair box genes that are involved in transcriptional regulation during embryogenesis.19, 20 PAX2 expression has been implicated in the normal development of the central nervous system, eye, ear and genitourinary tract. Its expression in the derivatives of the Wolffian ducts and the kidneys has been well characterized and it can be used as a diagnostic marker of renal cell carcinoma, Wilms' tumor, and nephrogenic adenoma. 21-24 More recently, its expression as a marker of the Mullerian duct derivatives (fallopian tubes, uterus, cervix and upper vagina) has been described. 25 According to Tong et al, normal endometrial glandular cells have nuclear expression of PAX2 by immunohistochemistry. Interestingly, experiments in cell culture suggest that the PAX2 gene is activated by estrogen and tamoxifen in endometrial carcinoma cell lines but not in normal endometrium, implicating PAX2 as a possible key regulator in endometrial carcinogenesis. 26
Monte et al recently described loss of PAX2 expression in endometrial pre-cancers using the endometrial intraepithelial neoplasia (EIN) diagnostic scheme and suggested its role as a tumor suppressor. 27 However, immunohistochemical studies looking at PAX2 expression using the WHO classification scheme of endometrial lesions, are lacking.
We examined PAX2 expression in cases of normal endometrium, simple hyperplasia and complex hyperplasia without atypia, atypical hyperplasia and FIGO grade 1 endometrioid carcinoma diagnosed using WHO criteria.
Materials and Methods
Study Population
A subsample of index endometrial biopsies from women with complex and atypical endometrial hyperplasia enrolled in a large endometrial hyperplasia cohort study28 were evaluated for PAX2 protein expression by immunohistochemistry (IHC). Methodologies for subject selection in the cohort study have been previously described.28,29 Of the 185 cases of complex and atypical hyperplasia, 140 had tissue available for PAX2 staining. 66 of 83 potential additional cases of normal proliferative (n=18), normal secretory (n=10), simple hyperplasia without atypia (n=23) and FIGO grade 1 endometrioid endometrial cancer (n=15) from the University of Washington Medical Center (UWMC) Department of Pathology database were identified and had sufficient tissue for additional immunohistochemistry. Insufficient cases with a diagnosis of simple hyperplasia with atypia were identified to include in this study.
Pathology Review
All eligible cases were reviewed independently, and in random order, by two University of Washington pathologists (RG, KA), using standard International Society of Gynecological Pathologists and World Health Organization criteria.15 The pathologists were masked to the original diagnosis. If the two pathologists did not agree on the diagnosis a third masked pathologist (DJ) reviewed the case and the most common diagnosis was used. If all three pathologists disagreed, the diagnosis was assigned by the senior pathologist (RG) or consensus review.
Immunohistochemical analysis
IHC stains were performed on unstained slides cut from formalin fixed, paraffin embedded tissue blocks. All tissues were deparaffinized followed by blockade of endogenous peroxidases and antigen retrieval using Antigen Unmasking Solution (Vector; USA). PAX-2 (Clone Z-RX2, dilution 1:100, 15 minute pre-treatment with EDTA, Zymed, CA) antibody stains were performed by the University of Washington Medical Center immunohistochemistry research laboratory. The slides were then counterstained in hematoxylin, dehydrated, and mounted. Positive and negative controls were performed to ensure the staining procedure was successful.
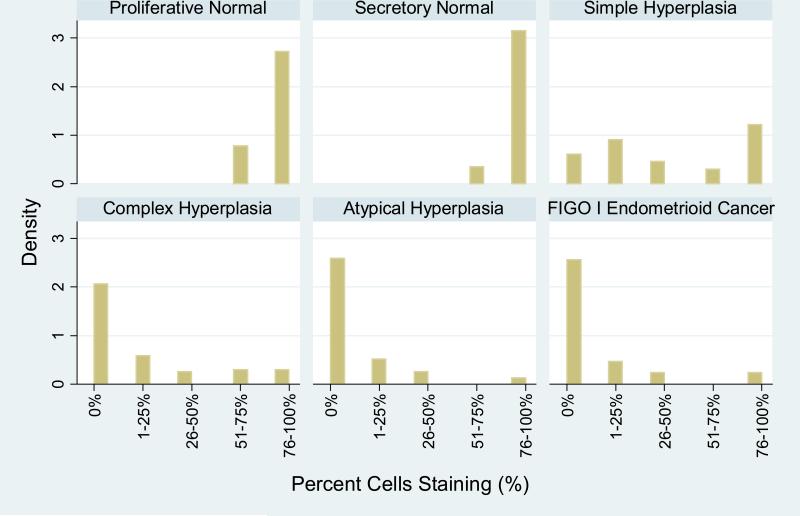
One of two study pathologists (KA, RG) scored the antibody staining in both the lesional tissue and the adjacent normal tissue (in cases that were not solely normal endometrium). Our initial scoring system used ranged of PAX2 expression as follows: 0%, 1-25%, 26-50%, 51-75%, or 76-100% (see Figure 1). Positive PAX2 expression was only considered with a nuclear staining pattern. After reviewing the distribution of PAX2 expression in the different diagnostic categories it became clear that PAX2 loss was more characteristic of hyperplasia and carcinoma. Therefore, we translated our findings for PAX2 expression into PAX2 loss as follows: 1) Complete loss (0% cells staining), 2) Partial loss (1-75% cells staining) and 3) Minimal to no loss (76-100% cells staining).
Figure 1.
Percent glandular cells with PAX2 staining.
Data Collection
Additional available information regarding medical and family history, demographic, reproductive, and physical characteristics, including height and weight at the time of the index biopsy, as well as recent use of hormones (dispensed within the six months prior to biopsy for at least 2 months) was collected from the medical record.
Statistical Analyses
We computed the frequency of PAX2 immunohistochemical marker loss by normal endometrium phase (proliferative and secretory) and lesional endometrium type (simple, complex, and atypical hyperplasia, and FIGO grade 1 endometrioid cancer) and compared the frequency of PAX2 loss for each diagnosis to that of proliferative normal endometrium using Fisher's exact test. We also compared the frequency of PAX2 loss between lesional endometrium and adjacent histologically normal endometrium within women (paired data) separately for simple, complex, and atypical hyperplasia and FIGO grade 1 endometroid cancer diagnosis using the Stuart-Maxwell Test for homogeneity of marginal distributions. We repeated all analyses to exclude women who had used hormonal therapy prior to index biopsy (oral contraceptives, combined hormone replacement therapy, estrogen only or progestin only therapy, or tamoxifen) or who were confirmed or suspected members of a single family. Additionally, among cases of complex and atypical hyperplasia, we repeated the analyses stratifying by 5-year increments of index year of biopsy. In this study, body mass index (BMI) was calculated as kg/m2. For the 17 UWMC cases for which weight was only available, weight ≥198 pounds was categorized as a BMI >30 kg/m2; weight ≤128 pounds was categorized <25 kg/m2; weight greater than 128 and less than198 pounds was categorized as missing. All analyses were performed using STATA 10.0 (STATA Corporation, College Station, Texas) with the level of significance set at α=0.05.
Results
Of the 207 cases eligible for this study, 4 cases were excluded due to being unable to complete PAX2 IHC analyses (1 simple hyperplasia, 1 complex hyperplasia, 2 atypical hyperplasia). A total of 203 women were included in our analyses. The average age of women in this study was 54 years, the majority were Caucasian; 37.9% were younger than 50 years of age, 10.2% were smokers, 26.8% were nulliparous, and 52.4% had BMIs of 30 kg/m2 or greater. Women with endometrial hyperplasia with atypia and endometrial carcinoma were more likely to be older and to have diabetes and the majority of women with normal proliferative or secretory endometrium were under the age of 50. Recent use of hormone therapies of any kind was more common in the women with complex hyperplasia (27%) than in other diagnostic categories (Table 1).
Table 1.
Patient Characteristics
| Normal Proliferative1 N=18 N(%) | Normal Secretory1 N=10 N(%) | Simple1 N=23 N(%) | Hyperplasia Complex2 N=83 N(%) | Atypia2 N=54 N(%) | Carcinoma1 N=15 N(%) | |
|---|---|---|---|---|---|---|
| Age (years) | ||||||
| ≤39 | 4 (22.2) | 4 (40.0) | 2 (8.7) | 8 (9.6) | 4 (7.4) | 1 (6.7) |
| 40-49 | 10 (55.6) | 5 (50.0) | 8 (34.8) | 22 (26.5) | 9 (16.7) | 0 (0.0) |
| 50-59 | 3 (16.7) | 1 (10.0) | 11 (47.8) | 22 (26.5) | 21 (38.9) | 7 (46.7) |
| 60-69 | 0 (0.0) | 0 (0.0) | 2 (8.7) | 21 (25.3) | 8 (14.8) | 4 (26.7) |
| ≥70 | 1 (5.6) | 0 (0.0) | 0 (0.0) | 10 (12.1) | 12 (22.2) | 3 (20.0) |
| Diabetes3 | 0 (0.0) | 0 (0.0) | 1 (4.4) | 7 (9.0) | 5 (10.0) | 4 (26.7) |
| Breast/Colon Cancer3 | 1 (5.6) | 0 (0.0) | 2 (8.7) | 2 (2.6) | 4 (8.0) | 0 (0.0) |
| Current Smoker3 | 1 (5.6) | 3 (30.0) | 1 (5.0) | 8 (11.0) | 4 (8.0) | 2 (13.3) |
| BMI (kg/m2)3 | ||||||
| <25 | 3 (21.4) | 3 (37.5) | 2 (9.5) | 20 (25.6) | 13 (26.5) | 1 (6.7) |
| 25-29.9 | 4 (28.6) | 2 (25.0) | 6 (28.6) | 18 (23.1) | 13 (26.5) | 3 (20.0) |
| ≥30 | 7 (50.0) | 3 (37.5) | 13 (61.9) | 40 (51.3) | 23 (46.9) | 11 (73.3) |
| Nulliparous3 | 6 (35.3) | 3 (30.0) | 10 (47.6) | 14 (18.2) | 15 (30.0) | 3 (20.0) |
| Oral contraceptive4 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 3 (3.6) | 0 (0.0) | 0 (0.0) |
| HT4,5 | 0 (0.0) | 0 (0.0) | 0 (0.0) | 9 (10.8) | 5 (9.3) | 2 (13.3) |
| Unopposed Estrogen4,6 | 0 (0.0) | 0 (0.0) | 1 (4.4) | 12 (14.5) | 3 (5.6) | 0 (0.0) |
| Progestin only4,7 | 0 (0.0) | 0 (0.0) | 2 (8.7) | 2 (2.4) | 1 (1.9) | 1 (6.7) |
| Index biopsy year | ||||||
| 1985-1989 | 4 (4.8) | 0 (0.0) | ||||
| 1990-1994 | 21 (25.3) | 13 (24.1) | ||||
| 1995-1999 | 24 (28.9) | 28 (51.9) | ||||
| 2000-2004 | 34 (41.0) | 13 (24.1) | ||||
| 2005-2009 | 18 (100.0) | 10 (100.0) | 23 (100.0) | 0 (0.0) | 0 (0.0) | 15 (100.0) |
Abbreviations: BMI=body mass index in kg per meters squared, HT = postmenopausal estrogen and progestin therapy
University of Washington Medical Center (UWMC) specimens
Endometrial Cohort (ECO) Study specimens
For ECO cohort, missing data on: diabetes - 9; history of breast/colon cancer, BMI, parity - 10; smoking - 14. For UWMC cohort, missing data on; smoke and parity - 3; BMI – 8, only weight available for 17 UWMC women, weight ≥198 categorized as BMI >30 kg/m2 (4 women), weight ≤128 categorized as <25 kg/m2 (6 women) and weight between 128 and 198 pounds categorized as BMI missing (7 women)
Dispensed in the six months preceding diagnosis of endometrial hyperplasia.
HT (estrogen plus progestin for 2 months or more and the progestin was dispensed for at least 1/3 of the time that estrogen was dispensed)
Unopposed estrogen = postmenopausal estrogen therapy (estrogen alone or estrogen plus progestin for 2 months or more and the progestin was dispensed less than 1/3 of the time that estrogen was dispensed)
Dispensed for at least 2 months.
PAX2 staining was nuclear in the glandular epithelium. The distribution of the percent cells with PAX2 expression in cases of normal proliferative, normal secretory, simple and complex hyperplasia without atypia, atypical hyperplasia and FIGO grade 1 endometrioid carcinoma varied by endometrial histology (Figure 1). In general, both normal proliferative and normal secretory endometrium had high levels of PAX2 expression, while simple hyperplasia had variable expression and complex and atypical hyperplasias and carcinoma were more likely to have complete loss of staining. Expression patterns in complex and atypical hyperplasia were also similar to the FIGO grade 1 endometrioid carcinomas. As described in methods above, PAX2 expression was then categorized into three categories: 1) Complete loss (0% cells staining), 2) Partial loss (1-76% cells staining) and 3) Minimal to no loss (76-100% cells staining).
The frequency of PAX2 loss using the three expression pattern categories varied by diagnosis (Table 2). Specifically, the percent of cases with complete PAX2 loss increased with increasing severity of hyperplasia: 0% of normal proliferative and secretory endometrium (n= 28), 17.4% of simple hyperplasia (n=23), 59.0% of complex hyperplasia (n=83), 74.1% of atypical hyperplasia (n=54 ) and 73.3% of FIGO grade 1 endometrioid cancers (n=15) had complete PAX2 loss. Partial loss of PAX2 expression did occur in normal endometrium (17.9%) but occurred in smaller proportions of tissue and was less frequent than in simple hyperplasia (47.8% with partial loss), complex hyperplasia (32.5%), atypical hyperplasia (22.2%) and FIGO grade 1 carcinomas (20.0%). No to minimal PAX2 loss was rare in complex (8.4%) and atypical hyperplasia (3.7%) and carcinoma (6.7%). There were statistically significant differences in PAX2 loss in complex hyperplasia, atypical hyperplasia and carcinomas when compared to separate normal proliferative cases (P< 0.001). Figure 2 shows examples of PAX2 staining in normal and lesional endometrium.
Table 2.
PAX2 Loss in Cases of Histologically Normal and Abnormal Endometrial Tissue using WHO Diagnostic Categories
| Percent PAX2 Loss | |||||
|---|---|---|---|---|---|
| Diagnostic Category | No/Minimal Loss | Partial Loss | Complete Loss | ||
| N | n (%) | n (%) | n (%) | P 2 | |
| Proliferative | 18 | 14 (77.8) | 4 (22.2) | 0 (0.0) | reference |
| Secretory | 10 | 9 (90.0) | 1 (10.0) | 0 (0.0) | 0.63 |
| Simple Hyperplasia | 23 | 8 (34.8) | 11 (47.8) | 4 (17.4) | 0.01 |
| Complex Hyperplasia1 | 84 | 7 (8.4) | 27 (32.5) | 49 (59.0) | <0.001 |
| Atypical Hyperplasia1 | 56 | 2 (3.7) | 12 (22.2) | 40 (74.1) | <0.001 |
| FIGO Grade 1 | |||||
| Endometrioid cancer | 15 | 1 (6.7) | 3 (20.0) | 11 (73.3) | <0.001 |
Abbreviations: WHO=World Health Organization; PAX2=Paired Box 2 gene; FIGO 1=International Federation of Gynecology and Obstetrics Grade 1
PAX2 data missing for 1 woman with complex hyperplasia and 2 women with atypical hyperplasia.
Fisher's Exact test used to calculate P values.
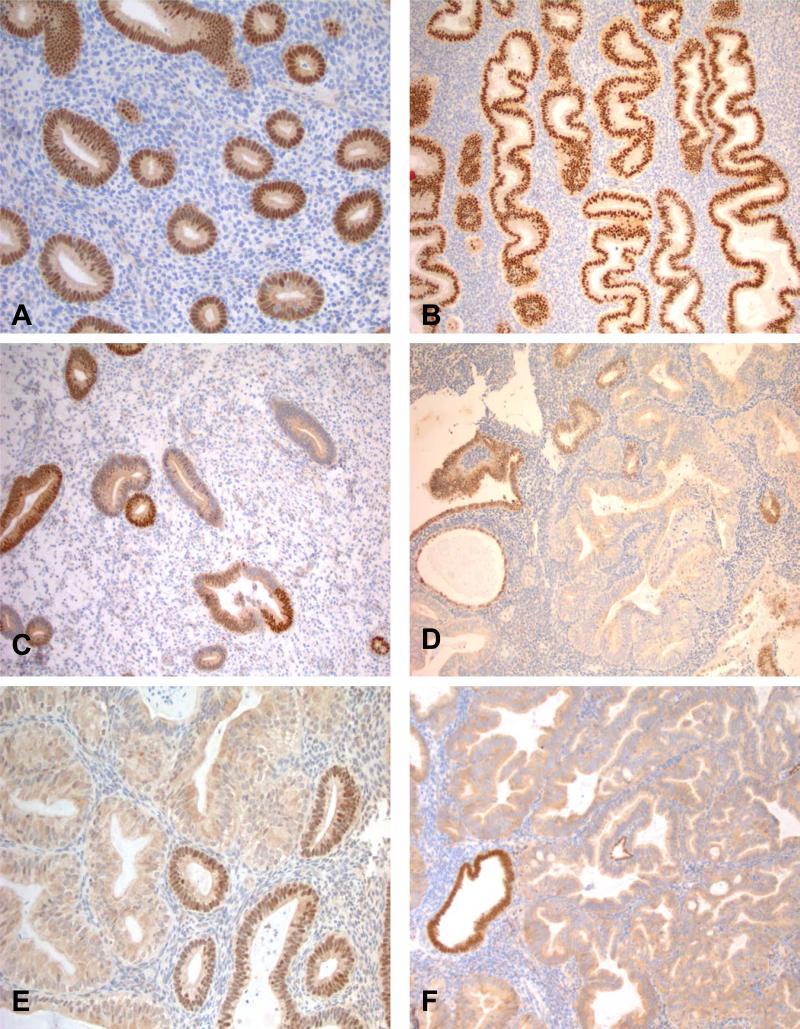
Figure 2.
Strong and uniform nuclear PAX2 expression was characteristic of normal proliferative endometrium (A) and secretory endometrium (B). Occasional partial loss of PAX2 expression did occur in background histologically normal endometrium (C). Complete loss of nuclear PAX2 expression was much more characteristic of complex hyperplasia (D), atypical hyperplasia (E) and FIGO grade 1 endometrioid carcinoma (F).
PAX2 loss was also evaluated in 144 cases in which histologically normal endometrium adjacent to lesional endometrium was available (Table 3). The difference in staining between lesional endometrium and adjacent histologically normal endometrium was statistically significant for all diagnoses considered: simple hyperplasia (p=0.011), complex hyperplasia (p<0.001), atypical hyperplasia (p<0.001) and FIGO grade 1 endometrioid cancer (p=0.003). Most notable in this paired analysis, PAX2 loss was the same or greater in lesional tissue than adjacent normal endometrium and no matched adjacent normal tissue had complete PAX2 loss. The pattern of complete PAX2 loss among lesional tissue and minimal to no PAX2 loss among adjacent histologically normal endometrium appeared more evident with increasing severity of diagnosis. Overall, partial PAX2 loss occurred in 16.7% of these matched normals (24/144 cases), a finding very similar to separate normal controls (17.5%).
Table 3.
Frequency of PAX2 Loss in Abnormal Endometrium verses Matched Adjacent Normal
| PAX2 loss in Adjacent Normal Endometrium | PAX2 loss in Simple Hyperplasia | ||||
|---|---|---|---|---|---|
| No/minimal loss | Partial loss | Complete loss | Total | ||
| No/minimal loss | 6 | 5 | 4 | 15 | P=0.011 |
| Partial loss | 0 | 4 | 0 | 4 | |
| Complete loss | 0 | 0 | 0 | 0 | |
| Total | 6 | 9 | 4 | 19 | |
| PAX2 loss in Complex Hyperplasia | |||||
|---|---|---|---|---|---|
| No/minimal loss | Partial loss | Complete loss | Total | ||
| No/minimal loss | 3 | 2 | 32 | 56 | P<0.001 |
| Partial loss | 0 | 4 | 12 | 16 | |
| Complete loss | 0 | 0 | 0 | 0 | |
| Total | 3 | 25 | 44 | 72 | |
| PAX2 loss in Atypical Hyperplasia | |||||
|---|---|---|---|---|---|
| No/minimal loss | Partial loss | Complete loss | Total | ||
| No/minimal loss | 1 | 7 | 29 | 37 | P<0.001 |
| Partial loss | 0 | 0 | 4 | 4 | |
| Complete loss | 0 | 0 | 0 | 0 | |
| Total | 1 | 7 | 33 | 41 | |
| PAX2 loss in FIGO Grade 1 Endometrial Cancer | |||||
|---|---|---|---|---|---|
| No/minimal loss | Partial loss | Complete loss | Total | ||
| No/minimal loss | 0 | 2 | 10 | 12 | P=0.003 |
| Partial loss | 0 | 0 | 0 | 0 | |
| Complete loss | 0 | 0 | 0 | 0 | |
| Total | 0 | 2 | 10 | 12 | |
Stuart-Maxwell Test used to calculate P values
When the analyses were repeated to exclude 43 women taking hormonal therapies at baseline, including oral contraceptives, combined hormonal replacement therapy, estrogen only, progestin only or tamoxifen treatment, our results did not substantially vary. This was also true after we excluded nine women who were confirmed or suspected members of the same family. Additionally, we found similar results when stratifying by 5-year index biopsy periods among women diagnosed with complex and atypical hyperplasia.
Discussion
We found that PAX2 loss in endometrial hyperplasia occurred early in the spectrum of hyperplasia and became more frequent and complete with increasing severity of diagnosis, using the WHO diagnostic categories. In addition, we found that complete PAX2 loss did not occur in normal proliferative or secretory endometrium but was common in complex and atypical hyperplasia as well as FIGO grade 1 endometrial carcinoma. While partial PAX2 loss did occur in cases of histologically normal endometrium, the total percent of cells with loss was much lower in normal than in hyperplastic lesions. However, because loss of expression was so frequent in hyperplastic tissue, it was not useful as a diagnostic marker in distinguishing between simple, complex and atypical endometrial hyperplasia. These results suggest that PAX2 loss occurs early in the biologic continuum of endometrial pre-cancers and may only be clinically useful in situations where it is challenging to determine whether an endometrial biopsy represents entirely normal or neoplastic tissue.
PAX2 expression in the endometrium was first described in endometrial cancer cell lines by Wu et al.26 In their study, tamoxifen and estrogen were found to activate PAX2 mRNA expression in endometrial cancer cell lines but not in normal endometrial samples. This increased expression was associated with cancer-linked hypomethylation of the PAX2 promoter. These results appeared to offer a mechanism to explain the increased incidence of endometrial cancers in women treated with tamoxifen or unopposed estrogen. However, normal PAX2 protein expression by IHC in paraffin embedded human endometrial samples had not been systematically evaluated until Tang et al described its expression by IHC in the gynecologic tract in 2007. In contrast to the initial data in cell lines, they noted that PAX2 was normally expressed in Müllerian-derived epithelium of the endometrium, fallopian tube and cervical glands but not in the non-Müllerian derived ovarian surface epithelium.
A study published to date only in abstract form by Cao and colleagues appears to support our findings for PAX2 using the WHO scheme for endometrial hyperplasia. 30 Their study evaluated PAX2 staining by IHC in 71 cases of endometrial carcinoma, 31 cases of atypical hyperplasia, and 22 cases of non-atypical hyperplasia (complex and simple). Similar to our study, they found PAX2 protein expression had progressive loss along the spectrum from hyperplasia to endometrioid cancers of increasing FIGO grade.
One of the major challenges with endometrial hyperplasia is the poor inter-observer diagnostic reproducibility of the WHO diagnostic categories. Unfortunately, we did not find PAX2 to be useful in distinguishing between WHO diagnostic categories. While we did not specifically examine how the inter-observer diagnostic variability of endometrial hyperplasia would be affected by concurrent review of a supplemental PAX2 stain, given the very high frequency of complete loss in complex hyperplasia with and without atypia and FIGO grade 1 cancers, it is unlikely to decrease variability in these diagnoses. However, complete (not partial) PAX2 loss in an endometrial lesion is perhaps better considered a potential marker of endometrial neoplasia in general. One could also argue, given the problems reproducibly distinguishing between these diagnostic categories using histologic features, in addition to the lack of robust biological markers distinguishing between them, that the division between these diagnostic categories is biologically arbitrary and as such may remain a gray zone in pathology as they are currently defined.
More recently, Monte et al have described PAX2 staining in endometrial lesions using the EIN scheme. 27 In their study, they used only 1% of glands with loss as a threshold for PAX2 loss. Using this very low threshold, they found loss of PAX2 expression in 36% of normal, 71% of EIN and 77% of endometrial adenocarcinomas compared to PTEN loss in 49% of normal, 44% of EIN and 68% of endometrial adenocarcinomas. The higher frequency of loss in normal endometrium in their study compared with ours is not surprising given their low threshold for PAX2 loss. Although the differences in thresholds used does not allow us to directly compare the degree of PAX2 loss and the frequency of more complete PAX2 loss in their data set with ours, they did note that the proportion of glands with PAX2 loss in normal proliferative endometrium was typically only a few glands in comparison with the high proportion of glands with loss in EIN or cancer. We had similar findings, using the WHO scheme. These results suggest that while there may be isolated glands with loss of PAX2 in histologically normal endometrium, histologically recognizable pre-cancers more frequently have complete to near complete PAX2 intra-lesional loss. Additional studies looking specifically at complete loss of PAX2 expression (using a higher threshold than 1% ) in the EIN scheme would be useful to further evaluate the utility of complete PAX2 loss as a marker of EIN.
Research to identify IHC markers in endometrial pre-cancers has yielded few, if any, clinically relevant and robust diagnostic markers. Probably the most well-established of these is PTEN, a tumor suppressor involved in controlling cell proliferation in the endometrium. One problem with PTEN is that it is normally expressed in proliferative endometrial glands and stroma but expression decreases in the normal secretory cycle, resulting in variable staining. 31 When considering the use of PAX2 and PTEN as a diagnostic marker, one advantage of PAX2 is that it is less frequently lost in both normal proliferative and secretory endometrium than PTEN. PAX2 staining may also be easier to evaluate because of its distinct nuclear staining (while PTEN expression is both cytoplasmic and nuclear).
The strength of this study is its sample size and careful description of the population studied. We obtained detailed information regarding BMI and comorbid conditions and the similarity in frequencies across diagnostic categories suggest that these factors did not drive our findings. One limitation of our study is that the methodology used initially to score PAX2 expression used ranges rather than continuous variables (as shown in Figure 1). When it became clear that PAX2 loss was a better marker for endometrial hyperplasias/carcinomas than expression, this resulted in tissue with 76-100% PAX2 expression (or 0-25% PAX2 loss) being categorized as a case with “no/minimal loss.” Although one could argue that ranges for IHC scoring may be more reproducible and practical than an exact percent point threshold, the finding of more complete PAX2 loss with increasing severity of the lesion was clear regardless of exact thresholds used. An additional limitation of this study is the relatedly small number of separate normal endometrium cases (n=28). However, we also scored PAX2 in the matched normal endometrium in 144 cases with lesional endometrium and had very similar results.
In summary, loss of PAX2 IHC staining occurs early and often in the spectrum of endometrial pre-cancers using the WHO diagnostic categories. PAX2 loss appears to be a sensitive marker of endometrial pre-cancers and carcinoma and is rarely completely lost in histologically normal controls. As such, it may prove to be useful diagnostically when the neoplastic nature of a given sample is in question. However, PAX2 is not likely to be diagnostically useful in distinguishing complex from atypical hyperplasia and endometrial carcinoma. Research using a combination of biomarkers, including PAX2 and PTEN, to predict risk of progression to carcinoma and response to progestin therapy is a potentially important future area of investigation.
Acknowledgments
Funding Sources:
NICHD 5 R01 HD44813-02
REFERENCES
- 1.Beutler HK, Dockerty MB, Randall LM. Precancerous lesions of the endometrium. Am J Obstet Gynecol. 1963;86:433–43. doi: 10.1016/0002-9378(63)90167-4. [DOI] [PubMed] [Google Scholar]
- 2.Campbell PE, Barter RA. The significance of a typical endometrial hyperplasia. J Obstet Gynaecol Br Commonw. 1961;68:668–72. doi: 10.1111/j.1471-0528.1961.tb02789.x. [DOI] [PubMed] [Google Scholar]
- 3.Gore H, Hertig AT. Carcinoma in situ of the endometrium. Am J Obstet Gynecol. 1966;94(1):134–55. doi: 10.1016/0002-9378(66)90391-7. [DOI] [PubMed] [Google Scholar]
- 4.Gusberg SB, Kaplan AL. Precursors of Corpus Cancer. Iv. Adenomatous Hyperplasia as Stage O Carcinoma of the Endometrium. Am J Obstet Gynecol. 1963;87:662–78. [PubMed] [Google Scholar]
- 5.Hendrickson MKR. Surgical Pathology of the Uterine Corpus Vol. WB Saunders Co.; Philadelphia: 1980. [Google Scholar]
- 6.Kurman RJ, Kaminski PF, Norris HJ. The behavior of endometrial hyperplasia. A long-term study of “untreated” hyperplasia in 170 patients. Cancer. 1985;56(2):403–12. doi: 10.1002/1097-0142(19850715)56:2<403::aid-cncr2820560233>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 7.Tavassoli F, Kraus FT. Endometrial lesions in uteri resected for atypical endometrial hyperplasia. Am J Clin Pathol. 1978;70(5):770–9. doi: 10.1093/ajcp/70.5.770. [DOI] [PubMed] [Google Scholar]
- 8.Zaino RJ, Kauderer J, Trimble CL, et al. Reproducibility of the diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106(4):804–11. doi: 10.1002/cncr.21649. [DOI] [PubMed] [Google Scholar]
- 9.Mutter GL. Endometrial intraepithelial neoplasia (EIN): will it bring order to chaos? The Endometrial Collaborative Group. Gynecol Oncol. 2000;76(3):287–90. doi: 10.1006/gyno.1999.5580. [DOI] [PubMed] [Google Scholar]
- 10.Mutter GL, Zaino RJ, Baak JP, Bentley RC, Robboy SJ. Benign endometrial hyperplasia sequence and endometrial intraepithelial neoplasia. Int J Gynecol Pathol. 2007;26(2):103–14. doi: 10.1097/PGP.0b013e31802e4696. [DOI] [PubMed] [Google Scholar]
- 11.Allison KH, Reed SD, Voigt LF, et al. Diagnosing endometrial hyperplasia: why is it so difficult to agree? Am J Surg Pathol. 2008;32(5):691–8. doi: 10.1097/PAS.0b013e318159a2a0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bergeron C, Nogales FF, Masseroli M, et al. A multicentric European study testing the reproducibility of the WHO classification of endometrial hyperplasia with a proposal of a simplified working classification for biopsy and curettage specimens. Am J Surg Pathol. 1999;23(9):1102–8. doi: 10.1097/00000478-199909000-00014. [DOI] [PubMed] [Google Scholar]
- 13.Kendall BS, Ronnett BM, Isacson C, et al. Reproducibility of the diagnosis of endometrial hyperplasia, atypical hyperplasia, and well-differentiated carcinoma. Am J Surg Pathol. 1998;22(8):1012–9. doi: 10.1097/00000478-199808000-00012. [DOI] [PubMed] [Google Scholar]
- 14.Skov BG, Broholm H, Engel U, et al. Comparison of the reproducibility of the WHO classifications of 1975 and 1994 of endometrial hyperplasia. Int J Gynecol Pathol 1997. 16(1):33–7. doi: 10.1097/00004347-199701000-00006. [DOI] [PubMed] [Google Scholar]
- 15.Tavassoli FADPE, editor. World Health Organization Classification of Tumors: Pathology and Genetics of Tumours of the Breast and Female Genital Organs. IARC Press; Lyon: 2003. [Google Scholar]
- 16.Horn LCSU, Bilek K, Hentschel B, Einenkel J. Risk of progression in complex and atypical endometrial hyperplasia: clinicopathologic analysis in cases with and without progestin treatment. International Journal of Gynecological Cancer. 2004;14:348–53. doi: 10.1111/j.1048-891x.2004.014220.x. [DOI] [PubMed] [Google Scholar]
- 17.Trimble CL, Kauderer J, Zaino R, et al. Concurrent endometrial carcinoma in women with a biopsy diagnosis of atypical endometrial hyperplasia: a Gynecologic Oncology Group study. Cancer. 2006;106(4):812–9. doi: 10.1002/cncr.21650. [DOI] [PubMed] [Google Scholar]
- 18.Lacey JV, Jr., Mutter GL, Nucci MR, et al. Risk of subsequent endometrial carcinoma associated with endometrial intraepithelial neoplasia classification of endometrial biopsies. Cancer. 2008;113(8):2073–81. doi: 10.1002/cncr.23808. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Gruss P, Walther C. Pax in development. Cell. 1992;69(5):719–22. doi: 10.1016/0092-8674(92)90281-g. [DOI] [PubMed] [Google Scholar]
- 20.Lang D, Powell SK, Plummer RS, Young KP, Ruggeri BA. PAX genes: roles in development, pathophysiology, and cancer. Biochem Pharmacol. 2007;73(1):1–14. doi: 10.1016/j.bcp.2006.06.024. [DOI] [PubMed] [Google Scholar]
- 21.Kuschert S, Rowitch DH, Haenig B, McMahon AP, Kispert A. Characterization of Pax-2 regulatory sequences that direct transgene expression in the Wolffian duct and its derivatives. Dev Biol. 2001;229(1):128–40. doi: 10.1006/dbio.2000.9971. [DOI] [PubMed] [Google Scholar]
- 22.Lechner MS, Dressler GR. The molecular basis of embryonic kidney development. Mech Dev. 1997;62(2):105–20. doi: 10.1016/s0925-4773(97)00667-9. [DOI] [PubMed] [Google Scholar]
- 23.Tong GX, Melamed J, Mansukhani M, et al. PAX2: a reliable marker for nephrogenic adenoma. Mod Pathol. 2006;19(3):356–63. doi: 10.1038/modpathol.3800535. [DOI] [PubMed] [Google Scholar]
- 24.Torres M, Gomez-Pardo E, Dressler GR, Gruss P. Pax-2 controls multiple steps of urogenital development. Development. 1995;121(12):4057–65. doi: 10.1242/dev.121.12.4057. [DOI] [PubMed] [Google Scholar]
- 25.Tong GX, Chiriboga L, Hamele-Bena D, Borczuk AC. Expression of PAX2 in papillary serous carcinoma of the ovary: immunohistochemical evidence of fallopian tube or secondary Mullerian system origin? Mod Pathol. 2007;20(8):856–63. doi: 10.1038/modpathol.3800827. [DOI] [PubMed] [Google Scholar]
- 26.Wu H, Chen Y, Liang J, et al. Hypomethylation-linked activation of PAX2 mediates tamoxifen-stimulated endometrial carcinogenesis. Nature. 2005;438(7070):981–7. doi: 10.1038/nature04225. [DOI] [PubMed] [Google Scholar]
- 27.Monte NM, Webster KA, Neuberg D, Dressler GR, Mutter GL. Joint loss of PAX2 and PTEN expression in endometrial precancers and cancer. Cancer Res. 2010;70(15):6225–32. doi: 10.1158/0008-5472.CAN-10-0149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Reed SD, Newton KM, Clinton WL, et al. Incidence of endometrial hyperplasia. Am J Obstet Gynecol. 2009;200(6):678, e1–6. doi: 10.1016/j.ajog.2009.02.032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Reed SDVL, Newton KM, Garcia RH, Allison HK, Epplein M, Jordan D, Swisher E, Weiss NS. Progestin therapy of complex endometrial hyperplasia with and without atypia. Obstet Gynecol. 2009;113(3):655–62. doi: 10.1097/AOG.0b013e318198a10a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cao DGA, Vang RS, Kurman RJ, Ronnett BM. Expression of PAX2 in endometrial hyerplasia and carcinomas: immunohistochemical analysis of 136 cases. Modern Pathology. 2008;20(Suppliment 2) Abstract #869. [Google Scholar]
- 31.Mutter GL, Lin MC, Fitzgerald JT, Kum JB, Eng C. Changes in endometrial PTEN expression throughout the human menstrual cycle. J Clin Endocrinol Metab. 2000;85(6):2334–8. doi: 10.1210/jcem.85.6.6652. [DOI] [PubMed] [Google Scholar]