Abstract
Biocompatible optical waveguides were constructed entirely of silk fibroin. A silk film (n=1.54) was encapsulated within a silk hydrogel (n=1.34) to form a robust and biocompatible waveguide. Such waveguides were made using only biologically and environmentally friendly materials without the use of harsh solvents. Light was coupled into the silk waveguides by direct incorporation of a glass optical fiber. These waveguides are extremely flexible, and strong enough to survive handling and manipulation. Cutback measurements showed propagation losses of approximately 2 dB/cm. The silk waveguides were found to be capable of guiding light through biological tissue.
OCIS codes: (160.1435) Biomaterials, (230.7370) Waveguides, (160.5470) Polymers, (170.3890) Medical optics instrumentation
1. Introduction
The delivery of light to a small volume within the body has tremendous potential to extend the utility of light-based imaging and therapy. Such delivery is challenging due to the absorption and scattering characteristics of biological tissue. In the visible spectrum penetration of light is limited to only a few hundred microns. Absorption of near infrared light by chromophores is minimized around 850 nm resulting in a maximum depth of penetration of several centimeters. However, scattering from cell nuclei and other structures causes light delivered at a point to rapidly diffuse through a large volume of tissue [1].
Optical fibers are an attractive method for delivery of light because they are small, inexpensive, and highly efficient. Although glass is biologically inert, these fibers are not well suited to biological applications. Broken fibers are sharp and can cause damage to surrounding tissue. Glass is also very stiff and this mechanical mismatch can damage surrounding soft tissue due to either fiber motion or natural body motions such as heartbeat and breathing. The mismatch in stiffness between the tissue and the fiber can result in scar formation [2] which may limit light delivery to the desired target. Implanted glass fibers are currently used to deliver light for treatment of malignant brain tumors [3], but this technique is limited to severe cases due to the risks outlined above. To wire the body with optical waveguides, a soft light guiding material is needed.
Hydrogels of biopolymers are used as materials for scaffolds in tissue engineering. They are easily interfaced with the biological environment due to their mechanical properties; porosity, which resembles that of extra cellular matrix; and their high water content. Hydrogels capable of transmitting light in vivo have previously been described that are capable of using encapsulated cells for sensing and therapy [4]. The index of refraction of these gels, however, was lower than that of the surrounding tissue making the constructs incapable of guiding light around bends in tissue. This severe limitation was recently addressed by the development of a step-index optical waveguide made solely from hydrogels that are capable of guiding light around curves in tissue [5]. However, dichloromethane, a harsh organic solvent, is used in their manufacture raising concerns about long term biocompatibility and preventing the encapsulation of cells or functional biomolecules.
The use of hydrogels as waveguides has also been investigated for sensing of various biomolecules [6, 7]. Many hydrogels are amenable to cell encapsulation which can then be used for light-mediated sensing or drug release [4, 8]. Other research into biocompatible optical waveguides have utilized a variety of other materials including cellulose [9], silicone [10], agarose [8], and even bacterial cells [11], but due to high propagation losses, these materials have not seen rapid adoption.
Silk fibroin has been under steady investigation for the last several decades as an attractive material for tissue engineering due to its all aqueous processing, biocompatibility, non-immunogenicity, and ability to stabilize labile compounds such as DNA and enzymes [12]. It has also been investigated as an attractive optical material due to its near complete transparency, and ability to conform to nano-scale structures such as photonic crystals and diffraction gratings [13]. Nearly all silk processing is done in water without any harsh chemicals making it possible to dope silk devices with cells [14], growth factors [15], and laser dyes [16]. By using a material able to degrade within the body after a period of time, as is possible with silk [17], the need for a second surgery to retrieve the implanted waveguide is eliminated.
Previous efforts have realized printed silk optical waveguides on quartz which predominantly used ambient air as a cladding layer. These waveguides had low loss (~0.5 dB/cm) propagation but were not free-standing limiting their utility for in vivo applications [18].
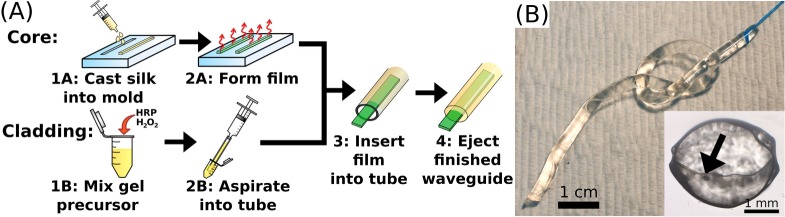
In this letter we present an optical waveguide made entirely of silk fibroin (Fig. 1(A)). The core of the waveguide was a long narrow strip of silk film with an index of refraction of 1.54 [18]. The silk film was surrounded by a silk hydrogel comprised of greater than 90% water resulting in a refractive index of around 1.34. The index of refraction difference was large enough to provide strong guidance and propagation of light within the core of the waveguide. The flexibility, biodegradablility, and low loss of these waveguides makes them ideally suited for biomedical applications.
Fig. 1.
Panel A: schematic representation of the construction of silk optical waveguides. To fabricate the core, silk solution is cast into molds (1A) and allowed to dry into films (1B). For the cladding, HRP and H2O2 are added to a silk solution (1B) and aspirated into a PTFE tube (2B). Prior to gelation, the film is inserted into the liquid gel precursor (3). After the gel precursor solidifies, pressure on the syringe ejects the completed waveguide (4). Panel B: photograph of a 9 cm long silk waveguide coupled to a glass optical fiber knotted to show flexibility. Scale bar indicates 1 cm. Inset: Brightfield microscope image of a cross-section of a 3 mm diameter silk waveguide. Scale bar indicates 1 mm. Arrow indicates the silk film core which is 2.9 mm wide and ∼40 μm thick.
2. Methods
Silk fibroin was extracted from raw Bombyx mori cocoons as previously described [19]. Succinctly, cocoons were cut and boiled in a 0.02 M solution of sodium carbonate to remove the gum-like serecin protein. The resulting fibroin was dissolved in 9.2 M lithium bromide and subsequently dialyzed against deionized water for 48 hours to remove the salt. Prior to use, the solution was filtered through a 0.22 μm filter to remove any dust or other impurities that would scatter light. To form the core film, 200 μL of 6.5% silk fibroin solution was cast into a poly-dimethylsiloxane (PDMS) mold measuring 2 mm wide by 14.5 cm long and left to dry in ambient conditions. To facilitate coupling into the silk waveguides, a hand-drawn glass fiber taper was inserted into the still liquid silk. The tapered geometry was formed by applying tensile stress on a multi-mode glass fiber (NA = 0.39) while holding it above a butane flame. After the liquid silk dried into a film, the fiber taper was fully encapsulated within the silk. Films with the encapsulated fiber were water-vapor annealed for 2 hours after drying by placing them in a vacuum chamber partially filled with water. This post treatment was necessary to render the initially water soluble films insoluble [20].
The silk film was then surrounded by a silk hydrogel to act as a cladding. The gels were formed by mixing silk fibroin solution, horseradish peroxidase (10 U/mL), and 10 μL/mL of 1% hydrogen peroxide. The subsequent reaction resulted in the formation of an elastic, optically transparent hydrogel [14]. To produce the desired cylindrical geometry, a 3.18 mm inner diameter Polytetrafluoroethylene (PTFE) tube was used as a mold. The tube was first filled with 10% Tween 20 to prevent the gels from sticking to the PTFE. The tube was cleared of Tween and then shaken vigorously to remove most of the residual surfactant from the inner walls. The silk hydrogel precursor solution was aspirated into the tube using a syringe. While the gel precursor solution was still in the liquid state the silk film core was inserted into the tube. Following gelation, gentle pressure applied to the syringe was sufficient to eject the completed waveguide from the PTFE tube (Fig. 1(B)). This entire procedure involves only environmentally and biologically friendly compounds and ambient temperatures making it suitable for cell growth or the inclusion of labile compounds.
The refractive index of the silk gels was measured using spectroscopic ellipsometry. 1 mm thick samples of gels (300 μL volume) were formed on glass slides. These gels were measured at wavelengths ranging from 400 nm to 1300 nm at 70 and 75 degree incident angles. The data were fit to Cauchy's equation to extract the refractive index of the gels. Ellipsometry data collected were found to be a good fit for the Cauchy model. The index of refraction of the gel was found to depend on the concentration of silk in the gel and ranged from 1.33 to 1.36 at 532 nm and varied roughly linearly with increasing silk concentration.
Optical losses in the silk film core were measured via the cutback technique [21]. 4 films with encapsulated optical couplers were prepared and 540 nm light was end-coupled into the glass optical fiber [21]. The silk waveguide was cut at regular intervals and the power output measured after each cut to calculate the loss per unit distance (Fig. 2).
Fig. 2.
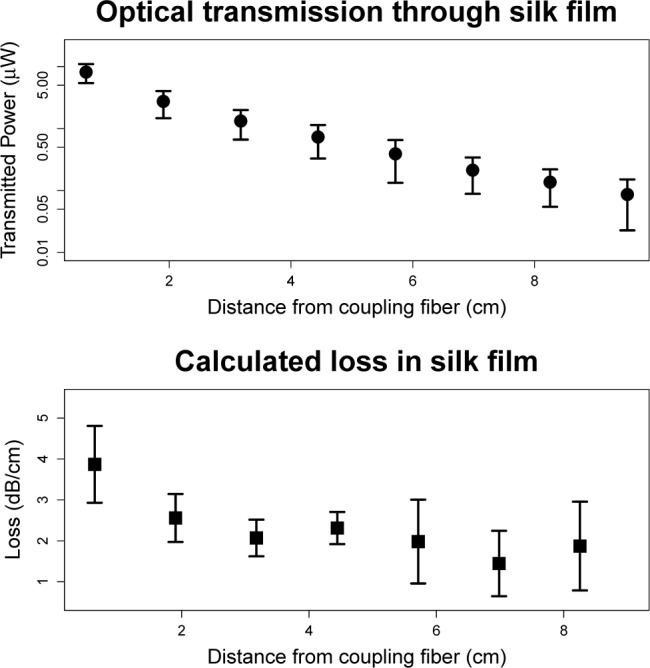
Top: Plot showing transmitted power as a function of distance from the coupling fiber. Y-axis is presented on a log scale so the straight line of the data indicates an exponential decline in transmitted power with a consistent decay constant. Error bars indicate 1 standard deviation, n=4. Bottom: Calculated loss per centimeter as a function of distance from the tip of the coupling fiber. High loss near the tip of the coupling fiber is likely due to unguided modes. Error bars indicate 1 standard deviation, n=4.
3. Results
The loss along the film was found to be relatively constant except high high loss was noted near the tip of the glass coupling fiber. The high initial loss is likely due to unguided modes. For this reason, the first centimeter was excluded from the reported waveguide propagation loss which was calculated based on an average of all the other locations to be 2.0 ± 0.7 dB/cm (mean ± standard deviation). This level of loss is comparable to other polymeric waveguides which range from 0.02 to 5 dB/cm [22]. Observing the films it was noted that most of the losses occurred along the relatively rough edges of the film. Little scattering was seen on the top and bottom surface because air dried silk films are very smooth (< 5 nm rms) [13].
To test whether the silk waveguides were capable of guiding light in biological tissue, the waveguide was inserted into bovine muscle. Two incisions were made in the tissue forming a right angle. The proximal end of the silk waveguide was placed in one of the incisions and threaded around the corner. The incisions were held closed using 23 gauge needles and care was taken to ensure that the tissue contacted the cladding of the waveguide to eliminate guidance due to the index difference between the cladding and air. The incisions were flooded with phosphate buffered saline to make sure that no cladding modes would be guided. Prior to the bend, light could be observed in both the cladding and the core. After the bend light could only be seen in the silk film core (Fig. 3).
Fig. 3.
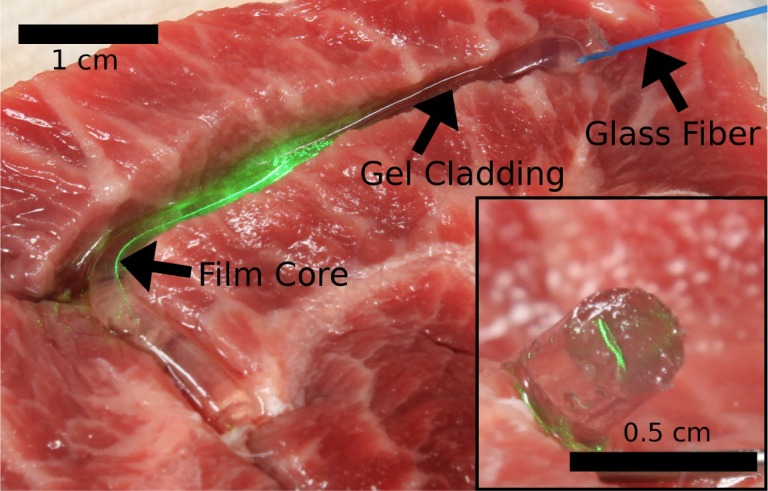
Silk waveguide guiding light in tissue. Light coupled from multimode glass fiber is confined within the silk film core of a 3.5 cm long waveguide. Inset shows the core of the waveguide glowing green after closure of the incisions.
We estimated the approximate light carrying capacity of these waveguides by transversely exposing the silk films to various intensities of green light for 10 s. Following exposure, the films were examined under brightfield microscopy for signs of damage. No damage was noted up to intensities of ∼10 W/cm2. Above 11 W/cm2, significant thermal damage was observed. These intensities far exceed the damage threshold for tissue indicating that photodamage to the fiber would not be a limiting factor in biomedical applications.
4. Discussion
We found that a silk film encapsulated in a silk hydrogel acts as an effective waveguide in tissue. These waveguides are relatively easy to produce, robust, and able to survive handling and placement within tissue. Utilizing a glass fiber taper to couple light into the silk waveguide provides a convenient interface between the biocompatible waveguides and conventional optical systems. Loss in the waveguide core was measured to be 2.0 dB/cm which is significantly higher than losses reported previously in silk [18]. We believe this is due to surface scattering by the rough edges of the film. When observing the waveguide nearly all of the lost light was seen from the edges. This implies that the loss could be significantly reduced by smoothing the edges or by using a cylindrical core geometry.
Silk is both biocompatible and biodegradable so once implanted waveguides could be left within the body. We estimate, based on data from other types of silk gels, that the cladding of these waveguides would be fully infiltrated by cells and blood vessels after approximately 12 weeks [23]. Once the cladding was infiltrated, the core of the film could last up to an additional year [17]. We expect no disruption of wave guiding properties until the cells reached the interface between the silk film core and the silk gel cladding. When this occurs there would be an increase in scattering losses. We believe losses in the waveguide could be monitored over time to estimate the degree of cellular infiltration into the waveguide.
Biocompatible waveguides capable of delivering light to a point deep within the body have many applications. One such is photodynamic therapy (PDT) which is currently used to treat tumors using photoactivated chemotheraputics. PDT illumination is typically provided transdermally, however, during tumor debulking surgery, it is frequently applied directly to the site of the tumor [24]. In this case, a silk waveguide could be left behind to deliver multiple PDT treatments over time to reduce the likelihood of recurrence. This type of staggered therapy has been shown to be more effective than a single dose of light [25]. After the conclusion of therapy the silk waveguide would degrade eliminating the need for a second surgery to retrieve it. The waveguides described in this letter are both environmentally and biologically friendly using only mild chemicals and producing no toxic byproducts. The ability to bring visible light to a small area deep within the body opens up new avenues of investigation in imaging and therapy.
Acknowledgments
MBA would like to acknowledge support from the National Defense Science and Engineering Graduate Fellowship and the Stern Fellowship at Tufts University.
References and links
- 1.Jacques S. L., “Optical properties of biological tissues: a review,” Phys. Med. Biol. 58, R37–R61 (2013). 10.1088/0031-9155/58/11/R37 [DOI] [PubMed] [Google Scholar]
- 2.Tien L. W., Wu F., Tang-Schomer M. D., Yoon E., Omenetto F. G., Kaplan D. L., “Silk as a multifunctional biomaterial substrate for reduced glial scarring around brain-penetrating electrodes,” Adv. Funct. Mater. 23, 3185–3193 (2013). 10.1002/adfm.201203716 [DOI] [Google Scholar]
- 3.Bechet D., Mordon S. R., Guillemin F., Barberi-Heyob M. a., “Photodynamic therapy of malignant brain tumours: A complementary approach to conventional therapies,” Cancer Treat. Rev. 40, 229–241 (2014). 10.1016/j.ctrv.2012.07.004 [DOI] [PubMed] [Google Scholar]
- 4.Choi M., Choi J. W., Kim S., Nizamoglu S., Hahn S. K., Yun S. H., “Light-guiding hydrogels for cell-based sensing and optogenetic synthesis in vivo,” Nat. Photonics 7, 987–994 (2013). 10.1038/nphoton.2013.278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Choi M., Humar M., Kim S., Yun S.-H., “Step-Index Optical Fiber Made of Biocompatible Hydrogels,” Adv. Mater. 27, 4081–4086 (2015). 10.1002/adma.201501603 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Valdastri P., Susilo E., Förster T., Strohhöfer C., Menciassi A., Dario P., “Wireless Implantable Electronic Platform for Chronic Fluorescent-Based Biosensors,” IEEE T. Bio-med. Eng., 58 1846–1854 (2011). 10.1109/TBME.2011.2123098 [DOI] [PubMed] [Google Scholar]
- 7.Wang Y., Huang C.-J., Jonas U., Wei T., Dostalek J., Knoll W., “Biosensor based on hydrogel optical waveguide spectroscopy,” Biosens. Bioelectron. 25, 1663–1668 (2010). 10.1016/j.bios.2009.12.003 [DOI] [PubMed] [Google Scholar]
- 8.Jain A., Yang A. H. J., Erickson D., “Gel-based optical waveguides with live cell encapsulation and integrated microfluidics,” Opt. Lett. 37, 1472–1474 (2012). 10.1364/OL.37.001472 [DOI] [PubMed] [Google Scholar]
- 9.Dupuis A., Guo N., Gao Y., Godbout N., Lacroix S., Dubois C., Skorobogatiy M., “Prospective for biodegradable microstructured optical fibers,” Opt. Lett. 32, 109–111 (2007). 10.1364/OL.32.000109 [DOI] [PubMed] [Google Scholar]
- 10.Ding L., Blackwell R. I., Künzler J. F., Knox W. H., “Femtosecond laser micromachining of waveguides in silicone-based hydrogel polymers,” Appl. Opt. 47, 3100–3108 (2008). 10.1364/AO.47.003100 [DOI] [PubMed] [Google Scholar]
- 11.Xin H., Li Y., Liu X., Li B., “Escherichia coli-Based Biophotonic Waveguides,” Nano Lett. 13, 3408–3413 (2013). 10.1021/nl401870d [DOI] [PubMed] [Google Scholar]
- 12.Tao H., Kaplan D. L., Omenetto F. G., “Silk materials - a road to sustainable high technology,” Adv. Mater. 24, 2824–2837 (2012). 10.1002/adma.201104477 [DOI] [PubMed] [Google Scholar]
- 13.Omenetto F. G., Kaplan D. L., “A new route for silk,” Nat. Photonics 2, 641–643 (2008). 10.1038/nphoton.2008.207 [DOI] [Google Scholar]
- 14.Partlow B. P., Hanna C. W., Rnjak-Kovacina J., Moreau J. E., Applegate M. B., Burke K. A., Marelli B., Mitropoulos A. N., Omenetto F. G., Kaplan D. L., “Highly Tunable Elastomeric Silk Biomaterials,” Adv. Funct. Mater. 24 4615–4624 (2014). 10.1002/adfm.201400526 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Karageorgiou V., Meinel L., Hofmann S., Malhotra A., Volloch V., Kaplan D., “Bone morphogenetic protein-2 decorated silk fibroin films induce osteogenic differentiation of human bone marrow stromal cells,” J. Biomed. Mater. Res. A. 71, 528–537 (2004). 10.1002/jbm.a.30186 [DOI] [PubMed] [Google Scholar]
- 16.Toffanin S., Kim S., Cavallini S., Natali M., Benfenati V., Amsden J. J., Kaplan D. L., Zamboni R., Muccini M., Omenetto F. G., “Low-threshold blue lasing from silk fibroin thin films,” Appl. Phys. Lett. 101, 091110 (2012). 10.1063/1.4748120 [DOI] [Google Scholar]
- 17.Wang Y., Rudym D., Walsh A., Abrahamsen L., “In vivo degradation of three-dimensional silk fibroin scaffolds,” Biomater. 29, 3415–3428 (2008). 10.1016/j.biomaterials.2008.05.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Parker S. T., Domachuk P., Amsden J., Bressner J., Lewis J. A., Kaplan D. L., Omenetto F. G., “Biocompatible Silk Printed Optical Waveguides,” Adv. Mater. 21, 2411–2415 (2009). 10.1002/adma.200801580 [DOI] [Google Scholar]
- 19.Rockwood D. N., Preda R. C., Yücel T., Wang X., Lovett M. L., Kaplan D. L., “Materials fabrication from Bombyx mori silk fibroin,” Nat. Protoc. 6, 1612–1631 (2011). 10.1038/nprot.2011.379 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hu X., Shmelev K., Sun L., Gil E. S., Park S. H., Cebe P., Kaplan D. L., “Regulation of silk material structure by temperature-controlled water vapor annealing,” Biomacromolecules 12, 1686–1696 (2011). 10.1021/bm200062a [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Characterization of Optical Fibers (John Wiley & Sons, 2007). [Google Scholar]
- 22.Ma B. H., Jen A. K., Dalton L. R., “Polymer-Based Optical Waveguides : Materials, Processing, and Devices,” Adv. Mater. 14, 1339–1365 (2002). [DOI] [Google Scholar]
- 23.Etienne O., Schneider A., Kluge J. A., Bellemin-Laponnaz C., Polidori C., Leisk G. G., Kaplan D. L., Garlick J. A., Egles C., “Soft Tissue Augmentation Using Silk Gels: An In Vitro and In Vivo Study,” J. Periodontol., 80, 1852–1888 (2009). 10.1902/jop.2009.090231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sindelar W. F., DeLaney T. F., Tochner Z., Thomas G. F., Dachoswki L. J., Smith P. D., Friauf W. S., Cole J. W., Glatstein E., “Technique of photodynamic therapy for disseminated intraperitoneal malignant neoplasms. Phase I study,” Arch. Surg.-Chicago 126, 318–324 (1991). 10.1001/archsurg.1991.01410270062011 [DOI] [PubMed] [Google Scholar]
- 25.Roozeboom M. H., Aardoom M. A., Nelemans P. J., Thissen M. R., Kelleners-Smeets N. W., Kuijpers D. I., Mosterd K., “Fractionated 5-aminolevulinic acid photodynamic therapy after partial debulking versus surgical excision for nodular basal cell carcinoma: a randomized controlled trial with at least 5-year follow-up,” J. Am. Acad. Dermatol. 69, 280–287 (2013). 10.1016/j.jaad.2013.02.014 [DOI] [PubMed] [Google Scholar]