Abstract
Scattering and absorption limit light penetration through inhomogeneous tissue. To reduce scattering, biochemists have shifted the wavelengths of excitation light for optogenetic actuators and fluorescent proteins to the orange-red range, while physicists have developed multiphoton technologies for deep tissue stimulation. We have built a rapid multiphoton spectroscopic screening system of genetically encoded red-activatable channelrhodopsin (ReaChR), and considered specific behaviors in transgenic Drosophila melanogaster as readouts to optimize the laser parameters for two-photon optogenetic activation. A wavelength-tunable optical parametric amplifier was adopted as the major light source for widefield two-photon excitation (TPE) of ReaChR. Our assays suggest that the optimized TPE wavelength of ReaChR is 1250 nm. Exploiting its capacity for optogenetic manipulation to induce macroscopic behavioral change, we realized rapid spectroscopic screening of genetically encoded effectors or indicators in vivo, and used modulation of ReaChR in the fly as a successful demonstration of such a system.
OCIS codes: (190.4970) Parametric oscillators and amplifiers, (320.7110) Ultrafast nonlinear optics, (000.1430) Biology and medicine
1. Introduction
Advances in optogenetics can help scientists to monitor and control neuron activity. For example, channelrhodopsin-2 (ChR2) is a light-gated ion channel that has already proven to be useful in the fruit fly [1–5]. The most efficient wavelength for activating ChR2 is 460 nm [6]. Because scattering effects are inversely proportional to the fourth power of the radiation wavelength, strong scattering and pigment absorption increase the power level required to activate ChR2 in vivo. Hence, even small improvements can facilitate in vivo experiments, such as optical transmission through the cuticles [4]. Longer wavelengths can be used to achieve deeper penetration in living animals and reduce scattering effects. Taking advantage of this knowledge, biochemists have developed several long-wavelength-activatable optogenetic tools for deep-tissue manipulation [7–11]. Red-activatable ChR (ReaChR), for example, which is optimally excited by light in the orange to red region (590–630 nm) [10,12], was developed to perform single-photon neuron activation in deep-tissue experiments. The feasibility of this approach has been experimentally demonstrated with the fruit fly [4,12]. On the other hand, a red-shifted cruxhalorhodopsin, Jaws, can perform single-photon neuron inhibition in deep-tissue experiments [13].
Although red-shifted optogenetic tools have shown their applicability in single-photon neuron activation or inhibition in deep-tissue experiments, a higher spatial precision in two-photon optogenetic activation, which can be combined with two-photon calcium imaging for optical manipulation and observation experiments [14], has motivated us to screen the optimized wavelength of ReaChR. The phenomenon of two-photon excitation (TPE) is a third-order nonlinear optical effect. Its efficiency is strongly correlated with the peak intensity, which varies with the divergence of the laser beam. For example, a 920-nm mode-locked Ti:Sapphire laser has been employed to achieve TPE of ion channels such as ChR2 for point manipulations [15,16]. In a previous cell-culture demonstration, the required laser intensity of two-photon neuron activation was a few gigawatts per square centimeter [16]. In general, the TPE spectrum, which shows the lowest-energy electronic transition of a fluorophore, is not identical to twice its one-photon excitation spectrum, although their ranges mostly overlap or are slightly blue shifted [17–19]. Therefore, the expected efficient TPE wavelength of ReaChR is ~1200 nm, which is twice the wavelength of light in the orange to red range (580–650 nm). Since the lasing wavelengths of a Ti:Sapphire laser are limited to 650–1100 nm, owing to the intrinsic energy levels of the laser gain media, optimal ReaChR TPE efficiency cannot be obtained using a mode-locked Ti:sapphire. However, new laser sources can present new opportunities for more effective excitation, therefore, new demand for a longer-wavelength femtosecond (fs) laser has developed.
The synchronously pumped optical parametric oscillator (SPOPO) is a commonly used ultrafast laser source. Because the pulse energy of a SPOPO is only in the tens of nanojoules, TPE is difficult unless a tightly focused condition is achieved using a high-numerical-aperture (NA) objective lens. Although the optimized TPE wavelength of optogenetic actuators and fluorescent proteins is normally deduced in vitro, several parameters, such as cuticle transmission and pigment absorption, must be considered for in vivo experiments. Therefore, we propose the use of specific behaviors in a transgenic Drosophila melanogaster as an indicator for optimizing the laser parameters. If the pulse energy of a laser source reaches 10,000 times the pulse energy of a SPOPO, TPE-induced changes in behavior can be directly observed with wide-field illumination. A cascaded multi-stage optical parametric amplification (OPA) system is typically used to generate long-wavelength signals and idler pulses of several microjoules [20–22]. In this study, a self-designed three-stage OPA system was built to generate laser pulses (1 kHz, ~30 fs, ~100 μJ) with central wavelengths of 1150–1565 nm. To determine the optimized wavelength of ReaChR, we established a wide-field TPE bending reflex assay in corazonin (Crz) transgenic flies [23] and a wide-field TPE proboscis extension reflex (PER) assay [24] in Gr5a transgenic flies using the self-built three-stage OPA to trigger ReaChR. We showed that, although Crz transgenic flies show robust bending behavior, flies in control experiments that utilized varying laser wavelength, pulse width, or transgene expression (via all-trans-retinal feeding or genetic modulation) exhibited low levels of bending. Our results showed that abdominal bending and ejaculation are most evident when a 1250-nm pulse, at an effective peak power of 0.98 GW, is used to activate ReaChR proteins in Crz neurons.
2. Light-source preparation: Femtosecond three-stage OPA system
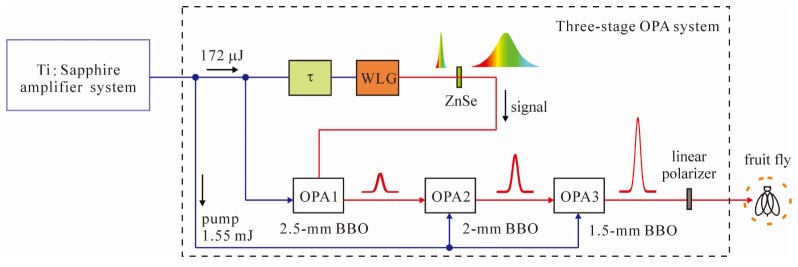
The experimental setup is shown in Fig. 1. A Ti:Sapphire amplifier (FemtopowerTM PRO HE CEP, Femtolasers) was used to provide 780-nm laser pulses (25 fs, 1.72 mJ), which were divided by 172 μJ and 1.55 mJ. A coherent white-light seeding pulse [900–1600 nm, Fig. 2(c)] was generated by focusing ~10-μJ Ti:Sapphire laser pulses through a 4-mm-thick yttrium-aluminum-garnet (YAG) window. A 10-mm-thick zinc selenide (ZnSe) plate was inserted to separate different wavelength components of the white-light seeding pulse. In the first-stage OPA, 162-μJ laser pulses pumped a 2.5-mm-thick type-II beta barium borate (BBO) crystal to amplify the white-light seeding pulse. The signal wavelength in the first-stage OPA was tuned by adjusting the BBO orientation angle and relative delay between the pump pulses and white-light seeding pulses.
Fig. 1.
Schematic of the experimental setup. OPA, optical parametric amplifier; BBO, beta barium borate; WLG, white-light generation.
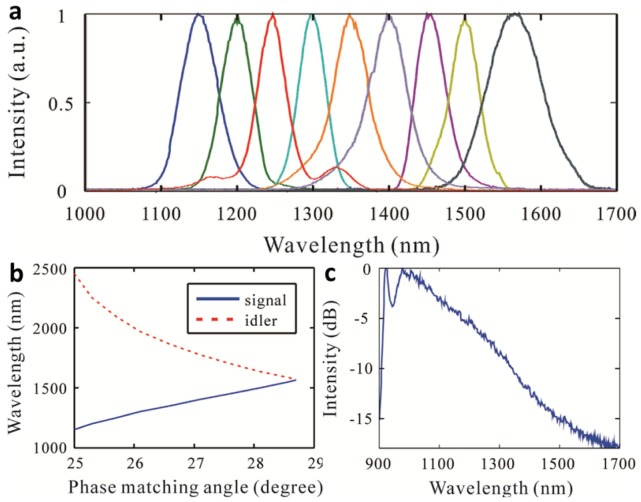
Fig. 2.
Characteristics of femtosecond three-stage OPA system. (a). Signal power spectra, centered at seven wavelengths from 1150−1565 nm, generated by our three-stage OPA system. (b). Calculated signal and idler wavelengths at different phase-matching angles. (c). White-light spectrum seeded to the first-stage OPA.
1.55-mJ laser pulses propagate through 2- and 1.5-mm-thick type-II BBO crystals as the second- and third-stage OPAs, respectively. The three-stage OPA system delivered signal laser pulses (~30 fs, ~100 μJ) with 1150–1565-nm wavelength tuning range. A linear polarizer separated the signal and idler pulses, which were orthogonally polarized in type-II phase-matching. For the behavioral experiments, the output beam (7.3 mm in diameter) was used to directly illuminate the fruit flies.
Figure 2(a) shows the signal power spectra at the identical peak power (2.9 GW) centered at 1150, 1200, 1250, 1300, 1350, 1400, 1450, 1500, and 1565 nm with a pulse width of 31 fs. The bandwidths (defined as the full width at half maximum) corresponding to these spectra were 12.9, 9.8, 7.8, 7.3, 8.9, 8.9, 6.5, 5.7, and 10 THz. Figure 2(b) shows the calculated signal (solid blue line) and idler (dashed red line) wavelengths at different type-II BBO phase-matching angles obtained by changing the phase-matching angle from 25.0° to 28.7°. Figure 2(c) shows the original white light supercontinuum spectrum that indicates continuum seeding from 900 to 1600 nm.
3. Fruit fly preparation and protocols
3.1 ReaChR transgenic flies for bending and proboscis extension assays
In the abdominal bending and proboscis extension assays, we expressed ReaChR proteins with the specific drivers (Crz-Gal4 > UAS-ReaChR) and (Gr5a-Gal4 > UAS- ReaChR), respectively. In the experimental group, Crz-Gal4>UAS-ReaChR and Gr5a-Gal4 > UAS-ReaChR flies were fed standard food with 100 μM of all-trans-retinal for 7 days before the experiments were conducted. In two control groups, Crz-Gal4>UAS-ReaChR flies without all-trans-retinal feeding and UAS-ReaChR/W flies with all-trans-retinal feeding for 7 days were tested. The ReaChR protein was not activated in the fruit flies that did not receive all-trans-retinal feeding, resulting in a very minimal response to laser stimulation [6].
3.2 Bending reflex and proboscis extension assay
For the bending reflex and proboscis extension assays, fruit flies were fixed on a cotton swab. Subsequently, the bending rate was calculated as the percentage of cycles with a response during 10 stimulation cycles in which the subject was manually moved into and out of the region of laser illumination for 3 and 5 s, respectively, in each cycle. Between-group differences were analyzed pair wise using the two-tailed Mann–Whitney test, and were considered statistically significant when P < 0.05. The data were represented as the mean ± SEM.
3.3 Sample preparation and immunohistochemistry for neuron pattern
The brains of adult flies were dissected in cold isotonic phosphate-buffered saline (PBS) and fixed with 30 min after dissection. The dissected brains were fixed in 4% paraformaldehyde and microwave-irradiated three times (2450 MHz, 1100 W) for 90 s with continuous rotation. After washing in PBS containing 1% Triton X-100 and 10% normal goat serum (PBS-T) for 30 min at room temperature, the samples were incubated in the same solution and degassed in a vacuum chamber to expel tracheal air with six cycles of vacuum (depressurized to −70 mmHg for 10 min). Next, the samples were blocked and penetrated in PBS-T at 4 °C overnight, and then incubated in PBS-T containing 1:50 mouse 4F3 anti-discs large monoclonal antibody at 4 °C for two days. After three washes in PBS-T, the samples were incubated in PBS-T containing 1:250 biotinylated goat anti-mouse IgG (Molecular Probes) at 4 °C for two days. The samples were then washed and incubated with 1:500 Alexa Fluor 635 streptavidin (Molecular Probes) at 4 °C overnight. Finally, the brain was cleared and mounted in FocusClearTM (CelExplorer, Taiwan) and imaged with a Zeiss LSM 710 confocal microscope. Amira 3.0 was used for 3D visualization.
3.4 Effective peak power
In this paper, we discuss the efficient TPE wavelength of ReaChR. The TPE effect is a kind of nonlinear optical effect, so the peak intensity is a more realistic measurement to describe laser conditions in different experiments. Biologists are more familiar with effective power in relation to biological samples; hence, we use the definition of the peak power being equal to the peak intensity multiplied by the illumination area in order to obtain the peak power from the peak intensity measurement. In our experiments, the laser beam area was always larger than the average fly body size for wide-field illumination, so the effective peak power on the samples themselves corresponded to the peak intensity multiplied by the average body sizes of the flies, rather than the laser beam area. The average fly body size can be approximated to an ellipse-shaped area having a 2.5-mm major axis and a 0.9-mm minor axis [25], and the peak intensity used in these experiments can be deduced accordingly.
4. Experimental results
4.1 Abdominal bending of ReaChR transgenic flies as a function of femtosecond laser wavelength
To determine the optimized TPE wavelength of ReaChR, we selected Crz-Gal4 to drive ReaChR expression in Crz neurons [Fig. 3(a)]. In general, neuron activity measurements need a microscope system to image and record samples; however, modification of a microscope system for a new laser wavelength is complex. A previous study showed that Crz neuron activation can cause abdominal bending and ejaculation [23]. Thus, we propose that this behavior can be considered as an indicator of two-photon optogenetic neuron activation. If the ~100-μJ infrared (IR) laser pulses can activate Crz neurons via TPE, the statistics of abdominal bending and ejaculation behavior tests in living fruit flies can be used to optimize the laser parameters. Figure 3(b) shows that experimental (Crz-Gal4 > UAS-ReaChR) flies fed 100 μM of all-trans-retinal (solid black bars) exhibited significantly higher bending rates than control flies not feeding all-trans-retinal (gray bars) or that did not express Crz neurons (white bars) at an identical effective peak power of 0.98 GW for all testing wavelengths. The highest bending rate of the experimental group was (86 ± 2%) at 1250 nm, compared to the bending rates at 780 (19 ± 3%), 1150 (63 ± 5%), 1200 (67 ± 2%), 1300 (59 ± 2%), and 1500 nm (35 ± 5%). Visualization 1 (3.9MB, AVI) is a video recording of the experimental group. The TPE efficiency is dependent on the square of the incident laser intensity [26]. To disprove the possibility that the different bending rates were due to differences in the effective peak power, the effective peak power of the OPA signal was fixed to 0.98 GW for all wavelengths. The results indicate that the ReaChR proteins on the Crz neuron membrane are activated by TPE and drive abdominal bending in the wavelength range of 1150–1300 nm, but not at 780 nm (see Section 4.3). However, all controlled experiments resulted in non-zero bending rates (< 20%), presumably because the flies were still sensing heat from the IR laser. According to previous reports, wavelengths longer than 600 nm are invisible to flies; hence, the visual response to the IR pulse could be weak [27]. These results suggest that ReaChR proteins were successfully stimulated by femtosecond laser pulses at an effective peak power of 0.98 GW, and that the optimized TPE wavelength is 1250 nm. From Fig. 3(b), the abdominal bending of 780 nm is low; hence, no apparent two-photon activation was observed at 780 nm, which corresponds to the higher-energy electronic transition of two-photon excited ReaChR.
Fig. 3.
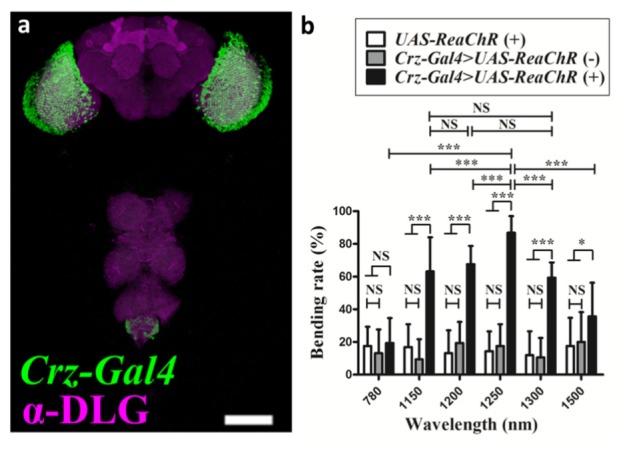
Fruit fly neuron map and bending rate as a function of wavelength. (a) Corazonin (Crz) neurons (green) were labeled in the Crz-GAL4> UAS-ReaChR transgenic fly. The brain and thoracic ganglia were immunostained with anti-discs large antibody (magenta). The scale bar represents 100 μm. (b) Bending rates at different wavelengths of the femtosecond three-stage OPA laser with an effective peak power of 0.98 GW. The optimized TPE wavelength of ReaChR is 1250 nm. Crz-Gal4>UAS-ReaChR and UAS-ReaChR/W flies were fed standard food with ( + ) or without (–) 100-μM all-trans-retinal, respectively, for 7 days before the experiment. The bending rates at wavelengths of 1150, 1200, and 1300 nm show no significant difference. The data represent mean ± SEM (N = 16). *, P < 0.05; ***, P < 0.005; NS, not significant.
4.2 Effectiveness of 1150-, 1250-, or 1300-nm femtosecond laser irradiation of ReaChR transgenic flies in bending assay as a function of effective peak power
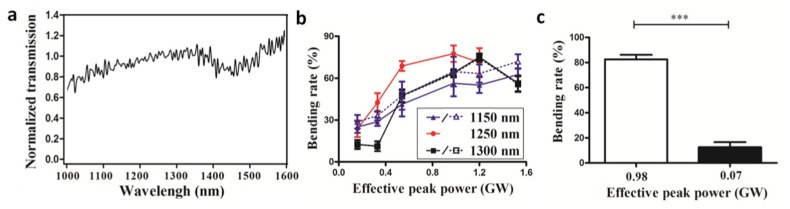
To investigate the optimal effective peak power of laser radiation for TPE, we irradiated different experimental (Crz-Gal4 > UAS-ReaChR) flies fed 100 μM of all-trans-retinal at different effective peak powers. According to Fig. 3(b), the optimized TPE wavelength is 1250 nm. The bending rates at wavelengths of 1150, 1200, and 1300 nm did not differ significantly [Fig. 3(b)]. Therefore, 1150- and 1300-nm irradiations were selected for comparison with 1250-nm irradiation. Since we measured TPE in living fruit flies, the optical attenuation of the cuticle should also be considered. The optical transmission of the dissected fly cuticle in the dorsal abdomen from 1000 to 1600 nm is shown in Fig. 4(a). A comparison between 1150, 1250, and 1300 nm is necessary when the difference of propagation attenuation is small. The effective peak power (0.16, 0.33, 0.54, 0.98, 1.20, and 1.53 GW) was controlled by a passive attenuator at the output of the three-stage OPA (Fig. 1). Figure 4(b) shows that the bending rate increases with the effective peak power, and this relationship is similar at all wavelengths tested. At 1250 nm, with a lower effective peak power of 0.98 GW, the maximum bending rate attained was 2.5% higher than the bending rate at 1300 nm. The maximum bending rate at 1150 nm was also less than that at 1250 nm.
Fig. 4.
Optical transmission spectrum of fly cuticle and laser effective peak power manipulations. (a) Optical transmission of a dissected fly cuticle in the dorsal abdomen. The optical transmission of 1250 nm is 10.8% and the optical transmissions of the other wavelengths were normalized to 1250 nm. The transmission at longer wavelengths is higher because of reduced scattering. (b) Bending rate as a function of effective peak power (0.16, 0.33, 0.54, 0.98, 1.20, and 1.53 GW) at different central wavelengths. Because the required effective peak power of the 1250-nm (red) laser is lower, its efficiency is higher than that of the 1150-nm (blue) and 1300-nm (black) lasers. The solid lines (filled triangle, filled round, and filled square) are the real measured curves, and the dotted lines (unfilled triangle, and unfilled square) are the curves which were adjusted relative to 1250 nm based on the optical transmission through the cuticles. (c) Bending rates for two-photon (effective peak power of 0.98 GW) and one-photon (effective peak power of 0.07 GW) excitations at the same effective average power, using a femtosecond three-stage OPA laser at a central wavelength of 1250 nm. In both panels, Crz-Gal4>UAS-ReaChR flies were fed standard food with 100 μM of all-trans-retinal for 7 days before the experiment. The data represent mean ± SEM (N = 8). ***, P < 0.005.
Second, the required effective peak power for increased bending rate (42 ± 6%) at 1250 nm was 0.33 GW, but the bending rates at 1150 and 1300 nm under the effective peak power of 0.33 GW were 28 ± 2 and 11 ± 3%, respectively. The irradiation was lethal when the effective peak power of the 1150-, 1250-, and 1300-nm femtosecond laser exceeded 1.53, 1.20, and 1.53 GW, respectively. When the effective peak power was above 0.33 GW, Crz-Ga4 > UAS-ReaChR flies fed with all-trans-retinal showed optimized two-photon optogenetic activation at 1250 nm.
4.3 Verification of one-photon excitation
In the assay presented in Section 4.2, the effective average power changed in proportion to the effective peak power. To further verify that the behavioral changes observed were due to TPE, we reduced the effective peak power from 0.98 to 0.07 GW by stretching the pulse width from 31 to 391 fs via the group delay dispersion. By doing this, we kept the effective average power constant, so that the single-photon phenomena could not be affected. We then repeated the bending reflex assay. Figure 4(c) shows that the bending rate at an effective peak power of 0.07 GW (12 ± 4%) was much less than that at 0.98 GW (82 ± 3%). The low non-zero bending rates (< 20%) at 0.07 GW are presumably due to heat from the IR laser. The results in Fig. 4(b) and Fig. 4(c) reveal a nonlinear optical process, presumably TPE, which is dominated by peak power rather than a process such as single-photon excitation, which depends on the average power.
4.4 Proboscis extension of ReaChR transgenic flies to femtosecond laser irradiation
We also examined whether the ReaChR expression system could be used to trigger another behavior, proboscis extension, in adult flies via TPE. Gr5a neurons in the sensillum, which have been reported to be the putative gustatory neurons, respond to sugars and induce a feeding behavior that involves proboscis extension [24]. Therefore, we used Gr5a-Gal4 to drive the expression of UAS-ReaChR in order to demonstrate that the femtosecond three-stage OPA system can activate specific neurons via TPE in a different response system of the living fruit fly. We can verify that all the behavioral changes are due to TPE activation. Activating ReaChR-expressing Gr5a neurons using a 1250-nm femtosecond three-stage OPA laser triggered proboscis extension at an effective peak power of 0.98 GW, as suggested by Fig. 4(b) and Visualization 2 (3.5MB, AVI) . Therefore, this behavior can also be used as a readout to characterize the optimized TPE wavelength of ReaChR.
5. Discussion and conclusion
In our experimental results [Fig. 4(b)], a 1250-nm laser pulse at an effective peak power of 0.33 GW increased the bending rate to 42 ± 6%. According to a previous study [16], a minimum effective peak power of 0.2–0.35 GW is required to measure the rate of photocurrent elevation in a cell-culture demonstration. Given that laser pulses must penetrate though cuticles and turbid tissue (e.g., adipose, muscle, and tracheal) in in vivo experiments, the measured TPE effective peak power in this paper is consistent with the cell-culture demonstrations once attenuation is considered. Because the bending rate of UAS-ReaChR/W flies remains below 20%, high-peak-power laser pulses cannot force the fly to bend its abdomen. At the same time, the behavioral assays of abdomen bending and proboscis extension established here show that the observed behavior changes with the genetic background. This suggests that these behaviors were not due to physiological damage caused by high-peak-power laser pulses, but due to the TPE of ReaChR proteins on specific neuronal circuits.
We demonstrated that stimulating the ReaChR protein triggered bending and proboscis extension via the TPE process. A custom-designed three-stage OPA system was constructed to produce a 31-fs laser with an effective peak power of 0.98 GW in the wavelength range of 1150–1565 nm. This laser was shown to be an appropriate tool for rapid screening of two-photon optogenetic activation in behavioral experiments. By tuning the central wavelength of the laser, we showed that the bending rate of the fruit fly was optimal at 1250 nm and an effective peak power of 0.98 GW. These results suggest that the ReaChR protein has an optimal TPE wavelength of 1250 nm. Exploiting its capacity for optogenetic manipulation to induce macroscopic behavioral change, we realized rapid spectroscopic screening of genetically encoded effectors or indicators in vivo, and used modulation of ReaChR in the fly as a successful demonstration of such a system.
Acknowledgments
This work was supported by grants from the Ministry of Science and Technology (103-2633-B-007-001 to A.-S. C. and Y.-Y L., 102-2112-M-007 −025 -MY3 to M.-C. C., 103-2221-E-007-056- to S.-D. Y., and 103-2221-E-007-055- to Y.-Y. L.), the Ministry of Education, and the University System of Taiwan. We thank Klemens F. Störtkuhl, Roger Y. Tsien, and David J. Anderson for providing the flies. We thank A. H. Kung for providing the Ti:Sapphire amplifier system for the three-stage OPA pump.
References and links
- 1.Schroll C., Riemensperger T., Bucher D., Ehmer J., Völler T., Erbguth K., Gerber B., Hendel T., Nagel G., Buchner E., Fiala A., “Light-induced activation of distinct modulatory neurons triggers appetitive or aversive learning in Drosophila larvae,” Curr. Biol. 16(17), 1741–1747 (2006). 10.1016/j.cub.2006.07.023 [DOI] [PubMed] [Google Scholar]
- 2.Suh G. S., Ben-Tabou de Leon S., Tanimoto H., Fiala A., Benzer S., Anderson D. J., “Light activation of an innate olfactory avoidance response in Drosophila,” Curr. Biol. 17(10), 905–908 (2007). 10.1016/j.cub.2007.04.046 [DOI] [PubMed] [Google Scholar]
- 3.Wu M. C., Chu L. A., Hsiao P. Y., Lin Y. Y., Chi C. C., Liu T. H., Fu C. C., Chiang A. S., “Optogenetic control of selective neural activity in multiple freely moving Drosophila adults,” Proc. Natl. Acad. Sci. U.S.A. 111(14), 5367–5372 (2014). 10.1073/pnas.1400997111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lin Y. Y., Wu M. C., Hsiao P. Y., Chu L. A., Yang M. M., Fu C. C., Chiang A. S., “Three-wavelength light control of freely moving Drosophila Melanogaster for less perturbation and efficient social-behavioral studies,” Biomed. Opt. Express 6(2), 514–523 (2015). 10.1364/BOE.6.000514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lin H. H., Chu L. A., Fu T. F., Dickson B. J., Chiang A. S., “Parallel neural pathways mediate CO2 avoidance responses in Drosophila,” Science 340(6138), 1338–1341 (2013). 10.1126/science.1236693 [DOI] [PubMed] [Google Scholar]
- 6.Nagel G., Szellas T., Huhn W., Kateriya S., Adeishvili N., Berthold P., Ollig D., Hegemann P., Bamberg E., “Channelrhodopsin-2, a directly light-gated cation-selective membrane channel,” Proc. Natl. Acad. Sci. U.S.A. 100(24), 13940–13945 (2003). 10.1073/pnas.1936192100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Zhang F., Wang L. P., Boyden E. S., Deisseroth K., “Channelrhodopsin-2 and optical control of excitable cells,” Nat. Methods 3(10), 785–792 (2006). 10.1038/nmeth936 [DOI] [PubMed] [Google Scholar]
- 8.Zhang F., Prigge M., Beyrière F., Tsunoda S. P., Mattis J., Yizhar O., Hegemann P., Deisseroth K., “Red-shifted optogenetic excitation: a tool for fast neural control derived from Volvox carteri,” Nat. Neurosci. 11(6), 631–633 (2008). 10.1038/nn.2120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Berndt A., Yizhar O., Gunaydin L. A., Hegemann P., Deisseroth K., “Bi-stable neural state switches,” Nat. Neurosci. 12(2), 229–234 (2009). 10.1038/nn.2247 [DOI] [PubMed] [Google Scholar]
- 10.Lin J. Y., Knutsen P. M., Muller A., Kleinfeld D., Tsien R. Y., “ReaChR: a red-shifted variant of channelrhodopsin enables deep transcranial optogenetic excitation,” Nat. Neurosci. 16(10), 1499–1508 (2013). 10.1038/nn.3502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Klapoetke N. C., Murata Y., Kim S. S., Pulver S. R., Birdsey-Benson A., Cho Y. K., Morimoto T. K., Chuong A. S., Carpenter E. J., Tian Z., Wang J., Xie Y., Yan Z., Zhang Y., Chow B. Y., Surek B., Melkonian M., Jayaraman V., Constantine-Paton M., Wong G. K., Boyden E. S., “Independent optical excitation of distinct neural populations,” Nat. Methods 11(3), 338–346 (2014). 10.1038/nmeth.2836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Inagaki H. K., Jung Y., Hoopfer E. D., Wong A. M., Mishra N., Lin J. Y., Tsien R. Y., Anderson D. J., “Optogenetic control of Drosophila using a red-shifted channelrhodopsin reveals experience-dependent influences on courtship,” Nat. Methods 11(3), 325–332 (2013). 10.1038/nmeth.2765 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Chuong A. S., Miri M. L., Busskamp V., Matthews G. A., Acker L. C., Sørensen A. T., Young A., Klapoetke N. C., Henninger M. A., Kodandaramaiah S. B., Ogawa M., Ramanlal S. B., Bandler R. C., Allen B. D., Forest C. R., Chow B. Y., Han X., Lin Y., Tye K. M., Roska B., Cardin J. A., Boyden E. S., “Noninvasive optical inhibition with a red-shifted microbial rhodopsin,” Nat. Neurosci. 17(8), 1123–1129 (2014). 10.1038/nn.3752 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Packer A. M., Russell L. E., Dalgleish H. W., Häusser M., “Simultaneous all-optical manipulation and recording of neural circuit activity with cellular resolution in vivo,” Nat. Methods 12(2), 140–146 (2014). 10.1038/nmeth.3217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Papagiakoumou E., Anselmi F., Bègue A., de Sars V., Glückstad J., Isacoff E. Y., Emiliani V., “Scanless two-photon excitation of channelrhodopsin-2,” Nat. Methods 7(10), 848–854 (2010). 10.1038/nmeth.1505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Rickgauer J. P., Tank D. W., “Two-photon excitation of channelrhodopsin-2 at saturation,” Proc. Natl. Acad. Sci. U.S.A. 106(35), 15025–15030 (2009). 10.1073/pnas.0907084106 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Xu C., Zipfel W., Shear J. B., Williams R. M., Webb W. W., “Multiphoton fluorescence excitation: new spectral windows for biological nonlinear microscopy,” Proc. Natl. Acad. Sci. U.S.A. 93(20), 10763–10768 (1996). 10.1073/pnas.93.20.10763 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Guild J. B., Xu C., Webb W. W., “Measurement of group delay dispersion of high numerical aperture objective lenses using two-photon excited fluorescence,” Appl. Opt. 36(1), 397–401 (1997). 10.1364/AO.36.000397 [DOI] [PubMed] [Google Scholar]
- 19.Drobizhev M., Makarov N. S., Tillo S. E., Hughes T. E., Rebane A., “Two-photon absorption properties of fluorescent proteins,” Nat. Methods 8(5), 393–399 (2011). 10.1038/nmeth.1596 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Li C., Wang D., Song L., Liu J., Liu P., Xu C., Leng Y., Li R., Xu Z., “Generation of carrier-envelope phase stabilized intense 1.5 cycle pulses at 1.75 μm,” Opt. Express 19(7), 6783–6789 (2011). 10.1364/OE.19.006783 [DOI] [PubMed] [Google Scholar]
- 21.Andriukaitis G., Balčiūnas T., Ališauskas S., Pugžlys A., Baltuška A., Popmintchev T., Chen M. C., Murnane M. M., Kapteyn H. C., “90 GW peak power few-cycle mid-infrared pulses from an optical parametric amplifier,” Opt. Lett. 36(15), 2755–2757 (2011). 10.1364/OL.36.002755 [DOI] [PubMed] [Google Scholar]
- 22.Ishii N., Kaneshima K., Kitano K., Kanai T., Watanabe S., Itatani J., “Sub-two-cycle, carrier-envelope phase-stable, intense optical pulses at 1.6 μm from a BiB3O6 optical parametric chirped-pulse amplifier,” Opt. Lett. 37(20), 4182–4184 (2012). 10.1364/OL.37.004182 [DOI] [PubMed] [Google Scholar]
- 23.Tayler T. D., Pacheco D. A., Hergarden A. C., Murthy M., Anderson D. J., “A neuropeptide circuit that coordinates sperm transfer and copulation duration in Drosophila,” Proc. Natl. Acad. Sci. U.S.A. 109(50), 20697–20702 (2012). 10.1073/pnas.1218246109 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wang Z., Singhvi A., Kong P., Scott K., “Taste representations in the Drosophila brain,” Cell 117(7), 981–991 (2004). 10.1016/j.cell.2004.06.011 [DOI] [PubMed] [Google Scholar]
- 25.Chakir M., Negoua H., Moreteau B., David J. R., “Quantitative morphometrical analysis of a North African population of Drosophila melanogaster: sexual dimorphism, and comparison with European populations,” J. Genet. 87(4), 373–382 (2008). 10.1007/s12041-008-0060-0 [DOI] [PubMed] [Google Scholar]
- 26.Y. R. Shen, “Two-photon absorption,” in The Principle of Nonlinear Optics, Y. R. Shen, ed. (Wiley-Interscience 1984) pp. 202–210. [Google Scholar]
- 27.Yamaguchi S., Desplan C., Heisenberg M., “Contribution of photoreceptor subtypes to spectral wavelength preference in Drosophila,” Proc. Natl. Acad. Sci. U.S.A. 107(12), 5634–5639 (2010). 10.1073/pnas.0809398107 [DOI] [PMC free article] [PubMed] [Google Scholar]