Abstract
Objective
Medicare Part D claims are commonly used for research, but missing claims could compromise their validity. This study assessed two possible causes of missing claims: veteran status and Generic Drug Discount Programs (GDDP).
Methods
We merged medication self-reports from telephone interviews in the Atherosclerosis Risk in Communities Study (ARIC) with Part D claims for six medications (three were commonly in GDDP in 2009). Merged records (4,468) were available for 2,905 ARIC participants enrolled in Part D. Multinomial logit regression provided estimates of the association of concordance (self-report & Part D, self-report only, or Part D only) with veteran and GDDP status, controlling for participant socio-demographics.
Results
Sample participants were 74±5 years of age, 68% white and 63% female; 19% were male veterans. Compared to females, male veterans were 11% (95% CI: 7%–16%) less likely to have matched medications in self-report & Part D and 11% (95% CI: 7%–16%) more likely to have self-report only. Records for GDDP versus non-GDDP medications were 4% (95% CI: 1%–7%) more likely to be in self-report & Part D and 3% (95% CI: 1%–5%) less likely to be in Part D only, with no difference in self-report only.
Conclusions
Part D claims were more likely to be missing for veterans, but claims for medications commonly available through GDDP were more likely to match with self-reports. While researchers should be aware of the possibility of missing claims, GDDP status was associated with a higher rather than lower likelihood of claims being complete in 2009.
INTRODUCTION
Prescription claims data are increasingly used by various organizations including pharmacy benefit managers, insurers, pay-for-performance contractors and researchers.1 The Centers for Medicare & Medicaid Services (CMS) adopted adherence quality measures, developed by the Pharmacy Quality Alliance, which measure patients’ adherence to long-term therapy with pharmacy claims.2 Medicare Part D sponsors receive financial incentives contingent on a star rating system.2 CMS star ratings include Core Measures that focus on prescribing selected medications for specific diseases.
Prescription claims undergo numerous audit and validity checks during the filing and billing processes to ensure accuracy.3 Despite the growing interest in using prescription medication claims for research and quality monitoring purposes, the completeness of Medicare Part D claims has yet to be fully investigated. Discrepancies between self-reported medication use and Part D claims may be due to different reasons: recall bias, free samples from providers, and provision or purchase from another source (e.g., the Veteran’s Administration Pharmacy Benefit, Generic Drug Discount Programs (GDDP), State Pharmaceutical Assistance Programs, out-of-pocket purchase, or mail order from foreign countries).4 Conversely, Part D claims may be found for drugs not reported by an individual for reasons including recall bias or filled prescriptions that are subsequently not taken.
While we are not aware of a data source that would allow a comprehensive assessment of the role of all causes of incomplete claims data, this study assesses the extent of two important potential deficiencies in Medicare Part D claims: veteran status and GDDP coverage. Although not all veterans are covered by the VA Pharmacy Benefit, some veterans may fill prescriptions through the VA Pharmacy Benefit even when they are enrolled in Medicare Part D, especially since the VA Pharmacy Benefit is considered more generous than Medicare Part D.5 The rapid increase in the offering of GDDPs, often referred to as “$4 generics,” by major pharmaceutical chains over the last decade may lead to under-representation of total medication consumption by Part D claims. Pharmacies are encouraged to submit GDDP claims to the Medicare program, but such submission is not required, and pharmacies generally do not receive additional reimbursement for submission. We first review issues and existing evidence about claims completeness. We then assess trends in claims submission using Medicare Part D claims from 2006–2009 for participants in the Atherosclerosis Risk in Communities Study (ARIC).6 The claims were merged with self-reported medications in 2009 to assess concordance between claims and self-reports. Concordance was assessed overall and using multinomial logit regression to identify the association of the two variables of interest with missing Part D claims.
Claims Completeness: Concerns and Prior Evidence
Since veterans may receive their medications through the VA Pharmacy Benefit, analyses of Part D claims have sometimes simply excluded veterans when veteran status was known,7 while medication adherence studies for veterans often use VA data.8 The completeness of prescription claims became a further concern for analysts over the last decade, due to the implementation of GDDPs by major retail chain pharmacies.9 The programs permit patients to purchase generic medications on a select list for a low out-of-pocket price (e.g., $4–$10 per month or $10–$12 for a 3-month supply). A 2011 survey of members of a university-affiliated health system found the use of GDDPs by its members increased from 5% to 32% between 2008 and 2010.10
Some researchers have expressed concerns that pharmacies may not submit prescription claims to the pharmacy benefit manager if a patient uses a GDDP and pays out-of-pocket.1 Gaps in claims data could substantially impact the validity of studies utilizing claims for various purposes.11, 12 However, Medicare beneficiaries may present their Medicare ID and Part D plan information even when paying cash to buy generic medications, and Part D plans often adjudicate the $4 claims to track total medication purchases.13 One study using the 2007 Medicare Current Beneficiary Survey (MCBS) found 97% of $4 medications on the GDDP list for a major pharmacy that were purchased out-of-pocket were adjudicated through Medicare Part D.9 This high rate may occur because pharmacies may have a customer’s Medicare information on file for other medication purchases and automatically file claims even if a GDDP covered the payment in full. In 2012, CMS issued a memo reinforcing its recommendation that Medicare Part D sponsors should encourage network pharmacies to submit all claims for medications provided to Part D beneficiaries.14
Several prior studies assessed the impact of GDDPs on the completeness of non-Medicare prescription claims; the results were conflicting but showed evidence of the potential for missing claims.4, 15, 16 Based on Medical Expenditure Panel Survey (MEPS) self-reports of medication fills/ expenditures merged with Part D claims, researchers concluded that people tended to over-report the number of fills per medication (possibly due to reporting free samples from providers) but to underreport the number of medications.17 However, the data were from 2006–2007, which was at the start of the expansion of GDDP plans, and the study excluded veterans since federal pharmacies (including VA pharmacies) do not file Part D claims. Other investigators found a high rate of Part D fills for MEPS respondents in 2007, though their conclusions were contested with anecdotal reports that smaller pharmacies are substantially less likely to file Part D claims.18, 19 A recent assessment using the 2009 MCBS found little evidence of out-of-plan use of discounted generics that was not adjudicated by Medicare and also showed that prescriptions filled at VA pharmacies or other sources only accounted for about 1% of total prescription fills.20 However, this study did not assess the association of veteran or GDDP status jointly with concordance while controlling for other enrollee characteristics.
In total, prior analyses do not provide a comprehensive assessment of the association of veteran status or GDDP coverage with Medicare Part D claims completeness. We therefore use Medicare claims data merged with medication self-reports to assess the extent of possible deficiencies in Part D claims for participants in community surveys such as ARIC from these two causes.
METHODS
Study Population
The Atherosclerosis Risk in Communities (ARIC) Study is an ongoing prospective population-based cohort study comprised of 15,792 adults aged 45–64 years at recruitment in 1987–1989.6 Cohort participants were selected from four US communities: Forsyth County, NC; Jackson, MS; Minneapolis, MN; and Washington County, MD. Participants completed five clinical exams between 1987 and 2013 and were contacted for annual follow-up telephone interviews. Ancillary to ARIC, 91% of the surviving cohort also participated in the Life Course Socioeconomic Status, Social Context and Cardiovascular Disease Study, which asked about veteran status.21 ARIC cohort participants were linked with CMS Medicare Part D claims from 2006–2009 for the Part D drug benefit that started in 2006.
During annual telephone interviews conducted by ARIC, the interviewer queried respondents about the names of all the medications used in the past two weeks. Merged Part D claims include the nonproprietary and proprietary names, date filled, and days supplied for each medication dispensed.
Medications Selected for Analysis
Six medications were selected based on high interview self-reporting frequency and likely inclusion versus non-inclusion on GDDP plan drug lists in 2009. To confirm broad use of the medications selected, we utilized web-based historical information to identify medications consistently found on GDDP lists in 2009 for 5 major pharmaceutical companies (Walmart, CVS, Walgreens, Target, and K-Mart); we also reviewed the medication lists and selected medications with two pharmacists. Table 1 lists the generic and brand names, drug class, and typical indications for the medications. The non-GDDP medications were primarily brand-name medications (except for amlodipine which was released as generic in 2007) that were not on the GDDP lists in these major pharmacies in 2009.
Table 1.
Selected Medications by GDDP Status (2009) and Part D Claim Filling Trends (2006 to 2009)
| GDDP status (2009) |
Generic drug name |
Type | Drug class | Typical indication |
Percent of claims where gross drug payment equals patient pay amount (%) |
Adjusted 30-day patient payment (claims where gross drug payment equals patient payment) |
||
|---|---|---|---|---|---|---|---|---|
| 2006 | 2009 | 2006 | 2009 | |||||
| Yes | Atenolol | Generic | Beta blocker | Hypertension | 55.1 | 57.9 | $4.80 | $3.70 |
| Yes | Lisinopril | Generic | ACE inhibitor | Hypertension | 23.6 | 34.0 | $8.90 | $5.30 |
| Yes | Metformin | Generic | Anti-diabetic agent | Type 2 Diabetes | 15.7 | 26.6 | $12.90 | $6.60 |
| No | Amlodipine | Generic | Calcium channel blocker | Hypertension | 7.1* | 15.9 | $37.10* | $8.60 |
| No | Amlodipine | Brand | Calcium channel blocker | Hypertension | 8.6 | 8.8 | $65.70 | $120.20 |
| No | Atorvastatin | Brand | HMG-CoA reductase inhibitors | Hyperlipidemia | 6.7 | 6.3 | $83.90 | $104.60 |
| No | Valsartan | Brand | Angiotensin receptor block | Hypertension | 10.9 | 8.2 | $63.60 | $78.60 |
Notes:
indicates 2007 value for Amlodipine Generic. Norvasc®, Lipitor®, Diovan®are the brand names of the drugsAmlodipine, Atorvastatin, Valsartan respectively.
Linkage for Assessing Medication Self-Report and Claims Concordance
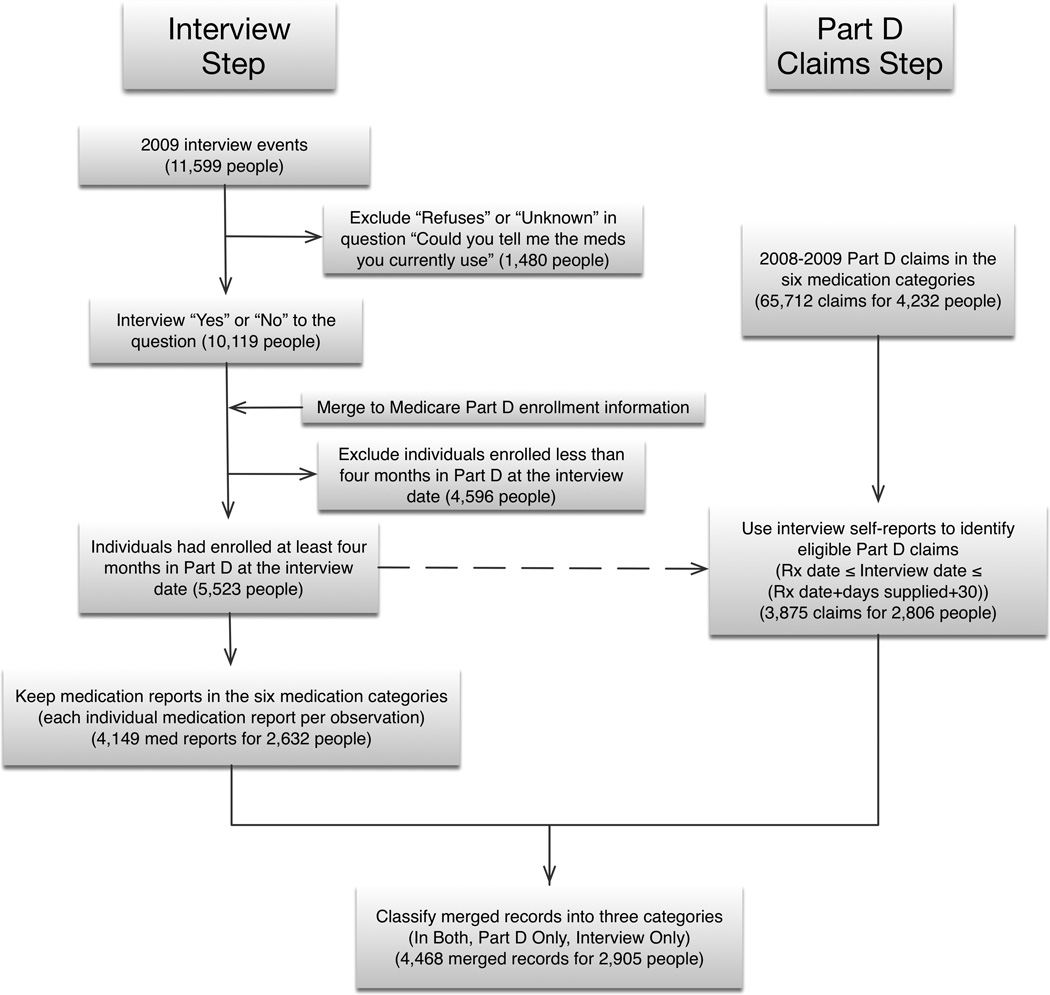
Figure 1 shows the steps in merging the medication self-reports and Part D claims. The 2009 telephone interview was administered to 11,599 ARIC participants. We excluded 1,480 people who refused to report medications used and 4,596 people who were enrolled in Part D for less than four months prior to the interview date (as Part D prescriptions are often filled for 90 days). Keeping only medication reports in the six selected medication categories resulted in 4,149 medication reports for 2,632 people.
Figure 1.
For Part D claims, we identified 65,712 Part D claims for 4,232 ARIC participants in 2008–2009 in the six medication categories. We then limited the sample to claims which were filled before the interview (with days supplied overlapping the interview date) or up to 30 days afterwards to allow for overstock due to refills or moderate adherence. This step resulted in 3,875 claims for 2,806 people.
Finally, we merged interview medication self-reports and Part D claims by medication name and classified records from the merge into one of three categories: in both self-report and Part D, only in self-report, or only in Part D. We excluded an additional 127 people who were missing veteran status. The final analysis file had 4,468 merged records for 2,905 people.
Analyses and Statistical Methods
We conducted three analyses to describe and assess the completeness of Medicare Part D claims. The first analysis used Part D claims from 2006–2009 to characterize the rate over time at which participants’ out-of-pocket payments constituted the full payment for the medication, which could reflect GDDP filings. Since prescriptions may be filled for varying amounts of time (e.g., 30 days, 90 days, etc.), we adjusted the measures in this analysis to reflect 30 day prescriptions. The second analysis used the merged Part D claims and interview self-reports in 2009 to calculate medication concordance statistics (overall and by GDDP status). The Kappa statistic provides an assessment of concordance (i.e., agreement from two different sources of information), with values as follows: poor (<0.20), fair (0.20–0.40), moderate (0.41–0.60), good (0.61–0.80), and very good (0.81–1.00).22, 23 The third analysis provided tests of two specific hypotheses:
H1: Relative to non-veteran males and females, male veterans are less likely to have matched claims and more likely to have medication reports that are self-report only.
H2: Medications typically on GDDP plans in 2009 are less likely to have matched claims and more likely to have medication reports that are self-report only.
The first hypothesis is based on the fact that veterans may obtain medications from the VA Pharmacy Benefit rather than through Part D. The second hypothesis will be supported if claims are less likely to be submitted to Part D when the patient’s payment for a GDDP plan is the total payment (e.g., for a $4 generic).
Multinomial logit regression was used to test these hypotheses by examining whether the likelihood of the trichotomous measure of concordance (in both, self-report only, or Part D only) differed in 2009 for veterans or for medications on the GDDP lists versus not on the GDDP lists. Covariates included in the regression were: age, gender, and ARIC site interacted with race. (The small number of study participants who were not white or black were excluded from the analysis.) All included ARIC participants in Jackson, MS are black, while all included participants in Minneapolis, MN and Washington County, MD are white. Forsyth, NC is the only site with variation in race. Only two females in the ARIC sample were identified as veterans; therefore, female veterans were grouped with female non-veterans and included in the referent group. The regression model is subject to the “Independence of Irrelevant Alternatives (IIA) assumption” which was tested using a Hausman test, with no evidence of violation of IIA assumption. We adjusted the standard errors for clustering on individuals, since many individuals had multiple medication reports in the file.24 Statistical tests of a model using a quadratic form for age showed that age had a linear rather than non-linear relationship with concordance.
The main regression model investigated the effect of veteran and GDDP status while controlling for study participant age, race and geographic location. Marginal effects for each variable, standard errors for the estimated marginal effects, and 95% confidence intervals were generated using the method of recycled prediction for categorical variables and the calculus method for the continuous variable age. To investigate the consistency of specific medication effects, we estimated two additional models using: (1) separate indicators for each GDDP medication (with overall non-GDDP status as the referent group); and (2) separate indicators for the non-GDDP medications (with overall GDDP status as the referent group).
The regressions control for key person or programmatic factors that may be associated with concordance between claims and self-report. Several important pieces of information were not available from either source, including: whether the individual actually purchased the medication through a GDDP plan, whether the person was using the Veteran pharmacy benefit, and whether the person had some other source of payment for medications (e.g., State pharmacy assistance benefit program). While these gaps in information limited definitive determination of sources of non-concordance, the identification of key factors associated with non-concordance is still instructive to researchers using claims or self-reports of medications.
RESULTS
Trends in Part D Claims Payments Over Time
The last four columns of Table 1 provide trends for Part D claims from 2006–2009 for the six medications. Compared to non-GDDP medications, a relatively high proportion of claims for the GDDP medications had patient pay amounts that equaled total payment for the medication, as might occur for medications obtained through GDDP plans (though we cannot rule out simple “payment in full” for the drugs under Part D). In contrast, the non-GDDP medications had a much lower rate of claims where the patient pay amount equaled the total payment amount. (For both categories, some claims may have been for higher payments while the beneficiary was in the Part D “donut hole” where the beneficiary is responsible for the full medication payment.) The percent of claims where gross drug payment equaled patient pay amount increased over time for the GDDP medications. whereas it decreased over time for the non-GDDP medications. Patient out-of-pocket payments (adjusted to reflect 30 day prescriptions) for the GDDP medications were relatively low and declined from 2006 to 2009. In contrast, the out-of-pocket payments were much higher and increased over time for the non-GDDP brand name medications.
Concordance Statistics between Part D Claims and Self-reports
Table 2 provides statistics on the concordance by GDDP status for the 4,468 medication reports for the 2,905 ARIC participants with self-reports and/or Part D claims for the selected medications in 2009. The first column of Table 3 provides descriptive statistics for the sample; the mean age was 74±5.4 years, 63% were female, and 68% were white. Almost 20% of participants were male veterans.
Table 2.
GDDP versus non-GDDP Medication Reporting by Medicare Part D and Patient Self-Report
| Overall (N=4468) |
In Both Sources (N= 3,347) |
Part D only (N= 420) |
Self-report only (N= 701) |
p value* | |
|---|---|---|---|---|---|
| GDDP (overall) | <0.001 | ||||
| Yes | 100% (2441) |
77.0% (1879) |
7.7% (189) |
15.3% (373) |
|
| No | 100% (2027) |
72.4% (1468) |
11.4% (231) |
16.2% (328) |
|
| By Drug name | <0.001 | ||||
| GDDP | |||||
| Atenolol | 100% (605) |
78.5% (475) |
4.5% (27) |
17.0% (103) |
|
| Lisinopril | 100% (1178) |
77.4% (912) |
7.6% (89) |
15.0% (177) |
|
| Metformin | 100% (658) |
74.8% (492) |
11.1% (73) |
14.1% (93) |
|
| Non-GDDP | |||||
| Atorvastatin | 100% (780) |
72.7% (567) |
3.7% (29) |
23.6% (184) |
|
| Amlodipine | 100% (918) |
71.8% (659) |
19.4% (178) |
8.8% (81) |
|
| Valsartan | 100% (329) |
73.6% (242) |
7.3% (24) |
19.2% (63) |
p value from a chi-square test. Numbers are presented as “% (count)”
Table 3.
Demographic Characteristics & Estimation Results from Multinomial Logit Model Estimation (Coefficients and Marginal Effects)
| Descriptive statistics |
Outcome = In Both | Outcome = Self-report only |
Outcome = Part D only | ||||
|---|---|---|---|---|---|---|---|
| N=2,905 people |
Coefficient | Marginal Effect (%) |
Coefficient | Marginal Effect (%) |
Coefficient (Reference) |
Marginal Effect (%) |
|
| Age | 73.8 (5.4) |
0.001 (0.011) |
0.1 (0.1) |
−0.006 (0.013) |
−0.1 (0.1) |
0.1 (0.1) |
|
| Gender & Veteran Status | |||||||
| Female | 1,844 (63.5%) |
Referent group | |||||
| Male Non-Vet | 507 (17.5%) |
−0.197 (0.139) |
−3.1 (1.9) |
−0.041 (0.169) |
1.6 (1.5) |
1.5 (1.2) |
|
| Male Vet | 554 (19.1%) |
−0.149 (0.163) |
−11.2*** (2.2) |
0.621** (0.191) |
11.3*** (2.1) |
0.2 (1.3) |
|
| Site : Race | |||||||
| Minneapolis:White | 534 (18.4%) |
Referent group | |||||
| Washington:White | 846 (29.1%) |
−0.757*** (0.184) |
−6.4*** (2.0) |
−0.576** (0.217) |
1.3 (1.7) |
5.0*** (1.3) |
|
| Forsyth:White | 611 (21.0%) |
−0.585** (0.188) |
−7.0*** (2.0) |
−0.246 (0.220) |
3.7* (1.8) |
3.3** (1.2) |
|
| Forsyth:Black | 72 (2.5%) |
−1.412*** (0.297) |
−19.5*** (4.9) |
−0.632 (0.331) |
8.5* (4.0) |
11.0*** (3.4) |
|
| Jackson:Black | 842 (29.0%) |
−1.045*** (0.168) |
−11.9*** (1.9) |
−0.596** (0.198) |
4.4** (1.7) |
7.5*** (1.2) |
|
| GDDP Status | |||||||
| Non-GDDP | Referent group | ||||||
| GDDP | 0.361*** (0.108) |
3.9** (1.3) |
0.238 (0.128) |
−1.1 (1.1) |
−2.9** (0.9) |
||
| Intercept | 2.575** (3.23) |
1.093 (1.13) |
|||||
For column 1, Presented as “count (%)” except for age (which has mean and standard deviation).
Standard errors are in parentheses below coefficients and marginal effects.
p<0.05,
p<0.01,
p<0.001
Interestingly, GPPD medications had a higher rate of concordance (i.e., being in both the self-reports and Part D claims) of 77.0% than non-GPPD drugs (72.4%), indicating good agreement. Overall, the GDDP medications (k=0.85) had higher concordance than non-GDDP medications (k=0.78). The specific medications all had kappa statistics indicating good to very good concordance.
Regression Analysis
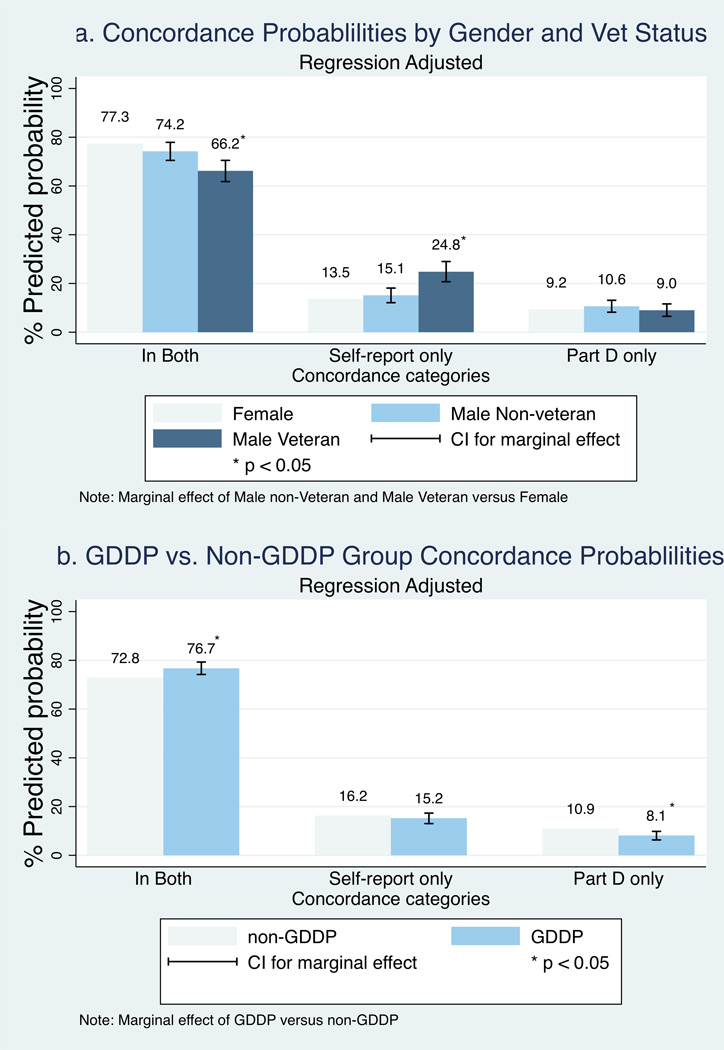
The regression coefficients for the main model in Table 3 provide estimates of the association between concordance and the two characteristics that are hypothesized to be associated with lower concordance (male veteran status and GDDP drug), while controlling for participant characteristics. Interpretation of the association is facilitated by the marginal effects. Figure 2 provides predicted values of the three concordance categories for male veteran status and GDDP medication status and the corresponding marginal effect from Table 3. Figure 2a shows that relative to females, male veterans were 11.1% (95% CI: 6.8%–15.5%) less likely in both the self-report & Part D and were 11.3% (95% CI: 7.2%–15.5%) more likely in self-report only. The probability of being Part D only did not vary significantly for male veterans versus females. Male non-veterans did not differ significantly from females for any of the three concordance categories, and the 95% confidence intervals for the marginal effects for male non-vets and male vets did not overlap. Therefore, the hypothesis that male veterans would be more likely to have a lower match rate and a greater rate of self-reports than females or male non-veterans is supported.
Figure 2.
Figure 2b, however, does not support the second hypothesis that Part D claims would be more likely to be missing for GDDP medications. Compared to non-GDDP medications, GDDP medications were 3.9% (95% CI: 1.4%–6.5%) more likely to be in both self-report and Part D and were not significantly more likely to be in self-report only. The higher match rate for GDDP medications is also reflected by the fact that GDDP medications were 2.8% (95% CI: 1.1%–4.6%) less likely to be in Part D only.
Other regression coefficients and estimated marginal effects in Table 3 show that concordance varied significantly by geographic site and, to some extent, race. As noted earlier, it is not possible to fully disentangle race and site effects in the ARIC sample because the Minneapolis, MN and Washington County, MD sites consist only of whites, while the Jackson site has only blacks. This site/race variation in concordance could be due to claim filing policies of individual pharmacies in the areas.
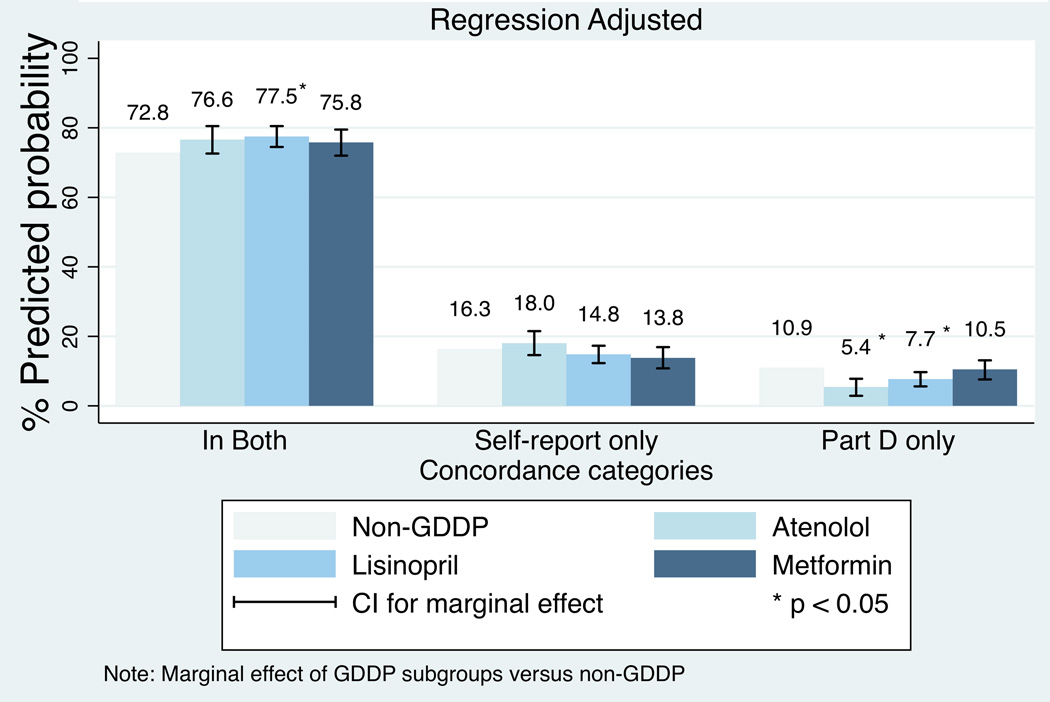
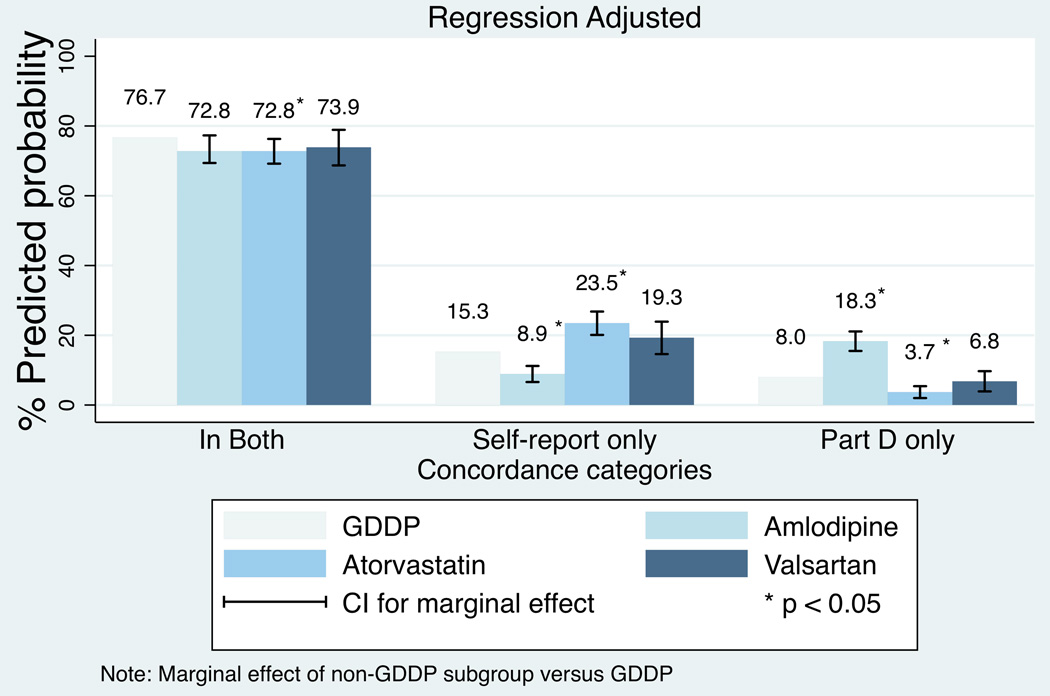
The regressions examining specific medications show consistent patterns for the likelihood of being in both the self-report and Part D claims for the three separate GDDP medications versus the combined group of non-GDDP medications. The regression results are available on request; Appendix Figures A1 and A2 show the marginal effects. Only lisinopril had a statistically significantly higher likelihood of being in both self-report and Part D as well as Part D only. Patterns for the three separate non-GDDP medications versus the combined group of GDDP medications were less consistent; the likelihood of being in self-report only or Part D only varied in direction and significance for some of the non-GDDP specific medications. In particular, amlodipine (combined brand name and generic) and atorvastatin had predicted likelihoods that were in the opposite directions for the self-report only and Part D only predicted likelihoods.
DISCUSSION
This study provides a relatively current assessment of the completeness of Medicare Part D claims based on comparisons to self-reported medication use for specific medications. The study analyzes several medications to assess the agreement between self-reported medication and Part D claims. The predicted probability and marginal effects for male veterans are consistent with the possibility that many veterans may fill their medications through the VA Pharmacy Benefit even when enrolled in Medicare Part D, resulting in medication reports by male veterans being less likely to be in concordance and more likely to be in interview self-reports only. Caution should be used, therefore, when analyzing Part D claims for study samples that may include veterans. Incomplete pharmacy claims may misclassify adherent users as non-adherent, or properly treated patients as under-treated, which in turn affects pharmaceutical quality measurement and improvement activities designed for improving medication utilization.1, 4 For veterans in particular, simultaneously using multiple medication measures may enhance analysis validity.8, 25
Consistent with the analysis by Roberto and Stuart,20 our results do not support that Medicare Part D claims were missing due to GDDP status in 2009. Instead, GDDP medications had a higher probability of being concordant than non-GDDP medications, with no statistical difference in the self-report only group. Our results provide assurance that the completeness and validity of Part D claims was not broadly compromised by GDDP status in 2009. However, we found that veteran status did account for a significantly higher percentage of medications that were self-report only. Roberto and Stuart’s analysis of a nationally representative data base found the proportion of prescriptions to be filed under VA coverage to be extremely small,20 so our finding of more sizeable differences in claim completeness by veteran status may be due to the fact that the ARIC study is conducted in only four geographical areas that may have utilization patterns specific to those areas. Alternatively, our regression-based approach controls for additional factors that may have enabled identification of the potential importance of veteran status for claims completeness.
The study is subject to several limitations. First, we were not able to identify specifically whether prescriptions were filled through GDDP plans. A study using 2007 Medicare Part D national sample claims showed that 80% of Part D filled prescriptions for generic medications from Medicare beneficiaries were available through GDDP, but only 16.3% were actually filled through GDDPs.13 However, the relatively high rate of claims where the patient payment is both relatively low and equal to the total payment is consistent with having a lot of Part D claims filed by GDDP pharmacies. Second, the use of GDDP plans has been increasing over time, so studies with more recent data may be needed to confirm whether the high rate of concordance for Part D claims and self-reports for GDDP drugs found in this study continues to hold over time. Third, our sample is not nationally representative. Given that prescription filing policies and mandates for different state plans or large pharmaceutical chains may vary, different geographic locations or certain pharmacies may have substantial discrepancies.18 Fourth, the self-reports are not a gold standard since subjects may fail to report medications they are taking, or may report medications previously prescribed that they are not currently taking. Fifth, we only assessed a modest number of common medications for chronic conditions; though we selected drugs from five classes, it is possible that the trends and findings could vary in other classes of drugs (e.g., for medications for acute conditions, such as antibiotics). Sixth, for the time period studied, we did not have information on whether study participants actually filled prescriptions through the VA. Finally, while veteran status and the GDDP categorization reflect important characteristics, veteran status and GDDP status are not the only reasons why Part D claims may be missing; for example, we lacked information on other sources of pharmacy coverage or medications.
Overall, this study increases our understanding of the completeness and validity of Part D prescription claims. This study is important because Part D claims are increasingly being utilized by organizations and researchers, as well as being used as a quality measure by CMS. Our analysis describes the most commonly used medication utilization patterns in Medicare Part D claims for ARIC participants. Concordance between medication self-reports with Medicare Part D was at least good to very good as measured by the Kappa statistic. We believe our study provides an important identification of the extent to which veteran status may be associated with missing claims in Part D; while Part D claims were available for many veterans, researchers may need to consider other options when using Part D claims for analysis of samples that include veterans. Such options might include controlling for veteran status, dropping veterans from the analysis, or seeking additional data from the Veteran’s Administration. Our study reduces concerns, however, about the extent to which Part D claim completeness may have been be compromised by the growth in generic drug plans over time for a set of frequently used chronic medications, at least through 2009.
Acknowledgments
The Atherosclerosis Risk in Communities Study is carried out as a collaborative study supported by National Heart, Lung, and Blood Institute contracts (HHSN268201100005C, HHSN268201100006C, HHSN268201100007C, HHSN268201100008C, HHSN268201100009C, HHSN268201100010C, HHSN268201100011C, and HHSN268201100012C). The authors thank the staff and participants of the ARIC study for their important contributions.
Appendix
REFERENCES
- 1.Choudhry NK, Shrank WH. Four-Dollar Generics -- Increased Accessibility, Impaired Quality Assurance. New England Journal of Medicine. 2010;363(20):1885–1887. doi: 10.1056/NEJMp1006189. [DOI] [PubMed] [Google Scholar]
- 2.Pharmacy Quality Alliance. PQA measures used by CMS in the star ratings ratings program. [Accessed 20 January 2014]; Available from: http://pqaalliance.org/measures/cms.asp. [Google Scholar]
- 3.Strom B, Kimmel S, Hennessy S, editors. Pharmacoepidemiology. 5th ed. John Wiley & Sons; 2011. [Google Scholar]
- 4.Lauffenburger JC, Balasubramanian A, Farley JF, et al. Completeness of prescription information in US commercial claims databases. Pharmacoepidemiology and Drug Safety. 2013;22(8):899–906. doi: 10.1002/pds.3458. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Medicare Interactive. Medicare and Veterans Affairs (VA) Benefits. [Accessed August 20, 2014]; http://www.medicareinteractive.org/page2.php?topic=counselor&page=script&script_id=331. [Google Scholar]
- 6.The ARIC Investigators. The Atherosclerosis Risk in Communities (ARIC) Study - Design and Objectives. American Journal of Epidemiology. 1989;129(4):687–702. [PubMed] [Google Scholar]
- 7.Hill SC, Zuvekas SH, Zodet MW. Implications of the Accuracy of MEPS Prescription Drug Data for Health Services Research. Inquiry-the Journal of Health Care Organization Provision and Financing. 2011;48(3):242–259. doi: 10.5034/inquiryjrnl_48.03.04. [DOI] [PubMed] [Google Scholar]
- 8.Thorpe CT, Bryson CL, Maciejewski ML, Bosworth HB. Medication Acquisition and Self-Reported Adherence in Veterans With Hypertension. Medical Care. 2009;47(4):474–481. doi: 10.1097/mlr.0b013e31818e7d4d. [DOI] [PubMed] [Google Scholar]
- 9.Stuart B, Loh EF. Medicare Part D Enrollees' Use of Out-of-Plan Discounted Generic Drugs. Journal of the American Geriatrics Society. 2012;60(2):387–388. doi: 10.1111/j.1532-5415.2011.03812.x. [DOI] [PubMed] [Google Scholar]
- 10.Gatwood J, Tungol A, Truong C, Kucukarslan SN, Erickson SR. Prevalence and Predictors of Utilization of Community Pharmacy Generic Drug Discount Programs. Journal of Managed Care Pharmacy. 2011;17(6):449–455. doi: 10.18553/jmcp.2011.17.6.449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Kronish IM, Ye SQ. Adherence to Cardiovascular Medications: Lessons Learned and Future Directions. Progress in Cardiovascular Diseases. 2013;55(6):590–600. doi: 10.1016/j.pcad.2013.02.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Zhang J, Yun HF, Wright NC, et al. Potential and Pitfalls of Using Large Administrative Claims Data to Study the Safety of Osteoporosis Therapies. Current Rheumatology Reports. 2011;13(3):273–282. doi: 10.1007/s11926-011-0168-8. [DOI] [PubMed] [Google Scholar]
- 13.Zhang YT, Gellad WF, Zhou L, Lin YJ, Lave JR. Access to and Use of $4 Generic Programs in Medicare. Journal of General Internal Medicine. 2012;27(10):1251–1257. doi: 10.1007/s11606-012-1993-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Centers for Medicare and Medicaid Services Prohibition on submitting Prescription Drug Events (PDEs) for non-part D prescriptions. [Accessed August 20, 2014]; Available from: http://www.healthlawyers.org/Members/PracticeGroups/PPMC/MAPD/Documents/HPMS%20Memoranda/May%202012/HPMSMemo_NonPartDRxPDEProhibition_05102012.pdf. [Google Scholar]
- 15.Tungol A, Starner CI, Gunderson BW, et al. Generic Drug Discount Programs: Are Prescriptions Being Submitted for Pharmacy Benefit Adjudication? Journal of Managed Care Pharmacy. 2012;18(9):690–700. doi: 10.18553/jmcp.2012.18.9.690. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhu VJ, Belsito A, Tu W, Overhage JM. Data for drugs available through low-cost prescription drug programs are available through pharmacy benefit manager and claims data. BMC Clin Pharmacol. 2012;12:12. doi: 10.1186/1472-6904-12-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Hill SC, Zuvekas SH, Zodet MW. Validity of Reported Medicare Part D Enrollment in the Medical Expenditure Panel Survey. Medical Care Research and Review. 2012;69(6):737–750. doi: 10.1177/1077558712457595. [DOI] [PubMed] [Google Scholar]
- 18.Harding J. Medicare Part D Enrollees' Use of Out-of-Plan Discounted Generic Drugs, Revisited. Journal of the American Geriatrics Society. 2013;61(2):309–310. doi: 10.1111/jgs.12076. [DOI] [PubMed] [Google Scholar]
- 19.Stuart B, Loh FE. Medicare Part D Enrollees' Use of Out-of-Plan Discounted Generic Drugs, Revisited Response. Journal of the American Geriatrics Society. 2013;61(2):310–310. doi: 10.1111/jgs.12076. [DOI] [PubMed] [Google Scholar]
- 20.Roberto PN, Stuart B. Out-of-Plan Medication in Medicare Part D. The American Journal of Managed Care. 2014;20(9):743–748. [PubMed] [Google Scholar]
- 21.Johnson AM, Rose KM, Elder GH, et al. Military Combat and Risk of Coronary Heart Disease and Ischemic Stroke in Aging Men: The Atherosclerosis Risk in Communities (ARIC) Study. Annals of Epidemiology. 2010;20(2):143–150. doi: 10.1016/j.annepidem.2009.10.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Altman DG. Practical statistics for medical research. London: 1991. [Google Scholar]
- 23.Viera AJ, Garrett JM. Understanding interobserver agreement: the kappa statistic. Family Medicine. 2005;37(5):360–363. [PubMed] [Google Scholar]
- 24.White H. A heteroskedasticity-consistent covariance matrix estimator and a direct test for heteroskedasticity. Econometrica. 1980;48:817–830. [Google Scholar]
- 25.Steiner JF. Self-reported Adherence Measures What Do They Assess and How Should We Use Them? Medical Care. 2012;50(12):1011–1012. doi: 10.1097/MLR.0b013e318270abaf. [DOI] [PubMed] [Google Scholar]