Abstract
Background
The composition of the diet is of increasing importance for the development and maturation of the ovarian follicles. In Polycystic Ovary Syndrome (PCOS) healthy dietary interventions improve the clinical spectrum. We hypothesized that dieting and diet inadequacy in the reproductive life course is associated with impaired programming of ovarian follicles and contributes to the severity of the PCOS phenotype.
Methods and Findings
To determine associations between the use of a self-initiated diet and diet inadequacy and the severity of the PCOS phenotype, we performed an explorative nested case control study embedded in a periconception cohort of 1,251 patients visiting the preconception outpatient clinic. 218 patients with PCOS and 799 subfertile controls were selected from the cohort and self-administered questionnaires, anthropometric measurements and blood samples were obtained. The Preconception Dietary Risk Score (PDR score), based on the Dutch dietary guidelines, was used to determine diet inadequacy in all women. The PDR score was negatively associated to cobalamin, serum and red blood cell folate and positively to tHcy. PCOS patients (19.9%), in particular the hyperandrogenic (HA) phenotype (22.5%) reported more often the use of a self-initiated diet than controls (13.1%; p = 0.023). The use of an inadequate diet was also significantly higher in PCOS than in controls (PDR score 3.7 vs 3.5; p = 0.017) and every point increase was associated with a more than 1.3 fold higher risk of the HA phenotype (adjusted OR 1.351, 95% CI 1.09–1.68). Diet inadequacy was independently associated with the anti-Müllerian Hormone (AMH) concentration (β 0.084; p = 0.044; 95% CI 0.002 to 0.165) and free androgen index (β 0.128; p = 0.013; 95% CI 0.028 to 0.229) in PCOS patients.
Conclusions
The use of a self-initiated diet and diet inadequacy is associated with PCOS, in particular with the severe HA phenotype. This novel finding substantiated by the association between diet inadequacy and AMH needs further investigation.
Introduction
Polycystic Ovary Syndrome (PCOS) is the most common endocrine disorder affecting young women of reproductive age, resulting in subfertility and increased risk of cardiovascular related disease in later life [1]. The estimated prevalence of PCOS varies between 5–15% [2,3]. PCOS is characterized by oligo- or anovulation (ovulatory dysfunction; OD), clinical and/or biochemical signs of hyperandrogenism (HA) and/or polycystic ovaries (Polycystic ovarian morphology; PCOM), and gene-environment interactions play a significant role in the variability within PCOS phenotypes [4,5]. The clinical spectrum and severity in PCOS is heterogeneous, including reproductive, metabolic and psychological features. Because of the profound cardiovascular and metabolic disturbances in hyperandrogenic PCOS patients, it is recommended to distinguish two subtypes of PCOS according to the severity of disease (HA-PCOS and non HA-PCOS) [6].
Personal behaviors, such as dieting, smoking, alcohol- and drug use, stress and exercise are considered behavioral factors that are important for reproductive functioning [7,8]. The composition of the preconception diet is of increasing interest, because over- and undernutrition derange metabolic and endocrine pathways, especially the B-vitamin dependent one-carbon (1-C) pathway [8–10]. During the development of the germ cells and the maturation of the ovarian follicles, the methyl groups mainly derived from B vitamins, methionine and choline are used for DNA synthesis and phospholipid and protein biosynthesis. Deficiencies of these nutrients are inversely associated with ovarian follicle development, the number of oocytes retrieved for IVF treatment, embryo quality and pregnancy outcome [8–10]. Worrisome is that in more than 50% of subfertile couples planning pregnancy the preconception diet is inadequate [11]. However it is promising that improvement of the diet can increase the chance of ongoing pregnancy up to 65% [12]. The prevalence of obesity and PCOS is increasing and although obesity is not the cause of PCOS it contributes to the clinical spectrum [13]. Moreover, weight loss can improve the clinical spectrum including menstrual regularity, insulin resistance and quality of life [14] and dietary interventions are therefore part of the clinical treatment of PCOS.
In the polycystic ovary, an arrest of follicular maturation results in the accumulation of small antral follicles that produce elevated levels of Anti-Müllerian Hormone (AMH) [15]. AMH concentration is therefore a marker of PCOS severity which is inversely associated with a healthy diet, irrespective of weight loss [16].
Psychological co-morbidities such as depression, eating and anxiety disorders, which can affect the nutritional state are also more prevalent in PCOS [17]. Another potential driver for differences in dieting could be obesity although previous research showed that dieting behavior is independent of current BMI, but strongly associated with negative emotions and problematic behaviors [18]. Dietary behavior is acquired during childhood and remains rather stabile over time only varying during episodes of illnesses, dieting and increased needs [19–22]. Dieting habits can be transferred unknowingly since the practices mothers adopt predict the children’s diet quality [23] and because parents contribute to adolescents' motivation to diet [24]. Here we hypothesize that regular self-initiated dieting and using an inadequate diet is associated with alterations in the 1-C pathway and with impaired functioning of ovarian follicles which contributes to the clinical spectrum of PCOS. We performed a case-cohort study embedded in a prospective periconception cohort study of couples referred for subfertility, to investigate whether the use of a self-initiated diet and dietary inadequacy are associated with PCOS severity and phenotype.
Materials and Methods
Study design
Between October 2007 and March 2011, all couples planning pregnancy and visiting the outpatient clinic of the Department of Obstetrics and Gynaecology at the Erasmus MC, University Medical Centre Rotterdam, a tertiary hospital in the Netherlands, were offered nutrition and lifestyle counselling at the preconception outpatient clinic ‘Achieving a Healthy Pregnancy’ [11,12].
In this study women completed a questionnaire from which we extracted general characteristics, such as age, ethnicity (Dutch or Non-Dutch), educational level (low, intermediate or high), the use of a diet (energy restricted, vegetarian, macrobiotic, vegan, other) and of six main food groups (Preconception Dietary Risk score), folic acid supplements (yes/no), vitamin supplements (yes/no), medication (yes/no), alcohol (yes/no), and smoking (yes/no), physical exercise (yes/no), and the experience of stress (yes/no). Ethnicity and education level were classified according to the definition of Statistics Netherlands (www.cbs.nl; 2012). The validated summary PDR score, based on the Dutch food based dietary guidelines was used to assess diet inadequacy in the outpatient clinical setting [12,25]. When patients did not meet the dietary guidelines for the food group, one point was administered. Since six guidelines were evaluated, the PDR score had a maximum of 6 points and a minimum of 0 points. Thus, the higher the score the more inadequate the intake according to the guideline. The adequate intake per food group was defined without a specific time frame by the following guidelines: 4–5 slices of whole wheat bread daily, the use of monounsaturated or polyunsaturated oils/fats, 200 grams of vegetables daily, 2 pieces of fruit daily, 3–4 servings of meat weekly and 2 servings of fish weekly [26]. During the visit at the outpatient clinic the questionnaires were verified by a trained counsellor.
Subsequently non-fasting blood samples were obtained by venipuncture and anthropometrics (i.e. height, weight and circumferences) were measured. Patients with oligo- or anovulation were screened for ovulatory dysfunction by trained professionals according to a standardized protocol.
The diagnosis of PCOS was based on the Rotterdam criteria [5] including the presence of oligo- or anovulation (i.e. menstrual bleeding interval between 35–182 days or absence of menstrual bleeding for more than 182 days), hyperandrogenism (Ferriman-Gallwey score ≥ 9 and/or a Free Androgen Index > 4.5), and/ or polycystic ovaries (volume exceeding 10 cm3 and/or the follicle count was ≥ 12).
Study population
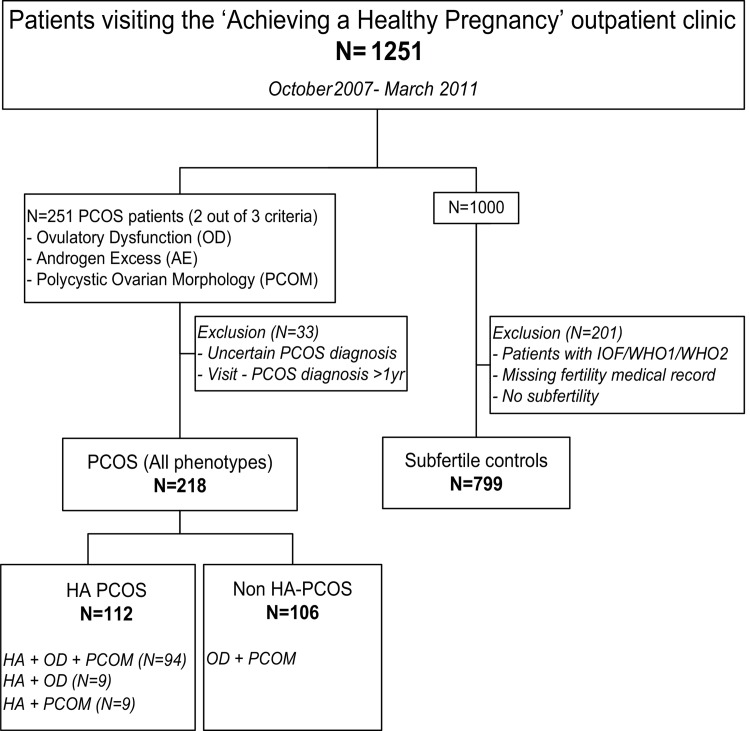
During the study period 1,348 couples visited the preconception outpatient clinic of which 1,251 participated in the study and provided a written informed consent. Two hundred and fifty one patients were diagnosed with PCOS and 33 patients were excluded because of an uncertain diagnosis or the period between enrolment and PCOS diagnosis comprised more than one year. From the remaining 1,000 patients we excluded patients with an incomplete fertility medical record (n = 80), patients with ovulatory disorders such as imminent ovarian failure, WHO 1 or WHO 2 disorders (n = 107) and fertile patients (n = 14) who were referred because of a complicated obstetrical history. This resulted in a control group of 799 subfertile patients (Fig 1). According to phenotype, we divided the PCOS group (n = 218) in the HA (n = 112) and non HA (n = 106) phenotype.
Fig 1. Flowchart of the selection of the study population.
IOF = imminent ovarian failure; WHO1 = hypogonadotropic hypogonadal anovulation; WHO2 = normogonadotropic normoestrogenic anovulation; HA = hyperandrogenic.
Biomarkers
On the day of the visit at the outpatient clinic blood samples were drawn and processed within 2 hours after withdrawal. Serum was stored at −20°C until assayed. In blood the biomarkers of 1-C metabolism serum and red blood cell (RBC) folate, serum cobalamin, and plasma total homocysteine (tHcy) were determined in all study participants. tHcy was measured using HPLC-TandemMS. For the determination of serum folate and cobalamin an immunoelectrochemoluminescence assay was used (E170; Roche Diagnostics GmbH, Mannheim, Germany) and for the determination of RBC folate the ADVIA 120 Hematology Analyzer was used (Bayer Diagnostics, Leverkusen, Germany).
For clinical purposes the patients with PCOS were additionally screened on hormonal and metabolic parameters. Testosterone was measured using radioimmunoassay (RIA) (Siemens DPC, Los Angeles, CA) and glucose levels were measured by using a Hitachi 917 analyzer (Roche Diagnostics, Almere, The Netherlands). The Free Androgen Index (FAI) was calculated as follows; 100 x [T (nmol/L) / Sex hormone-binding globulin (SHBG) (nmol/L)]. An immunoluminometric assay (Immulite® platform, Siemens Diagnostic Product Corporation, Los Angeles, CA) was used to measure LH, FSH, SHBG, androstenedione, insulin and DHEAS. Serum AMH (Anti-Müllerian Hormone) levels were measured by using an in-house double-antibody ELISA (GenII; Beckman Coulter) as previously described [27]. Intra- and interassay coefficients of variation were, respectively, <5% and <15% for LH, <3% and <18% for FSH, <3% and >5% for T, <8% and >11% for AD, <4% and >5% for SHBG, <9% and >11% for DHEAS, <5% and >8% for AMH, and <6% and >8% for insulin.
Statistical analyses
Continuous variables are presented as median with interquartile range. Dichotomous and categorical variables are presented as count and proportions. Chi Square tests with comparison of column proportions were performed with a Bonferroni correction to compare the distribution of categorical variables between PCOS phenotypes and subfertile controls. Mann Whitney U Tests were performed to compare the total group of PCOS with the subfertile controls. Kruskal Wallis tests with pairwise comparisons adjusted for the number of pairwise tests were performed for comparison of controls, HA-PCOS patients and non HA-PCOS patients. Simple linear regression analysis were conducted to establish associations between the PDR score and the biomarkers of 1-C metabolism. To achieve normality a natural log transformation was performed for cobalamin, RBC Folate and tHcy and a square root transformation for serum folate. Multivariable logistic regression analysis was applied to calculate the risk of PCOS after one point increase of the PDR score for each phenotype separately with adjustment for the potential confounders body mass index (BMI), age, educational level, ethnicity, medication use and vitamin supplement use. These covariates were selected because of the significant association with the PDR score or when significant differences revealed between PCOS and controls. Multivariable linear regression analysis were performed to investigate associations between the PDR score and hormonal parameters in PCOS only with adjustments. Statistical analyses were performed using SPSS software for Windows (version 21.0, IBM SPSS, Statistics for Windows, Armonk, NY: IBM Corp). The level of significance was set to 0.05 for all analyses.
Ethical approval
All questionnaire data and materials were processed anonymously. This study was conducted according to the guidelines of the Declaration of Helsinki, and all procedures involving patients were approved by the Medical Ethical and Institutional Review Board at the Erasmus MC, University Medical Centre in Rotterdam, the Netherlands. Written informed consent was obtained from all patients.
Results
To evaluate selection bias we present the general characteristics of the included and excluded patients in S1 Table. The general characteristics were comparable in both groups except the slightly younger age, higher percentage of smoking and alcohol use and slightly smaller waist circumference and waist-hip ratio in the study group.
PCOS study population
In Table 1 the general characteristics of PCOS patients (n = 218) and subfertile control patients (n = 799) are shown. The PCOS group was significantly younger and used less often alcohol, folic acid and vitamin supplements than subfertile controls, which is validated with the lower concentrations of serum and RBC folate observed in the PCOS group. They also used more often a self-initiated diet and were more frequently obese (BMI≥30).
Table 1. Preconception general characteristics.
| Total PCOS (n = 218) (Group A) | HA-PCOS (n = 112) (Group A1) | non HA-PCOS (n = 106) (Group A2) | Subfertile controls (n = 799) (Group B) | P value (Group A, B) | P value (Group A1, A2, B) | |
|---|---|---|---|---|---|---|
| Age (years) | 28.5 (25.5–31.3) | 27.7 (24.5–30.0)a | 29.0 (27.2–32.2)a | 33.1 (29.6–36.4)b | <0.01 | <0.01 |
| Ethnicity | ||||||
| Dutch | 122 (56.0%) | 54 (48.2%) | 68 (64.2%) | 445 (56.0%) | 1.00 | 0.06 |
| Other | 96 (44.0%) | 58 (51.8%) | 38 (35.8%) | 350 (44.0%) | - | - |
| Educational level | ||||||
| Low | 30 (14.6%) | 20 (18.7%) | 10 (10.1%) | 120 (15.8%) | 0.75 | 0.09 |
| Intermediate | 97 (47.1%) | 55 (51.4%) | 42 (42.4%) | 336 (44.2%) | - | - |
| High | 79 (38.3%) | 32 (29.9%) | 47 (47.5%) | 304 (40.0%) | - | - |
| Lifestyle Parameters: | ||||||
| Diet (Yes) | 43 (19.9%) | 25 (22.5%)b | 18 (17.1%)a, b | 105 (13.1%)a | 0.01 | 0.02 |
| PDR score (mean; sd) | 3.7 (1.14) | 3.9 (1.12)a | 3.5 (1.13)b | 3.5 (1.13)b | 0.02 | <0.01 |
| Folic acid supplement use (No) | 92 (42.4%) | 55 (49.1%)b | 37 (35.2%)a, b | 268 (33.5%)a | 0.02 | <0.01 |
| Vitamin supplement use (No) | 145 (67.4%) | 78 (70.3%) | 67 (64.4%) | 471 (59.5%) | 0.03 | 0.07 |
| Medication use (Yes) | 75 (34.6%) | 41 (36.6%) | 34 (32.4%) | 255 (32.0%) | 0.48 | 0.63 |
| Alcohol (Yes) | 101 (46.3%) | 39 (34.8%)b | 62 (58.5%)a | 439 (54.9%)a | 0.02 | <0.01 |
| Smoking (Yes) | 51 (23.5%) | 29 (26.1%) | 22 (20.8%) | 175 (22.3%) | 0.70 | 0.59 |
| Physical exercise (No) | 98 (51.0%) | 56 (60.2%)a | 42 (42.4%)b | 366 (49.4%)a, b | 0.68 | 0.04 |
| Stress (Yes) | 64 (34.4%) | 30 (33.7%) | 34 (35.1%) | 245 (33.6%) | 0.83 | 0.96 |
| Measurements: | ||||||
| BMI (kg/m2) | 25.6 (22.0–31.2) | 29.2 (24.9–33.2)a | 23.1 (20.6–26.6)b | 24.5 (22.0–28.3)c | 0.03 | <0.01 |
| BMI categories: | ||||||
| <20 | 21 (9.6%) | 3 (2.7%)a | 18 (17.0%)b | 54 (6.8%)a | <0.01 | <0.01 |
| ≥20 <25 | 82 (37.6%) | 27 (24.1%)b | 55 (51.9%)a | 373 (47.0%)a | - | - |
| ≥25 <30 | 52 (23.9%) | 31 (27.7%)a | 21 (19.8%)a | 228 (28.8%)a | - | - |
| ≥30 | 63 (28.9%) | 51 (45.5%)b | 12 (11.3%)a | 138 (17.4%)a | - | - |
| Waist circumference (cm) | 85 (74–97) | 93 (83–105)a | 77 (71–89)b | 84 (75–93)c | 0.28 | <0.01 |
| Waist hip ratio | 0.81 (0.75–0.87) | 0.84 (0.80–0.91)a | 0.78 (0.74–0.83)b | 0.81 (0.75–0.87)c | 0.66 | <0.01 |
| Biochemical Parameters: | ||||||
| Cobalamin (pmol/L) | 305 (232–392) | 282 (215–371) | 322 (254–416) | 310 (237–411) | 0.51 | 0.09 |
| RBC Folate (nmol/L) | 960 (769–1182) | 861 (718–1067)a | 1059 (869–1380)b | 1037 (831–1328)b | <0.01 | <0.01 |
| Folate (nmol/L) | 25.1 (15.6–36.1) | 21.3 (13.4–30.9)a | 29.1 (17.8–43.4)b | 30.1 (19.0–41.3)b | <0.01 | <0.01 |
| tHcy (μmol/L) | 8.8 (7.4–10.2) | 8.5 (7.3–10.2) | 9.1 (7.7–10.2) | 8.4 (7.1–9.9) | 0.08 | 0.12 |
Note: Values are expressed as median (interquartile range) or number (%). HA = Hyperandrogenic; PDR score = Preconception Dietary Risk score; BMI = Body Mass Index; RBC Folate = Red Blood Cell Folate; tHcy = homocysteine. Normal range biochemical parameters; Folate ≥8 nmol/L, RBC folate ≥500 nmol/L, Cobalamin ≥145 pmol/L, tHcy <15 μmol/L. For pairwise comparisons; each subscript letter denotes a subset of categories whose column proportions do not differ significantly from each other.
PCOS phenotypes
In Table 1 we depict the PCOS group divided into the HA (n = 112) and non HA phenotype (n = 106) and compare the general characteristics with subfertile controls (n = 799). The HA and non HA phenotype patients were both significantly younger than subfertile controls. Patients with the HA phenotype exercised less than non HA-PCOS patients and they used less often alcohol than non HA-PCOS patients or subfertile controls. Patients with the HA phenotype were also more likely to use a self-initiated diet and used less often folic acid supplements than subfertile controls and showed a significantly higher BMI and waist circumference compared to the non HA phenotype and subfertile controls. Moreover, the waist hip ratio, used as indicator of central obesity, was significantly lower in the non HA phenotype, compared to the HA phenotype and subfertile controls. Patients with the HA phenotype showed lower levels of serum and RBC folate than the non HA phenotype and subfertile controls.
Dietary inadequacy assessed with the PDR score
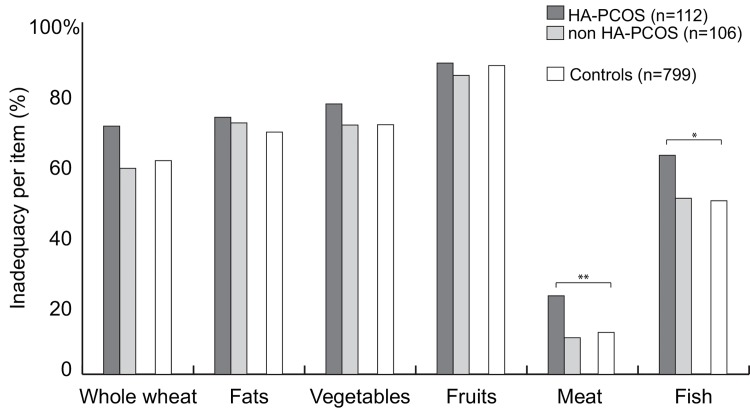
A higher mean PDR score was observed in the PCOS group than in the subfertile controls (3.7 vs 3.5; p = 0.017). Patients with the HA phenotype also showed a higher mean PDR score (3.9; p <0.001) than the non HA phenotypes (3.5) and the subfertile controls (3.5). The PDR score per food group was comparable between the groups, except for a higher percentage of inadequate meat and fish intake in the HA phenotype (Fig 2).
Fig 2. The items of the Preconception Dietary risk score.
Depicted are the percentages of inadequate dietary intake for each food group. Meat* and fish** intake differed significantly between the controls, HA and non HA phenotype (respectively p 0.006 and p 0.039).
Table 2 shows that one point increase in the PDR score was associated with a 1.4 fold higher risk of the HA phenotype (unadjusted OR 1.427, 95% CI 1.183 to 1.721). The PDR score was inversely associated with age (β -0.037, 95% CI -0.051 to -0.023, p = <0.001), educational level (category high β -0.311, 95% CI -0.522 to -0.100, p = 0.004) and the use of vitamin supplements (β -0.192, 95% CI -0.336 to -0.048, p = 0.009) and therefore these covariates were selected for adjustment of the analyses. Age was a confounder and inversely associated with the severity of the PCOS phenotype and positively associated with the PDR score. After adjustment in model 1 the risk of the HA phenotype slightly increased (OR 1.520, 95% CI 1.240 to 1.863) and attenuated in model 2 (OR 1.351, 95% CI 1.087 to 1.679), but remained significant. The PDR score was not associated with the risk of the non HA phenotype.
Table 2. Multivariable logistic regression analyses; The risk of PCOS after one point increase in the Preconception Dietary Risk score compared to subfertile controls.
| HA-PCOS | non HA-PCOS | |||||
|---|---|---|---|---|---|---|
| OR | P value | 95% CI | OR | P value | 95% CI | |
| Unadjusted: | 1.427 | 0.000 | 1.183–1.721 | 0.978 | 0.805 | 0.817–1.170 |
| Model I: Adjusted for BMI, ethnicity, education, vitamin supplement use and medication use | 1.520 | 0.000 | 1.240–1.863 | 0.937 | 0.520 | 0.767–1.143 |
| Model II: Adjusted for age, BMI, ethnicity, education, vitamin supplement use and medication use | 1.351 | 0.007 | 1.087–1.679 | 0.866 | 0.176 | 0.703–1.067 |
HA = Hyperandrogenic; OR = Odds Ratio; CI = Confidence Interval; BMI = Body Mass Index.
The PDR score and biomarkers of 1-C metabolism
To investigate the association between dietary inadequacy and the markers of 1-C metabolism, we subsequently performed linear regression analyses in the PCOS patients and subfertile controls with the separate biomarkers cobalamin, RBC folate, serum folate and tHcy. A negative linear association was established between the PDR score and cobalamin (unadjusted β -0.052; p < 0.001; 95% CI -0.074 to -0.030), RBC folate (unadjusted β -0.045; p < 0.001; 95% CI -0.067 to -0.023) and serum folate (unadjusted β -0.193; p < 0.001; 95% CI -0.295 to -0.091). A positive linear association was observed between the PDR score and tHcy (unadjusted β 0.030; p < 0.001; 95% CI 0.014 to 0.045). Additional adjustment for age, BMI, ethnicity, education, vitamin supplement use and medication use slightly attenuated the effect estimates of the four biomarkers and remained significant.
Diet and hormonal and metabolic parameters in PCOS patients
In Table 3 the serum characteristics of the PCOS phenotypes are depicted. The PDR score was positively associated with AMH and FAI. A positive linear association was established between the PDR score and AMH (unadjusted β 0.084; p = 0.044; 95% CI 0.002 to 0.165), however after adjustment for age and BMI this association was no longer significant.
Table 3. Serum characteristics according to PCOS phenotype.
| HA-PCOS (n = 112) | non HA-PCOS (n = 106) | |
|---|---|---|
| Hormonal Parameters: | ||
| AMH (μg/L) | 10.3 (6.7–14.8) | 7.6 (4.8–11.2)** |
| FSH (U/l) | 6.1 (5.2–7.2) | 5.9 (3.9–7.8) |
| LH (U/l) | 9.8 (6.7–14.6) | 6.6 (4.4–11.7)** |
| Progesterone (nmol/l) | 1.2 (0.9–1.9) | 1.5 (0.9–10.2)* |
| 17- hydroxyprogesterone (nmol/l) | 2.9 (2.2–4.4) | 2.7 (1.9–5.8) |
| Estradiol (pmol/L) | 225.5 (181.0–300.5) | 234.0 (143.0–363.0) |
| SHBG (nmol/l) | 27.0 (21.4–39.7) | 56.4 (45.3–81.6)** |
| Testosterone (nmol/l) | 2.0 (1.6–2.8) | 1.2 (0.9–1.6)** |
| FAI | 7.0 (5.5–10.7) | 2.2 (1.4–3.0)** |
| Androstenedione (nmol/l) | 11.8 (9.7–17.4) | 8.6 (6.4–10.4)** |
| DHEAS(μmol/L) | 5.7 (3.9–7.4) | 4.8 (3.3–6.1)** |
| Metabolic Parameters: | ||
| Insulin (pmol/l) | 67.0 (40.0–118.0) | 34.0 (17.0–57.0)** |
| Glucose (mmol/l) | 4.9 (4.6–5.3) | 4.7 (4.5–5.0)** |
| TG (mmol/L) | 1.0 (0.8–1.6) | 0.9 (0.7–1.2)* |
| Total–C (nmol/L) | 5.4 (4.6–6.7) | 5.2 (4.5–6.7) |
| HDL-C (nmol/L) | 1.4 (1.1–1.8) | 1.7 (1.4–2.2)** |
| LDL-C (nmol/L) | 3.8 (3.1–4.6) | 3.4 (2.8–4.4)* |
| Apolipoprotein A1 | 209.6 (183.3–256.7) | 239.4 (198.0–281.8)** |
| Apolipoprotein B | 124.1 (103.6–148.8) | 115.2 (84.1–136.1)** |
Note: Values are expressed as median (interquartile range)
* = p <0.05
** = p <0.01.
HA = Hyperandrogenic; FSH = Follicle Stimulating Hormone; LH = Luteinizing Hormone; SHBG = Sex Hormone Binding Globulin; FAI = Free Androgen Index; DHEAS = dehydroepiandrosterone sulfate; TG = Triglycerides; Total-C = total Cholesterol; HDL-C = HDL-cholesterol; LDL-C = LDL-cholesterol. Mann Whitney U Tests were performed.
This association was stronger when repeated only in the HA+OD+PCOM and OD+PCOM PCOS patients (unadjusted β 0.109; p = 0.011, 95% CI 0.025 to 0.193) and remained significant after adjustment for age (β 0.100; p = 0.022, 95% CI 0.015 to 0.185) and BMI (β 0.102; p = 0.018, 95% CI 0.018 to 0.186).
The PDR score was also positively associated with FAI (unadjusted β 0.128; p = 0.013; 95% CI 0.028 to 0.229), but lost significance after adjustment for age and BMI.
Discussion
This explorative study demonstrates that patients with PCOS more often use a self-initiated diet and an inadequate diet than subfertile controls. This is substantiated by the use of a more inadequate diet in patients with the more severe HA phenotype and the positive association between diet inadequacy and the AMH concentration in PCOS patients.
The strength of the study is the large sample size and case-cohort design to reduce selection and information bias. Furthermore, standardized anthropometric measurements were performed by trained counsellors who verified all questionnaire data individually during the preconception visit at the outpatient clinic. Several adjustments were made including BMI to minimize the presence of underreporting and overreporting of dietary intake. The PDR score was previously validated in the same study population and is shown to be a sensitive and simple tool to assess diet inadequacy [25]. Another strength of the study is that biomarkers were measured to validate the PDR score and that all laboratory determinations were measured in the same hospital. The PDR score estimates dietary inadequacy and not unhealthy overconsumption which is a limitation [25]. We are aware that although the PDR score covers the six main food groups, it does not reflect the total nutrient and energy intake. The control group consisted of patients referred for subfertility which reduces the external validity of the study. We were able to use clinical and laboratory data for PCOS diagnosis, which were not available for subfertile controls.
Despite numerous efforts, genetic research does not elucidate the developmental origin of PCOS. We suggest that this can be due to a more significant role of gene-nutrient interactions affecting epigenetic mechanisms of multiple pathways regulating ovarian function during the course of life. Previous research demonstrated that genes are prenatally programmed by androgen exposure in utero, resulting in disturbed ovarian and adrenal steroidogenesis [28], impaired oocyte development [29] and PCOM [30]. The diet is important in epigenetics by supplying substrates and methyl groups that are important for gene methylation, programming and subsequent expression and silencing of the genome. Dietary inadequacies can therefore lead amongst other pathways to derangements in the 1-C pathway, resulting in hypomethylation and altered gene expression [8,31]. As a first hit the nutritional environment in-utero can affect reproductive function in the offspring through impaired gonadal organogenesis before birth [31–33]. In rabbits, Leveille et al showed prenatal and postnatal effects of the diet on ovarian function and morphology by the number of atretic follicles [34]. Not only during early life but also postnatally through puberty and adolescence, diet can affect gene expression as second or third hit influencing disease occurrence and severity. Adolescents suffering from overweight and psychological problems are at risk to develop nutritional inadequacies [17,35]. Therefore these data support our hypothesis that the use of a self-initiated diet and dietary inadequacies can contribute to a clinical heterogeneous spectrum of PCOS phenotype development.
This study showed an association between the use of a more inadequate diet in PCOS patients and disease severity which is further underlined by a lower serum and RBC folate. Homocysteine concentrations were higher as well, although this did not reach statistical significance. The study of Rodrigues et al supports this finding showing a poor diet quality in PCOS patients (56% inadequacy according to the Brazilian healthy eating index) [36]. Studies comparing diet quality in PCOS to controls are contradictory. Comparable dietary compositions in PCOS and controls are reported [37–39], whereas the group of Moran et al observed a higher diet quality in PCOS [40]. A high prevalence of folate deficiency and increased levels of homocysteine is previously observed in PCOS substantiating our results and the association with the involvement of the one carbon pathway [41,42]. An explanation for the different findings in other studies is that in all other studies the assessment of diet inadequacy was performed always after diagnosing PCOS. Moreover, due to the exclusion of dieting, non-stabled weighted or normal weighted PCOS patients in other studies, selection bias could explain the different results. Information bias due to the awareness of PCOS and the intention to treat can also explain the differences with other studies. Finally, in contrast to other studies, we also investigated the association with the severity of the PCOS phenotypes and compared them with subfertile controls.
Our results are in line with studies showing the importance of dietary treatment in PCOS for reproductive and metabolic outcome [16,43,44]. Furthermore, in overweighed PCOS patients it is observed that folate supplement use had beneficial effects on metabolic profiles [45], which indicates the involvement of the 1-C cofactor folate in PCOS.
The positive linear association between the PDR score and AMH concentration, and PCOS severity very much supports the function of AMH as marker of the spectrum [46]. This is supported by Nybacka et al showing a decrease in AMH in PCOS patients following a 4 month calorie restricted, well-balanced diet [16]. Another observation is that PCOS gradually disappears during ageing [47,48]. We suggest that the known improvement of the diet during the course of life also contributes to its disappearance which is supported by the gradual decrease of AMH during ageing [49]. FAI as marker for androgen excess was also positively associated with diet inadequacy, which is in line with the findings of others. [43]. According to the biological gradient criteria of Hill, the associations of AMH and FAI with the PDR score strengthen the relationship between diet inadequacy and PCOS severity. This is substantiated by a stronger linear association between the PDR score and AMH after exclusion of the subpopulation without PCOM (HA+OD) or without OD (HA+PCOM). After adjustment for age and BMI this association remained significant, demonstrating the heterogeneity of the hyperandrogenic phenotype.
To conclude, this explorative study shows for the first time that the use of a self-initiated diet and diet inadequacy are associated with PCOS as well as PCOS phenotype. These findings emphasize the need for prospective research from the reproductive life course onwards with a focus on the role of the inadequacy of the diet in the developmental origins of PCOS. Moreover, in PCOS treatment more attention should be given to the adherence of an adequate diet.
Supporting Information
(SAV)
Note: Values are expressed as median (interquartile range) or number (%),* = p <0.05; ** = p <0.01. PDR score = Preconception Dietary Risk score; BMI = Body Mass Index; RBC Folate = Red Blood Cell Folate; tHcy = homocysteine. Normal range biochemical parameters; Folate ≥8 nmol/L, RBC folate ≥500 nmol/L, Cobalamin ≥145 pmol/L, tHcy <15 μmol/L. Chi Square tests and Mann Whitney U Tests were performed.
(DOC)
Acknowledgments
We would like to thank the team of the outpatient clinic of the Division of Reproductive Medicine and Obstetrics and Prenatal Medicine of the Department of Obstetrics and Gynaecology at the Erasmus MC, University Medical Centre in Rotterdam, the Netherlands for their significant clinical and research contributions.
Abbreviations
- 1-C
One-Carbon
- AMH
Anti-Müllerian Hormone concentration
- BMI
Body Mass Index
- FAI
Free Androgen Index
- HA
Hyperandrogenism
- OD
Ovulatory Dysfunction
- PCOM
Polycystic Ovarian Morphology
- PCOS
Polycystic Ovary Syndrome
- PDR score
Preconception Dietary Risk Score
- RBC folate
Red Blood Cell folate
- RIA
Radioimmunoassay
- SHBG
Sex Hormone-Binding Globulin
- tHcy
plasma total homocysteine
Data Availability
All relevant data are within the paper and its Supporting Information files. Further data can be requested from the corresponding author: r.steegers@erasmusmc.nl.
Funding Statement
The authors have no support or funding to report.
References
- 1. Talbott EO, Zborowski JV, Rager JR, Boudreaux MY, Edmundowicz DA, Guzick DS Evidence for an association between metabolic cardiovascular syndrome and coronary and aortic calcification among women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2004;89: 5454–5461. [DOI] [PubMed] [Google Scholar]
- 2. Azziz R, Woods KS, Reyna R, Key TJ, Knochenhauer ES, Yildiz BO The prevalence and features of the polycystic ovary syndrome in an unselected population. J Clin Endocrinol Metab. 2004;89: 2745–2749. [DOI] [PubMed] [Google Scholar]
- 3. Knochenhauer ES, Key TJ, Kahsar-Miller M, Waggoner W, Boots LR, Azziz R Prevalence of the polycystic ovary syndrome in unselected black and white women of the southeastern United States: a prospective study. J Clin Endocrinol Metab. 1998;83: 3078–3082. [DOI] [PubMed] [Google Scholar]
- 4. Kosova G, Urbanek M Genetics of the polycystic ovary syndrome. Mol Cell Endocrinol. 2013;373: 29–38. 10.1016/j.mce.2012.10.009 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Rotterdam EA-SPCWG Revised 2003 consensus on diagnostic criteria and long-term health risks related to polycystic ovary syndrome. Fertil Steril. 2004;81: 19–25. [DOI] [PubMed] [Google Scholar]
- 6. Daan NM, Louwers YV, Koster MP, Eijkemans MJ, de Rijke YB, Lentjes EW, et al. Cardiovascular and metabolic profiles amongst different polycystic ovary syndrome phenotypes: who is really at risk? Fertil Steril. 2014;102: 1444–1451 e1443. 10.1016/j.fertnstert.2014.08.001 [DOI] [PubMed] [Google Scholar]
- 7. Anderson K, Nisenblat V, Norman R Lifestyle factors in people seeking infertility treatment—A review. Aust N Z J Obstet Gynaecol. 2010;50: 8–20. 10.1111/j.1479-828X.2009.01119.x [DOI] [PubMed] [Google Scholar]
- 8. Steegers-Theunissen RP, Twigt J, Pestinger V, Sinclair KD The periconceptional period, reproduction and long-term health of offspring: the importance of one-carbon metabolism. Hum Reprod Update. 2013;19: 640–655. 10.1093/humupd/dmt041 [DOI] [PubMed] [Google Scholar]
- 9. Boxmeer JC, Steegers-Theunissen RP, Lindemans J, Wildhagen MF, Martini E, Steegers EA, et al. Homocysteine metabolism in the pre-ovulatory follicle during ovarian stimulation. Hum Reprod. 2008;23: 2570–2576. 10.1093/humrep/den292 [DOI] [PubMed] [Google Scholar]
- 10. Kralikova M, Melounova J, Crha I, Matejovicova M, Zakova J, Jarkovsky J, et al. Intraindividual variability of homocysteine and related thiols concentrations in follicular fluid. J Assist Reprod Genet. 2011;28: 863–868. 10.1007/s10815-011-9606-9 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Hammiche F, Laven JS, van Mil N, de Cock M, de Vries JH, Lindemans J, et al. Tailored preconceptional dietary and lifestyle counselling in a tertiary outpatient clinic in The Netherlands. Hum Reprod. 2011;26: 2432–2441. 10.1093/humrep/der225 [DOI] [PubMed] [Google Scholar]
- 12. Twigt JM, Bolhuis ME, Steegers EA, Hammiche F, van Inzen WG, Laven JS, et al. The preconception diet is associated with the chance of ongoing pregnancy in women undergoing IVF/ICSI treatment. Hum Reprod. 2012;27: 2526–2531. 10.1093/humrep/des157 [DOI] [PubMed] [Google Scholar]
- 13. Legro RS Obesity and PCOS: implications for diagnosis and treatment. Semin Reprod Med. 2012;30: 496–506. 10.1055/s-0032-1328878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Moran LJ, Ko H, Misso M, Marsh K, Noakes M, Talbot M, et al. Dietary composition in the treatment of polycystic ovary syndrome: a systematic review to inform evidence-based guidelines. J Acad Nutr Diet. 2013;113: 520–545. 10.1016/j.jand.2012.11.018 [DOI] [PubMed] [Google Scholar]
- 15. Durlinger AL, Visser JA, Themmen AP Regulation of ovarian function: the role of anti-Mullerian hormone. Reproduction. 2002;124: 601–609. [DOI] [PubMed] [Google Scholar]
- 16. Nybacka A, Carlstrom K, Fabri F, Hellstrom PM, Hirschberg AL Serum antimullerian hormone in response to dietary management and/or physical exercise in overweight/obese women with polycystic ovary syndrome: secondary analysis of a randomized controlled trial. Fertil Steril. 2013;100: 1096–1102. 10.1016/j.fertnstert.2013.06.030 [DOI] [PubMed] [Google Scholar]
- 17. Moran L, Gibson-Helm M, Teede H, Deeks A Polycystic ovary syndrome: a biopsychosocial understanding in young women to improve knowledge and treatment options. J Psychosom Obstet Gynaecol. 2010;31: 24–31. 10.3109/01674820903477593 [DOI] [PubMed] [Google Scholar]
- 18. Ackard DM, Croll JK, Kearney-Cooke A Dieting frequency among college females: association with disordered eating, body image, and related psychological problems. J Psychosom Res. 2002;52: 129–136. [DOI] [PubMed] [Google Scholar]
- 19. Devine CM, Bove CF, Olson CM Continuity and change in women's weight orientations and lifestyle practices through pregnancy and the postpartum period: the influence of life course trajectories and transitional events. Soc Sci Med. 2000;50: 567–582. [DOI] [PubMed] [Google Scholar]
- 20. Riboli E, Toniolo P, Kaaks R, Shore RE, Casagrande C, Pasternack BS Reproducibility of a food frequency questionnaire used in the New York University Women's Health Study: effect of self-selection by study subjects. Eur J Clin Nutr. 1997;51: 437–442. [DOI] [PubMed] [Google Scholar]
- 21. Willett WC. Nutritional Epidemiology. 2nd ed. New York: Oxford University Press, Inc.; 1998. [Google Scholar]
- 22. Willett WC Nutritional epidemiology issues in chronic disease at the turn of the century. Epidemiol Rev. 2000;22: 82–86. [DOI] [PubMed] [Google Scholar]
- 23. Jarman M, Ogden J, Inskip H, Lawrence W, Baird J, Cooper C, et al. How do mothers manage their preschool children's eating habits and does this change as children grow older? A longitudinal analysis. Appetite. 2015;95: 466–474. 10.1016/j.appet.2015.08.008 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Katz I, Madjar N, Harari A Parental support and adolescent motivation for dieting: the self-determination theory perspective. J Psychol. 2015;149: 461–479. 10.1080/00223980.2014.903890 [DOI] [PubMed] [Google Scholar]
- 25. Huijgen N, van de Kamp M, Twigt j, de Vries J, Eilers P, Steegers E, et al. The Preconception Dietary Risk score; a simple tool to assess an inadequate habitual diet for clinical practice. e-SPEN Journal. 2013;9: e13–e19. [Google Scholar]
- 26.The Netherlands Nutrition Centre. Richtlijnen voedselkeuze [in Dutch] (2011) http://www.voedingscentrum.nl/Assets/Uploads/Documents/Voedingsvoorlichters/Richtlijnen_voedselkeuze_2011.pdf.
- 27. de Vet A, Laven JS, de Jong FH, Themmen AP, Fauser BC Antimullerian hormone serum levels: a putative marker for ovarian aging. Fertil Steril. 2002;77: 357–362. [DOI] [PubMed] [Google Scholar]
- 28. Eisner JR, Barnett MA, Dumesic DA, Abbott DH Ovarian hyperandrogenism in adult female rhesus monkeys exposed to prenatal androgen excess. Fertil Steril. 2002;77: 167–172. [DOI] [PubMed] [Google Scholar]
- 29. Dumesic DA, Schramm RD, Peterson E, Paprocki AM, Zhou R, Abbott DH Impaired developmental competence of oocytes in adult prenatally androgenized female rhesus monkeys undergoing gonadotropin stimulation for in vitro fertilization. J Clin Endocrinol Metab. 2002;87: 1111–1119. [DOI] [PubMed] [Google Scholar]
- 30. Abbott DH, Dumesic DA, Franks S Developmental origin of polycystic ovary syndrome—a hypothesis. J Endocrinol. 2002;174: 1–5. [DOI] [PubMed] [Google Scholar]
- 31. Sinclair KD, Watkins AJ Parental diet, pregnancy outcomes and offspring health: metabolic determinants in developing oocytes and embryos. Reprod Fertil Dev. 2013;26: 99–114. 10.1071/RD13290 [DOI] [PubMed] [Google Scholar]
- 32. Dupont C, Cordier AG, Junien C, Mandon-Pepin B, Levy R, Chavatte-Palmer P Maternal environment and the reproductive function of the offspring. Theriogenology. 2012;78: 1405–1414. 10.1016/j.theriogenology.2012.06.016 [DOI] [PubMed] [Google Scholar]
- 33. Sloboda DM, Hickey M, Hart R Reproduction in females: the role of the early life environment. Hum Reprod Update. 2011;17: 210–227. 10.1093/humupd/dmq048 [DOI] [PubMed] [Google Scholar]
- 34. Leveille P, Tarrade A, Dupont C, Larcher T, Dahirel M, Poumerol E, et al. Maternal high-fat diet induces follicular atresia but does not affect fertility in adult rabbit offspring. J Dev Orig Health Dis. 2014;5: 88–97. 10.1017/S2040174414000014 [DOI] [PubMed] [Google Scholar]
- 35. Sanchez N A life course perspective on polycystic ovary syndrome. Int J Womens Health. 2014;6: 115–122. 10.2147/IJWH.S55748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Rodrigues AM, Martins LB, Franklin AM, Candido AL, Santos LC, Ferreira AV Poor quality diet is associated with overweight status and obesity in patients with polycystic ovary syndrome. J Hum Nutr Diet. 2014;28: 94–101. 10.1111/jhn.12205 [DOI] [PubMed] [Google Scholar]
- 37. Altieri P, Cavazza C, Pasqui F, Morselli AM, Gambineri A, Pasquali R Dietary habits and their relationship with hormones and metabolism in overweight and obese women with polycystic ovary syndrome. Clin Endocrinol (Oxf). 2013;78: 52–59. [DOI] [PubMed] [Google Scholar]
- 38. Douglas CC, Norris LE, Oster RA, Darnell BE, Azziz R, Gower BA Difference in dietary intake between women with polycystic ovary syndrome and healthy controls. Fertil Steril. 2006;86: 411–417. [DOI] [PubMed] [Google Scholar]
- 39. Wright CE, Zborowski JV, Talbott EO, McHugh-Pemu K, Youk A Dietary intake, physical activity, and obesity in women with polycystic ovary syndrome. Int J Obes Relat Metab Disord. 2004;28: 1026–1032. [DOI] [PubMed] [Google Scholar]
- 40. Moran LJ, Ranasinha S, Zoungas S, McNaughton SA, Brown WJ, Teede HJ The contribution of diet, physical activity and sedentary behaviour to body mass index in women with and without polycystic ovary syndrome. Hum Reprod. 2013;28: 2276–2283. 10.1093/humrep/det256 [DOI] [PubMed] [Google Scholar]
- 41. Ciotta L, Stracquadanio M, Pagano I, Carbonaro A, Palumbo M, Gulino F Effects of myo-inositol supplementation on oocyte's quality in PCOS patients: a double blind trial. Eur Rev Med Pharmacol Sci. 2011;15: 509–514. [PubMed] [Google Scholar]
- 42. Guzelmeric K, Alkan N, Pirimoglu M, Unal O, Turan C Chronic inflammation and elevated homocysteine levels are associated with increased body mass index in women with polycystic ovary syndrome. Gynecol Endocrinol. 2007;23: 505–510. [DOI] [PubMed] [Google Scholar]
- 43. Nybacka A, Carlstrom K, Stahle A, Nyren S, Hellstrom PM, Hirschberg AL Randomized comparison of the influence of dietary management and/or physical exercise on ovarian function and metabolic parameters in overweight women with polycystic ovary syndrome. Fertil Steril. 2011;96: 1508–1513. 10.1016/j.fertnstert.2011.09.006 [DOI] [PubMed] [Google Scholar]
- 44. Thomson RL, Buckley JD, Noakes M, Clifton PM, Norman RJ, Brinkworth GD The effect of a hypocaloric diet with and without exercise training on body composition, cardiometabolic risk profile, and reproductive function in overweight and obese women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93: 3373–3380. 10.1210/jc.2008-0751 [DOI] [PubMed] [Google Scholar]
- 45. Bahmani F, Karamali M, Shakeri H, Asemi Z The effects of folate supplementation on inflammatory factors and biomarkers of oxidative stress in overweight and obese women with polycystic ovary syndrome: a randomized, double-blind, placebo-controlled clinical trial. Clin Endocrinol (Oxf). 2014;81: 582–587. [DOI] [PubMed] [Google Scholar]
- 46. Homburg R, Ray A, Bhide P, Gudi A, Shah A, Timms P, et al. The relationship of serum anti-Mullerian hormone with polycystic ovarian morphology and polycystic ovary syndrome: a prospective cohort study. Hum Reprod. 2013;28: 1077–1083. 10.1093/humrep/det015 [DOI] [PubMed] [Google Scholar]
- 47. Brown ZA, Louwers YV, Fong SL, Valkenburg O, Birnie E, de Jong FH, et al. The phenotype of polycystic ovary syndrome ameliorates with aging. Fertil Steril. 2011;96: 1259–1265. 10.1016/j.fertnstert.2011.09.002 [DOI] [PubMed] [Google Scholar]
- 48. Laven JS, Mulders AG, Visser JA, Themmen AP, De Jong FH, Fauser BC Anti-Mullerian hormone serum concentrations in normoovulatory and anovulatory women of reproductive age. J Clin Endocrinol Metab. 2004;89: 318–323. [DOI] [PubMed] [Google Scholar]
- 49. Mulders AG, Laven JS, Eijkemans MJ, de Jong FH, Themmen AP, Fauser BC Changes in anti-Mullerian hormone serum concentrations over time suggest delayed ovarian ageing in normogonadotrophic anovulatory infertility. Hum Reprod. 2004;19: 2036–2042. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(SAV)
Note: Values are expressed as median (interquartile range) or number (%),* = p <0.05; ** = p <0.01. PDR score = Preconception Dietary Risk score; BMI = Body Mass Index; RBC Folate = Red Blood Cell Folate; tHcy = homocysteine. Normal range biochemical parameters; Folate ≥8 nmol/L, RBC folate ≥500 nmol/L, Cobalamin ≥145 pmol/L, tHcy <15 μmol/L. Chi Square tests and Mann Whitney U Tests were performed.
(DOC)
Data Availability Statement
All relevant data are within the paper and its Supporting Information files. Further data can be requested from the corresponding author: r.steegers@erasmusmc.nl.