Abstract
Piezosurgery, or the use of piezoelectric devices, is being applied increasingly in oral and maxillofacial surgery. The main advantages of this technique are precise and selective cuttings, the avoidance of thermal damage, and the preservation of soft-tissue structures. Through the application of piezoelectric surgery, implant-site preparation, bone grafting, sinus-floor elevation, edentulous ridge splitting or the lateralization of the inferior alveolar nerve are very technically feasible. This clinical overview gives a short summary of the current literature and outlines the advantages and disadvantages of piezoelectric bone surgery in implant dentistry. Overall, piezoelectric surgery is superior to other methods that utilize mechanical instruments. Handling of delicate or compromised hard- and soft-tissue conditions can be performed with less risk for the patient. With respect to current and future innovative surgical concepts, piezoelectric surgery offers a wide range of new possibilities to perform customized and minimally invasive osteotomies.
Keywords: implantology, piezoelectric device, piezosurgery, maxillary sinus elevation, bone grafting, osteotomy, edentulous ridge splitting
Historical background and technical characteristics
The term “piezo” originates from the Greek word piezein, and means “to press tight, squeeze”.1 In 1880, the Curie brothers Jacques and Pierre discovered “ piezoelectricity”. They found that putting pressure on various crystals, ceramics, or bone created electricity. A year later, Gabriel Lippmann found the converse piezoelectric effect. He demonstrated that if an electric field is applied to a crystal, the material will deform.2 These effects were further investigated by different scientists, and in 1953 Catuna published an article on the use of ultrasound on hard tissue.2,3 In the following decades, the application of ultrasonic vibrating technology for cutting mineralized tissue was demonstrated by different work groups.4–6 One of the groups was McFall et al.5 They investigated the distinction of healing by comparing rotating instruments with an oscillating scalpel blade. The healing was slightly slower in the oscillating scalpel blade group, but overall no severe complications occurred.5 Horton et al described that on alveolar bones in dogs, a smoother surface occurred with rotating instruments in comparison with ultrasound. However, in this publication, the bone regeneration was better using the ultrasound device.6
Almost another two decades passed before the first clinical study was published. A technical note was published by Torrella et al in 1998,7 and in 2000, Vercellotti published the first human clinical study about “piezoelectric bone surgery”.8 It was the first time a case was reported on a split ridge in which an edentulous ridge was split even though the ridge was very narrow. With other cutting instruments, it would not have been possible to keep its integrity. In 2001, the Piezosurgery® was introduced, a tool that combines the ultrasound and the piezo effect.9
Nowadays, piezosurgery is widely used, and different devices are available. To compare six devices – Piezosurgical Piezotom, SurgySonic, Piezon Master Surgery, VarioSurg, Surgybone, and Piezosurgery – osteotomies were performed on nine freshly slaughtered cattle ribs. It was concluded that the morphological characteristics of the produced piezosurgical osteotomies varied depending on the piezosurgical unit and tip.10 The bone-cutting technique of the piezoelectric device works due to the use of microvibrations at a specific ultrasonic frequency modulated by sonic waves.11 The sonic and ultrasonic frequency (25–30 kHz) is produced by a mechanical shock wave that vibrates in a linear manner. The cutting tip works with a reduced vibration amplitude (horizontal 20–200 µm, vertical 20–60 µm).11 This allows for the main advantages of this device, which are precise and selective cutting, the avoidance of thermal damage, and safety for the patient.11,12 The selective cutting is the result of the limited amplitude. At this amplitude, only mineralized tissue will be cut, because soft tissue requires frequencies of greater than 50 kHz.13 Therefore, the use of piezoelectric instruments will reduce the risk of nerve damage. The reduction of overheating is explained by the generation of a cavitation effect in the irrigation solution due to the mechanical micromovements at a frequency of approximately 25–30 kHz. This also accounts for reduced bleeding, which means better surgical visibility and increased safety.11
Biological aspects
With rising technologies, less invasive surgery is a major aim. Piezoelectric surgery is heading in this direction, not only due to the advantage of very precise customized cutting but also due to factors associated with the healing process. The reduced blood loss improves healing conditions,11 and the constant irrigation helps to reduce thermal damage and thus reduces the risk of bone necrosis. Overheating during implant-site preparation negatively affects the osseointegration process, as well as the final outcome of implant rehabilitations. Different tips generate different temperatures, with the smooth tips creating the lowest temperature. There are other factors that will influence the temperature rise as well, such as the manner in which the cutting is performed and the particular features of the bone itself.14 In this regard, Heinemann et al compared different sonic and ultrasonic devices with rotary burs in parts of porcine jaws. In this study, piezosurgery showed the highest temperature rise, but as in the other devices, the osteocytes and the trabecular bone seemed to be intact.15
Moreover, piezoelectric bone cutting does not influence bone remodeling or cell viability.16,17 Chiriac et al showed that bone chips harvested by piezoelectric surgery, as well as bone chips harvested with a conventional rotating drill, contained vital cells that would differentiate into osteoblasts in vitro.17 von See et al showed that if the bone was harvested with a scraper or piezoelectric device, the cell count contained more osteoblast-like cells in the harvested samples.18
In addition, Esteves et al focused on the dynamics of bone healing. They compared the differences of osteotomies performed with piezosurgery or a conventional drill in regard to “histomorphometrical, immunohistochemical and molecular analysis”.16 They showed that histologically and histomorphometrically, the bone healing showed no differences between the two groups, except for a slightly higher amount of newly formed bone observed 30 days after the use of the piezosurgery device.16 Comparing the bone healing after osteotomies performed either with piezosurgery or with an oscillatory saw in rabbits, Ma et al found no significant differences with regard to histomorphometry, but they found slightly more bone formation.19
Only a few studies have been published on the effect of the piezoelectric device concerning soft-tissue changes. Stoetzer et al published an example showing that the use of piezoelectric technology creates less soft-tissue damage for subperiosteal preparation.20 They performed an animal study on rats with regard to microcirculation after subperiosteal preparation, which led to the disturbance of local periosteal microcirculation, with either a piezoelectric device or periosteal elevator. Higher levels of periosteal perfusion in the piezosurgery group were found, and thus this group demonstrated better periosteal microcirculation. This can be an incentive for enhanced bone metabolism.20
Different applications in implantology
Preparation of the implant site
The different aspects of the piezoelectric device were mentioned before. The use of it for implantology will be described in detail in the following sections. Edentulous patients will benefit from implants, and these implants have appreciable outcomes.21,22 The piezoelectric device can be used for different clinical applications in implantology (Figures 1 and 2). In healthy bony conditions, it can be employed for the preparation of the implant site.23 By the use of a special tip, which allows for drilling of a precise implant hole, thermal and mechanical damage to the bone will be reduced. In 2007, Preti et al assessed the difference between the use of piezosurgery and a conventional drill in regard to the neo-osteogenesis and inflammatory reaction after implant-site preparation.24 They discovered that more newly formed bone with an increased amount of osteoblasts was visible on the piezoelectric implant site during the early phase (7–14 days). They investigated the following factors in detail: BMP-4, TGF-β2, TNFα, IL-1β, and IL-10. During this early period, BMP-4, TGF-β2, and IL-10 were increased in the piezoelectric group, while IL-1β and TNFα were not.24 In conclusion, the piezoelectric device stimulated peri-implant osteogenesis, and a reduction of proinflammatory cytokines. Stübinger et al reported similar results for implant-site preparation. Their pelvic sheep model revealed good biological and biomechanical results.25 da Silva Neto et al conducted a prospective study design with 30 patients (bilateral edentulous areas in the maxillary premolar region) who received dental implants using either conventional drilling or piezoelectric tips.26 Resonance-frequency analysis was used to evaluate the implant-stability quotient in sites prepared by either conventional drilling or piezoelectric tips, showing significant increases in quotient values for the piezosurgery group. Therefore, the stability of implants placed using the piezoelectric method was greater than that of implants placed using the conventional technique.26
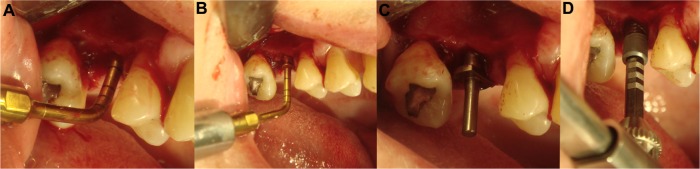
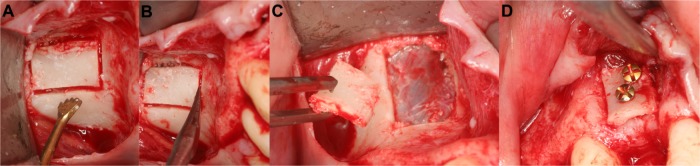
Figure 1.
Piezoelectric preparation of an implant site (right maxilla).
Notes: After definition of the initial implant length, widening of the implant hole, using different tips in an ascending order (A–C). Finally, control of the angulation and implant placement (D).
Figure 2.
Removal of an infected blade implant (left mandible).
Notes: Following tissue-protective piezo-osteotomy (A and B), the blade implant could be safely removed (C and D). The vestibular bone was used for bony reconstruction of the defect.
If the donor site is unsuitable, different alternatives depending on the location and amount of bone deficiency are possible. In the upper jaw, the use of the piezoelectric device for sinus-floor elevation is a perfect example.
Sinus-floor elevation
In edentulous patients with insufficient bone volume and therefore reduced height of the alveolar crest, a sinus-floor elevation is often the most suitable solution to prepare a sufficient donor site for implant insertion (Figures 3 and 4).
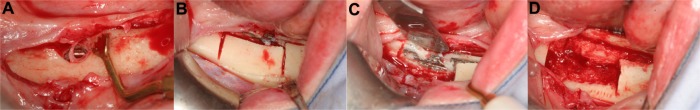
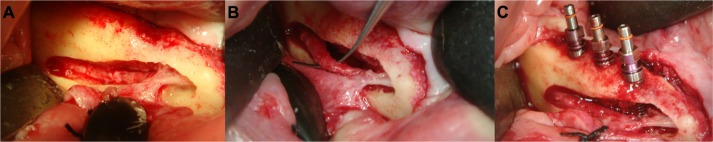
Figure 3.
Sinus elevation with simultaneous implant placement.
Notes: Removal of the vestibular alveolar wall (A), elevation of the Schneiderian membrane and dental implant placement (B). The sinus cavity was filled with bone substitutes and bone chips. Use of the buccal bone for additional stabilization and protection (C and D). The complete area was finally covered with a collagen membrane (E).
Figure 4.
Removal of sinus septum.
Notes: During a sinus elevation (A), a septum in the sinus was meticulously removed with a piezoelectric device (B). The thin and small tips allowed gentle removal of the septum. Finally, the sinus membrane could be elevated without problems (C). The septum was used for augmentation (D).
The surgical procedure includes the removal of a bony window of the anterior sinus maxillary wall. A precise cutting device that does not perforate the Schneiderian membrane is preferable to conventional methods. The perforation of the Schneiderian membrane can occur during the removal of the bony window and during the elevation itself. If a perforation occurs and bone grafting is completed, there is a risk for an inflammatory complication, which can necessitate further surgical procedures, including revision of the maxillary sinus. Al-Dajani found that a perforation of the Schneiderian membrane doubles the risk for the incidence of sinusitis or infection.27 Therefore, it is of great importance that any perforation should be avoided. Seoane et al showed that the use of the piezoelectric device reduces the frequency of membrane perforation among surgeons with limited experience.28 Specific tips can even decrease the risk of accidental or iatrogenic perforations.
Vercellotti et al published a surgical protocol using piezoelectric surgery showing a clear reduction (5%) of membrane perforation.29 In comparison, the prevalence with rotary instrumentation varies between 5% and 56%.30,31 Another clear advantage is the thin cut of the piezoelectric device. Sohn et al showed that the replacement of the bony lateral window into the former defect is possible when using the piezoelectric device.32
There are more articles published about the use of the piezoelectric device for lateral window sinus augmentation.33,34 Although the lateral window is probably the most commonly used method, other techniques, including the approach from the crestal and palatal side, have been described.35–38 Piezoelectric surgery has gained wide approval for sinus lift evaluation; moreover, many people are of the opinion that it does not show a clear benefit.39 Furthermore, another striking advantage of piezoelectric surgery is its use during the same surgical session for harvesting bone. Stacchi et al published a scraping–pulling fashion,40 in which the gained bone chips can then be used for the augmentation, or they can be mixed with various nonautologous materials and placed in the sinus. The successful use of the piezoelectric device for sinus grafting has been published previously.41–43
Bone grafting
Dental implants are only possible if sufficient residual bone volume is available. Different techniques for ridge augmentation have been published and proven to be very sufficient. Autogenous bone grafts from the chin or the ramus are the most common choices if only a limited amount of bone is needed (Figures 5 and 6). For larger bone volumes, other donor sites, such as the iliac crest, have to be considered. Bone grafts from the jaw region show good osteogenic properties, little resorption, and thus stable conditions. Mouraret et al compared the piezoelectric device with a conventional bur in an in vivo mouse model. Osteotomies performed with the piezoelectric device revealed greater osteocyte viability and reduced cell death.44 With the piezoelectric device, bone grafts exhibited greater short-term cell viability and showed slightly more new bone deposition and bone remodeling.44 Miron et al found in a porcine bone-graft model that “cell viability and the release of molecules affecting bone formation were higher in samples harvested by bone mill and bone scraper when compared with samples prepared by bone drilling and piezosurgery”.45 By use of the piezoelectric device, precise cutting of the graft is easily possible. Piezosurgery requires much less hand pressure than traditional rotary instruments.46 The shape of the graft can be accurately removed from the donor site, and donor-site morbidity can be kept as low as possible. Majewski investigated the possibility of harvesting individual bone blocks with an individual piezoelectric cut design.47 This also enables surgeons to remove grafts from regions that are more difficult to reach, eg, the zygomaticomaxillary region or the lateral wall of the maxillary sinus (Figure 7).48,49 Anitua et al used an onlay bone graft from the lateral wall of the maxillary sinus for augmentation.49 This is a good example indicating that the use of a piezoelectric device is not difficult. It is a safe method (preventing soft-tissue and nerve damage) with minimal surgical morbidity. Altiparmak et al recently evaluated donor-site morbidity following bone harvesting with piezoelectric and/or conventional surgical techniques.50 They investigated the ramus and symphysis as donor sites. They found that temporary paresthesia in the mucosa was significantly higher in the symphysis group than in the ramus group (P=0.004), and they showed that temporary skin and mucosa paresthesia was lower (P=0.006 and P=0.001) in the piezoelectric group in comparison to in the conventional group. Importantly, no permanent paresthesia of any region of the skin occurred in either donor-site group.50
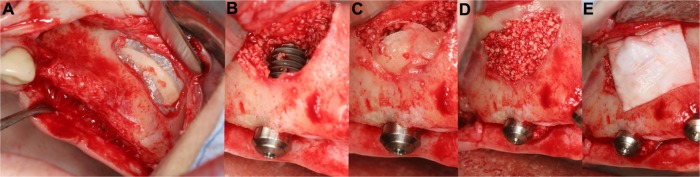
Figure 5.
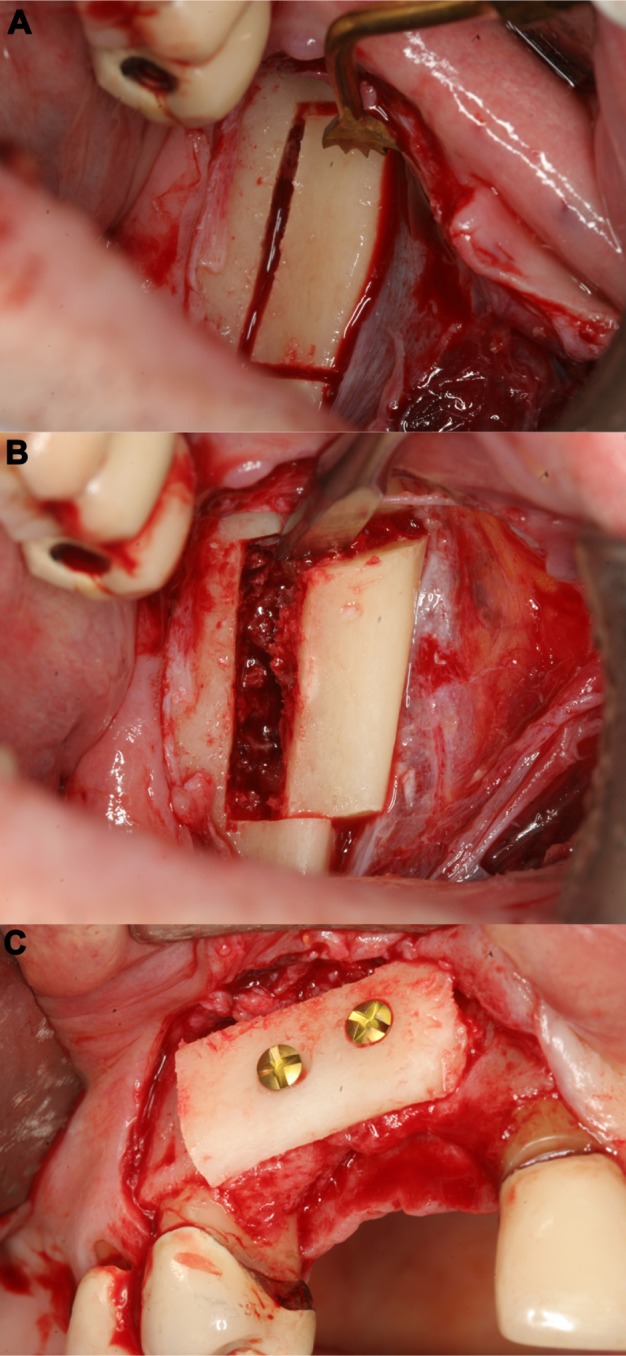
Harvesting of a corticocancellous ramus bone graft (right mandible).
Notes: The osteotomy of the bone graft could be easily performed with the piezoelectric device, after preparation of a mucoperiosteal flap (A and B). The bone graft was secured with two titanium screws (right upper jaw) (C).
Figure 6.
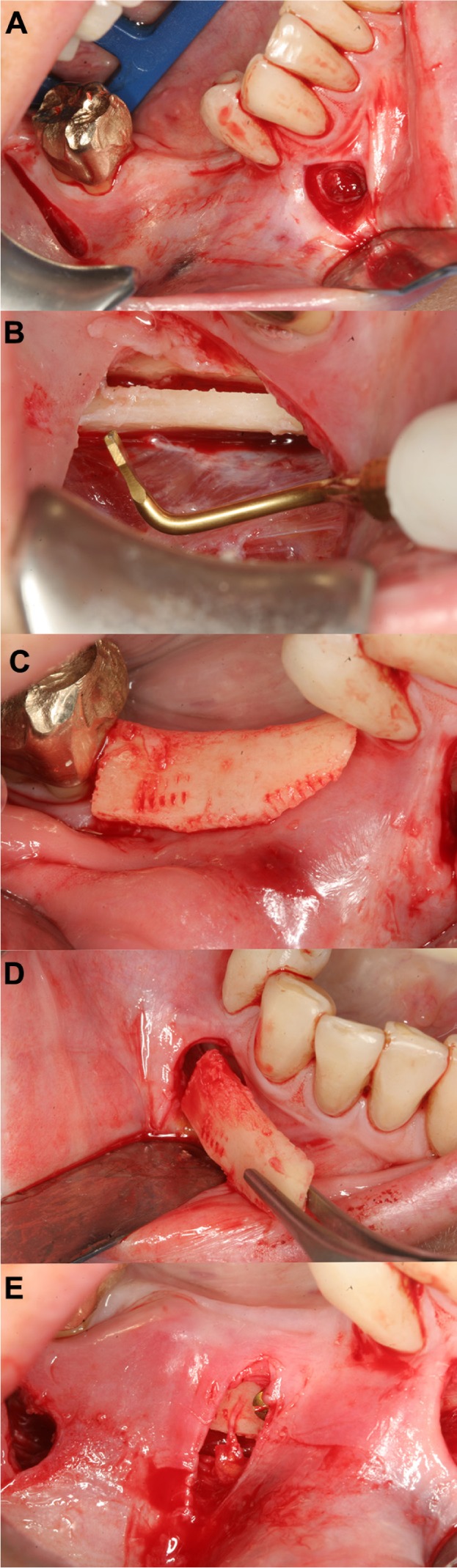
Minimally invasive augmentation procedure (right mandible).
Notes: Two vertical incisions (A) were performed (mesial and distal ends of the intended region for augmentation), followed by elevation of a mucoperiosteal flap. A bone graft from the contralateral side was harvested (B). The block was adapted to the defect (C), and inserted under the flap accessed from the mesial incision (D). The block was secured with two titanium screws onto the alveolar crest (E).
Figure 7.
Harvesting of ideally shaped bone graft from the zygomatic bone.
Notes: In a similar way to a sinus elevation, the bone graft was carefully removed without damaging the underlying sinus membrane (A–C). The slightly curved bone graft was placed in a vestibular bone defect (maxilla) and secured with two screws (D).
Another aspect is the removal of the graft itself. If it is performed with a conventional bur or saw, normally a chisel has to be used to remove the graft. By using a hammer and chisel, the risk of damaging teeth roots and soft-tissue structures increases. Therefore, in this regard, the use of the piezoelectric device is a safer option, because movement of the patient can lead to iatrogenic slipping and serious complications, even damage of the inferior alveolar nerve. If bone augmentation is avoided, edentulous ridge splitting is an option.
Edentulous ridge splitting
In insufficient width of the alveolar ridge, the edentulous ridge-splitting technique can be applied. For this procedure, the lingual plate is separated from the buccal plate of the edentulous ridge (Figure 8). Because bland tips are available, the procedure is very safe when using the piezoelectric device, even if the inferior alveolar nerve is accidentally touched. In the available space, the implant will be inserted. If required, alloplastic material can be inserted as well. One of the major advantages of edentulous ridge splitting is the avoidance of donor-site morbidity, because no graft is needed. Amato et al revealed that the maxilla allows an effective and fast osteotomy with atraumatic ridge expansion.51 The ridge splitting of the mandible can raise complications due to the inferior alveolar nerve, particularly if a significant amount of bone is lost. Furthermore, the risk of fracturing the bone segments in the cortical mandible is an issue. Edentulous ridge splitting is possible with conventional instruments,52,53 but the piezoelectric device showed a different dimension. Bone separation using the piezoelectric device is even possible in difficult bony situations, due to the exact and well-defined cutting abilities without macrovibrations. Case reports and studies demonstrate the successful use of the piezosurgical device, even with a modified protocol, to lateralize the inferior alveolar nerve.54–57
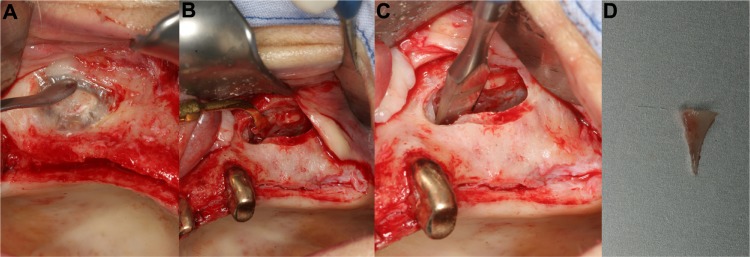
Figure 8.
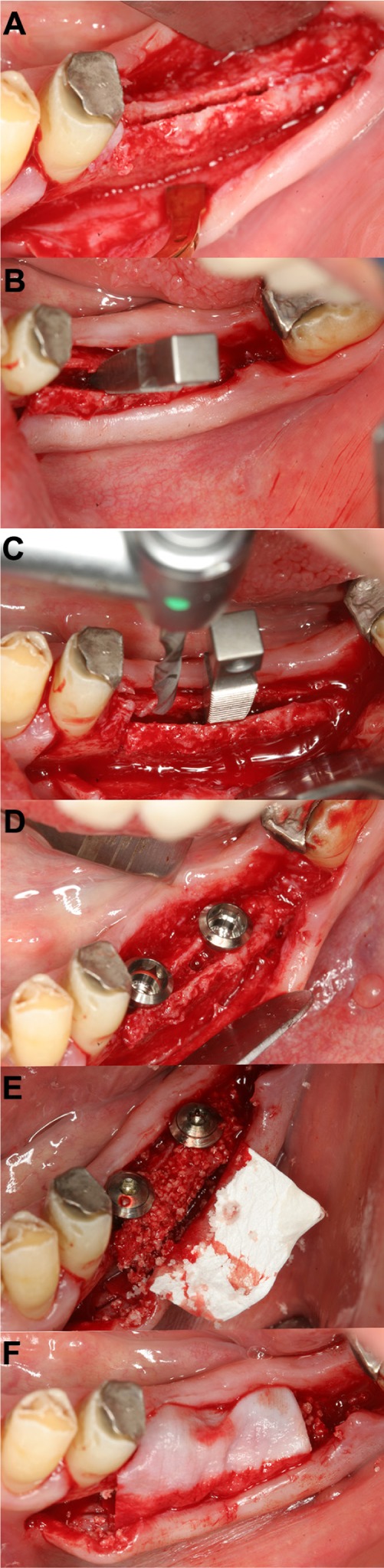
Ridge splitting (right mandible).
Notes: The transversally thin alveolar ridge was osteotomized with an OT7 piezo tip (A). After careful expansion and placement of titanium wedges, the implant holes were drilled (B and C). Two dental implants were placed in the widened alveolar ridge (D). The remaining space was augmented with bone substitutes (E). Finally, the complete area was covered with a collagen membrane (F).
Lateralization of the inferior alveolar nerve
To keep the inferior alveolar nerve intact is essential for the patient’s quality of life. The localization of the inferior alveolar nerve can vary distinctively in the edentulous mandible. The localization in the horizontal layer seems to be fairly stable (Figure 9). In a cadaver study conducted by Gowgiel, “the distance from the lateral border of the neurovascular bundle to the external surface of the buccal plate was usually half a centimeter in the molar and premolar regions”.58 Hur et al managed to find the most common patterns of nerve-fascicle innervation to the mandibular teeth, although they stated it only as a rough classification based on 30 hemifaces of cadavers. With their anatomical study, it was possible to vaguely detect the region where the damage occurred.59 Particularly in regions with a limited view, it is essential to perform the osteotomies with a tool that reduces the risk of nerve damage. This is possible with the piezoelectric device, because the shape of the tip, surgical control, and the cavitation effect60 support the surgeon in interventions close to the inferior alveolar nerve. This accounts for the removal of deeply impacted wisdom teeth, which are often located close to the inferior alveolar nerve, as well as for the lateralization of the inferior alveolar nerve. This procedure is an alternative to the augmentation technique if implants are planned in an edentulous jaw.61 For this, free and clear access to the nerve is desirable. This can be achieved by performing cuts with the piezoelectric device, so that the cortical lateral bone lid is replaceable over the neurovascular bundle. This procedure protects the nerve structure after nerve retraction and transposition.11 In situations where nerve contact cannot be avoided, Salami et al reported that the negative side effects are much higher if a rotating instrument comes into contact with the nerve.62
Figure 9.
Lateralization of the inferior alveolar nerve (right mandible).
Notes: Complete removal of the vestibular bone in that area and gentle loosening of the nerve (from the remaining nerve canal walls) (A). The nerve was carefully kept away from the osteotomy site (B). After implant insertions, the nerve was returned to its original place (C).
Another advantage of the piezoelectric device is that patients experience less stress and fear because it produces less noise. The microvibrations of the piezoelectric device in comparison to a conventional bur appear to be less stressful for the patient.11 The only known disadvantage we are aware of is the slightly longer operating time, but this can be accepted considering all of the advantages.
Clinical applications
The piezoelectric device is widely used in all fields of dentistry. In the field of orthodontic treatment, there are published reports regarding orthodontic traction of mandibular third molars,63 orthodontic closure of edentulous spaces,64 and “surgical cortical micro-incisions”.65 The piezosurgery technique can also be combined with endoscopic assistance for corticotomies.66 The use of piezosurgery and endoscopy is also described for other scenarios, such as when displaced root fragments from the maxillary sinus need to be removed.67 Other indications in the field of oral surgery are the use of the piezosurgical device for the removal of the third molar,68–72 and additionally even for the removal of an osteoma associated with a third molar,73 or lower third molar germectomy.74 There are many other indications for the use of the piezoelectric device in maxillofacial surgery.75 An increasing number of studies show the use of the piezoelectric device in orthognathic surgery,76–80 and even research on the use of computer-assisted piezoelectric surgery for osteotomies has been published.81 The advantage of high-precision cutting and reduced risk of nerve damage are very convincing arguments to use the piezoelectric device. Using the device for unilateral condylar hyperplasia can also be safer and less invasive when a high condylectomy is performed.82 Another field in which the piezosurgical device is applied nowadays is the harvesting of microvascular free bone flaps.83 An interdisciplinary use of the piezoelectric device is for orbital surgery84–87 or around the optic nerve canal.88 The piezoelectric device is also implemented in ear, nose, and throat surgery,89–94 hand surgery,95,96 and thoracic surgery.97 Another field in which piezosurgery is becoming increasingly attractive and accepted is bone surgery in children.82,98–101 Complex anatomical structures in children are at even higher risk due to the small size; therefore, the piezosurgery device is indispensable in these situations.
Conclusion
The application of piezoelectric surgery is an excellent tool to handle delicate or compromised hard- and soft-tissue conditions with less risk for the patient. Minimal accidental damage to adjacent soft-tissue structures allows for a safe and gentle surgical approach, particularly to thin and fragile bony structures. The slightly longer amount of time required if the piezoelectric tool is used for cutting large or extensive bone volumes is acceptable, keeping in mind the overall advantages of precise cutting. With respect to current and future minimally invasive and innovative surgical concepts, piezoelectric surgery offers a wide range of new possibilities to perform customized osteotomies for bone reconstruction and placement of smart implants.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.The Free Dictionary [homepage on the Internet] [Accessed July 15, 2015]. Available from: http://www.thefreedictionary.com.
- 2.American Physical Society This month in physics history: March 1880–the Curie brothers discover piezoelectricity. 2014. [Accessed July 10, 2015]. Available from: http://www.aps.org/publications/apsnews/201403/physicshistory.cfm.
- 3.Catuna MC. Sonic energy: a possible dental application, Preliminary report of an ultrasonic cutting method. Ann Dent. 1953;12:100–101. [Google Scholar]
- 4.Mazorow HB. Bone repair after experimental produced defects. J Oral Surg Anesth Hosp Dent Serv. 1960;18:107–115. [Google Scholar]
- 5.McFall TA, Yamane GM, Burnett GW. Comparison of the cutting effect on bone of an ultrasonic cutting device and rotary burs. J Oral Surg Anesth Hosp Dent Serv. 1961;19:200–209. [PubMed] [Google Scholar]
- 6.Horton JE, Tarpley TM, Jr, Wood LD. The healing of surgical defects in alveolar bone produced with ultrasonic instrumentation, chisel, and rotary bur. Oral Surg Oral Med Oral Pathol. 1975;39:536–546. doi: 10.1016/0030-4220(75)90192-9. [DOI] [PubMed] [Google Scholar]
- 7.Torrella F, Pitarch J, Cabanes G, Anitua E. Ultrasonic ostectomy for the surgical approach of the maxillary sinus: a technical note. Int J Oral Maxillofac Implants. 1998;13:697–700. [PubMed] [Google Scholar]
- 8.Vercellotti T. Piezoelectric surgery in implantology: a case report – a new piezoelectric ridge expansion technique. Int J Periodontics Restorative Dent. 2000;20:358–365. [PubMed] [Google Scholar]
- 9.Vercellotti T, Crovace A, Palermo A, Molfetta A. The piezoelectric osteotomy in orthopedics: clinical and histological evaluations (pilot study in animals) Mediterranean J Surg Med. 2001;9:89–95. [Google Scholar]
- 10.Bauer SE, Romanos GE. Morphological characteristics of osteotomies using different piezosurgical devices. A scanning electron microscopic evaluation. Implant Dent. 2014;23:334–342. doi: 10.1097/ID.0000000000000077. [DOI] [PubMed] [Google Scholar]
- 11.Stübinger S, Landes C, Seitz O, Zeilhofer HF, Sader R. Ultrasonic bone cutting in oral surgery: a review of 60 cases. Ultraschall Med. 2008;29:66–71. doi: 10.1055/s-2007-963507. German. [DOI] [PubMed] [Google Scholar]
- 12.Grötz KA. Die entwicklung der piezosurgery in der oralchirurgie. Oralchir J. 2010;2:14–17. [Google Scholar]
- 13.Labanca M, Azzola F, Vinci R, Rodella LF. Piezoelectric surgery: twenty years of use. Br J Oral Maxillofac Surg. 2008;46:265–269. doi: 10.1016/j.bjoms.2007.12.007. [DOI] [PubMed] [Google Scholar]
- 14.Lamazza L, Laurito D, Lollobrigida M, Brugnoletti O, Garreffa G, De Biase A. Identification of possible factors influencing temperatures elevation during implant site preparation with piezoelectric technique. Ann Stomatol (Roma) 2015;5:115–122. [PMC free article] [PubMed] [Google Scholar]
- 15.Heinemann F, Hasan I, Kunert-Keil C, et al. Experimental and histological investigations of the bone using two different oscillating osteotomy techniques compared with conventional rotary osteotomy. Ann Anat. 2012;194:165–170. doi: 10.1016/j.aanat.2011.10.005. [DOI] [PubMed] [Google Scholar]
- 16.Esteves JC, Marcantonio E, Jr, de Souza Faloni AP, et al. Dynamics of bone healing after osteotomy with piezosurgery or conventional drilling – histomorphometrical, immunohistochemical, and molecular analysis. J Transl Med. 2013;11:221. doi: 10.1186/1479-5876-11-221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chiriac G, Herten M, Schwarz F, Rothamel D, Becker J. Autogenous bone chips: influence of a new piezoelectric device (Piezosurgery) on chip morphology, cell viability and differentiation. J Clin Periodontol. 2005;32:994–999. doi: 10.1111/j.1600-051X.2005.00809.x. [DOI] [PubMed] [Google Scholar]
- 18.von See C, Rücker M, Kampmann A, Kokemüller H, Bormann KH, Gellrich NC. Comparison of different harvesting methods from the flat and long bones of rats. Br J Oral Maxillofac Surg. 2010;48:607–612. doi: 10.1016/j.bjoms.2009.09.012. [DOI] [PubMed] [Google Scholar]
- 19.Ma L, Stübinger S, Liu XL, Schneider UA, Lang NP. Healing of osteotomy sites applying either piezosurgery or two conventional saw blades: a pilot study in rabbits. Int Orthop. 2013;37:1597–1603. doi: 10.1007/s00264-013-1908-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Stoetzer M, Felgenträger D, Kampmann A, et al. Effects of a new piezoelectric device on periosteal microcirculation after subperiosteal preparation. Microvasc Res. 2014;94:114–118. doi: 10.1016/j.mvr.2014.06.003. [DOI] [PubMed] [Google Scholar]
- 21.Adell R, Eriksson B, Lekholm U, Brånemark PI, Jemt T. Long-term follow-up study of osseointegrated implants in the treatment of totally edentulous jaws. Int J Oral Maxillofac Implants. 1990;5:347–359. [PubMed] [Google Scholar]
- 22.Blanes RJ, Bernard JP, Blanes ZM, Belser UC. A 10-year prospective study of ITI dental implants placed in the posterior region. I: Clinical and radiographic results. Clin Oral Implants Res. 2007;18:699–706. doi: 10.1111/j.1600-0501.2006.01306.x. [DOI] [PubMed] [Google Scholar]
- 23.Vercellotti T, Stacchi C, Russo C, et al. Ultrasonic implant site preparation using piezosurgery: a multicenter case series study analyzing 3,579 implants with a 1- to 3-year follow-up. Int J Periodontics Restorative Dent. 2014;34:11–18. doi: 10.11607/prd.1860. [DOI] [PubMed] [Google Scholar]
- 24.Preti G, Martinasso G, Peirone B, et al. Cytokines and growth factors involved in the osseointegration of oral titanium implants positioned using piezoelectric bone surgery versus a drill technique: a pilot study in minipigs. J Periodontol. 2007;78:716–722. doi: 10.1902/jop.2007.060285. [DOI] [PubMed] [Google Scholar]
- 25.Stübinger S, Biermeier K, Bächi B, Ferguson SJ, Sader R, von Rechenberg B. Comparison of Er:YAG laser, piezoelectric, and drill osteotomy for dental implant site preparation: a biomechanical and histological analysis in sheep. Lasers Surg Med. 2010;42:652–661. doi: 10.1002/lsm.20944. [DOI] [PubMed] [Google Scholar]
- 26.da Silva Neto UT, Joly JC, Gehrke SA. Clinical analysis of the stability of dental implants after preparation of the site by conventional drilling or piezosurgery. Br J Oral Maxillofac Surg. 2014;52:149–153. doi: 10.1016/j.bjoms.2013.10.008. [DOI] [PubMed] [Google Scholar]
- 27.Al-Dajani M. Recent trends in sinus lift surgery and their clinical implications. Clin Implant Dent Relat Res. 2014 Oct 2; doi: 10.1111/cid.12275. Epub. [DOI] [PubMed] [Google Scholar]
- 28.Seoane J, López-Niño J, García-Caballero L, Seoane-Romero JM, Tomás I, Varela-Centelles P. Membrane perforation in sinus floor elevation – piezoelectric device versus conventional rotary instruments for osteotomy: an experimental study. Clin Implant Dent Relat Res. 2013;15:867–873. doi: 10.1111/j.1708-8208.2012.00447.x. [DOI] [PubMed] [Google Scholar]
- 29.Vercellotti T, De Paoli S, Nevins M. The piezoelectric bony window osteotomy and sinus membrane elevation: introduction of a new technique for simplification of the sinus augmentation procedure. Int J Periodontics Restorative Dent. 2001;21:561–567. [PubMed] [Google Scholar]
- 30.van den Bergh JP, ten Bruggenkate CM, Krekeler G, Tuinzing DB. Sinusfloor elevation and grafting with autogenous iliac crest bone. Clin Oral Implants Res. 1998;9:429–435. doi: 10.1034/j.1600-0501.1996.090608.x. [DOI] [PubMed] [Google Scholar]
- 31.Kasabah S, Krug J, Simůnek A, Lecaro MC. Can we predict maxillary sinus mucosa perforation? Acta Medica (Hradec Kralove) 2003;46:19–23. [PubMed] [Google Scholar]
- 32.Sohn DS, Moon JW, Lee HW, Choi BJ, Shin IH. Comparison of two piezoelectric cutting inserts for lateral bony window osteotomy: a retrospective study of 127 consecutive sites. Int J Oral Maxillofac Implants. 2010;25:571–576. [PubMed] [Google Scholar]
- 33.Peñarrocha-Diago M, Peñarrocha-Diago M, Sanchez-Recio C, Peñarrocha-Oltra D, Romero-Millán J. Osteotomy in direct sinus lift. A comparative study of the rotary technique and ultrasound. Med Oral Patol Oral Cir Bucal. 2012;17:e457–e61. doi: 10.4317/medoral.17599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Delilbasi C, Gurler G. Comparison of piezosurgery and conventional rotative instruments in direct sinus lifting. Implant Dent. 2013;22:662–665. doi: 10.1097/ID.0000000000000001. [DOI] [PubMed] [Google Scholar]
- 35.Stübinger S, Saldamli B, Seitz O, Sader R, Landes CA. Palatal versus vestibular piezoelectric window osteotomy for maxillary sinus elevation: a comparative clinical study of two surgical techniques. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2009;107:648–655. doi: 10.1016/j.tripleo.2008.12.016. [DOI] [PubMed] [Google Scholar]
- 36.Baldi D, Menini M, Pera F, Ravera G, Pera P. Sinus floor elevation using osteotomes or piezoelectric surgery. Int J Oral Maxillofac Surg. 2011;40:497–503. doi: 10.1016/j.ijom.2011.01.006. [DOI] [PubMed] [Google Scholar]
- 37.Cassetta M, Ricci L, Iezzi G, Calasso S, Piattelli A, Perrotti V. Use of piezosurgery during maxillary sinus elevation: clinical results of 40 consecutive cases. Int J Periodontics Restorative Dent. 2012;32:e182–e188. [PubMed] [Google Scholar]
- 38.Kühl S, Kirmeier R, Platzer S, Bianco N, Jakse N, Payer M. Transcrestal maxillary sinus augmentation: Summers’ versus a piezoelectric technique – an experimental cadaver study. Clin Oral Implants Res. 2015 Feb 16; doi: 10.1111/clr.12546. Epub. [DOI] [PubMed] [Google Scholar]
- 39.Rickert D, Vissink A, Slater JJ, Meijer HJ, Raghoebar GM. Comparison between conventional and piezoelectric surgical tools for maxillary sinus floor elevation. A randomized controlled clinical trial. Clin Implant Dent Relat Res. 2013;15:297–302. doi: 10.1111/j.1708-8208.2011.00364.x. [DOI] [PubMed] [Google Scholar]
- 40.Stacchi C, Vercellotti T, Toschetti A, Speroni S, Salgarello S, Di Lenarda R. Intraoperative complications during sinus floor elevation using two different ultrasonic approaches: a two-center, randomized, controlled clinical trial. Clin Implant Dent Relat Res. 2015;17(Suppl 1):e117–e125. doi: 10.1111/cid.12136. [DOI] [PubMed] [Google Scholar]
- 41.Wallace SS, Mazor Z, Froum SJ, Cho SC, Tarnow DP. Schneiderian membrane perforation rate during sinus elevation using piezosurgery: clinical results of 100 consecutive cases. Int J Periodontics Restorative Dent. 2007;27:413–419. [PubMed] [Google Scholar]
- 42.Cortes AR, Cortes DN, Arita ES. Effectiveness of piezoelectric surgery in preparing the lateral window for maxillary sinus augmentation in patients with sinus anatomical variations: a case series. Int J Oral Maxillofac Implants. 2012;27:1211–1215. [PubMed] [Google Scholar]
- 43.Wallace SS, Tarnow DP, Froum SJ, et al. Maxillary sinus elevation by lateral window approach: evolution of technology and technique. J Evid Based Dent Pract. 2012;12:161–171. doi: 10.1016/S1532-3382(12)70030-1. [DOI] [PubMed] [Google Scholar]
- 44.Mouraret S, Houschyar KS, Hunter DJ, et al. Cell viability after osteotomy and bone harvesting: comparison of piezoelectric surgery and conventional bur. Int J Oral Maxillofac Surg. 2014;43:966–971. doi: 10.1016/j.ijom.2013.11.018. [DOI] [PubMed] [Google Scholar]
- 45.Miron RJ, Gruber R, Hedbom E, et al. Impact of bone harvesting techniques on cell viability and the release of growth factors of autografts. Clin Implant Dent Relat Res. 2013;15:481–489. doi: 10.1111/j.1708-8208.2012.00440.x. [DOI] [PubMed] [Google Scholar]
- 46.Lakshmiganthan M, Gokulanathan S, Shanmugasundaram N, Daniel R, Ramesh SB. Piezosurgical osteotomy for harvesting intraoral block bone graft. J Pharm Bioallied Sci. 2012;4(Suppl 2):165–168. doi: 10.4103/0975-7406.100260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Majewski P. Autogenous bone grafts in the esthetic zone: optimizing the procedure using piezosurgery. Int J Periodontics Restorative Dent. 2012;32:210–217. [PubMed] [Google Scholar]
- 48.Stübinger S, Robertson A, Zimmerer KS, Leiggener C, Sader R, Kunz C. Piezoelectric harvesting of an autogenous bone graft from the zygomaticomaxillary region: case report. Int J Periodontics Restorative Dent. 2006;26:453–457. [PubMed] [Google Scholar]
- 49.Anitua E, Alkhraisat MH, Miguel-Sánchez A, Orive G. Surgical correction of horizontal bone defect using the lateral maxillary wall: outcomes of a retrospective study. J Oral Maxillofac Surg. 2014;72:683–693. doi: 10.1016/j.joms.2013.11.022. [DOI] [PubMed] [Google Scholar]
- 50.Altiparmak N, Soydan SS, Uckan S. The effect of conventional surgery and piezoelectric surgery bone harvesting techniques on the donor site morbidity of the mandibular ramus and symphysis. Int J Oral Maxillofac Surg. 2015;44:1131–1137. doi: 10.1016/j.ijom.2015.04.009. [DOI] [PubMed] [Google Scholar]
- 51.Amato F, Mirabella AD, Borlizzi D. Rapid orthodontic treatment after the ridge-splitting technique – a combined surgical-orthodontic approach for implant site development: case report. Int J Periodontics Restorative Dent. 2012;32:395–402. [PubMed] [Google Scholar]
- 52.Simion M, Baldoni M, Zaffe D. Jawbone enlargement using immediate implant placement associated with a split-crest technique and guided tissue regeneration. Int J Periodontics Restorative Dent. 1992;12:462–473. [PubMed] [Google Scholar]
- 53.Scipioni A, Bruschi GB, Calesini G, Bruschi E, De Martino C. Bone regeneration in the edentulous ridge expansion technique: histologic and ultrastructural study of 20 clinical cases. Int J Periodontics Restorative Dent. 1999;19:269–277. [PubMed] [Google Scholar]
- 54.Rahnama M, Czupkałło L, Czajkowski L, Grasza J, Wallner J. The use of piezosurgery as an alternative method of minimally invasive surgery in the authors’ experience. Wideochir Inne Tech Maloinwazyjne. 2013;8:321–326. doi: 10.5114/wiitm.2011.35144. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Brugnami F, Caiazzo A, Mehra P. Piezosurgery-assisted, flapless split crest surgery for implant site preparation. J Maxillofac Oral Surg. 2014;13:67–72. doi: 10.1007/s12663-012-0377-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Rodriguez JG, Eldibany RM. Vertical splitting of the mandibular body as an alternative to inferior alveolar nerve lateralization. Int J Oral Maxillofac Surg. 2013;42:1060–1066. doi: 10.1016/j.ijom.2013.04.014. [DOI] [PubMed] [Google Scholar]
- 57.Eldibany R, Rodriguez JG. Immediate loading of one-piece implants in conjunction with a modified technique of inferior alveolar nerve lateralization: 10 years follow-up. Craniomaxillofac Trauma Reconstr. 2014;7:55–62. doi: 10.1055/s-0033-1364198. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Gowgiel JM. The position and course of the mandibular canal. J Oral Implantol. 1992;18:383–385. [PubMed] [Google Scholar]
- 59.Hur MS, Kim HC, Won SY, et al. Topography and spatial fascicular arrangement of the human inferior alveolar nerve. Clin Implant Dent Relat Res. 2013;15:88–95. doi: 10.1111/j.1708-8208.2011.00335.x. [DOI] [PubMed] [Google Scholar]
- 60.Bovi M. Mobilization of the inferior alveolar nerve with simultaneous implant insertion: a new technique. Case report. Int J Periodontics Restorative Dent. 2005;25:375–383. [PubMed] [Google Scholar]
- 61.Metzger MC, Bormann KH, Schoen R, Gellrich NC, Schmelzeisen R. Inferior alveolar nerve transposition – an in vitro comparison between piezosurgery and conventional bur use. J Oral Implantol. 2006;32:19–25. doi: 10.1563/1548-1336(2006)32[19:IANTIV]2.0.CO;2. [DOI] [PubMed] [Google Scholar]
- 62.Salami A, Dellepiane M, Mora R. A novel approach to facial nerve decompression: use of piezosurgery. Acta Otolaryngol. 2008;128:530–533. doi: 10.1080/00016480701635175. [DOI] [PubMed] [Google Scholar]
- 63.Ma Z, Xu G, Yang C, Xie Q, Shen Y, Zhang S. Efficacy of the technique of piezoelectric corticotomy for orthodontic traction of impacted mandibular third molars. Br J Oral Maxillofac Surg. 2015;53:326–331. doi: 10.1016/j.bjoms.2015.01.002. [DOI] [PubMed] [Google Scholar]
- 64.Ozer M, Akdeniz BS, Sumer M. Alveolar ridge expansion-assisted orthodontic space closure in the mandibular posterior region. Korean J Orthod. 2013;43:302–310. doi: 10.4041/kjod.2013.43.6.302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Cassetta M, Pandolfi S, Giansanti M. Minimally invasive corticotomy in orthodontics: a new technique using a CAD/CAM surgical template. Int J Oral Maxillofac Surg. 2015;44:830–833. doi: 10.1016/j.ijom.2015.02.020. [DOI] [PubMed] [Google Scholar]
- 66.Hernández-Alfaro F, Guijarro-Martínez R. Endoscopically assisted tunnel approach for minimally invasive corticotomies: a preliminary report. J Periodontol. 2012;83:574–580. doi: 10.1902/jop.2011.110233. [DOI] [PubMed] [Google Scholar]
- 67.Hu YK, Yang C, Zhou Xu G, Wang Y, Abdelrehem A. Retrieval of root fragment in maxillary sinus via anterolateral wall of the sinus to preserve alveolar bone. J Craniofac Surg. 2015;26:81–84. doi: 10.1097/SCS.0000000000001286. [DOI] [PubMed] [Google Scholar]
- 68.Mantovani E, Arduino PG, Schierano G, et al. A split-mouth randomized clinical trial to evaluate the performance of piezosurgery compared with traditional technique in lower wisdom tooth removal. J Oral Maxillofac Surg. 2014;72:1890–1897. doi: 10.1016/j.joms.2014.05.002. [DOI] [PubMed] [Google Scholar]
- 69.Mozzati M, Gallesio G, Russo A, Staiti G, Mortellaro C. Third-molar extraction with ultrasound bone surgery: a case-control study. J Craniofac Surg. 2014;25:856–859. doi: 10.1097/SCS.0000000000000825. [DOI] [PubMed] [Google Scholar]
- 70.Pippi R, Alvaro R. Piezosurgery for the lingual split technique in mandibular third molar removal: a suggestion. J Craniofac Surg. 2013;24:531–533. doi: 10.1097/SCS.0b013e31826463f7. [DOI] [PubMed] [Google Scholar]
- 71.Rullo R, Addabbo F, Papaccio G, D’Aquino R, Festa VM. Piezoelectric device vs conventional rotative instruments in impacted third molar surgery: relationships between surgical difficulty and postoperative pain with histological evaluations. J Craniomaxillofac Surg. 2013;41:33–38. doi: 10.1016/j.jcms.2012.07.007. [DOI] [PubMed] [Google Scholar]
- 72.Itro A, Lupo G, Marra A, et al. The piezoelectric osteotomy technique compared to the one with rotary instruments in the surgery of included third molars. A clinical study. Minerva Stomatol. 2012;61:247–253. [PubMed] [Google Scholar]
- 73.D’Amato S, Sgaramella N, Vanore L, Piombino P, Orabona GD, Santagata M. Piezoelectric bone surgery in the treatment of an osteoma associated with an impacted inferior third molar: a case report. Clin Cases Miner Bone Metab. 2014;11:73–76. [PMC free article] [PubMed] [Google Scholar]
- 74.Sivolella S, Berengo M, Bressan E, Di Fiore A, Stellini E. Osteotomy for lower third molar germectomy: randomized prospective crossover clinical study comparing piezosurgery and conventional rotatory osteotomy. J Oral Maxillofac Surg. 2011;69:15–23. doi: 10.1016/j.joms.2010.12.036. [DOI] [PubMed] [Google Scholar]
- 75.Berg BI, Hilber N, Hille K, Kunz C, Goldblum D. Der zahn im auge – die erste in der Schweiz eingebrachte osteo-odonto-keratoprothese. Praxis (Bern 1994) 2013;102:545–548. doi: 10.1024/1661-8157/a001278. [DOI] [PubMed] [Google Scholar]
- 76.Brockmeyer P, Hahn W, Fenge S, Moser N, Schliephake H, Gruber RM. Reduced somatosensory impairment by piezosurgery during orthognathic surgery of the mandible. Oral Maxillofac Surg. 2015;19:301–307. doi: 10.1007/s10006-015-0499-0. [DOI] [PubMed] [Google Scholar]
- 77.Olate S, Pozzer L, Unibazo A, Huentequeo-Molina C, Martinez F, de Moraes M. LeFort I segmented osteotomy experience with piezosurgery in orthognathic surgery. Int J Clin Exp Med. 2014;7:2092–2095. [PMC free article] [PubMed] [Google Scholar]
- 78.Spinelli G, Lazzeri D, Conti M, Agostini T, Mannelli G. Comparison of piezosurgery and traditional saw in bimaxillary orthognathic surgery. J Craniomaxillofac Surg. 2014;42:1211–1220. doi: 10.1016/j.jcms.2014.02.011. [DOI] [PubMed] [Google Scholar]
- 79.Bertossi D, Lucchese A, Albanese M, et al. Piezosurgery versus conventional osteotomy in orthognathic surgery: a paradigm shift in treatment. J Craniofac Surg. 2013;24:1763–1766. doi: 10.1097/SCS.0b013e31828f1aa8. [DOI] [PubMed] [Google Scholar]
- 80.Geha HJ, Gleizal AM, Nimeskern NJ, Beziat JL. Sensitivity of the inferior lip and chin following mandibular bilateral sagittal split osteotomy using piezosurgery. Plast Reconstr Surg. 2006;118:1598–1607. doi: 10.1097/01.prs.0000232360.08768.de. [DOI] [PubMed] [Google Scholar]
- 81.Bianchi A, Badiali G, Piersanti L, Marchetti C. Computer-assisted piezoelectric surgery: a navigated approach toward performance of craniomaxillofacial osteotomies. J Craniofac Surg. 2015;26:867–872. doi: 10.1097/SCS.0000000000001360. [DOI] [PubMed] [Google Scholar]
- 82.Chiarini L, Albanese M, Anesi A, et al. Surgical treatment of unilateral condylar hyperplasia with piezosurgery. J Craniofac Surg. 2014;25:808–810. doi: 10.1097/SCS.0000000000000699. [DOI] [PubMed] [Google Scholar]
- 83.Nocini PF, Turra M, Valsecchi S, Blandamura S, Bedogni A. Microvascular free bone flap harvest with piezosurgery. J Oral Maxillofac Surg. 2011;69:1485–1492. doi: 10.1016/j.joms.2009.10.016. [DOI] [PubMed] [Google Scholar]
- 84.De Castro DK, Fay A, Wladis EJ, et al. Self-irrigating piezoelectric device in orbital surgery. Ophthal Plast Reconstr Surg. 2013;29:118–122. doi: 10.1097/IOP.0b013e31827f59d4. [DOI] [PubMed] [Google Scholar]
- 85.Kalwerisky K, Hill R, Czyz C, Foster J, Everman K, Cahill K. Piezoelectric-assisted removal of the lateral orbital rim in lateral orbital rim advancement. Orbit. 2012;31:63. doi: 10.3109/01676830.2011.605504. [DOI] [PubMed] [Google Scholar]
- 86.Ponto KA, Zwiener I, Al-Nawas B, et al. Piezosurgery for orbital decompression surgery in thyroid associated orbitopathy. J Craniomaxillofac Surg. 2014;42:1813–1820. doi: 10.1016/j.jcms.2014.06.020. [DOI] [PubMed] [Google Scholar]
- 87.Iacoangeli M, Di Rienzo A, Nocchi N, et al. Piezosurgery as a further technical adjunct in minimally invasive supraorbital keyhole approach and lateral orbitotomy. J Neurol Surg A Cent Eur Neurosurg. 2015;76:112–118. doi: 10.1055/s-0034-1368685. [DOI] [PubMed] [Google Scholar]
- 88.Heredero Jung S, Dean Ferrer A, Solivera Vela J, Alamillos Granados F. Spheno-orbital meningioma resection and reconstruction: the role of piezosurgery and premolded titanium mesh. Craniomaxillofac Trauma Reconstr. 2011;4:193–200. doi: 10.1055/s-0031-1286113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 89.Salami A, Mora R, Dellepiane M, Guastini L. Piezosurgery for removal of symptomatic ear osteoma. Eur Arch Otorhinolaryngol. 2010;267:1527–1530. doi: 10.1007/s00405-010-1289-9. [DOI] [PubMed] [Google Scholar]
- 90.Salami A, Mora R, Dellepiane M. Piezosurgery in the exeresis of glomus tympanicum tumours. Eur Arch Otorhinolaryngol. 2008;265:1035–1038. doi: 10.1007/s00405-007-0567-7. [DOI] [PubMed] [Google Scholar]
- 91.Salami A, Mora R, Dellepiane M, Crippa B, Santomauro V, Guastini L. Piezosurgery versus microdrill in intact canal wall mastoidectomy. Eur Arch Otorhinolaryngol. 2010;267:1705–1711. doi: 10.1007/s00405-010-1308-x. [DOI] [PubMed] [Google Scholar]
- 92.Crippa B, Salzano FA, Mora R, Dellepiane M, Salami A, Guastini L. Comparison of postoperative pain: piezoelectric device versus microdrill. Eur Arch Otorhinolaryngol. 2011;268:1279–1282. doi: 10.1007/s00405-011-1520-3. [DOI] [PubMed] [Google Scholar]
- 93.Salami A, Mora R, Mora F, Guastini L, Salzano FA, Dellepiane M. Learning curve for piezosurgery in well-trained otological surgeons. Otolaryngol Head Neck Surg. 2010;142:120–125. doi: 10.1016/j.otohns.2009.10.013. [DOI] [PubMed] [Google Scholar]
- 94.Salami A, Dellepiane M, Proto E, Mora R. Piezosurgery in otologic surgery: four years of experience. Otolaryngol Head Neck Surg. 2009;140:412–418. doi: 10.1016/j.otohns.2008.11.013. [DOI] [PubMed] [Google Scholar]
- 95.Hoigné D, Hug U, von Wartburg U. Piezoelectric osteotomy in hand surgery: the autologous osteocartilage transplantation for joint reconstruction. Handchir Mikrochir Plast Chir. 2011;43:319–320. doi: 10.1055/s-0031-1285885. German. [DOI] [PubMed] [Google Scholar]
- 96.Hoigne DJ, Stübinger S, Von Kaenel O, Shamdasani S, Hasenboehler P. Piezoelectric osteotomy in hand surgery: first experiences with a new technique. BMC Musculoskelet Disord. 2006;7:36. doi: 10.1186/1471-2474-7-36. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Santini M, Fiorelli A, Santagata M, Tartaro GP. Resection of costal exostosis using piezosurgery associated with uniportal video-assisted thoracoscopy. Ann Thorac Surg. 2015;99:1080–1082. doi: 10.1016/j.athoracsur.2014.04.132. [DOI] [PubMed] [Google Scholar]
- 98.Ramieri V, Saponaro G, Lenzi J, et al. The use of piezosurgery in cranial surgery in children. J Craniofac Surg. 2015;26:840–842. doi: 10.1097/SCS.0000000000001574. [DOI] [PubMed] [Google Scholar]
- 99.Robiony M, Polini F. Piezosurgery: a safe method to perform osteotomies in young children affected by hemifacial microsomia. J Craniofac Surg. 2010;21:1813–1815. doi: 10.1097/SCS.0b013e3181f43e03. [DOI] [PubMed] [Google Scholar]
- 100.de Castro e Silva LM, Pereira Filho VA, Vieira EH, Gabrielli MF. Tracheostomy-dependent child with temporomandibular ankylosis and severe micrognathia treated by piezosurgery and distraction osteogenesis: case report. Br J Oral Maxillofac Surg. 2011;49:47–49. doi: 10.1016/j.bjoms.2010.10.007. [DOI] [PubMed] [Google Scholar]
- 101.Jose A, Nagori SA, Virkhare A, Bhatt K, Bhutia O, Roychoudhury A. Piezoelectric osteoarthrectomy for management of ankylosis of the temporomandibular joint. Br J Oral Maxillofac Surg. 2014;52:624–628. doi: 10.1016/j.bjoms.2014.04.012. [DOI] [PubMed] [Google Scholar]