Abstract
Objective
Epidemiological studies relating maternal 25-hydroxyvitamin D (25OHD) with gestational diabetes mellitus (GDM) and mode of delivery have shown controversial results. We examined if maternal 25OHD status was associated with plasma glucose concentrations, risks of GDM and caesarean section in the Growing Up in Singapore Towards healthy Outcomes (GUSTO) study.
Methods
Plasma 25OHD concentrations, fasting glucose (FG) and 2-hour postprandial glucose (2HPPG) concentrations were measured in 940 women from a Singapore mother-offspring cohort study at 26–28 weeks’ gestation. 25OHD inadequacy and adequacy were defined based on concentrations of 25OHD ≤75nmol/l and >75nmol/l respectively. Mode of delivery was obtained from hospital records. Multiple linear regression was performed to examine the association between 25OHD status and glucose concentrations, while multiple logistic regression was performed to examine the association of 25OHD status with risks of GDM and caesarean section.
Results
In total, 388 (41.3%) women had 25OHD inadequacy. Of these, 131 (33.8%), 155 (39.9%) and 102 (26.3%) were Chinese, Malay and Indian respectively. After adjustment for confounders, maternal 25OHD inadequacy was associated with higher FG concentrations (β = 0.08mmol/l, 95% Confidence Interval (CI) = 0.01, 0.14), but not 2HPPG concentrations and risk of GDM. A trend between 25OHD inadequacy and higher likelihood of emergency caesarean section (Odds Ratio (OR) = 1.39, 95% CI = 0.95, 2.05) was observed. On stratification by ethnicity, the association with higher FG concentrations was significant in Malay women (β = 0.19mmol/l, 95% CI = 0.04, 0.33), while risk of emergency caesarean section was greater in Chinese (OR = 1.90, 95% CI = 1.06, 3.43) and Indian women (OR = 2.41, 95% CI = 1.01, 5.73).
Conclusions
25OHD inadequacy is prevalent in pregnant Singaporean women, particularly among the Malay and Indian women. This is associated with higher FG concentrations in Malay women, and increased risk of emergency caesarean section in Chinese and Indian women.
Introduction
Vitamin D inadequacy, which is defined as serum 25-hydroxyvitamin D (25OHD) <75nmol/l in some studies [1–2] and <50nmol/l in others [2–3], is common among pregnant women and has become a global public health problem [4]. During pregnancy, the active form of vitamin D, 1,25-dihydroxyvitamin D (1,25(OH)2D) concentrations increase by 100% or more [5]. Recently, it has been shown that production of 20-hydroxyvitamin D (20OHD) was higher than 25OHD in placenta [6–7]. However, these hydroxyvitamin D metabolites are not routinely measured to reflect vitamin D pool of the body. Serum 25OHD which is the major circulating form of vitamin D, is currently used as the best determinant of vitamin D status [5]. Associations have been found between serum 25OHD with various pregnancy outcomes such as gestational diabetes mellitus (GDM), hyperglycaemia and caesarean section (S1 Table), but results have been inconclusive.
25OHD involves in glucose homeostasis via different mechanisms. In its active form, it improves insulin sensitivity of the target cells (liver, skeletal muscle and adipose tissue) [8]. It also improves β-cell function [9], protects β-cell from immune attacks and reduces insulin resistant through immunoregulatory and anti-inflammatory effects [8]. Serum 25OHD has been shown to be inversely associated with maternal fasting glucose [10–14] and postprandial glucose concentrations [15–16] during pregnancy, but there are conflicting data with respect to the risk of developing GDM. Some studies have suggested that low serum 25OHD was associated with GDM [12–13,16–18], while other studies found no significant associations with GDM [1,10,15,19–20].
It has been shown that both skeletal and uterine smooth muscle contain vitamin D receptors [21–22], with more recent data implicating 25OHD and regulation of contractile proteins in human uterine myometrial cells [23]. The relationship of 25OHD with labour and delivery outcomes can thus be related to both muscle performance and uterine contraction. Higher likelihood of caesarean delivery has been observed in pregnant women with low serum 25OHD concentrations [24–25], although this had not been the case in several other studies [1,19,26–27].
Serum 25OHD levels vary according to geographical location and sunlight exposure [28]. In fact, striking ethnicity disparities in the prevalence of 25OHD deficiency has been reported within the same country or even city [29–30]. The Third National Health and Nutrition Examination Survey (NHANES III) in United States found that serum 25OHD levels were inversely associated with diabetes risk in white, but not black populations [29]. However, an Australian study reported no association between serum 25OHD and GDM risk in any ethnic subgroups [10]. These studies between 25OHD, ethnicity and disease outcomes were mainly conducted in Western setting, but none have been designed to describe the association in a multi-ethnic Asian setting.
The Singapore Growing Up in Singapore Towards healthy Outcomes (GUSTO) study, consisting of mothers from three ethnic groups, namely the Chinese, Malays and Indians, provides a unique opportunity to evaluate pregnancy outcomes associated with 25OHD status across ethnic groups with the absence of seasonal variation in sunlight exposure. This study aimed to examine the association of maternal 25OHD status in the second trimester of pregnancy with plasma glucose concentrations, risks of GDM and caesarean section. We hypothesized that 25OHD inadequacy was associated with higher fasting glucose (FG) and 2-hour postprandial glucose (2HPPG) concentrations, increased risks of GDM and emergency caesarean section due to prolonged labour and foetal distress.
Methods
Study design and participants
Women were drawn from the GUSTO mother-offspring cohort study, which involved detailed assessment of pregnant women and characteristics of their offspring from birth onwards [31]. The GUSTO study was designed to investigate the effects of early life events on the risk of developing metabolic diseases in later life. This study was conducted according to the guidelines laid down in the Declaration of Helsinki. Ethical approval was obtained from the Domain Specific Review Board of Singapore National Healthcare Group (reference D/09/021) and the Centralised Institutional Review Board of SingHealth (reference 2009/280/D).
Pregnant women attending antenatal care (<14 weeks’ gestation) from June 2009 to September 2010 in KK Women’s and Children’s Hospital (KKH) and National University Hospital (NUH), which house the major public maternity units in Singapore, were recruited into the GUSTO study. The inclusion criteria included age range between 18 and 50 years, intention to reside in Singapore for the next five years, intention to deliver in KKH and NUH, and willingness to donate cord, cord blood and placenta. Only Chinese, Malay and Indian women whose parents and whose husband’s parents were of same ethnicity were included in the study. Women receiving chemotherapy, psychotropic drugs or with type 1 diabetes mellitus were excluded. Informed written consent was obtained from all women.
Data collection
Women recruited in their first trimester returned to the hospitals at 26–28 weeks’ gestation for a follow-up visit. Detailed interviews were conducted in the clinics at recruitment and at 26–28 weeks’ gestation. Data on socioeconomic status, educational attainment, personal health, dietary supplement intake, smoking status and physical activity were collected. Smoking exposure was defined as current smoking or exposed to second hand smoke on a daily basis. After delivery, data on mode of delivery and complications were retrieved from the hospital case notes by trained health personnel.
Physical activity assessment
Three types of physical activity were assessed, including light-moderate, moderate and vigorous intensity activities. Total level of physical activity was computed from the summation of the duration (in minutes) and frequency (days) of these three types of activity. Physical activity was expressed in metabolic equivalents (MET-minutes/week) and classified as not highly active (<3000 MET-minutes/week) and highly active (≥3000 MET minutes/week) levels [32].
Anthropometric measurement
Maternal height was measured with a stadiometer (Seca 206, Hamburg, Germany). Maternal weight was based on body weight measured at first antenatal clinic visit during the first trimester of pregnancy. Body mass index (BMI) was computed from the formula: weight (kg)/ height (m2). Because obesity is a risk factor of low 25OHD [5], GDM and caesarean section [33], we therefore categorized the continuous values of BMI for analysis. The BMI was classified according to World Health Organization ranges: underweight <18.5kgm-2, normal weight 18.5–24.9kgm-2, overweight 25–29.9kgm-2 and obese ≥30.0kgm-2 [34].
Plasma glucose and 25OHD concentrations
An overnight fasting blood samples were drawn at 26–28 weeks’ gestation for glucose and 25OHD analyses. At the same visit, women underwent 75g Oral Glucose Tolerance Test (OGTT) for GDM diagnosis using World Health Organization criteria (FG or 2HPPG concentrations ≥7.0 or ≥7.8mmol/l respectively) [35]. Plasma FG and 2HPPG concentrations were measured by colorimetry [Advia 2400 Chemistry system (Siemens Medical Solutions Diagnostics) and Beckman LX20 Pro analyzer (Beckman Coulter)].
Plasma 25OHD was analysed as 25-hydroxyvitamin D2 (25OHD2) and 25-hydroxyvitamin D3 (25OHD3) by isotope-dilution liquid chromatography–tandem mass spectrometry (ID-LC-MS/MS) [36]. The intra- and inter-assay CVs for 25OHD2 and 25OHD3 were ≤10.3%, and the detection limit was <4nmol/l for both metabolites. Women were categorized as having 25OHD inadequacy and 25OHD adequacy based on concentrations of 25OHD ≤75nmol/l and >75nmol/l respectively [37–38]. The cut-off of 25OHD deficiency at <50nmol/l was not adopted due to small sample size of pregnant women in this category when stratified by ethnicity, which would reduce the power of analysis (S2 Table).
Statistical analysis
Categorical data were presented as frequencies and percentages, while continuous data were presented as means and standard deviations. Comparisons between maternal characteristics and 25OHD status were performed using Pearson’s Chi-square test for categorical variables and independent t-test for continuous variables. Multiple logistic regression analysis was used to assess the association of 25OHD status with risks of GDM and caesarean sections (total caesarean section, emergency caesarean section and related indications, and elective caesarean section). Multiple linear regression analysis was used to assess the association of 25OHD status with FG and 2HPPG concentrations.
Both logistic and linear regression models were adjusted for confounding variables, which included maternal age, parity, ethnicity, education, body mass index, smoking exposure, physical activity during pregnancy, and pre-existing diabetes and / or hypertension. These confounders were selected based on literature review [25,39–40]. Because neonatal sex has been reported to be associated with risk of maternal GDM [41–42] and since an association was found between 25OHD status and neonatal sex (p = 0.036), we therefore further adjusted for this confounding variable in the final model. All statistical analyses were performed using IBM SPSS statistics, Version 20 (USA). Two-sided tests were used. A value of P<0.05 was considered statistically significant.
Results
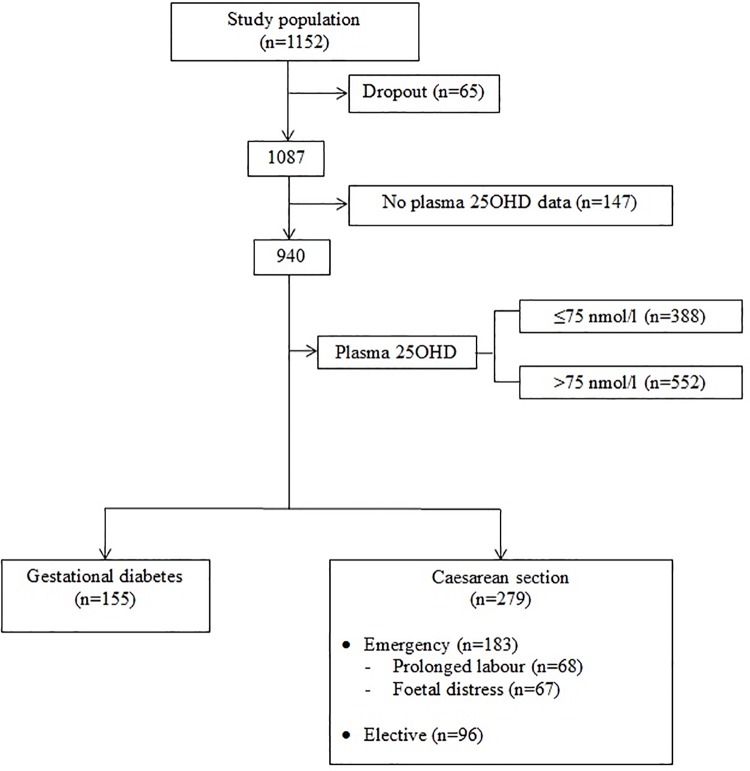
Of 1152 pregnant women who were recruited in the study at <14 weeks’ gestation, 1087 (94.4%) of them remained until the delivery stage (Fig 1). A total of 940 (86.5%) women with adequate amount of plasma samples were successfully analysed for 25OHD concentrations. Of this, 155 women had GDM, 279 women delivered via caesarean section, 183 and 96 women underwent emergency and elective caesarean sections respectively. Sixty eight women were delivered by emergency caesarean for a prolonged labour and another 67 for foetal distress. These two indications together accounted for almost three-quarters (73.8%) of delivery by emergency caesarean section (Table 1).
Fig 1. Study profile.
Table 1. Characteristics of participants according to 25OHD status (n = 940) a .
| Characteristics | Total (n = 940) | 25OHD inadequacy (n = 388) | 25OHD adequacy (n = 552) | p b |
|---|---|---|---|---|
| Plasma 25OHD, nmol/l | 81.03 (27.18) | 55.43 (13.81) | 99.02 (18.40) | <0.001 |
| Age, years | 30.53 (5.11) | 29.54 (5.12) | 31.24 (4.99) | <0.001 |
| Ethnicity, n (%) | ||||
| Chinese | 519 (55.2) | 131 (25.2) | 388 (74.8) | <0.001 |
| Malay | 247 (26.3) | 155 (62.8) | 92 (37.2) | |
| India | 174 (18.5) | 102 (58.6) | 72 (41.4) | |
| Parity, n (%) | ||||
| Nulliparous | 402 (42.8) | 162 (40.3) | 240 (59.7) | 0.599 |
| Multiparous | 538 (57.2) | 226 (42.0) | 312 (58.0) | |
| Body mass index (kg/m-2) | ||||
| Underweight, n (%) | 77 (8.2) | 30 (39.0) | 47 (61.0) | 0.008 |
| Normal weight, n (%) | 571 (61.1) | 222 (38.9) | 349 (61.1) | |
| Overweight, n (%) | 186 (19.9) | 77 (41.4) | 109 (58.6) | |
| Obese, n (%) | 100 (10.7) | 57 (57.0) | 43 (43.0) | |
| Education, n (%) | ||||
| None/ Primary/ Secondary | 284 (30.6) | 117 (41.2) | 167 (58.8) | 0.007 |
| Post-secondary | 326 (35.2) | 154 (47.2) | 172 (52.8) | |
| University and others | 317 (34.2) | 111 (35.0) | 206 (65.0) | |
| Smoking exposure, n (%) | 344 (36.8) | 163 (42.1) | 181 (33.0) | 0.004 |
| Intake of supplement containing vitamin D and Calcium, n (%) | 635 (74.6) | 228 (68.1) | 407 (78.9) | <0.001 |
| Physical activity, n (%) | ||||
| Not highly active | 746 (80.6) | 308 (41.3) | 438 (58.7) | 0.881 |
| Highly active | 179 (19.4) | 75 (41.9) | 104 (58.1) | |
| Pre-existing diabetes and/ or hypertension, n (%) | 19 (2.0) | 8 (2.1) | 11 (2.0) | 0.941 |
| Neonatal sex, n (%) | ||||
| Boys | 489 (52.0) | 186 (38.0) | 303 (62.0) | 0.036 |
| Girls | 451 (48.0) | 202 (44.8) | 249 (55.2) | |
| FG concentrations, mmol/l | 4.35 (0.47) | 4.39 (0.54) | 4.31 (0.42) | 0.020 |
| 2HPPG concentrations, mmol/l | 6.49 (1.44) | 6.43 (1.51) | 6.53 (1.40) | 0.303 |
| GDM, n (%) | 155 (17.7) | 59 (16.8) | 96 (18.3) | 0.570 |
| Caesarean section, c n (%) | 279 (29.7) | 122 (31.4) | 157 (28.4) | 0.321 |
| Emergency caesarean section, n (%) | 183 (21.7) | 86 (24.4) | 97 (19.7) | 0.101 |
| Prolonged labour, n (%) | 68 (8.1) | 30 (8.5) | 38 (7.7) | 0.674 |
| Foetal distress, n (%) | 67 (7.9) | 32 (9.1) | 35 (7.1) | 0.295 |
| Elective caesarean section, n (%) | 96 (10.2) | 36 (11.9) | 60 (13.2) | 0.608 |
a Total sample size is not always n = 940 due to the missing values. Data are presented as mean (standard deviation) or number (percentage). 25OHD inadequacy = 25OHD ≤75nmol/l; 25OHD adequacy = 25OHD>75nmol/l.
b p values are determined by independent t-test or Pearson chi-square test.
c Included both emergency and elective caesarean sections.
25OHD = 25-hydroxyvitamin D; FG = fasting glucose; 2HPPG = 2-hour postprandial glucose; GDM = gestational diabetes mellitus
Table 1 shows the characteristics of the participants. The mean 25OHD concentration for all women (n = 940) was 81.0nmol/l (standard deviation = 27.2). A total of 388 (41.3%) women had 25OHD inadequacy. Compared to women with adequate 25OHD status, this group of women were younger (p<0.001), comprised of more Malays (p<0.001), heavier (p = 0.008), attained lower educational levels (p = 0.007), reported higher smoking exposures during pregnancy (p = 0.004) and less likely to take vitamin D and calcium supplements during pregnancy (p<0.001). No significant differences in maternal 25OHD status were observed when analysed for parity, physical activity and pre-existing diabetes and/ or hypertension. Higher FG concentrations were found in women with 25OHD inadequacy compared to those with 25OHD adequacy (p = 0.020). Upon stratification by ethnicity, FG concentrations and incidence of emergency caesarean sections differed by 25OHD status in only the Malay (p = 0.027) and Indian women (p = 0.034) (Table 2).
Table 2. Pregnancy outcomes according to 25OHD status and ethnicity.
| Variables | Chinese (n = 519) | Malay (n = 247) | Indian (n = 174) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| 25OHD inadequacy (n = 131) | 25OHD adequacy (n = 388) | p a | 25OHD inadequacy (n = 155) | 25OHD adequacy (n = 92) | p a | 25OHD inadequacy (n = 102) | 25OHD adequacy (n = 72) | p a | |
| FG concentrations, mmol/l | 4.33 (0.51) | 4.31 (0.42) | 0.741 | 4.39 (0.60) | 4.25 (0.38) | 0.027 | 4.48 (0.49) | 4.39 (0.46) | 0.266 |
| 2HPPG concentrations, mmol/l | 6.67 (1.43) | 6.60 (1.40) | 0.622 | 6.20 (1.49) | 6.20 (1.31) | 0.995 | 6.47 (1.62) | 6.59 (1.46) | 0.629 |
| GDM | 24 (20.0) | 75 (20.3) | 0.949 | 14 (9.8) | 8 (9.0) | 0.839 | 21 (23.6) | 13 (19.4) | 0.530 |
| Caesarean section b | 42 (32.1) | 104 (26.8) | 0.247 | 40 (25.8) | 30 (32.6) | 0.251 | 40 (39.2) | 23 (31.9) | 0.326 |
| Emergency caesarean section | 27 (23.3) | 65 (18.6) | 0.276 | 27 (19.0) | 21 (25.3) | 0.267 | 32 (34.0) | 11 (18.3) | 0.034 |
| Prolonged labour | 10 (8.6) | 27 (7.7) | 0.760 | 13 (9.2) | 8 (9.6) | 0.904 | 7 (7.4) | 3 (5.0) | 0.548 |
| Foetal distress | 8 (6.9) | 21 (6.0) | 0.734 | 14 (9.9) | 9 (10.8) | 0.814 | 10 (10.6) | 5 (8.3) | 0.638 |
| Elective caesarean section | 15 (14.4) | 39 (12.1) | 0.531 | 13 (10.2) | 9 (12.7) | 0.587 | 8 (11.4) | 12 (19.7) | 0.191 |
Data are presented as mean (standard deviation) or number (percentage). 25OHD inadequacy = 25OHD ≤75nmol/l; 25OHD adequacy = 25OHD>75nmol/l. The values in bold indicate p<0.05.
a p values are determined by chi-square test or independent t-test.
b Included both emergency and elective caesarean sections.
25OHD = 25-hydroxyvitamin D; FG = fasting glucose; 2HPPG = 2-hour postprandial glucose; GDM = gestational diabetes mellitus.
Table 3 presents the associations between 25OHD status and related outcomes before and after adjusting for potential confounding. Compared to women with adequate 25OHD status, women with 25OHD inadequacy had higher FG concentrations (β = 0.08mmol/l, 95% Confidence Interval (CI) = 0.02, 0.14). This effect estimate did not change even after adjustment for confounders (β = 0.08mmol/l, 95% CI = 0.01, 0.14). Women with 25OHD inadequacy showed a trend towards a higher likelihood of emergency caesarean section in both unadjusted (Odds Ratio (OR) = 1.32, 95% CI = 0.95, 1.83) and adjusted (OR = 1.39, 95% CI = 0.95, 2.05) models. The 2HPPG concentrations, GDM and non-emergency caesarean section rates were not found to be associated with 25OHD status in both unadjusted and adjusted models.
Table 3. Associations between 25OHD status and pregnancy outcomes.
| Crude | Adjusted | |||||
|---|---|---|---|---|---|---|
| Maternal outcomes | 25OHD adequacy | 25OHD inadequacy | 25OHD adequacy | 25OHD inadequacy | ||
| OR (95% CI) a | p | OR (95% CI) a | p | |||
| FG concentrations, mmol/l | reference | 0.08 (0.02, 0.14) b | 0.014 | Reference | 0.08 (0.01, 0.14) b | 0.025 |
| 2HPPG concentrations, mmol/l | reference | -0.10 (-0.30, 0.09) b | 0.303 | Reference | 0.05 (-0.15, 0.25) b | 0.631 |
| GDM | reference | 0.90 (0.63, 1.29) | 0.571 | Reference | 1.02 (0.68, 1.53) | 0.938 |
| Caesarean section c | reference | 1.15 (0.87, 1.53) | 0.322 | Reference | 1.15 (0.83, 1.58) | 0.406 |
| Emergency caesarean section | reference | 1.32 (0.95, 1.83) | 0.102 | Reference | 1.39 (0.95, 2.05) | 0.092 |
| Prolonged labour | reference | 1.11 (0.68, 1.83) | 0.674 | Reference | 1.24 (0.68, 2.27) | 0.480 |
| Foetal distress | reference | 1.31 (0.79, 2.15) | 0.296 | Reference | 1.08 (0.61, 1.91) | 0.788 |
| Elective caesarean section | reference | 0.89 (0.57, 1.39) | 0.608 | Reference | 0.76 (0.47, 1.24) | 0.277 |
Adjusted for maternal age, parity, ethnicity, education, body mass index, smoking exposure, physical activity, pre-existing diabetes and/ or hypertension, neonatal sex. 25OHD inadequacy = 25OHD ≤75nmol/l; 25OHD adequacy = 25OHD>75nmol/l. The values in bold indicate p<0.05.
a Data are presented as Odds Ratio (95% Confidence Interval), unless otherwise indicated.
b Data are presented as β regression coefficient (95% Confidence Interval)
c Included both emergency and elective caesarean sections.
25OHD = 25-hydroxyvitamin D; FG = fasting glucose; 2HPPG = 2-hour postprandial glucose; GDM = gestational diabetes mellitus
When analyses were stratified by ethnicity (Table 4), the association between inadequate 25OHD status and higher FG concentrations was significant in Malay women (β = 0.19 mmol/l, 95% CI = 0.04, 0.33), but not in Chinese and Indian women, while the odds of having emergency caesarean section were approximately two times greater in Chinese (OR = 1.90, 95% CI = 1.06, 3.43) and Indian women (OR = 2.41, 95% CI = 1.01, 5.73) with 25OHD inadequacy compared to those with 25OHD adequacy.
Table 4. Associations between 25OHD status and pregnancy outcomes according to ethnicity.
| Chinese | Malay | Indian | |||||||
|---|---|---|---|---|---|---|---|---|---|
| Pregnancy outcomes | 25OHD adequacy | 25OHD inadequacy | 25OHD adequacy | 25OHD inadequacy | 25OHD adequacy | 25OHD inadequacy | |||
| OR a (95% CI) | P | OR a (95% CI) | p | OR a (95% CI) | p | ||||
| FG concentrations, mmol/l | reference | 0.03 (-0.06, 0.12) b | 0.507 | Reference | 0.19 (0.04, 0.33) b | 0.013 | reference | 0.09 (-0.07, 0.24) b | 0.268 |
| 2HPPG concentrations, mmol/l | reference | 0.10 (-0.21, 0.36) b | 0.616 | Reference | 0.12 (-0.24, 0.49) b | 0.509 | reference | 0.08 (-0.42, 0.59) b | 0.747 |
| GDM | reference | 0.92 (0.54, 1.59) | 0.771 | Reference | 1.36 (0.50, 3.72) | 0.546 | reference | 01.34 (0.55, 3.24) | 0.520 |
| Caesarean section c | reference | 1.46 (0.91, 2.35) | 0.117 | Reference | 0.67 (0.37, 1.23) | 0.194 | reference | 1.42 (0.70, 2.89) | 0.337 |
| Emergency caesarean section | reference | 1.90 (1.06, 3.43) | 0.033 | Reference | 0.56 (0.28, 1.15) | 0.115 | reference | 2.41 (1.01, 5.73) | 0.048 |
| Prolonged labour | reference | 1.91 (0.80, 4.58) | 0.146 | Reference | 0.76 (0.27, 2.14) | 0.599 | reference | 1.50 (0.32, 7.04) | 0.608 |
| Foetal distress | reference | 1.41 (0.55, 3.59) | 0.477 | Reference | 0.73 (0.28, 1.94) | 0.533 | reference | 1.33 (0.33, 5.29) | 0.690 |
| Elective caesarean section | reference | 1.02 (0.52, 2.01) | 0.948 | Reference | 0.98 (0.37, 2.61) | 0.967 | reference | 0.35 (0.12, 1.01) | 0.052 |
Adjusted for maternal age, parity, education, body mass index, smoking exposure, physical activity, pre-existing diabetes and/ or hypertension, neonatal sex. 25OHD inadequacy = 25OHD ≤75nmol/l; 25OHD adequacy = 25OHD>75nmol/l. The values in bold indicate p<0.05.
a Data are presented as Odds Ratio (95% Confidence Interval), unless otherwise indicated.
b Data are presented as β regression coefficient (95% Confidence Interval)
c Included both emergency and elective caesarean sections.25OHD = 25-hydroxyvitamin D; FG = fasting glucose; 2HPPG = 2-hour postprandial glucose; GDM = gestational diabetes mellitus.
Discussion
In this multi-ethnic cohort, 41% of pregnant women were found to have inadequate plasma levels of 25OHD in the second trimester, with substantially higher rates found in Malay and Indian women compared to Chinese women. Overall, maternal 25OHD inadequacy was significantly associated with higher FG concentrations, and a trend towards higher likelihood of emergency caesarean delivery. By ethnicity, the association between inadequate 25OHD status and higher FG concentrations was found to be significant only in Malay women, while the odds of having emergency caesarean section were approximately two times greater in Chinese and Indian women with 25OHD inadequacy. We found no association of maternal 25OHD status with 2HPPG concentrations, risks of GDM and non-emergency caesarean sections in the overall cohort as well as within any ethnic group.
Our observation mostly supports the findings of previous studies regarding the association between 25OHD and glucose metabolism in obstetric populations. In line with previous studies [10–14], we found an inverse association between 25OHD and FG concentrations, but no association with 2HPPG concentrations was observed [11,13]. This suggests that 2HPPG may be less likely to be influenced by 25OHD concentrations although its variability is larger than FG. The reasons for the lack of an association between 25OHD and 2HPPG are unclear. It has been reported that 1- and 2-hour postprandial glucose values are largely driven by insulin resistant state [11]. One possibility is that 25OHD may not influence glucose metabolism via modulation of insulin sensitivity, but through other pathways such as modulating pancreatic beta-cell function or cytokines generation [43]. Compared to 2HPPG, FG during pregnancy has been shown as a stronger predictor of foetal serum C-peptide levels [44] and adiposity [45], suggesting a potential link between maternal 25OHD inadequacy and impaired metabolic outcomes in the offspring.
While two previous studies from Australia involving approximately 300 women have shown an inverse association between 25OHD and FG independent of ethnicity [10–11], we found that similar to the NHANES III among non-pregnant population [29], ethnicity can modify the association between 25OHD and glucose metabolism. When ethnic subgroups were analysed separately, the inverse association between 25OHD and FG only appeared significant in Malay women, but not in Chinese and Indian women, suggesting the importance of vitamin D adequacy to optimise FG concentrations in Malay pregnant women. The inverse association between 25OHD and FG in Malay women could also be attributable to their relatively higher rates of 25OHD deficiency (25OHD<50nmol/l) compared to other ethnic groups. However, the contrasting lack of any inverse association of 25OHD with FG in Indian women was unexpected given their relatively lower 25OHD concentrations and higher FG concentrations. It is possible that this group of Indian women were predisposed to the risk of fasting hyperglycaemia through pathway which is independent from the influence of 25OHD concentrations [10].
The association between serum 25OHD and GDM in the literature is conflicting [46]. In general, the association between 25OHD and GDM was more commonly shown in populations with high prevalence of 25OHD deficiency. In a Turkish study, Zuhur and colleagues [13] showed significantly lower 25OHD levels in GDM women (n = 234, mean = 30.8nmol/l) than the controls (n = 168, mean = 36.0nmol/l). However, when subgroups of 25OHD levels were analysed, only women with severely deficient 25OHD (n = 64, <12.5nmol/l) showed an increased risk of GDM [13]. Similarly, in a Chinese study of 400 pregnant women with 54% 25OHD deficiency (<25nmol/l), a significant association between 25OHD at 26–28 weeks’ gestation and risk of developing GDM was found [12]. Similar observations were shown by other studies which reported a high prevalence (>25%) of vitamin D deficiency with 25OHD<50nmol/l [16–18]. In our cohort, only 0.5% of women had 25OHD<25nmol/l and 13.4% had 25OHD<50nmol/l, and this may explain the lack of association between 25OHD status and GDM. In fact, several other studies performed in populations where 25OHD deficiency is low [15,20] shared the same finding as ours. We recognized that some of the discrepancies in findings between 25OHD and GDM could also be due to the use of different GDM diagnosis criteria across studies.
In a cross-sectional study of 253 women, 25OHD<37.5nmol/l measured at birth was associated with a fourfold increased risk of caesarean section [24]. This finding was replicated by Scholl et al. [25] who reported a less than twofold (66%) increased risk of caesarean birth for women with 25OHD<30nmol/l measured at 14 weeks’ gestation. When specific indications were examined, 25OHD<30nmol/l was linked to a twofold increased risk of caesarean for prolonged labour [25]. While our data did not show any significant association between 25OHD status and total caesarean section, we found that Chinese and Indian women with 25OHD inadequacy were approximately two times more likely to have emergency caesarean section than those with 25OHD adequacy. A prospective cohort which studied 995 women in UK reported no association of 25OHD in the first trimester with emergency or elective caesarean sections, and related indications such as failure to progress and foetal distress in labour [26]. Similarly, we also did not observe any significant association of 25OHD status with risks of prolonged labour and foetal distress which could be due to small sample size. These null findings were supported by others [1,19,27]. In these studies, 25OHD assessment was done in early pregnancy [19,26] and the effect on mode of delivery may become apparent only in late pregnancy [26].
The strengths of this study include its prospective study design [31] and the use of liquid chromatography–tandem mass spectrometry which is a more accurate method compared to other techniques in measuring 25OHD concentrations [47]. Our study was limited for not being able to adjust for residual confounding. Only one blood sample during mid-late gestation was obtained which restricts our ability to evaluate 25OHD concentrations in other windows that could have had a profound impact on maternal plasma glucose, GDM risk or mode of delivery. Other pregnancy outcomes such as preeclampsia or infections were not examined due to their relatively low prevalence rates in our study. A growing body of literature has now documented an association of genetic variation in cytochrome P450, vitamin D binding protein [48] and vitamin D receptor [49] with 25OHD concentrations that could impact on vitamin D metabolism and disease susceptibility. These genetic factors might shed some light in the ethnicity susceptibility in the association between 25OHD and different outcome measures. Thus, assessment of vitamin D related genotype and stratification of cases by both serum levels and genetic polymorphisms are warranted in future studies.
Conclusions
In conclusion, while prevalence of 25OHD deficiency is low, 25OHD inadequacy is highly prevalent during pregnancy in Singaporean women, particularly among Malay and Indian women. This is associated with higher FG concentrations in Malay women and an increased risk of emergency caesarean section in Chinese and Indian women. This may suggest varying threshold effects of 25OHD sensitivity on pregnancy outcomes among ethnic groups. Further investigations on biological components, social, nutritional practices and cultural differences are required to explain the mechanism of ethnicity disparity in 25OHD effects. Nevertheless, the present findings are important to provide evidence for clinical recommendations regarding potential screening of 25OHD inadequacy during prenatal care and the need for vitamin D supplementation in at risk groups.
Supporting Information
(DOCX)
(DOC)
Acknowledgments
The GUSTO study group includes:
Allan Sheppard, Developmental Epigenetics Group, The Liggins Institute, University of Auckland, New Zealand
Amutha Chinnadurai, Department of Neonatology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Anne Eng Neo Goh, Allergy Service, Department of Paediatrics, KK Women's and Children's Hospital, Singapore
Anne Rifkin-Graboi, Singapore Institute for Clinical Sciences, the Agency for Science, Technology and Research, Singapore
Anqi Qiu, Department of Biomedical Engineering, National University of Singapore, Singapore; Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research, Singapore; Clinical Imaging Research Centre, National University of Singapore, Singapore
Arijit Biswas, Obstetrics & Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Bee Wah Lee, Department of Paediatrics, University Children’s Medical Institute, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Birit F.P. Broekman, Singapore Institute for Clinical Sciences, the Agency for Science, Technology and Research, Singapore; Department of Psychological Medicine, Yong Loo Lin, School of Medicine, National University of Singapore and National University Health System, Singapore
Boon Long Quah, Singapore National Eye Centre, Singapore; Department of Ophthalmology, KK Women’s and Children’s Hospital, Singapore
Borys Shuter, Department of Diagnostic Radiology, National University of Singapore, Singapore
Chai Kiat Chng, Dental Service, KK Women's and Children's Hospital, Singapore
Cheryl Ngo, Department of Opthalmology, National University Hospital, Singapore
Stephen Chin-Ying Hsu, Department of Preventive Dentistry, Faculty of Dentistry, National University of Singapore, Singapore
Choon Looi Bong, Paediatric Anaesthesia, KK Women's and Children's Hospital, Singapore
Christiani Jeyakumar Henry, Clinical Nutrition Research Centre, Singapore Institute for Clinical Sciences, Singapore
Cornelia Yin Ing Chee, Department of Psychological Medicine, Yong Loo Lin School of Medicine, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Doris Fok, Obstetrics & Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Fabian Yap, Paediatric Endocrinology, KK Women’s and Children’s Hospital, Singapore; Duke-NUS Graduate Medical School, Singapore
George Seow Heong Yeo, Department of Maternal Fetal Medicine, KK Women’s and Children’s Hospital, Singapore
Hazel Inskip, MRC Life-course Epidemiology Unit, University of Southampton, Southampton General Hospital, Southampton, United Kingdom
Helen Chen, Mental Wellness Service, Department of Psychological Medicine, KK Women’s and Children’s Hospital, Singapore
Hugo P S van Bever, Department of Paediatrics, Children’s Medical Institute, National University Hospital, National University Health System, Singapore
Iliana Magiati, Department of Psychology, National University of Singapore, Singapore
Inez Bik Yun Wong, Paediatric Ophthalmology and Strabismus Service, Department of Ophthalmology, National University Hospital, Singapore
Ivy Yee-Man Lau, School of Social Sciences, Singapore Management University, Singapore
Jeevesh Kapur, Department of Diagnostic Imaging, National University Hospital, Singapore
Jenny L. Richmond, School of Psychology, University of New South Wales, Sydney, NSW, Australia
Jerry Kok Yen Chan, KK Research Centre, KK Women’s and Children’s Hospital, Singapore; Department of Reproductive Medicine, KK Women’s and Children’s Hospital, Singapore; Duke-NUS Graduate Medical School, Singapore
Joanna D. Holbrook, Growth, Development and Metabolism Programme, Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore
Joshua J. Gooley, Program in Neuroscience and Behavioral Disorders, Duke-NUS Graduate Medical School, Singapore; Division of Sleep and Circadian Disorders, Departments of Medicine and Neurology, Brigham and Women’s Hospital, Boston USA; Division of Sleep Medicine, Harvard Medical School, Boston, USA
Keith M. Godfrey, MRC Lifecourse Epidemiology Unit, University of Southampton, Southampton, United Kingdom; NIHR Southampton Biomedical Research Centre, University of Southampton and University Hospital Southampton NHS Foundation Trust, Southampton, United Kingdom
Kenneth Kwek, Department of Maternal Fetal Medicine, KK Women’s and Children’s Hospital, Singapore
Kok Hian Tan, Department of Maternal Fetal Medicine, KK Women’s and Children’s Hospital, Singapore
Krishnamoorthy Niduvaje, Department of Neonatology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Leher Singh, Department of Psychology, National University of Singapore, Singapore
Lin Lin Su, Department of Obstetrics and Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, SingaporeLourdes Mary Daniel, Department of Neonatology, KK Women’s and Children’s Hospital, Singapore
Lynette Pei-Chi Shek, Department of Paediatrics, University Children’s Medical Institute, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Marielle V. Fortier, Department of Diagnostic Imaging, KK Women’s and Children’s Hospital, Singapore
Mark Hanson, Institute of Developmental Sciences, Faculty of Medicine, University of Southampton; NIHR Nutrition Biomedical Research Centre, University Hospital Southampton, United Kingdom
Mary Foong-Fong Chong, Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research, Singapore; Clinical Nutrition Research Centre, Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore; Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Mary Rauff, Obstetrics & Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
Mei Chien Chua, Department of Neonatology, KK Women’s and Children’s Hospital, Singapore; Duke-NUS Graduate Medical School, Singapore
Michael Meaney, Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore; Departments of Psychiatry and Neurology & Neurosurgery, McGill University, Montreal, Canada
Mya Thway Tint, Obstetrics & Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore, National University of Singapore and National University Health System, Singapore
Ngee Lek, Paediatric Endocrinology, KK Women’s and Children’s Hospital, Singapore; Duke-NUS Graduate Medical School, Singapore
Oon Hoe Teoh, Respiratory Medicine Service, Department of Paediatric Medicine, KK Women’s and Children’s Hospital, Singapore
Peng Cheang Wong, Department of Obstetrics & Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Peter D. Gluckman, Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore; Liggins Institute, University of Auckland, Auckland, New Zealand
Pratibha Agarwal, Department of Neonatology, KK Women’s and Children’s Hospital, Singapore
Rob M. van Dam, Saw Swee Hock School of Public Health and Department of Medicine, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Salome A. Rebello, Life Sciences Institute, Centre for Life Sciences, National University of Singapore, Singapore
Seang-Mei Saw, Saw Swee Hock School of Public Health, National University of Singapore, Singapore and Myopia Unit, Singapore Eye Research Institute, Singapore
Shang Chee Chong, Division of Paediatric Neurology, Developmental and Behavioural Paediatrics, University Children’s Medical Institute, National University of Singapore and National University Health System, Singapore
Shirong Cai, Obstetrics & Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Shu-E Soh, Singapore Institute for Clinical Sciences, Agency for Science, Technology and Research, Singapore
Sok Bee Lim, Department of Child Development, KK Women’s & Children’s Hospital, Singapore
Victor Samuel Rajadurai, Department of Neonatology, KK Women's & Children's Hospital, Singapore
Walter Stunkel, Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore
Wee Meng Han, Department of Nutrition and Dietetics, KK Women’s and Children’s Hospital, Singapore
Wei Wei Pang, Obstetrics & Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Yam Thiam Daniel Goh, Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Yap-Seng Chong, Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore; Obstetrics & Gynaecology, Yong Loo Lin School of Medicine, National University of Singapore and National University Health System, Singapore
Yin Bun Cheung, Center for Quantitative Medicine, Duke-NUS Graduate Medical School, Singapore; Department for International Health, University of Tampere, Finland
Yiong Huak Chan, Medicine Dean's Office, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
Yung Seng Lee, Growth, Development and Metabolism Programme, Singapore Institute for Clinical Sciences, Agency for Science Technology and Research, Singapore; Paediatric Endocrinology and Diabetes, University Children's Medical Institute, National University Health System, Singapore; Department of Paediatrics, Yong Loo Lin School of Medicine, National University of Singapore, Singapore
Lead author GUSTO group: A/Prof Yap-Seng Chong, email: yap_seng_chong@nuhs.edu.sg
Data Availability
Due to an ethical restriction (patient confidentiality), data are available upon request to the GUSTO team. Please contact the GUSTO data team at Chang_Mei_Ling@sics.a-star.edu.sg to request access to the data.
Funding Statement
This study was supported by the Singapore National Research Foundation under its Translational and Clinical Research (TCR) Flagship Programme and administered by the Singapore Ministry of Health’s National Medical Research Council (NMRC), Singapore—NMRC/TCR/004-NUS/2008; NMRC/TCR/012-NUHS/2014. Additional funding is provided by the Singapore Institute for Clinical Sciences, Agency for Science Technology and Research (A*STAR), Singapore. KMG is supported by the National Institute for Health Research through the NIHR Southampton Biomedical Research Centre and by the European Union's Seventh Framework Programme (FP7/2007-2013), project EarlyNutrition under grant agreement number 289346. JKYC received salary support from the Ministry of Health’s National Medical Research Council, Singapore (NMRC/CSA/043/2012). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Charatcharoenwitthaya N, Nanthakomon T, Somprasit C, Chanthasenanont A, Chailurkit LO, Pattaraarchachai J, et al. Maternal vitamin D status, its associated factors and the course of pregnancy in Thai women. Clin Endocrinol (Oxf). 2013;78(1):126–133. [DOI] [PubMed] [Google Scholar]
- 2. Bruyère O, Slomian J, Beaudart C, Buckinx F, Cavalier E, Gillain S, et al. Prevalence of vitamin D inadequacy in European women aged over 80 years. Arch Gerontol Geriatr. 2014; 59(1):78–82. 10.1016/j.archger.2014.03.010 [DOI] [PubMed] [Google Scholar]
- 3. Holick MF. High prevalence of vitamin D inadequacy and implications for health. Mayo Clin Proc. 2006;81(3):353–373. [DOI] [PubMed] [Google Scholar]
- 4. van Schoor NM, Lips P. Worldwide vitamin D status. Best Pract Res Clin Endocrinol Metab. 2011;25(4):671–680. 10.1016/j.beem.2011.06.007 [DOI] [PubMed] [Google Scholar]
- 5. Christesen HT, Falkenberg T, Lamont RF, Jørgensen JS. The impact of vitamin D on pregnancy: a systematic review. Acta Obstet Gynecol Scand. 2012;91(12):1357–1367. 10.1111/aogs.12000 [DOI] [PubMed] [Google Scholar]
- 6. Slominski AT, Kim TK, Shehabi HZ, Semak Igor, Tang EKY, Nguyen MN, et al. In vivo evidence for a novel pathway of vitamin D3 metabolism initiated by P450scc and modified by CYP27B1. FASEB J. 2012; 26:3901–3915. 10.1096/fj.12-208975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Slominski AT, Kim TK, Shehabi HZ, Tang E, Benson HAE, Semak I, et al. In vivo production of novel vitamin D2 hydroxy-derivatives by human placentas, epidermal keratinocytes, Caco-2 colon cells and the adrenal gland. Mol Cell Endocrinol. 2014; 383(0): 181–192. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Sung CC, Liao MT, Lu KC, Wu CC. Role of vitamin D in insulin resistance. J Biomed Biotechnol. 2012;2012:634195 10.1155/2012/634195 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Chiu KC, Chu A, Go VLW, Saad MF. Hypovitaminosis D is associated with insulin resistance and β cell dysfunction. Am J Clin Nutr. 2004;79(5):820–825. [DOI] [PubMed] [Google Scholar]
- 10. Clifton-Bligh RJ, McElduff P, McElduff A. Maternal vitamin D deficiency, ethnicity and gestational diabetes. Diabet Med. 2008;25(6):678–684. 10.1111/j.1464-5491.2008.02422.x [DOI] [PubMed] [Google Scholar]
- 11. McLeod DS, Warner JV, Henman M, Cowley D, Gibbons K, McIntyre HD, et al. Associations of serum vitamin D concentrations with obstetric glucose metabolism in a subset of the Hyperglycemia and Adverse Pregnancy Outcome (HAPO) study cohort. Diabet Med. 2012;29(8):e199–204. 10.1111/j.1464-5491.2011.03551.x [DOI] [PubMed] [Google Scholar]
- 12. Wang O, Nie M, Hu YY, Zhang K, Li W, Ping F, et al. Association between vitamin D insufficiency and the risk for gestational diabetes mellitus in pregnant Chinese women. Biomed Environ Sci. 2012;25(4):399–406. 10.3967/0895-3988.2012.04.004 [DOI] [PubMed] [Google Scholar]
- 13. Zuhur SS, Erol RS, Kuzu I, Altuntas Y. The relationship between low maternal serum 25-hydroxyvitamin D levels and gestational diabetes mellitus according to the severity of 25-hydroxyvitamin D deficiency. Clinics (Sao Paulo). 2013;68(5):658–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. El Lithy A, Abdella RM, El-Faissal YM, Sayed AM, Samie RM. The relationship between low maternal serum vitamin D levels and glycemic control in gestational diabetes assessed by HbA1c levels: an observational cross-sectional study. BMC Pregnancy Childbirth. 2014;14:362 10.1186/1471-2393-14-362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Burris HH, Rifas-Shiman SL, Kleinman K, Litonjua AA, Huh SY, Rich-Edwards JW, et al. Vitamin D deficiency in pregnancy and gestational diabetes mellitus. Am J Obstet Gynecol. 2012;207(3):182.e1–8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Parlea L, Bromberg IL, Feig DS, Vieth R, Merman E, Lipscombe LL. Association between serum 25-hydroxyvitamin D in early pregnancy and risk of gestational diabetes mellitus. Diabet Med. 2012;29(7):e25–32. 10.1111/j.1464-5491.2011.03550.x [DOI] [PubMed] [Google Scholar]
- 17. Cho GJ, Hong SC, Oh MJ, Kim HJ. Vitamin D deficiency in gestational diabetes mellitus and the role of the placenta. Am J Obstet Gynecol. 2013;209(6):560.e1–8. [DOI] [PubMed] [Google Scholar]
- 18. Lacroix M, Battista MC, Doyon M, Houde G, Menard J, Ardilouze JL, et al. Lower vitamin D levels at first trimester are associated with higher risk of developing gestational diabetes mellitus. Acta Diabetol. 2014;51(4):609–616. 10.1007/s00592-014-0564-4 [DOI] [PubMed] [Google Scholar]
- 19. Fernández-Alonso AM, Dionis-Sanchez EC, Chedraui P, Gonzalez-Salmeron MD, Perez-Lopez FR, The Spanish Vitamin D and Women's Health Research Group. First-trimester maternal serum 25-hydroxyvitamin D(3) status and pregnancy outcome. Int J Gynaecol Obstet. 2012;116 (1):6–9. 10.1016/j.ijgo.2011.07.029 [DOI] [PubMed] [Google Scholar]
- 20. Schneuer FJ, Roberts CL, Guilbert C, Simpson JM, Algert CS, Khambalia AZ, et al. Effects of maternal serum 25-hydroxyvitamin D concentrations in the first trimester on subsequent pregnancy outcomes in an Australian population. Am J Clin Nutr. 2014;99(2):287–295. 10.3945/ajcn.113.065672 [DOI] [PubMed] [Google Scholar]
- 21. Bischoff HA, Borchers M, Gudat F, Duermueller U, Theiler R, Stahelin HB, et al. In situ detection of 1,25-dihydroxyvitamin D3 receptor in human skeletal muscle tissue. Histochem J. 2001;33(1):19–24. [DOI] [PubMed] [Google Scholar]
- 22. Vienonen A, Miettinen S, Blauer M, Martikainen PM, Tomas E, Heinonen PK, et al. Expression of nuclear receptors and cofactors in human endometrium and myometrium. J Soc Gynecol Investig. 2004;11(2):104–112. [DOI] [PubMed] [Google Scholar]
- 23. Thota C, Laknaur A, Farmer T, Ladson G, Al-Hendy A, Ismail N. Vitamin D regulates contractile profile in human uterine myometrial cells via NF-kappaB pathway. Am J Obstet Gynecol. 2014;210(4):347e1-10. 10.1016/j.ajog.2013.11.027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24. Merewood A, Mehta SD, Chen TC, Bauchner H, Holick MF. Association between vitamin D deficiency and primary cesarean section. J Clin Endocrinol Metab. 2009;94(3):940–945. 10.1210/jc.2008-1217 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Scholl TO, Chen X, Stein P. Maternal vitamin D status and delivery by cesarean. Nutrients. 2012;4(4):319–330. 10.3390/nu4040319 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Savvidou MD, Makgoba M, Castro PT, Akolekar R, Nicolaides KH. First-trimester maternal serum vitamin D and mode of delivery. Br J Nutr. 2012;108(11):1972–1975. 10.1017/S0007114512000207 [DOI] [PubMed] [Google Scholar]
- 27. Gernand AD, Klebanoff MA, Simhan HN, Bodnar LM. Maternal vitamin D status, prolonged labor, cesarean delivery and instrumental delivery in an era with a low cesarean rate. J Perinatol. 2014;35(1):23–28. 10.1038/jp.2014.139 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Mulligan ML, Felton SK, Riek AE, Bernal-Mizrachi C. Implications of vitamin D deficiency in pregnancy and lactation. Am J Obstet Gynecol. 2010;202(5):429.e1–9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Scragg R, Sowers M, Bell C. Serum 25-hydroxyvitamin D, diabetes, and ethnicity in the Third National Health and Nutrition Examination Survey. Diabetes Care. 2004;27(12):2813–2818. [DOI] [PubMed] [Google Scholar]
- 30. Tan KM, Saw S, Sethi SK. Vitamin D and its relationship with markers of bone metabolism in healthy Asian women. J Clin Lab Anal. 2013;27(4):301–304. 10.1002/jcla.21602 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Soh SE, Tint MT, Gluckman PD, Godfrey KM., Rifkin-Graboi A, Chan YH, et al. Cohort Profile: Growing Up in Singapore Towards healthy Outcomes (GUSTO) birth cohort study. Int J Epidemiol. 2014;43(5):1401–1409. 10.1093/ije/dyt125 [DOI] [PubMed] [Google Scholar]
- 32. Padmapriya N, Shen L, Soh SE, Shen Z, Kwek K, Godfrey KM, et al. Physical Activity and Sedentary Behavior Patterns Before and During Pregnancy in a Multi-ethnic Sample of Asian Women in Singapore. Matern Child Health J. 2015; 10.1007/s10995-015-1773-3 [DOI] [PubMed] [Google Scholar]
- 33. Mission JF, Marshall NE, Caughey AB. Pregnancy risks associated with obesity. Obstet Gynecol Clin North Am. 2015;42(2):335–353. 10.1016/j.ogc.2015.01.008 [DOI] [PubMed] [Google Scholar]
- 34. World Health Orgnization. Obesity:Preventing and Managing a Global Epidemic, Report of a WHO Consultant on Obesity. 1998. Geneva: World Health Organization. [PubMed] [Google Scholar]
- 35. Alberti KG, Zimmet PZ. Definition, diagnosis and classification of diabetes mellitus and its complications. Part 1: diagnosis and classification of diabetes mellitus. Provisional report of a WHO Consultation. Diabet Med 1998;15(7):539–553. [DOI] [PubMed] [Google Scholar]
- 36. Maunsell Z, Wright DJ, Rainbow SJ. Routine isotope-dilution liquid chromatography-tandem mass spectrometry assay for simultaneous measurement of the 25-hydroxy metabolites of vitamins D2 and D3. Clin Chem. 2005;51(9):1683–1690. [DOI] [PubMed] [Google Scholar]
- 37. Aghajafari F, Nagulesapillai T, Ronksley PE, Tough SC, O'Beirne M, Rabi DM. Association between maternal serum 25-hydroxyvitamin D level and pregnancy and neonatal outcomes: systematic review and meta-analysis of observational studies. BMJ. 2013;346:f1169 10.1136/bmj.f1169 [DOI] [PubMed] [Google Scholar]
- 38. Wei SQ, Qi HP, Luo ZC, Fraser WD. Maternal vitamin D status and adverse pregnancy outcomes: a systematic review and meta-analysis. J Matern Fetal Neonatal Med. 2013; 26(9):889–899. 10.3109/14767058.2013.765849 [DOI] [PubMed] [Google Scholar]
- 39. Abboud M, Puglisi DA, Davies BN, Rybchyn M, Whitehead NP, Brock KE, et al. Evidence for a specific uptake and retention mechanism for 25-hydroxyvitamin D (25OHD) in skeletal muscle cells. Endocrinology. 2013;154(9):3022–3030. 10.1210/en.2012-2245 [DOI] [PubMed] [Google Scholar]
- 40. Nimitphong H, Holick MF. Vitamin D status and sun exposure in Southeast Asia. Dermatoendocrinol. 2013;5(1):34–37. 10.4161/derm.24054 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Retnakaran R, Kramer CK, Ye C, Kew S, Hanley AJ, Connelly PW, et al. Fetal sex and maternal risk of gestational diabetes mellitus: the impact of having a boy. Diabetes Care 2015; 38:844–851. 10.2337/dc14-2551 [DOI] [PubMed] [Google Scholar]
- 42. Retnakaran R, Shah BR. Fetal Sex and the Natural History of Maternal Risk of Diabetes During and After Pregnancy. J Clin Endocrinol Metab. 2015;100(7):2574–2580. 10.1210/jc.2015-1763 [DOI] [PubMed] [Google Scholar]
- 43. Pittas AG, Lau J, Hu FB, Dawson-Hughes B. The role of vitamin D and calcium in type 2 diabetes. A systematic review and meta-analysis. J Clin Endocrinol Metab. 2007;92(6):2017–2029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. The HAPO Study Cooperative Research Group. Hyperglycemia and adverse pregnancy outcomes. N Engl J Med. 2008;358:1991–2002. 10.1056/NEJMoa0707943 [DOI] [PubMed] [Google Scholar]
- 45. Aris IM, Soh SE, Tint MT, Liang S, Chinnadurai A, Saw SM, et al. Effect of maternal glycemia on neonatal adiposity in a multiethnic Asian birth cohort. J Clin Endocrinol Metab. 2014;99:240–247. 10.1210/jc.2013-2738 [DOI] [PubMed] [Google Scholar]
- 46. Joergensen JS, Lamont RF, Torloni MR. Vitamin D and gestational diabetes: an update. Curr Opin Clin Nutr Metab Care. 2014;17(4):360–367. 10.1097/MCO.0000000000000064 [DOI] [PubMed] [Google Scholar]
- 47. Hollis BW. Measuring 25-hydroxyvitamin D in a clinical environment: challenges and needs. Am J Clin Nutr. 2008;88(2):507S–510S. [DOI] [PubMed] [Google Scholar]
- 48. Robien K, Butler LM, Wang R, Beckman KB, Walek D, Koh WP, et al. Genetic and environmental predictors of serum 25-hydroxyvitamin D concentrations among middle-aged and elderly Chinese in Singapore. Br J Nutr. 2013;109(3):493–502. 10.1017/S0007114512001675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Levin GP, Robinson-Cohen C, de Boer IH, Houston DK, Lohman K, Liu Y, et al. Genetic variants and associations of 25-hydroxyvitamin D concentrations with major clinical outcomes. JAMA. 2012;308(18):1898–1905. 10.1001/jama.2012.17304 [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
(DOCX)
(DOC)
Data Availability Statement
Due to an ethical restriction (patient confidentiality), data are available upon request to the GUSTO team. Please contact the GUSTO data team at Chang_Mei_Ling@sics.a-star.edu.sg to request access to the data.