Abstract
Objective
The purpose of this study was to report optical coherence tomography (OCT) and angiographic findings in a patient with pregnancy-induced hypertension (PIH).
Case report
A 39-year-old woman, who was diagnosed with PIH, reported blurred and distorted vision at 5 days after an emergency cesarean delivery. OCT revealed a large serous retinal detachment (SRD) that included areas in the macula, along with an increased choroidal thickness noted in both eyes. Indocyanine green angiograms indicated delayed filling of the choroidal circulation in the early phase but choroidal hyperpermeability in the mid-phase. The SRD was gradually resolving without any treatment except for antihypertensive drugs. At 40 days after the initial examination, OCT revealed both the disappearance of the SRD and marked improvement of the choroidal thickening.
Conclusion
Ophthalmologists need to be aware that PIH can cause choroidal ischemia, a breakdown of the outer blood–retinal barrier, and lead to the development of SRD.
Keywords: pregnancy, hypertension, serous retinal detachment, choroidal thickening
Introduction
Pregnancy-induced hypertension (PIH) is responsible for high maternal and neonatal morbidity and mortality rates. Hypertensive choroidopathy is a manifestation of vasogenic edema in the choroid and has been reported to be associated with malignant hypertension, renal disease, pheochromocytoma, and PIH.1,2 In the majority of hypertensive choroidopathy patients, subjects do regain normal vision.2 Use of an experimental malignant hypertension model has demonstrated that severe high blood pressure (BP) can lead to choroidal fibrinoid necrosis, choriocapillaris nonperfusion, retinal pigment epithelium (RPE) ischemic necrosis, compromise of the outer blood–retinal barrier, and localized RPE detachment and/or serous retinal detachment (SRD).3 Presently, there have been few studies that have described how to effectively use combined angiographic and optical coherence tomography (OCT) findings when evaluating PIH patients.
Here, we describe the OCT and angiographic findings in a female patient with PIH who developed bilateral SRD immediately after delivery.
Case report
Institutional Review Board approval was not obtained as it was not deemed necessary, this being a retrospective case report. The Declaration of Helsinki was followed in this case report. At 24 weeks of pregnancy, a 39-year-old woman was examined by her obstetrician–gynecologist during a regular checkup. Although her BP was 140/90 mmHg, proteinuria was not found. At 26 weeks of pregnancy, BP was 180/110 mmHg, and she was positive for proteinuria, which led to a diagnosis of severe PIH/preeclampsia and the necessity of an emergency cesarean delivery. At delivery, her BP was 190/100 mmHg and urinary protein was 4+. Two days after starting treatment with oral medications (nifedipine and enalapril), she reported blurred and distorted vision.
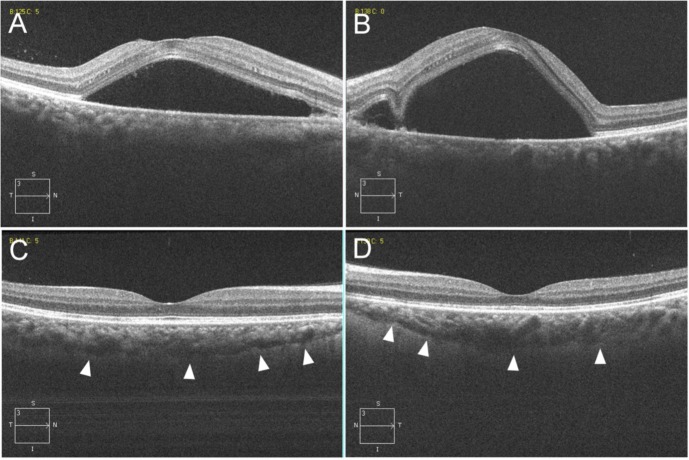
After her referral to the Department of Ophthalmology at 5 days after the delivery, her BP had decreased to 150/90 mmHg. Although there was a previous history of chronic thyroiditis, she reported having neither vertigo, tinnitus, hearing loss, nor headache. At her initial examination, corrected visual acuities (CVA) were 1.0 and 0.6, and intraocular pressures were 15 and 17 mmHg in her right and left eye, respectively. No inflammatory cells were observed in the anterior segment or vitreous of either eye. Ophthalmoscopy showed SRD in the posterior poles of both eyes (Figure 1A and B). We performed fluorescein and indocyanine green angiographies (FA and ICGA) using a Spectralis HRA device (Heidelberg Engineering, Heidelberg, Germany). Bilateral FA images showed multiple foci of fluorescein leakage during the mid-phase (4 minutes after injection) (Figure 1C and D) and pooling of the dye in the subretinal spaces during the late phase (25 minutes after injection) (Figure 1E and F). ICGA showed delayed filling of the choroidal circulation in the early-phase images (Figure 1G and H), which subsequently became mottled with hyperfluorescent lesions during the mid-phase (7 minutes) (Figure 1I and J), suggesting choroidal hyperpermeability. Unlike the findings characteristically observed in Vogt–Koyanagi–Harada disease (VKH), no hypofluorescent dark dots were seen in the posterior poles during the late phase of ICGA. Spectral-domain OCT (Cirrus HD OCT, Carl Zeiss Meditec AG, Dublin, CA, USA) indicated there was a large SRD that included areas in the macula, along with an increased choroidal thickness (which could be observed because the choroid–scleral interface was not visible) noted in both eyes (Figure 2A and B). Blood test findings revealed elevated fibrin/fibrinogen degradation products (12 µg/mL; normal range: <5 µg/mL) and D-dimer levels (5.2 µg/mL; normal range: <1 µg/mL), which indicated mild hypercoagulation.
Figure 1.
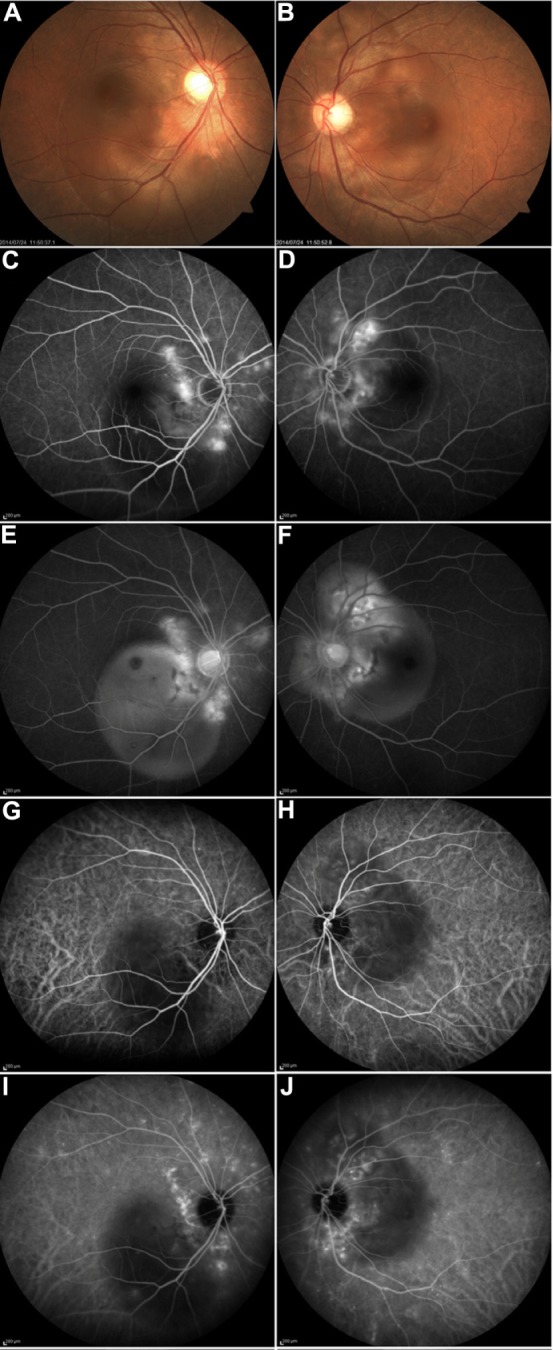
Fundus findings at the initial visit.
Notes: Fundus images show serous retinal detachment in the right (A) and left eyes (B). Mid-phase FA (4 minutes) show multiple punctuate hyperfluorescent lesions in the right (C) and left eyes (D). Late phase FA (25 minutes) shows multilobular pooling of the dye in the right (E) and left eyes (F). Early-phase ICGA (15 seconds) show a filling delay in the choroid of the right (G) and left eyes (H). Mid-phase ICGA (7 minutes) shows focal areas of hyperfluorescence in the right (I) and left eyes (J).
Abbreviations: FA, fluorescein angiograms; ICGA, indocyanine green angiograms.
Figure 2.
Horizontal scan images (6.0 mm) from the spectral-domain OCT of the right (A and C) and left eyes (B and D).
Notes: At the initial visit, OCT through the fovea shows a large SRD that included areas of the macula and increased choroidal thickness (which can be observed because of the invisible choroid–scleral interface) in both eyes (A and B). At 40 days after the initial visit, OCT shows not only the disappearance of the SRD, but also marked improvement of the choroidal thickening (which can be observed because of the visible choroid–scleral interface depicted by the arrowheads) (C and D).
Abbreviations: OCT, optical coherence tomography; SRD, serous retinal detachment.
The patient’s BP gradually began to decline once she started taking the medications prescribed by her obstetrician, with her SRD also gradually resolving. At 14 days after the initial examination (20 days after starting the medication), the patient’s BP decreased to 117/80 mmHg, an OCT performed on the same day showed large improvement in the SRD, and her CVA were 1.2 and 0.8 in her right and left eyes. At 40 days after the initial examination, her CVA were 1.2 in both eyes, and OCT revealed both the disappearance of the SRD and marked improvement of the choroidal thickening (which could be observed because of the visible choroid–scleral interface) (Figure 2C and D).
Discussion
This case report provides details on a female patient with PIH who developed bilateral SRD. ICGA findings showed there was a filling delay during the early phase, which was indicative of choriocapillaris occlusion, and hyperfluorescence during the mid-phase, which suggests choroidal hyperpermeability. The OCT images confirmed there was a large SRD and increased choroidal thickening during the acute phase, which resembles the characteristics observed in VKH disease.
PIH is believed to cause acute retinal pigment epitheliopathy due to choroidal ischemia. Constriction and occlusion of the choroidal artery in an experimental model of malignant hypertension has been shown to cause choriocapillary occlusion and RPE necrosis.3 Endothelial damage of the choroidal vasculature can also lead to fibrinoid necrosis of the choroidal arterioles with choriocapillary occlusion. Other studies have also demonstrated that nonperfusion of the choriocapillaris can cause necrosis of the overlying RPE, which leads to the breakdown of the outer blood–retinal barrier and the development of SRD.1,3,4
Saito5 reported that retinochoroidal lesions associated with PIH can be classified into three types: a retinal circulation disorder (retinal type) that is primarily characterized by cotton-wool patches, a choroidal circulation disorder (choroidal type) that is primarily characterized by SRD, and a combined disorder of the first two types (combined type). When both the systolic and diastolic BPs are high, the retinal type is more prominent than the choroidal type, whereas the choroidal type can occur even when systolic pressures are <160 mmHg. This suggests that in addition to hypertension itself, these hypercoagulation factors may play a greater role in the choroidal type of disorder. These manifestations can be attributed to the physiological changes that occur during pregnancy, and the chronic disseminated intravascular coagulation (DIC)-like changes in the blood properties in which the RPE is disrupted over the choriocapillary occlusion.6
Uto and Uemura7 reported that retinal circulation disorder (the previously designated retinal type) exhibited normal platelet and fibrinogen levels, whereas the choroidal circulation disorder (the previously designated choroidal type) had reduced levels. These results support the findings of Saito,5 who demonstrated the involvement of chronic DIC. We were able to similarly diagnose our patient with the choroidal circulation disorder type based on both her clinical findings and the blood test results that showed elevated fibrin/fibrinogen degradation products and D-dimer levels indicating mild hypercoagulation.
It has been reported that not only delayed choroidal filling but also choroidal hyperpermeability may impair the RPE function, thereby leading to SRD in patients with PIH.8 Our current findings support this, as our ICGA evaluation demonstrated that our patient exhibited bilateral choroidal hyperpermeability (Figure 1I and J). In addition, FA revealed multifocal areas of pinpoint leakage in this patient (Figure 1C and D), while OCT images indicated the presence of bilateral multifocal SRD and choroidal thickening in the acute phase (Figure 2A and B), which resembles the typical characteristics seen in VKH. In VKH patients, inflammation and infiltration of inflammatory cells are triggers for the vascular disease, which in turn causes a vascular permeability that ultimately results in marked, choroidal thickening during the acute phase.8 Although, the cause of the disorder in our patient differed from VKH, we were still able to demonstrate similar pathological findings, as our case exhibited choroidal hyperpermeability that subsequently induced a marked, acute-phase choroidal thickening. Also, our case and VKH were similar in that both are characterized by breakdown of the outer blood–retinal barrier as previously reported.9
More than 70% of the eyes of VKH patients have folds of the RPE,10,11 with OCT demonstrating the highest sensitivity and specificity for the detection of RPE folds. However, our patient did not have any RPE folds in either eye (Figure 2A and B). ICGA has shown that both VKH and PIH exhibit delayed choroidal circulation and choroidal hyperpermeability. The characteristic VKH multiple dark dots seen with ICGA during the late phase are caused by filling defects induced by inflammatory granuloma. The ICGA evaluation in our patient demonstrated that she did not exhibit any multiple dark dots in either the middle (Figure 1I and J) or the late phases (data not shown). Even though there is a similar choroidal circulatory disturbance between VKH and PIH, fibrinoid necrosis of the choroidal arterioles and spasms of the choroidal artery are thought to be the primary causes in PIH.1,3
In terms of the clinical course, our patient promptly recovered spontaneously without the need for any further treatments, with the exception of antihypertensive drugs, which made it easy to discern the cause of the illness. Although we did not reevaluate the choroidal circulation by ICGA at 40 days after the initial examination, it was assumed that the choroidal ischemia would be transient and improved. Since both VKH disease and PIH have a common ability to rupture the outer blood–retinal barrier, similar clinical findings are observed during the acute phase in both conditions. There have been many reports of cases with VKH disease that occurred during pregnancy, with the timing of the onset similar to that seen for PIH. The main treatment in PIH is obstetrically guided BP management, with the role of the ophthalmologist limited to patient follow-up. Thus, for VKH, it is essential that both medical experts provide differential and confirmatory diagnoses on an early and expedited basis.
Conclusion
In order to prevent a potential misdiagnosis, ophthalmologists need to be aware that similar to the clinical findings observed during the acute phase in VKH, choroidal ischemia in PIH can also cause a breakdown of the outer blood–retinal barrier and lead to the development of SRD.
Acknowledgments
This case study was supported by a grant from the Vehicle Racing Commemorative Foundation.
Footnotes
Disclosure
The authors report no conflicts of interest in this work.
References
- 1.Tso MO, Jampol LM. Pathophysiology of hypertensive retinopathy. Ophthalmology. 1982;89:1132–1145. doi: 10.1016/s0161-6420(82)34663-1. [DOI] [PubMed] [Google Scholar]
- 2.Kinyoun JL, Kalina RE. Visual loss from choroidal ischemia. Am J Ophthalmol. 1986;101:650–656. doi: 10.1016/0002-9394(86)90764-6. [DOI] [PubMed] [Google Scholar]
- 3.Kishi S, Tso MO, Hayreh SS. Fundus lesions in malignant hypertension. I. A pathologic study of experimental hypertensive choroidopathy. Arch Ophthalmol. 1985;103:1189–1197. doi: 10.1001/archopht.1985.01050080101029. [DOI] [PubMed] [Google Scholar]
- 4.Bourke K, Patel MR, Prisant LM, Marcus DM. Hypertensive choroidopathy. J Clin Hypertens (Greenwich) 2004;6:471–472. doi: 10.1111/j.1524-6175.2004.3749.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saito Y. Retinochoroidal changes in toxemia of pregnancy with the relation to hypertensive retinopathy and choroidopathy. Nippon Ganka Gakkai Zasshi. 1990;94:748–755. Japanese. [PubMed] [Google Scholar]
- 6.Cogan DG. Ocular involvement in disseminated intravascular coagulopathy. Arch Ophthalmol. 1975;93:1–8. doi: 10.1001/archopht.1975.01010020005001. [DOI] [PubMed] [Google Scholar]
- 7.Uto M, Uemura A. Retinochoroidopathy and systemic state in toxemia of pregnancy. Nippon Ganka Gakkai Zasshi. 1991;95:1016–1019. Japanese. [PubMed] [Google Scholar]
- 8.Iida T, Hagimura N, Otani T, Ikeda F, Muraoka K. Choroidal vascular lesions in serous retinal detachment viewed with indocyanine green angiography. Nippon Ganka Gakkai Zasshi. 1996;100:817–824. Japanese. [PubMed] [Google Scholar]
- 9.Song YS, Kinouchi R, Ishiko S, Fukui K, Yoshida A. Hypertensive choroidopathy with eclampsia viewed on spectral-domain optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2013;251:2647–2650. doi: 10.1007/s00417-013-2462-9. [DOI] [PubMed] [Google Scholar]
- 10.Ishihara K, Hangai M, Kita M, Yoshimura N. Acute Vogt-Koyanagi-Harada disease in enhanced spectral-domain optical coherence tomography. Ophthalmology. 2009;116:1799–1807. doi: 10.1016/j.ophtha.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 11.Kato Y, Yamamoto Y, Tabuchi H, Fukushima A. Retinal pigment epithelium folds as a diagnostic finding of Vogt-Koyanagi-Harada disease. Jpn J Ophthalmol. 2013;57:90–94. doi: 10.1007/s10384-012-0212-x. [DOI] [PubMed] [Google Scholar]