Abstract
Introduction
The phosphatidylinositol 3-kinase (PI3K) pathway promotes tumor growth and treatment resistance in non-small cell lung cancer (NSCLC). The aim of the open-label, two-stage, Phase II study BASALT-1 (NCT01820325) was to investigate the pan-PI3K inhibitor buparlisib (BKM120) in patients with PI3K pathway-activated, relapsed NSCLC.
Methods
After prescreening for PI3K pathway activation, patients with PI3K pathway-activated, metastatic, squamous or nonsquamous NSCLC, who had relapsed after prior systemic antineoplastic therapy, were enrolled. In Stage 1, patients received single-agent buparlisib (100 mg/day). A futility analysis was performed independently in each histology group, based on the 12-week progression-free survival rate for the first 30 patients treated in each group being less than 50%. Exploratory biomarker analyses were performed in archival tissue samples and circulating tumor DNA (ctDNA).
Results
Of 1242 prescreened patients, 13.5% exhibited PI3K pathway activation. As of June 5, 2014, 63 patients (30 squamous and 33 nonsquamous) were treated in Stage 1. The 12-week progression-free survival rates were 23.3% (95% confidence interval: 9.9–42.3) and 20.0% (95% confidence interval: 7.7–38.6) in the squamous and nonsquamous groups, respectively. Stage 2 was therefore not initiated in either group. PI3K pathway mutations in ctDNA were more concordant with metastatic tissue than with primary biopsies.
Conclusions
Despite preselecting patients for targeted treatment, BASALT-1 did not meet its primary objective during Stage 1. PI3K pathway activation can be detected using ctDNA, but may not be the main oncogenic driver in NSCLC. Combinations of PI3K inhibitors with other agents may demonstrate greater efficacy than monotherapy.
Keywords: Buparlisib/BKM120, PI3K pathway activation, Squamous NSCLC, Nonsquamous NSCLC.
Lung cancer is the leading cause of cancer-related death in the European Union, responsible for an estimated 270,000 deaths in 2014.1 Non-small cell lung cancer (NSCLC) accounts for 80%–85% of all lung cancer cases, and can be classified by histology into squamous carcinoma (20% of all lung cancer) and nonsquamous carcinoma (including adeno-carcinoma; 40% of all lung cancer).2,3
The paradigm for treatment of advanced NSCLC has evolved from an approach based on a patient's ability to withstand the toxicities of chemotherapy to one that incorporates disease histology and molecular genotype as predictive markers of benefit from targeted agents.4 In patients with advanced, recurrent NSCLC, single-agent docetaxel, or pemetrexed (for nonsquamous NSCLC), are established second-line agents.4 However, the clinical outcome in this setting is poor, with a response rate of less than 10%, and progression-free survival (PFS) and overall survival (OS) in the range of 2.7–3.0 months and 7.4–8.0 months, respectively.5–7 In patients without oncogenic drivers (e.g., EGFR activating mutations or ALK translocation), the outcome with targeted agents, such as erlotinib is similarly poor, if not inferior.8 For both squamous and nonsquamous metastatic, recurrent NSCLC, treatment options are limited.
The phosphatidylinositol 3-kinase (PI3K)/AKT/mammalian target of rapamycin pathway is frequently dysregulated in cancer and is implicated in oncogenesis and tumor progression, as well as resistance to standard anticancer therapies.9 Previous studies have reported activating mutations in PIK3CA (encoding the catalytic subunit PI3Kα)10–12 and alterations (mutation or loss of expression) of the tumor suppressor phosphatase and tensin homolog (PTEN)13–16 in a clinically meaningful proportion of patients with squamous and nonsquamous NSCLC.
Preclinical studies suggest that targeting the PI3K pathway could be an effective treatment strategy in patients with NSCLC—simultaneous inhibition of multiple PI3K pathway components blocks the growth of PI3K-dependent NSCLC cell lines, and induces tumor regression in mouse xenograft models of PIK3CA-mutant lung cancer.17–19
Buparlisib (BKM120) is an oral PI3K inhibitor that selectively targets all four isoforms of Class I PI3K (α, β, γ, and δ).20 Single-agent buparlisib has demonstrated antiproliferative, proapoptotic, and antitumor activity in a variety of cell lines and xenograft models from cancers with and without aberrant PI3K pathway activation.20–24 In preclinical studies, approximately half of NSCLC cell lines (three of seven squamous and 15 of 28 nonsquamous) were sensitive to buparlisib (data on file), suggesting that buparlisib treatment may be beneficial in patients with NSCLC.
The aim of BASALT-1, an open-label, two-stage, Phase II study (NCT01820325≥), was to assess the safety and efficacy of buparlisib in patients with relapsed NSCLC harboring documented aberrations in the PI3K pathway. Here, we present biomarker data detailing the frequency of PIK3CA and PTEN alterations in 1242 patients prescreened for the BASALT-1 study, as well as further characterization of the molecular underpinnings of PI3K pathway activation in NSCLC using circulating tumor DNA (ctDNA) for PIK3CA mutation analysis. We also present safety and efficacy results from the first 63 patients enrolled and treated in Stage 1.
MATERIALS AND METHODS
PI3K Pathway Analysis
Before screening and enrollment for BASALT-1, PI3K pathway activation status was measured in archival or fresh biopsy tissue in patients with previously treated, metastatic squamous and nonsquamous NSCLC. Pathway activation was defined as PIK3CA mutation, PTEN mutation, or PTEN negative (less than 10% protein expression by immunohistochemistry).
PIK3CA mutations (exons 1, 5, 7, 9, and 20) and PTEN mutations (exons 1–9) were identified by Sanger sequencing at central facilities; local PIK3CA mutation analysis was permitted at validated sites, and was most commonly performed by SNaPshot assay.25 PTEN expression was assessed centrally by immunohistochemistry using a PTEN antibody (Cell Signaling Technology, Inc., Danvers, MA).
Patient Eligibility
Adult patients (≥18 years of age) with metastatic NSCLC previously treated with one prior platinum-based chemotherapy line (squamous histology), or one or two prior systemic anti-neoplastic therapy lines (nonsquamous histology), were eligible for the study. Only patients with confirmed PI3K pathway activation at prescreening, as defined above, were considered. Patients with a known EGFR activating mutation must have been previously treated with at least one EGFR tyrosine kinase inhibitor. Other key inclusion criteria included having Eastern Cooperative Oncology Group (ECOG) performance status less than or equal to two, measurable and/or nonmeasureable disease per Response Evaluation Criteria In Solid Tumors (RECIST) v1.1, and laboratory values within normal ranges.
Patients previously treated with a PI3K inhibitor or currently receiving an approved or investigational antineoplastic drug were excluded from the trial, as were patients with a history of cardiac ≥dysfunction or those taking medication with a known risk of prolonging QT interval or inducing Torsades de Pointes. As hyperglycemia is a class effect of PI3K inhibitors, patients with poorly controlled diabetes mellitus (HbA1c greater than 8%) were excluded. Patients with any of the following mood disorders were also excluded: history of major depressive episode, bipolar disorder, obsessive–compulsive disorder, schizophrenia, suicidal attempt or ideation, homicidal ideation, or anxiety Grade ≥3 per Common Terminology Criteria for Adverse Events (CTCAE) v4.03. Mood disorders were assessed by the investigator or a psychiatrist, and by patient-rated questionnaires (a score of ≥12 on the 9-item patient health questionnaire [PHQ-9] or ≥15 on the 7-item generalized anxiety disorder scale [GAD-7]).
Ethics
The protocol, informed consent form, and any protocol amendments were approved by an Independent Ethics Committee. All participating patients provided written informed consent and agreed to comply with the protocol. The study was conducted in accordance with the International Conference on Harmonization's Harmonized Tripartite Guidelines for Good Clinical Practice, with applicable local regulations (including European Directive 2001/≥20/≥EC and US Code of Federal Regulations Title 21), and with the ethical principles laid down in the Declaration of Helsinki.
Study Design and Treatment
Eligible patients were enrolled in two groups defined by squamous and nonsquamous tumor histology. In Stage 1, the first 30 patients in each group with confirmed PI3K pathway activation status and meeting the eligibility criteria received single-agent oral buparlisib at the maximum tolerated dose of 100 mg/day26 on a continuous schedule in 21-day treatment cycles (Supplementary Figure 1, Supplemental Digital Content, http://links.lww.com/JTO/A850). Dosing continued until disease progression, intolerable toxicity, death, start of a new antineoplastic medication, or until the patient discontinued for any reason. In Stage 2, approximately 60 patients in each group would be randomized in a 2:1 ratio to receive either buparlisib (100 mg/day) or chemotherapy (3-weekly docetaxel 75 mg/m2 or pemetrexed 500 mg/m2).
A futility analysis was performed in Stage 1 independently for each group, after 30 patients had been enrolled and observed for 12 weeks from the first day of treatment. The futility criterion was defined as a 12-week PFS rate (based on local investigator assessment per RECIST v1.1) less than 50% in the intent-to-treat population, and was based on the median PFS of approved agents in this setting.5–7 Thirty patients were enrolled in each group to estimate the 12-week PFS rate with adequate precision. If the futility criterion was met, enrollment would be stopped and Stage 2 would not be initiated for that group.
Study Objectives
In Stage 1, the primary objective was to assess preliminary activity of buparlisib independently for each histology group, based on the 12-week PFS rate. In Stage 2, the primary objective was to evaluate the efficacy of buparlisib compared with chemotherapy in each histology group, based on PFS per RECIST v1.1.
Secondary objectives were to evaluate OS, overall response rate (ORR), disease control rate, time to response, and duration of response; and to characterize safety, based on the frequency and severity of adverse events (AEs) per CTCAE v4.03.
Key exploratory objectives were to evaluate individual components of the PI3K pathway in patients with squamous and nonsquamous NSCLC, to assess the impact of PIK3CA mutations or PTEN alterations on PFS, and to identify other biomarkers predictive of response to buparlisib.
Safety and Efficacy Assessments
Radiological assessments (per RECIST v1.1) were performed at 6-week intervals (±7 days) until disease progression. Laboratory evaluations were performed throughout the study, and included hematology, biochemistry, glucose monitoring, coagulation, lipase, and urinalysis. Safety was monitored by physical examination, vital signs, weight, ECOG performance status, electrocardiogram, cardiac imaging, and assessment of patient-rated questionnaires (PHQ-9 and GAD-7). Serious and nonserious AEs were monitored throughout the study, described per the Medical Dictionary for Regulatory Activities (MedDRA) v17.0, and graded per CTCAE v4.03.
Additional Biomarker Assessments
In addition to PIK3CA and PTEN assessment of tumor tissue at prescreening, mutation analysis of ctDNA was performed using BEAMing technology (Sysmex Inostics, Inc., Mundelein, IL). A wider set of genetic alterations was assessed in archival tissue samples by next-generation sequencing (NGS; Illumina HiSeq, Illumina, Inc., San Diego, CA).
RESULTS
PI3K Pathway Analysis
Assessment of PIK3CA and PTEN alterations in 1242 patients with NSCLC at the prescreening stage (668 squamous and 574 nonsquamous) showed that 13.5% of patients exhibited PI3K pathway activation (Supplementary Table 1, Supplemental Digital Content, http://links.lww.com/JTO/A850).
Patient Characteristics
As of June 5, 2014, 63 patients were recruited onto Stage 1 of this Phase II study—30 patients in the squamous group and 33 patients in the nonsquamous group (Supplementary Figure 1, Supplemental Digital Content, http://links.lww.com/JTO/A850). All patients received at least one dose of buparlisib (100 mg/day) and had a post-baseline safety assessment, hence were evaluable for safety and efficacy (Table 1). Chemotherapy was the most common last prior therapy in 53.3% and 54.5% of patients in the squamous and nonsquamous groups, respectively. In the squamous group, radiotherapy was the second-most common (33.3% of patients), whereas in the nonsquamous group, patients had received radiotherapy (15.2%), targeted therapy, or surgery (12.1% each) as last therapy. Best response at last therapy was partial response (PR) in 43.3% of squamous patients and stable disease in 48.5% of nonsquamous patients.
TABLE 1.
Patient Characteristics at Baseline
| Characteristic | Squamous n = 30 | Nonsquamous n = 33 | Total N = 63 |
|---|---|---|---|
| Median age, years (range) | 65.5 (46.0–78.0) | 63.0 (39.0–77.0) | 65.0 (39.0–78.0) |
| Male patients, n (%) | 21 (70.0) | 19 (57.6) | 40 (63.5) |
| Race, n (%) | |||
| Caucasian | 20 (66.7) | 25 (75.8) | 45 (71.4) |
| Black | 1 (3.3) | 1 (3.0) | 2 (3.2) |
| Asian | 4 (13.3) | 3 (9.1) | 7 (11.1) |
| Unknown | 1 (3.3) | 4 (12.1) | 5 (7.9) |
| Other | 4 (13.3) | 0 | 4 (6.3) |
| Performance status, n (%) | |||
| 0 | 5 (16.7) | 14 (42.4) | 19 (30.2) |
| 1 | 23 (76.7) | 18 (54.5) | 41 (65.1) |
| 2 | 2 (6.7) | 1 (3.0) | 3 (4.8) |
| Patients with prior antineoplastic therapies, n (%) | 30 (100.0) | 33 (100.0) | 63 (100.0) |
| Type of last therapy, n (%) | |||
| Chemotherapy | 16 (53.3) | 18 (54.5) | 34 (54.0) |
| Targeted therapy | 2 (6.7) | 4 (12.1) | 6 (9.5) |
| Radiotherapy | 10 (33.3) | 5 (15.2) | 15 (23.8) |
| Surgery | 0 | 4 (12.1) | 4 (6.3) |
| Other | 0 | 5 (15.2) | 5 (7.9) |
| Missing | 4 (13.3) | 0 | 4 (6.3) |
| Best response at last therapy, n (%) | |||
| Partial response | 13 (43.3) | 5 (15.2) | 18 (28.6) |
| Stable disease | 5 (16.7) | 16 (48.5) | 21 (33.3) |
| Progressive disease | 9 (30.0) | 9 (27.3) | 18 (28.6) |
| Unknown | 2 (6.7) | 2 (6.1) | 4 (6.3) |
| Not applicable | 1 (3.3) | 1 (3.0) | 2 (3.2) |
| Predominant histology, n (%) | |||
| Adenocarcinoma | 0 | 29 (87.9) | 29 (46.0) |
| Squamous cell carcinoma | 29 (96.7) | 0 | 29 (46.0) |
| Large cell carcinoma | 0 | 3 (9.1) | 3 (4.8) |
| Large cell neuroendocrine carcinoma | 0 | 1 (3.0) | 1 (1.6) |
| Othera | 1 (3.3) | 0 | 1 (1.6) |
| Stage at study entry, n (%) | |||
| Stage IIIA | 1 (3.3) | 0 | 1 (1.6) |
| Stage IIIB | 1 (3.3) | 2 (6.1) | 3 (4.8) |
| Stage IV | 28 (93.3) | 31 (93.9) | 59 (93.7) |
Other histologies include large cell endocrine, adenoid, or squamous tumors.
All patients enrolled (N = 63) had tumors with confirmed PI3K pathway activation (Table 2). PIK3CA mutations (regardless of PTEN alterations) were more common in the nonsquamous than the squamous group (45.2% vs. 20.7% of tumors assessed). The most common mechanism of PI3K pathway activation in both histotypes was PTEN alteration in the absence of PIK3CA mutations, occurring in 63.3% of squamous tumors and 36.4% of nonsquamous tumors. Concomitant PIK3CA mutation and PTEN alteration was infrequent, occurring in only 3.3% of the squamous group and 9.1% of the nonsquamous group. Among patients with known PTEN mutation and expression status, simultaneous PTEN mutation and PTEN loss occurred in 8.0% of patients (2 of 25) in the squamous group and 5.6% of patients (1 of 18) in the nonsquamous group.
TABLE 2.
Summary of Tumor Molecular Alterations at Baseline for Enrolled Patients
| Squamous n = 30 | Nonsquamous n = 33 | Total N = 63 | |
|---|---|---|---|
| PIK3CA mutation status, n (%) | |||
| n | 29 | 31 | 60 |
| PIK3CA mutation | 6 (20.7) | 14 (45.2) | 20 (33.3) |
| PIK3CA wild-type | 19 (65.5) | 13 (41.9) | 32 (53.3) |
| Unknown | 4 (13.8) | 4 (12.9) | 8 (13.3) |
| PTEN mutation status, n (%) | |||
| n | 29 | 30 | 59 |
| PTEN mutation | 8 (27.6) | 12 (40.0) | 20 (33.9) |
| PTEN wild-type | 18 (62.1) | 14 (46.7) | 32 (54.2) |
| Unknown | 3 (10.3) | 4 (13.3) | 7 (11.9) |
| PTEN expression, n (%) | |||
| n | 29 | 25 | 54 |
| PTEN negative (<10% IHC) | 18 (62.1) | 6 (24.0) | 24 (44.4) |
| PTEN positive | 11 (37.9) | 16 (64.0) | 27 (50.0) |
| Unknown | 0 | 3 (12.0) | 3 (5.6) |
| PI3K pathway activation status, n (%) | |||
| n | 30 | 33 | 63 |
| PIK3CA mutation only | 4 (13.3) | 8 (24.2) | 12 (19.0) |
| PTEN mutation only | 5 (16.7) | 5 (15.2) | 10 (15.9) |
| PTEN negative only | 12 (40.0) | 2 (6.1) | 14 (22.2) |
| PIK3CA wild-type and PTEN alterationa | 19 (63.3) | 12 (36.4) | 31 (49.2) |
| PIK3CA mutation and PTEN alterationa | 1 (3.3) | 3 (9.1) | 4 (6.3) |
| PIK3CA mutation or PTEN alterationa | 29 (96.7) | 28 (84.8) | 57 (90.5) |
| Patients with known PTEN mutation and expression status, n (%) | |||
| n | 25 | 18 | 43 |
| PTEN mutation and PTEN positive | 6 (24.0) | 6 (33.3) | 12 (27.9) |
| PTEN wild-type and PTEN negative | 13 (52.0) | 3 (16.7) | 16 (37.2) |
| PTEN mutation and PTEN negative | 2 (8.0) | 1 (5.6) | 3 (7.0) |
| PTEN wild-type and PTEN positive | 4 (16.0) | 8 (44.4) | 12 (27.9) |
Percentages were calculated from the number of samples assessed, n. “Unknown” includes patients whose tumors were assessed, but whose results were not evaluable. Concomitant mutations were calculated omitting samples with “unknown” or “missing” status as appropriate.
PTEN alteration is defined as either PTEN mutation or PTEN negative.
IHC, immunohistochemistry; PI3K, phosphatidylinositol 3-kinase; PTEN, phosphatase and tensin homolog.
KRAS mutations were analyzed by Sanger sequencing at baseline in all patients for whom sufficient biopsy tissue was available. KRAS mutations were observed in 12.0% of tumor samples (3 of 25) in the nonsquamous group, and in none of the tumor samples (0 of 14) in the squamous group.
NGS analysis was performed in 23 (11 squamous and 12 nonsquamous) of the 63 enrolled patients, producing a genetic profile broadly in line with existing data published by The Cancer Genome Atlas (TCGA). The most commonly altered genes were TP53, followed by PIK3CA and EGFR.
Patient Disposition
As of June 5, 2014, all 30 patients enrolled in the squamous group, and 32 patients in the nonsquamous group, had discontinued study treatment. In the squamous group, AEs and progressive disease were the most common reasons for treatment discontinuation (36.7% of patients each), and two patients (6.7%) discontinued due to death (one due to sepsis and one due to rapidly progressive NSCLC). In the nonsquamous group, progressive disease was the most common reason for treatment discontinuation (57.6% of patients) followed by AEs (18.2%), and one patient (3.0%) discontinued due to death (due to disease progression). One patient in the nonsquamous group continued to receive treatment at the cut-off date.
Clinical Activity
The PFS rate in Stage 1 was calculated after 30 patients in each group had received treatment for 12 weeks. The observed 12-week PFS rates were 23.3% (95% confidence interval [CI]: 9.9–42.3) in the squamous group (n = 30) and 20.0% (95% CI: 7.7–38.6) in the nonsquamous group (n = 30). The futility criterion (12-week PFS less than 50%) was met in both histology groups, and Stage 2 was therefore not initiated.
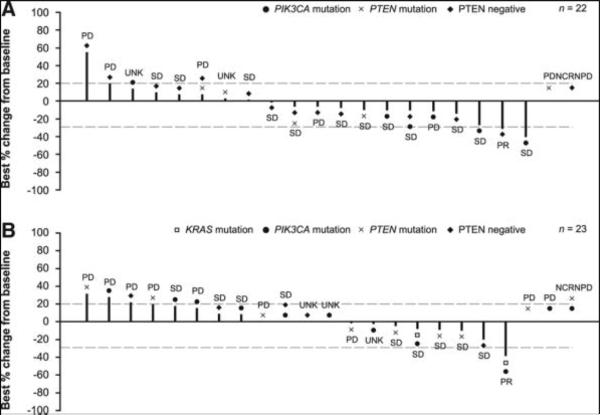
Two patients achieved a PR in each of the squamous and nonsquamous histology groups. The ORR (complete response + PR) was 3.3% (95% CI: 0.1–17.2) in the squamous group and 3.0% (95% CI: 0.1–15.8) in the nonsquamous group (Table 3). Disease control rate was 46.7% (95% CI: 28.3–65.7) in the squamous group and 45.5% (95% CI: 28.1–63.6) in the nonsquamous group. Waterfall plots are shown in Figure 1. The first patient with a PR had PTEN-negative squamous NSCLC, and experienced a 31% reduction in size of target lesions. This patient had previously progressed on carboplatin–gemcitabine. Time to response was 41 days and duration of response was 73 days. The second patient with a PR had nonsquamous NSCLC harboring mutations in PIK3CA and KRAS, and experienced a 38% reduction in size of target lesions. This patient had progressed after a lung lobectomy followed by cisplatin–pemetrexed chemotherapy. Time to response was 42 days and duration of response was 85 days.
TABLE 3.
Best Overall Response per Investigator Assessment
| Squamous n = 30 | Nonsquamous n = 33 | |
|---|---|---|
| Best overall response, n (%) | ||
| CR | 0 | 0 |
| PR | 1 (3.3) | 1 (3.0) |
| SD | 12 (40.0) | 12 (36.4) |
| PD | 7 (23.3) | 9 (27.3) |
| Not CR, not PD (NCRNPD) | 1 (3.3) | 2 (6.1) |
| UNK | 9 (30.0) | 9 (27.3) |
| ORR (CR + PR), n (%) [95% CI] | 1 (3.3) [0.1–17.2] | 1 (3.0) [0.1–15.8] |
| DCR (CR + PR + SD + NCRNPD), n (%) [95% CI] | 14 (46.7) [28.3–65.7] | 15 (45.5) [28.1–63.6] |
CI, confidence interval; DCR, disease control rate; CR, complete response; PR, partial response; SD, stable disease; PD, progressive disease; UNK, unknown; ORR, overall response rate; NCRNPD, not complete response, not progressive disease.
FIGURE 1.
Waterfall plot of best percentage change in sum of diameters and best overall response, in (A) squamous and (B) nonsquamous groups. UNK indicates patients not qualifying for confirmed CR or PR and without SD after more than 5 weeks from the start of treatment. Patients assessed for best overall response but missing best percentage change from baseline are shown to the right; patients with unknown overall response and missing best percentage change from baseline are not included. One patient in the squamous group had a decrease in tumor measurement of greater than 30%, which was not confirmed at a subsequent assesment at least 4 weeks later, and was therefore classed as SD per RECIST v1.1. CR, complete response; NCRNPD, not complete response, not progressive disease; PD, progressive disease; PR, partial response; PTEN, phosphatase and tensin homolog; SD, stable disease; UNK, unknown.
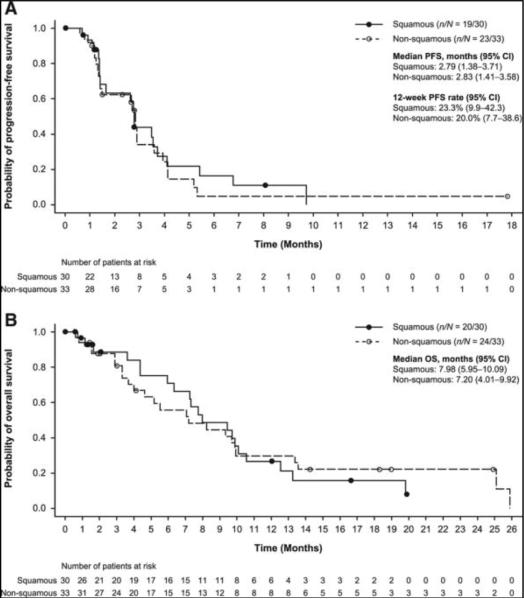
Subsequent safety and efficacy data are reported for all 63 patients enrolled in Stage 1, as of June 5, 2014. Median PFS was 2.79 months (95% CI: 1.38–3.71) in the squamous group, and 2.83 months (95% CI: 1.41–3.58) in the nonsquamous group (Fig. 2A). Median OS was 7.98 months (95% CI: 5.95–10.09) in the squamous group, and 7.20 months (95% CI: 4.01–9.92) in the nonsquamous group (Fig. 2B).
FIGURE 2.
Kaplan–Meier plots of (A) PFS and (B) OS in squamous and nonsquamous groups. Censoring times are shown as filled or open circles. CI, confidence interval; OS, overall survival; PFS, progression-free survival.
Safety and Tolerability
Median duration of exposure to buparlisib was 6.9 weeks (range: 0.1–36.0) in the squamous group, and 6.7 weeks (range: 1.4–79.6) in the nonsquamous group. Eight patients in each histology group remained on treatment for at least 12 weeks.
The most common AEs (≥25% of patients) regardless of relationship to study drug treatment were nausea, vomiting, and diarrhea (61.9%), asthenia/fatigue (49.2%), hyperglycemia (39.7%), liver toxicity (31.7%), and hypersensitivity/rash (28.6%; Table 4). A summary of AEs suspected to be related to study drug treatment experienced by patients in each histology group is presented in Supplementary Table 2 (Supplemental Digital Content, http://links.lww.com/JTO/A850).
TABLE 4.
Adverse Events Observed in Patients per Specific Safety Event Category
| Preferred Term, n (%) | Squamous n = 30 | Nonsquamous n = 33 | Total N = 63 |
|---|---|---|---|
| Nausea, vomiting, diarrhea | 22 (73.3) | 17 (51.5) | 39 (61.9) |
| Asthenia/fatigue | 14 (46.7) | 17 (51.5) | 31 (49.2) |
| Hyperglycemia | 13 (43.3) | 12 (36.4) | 25 (39.7) |
| Liver toxicity | 11 (36.7) | 9 (27.3) | 20 (31.7) |
| Hypersensitivity/rash | 10 (33.3) | 8 (24.2) | 18 (28.6) |
| Mood disorders | 3 (10.0) | 5 (15.2) | 8 (12.7) |
| Pneumonitis | 3 (10.0) | 0 | 3 (4.8) |
| QTc prolongation | 1 (3.3) | 0 | 1 (1.6) |
Adverse events regardless of relationship to study drug treatment were described according to MedDRA v17.0, and are listed by specific safety event category in order of descending frequency in the “Total” column.
MedDRA, Medical Dictionary for Regulatory Activities.
The most common Grade 3/4 AEs (≥5% of patients) suspected to be related to study drug treatment were hyperglycemia (23.3%), asthenia, and fatigue (6.7% each) in the squamous group, and increased alanine aminotransferase (15.2%), increased aspartate aminotransferase, hyperglycemia (12.1% each), asthenia, and rash (6.1% each) in the nonsquamous group.
Of note, Grade 3 psychiatric AEs of anxiety, confusional state, and visual hallucination (3.3% each) were observed in the squamous group, and depression and altered mood (3.0% each) in the nonsquamous group. No Grade 4 psychiatric AEs were reported in either group. The most common AEs leading to discontinuation (≥5% of patients) were hyperglycemia (13.3%) in the squamous group, and dyspnea (9.1%), increased alanine aminotransferase, and depression (6.1% each) in the nonsquamous group. The most common serious AEs (≥5% of patients) were general physical health deterioration (13.3%), pneumonia (10.0%), dehydration, and hyperglycemia (6.7% each) in the squamous group, and dyspnea, hyperglycemia, and respiratory failure (9.1% each) in the nonsquamous group.
In total, there were nine on-treatment deaths. In the squamous group, four deaths were reported (three with study indication as the primary cause, and one due to sepsis, suspected to be related to study treatment). In the nonsquamous group, there were five deaths (four due to study indication or disease progression, and one due to cardiogenic shock, not suspected to be related to study treatment).
Biomarker Analysis
Exploratory biomarker analyses were performed on the subset of tumor samples with sufficient material available. There was no evidence of a difference in median PFS between patients with PIK3CA mutation (20 patients) and PTEN alteration (41 patients; Supplementary Figure 2, Supplemental Digital Content, http://links.lww.com/JTO/A850). Furthermore, when exploring the role of individual mutations within the PIK3CA gene, there was no evidence of a difference in median PFS between the 32 patients with wild-type PIK3CA and the eight patients with “hot-spot” mutations (defined as E542K/V, E545K/A/G, and H1047R/L/Y/T,27 most likely to drive tumor growth and treatment resistance). Analysis of a panel of approximately 600 cancer-associated genes by NGS in 23 patients showed no evidence of association between PFS and the total tumor mutation burden (Supplementary Figure 3, Supplemental Digital Content, http://links.lww.com/JTO/A850). The subset of patients with alterations in either KRAS or EGFR appears to have a somewhat longer median PFS than wild-type patients, although the limited number of patients included in this analysis (three patients with KRAS mutation and five with EGFR mutation) should be noted.
PIK3CA mutations were also investigated in ctDNA. In 38 patients for whom PIK3CA mutation status was confirmed in both tissue and ctDNA samples, the concordance for PIK3CA mutation was 55.3%.9 The concordance was 81.8% (9 of 11 samples) between ctDNA and metastatic tissue, compared with 44.4% (12 of 27 samples) between ctDNA and primary tissue.
DISCUSSION
BASALT-1 is the first study to assess an oral pan-Class I PI3K inhibitor in patients with squamous and nonsquamous metastatic NSCLC which exhibits PI3K pathway activation. Before screening and enrollment for BASALT-1, 1242 patients were prescreened for PI3K pathway activation between May 2011 and September 2013—the largest such screen in squamous and nonsquamous NSCLC to date.28 Among patients with known alteration status, PI3K pathway activation (defined as PIK3CA mutation, PTEN mutation, or PTEN expression negative) was exhibited in 15% and 11% of squamous and nonsquamous NSCLC, respectively; alterations in PIK3CA and PTEN were broadly mutually exclusive in both histology types. The observed frequency of PI3K pathway alterations in squamous NSCLC was lower than that reported by a previous TCGA study, in which approximately 30% of 178 patients exhibited PIK3CA mutations or PTEN alterations.10 Meanwhile, our results in the nonsquamous group are in line with a previous TCGA study reporting that 10% of 230 patients with adeno-carcinoma exhibited PIK3CA mutations or PTEN alterations.29 The large, globally distributed population investigated in this study provides a good opportunity to estimate the actual frequency of PIK3CA mutations and PTEN alterations in NSCLC; no differences were identified between ethnic groups.
All patients entered the study with advanced disease and had received prior antineoplastic treatment. At the cut-off date (June 5, 2014), all but one patient in the nonsquamous group had discontinued from treatment. The most common reasons for treatment discontinuation included AEs in the squamous group (36.7% of patients) and disease progression in the nonsquamous group (57.6% of patients). This difference may be explained by the higher associated comorbidity in patients with squamous NSCLC, as demonstrated by the higher average ECOG performance status. Of note, treatment exposure was short in both groups (6.9 and 6.7 weeks in squamous and nonsquamous NSCLC, respectively), and was curtailed due to rapid disease progression in almost half (47.6%) of the patients.
The safety and tolerability profile of buparlisib presented here is consistent with that observed in previous single-agent buparlisib trials—of particular interest, hyperglycemia and liver toxicities occurred in similar numbers of patients, and mood disorders were slightly less frequent than in previous trials.
Despite the rationale for BASALT-1—treating a selected patient population (NSCLC exhibiting PI3K pathway activation) with a targeted agent (the pan-PI3K inhibitor buparlisib)9—the expected improvement in clinical outcome compared with chemotherapeutic agents in unselected patient populations was not observed: median PFS (2.79–2.83 months), median OS (7.20–7.98 months), and ORR (~3%) were similar to that observed with single-agent docetaxel.7 More recently, two targeted agents, afatinib and erlotinib, as second-line treatments for squamous NSCLC have demonstrated a median PFS of 2.4 and 1.9 months, respectively.30 We therefore investigated whether the tissue samples used for determination of PI3K pathway activation were appropriate, and whether the detailed molecular profile revealed by NGS would identify potential biomarkers for lack of response to therapy.
Only two new biopsies were taken at study entry (one primary and one metastatic tumor), whereas 62 samples were from archival tissue (47 primary and 15 metastatic tumors). Hence, the majority of samples used for the determination of pathway activation were from archival primary tumors. An exploratory analysis of PIK3CA mutations in ctDNA in this limited sample set revealed a greater concordance with metastatic tissue than with primary tissue. Our results suggest either that metastases have acquired alterations not present in the primary tumor or that the biopsy tissue used for sequencing may not be representative of the primary tumor. In both cases, the activation status in archival primary tissue samples may not match that at metastatic sites; hence sequencing of ctDNA may present an opportunity for more accurate characterization of tumor molecular status. However, even though ctDNA can be used to identify patients with PIK3CA mutations, the clinical outcome of this study does not support the use of PIK3CA mutation as a predictive marker for response to buparlisib therapy.
Further investigation of 23 patients with sufficient tumor tissue for NGS analysis did not identify any particular gene alteration associated with a lack of response to PI3K inhibition (such as members of the mitogen-activated protein kinase pathway).31 The observed frequencies of PIK3CA amplification and PTEN homozygous deletion detected were lower here than reported by a previous TCGA study,10 which could be due to the selection of patients with confirmed PIK3CA or PTEN alterations, or due to the different methodology used to detect copy number variations. KRAS alterations in this patient population were rare (only three patients with nonsquamous NSCLC harbored KRAS alterations), and in contrast to colorectal cancer, did not appear to be associated with a worse response to therapy.32 Of note, TP53 mutation, previously reported to activate the PI3K pathway, was the most common alteration detected by NGS, but showed no evidence of association with improved PFS. Overall, PI3K pathway activation has been observed in a clinically relevant proportion of patients, and no alterations were identified in genes known to negatively regulate the PI3K pathway. Therefore, the dependence on PI3K pathway activation of tumor growth and survival in NSCLC remains unclear.
In conclusion, despite preselecting patients with PI3K pathway-activated NSCLC, BASALT-1 did not meet its primary objective during Stage 1; the futility criterion was met in both squamous and nonsquamous groups. Although preclinical evidence supported the rationale for PI3K inhibition in advanced NSCLC,9 alternative pathways may play an oncogenic role in this setting; however, given the involvement of PI3K pathway activation in treatment resistance,9 combining PI3K inhibitors with chemotherapy, immunotherapy, or other targeted agents may improve efficacy compared to monotherapy, and warrants further investigation in future studies.
Supplementary Material
ACKNOWLEDGMENTS
The authors thank the patients who took part in the trial and their families, as well as the staff who assisted with the study at each site. This study was sponsored by Novartis Pharmaceuticals, who also provided financial support for medical editorial assistance. We thank Nadia Solovieff and Mike Morrissey (Novartis) for assistance with NGS experiments, and Nirmal Jethwa, PhD (Articulate Science Ltd.) for medical editorial assistance with this manuscript.
Footnotes
Disclosure: Cesare Gridelli has served as a board member for Novartis. Enriqueta Felip has served as a board member for BMS and AstraZeneca, and as a consultant for Eli Lilly, Pfizer, Roche, and Boehringer-Ingelheim. Filippo De Braud has served as a board member for Novartis, MSD, GSK, IRIS, Philogen, Eisai,Boehringer-Ingelheim, Eli Lilly, and Merck-Serono), and his institution has received grants from GSK, BMS, Merck, Sharp, Boehringer-Ingelheim, Servier, Novartis, and Celgene. Jean-Charles Soria has received honoraria from Novartis. The institutions of Jean-Luc Canon and Jhanelle E. Gray have received grants from Novartis. Johan F. Vansteenkiste has served as a consultant for Novartis. Martin Reck has served as a consultant for Hoffmann-La Roche, Eli Lilly, MSD, BMS, AstraZeneca, Boehringer-Ingelheim, Pfizer, and Novartis), and has participated in lectures and speakers’ bureaus for Hoffman-La Roche, Eli Lilly, MSD, BMS, AstraZeneca, Pfizer, and Boehringer-Ingelheim. Paola Aimone is an employee of Novartis. Emmanuelle Di Tomaso, Gena Atalla Vidam, Pantelia Roussou, and Ying A. Wang are employees and shareholders of Novartis. The remaining authors declare no conflicts of interest.
REFERENCES
- 1.Malvezzi M, Bertuccio P, Levi F, La Vecchia C, Negri E. European cancer mortality predictions for the year 2014. Ann Oncol. 2014;25:1650–1656. doi: 10.1093/annonc/mdu138. [DOI] [PubMed] [Google Scholar]
- 2.Howlader N, Noone A, Krapcho M, et al. SEER cancer statistics review, 1975–2008. Bethesda MD Natl Cancer Inst. 2011:19. [Google Scholar]
- 3.National Comprehensive Cancer Network NCCN Clinical Practice Guidelines in Oncology: Non-small cell lung cancer, Version 4. 2014 Available from: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp. Accessed February 7, 2015.
- 4.Leighl NB. Treatment paradigms for patients with metastatic non-small-cell lung cancer: First-, second-, and third-line. Curr Oncol. 2012;19(Suppl 1):S52–S58. doi: 10.3747/co.19.1114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Hanna N, Shepherd FA, Fossella FV, et al. Randomized phase III trial of pemetrexed versus docetaxel in patients with non-small-cell lung cancer previously treated with chemotherapy. J Clin Oncol. 2004;22:1589–1597. doi: 10.1200/JCO.2004.08.163. [DOI] [PubMed] [Google Scholar]
- 6.Shepherd FA, Rodrigues Pereira J, Ciuleanu T, et al. National Cancer Institute of Canada Clinical Trials Group. Erlotinib in previously treated non-small-cell lung cancer. N Engl J Med. 2005;353:123–132. doi: 10.1056/NEJMoa050753. [DOI] [PubMed] [Google Scholar]
- 7.Scagliotti G, Hanna N, Fossella F, et al. The differential efficacy of pemetrexed according to NSCLC histology: A review of two Phase III studies. Oncologist. 2009;14:253–263. doi: 10.1634/theoncologist.2008-0232. [DOI] [PubMed] [Google Scholar]
- 8.Garassino MC, Martelli O, Broggini M, et al. TAILOR trialists. Erlotinib versus docetaxel as second-line treatment of patients with advanced non-small-cell lung cancer and wild-type EGFR tumours (TAILOR): A randomised controlled trial. Lancet Oncol. 2013;14:981–988. doi: 10.1016/S1470-2045(13)70310-3. [DOI] [PubMed] [Google Scholar]
- 9.Engelman JA. Targeting PI3K signalling in cancer: opportunities, challenges and limitations. Nat Rev Cancer. 2009;9:550–562. doi: 10.1038/nrc2664. [DOI] [PubMed] [Google Scholar]
- 10.The Cancer Genome Atlas Research Network. Comprehensive genomic characterization of squamous cell lung cancers. Nature. 2012;489:519–525. doi: 10.1038/nature11404. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yamamoto H, Shigematsu H, Nomura M, et al. PIK3CA mutations and copy number gains in human lung cancers. Cancer Res. 2008;68:6913–6921. doi: 10.1158/0008-5472.CAN-07-5084. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Perez-Moreno P, Brambilla E, Thomas R, Soria JC. Squamous cell carcinoma of the lung: Molecular subtypes and therapeutic opportunities. Clin Cancer Res. 2012;18:2443–2451. doi: 10.1158/1078-0432.CCR-11-2370. [DOI] [PubMed] [Google Scholar]
- 13.Courtney KD, Corcoran RB, Engelman JA. The PI3K pathway as drug target in human cancer. J Clin Oncol. 2010;28:1075–1083. doi: 10.1200/JCO.2009.25.3641. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Bianco R, Shin I, Ritter CA, et al. Loss of PTEN/MMAC1/TEP in EGF receptor-expressing tumor cells counteracts the antitumor action of EGFR tyrosine kinase inhibitors. Oncogene. 2003;22:2812–2822. doi: 10.1038/sj.onc.1206388. [DOI] [PubMed] [Google Scholar]
- 15.Jin G, Kim MJ, Jeon HS, et al. PTEN mutations and relationship to EGFR, ERBB2, KRAS, and TP53 mutations in non-small cell lung cancers. Lung Cancer. 2010;69:279–283. doi: 10.1016/j.lungcan.2009.11.012. [DOI] [PubMed] [Google Scholar]
- 16.Marsit CJ, Zheng S, Aldape K, et al. PTEN expression in non-small-cell lung cancer: Evaluating its relation to tumor characteristics, allelic loss, and epigenetic alteration. Hum Pathol. 2005;36:768–776. doi: 10.1016/j.humpath.2005.05.006. [DOI] [PubMed] [Google Scholar]
- 17.Sos ML, Fischer S, Ullrich R, et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci USA. 2009;106:18351–18356. doi: 10.1073/pnas.0907325106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Zito CR, Jilaveanu LB, Anagnostou V, et al. Multi-level targeting of the phosphatidylinositol-3-kinase pathway in non-small cell lung cancer cells. PLoS One. 2012;7:e31331. doi: 10.1371/journal.pone.0031331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Engelman JA, Chen L, Tan X, et al. Effective use of PI3K and MEK inhibitors to treat mutant Kras G12D and PIK3CA H1047R murine lung cancers. Nat Med. 2008;14:1351–1356. doi: 10.1038/nm.1890. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Maira SM, Pecchi S, Huang A, et al. Identification and characterization of NVP-BKM120, an orally available pan-class I PI3-kinase inhibitor. Mol Cancer Ther. 2012;11:317–328. doi: 10.1158/1535-7163.MCT-11-0474. [DOI] [PubMed] [Google Scholar]
- 21.Koul D, Shen R, LaFortune TA, et al. NVP-BKM120: A selective pan-PI3 kinase inhibitor induces G2/M arrest in glioma cell lines via FOXO3a and GADD45a loop. Cancer Res. 2010;70(Suppl 8) abstr 350. [Google Scholar]
- 22.Schnell CR, Arnal S, Becquet M, et al. NVP-BKM120, a pan class I PI3K inhibitor impairs microvascular permeability and tumor growth as detected by DCE-MRI and IFP measurements via radio-telemetry: Comparison with NVP-BEZ235. Cancer Res. 2010;70(Suppl 8) abstr 4472. [Google Scholar]
- 23.Maira S-M, Menezes D, Pecchi S, et al. NVP-BKM120, a novel inhibitor of phosphoinosotide 3-kinase in Phase I/II clinical trials, shows signifi-cant antitumor activity in xenograft and primary tumor models. Cancer Res. 2010;70(Suppl 8) abstr 4497. [Google Scholar]
- 24.Voliva CF, Pecchi S, Burger M, et al. Biological characterization of NVPBKM120, a novel inhibitor of phosphoinosotide 3-kinase in Phase I/II clinical trials. Cancer Res. 2010;70(Suppl 8) abstr 4498. [Google Scholar]
- 25.Hurst CD, Zuiverloon TC, Hafner C, Zwarthoff EC, Knowles MA. A SNaPshot assay for the rapid and simple detection of four common hotspot codon mutations in the PIK3CA gene. BMC Res Notes. 2009;2:66. doi: 10.1186/1756-0500-2-66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Rodon J, Braña I, Siu LL, et al. Phase I dose-escalation and -expansion study of buparlisib (BKM120), an oral pan-Class I PI3K inhibitor, in patients with advanced solid tumors. Invest New Drugs. 2014;32:670–681. doi: 10.1007/s10637-014-0082-9. [DOI] [PubMed] [Google Scholar]
- 27.Saal LH, Holm K, Maurer M, et al. PIK3CA mutations correlate with hormone receptors, node metastasis, and ERBB2, and are mutually exclusive with PTEN loss in human breast carcinoma. Cancer Res. 2005;65:2554–2559. doi: 10.1158/0008-5472-CAN-04-3913. [DOI] [PubMed] [Google Scholar]
- 28.Dy GK, Vansteenkiste J, Thomas M, et al. Epidemiology of PI3K pathway alterations in patients with metastatic non-small cell lung cancer (NSCLC): Findings from the international BASALT-1 study. J Thorac Oncol. 2013;8(Suppl 2) abstr O04.05. [Google Scholar]
- 29.The Cancer Genome Atlas Research Network Comprehensive molecular profiling of lung adenocarcinoma. Nature. 2014;511:543–550. doi: 10.1038/nature13385. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Goss G, Felip E, Cobo M, et al. A randomized, open-label, phase III trial of afatinib (A) Vs erlotinib (E) as second-line treatment of patients (pts) with advanced squamous cell carcinoma (SCC) of the lung following first-line platinum-based chemotherapy: Lux-Lung 8 (LL8). Ann Oncol. 2014;25(Suppl 4) abstr 1222O. [Google Scholar]
- 31.McCubrey JA, Steelman LS, Chappell WH, et al. Roles of the Raf/MEK/ ERK pathway in cell growth, malignant transformation and drug resistance. Biochim Biophys Acta. 2007;1773:1263–1284. doi: 10.1016/j.bbamcr.2006.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Fruman DA, Rommel C. PI3K and cancer: Lessons, challenges and opportunities. Nat Rev Drug Discov. 2014;13:140–156. doi: 10.1038/nrd4204. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.