Abstract
Podoplanin is a small transmembrane glycoprotein widely known to be a marker for lymphatic endothelial cells. In this study, we identify a novel localization of podoplanin in the retinal pigment epithelium (RPE), a cellular monolayer critically involved in the visual process. Using a small interfering RNA (siRNA)-mediated gene silencing approach, we have also demonstrated, for the first time, that podoplanin depletion in human RPE cells leads to a marked reduction of cell aggregates and tight junctions. Additionally, the podoplanin-depleted cells also exhibit a significantly lower rate of proliferation. These data together indicate that podoplanin plays a crucial role in RPE cell functions. Further investigation on this factor may reveal novel mechanisms and therapeutic strategies for RPE-related eye diseases, such as proliferative retinopathy and age-related macular degeneration.
Keywords: podoplanin, retinal pigment epithelium, lymphatic, siRNA
Podoplanin is a mucin-like transmem-brane protein which has been widely used as a molecular marker for lymphatic endothelial cells due to its absence in blood endothelial cells (1). Podoplanin gene knockout mice exhibit a dysfunctional lymphatic system and die as a result of respiratory failure (2). Podoplanin expression has also been detected in several other cell types, including kidney podocytes, alveolar type I cells, choroid plexuses, and osteocytes (3-5). However, its precise physiological role still remains largely unknown. To date, there is no report of the podoplanin expression in the retinal pigment epithelium (RPE) of the eye.
RPE is a monolayer of pigmented cells situated between the neuroretina and the choroids. It serves many functions that are critical to the eye, which include the formation of a blood-brain barrier, maintenance of an immune-privileged subretinal space, phagocytosis of the photoreceptor outer segments, recycling of the visual pigments, and secretion of essential factors for the structural integrity of the retina (6-8). RPE cells in the adult normal eyes are in a differentiated and quiescent state, however, the cells can become activated and enter the cell cycle after a pathological stimulation. Alterations in the blood-retinal barrier are also found in several retinal disorders, such as the heredity disorder of retinitis pigmentosa, as well as diabetic macular edema, and proliferative vitreoretinopathy (PVR) (8).
The purpose of this study was to characterize the expression of podoplanin in the RPE both in vivo and in vitro. We further investigated the functional roles of podoplanin in human RPE cells in vitro using a small interfering RNA (siRNA) mediated gene silencing approach. Our results provide the first evidence that podoplanin is highly expressed in the RPE, and that it also functions as a pro-adhesive molecule and a key regulator of proliferation for RPE cells.
METHODS
Animals
Normal adult male BALB/c mice were purchased from Taconic Farms (Germantown, New York). All mice were treated according to ARVO statement for the Use of Animals in Ophthalmic and Vision Research, and all protocols were approved by the Animal Care and Use Committee, University of California, Berkeley.
Cells and Antibodies
Human ARPE-19 cells were purchased from ATCC (Manassas, VA) and maintained in culture according to the manufacturer’s instructions. The following primary anti-bodies were used: anti-mouse podoplanin (Abcam, Cambridge, MA), anti-mouse RPE-65 (Novus Biologicals, Littleton, CO), anti-human podoplanin (Covance, Emeryville, CA), and anti-human ZO-1 (Invitrogen, Camarillo, CA). The secondary antibodies were Cy-3 conjugated anti-hamster IgG (Jackson Immunoresearch, West Grove, PA) and FITC-conjugated anti-mouse IgG (Millipore, Temecula, CA).
Immunofluorescent Microscopic Assay
Experiments were performed according to our standard protocol, as previously described (9-11). Briefly, 8 µm frozen sections of eyes were fixed in acetone and blocked with 10% goat serum to reduce non-specific staining. Sections were then incubated with primary antibodies overnight at 4ºC and visualized by subsequent staining with secondary antibodies for 1 hour at room temperature. Samples were mounted with Vectashield mounting medium with DAPI (Vector Laboratories, Burlingame, CA) and examined by an epifluorescence microscope (Zeiss Axioplan 2; Carl Zeiss, Germany). Digital images were taken using iVision-Mac software (BioVision Technologies Inc., Exton, PA).
Immunocytofluorescent Microscopic Assay
Cells were grown on coverslips, fixed with 4% paraformaldehyde, and blocked with 3% bovine serum albumin. Samples were incubated with primary antibodies overnight at 4°C, and visualized by subsequent staining with secondary antibodies for 1 hour at room temperature. Samples were mounted and examined as above.
Reverse-Transcriptase PCR
Total RNA was extracted and purified from cultured ARPE-19 cells using the RNeasy mini-kit (Qiagen, Valencia, CA). Reverse transcription was performed using the SuperScript® VILO™ cDNA Synthesis Kit (Invitrogen, Carlsbad, CA). PCR amplification was done with the PCR mastermix (Promega, Madison, WI) and primer sequences were as follows: Podoplanin forward 5’-GGAGAAAGTGGATGGAGA-3’, reverse 5’-AATGGTCAGACGGAGACA-3’; GAPDH forward 5’ACCACAGTCCA TGCCATCAC-3’, reverse 5’-TCCACCACCCTGTTGCTGT-3’.
siRNA Transfection
The podoplanin siRNA was synthesized by Qiagen (Valencia, CA). The sequences were designed against human podoplanin mRNA: 5’-GCGAAGACCGCTATAAGTC-3’, as previously characterized (12). A scrambled siRNA control was purchased from Ambion (Austin, TX). Transfection was carried out in opti-MEM reduced serum medium overnight at 37ºC using RNAiMax (Invitrogen, Carlsbad, CA) according to manufacturer’s instructions.
Proliferation Assay
Cells were subjected to a BRDU proliferation assay from EMD chemicals (Gibbstown, NJ) according to the manufacturer’s protocol. Briefly, after an overnight incubation with BRDU nucleotides, cells were fixed and incorporated BRDU was detected by ELISA. Optical density (OD) was measured using an M5e microplate reader (Molecular Devices, Sunnyvale, CA).
RESULTS
Podoplanin Expression in the Retinal Pigment Epithelium Layer In Vivo
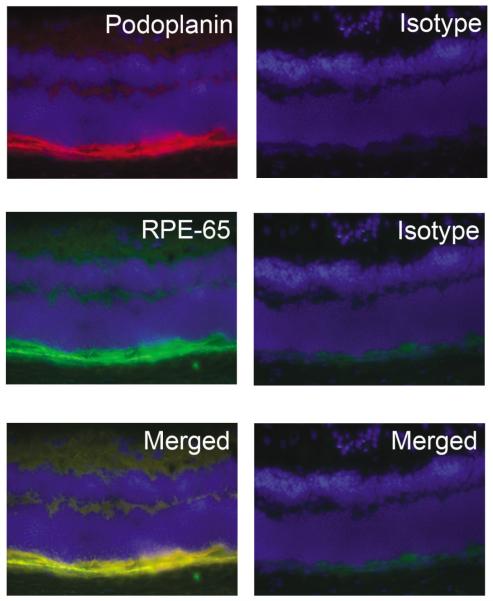
We first investigated whether podoplanin is expressed in the RPE cell layer in mice. As shown in Fig. 1. Immunofluorescent microscopic assay results revealed a high expression of podoplanin in the posterior segment of the eye. This linear expression also co-localized with RPE-65, a specific marker for RPE cells. These data together indicate a novel expression pattern of podoplanin in the RPE cells in vivo.
Fig. 1.
Podoplanin expression in murine RPE layer. (A) Representative cross-sectional micrographs demonstrating co-expression of podoplanin (top panel) and RPE-65 (middle panel) in the murine RPE layer. Right panels: isotype control staining. Bottom panels: merged images. Podoplanin: red; RPE-65: green; DAPI nuclear staining: Blue. Original magnification: X200.
Podoplanin Expression in the Retinal Pigment Epithelial Cells In Vitro
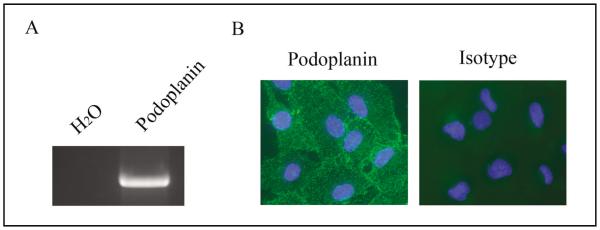
To corroborate our in vivo results, we next investigated the expression of podoplanin in human RPE cells in culture. Both reverse transcriptase (RT)-PCR and immunocyto-fluorescent microscopic assays confirmed that podoplanin was also highly expressed in RPE cells in culture, at both mRNA (Fig. 2A) and protein (Fig. 2B) levels.
Fig. 2.
Podoplanin expression in human RPE cells. RT-PCR (A) and immunocytofluorescent microscopic analysis (B) of podoplanin expression in the human RPE cells in culture. Podoplanin: green; DAPI nuclear staining: Blue. Original magnification: X200.
Podoplanin Inhibition by siRNA
We then investigated whether the expression of podoplanin in the RPE cells can be down-regulated by a siRNA-mediated gene silencing approach. As demonstrated in Fig. 3A, podoplanin targeting led to an approximately 50% reduction of its mRNA expression 24 hours following the transfection when compared to control. Furthermore, protein levels of podoplanin in RPE cells also became marginally detectable 48 hours following the transfection as assessed by the immunocytofluorescent microscopic assay (Fig. 3B). These results indicate that our siRNA approach is effective in modulating the podoplanin expression in the cells and thereby provide a useful tool for further indepth studies on the functional roles of this molecule in RPE cells.
Fig. 3.
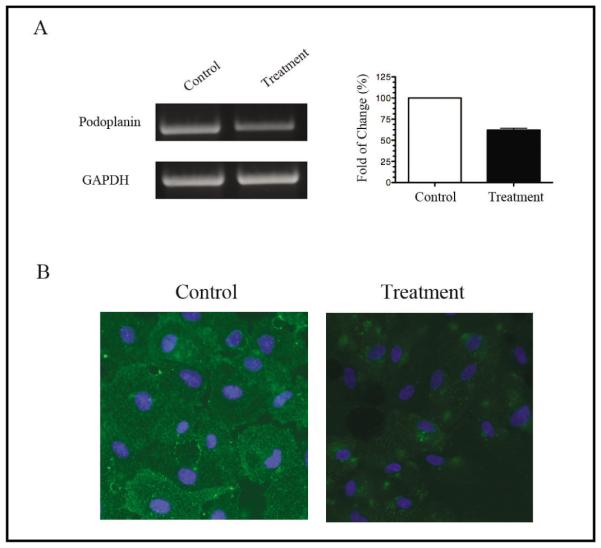
Podoplanin depletion from RPE cells in vitro. (A) Semi-quantitative RT-PCR analysis of the podoplanin gene in RPE cells 24 hours after the siRNA transfection. Right panel: band density analysis from repetitive assays. The mRNA levels of the control groups were presented as 100 percent. (B) Representative micrographs from immunocytofluorescent microscopic assays showing a significant reduction in podoplanin protein expression in the RPE cells 48 hours after the siRNA transfection. Podoplanin: green; DAPI nuclear staining: Blue. Original magnification: X200.
Decreased Cell-Cell Contacts by Podoplanin Depletion
Following the podoplanin siRNA treatments, we observed an obvious change in RPE cell morphology. As shown in the phase-contrast images (Fig. 4A), podoplanin-depleted cells appeared scattered with a lack of the ability to aggregate into cell clusters. Further investigation on the cell-cell adhesion using the tight junction specific marker ZO-1, also confirmed a significant reduction of ZO-1 expression in the podoplanin-depleted cells (Fig. 4B). These data suggest that podoplanin may have a specific role in maintaining cell-cell adhesion between RPE cells.
Fig. 4.
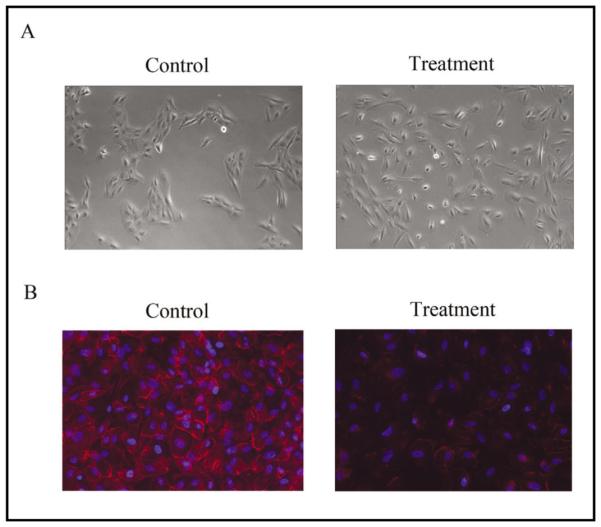
Podoplanin depletion disrupts RPE cell-cell contacts. (A) Representative phase-contrast images showing scattered RPE cells 72 hours after the podoplanin siRNA transfection. Original magnification: X200. (B) Representative immunocytofluorescent micrographs showing marginal expression of ZO-1 in the RPE cells 72 hours after the podoplanin siRNA transfection. ZO-1: red; DAPI nuclear staining: Blue. Original magnification: X200.
Reduced Cell Proliferation by Podoplanin Depletion
To further investigate the effect of podoplanin depletion in RPE cells, we also measured cell proliferation using a BRDU incorporation assay. Compared to control, podoplanin depleted cells exhibited an approximately 50% reduction in proliferation rate, suggesting that podoplanin is also a key regulator of the proliferation process (Fig. 5).
Fig. 5.
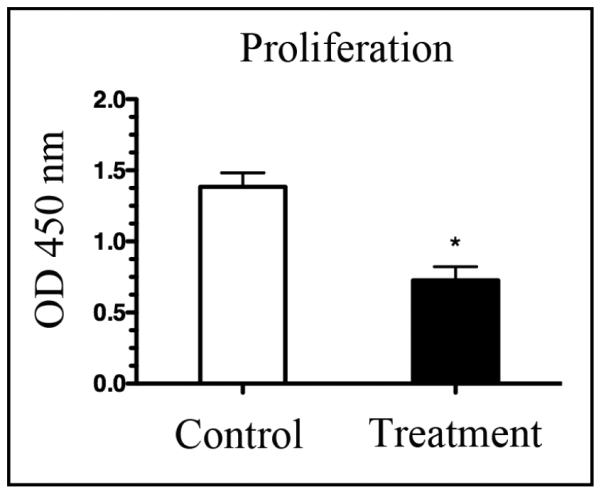
Podoplanin depletion inhibits RPE cell proliferation. Summarized data from repetitive BRDU incorporation assays showing a significant inhibition of RPE cell proliferation 48 hours following the transfection with the podoplanin siRNA. *P < 0.05.
DISCUSSION
In this study we identified a novel expression pattern of podoplanin in the RPE. Additionally, we have also defined specific roles of this molecule in RPE cell functions, including cell-cell adhesion and cell proliferation. Since both cell-cell adhesion and proliferation play major roles in the biology and disease states of the RPE, our results indicate that further investigation of podoplanin may provide a novel diagnostic marker or a therapeutic target for RPE-related diseases.
Our findings bear several important implications. Firstly, membrane-bound mucin glycoproteins are well known to have adhesive properties – yet they can be pro-adhesive or anti-adhesive (13). Our results depict a pro-adhesive role of podoplanin in RPE cell-cell contacts and tight junctions. Allied to this notion is a previous report showing that podoplanin facilitates lymphatic endothelial cell adhesion to the extracellular matrix (2). Though it is yet to be determined, it is plausible to speculate that podoplanin has a pro-adhesive effect among different cell types.
Secondly, the integrity of the RPE monolayer is vital for maintaining the blood-retinal barrier and normal functioning of the neural retina. This integrity is highly dependent on the intracellular tight junctions, which act to seal the intercellular space while maintaining cell-cell attachments (14). Several factors are known to regulate the RPE permeability, such as interluekin-β, hepatocyte growth factor, placental growth factor-1 and vascular endothelial growth factor, though their specific actions differ (15-17). Our finding that podoplanin depletion disrupts tight junctions between the RPE cells suggests that this factor is also critically involved in the process. Further investigation on podoplanin may therefore reveal novel mechanisms linking it to other factors in the pathway, shedding some new light on our understanding of the RPE cells.
Thirdly, it has been reported that though arrested in a G0 state, adult RPE cells can be activated into a proliferative and migratory state in diseases, such as PVR, proliferative diabetic retinopathy, and age-related macular degeneration (AMD) (18-20). Vision loss and blindness caused by these diseases affect hundreds of millions of people worldwide and impose a great social and economic burden on individuals and the society. There is, therefore, an urgent demand to develop novel strategies to prevent or treat these diseases. In the treatment of PVR, the anti-proliferative approach with use of chemotherapeutic agents such as 5-fluorouracil (5-FU) was initially promising (21,22). However, the 5-FU approach proved to be unsuccessful in the clinical setting due to its temporary effect (22). Currently, treatment for PVR still heavily relies on the surgical removal of the membranes even though the visual results are often disappointing (23). Our finding that podoplanin is a key regulator of RPE cell proliferation may provide a promising therapeutic candidate for treatment of proliferative diseases.
Lastly, but more elusively, since podoplanin is expressed in lymphatic but not blood endothelial cells, its expression in RPE cells may indicate a correlation or similarity between lymphatic endothelial cells and RPE cells. For example, both types of cell have functions in phagocytosis, immune responses, and tissue fluid regulation. RPE transport is also the major force eliminating water from the sub-retinal space in a normal eye. Because the retina is endowed with blood vessels but devoid of lymphatic supply (24), a bold hypothesis is that the RPE cells may partially assume the lymphatic function at the local site, which merits further investigation.
ACKNOWLEDGMENTS
This work is supported in part by research grants from National Institutes of Health and the Hellman Family Faculty Fund Award, University of California at Berkeley.
REFERENCES
- 1.Breiteneder-Geleff S, Soleiman A, Kowalski H, et al. Angiosarcomas express mixed endothelial phenotypes of blood and lymphatic capillaries: Podoplanin as a specific marker for lymphatic endothelium. Am. J. Pathol. 1999;154:385–394. doi: 10.1016/S0002-9440(10)65285-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Schacht V, Ramirez M, Hong Y, et al. T1alpha/podoplanin deficiency disrupts normal lymphatic vasculature formation and causes lymphedema. EMBO J. 2003;22:3546–3556. doi: 10.1093/emboj/cdg342. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Breiteneder-Geleff S, Matsui K, Soleiman A, et al. Podoplanin, novel 43-kd membrane protein of glomerular epithelial cells, is down-regulated in puromycin nephrosis. Am. J. Pathol. 1997;151:1141–1152. [PMC free article] [PubMed] [Google Scholar]
- 4.Gonzalez R, Dobbs L. Purification and analysis of RTI40, a type I alveolar epithelial cell apical membrane protein. Biochim. Biophys. Acta. 1998;1429:208–216. doi: 10.1016/s0167-4838(98)00231-3. [DOI] [PubMed] [Google Scholar]
- 5.Williams M, Cao Y, Hinds A, et al. T1 alpha protein is developmentally regulated and expressed by alveolar type I cells, choroid plexus, and ciliary epithelia of adult rats. Am. J. Respir. Cell. Mol. Biol. 1996;14:577–585. doi: 10.1165/ajrcmb.14.6.8652186. [DOI] [PubMed] [Google Scholar]
- 6.Simo R, Villarroel M, Corraliza L, et al. The retinal pigment epithelium: Something more than a constituent of the blood-retinal barrier—implications for the pathogenesis of diabetic retinopathy. J. Biomed. Biotechnol. 2010;2010:190724. doi: 10.1155/2010/190724. Epub 2010 Feb 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Erickson K, Sundstrom J, Antonetti D. Vascular permeability in ocular disease and the role of tight junctions. Angiogenesis. 2007;10:103–117. doi: 10.1007/s10456-007-9067-z. [DOI] [PubMed] [Google Scholar]
- 8.Strauss O. The retinal pigment epithelium in visual function. Physiol. Rev. 2005;85:845–881. doi: 10.1152/physrev.00021.2004. [DOI] [PubMed] [Google Scholar]
- 9.Ecoiffier T, Yuen D, Chen L. Differential distribution of blood and lymphatic vessels in the murine cornea. Invest. Ophthalmol. Vis. Sci. 2010;51:2436–2440. doi: 10.1167/iovs.09-4505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Zhang H, Tse J, Hu X, et al. Novel discovery of LYVE-1 expression in the hyaloid vascular system. Invest. Ophthalmol. Vis. Sci. 2010 Aug;:4. doi: 10.1167/iovs.10-5205. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Zhang H, Hu X, Tse J, et al. Spontaneous lymphatic vessel formation and regression in the murine cornea. Invest Ophthalmol. Vis. Sci. 2010 Aug;:25. doi: 10.1167/iovs.10-5404. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Schacht V, Dadras S, Johnson L, et al. Up-regulation of the lymphatic marker podoplanin, a mucin-type transmembrane glycoprotein, in human squamous cell carcinomas and germ cell tumors. Am. J. Pathol. 2005;166:913–921. doi: 10.1016/S0002-9440(10)62311-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hilkens J, Ligtenberg M, Vos H, et al. Cell membrane-associated mucins and their adhesion-modulating property. Trends Biochem. Sci. 1992;17:359–363. doi: 10.1016/0968-0004(92)90315-z. [DOI] [PubMed] [Google Scholar]
- 14.Matter K, Balda M. Occludin and the functions of tight junctions. Int. Rev. Cytol. 1999;186:117–146. doi: 10.1016/s0074-7696(08)61052-9. [DOI] [PubMed] [Google Scholar]
- 15.Abe T, Sugano E, Saigo Y, et al. Interleukin-1beta and barrier function of retinal pigment epithelial cells (ARPE-19): Aberrant expression of junctional complex molecules. Invest. Ophthalmol. Vis. Sci. 2003;44:4097–4104. doi: 10.1167/iovs.02-0867. [DOI] [PubMed] [Google Scholar]
- 16.Jin M, Barron E, He S, et al. Regulation of RPE intercellular junction integrity and function by hepatocyte growth factor. Invest. Ophthalmol. Vis. Sci. 2002;43:2782–2790. [PubMed] [Google Scholar]
- 17.Miyamoto N, de Kozak Y, Jeanny J, et al. Placental growth factor-1 and epithelial haemato-retinal barrier breakdown: Potential implication in the pathogenesis of diabetic retinopathy. Diabetologia. 2007;50:461–470. doi: 10.1007/s00125-006-0539-2. [DOI] [PubMed] [Google Scholar]
- 18.Campochiaro P. Pathogenic mechanisms in proliferative vitreoretinopathy. Arch. Ophthalmol. 1997;115:237–241. doi: 10.1001/archopht.1997.01100150239014. [DOI] [PubMed] [Google Scholar]
- 19.Esser P, Heimann K, Bartz-Schmidt K, et al. Apoptosis in proliferative vitreoretinal disorders: Possible involvement of TGF-beta-induced RPE cell apoptosis. Exp. Eye Res. 1997;65:365–378. doi: 10.1006/exer.1997.0341. [DOI] [PubMed] [Google Scholar]
- 20.Miller H, Miller B, Ryan S. The role of retinal pigment epithelium in the involution of subretinal neovascularization. Invest Ophthalmol. Vis. Sci. 1986;27:1644–1652. [PubMed] [Google Scholar]
- 21.Blumenkranz M, Ophir A, Claflin A, et al. Fluorouracil for the treatment of massive periretinal proliferation. Am. J. Ophthalmol. 1982;94:458–467. doi: 10.1016/0002-9394(82)90239-2. [DOI] [PubMed] [Google Scholar]
- 22.Stern W, Lewis G, Erickson P, et al. Fluorouracil therapy for proliferative vitreoretinopathy after vitrectomy. Am. J Ophthalmol. 1983;96:33–42. doi: 10.1016/0002-9394(83)90452-x. [DOI] [PubMed] [Google Scholar]
- 23.Charteris D, Sethi C, Lewis G, et al. Proliferative vitreoretinopathy-developments in adjunctive treatment and retinal pathology. Eye (Lond) 2002;16:369–374. doi: 10.1038/sj.eye.6700194. [DOI] [PubMed] [Google Scholar]
- 24.Chen L. Ocular lymphatics: State-of-the-art review. Lymphology. 2009;42:66–76. [PMC free article] [PubMed] [Google Scholar]