Abstract
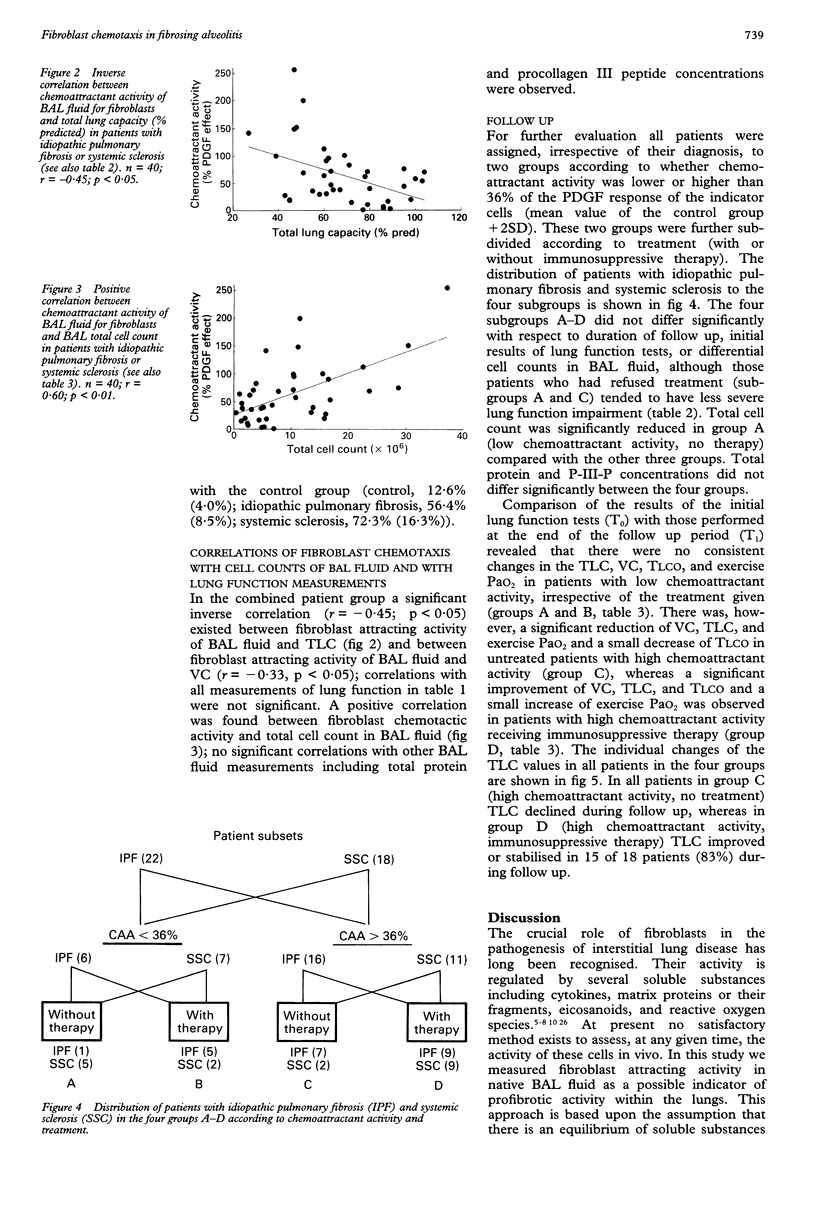
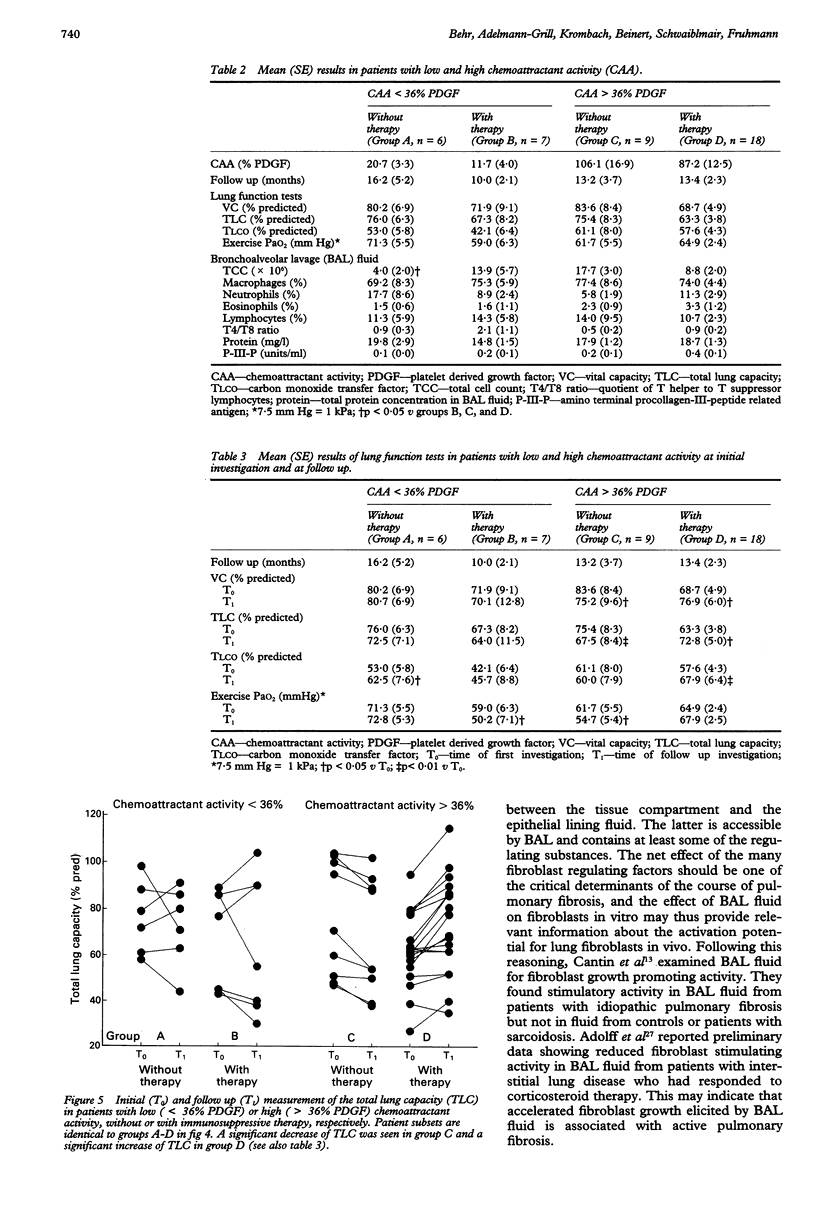
BACKGROUND--In fibrosing alveolitis activation of lung fibroblasts is the decisive event in the pathogenetic sequence leading to pulmonary fibrosis. Fibroblast stimulating activity was measured in bronchoalveolar lavage (BAL) fluid to assess its relationship to the activity of fibrosing alveolitis. METHODS--Nine control subjects and 40 patients with fibrosing alveolitis caused by idiopathic pulmonary fibrosis (n = 22) or pulmonary involvement in systemic sclerosis (n = 18) were studied. All patients were followed up by lung function testing for a minimum of six months (mean (SE) 13.3 (1.4) months). Twenty five patients received immunosuppressive therapy and 15 refused. At the beginning of follow up BAL was performed and, as a possible indicator of fibroblast stimulating mediators within the lungs, chemotactic migration of cultured human fibroblasts elicited by native BAL fluid was measured in Boyden-type chambers and expressed as a percentage of the chemoattractant effect of 25 ng/ml platelet derived growth factor. The procollagen III peptide level in BAL fluid served as a marker for collagen synthesis. RESULTS--Chemoattractant activity was elevated in the patients with idiopathic pulmonary fibrosis and systemic sclerosis compared with the control group, (mean (SE) 56.4% (8.5%)) and 72.3% (16.3%) v 12.6% (4.0%). Chemoattractant activity was inversely correlated with total lung capacity (TLC) (r = -0.45) and with vital capacity (VC) (r = -0.33). Procollagen III peptide concentrations in BAL fluid and chemoattractant activity were not significantly correlated. For further evaluation chemoattractant activity of 36% (mean value of controls +2 SD) was used to separate normal (< 36%) from elevated (> or = 36%) activity. At the end of follow up, untreated patients with high chemoattractant activity (> or = 36%) showed a significant reduction of VC, TLC, and exercise arterial oxygen tension (PaO2) and a small decrease in carbon monoxide transfer factor (TLCO), whereas a significant improvement in VC, TLC, and TLCO and a small increase of exercise PaO2 occurred in treated patients with high chemoattractant activity. Patients with low chemoattractant activity (< 36%) showed no consistent change in lung function measurements, irrespective of treatment. In contrast, lung function results and differential cell counts in BAL fluid failed to identify progressive disease. CONCLUSIONS--In patients with fibrosing alveolitis the chemoattractant activity of BAL fluid seems to be an independent indicator of lung fibroblast stimulating activity providing relevant information about disease activity, and may help to improve the clinical management of these patients.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Adelmann-Grill B. C., Cully Z. Signal perception of fibroblasts for directional migration to platelet-derived growth factor in Boyden-type chambers. J Cell Physiol. 1990 Apr;143(1):172–177. doi: 10.1002/jcp.1041430124. [DOI] [PubMed] [Google Scholar]
- Adolff C. A., Golden J. A., Gamsu G., Gøetzl E. J., Turck C. W. Reduction in pulmonary fibroblast-stimulating activity as an index of response to treatment of interstitial lung diseases. N Engl J Med. 1990 Jun 28;322(26):1890–1891. doi: 10.1056/NEJM199006283222618. [DOI] [PubMed] [Google Scholar]
- Albini A., Adelmann-Grill B. C., Müller P. K. Fibroblast chemotaxis. Coll Relat Res. 1985 Jun;5(3):283–296. doi: 10.1016/s0174-173x(85)80018-2. [DOI] [PubMed] [Google Scholar]
- Bjermer L., Lundgren R., Hällgren R. Hyaluronan and type III procollagen peptide concentrations in bronchoalveolar lavage fluid in idiopathic pulmonary fibrosis. Thorax. 1989 Feb;44(2):126–131. doi: 10.1136/thx.44.2.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bjermer L., Thunell M., Hällgren R. Procollagen III peptide in bronchoalveolar lavage fluid. A potential marker of altered collagen synthesis reflecting pulmonary disease in sarcoidosis. Lab Invest. 1986 Dec;55(6):654–656. [PubMed] [Google Scholar]
- Cantin A. M., Boileau R., Bégin R. Increased procollagen III aminoterminal peptide-related antigens and fibroblast growth signals in the lungs of patients with idiopathic pulmonary fibrosis. Am Rev Respir Dis. 1988 Mar;137(3):572–578. doi: 10.1164/ajrccm/137.3.572. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Bitterman P. B., Rennard S. I., Hance A. J., Keogh B. A. Interstitial lung diseases of unknown cause. Disorders characterized by chronic inflammation of the lower respiratory tract (first of two parts). N Engl J Med. 1984 Jan 19;310(3):154–166. doi: 10.1056/NEJM198401193100304. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Fulmer J. D., Roberts W. C., Moss M. L., Line B. R., Reynolds H. Y. Idiopathic pulmonary fibrosis. Clinical, histologic, radiographic, physiologic, scintigraphic, cytologic, and biochemical aspects. Ann Intern Med. 1976 Dec;85(6):769–788. doi: 10.7326/0003-4819-85-6-769. [DOI] [PubMed] [Google Scholar]
- Crystal R. G., Gadek J. E., Ferrans V. J., Fulmer J. D., Line B. R., Hunninghake G. W. Interstitial lung disease: current concepts of pathogenesis, staging and therapy. Am J Med. 1981 Mar;70(3):542–568. doi: 10.1016/0002-9343(81)90577-5. [DOI] [PubMed] [Google Scholar]
- Dill J., Ghose T., Landrigan P., MacKeen A. D., Macneil A. R. Cryptogenic fibrosing alveolitis. Chest. 1975 Apr;67(4):411–416. doi: 10.1378/chest.67.4.411. [DOI] [PubMed] [Google Scholar]
- Elias J. A., Freundlich B., Kern J. A., Rosenbloom J. Cytokine networks in the regulation of inflammation and fibrosis in the lung. Chest. 1990 Jun;97(6):1439–1445. doi: 10.1378/chest.97.6.1439. [DOI] [PubMed] [Google Scholar]
- Goldstein R. H., Fine A. Fibrotic reactions in the lung: the activation of the lung fibroblast. Exp Lung Res. 1986;11(4):245–261. doi: 10.3109/01902148609062828. [DOI] [PubMed] [Google Scholar]
- Hunninghake G. W., Gadek J. E., Kawanami O., Ferrans V. J., Crystal R. G. Inflammatory and immune processes in the human lung in health and disease: evaluation by bronchoalveolar lavage. Am J Pathol. 1979 Oct;97(1):149–206. [PMC free article] [PubMed] [Google Scholar]
- Kelley J. Cytokines of the lung. Am Rev Respir Dis. 1990 Mar;141(3):765–788. doi: 10.1164/ajrccm/141.3.765. [DOI] [PubMed] [Google Scholar]
- Keogh B. A., Crystal R. G. Clinical significance of pulmonary function tests. Pulmonary function testing in interstitial pulmonary disease. What does it tell us? Chest. 1980 Dec;78(6):856–865. doi: 10.1378/chest.78.6.856. [DOI] [PubMed] [Google Scholar]
- Lemaire I., Beaudoin H., Dubois C. Cytokine regulation of lung fibroblast proliferation. Pulmonary and systemic changes in asbestos-induced pulmonary fibrosis. Am Rev Respir Dis. 1986 Oct;134(4):653–658. doi: 10.1164/arrd.1986.134.4.653. [DOI] [PubMed] [Google Scholar]
- Low R. B., Cutroneo K. R., Davis G. S., Giancola M. S. Lavage type III procollagen N-terminal peptides in human pulmonary fibrosis and sarcoidosis. Lab Invest. 1983 Jun;48(6):755–759. [PubMed] [Google Scholar]
- Martinet Y., Rom W. N., Grotendorst G. R., Martin G. R., Crystal R. G. Exaggerated spontaneous release of platelet-derived growth factor by alveolar macrophages from patients with idiopathic pulmonary fibrosis. N Engl J Med. 1987 Jul 23;317(4):202–209. doi: 10.1056/NEJM198707233170404. [DOI] [PubMed] [Google Scholar]
- Murrell G. A., Francis M. J., Bromley L. Modulation of fibroblast proliferation by oxygen free radicals. Biochem J. 1990 Feb 1;265(3):659–665. doi: 10.1042/bj2650659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- O'Connor C., Ward K., van Breda A., McIlgorm A., FitzGerald M. X. Type 3 procollagen peptide in bronchoalveolar lavage fluid. Poor indicator of course and prognosis in sarcoidosis. Chest. 1989 Aug;96(2):339–344. doi: 10.1378/chest.96.2.339. [DOI] [PubMed] [Google Scholar]
- Reynolds H. Y. Bronchoalveolar lavage. Am Rev Respir Dis. 1987 Jan;135(1):250–263. doi: 10.1164/arrd.1987.135.1.250. [DOI] [PubMed] [Google Scholar]
- Rohde H., Vargas L., Hahn E., Kalbfleisch H., Bruguera M., Timpl R. Radioimmunoassay for type III procollagen peptide and its application to human liver disease. Eur J Clin Invest. 1979 Dec;9(6):451–459. doi: 10.1111/j.1365-2362.1979.tb00912.x. [DOI] [PubMed] [Google Scholar]
- Scadding J. G., Hinson K. F. Diffuse fibrosing alveolitis (diffuse interstitial fibrosis of the lungs). Correlation of histology at biopsy with prognosis. Thorax. 1967 Jul;22(4):291–304. doi: 10.1136/thx.22.4.291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Turner-Warwick M., Burrows B., Johnson A. Cryptogenic fibrosing alveolitis: clinical features and their influence on survival. Thorax. 1980 Mar;35(3):171–180. doi: 10.1136/thx.35.3.171. [DOI] [PMC free article] [PubMed] [Google Scholar]



