Abstract
We have examined the early events in Cai2+-induced fusion of large (0.2 μm diameter) unilamellar cardiolipin/phosphatidylcholine and phosphatidylserine/phosphatidylethanolamine vesicles by quick-freezing freeze-fracture electron microscopy, eliminating the necessity of using glycerol as a cryoprotectant. Freeze-fracture replicas of vesicle suspensions frozen after 1-2 s of stimulation revealed that the majority of vesicles had already undergone membrane fusion, as evidenced by dumbbeII-shaped structures and large vesicles. In the absence of glycerol, lipidic particles or the hexagonal H 11phase, which have been proposed to be intermediate structures in membrane fusion, were not observed at the sites of fusion. Lipidic particles were evident in less than 5% of the cardiolipin/phosphatidylcholine vesicles after long-term incubation with Ca2+, and the addition of glycerol produced more vesicles displaying the particles. We have also shown that rapid fusion occurred within seconds of Ca2+ addition by the time-course of fluorescence emission produced by the intermixing of aqueous contents of two separate vesicle populations. These studies therefore have produced no evidence that lipidic particles are necessary intermediates for membrane fusion. On the contrary, they indicate that lipidic particles are structures obtained at equilibrium long after fusion has occurred and they become particularly prevalent in the presence of glycerol.
Keywords: Membrane fusion, Lipidic particle, Phospholipid vesicle, Quick-freezing, Fusion intermediate, Freeze-fracture
Introduction
Reiss-Husson in 1967 [1] and Luzzatti et al. in 1968 [2] first described the structural characteristics of the hexagonal (HII) phase of phospholipids such as phosphatidylethanolamine (PE), and phosphatidylcholine (PC). The Hu phase is composed of hexagonally packed rod-like structures in which head-groups line the inside of the rod and acyl tails radiate outward. Other investigators later reported that cardiolipin (CL) and phosphatidic acid (PA) membranes undergo a phase transition from lamellar (Lα) to HII in the presence of divalent cations [3–5]. Recently, it has been claimed that the fusion of model and natural membranes proceeds via the formation of the Hu phase or inverted micelle structures [6–8]. More specifically, it has been proposed that the formation of lipidic particles [9–11], which have been observed with freeze-fracture preparations in the presence of glycerol, are essential for initiation of fusion between membranes [12,13].
In contrast to the above, we have already demonstrated that fusion can be induced under conditions which do not result in the formation of the H II phase or lipidic particles. For example, vesicles composed of phosphatidylserine (PS) do not undergo H II phase transitions, but fuse extensively in the presence of Ca2+ [14,15]. Furthermore, PS :PE (transphosphatidylated from egg PC) vesicles undergo Ca2+-induced fusion at temperatures well below the lamellar-to-H II transition temperature of PE[16,17]. These vesicles, or PS : egg PE vesicles, also fuse in the presence of Mg 2+ (Ref. 16 and Düzgüne§, N., unpublished data), which does not induce the H II phase formation in mixtures of PS with egg PE or soya PE [7,18]. We have also shown that membranes made of a mixture of phosphatidylinositol and dimyristoylphosphatidylethanolamine, which does not transform into the H II phase over the physiological temperature range [19-21], can undergo Ca2+ -induced fusion as long as the membrane is above the gel-to-liquid-crystalline transition temperature [22].
In order to re-examine the role of lipidic particles or the Lα → H II transition in fusion, we have undertaken to study the fusion characteristics of two membrane systems by morphologic and fluorometric techniques. We have employed the quick-freeze device of Heuser et al. [23] to observe the early stages of vesicle fusion following Ca2+ addition. This technique also permitted us to assess the role of glycerol in the production of lipidic particles. Further, we have used a fluorescence assay that allows a quantitation of the mixing of vesicle contents during fusion [24].
Our observations with both techniques demonstrate that although large unilamellar vesicles composed of CL:PC (1:1) and PS:PE (1:1) did indeed fuse within seconds of Ca2+ addition, no lipidic particles were present in these vesicles. However, lipidic particles were evident after long incubations (1–2 h) in the presence of calcium and glycerol and, to a limited extent, Ca2+ alone, as recently noted by Rand et al. [25].
Materials and Methods
Beef heart cardiolipin, soya PE, and egg PE were obtained from Avanti Polar Lipids (Bir-mingham, AL). Bovine brain PS and egg PC were prepared as described earlier [15,16]. Unilamellar vesicles were made from CL: PC (in a molar ratio of 1: 1), egg PE: bovine brain PS (1:1) or soya PE:PS(l: 1) by reverse-phase evaporation [24,26] and were diluted to 1O μmol/ml in 100 mM NaCl/O.l mM EDTA/2 mM histidine/2 mM Tes buffer (pH 7.4) (buffer A) and sized by extrusion through polycarbonate membranes with a 0.2 μm pore diameter [27]. Vesicles from any given preparation were divided into separate test-tubes and prepared in the following manner. 1O-μ1 droplets of untreated liposomes were mounted directly on filter paper (Whatman No. 42) glued to an aluminum platform of a quick-freeze device and frozen on a liquid-helium-cooled copper block according to the method of Heuser et al. [23]. In this procedure, the filter paper acts only as an adhesive support for the droplet and does not cause drying of the sample. For observing fusion, we injected 8-μl droplets with 2 μl of a 25 mM Ca2+ solution (in buffer A) at 25°C, l–2 s before freezing. Liposomes from the same initial batch were incubated for 1–2 h at 25°C in either 5 mM Ca2+ alone, or in Ca2+ and 25% glycerol, and frozen in a similar manner. Except for the use of quick-freezing, these latter preparations duplicated previously reported experiments (9,11,12,28), and displayed the lipidic particles which have been described. To determine whether the lipidic particles were a product of long incubations or could appear during fusion in the presence of glycerol, glycerinated vesicles were also treated exactly like untreated vesicles and stimulated with Ca2+ 1 s before freezing. All samples were fractured and replicated at l15°C in a Balzers freeze-fracture device. The replicas were then cleaned and examined in a Siemens 1O1 electron microscope. Fusion of CL:PC vesicles was studied independently by assaying the intermixing of aqueous contents from two populations of vesicles, using the fluorescence technique developed by Wilschut et al. [24]. A preliminary account of some of this work has been published [29].
Results and Discussion
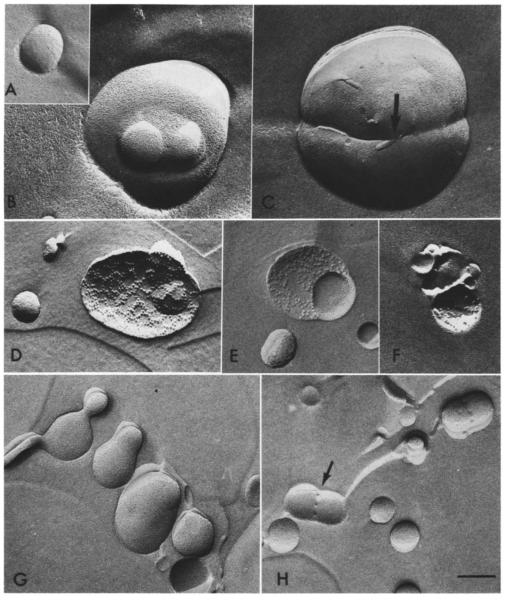
The CL:PC vesicles not stimulated with Ca2+ remained smooth-surfaced with a 0.2 μM diameter after quick-freezing (Fig. lA). Within I s of injection, Ca2+ triggered dumbbell and egg shapes as well as larger, round vesicles in the process of fusing with smaller ones, such as those in Fig. 1B. The fusion of two or more vesicles could clearly be discerned by the continuity of fracture surfaces between vesicles. However, the liposome mem-brane faces were smooth, without any easily identifiable structure, such as lipidic particles. Large unilamellar egg PE: PS (and soya PE: PS) vesicles also underwent fusion within 1 s of Ca2+ injection without any indication of lipidic particles. No gross morphological differences were apparent between the PE: PS and CL: PC vesicles. In Fig. 1C. obtained with PE: PS vesicles, there is an anomaly (to the left of the arrow) in the continuity between the membranes of two vesicles. We suggest that this may represent a stage prior to complete fusion of the two membranes. This has been observed infrequently but with both types of vesicle. When CL: PC liposomes were treated for l min with Ca2+ (5 mM) and then incubated for l–2 h in glycerol, many large vesicles emerged, 10–20% of which were embossed with 9–10 nm particles (Fig. 1D). Some of the particles described circles about 0.2 μmin diameter with a smooth surface, possibly delineating the margins of another vesicle (Fig. 1E). Barren circles like these are reminiscent of the ‘clearings’ of intramembranous particles observed in glycerinated eukaryotic cells such as β-islet cells [30] and guinea-pig sperm (Fig. 2A and B) [31]. in which the larger intramembranous particles also measure 9 nm. Such clearings, common in stimulated mast cells treated with glycerol, no longer appear when the cells have been quick-frozen in the absence of glycerol [32]. We found that long incubation (2 h at 25°C) of CL:PC vesicles in Ca2+ alone resulted in far fewer vesicles embellished with lipidic particles (1–5% of total vesicles present) than incubation in glycerol and Ca2+ (Fig. IF). Suspensions incubated first in glycerol for 2 h and then injected with Ca2+ within seconds of freezing revealed more extensive fusion than in the absence of glycerol. Liposomes in various stages of fusion could be seen within μm of each other (Fig. 1G) but only very few of all Ca2+-injected vesicles that had been pre-incubated with glycerol exhibited structures resembling particles (Fig. 1H). These particles were visible at points between the vesicles where the two bilayers had achieved at least partial continuity. That this was so rare during initial fusion even in the presence of glycerol but proliferate as the suspension attains equilibrium suggests that these are stable structures and not destabilizing centers. The requirement for glycerol in eliciting lipidic particles during the rapid initial phase of fusion intimates that glycerol contributes to their formation and stabilization. A recent study by Sen et al. [33] has also produced evidence on the role of cryoprotec-tants in stabilizing the formation of lipidic particles in galactolipid membranes.
Fig. 1.
Quick-frozen vesicles. X60000. Bar = 0.2 μm. A. A 0.2 μm vesicle, quick-frozen. The diameter of the vesicles ranges from 0.08 to 0.2 μm. B. Several PC:CL vesicles fusing after being injected with 5 mM CaC1 2 (final concentration) 1 s prior to quick-freezing. The average diameter of fused vesicles was 0.4 μm. C. Two large egg PE:PS vesicles captured by quick-freezing in the process of fusion . D. Lipidic particles decorate this PC:CL vesicle incubated for 2 h in Ca2+ and glycerol. E. The particles apparent following a 2 h incubation in Ca2+/glycerol outline a cleared circle the same size (0.2 μm) as unfused vesicles. F. A few clustered particles adorn some PC:CL vesicles after a 2 h incubation in Ca2+ alone. G. The sequence of fusion events in the outer leaflet of PC:CL vesicles pre-incubated with glycerol for 2 h and then injected with Cai2+ 1 s prior to0 quick-freezing. Two vesicles on the bottom right have aggregated. At the top left, three fused vesicles remain separated by narrow necks, which widen in the adjacent vesicle. H. PC:CL vesicles incubated for 2 h in glycerol alone and then injected with Ca2+ 1 s before quick-freezing, fuse, displaying a few particles in the region of confluence. These particles embellish both P (arrow) and E fracture faces.
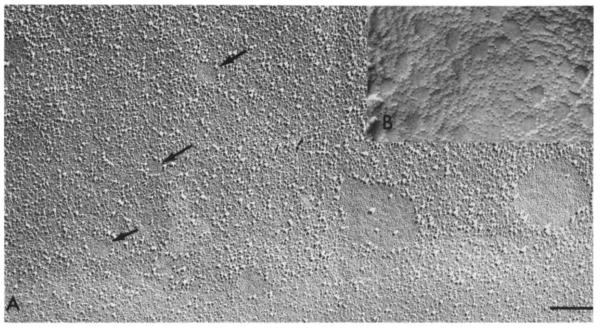
Fig. 2.
Comparison of lipidic particles and the intramembranous particles of cells. X60000 . Bar = 2 μm. A. A guinea-pig sperm cell, fixed, cryoprotected with 25% glycerol for 2 h quenched in liquid Freon 22 and frozen in liquid N2, as described [30]. Both large and small (arrows) clearings occupy the site of future sperm-egg fusion . The size of the larger intramembranous particles ringing the clearings as well as the size of the smaller clearings is similar lo the particles and clearings seen in glycerinated, Ca2+ incubated PC : CL liposomes (B).
Measurements of fluorescence emission created by the intermixing of aqueous contents (dipicolinic acid encapsulated in one population of vesicles and terbium in another) confirmed that fusion occurred in seconds, in agreement with the results of Wilschut et al. [34] on 0.1 μm diameter vesicles. The technical limitations of these two methods, quick-freeze and fluorescent emission, necessitated the use of two different concentrations of vesicles: in the fluorescence assay, the vesicle suspensions was considerably more dilute (0.05 μmol lipid/ml). However, since the rate of fusion increases with vesicle concentration [24], the quick-frozen vesicles would be expected to undergo fusion even more quickly than the more dilute preparation. As vesicles were sized to 0.2 μm before Caz+ stimulation, the presence of vesicles larger than 0.2 μmin the freeze-fracture replicas further confirmed that fusion had indeed occurred during the 1 s of stimulation.
Thus, Ca2+-induced fusion of large unilamellar vesicles can be captured by quick-freezing within seconds of stimulation. Lipidic particles, on the other hand, appear only during prolonged incuba-tions. We conclude that lipidic particles (as defined by their morphology in freeze-fracture elec-tron microscopy) are not involved as an intermediate in the stages of fusion, even in membrane types which prominently display these structures after prolonged incubations with Ca2+ or in the presence of both Ca2+ and glycerol.
This conclusion, however, does not exclude the possibility of a transitory intermediate at the site of membrane fusion which involves a lipid conformation distinguishable from the unmodified stable bilayer configuration. This ‘elusive’ intermediate, which is not visualized at present in any morphological studies, could be an inverted micellar or some other nonbilayer structure or a small domain of a more condensed or crystalline lipid bilayer. For lack of concrete evidence at this point, this ‘intermediate’ could be characterized simply as a local perturbation of the lipid bilayer structure, which allows mixing of lipid molecules between the two closely apposed membranes. Local dehydration [35] and specific contact-dependent Ca2+ complexes [36] could be the driving force for such perturbations. These intermediates would be unstable and convert with time to more stable structures, such as lipidic particles [9,10], the hexagonal (HII) phase [3,13] or the crystalline bilayer [15,35] depending on the phospholipid species.
Acknowledgements
We acknowledge the support of Public Health Service training grant GMO 7618-04 (E.B.), National Institutes of Health grant HD 10445 (D.S.F.), National Institutes of Health grant GM 28117 (D.P.) and Fellowship CA 06190 (N.D.). We are indebted to Rosamond Michael for her invaluable editing, Ivy Hsieh for technical assistance, and Susan Turner for secretarial assistance.
Abbreviations
- PE
phosphatidylethanolamine
- PC
phosphatidylcholine
- PA
phosphatidic acid
- PS
phosphatidylserine
- PG
phosphatidylglycerol
- CL
cardiolipin
- Tes
2{[2-hydroxy-I, I-bis(hydroxymethyl)ethyl]-amino}ethanesulfonicacid
References
- 1.Reiss-Husson F. J. Mol. Biol. 1967;25:363–382. doi: 10.1016/0022-2836(67)90192-1. [DOI] [PubMed] [Google Scholar]
- 2.Luzzati V, Gulik-Krzywicki T, Tardieu A. Nature. 1968;218:1031–1034. doi: 10.1038/2181031a0. [DOI] [PubMed] [Google Scholar]
- 3.Rand RP, Sengupta S. Biochim. Biophys. Acta. 1972;255:484–492. doi: 10.1016/0005-2736(72)90152-6. [DOI] [PubMed] [Google Scholar]
- 4.Papahadjopoulos D, Vail WJ, Pangborn WA, Poste G. Biochim. Biophys. Acta. 1976;448:265–283. doi: 10.1016/0005-2736(76)90241-8. [DOI] [PubMed] [Google Scholar]
- 5.Deamer DW, Leonard R, Tardieu A, Branton D. Biochim. Biophys. Acta. 1970;219:47–60. doi: 10.1016/0005-2736(70)90060-x. [DOI] [PubMed] [Google Scholar]
- 6.Cullis PR, De Kruijff B. Biochim. Biophys. Acta. 1979;559:399–420. doi: 10.1016/0304-4157(79)90012-1. [DOI] [PubMed] [Google Scholar]
- 7.Cullis PR, Verkleij AJ. Biochim. Biophys. Acta. 1979;552:546–551. doi: 10.1016/0005-2736(79)90200-1. [DOI] [PubMed] [Google Scholar]
- 8.Cullis PR, Hope MJ. Nature. 1978;271:672–674. doi: 10.1038/271672a0. [DOI] [PubMed] [Google Scholar]
- 9.Verkleij AJ, Mombers C, Leunissen-Bijvelt J, Ververgaert P.H.J.Th. Nature. 1979;279:162–163. doi: 10.1038/279162a0. [DOI] [PubMed] [Google Scholar]
- 10.Vail WJ, Stollery JG. Biochim. Biophys. Acta. 1979;551:74–84. doi: 10.1016/0005-2736(79)90354-7. [DOI] [PubMed] [Google Scholar]
- 11.De Kruijff B, Verkleij AJ, Van Echteld CJA, Gerrit-sen WJ, Mombers C, Noordam PC, De Gier J. Biochim. Biophys. Acta. 1979;555:200–209. doi: 10.1016/0005-2736(79)90160-3. [DOI] [PubMed] [Google Scholar]
- 12.Verkleij AJ, Mombers C, Gerritsen WJ, Leunissen-Bi-jvelt L, Cullis PR. Biochim. Biophys. Acta. 1979;555:358–361. doi: 10.1016/0005-2736(79)90175-5. [DOI] [PubMed] [Google Scholar]
- 13.Verkleij AJ, Van Echteld CJA, Gerritsen WJ, Cullis PR, De Kruijff B. Biochim. Biophys. Acta. 1980;600:620–624. doi: 10.1016/0005-2736(80)90465-4. [DOI] [PubMed] [Google Scholar]
- 14.Papahadjopoulos D, Vail WJ, Jacobson K, Poste G. Biochim. Biophys. Acta. 1975;394:483–491. doi: 10.1016/0005-2736(75)90299-0. [DOI] [PubMed] [Google Scholar]
- 15.Papahadjopoulos D, Vail WJ, Newton C, Nir S, Jacobson K, Poste G, Lazo T. Biochim. Biophys. Acta. 1977;465:579–598. doi: 10.1016/0005-2736(77)90275-9. [DOI] [PubMed] [Google Scholar]
- 16.Düzgüneş N, Wilschut J, Fraley R, Papahadjopou-los D. Biochim. Biophys. Acta. 1981;642:182–195. doi: 10.1016/0005-2736(81)90148-6. [DOI] [PubMed] [Google Scholar]
- 17.Düzgüneş N, Hong K, Papahadjopoulos D. In: Calcium Binding Proteins: Structure and Function. Siegel FL, Carafoli E, Kretsinger RN, Mac Lennan DH, Wasserman RH, editors. Elsevier/North-Hol-land; New York: 1980. pp. 17–22. [Google Scholar]
- 18.Tilcock CPS, Cullis PR. Biochim. Biophys. Acta. 1981;641:189–201. doi: 10.1016/0005-2736(81)90583-6. [DOI] [PubMed] [Google Scholar]
- 19.Harlos K, Eibl H. Biochemistry. 1981;20:2888–2892. doi: 10.1021/bi00513a027. [DOI] [PubMed] [Google Scholar]
- 20.Wilkinson DA, Nagle JF. Biochemistry. 1981;20:187–192. doi: 10.1021/bi00504a031. [DOI] [PubMed] [Google Scholar]
- 21.Cullis PR, De Kruijff B. Biochim. Biophys. Acta. 1976;436:523–540. doi: 10.1016/0005-2736(76)90438-7. [DOI] [PubMed] [Google Scholar]
- 22.Sundler R, Düzgüne§ N, Papahadjopoulos D. Biochim. Biophys. Acta. 1981;640:751–758. doi: 10.1016/0005-2736(81)90180-2. [DOI] [PubMed] [Google Scholar]
- 23.Heuser JE, Reese TS, Landis DM. Cold Spring Harbor Symp. Quant. Biol. 1976;40:17–24. doi: 10.1101/sqb.1976.040.01.004. [DOI] [PubMed] [Google Scholar]
- 24.Wilschut J, Düzgüne§ N, Fraley R, Papahadjopou-los D. Biochemistry. 1980;19:6011–6021. doi: 10.1021/bi00567a011. [DOI] [PubMed] [Google Scholar]
- 25.Rand RP, Reese TS, Miller RG. Nature. 1981;293:237–238. doi: 10.1038/293237a0. [DOI] [PubMed] [Google Scholar]
- 26.Szoka F, Jr., Papahadjopoulos D. Annu. Rev. Biophys. Bioeng. 1980;9:467–508. doi: 10.1146/annurev.bb.09.060180.002343. [DOI] [PubMed] [Google Scholar]
- 27.Olson F, Hunt CA, Szoka FC, Vail WJ, Papa-hadjopoulos C. Biochim. Biophys. Acta. 1979;557:9–23. doi: 10.1016/0005-2736(79)90085-3. [DOI] [PubMed] [Google Scholar]
- 28.Miller RG. Nature. 1980;287:166–167. doi: 10.1038/287166a0. [DOI] [PubMed] [Google Scholar]
- 29.Düzgüneş N, Bearer E, Papahadjopoulos D. Biophys. J. 1982;37:25a. doi: 10.1016/S0006-3495(82)84678-X. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Orci L, Perrelet A, Friend DS. J. Cell Biol. 1977;75:23–30. doi: 10.1083/jcb.75.1.23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Friend DS, Orci L, Perrelet A, Yanagimachi R. J. Cell Biol. 1977;74:561–577. doi: 10.1083/jcb.74.2.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Chandler DE, Heuser J. J. Cell Biol. 1980;86:666–674. doi: 10.1083/jcb.86.2.666. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sen A, Brain APR, Quinn PJ, Williams WP. Biochim. Biophys. Acta. 1982;686:215–224. doi: 10.1016/0005-2736(82)90115-8. [DOI] [PubMed] [Google Scholar]
- 34.Wilschut J, Holsappel M, Jansen R. Biochim. Biophys. Acta. 1982;690:297–301. doi: 10.1016/0005-2736(82)90334-0. [DOI] [PubMed] [Google Scholar]
- 35.Portis A, Newton C, Pangborn W, Papahadjopoulos D. Biochemistry. 1979;18:780–790. doi: 10.1021/bi00572a007. [DOI] [PubMed] [Google Scholar]
- 36.Ekerdt R, Papahadjopoulos D. Proc. Natl. Acad. Sci. U.S.A. 1982;79:2273–2277. doi: 10.1073/pnas.79.7.2273. [DOI] [PMC free article] [PubMed] [Google Scholar]