Abstract
Objective
To determine whether knee cartilage composition differs between African-American and Caucasian-American women at risk for Osteoarthritis using in-vivo 3 Tesla MRI T2 relaxation time measurements.
Methods
Right knee MRI studies of 200 subjects (100 African-American women, and 100 closely matched Caucasian-American women) were selected from the Osteoarthritis Initiative. Knee cartilage was segmented in the patellar (PAT), medial and lateral femoral (MF/LF), and medial and lateral tibial compartments (MT/LT)). Mean T2 relaxation time values per compartment and per whole joint cartilage were generated and analyzed spatially via laminar and grey-level co-occurrence matrix texture methods. Presence and severity of cartilage lesions per compartment were graded using a modified WORMS grading. Statistical analysis employed paired t- and McNemar testing.
Results
While African-American women and Caucasian-Americans had similar WORMS cartilage lesion scores (p=0.970), African-Americans showed significantly lower mean T2 values (~1ms difference; ~0.5SD) than Caucasian-Americans in the whole knee cartilage (p<0.001), and in the subcompartments (LF: p=0.001, MF: p<0.001, LT: p=0.019, MT: p=0.001) and particularly in the superficial cartilage layer (whole cartilage: p<0.001, LF: p<0.001, MF: p<0.001, LT: p=0.003, MT: p<0.001). T2 texture parameters were also significantly lower in the whole joint cartilage of African-Americans than in Caucasian-Americans (variance: p=0.001; contrast: p=0.018). In analyses limited to matched pairs with no cartilage lesions in a given compartment, T2 values remained significantly lower in African-Americans.
Conclusion
Using T2 relaxation time as a biomarker for the cartilage collagen network, our findings suggest racial differences in the biochemical knee cartilage composition between African-American and Caucasian-American women.
Keywords: MRI, T2 relaxation time, cartilage, knee, race
INTRODUCTION
Osteoarthritis (OA) is the most common form of arthritis and is characterized by progressive cartilage loss, osteophyte formation, subchondral bone changes, and synovitis [1]. It is a chronic musculoskeletal disorder with an increasing prevalence worldwide [2]. Estimates suggest that by the year 2020, about 59.4 million people will suffer from OA in the United States, accounting for about 18% of the population [3, 4], and similar numbers are projected for Europe [5]. OA can affect every joint, but is specifically predominant at knee, hips and hands causing substantial pain and disability [6]. Several factors have been identified that play a role in OA risk including age, gender, genetics, behavioral factors and ethnicity [7]. Among those, the risk factor ethnicity has attracted limited research attention so far, although several radiographic studies demonstrated that African-Americans and in particular African-American women showed higher prevalence of radiographic knee OA than Caucasians [8–10]. The reasons for this ethnic difference in OA development are currently unclear, but could involve ethnic differences in cartilage composition, in cartilage degradation, or in sociocultural behavior, such as different coping[11] and belief-systems[12] leading to a higher prevalence of OA in African-American women. First epidemiologic evidence evolving from the Johnston County Osteoarthritis Project suggests racial differences in cartilage composition or degradation, but further data are lacking. In this cohort, African-American women were found to have higher serum levels of cartilage oligomeric matrix protein (COMP) compared to Caucasian women [13], a glycoprotein that is predominantly synthesized in articular cartilage [14]. Another study emerging from the same population-based cohort reported differences in serum hyaluronan levels among African-American and Caucasian-Americans [15], providing further clues that the composition of cartilage might differ by race.
In the past, analysis of cartilage composition was challenging, as it required the harvesting of biological specimens during arthroscopy or in cadaveric specimens. With the advent of quantitative MRI techniques such as cartilage T2 mapping, an effective tool has emerged allowing for the non-invasive assessment of structural and biochemical cartilage composition and integrity [16]. Several studies have demonstrated that MRI T2 mapping is particularly sensitive to the cartilage water content [17], and serves in first line as a measure of collagen network integrity [18] which accounts for approximately 15–20% weight of the extracellular cartilage matrix (ECM) [19]. In contrast, T2 mapping is relatively insensitive to the change in proteoglycans content that account for about 3–6% of the weight of the ECM[19]. It has been demonstrated that cartilage damage due to degeneration of the collagen matrix is associated with elevated water content within the cartilage and therefore will increase cartilage T2 relaxation time measurements [20, 21].
Unlike standard T2 relaxation time techniques, advanced methods such as laminar [22] and texture grey-level co-ocurrence matrix (GLCM) analyses [23, 24] can be utilized to better understand the spatial and laminar distribution of T2 values within the cartilage. Cartilage T2 laminar analysis generates information on the horizontal organization of articular cartilage by averaging T2 values over a superficial layer, adjacent to the joint fluid and over a deep cartilage layer adjacent to the subchondral bone [25]. Texture analysis via grey-level co-occurrence matrices allows to extract information on the organizational relationship of neighbouring pixels by computing features such as entropy, contrast and variance [25]. Using T2 MRI relaxation time measurements of knee cartilage as a sensitive measure of cartilage collagen network, this is the first human in vivo and MRI study aiming to investigate ethnic differences in knee cartilage composition, texture and laminar structure between African-American and Caucasian women. Our study focused on women as they have a higher disease burden of OA than men [26].
We hypothesized that knees without radiographic OA in African-American women and Caucasian women would differ in their knee cartilage composition as assessed by MRI T2 relaxation time measurements. Furthermore, we hypothesized that the knee cartilage in African-American women would exhibit a different texture than knee cartilage in Caucasian-American women.
MATERIAL AND METHODS
Subjects
A subset of 200 African-American and Caucasian-American women was identified from the Osteoarthritis Initiative (OAI) incidence and progression subcohorts as illustrated in Figure 1. The OAI is a large-scale, multi-center, longitudinal cohort study which is dedicated to investigate the role of MRI-based imaging biomarkers in knee OA. “It consists of 4796 participants who either have at baseline no OA and no OA risk factors (=normal cohort), or have no OA but possess risk factors to develop OA (=incidence cohort), or experienced frequent knee symptoms in the past 12 months and have radiographic evidence of OA (progression cohort, Kellgren-Lawrence -score of ≥ 2). The OAI is set up as a huge publicly accessible repository providing collected imaging and clinical data, such as e.g. data on physical activity (PASE) or on OA risk factors for further research use [27]. The study protocol, amendments, and informed consent documentation including analysis plans were reviewed and approved by local institutional review boards.
Fig. 1.
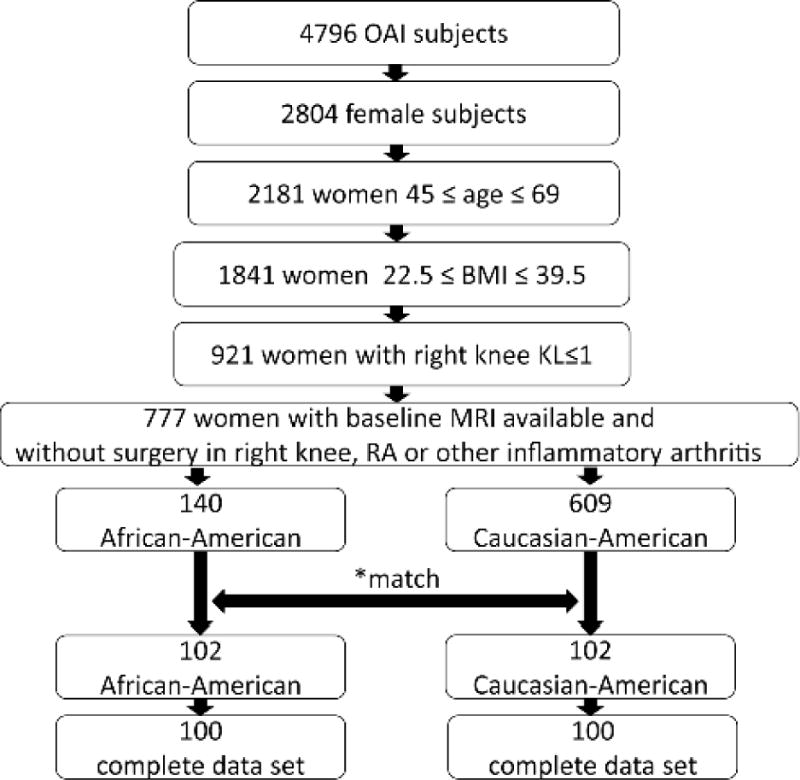
Schematic illustration showing selection of subjects. 100 African-American women and 100 Caucasian-American women fulfilled the inclusion and exclusion criteria.
* Subjects were matched by cohort, KL score, site, baseline age and by BMI strata, and randomly selected from the eligible knees in each stratum. Thirty-eight African-Americans failed to match.
To be included in the study, all women had to be non-Hispanic and had to self-report their racial background as either Black or African-American or Caucasian or White. The 45 Asians enrolled in the OAI were not included in this study. As OA prevention is most effective in younger individuals and as T2 measurements have been shown to be less useful in subjects with more advanced OA, we only included women aged less than 70 years old [28]. A body mass index between of 22.5–39.5 kg/m2 was also required. To allow for a sufficiently large sample size we included subjects with no or doubtful OA in right baseline knee radiographs as defined by Kellgren-Lawrence (KL) scores [29] of ≤1 (71% KL0 subjects, 29% KL1 subjects). The purpose of this latter inclusion criterion was to identify participants with no OA or in the very early stage of the OA disease process, when cartilage was still well-preserved and matrix imaging biomarkers, such as T2 relaxation time, could be used to measure differences in cartilage composition. Exclusion criteria comprised all women with a positive history of rheumatoid arthritis, other inflammatory arthritis, or knee surgery at the right knee. Using these inclusion and exclusion criteria, 140 African-American women were available in the overall OAI cohort. These were matched by KL grade (0 or 1), baseline age (45–54, 55–64, 65–69 years) and BMI strata (≥20–25, ≥25–30, ≥30–35, ≥35–40kg/m2), subcohort and clinical site. Caucasian-American controls were randomly selected from each stratum. 38 African-American women failed to have a Caucasian match and therefore were not included in the study. An additional two subjects could not be analyzed due to the incomplete MRI imaging data set. In total 100 subjects per group were finally studied.
Imaging
Radiographs
Baseline standing postero-anterior fixed flexion knee radiographs were acquired as described in detail in the OAI Radiographic Procedure Manual freely accessible at http://www.oai.ucsf.edu. A Plexiglas frame (SynaFlexer, CCBR-Synarc, Newark, CA, USA) was used for image acquisition. Knees were placed in 20–30° flexion and 10° internal rotation of the feet. A focus-to-film distance of 72 inches was used. All knee radiographs were graded by a central reading center for Kellgren-Lawrence (KL) scores [29, 30].
MR imaging protocol
MR images of the right knee were obtained in all subjects, using identical 3.0 Tesla scanners (Siemens Magnetom Trio, Erlangen, Germany) and quadrature transmit-receive coils (USA Instruments, Aurora, Oh, USA) at four clinical sites. A standardized [31, 32] sagittal T2 map 2-D Multi-Slice Multi-Echo (MSME) spin-echo sequence (TR 2700ms, TE1-TE7 10ms, 20ms, 30ms, 40ms, 50ms, 60ms, and 70ms, in-plane spatial resolution of 0.313mm × 0.446mm (0.313mm × 0.313mm after reconstruction), slice thickness 3.0mm, gap 0.5mm) was used for measuring T2 relaxation times. For WORMS cartilage scoring, a coronal intermediate-weighted (IW) 2D fast spin-echo (FSE) sequence (TE/T2 29/3700, flip angle 180°), a sagittal 3D dual-echo in steady state (DESS) with selective water excitation (WE) (TE/TR 4.7/16.3, flip angle 25°) and a sagittal 2D IW fat suppressed FSE sequence (FS) (TE/TR 30/3200, flip angle 180°) were analyzed. More detailed information on the OAI MRI sequence parameters can be found in Peterfy. et al.[31].
Quantitative T2 relaxation time measurements
T2 relaxation time measurements were carried out in all 200 subjects as described in detail previously [33]: Articular knee cartilage of the right knee was first segmented on MRI T2 sequences by two board certified musculoskeletal radiologists using an in-house developed, semi-automated, spline-based software implemented in MATLAB (The Mathworks Inc., Natick, MA) [34]. For each knee, five cartilage compartments consisting of the patellar, lateral/medial femoral and lateral/medial tibia cartilage were segmented on all image slices throughout the sagittal image stack in which the cartilage was clearly depictable and free of partial-volume effects. The trochlea was excluded because of interfering flow artifacts from the popliteal artery. In a second step, T2 relaxation time measurements were calculated based on the Levenberg-Marquardt algorithm [22]. This algorithm uses a mono-exponential decay model as fitting function. The first echo time was dropped as suggested by recent studies to optimize signal-to-noise ratio [35, 36]. Mean T2 relaxation time measures were generated for the cartilage of each compartment (patella, medial and lateral femur, medial and lateral tibia). In addition, a global T2 value for the overall cartilage of the joint was obtained by calculating the mean of all compartments.
Laminar and GLCM texture analysis
Cartilage laminar analysis was performed in all 200 women using in house-software that has been utilized in previous studies [22]. This technique separates the cartilage on a slice-by-slice basis into a deep layer adjacent to the bone cartilage interface and a superficial articular layer of equal thickness resulting in an average layer thickness of about 3.5 (medial tibia) to 7 pixels (patella) per layer (Figure 2). Furthermore, cartilage grey-level co-occurrence matrix (GLCM) texture analysis was performed to evaluate the spatial distribution of cartilage T2 values within each compartment, based on the method as described by Haralick et al [23]. GLCM texture parameters including variance, contrast and entropy were calculated in each cartilage region. The GLCM parameters reflect heterogeneity of T2 values throughout the cartilage matrix [20, 37]. GLCM variance indicates how much pixel values vary from the compartment mean. The higher the variance the more T2 co-occurences are dispersed from the GLCM mean. The parameter contrast is defined as the probability of finding neighbouring pixels with a large T2 difference. Therefore an elevated contrast signifies that there is a high probability of finding neighbouring pixels with large T2 differences. The GLCM parameter entropy is a measure of disorder in an image and indicates how irregular pixel pairs occur within an image. The higher the entropy the less organized an image and the rarer to find common pixel pairs [38].
Fig. 2.
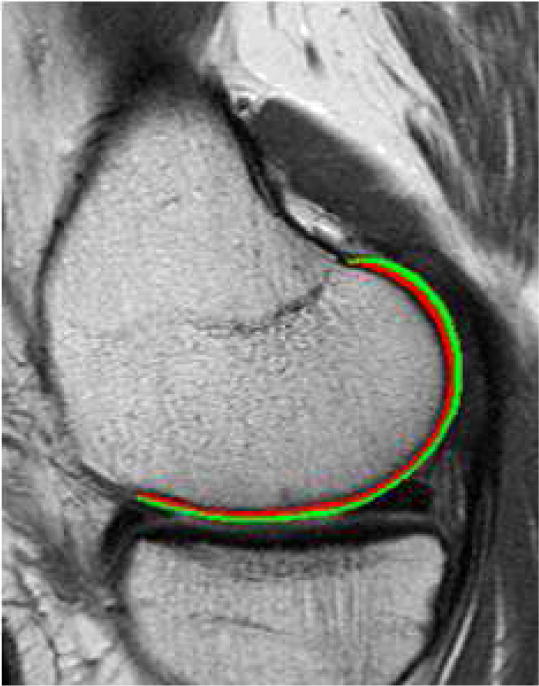
Representative example of laminar regions of interest shown for the medial femoral cartilage. The green region corresponds to the superficial layer, while the red region corresponds to the deep cartilage layer.
Whole-Organ Magnetic Resonance (WORMS) cartilage lesion scoring
MR images were evaluated independently by two board-certified radiologists for presence and severity of cartilage lesions using a semi-quantitative modified Whole-Organ Magnetic Resonance Imaging Score (WORMS) [32, 39]. In case of disagreement, a consensus reading was performed with a senior musculoskeletal radiologist with 24-years of experience (TML). Cartilage lesions were assessed in 5 compartments (patella, medial/lateral femur, and medial/lateral tibia) using an 8-point scale as outlined in Table 1.
Table 1. WORMS cartilage lesion scoring system.
adopted from Peterfy et al. and utilized to score the frequency and severity of cartilage lesions in the five knee cartilage compartments (lateral femur, medial femur lateral tibia, medial tibia and patella). Each cartilage lesion was scored on the following eight-point scale:
| 0 | normal cartilage thickness and signal intensity |
| 1 | normal cartilage thickness or swelling with abnormal signal on fluid-sensitive sequences |
| 2 | single partial-thickness focal cartilage lesion < 1 cm in greatest width |
| 2.5 | single full-thickness focal cartilage lesion < 1 cm in greatest width |
| 3 | multiple areas of partial-thickness (grade 2) cartilage lesions intermixed with areas of normal cartilage thickness or a grade 2 cartilage lesion wider than 1 cm but < 75% of the region |
| 4 | diffuse (≥75% of the region) partial-thickness cartilage loss |
| 5 | multiple areas of full-thickness loss (grade 2.5) or a grade 2.5 lesion wider than 1 cm but < 75% of the region |
| 6 | diffuse (≥75% of the region) full-thickness cartilage loss |
Statistical analysis
Statistical analysis was performed using STATA version 12 software (StataCorp LP, College Station, TX) and SPSS 20 (SPSS Inc., Chicago, IL, USA). Normal distribution of numeric variables was explored by visualization of histograms and Shapiro Wilk tests. Paired t-tests were used to assess differences in numeric variables between pairs of African-American and Caucasian-American women. To determine intergroup differences in categorical variables such as knee alignment (evaluated in physical exam via goniometer), physical activity score and OA risk factors, McNemar’s tests were used. Statistical significance was defined as p< 0.05.
We assessed interracial differences in mean cartilage T2 and in mean GLCM variance as primary outcomes. GLCM variance was chosen over other texture parameters based on the rationale that it is highly correlated with GLCM contrast [38] and has proven to be a useful and sensitive biomarker for detection of early extracellular matrix changes in patients at risk for OA [37]. As an exploratory secondary outcome and to verify our results we calculated and reported the differences between African-American and Caucasian-American cartilage T2 values in the superficial and deep cartilage layer, as well as between other texture parameters such as entropy and contrast.
For WORMS grading analysis we treated the score as a numeric outcome. As sensitivity analyses we also analyzed cartilage damage on MRI in each of the five compartments as dichotomous outcomes (first as abnormality present if WORMS grade≥2 and secondly using the more stringent definition as abnormalities present if WORMS grade ≥1). We additionally performed a sub-analysis in all matched pairs that were free of any cartilage lesion in a given compartment (WORMS=0 or 1).
Reproducibility measurements
Previous studies on T2 relaxation time measurements from our group using this segmentation technique found minimal reproducibility errors in the same dataset. For intra-reader reproducibility, the mean T2 RMS (root mean square) errors by compartment were as follows: lateral femur (LF) 1.52%, lateral tibia (LT) 1.02%, medial femur (MF) 1.18%, medial tibia (MT) 2.36%, patella (Pat) 1.19%, and mean of all compartments 1.46%. For inter-reader reproducibility, the mean T2 RMS errors by compartment were as follows: LF 1.39%, LT 1.86%, MF 1.63%, MT 1.45%, Pat 1.22%, and mean of all compartments 1.57% [34].
RESULTS
Subject characteristics
African-American women and matched Caucasian-American women exhibited comparable age, BMI, and physical activity levels and demonstrated similar knee alignment (Table 2). Both racial groups consisted to 71% of subjects without any sign of radiographic OA (KL=0). 29% of subjects of both groups had doubtful OA on knee radiographs (KL=1). WORMS cartilage lesion scoring revealed that cartilage in both racial groups had no or a very low and similar prevalence of cartilage lesions in all tibiofemoral compartments and in the whole joint cartilage (medial femur: p=0.306, lateral femur: p=0.804; medial tibia: p=0.150; lateral tibia: p=0.686, whole joint cartilage: p=0.970, WORMS grade 0, Table 3). This remained true, even when we subdivided the groups according to their WORMS cartilage grading (Total WORMS, WORMS grade 0 and 1, and WORMS grade≥ 2). Most lesions were found in the patella, but were similar in severity and frequency in African-American women and Caucasians (p=0.337). African-American women exhibited higher percentages of knee symptoms compared to Caucasian-American women (p=0.029). With respect to other OA risk factors such as history of knee injury and family history of knee replacement surgery, no differences between African-American and Caucasian-American females were detected (p>0.05).
Table 2.
Subject characteristics at time of baseline visit
| Parameter | Subjects
|
p-value | |
|---|---|---|---|
| African American n = 100 |
Caucasian American n = 100 |
||
| Ageˆ | 55.89±6.02 | 55.32±6.46 | 0.138 |
| BMIˆ | 29.20±4.02 | 29.16±3.81 | 0.816 |
| Physical Activity Score (average PASE)ˆ | 151.2±80.4 | 167.1±78.7 | 0.160 |
| Alignment* | |||
| Neither | 32(32.0) | 32/98 (32.7) | |
| Varus | 16(16.0) | 19/98 (19.4) | 0.869 |
| Valgus | 52(52.0) | 47/98 (48.0) | |
| OA risk factors* | |||
| History of knee injury | 26(26.0) | 31(31.0) | 0.423 |
| Knee symptoms in the past 12 months | 47/99(47.5) | 31/99 (31.3) | 0.029† |
| Family history of knee replacement surgery | 15/99(15.2) | 15/99(15.2) | 0.842 |
Values are given as mean ± SD
Values are number (%)
OA osteoarthritis; BMI body mass index
Statistically significant (p < 0.05)
Table 3.
WORMS cartilage lesion scoring presented for both cohorts. The cartilage was scored per compartment on an 8-point scale from 0 to 6, with 0 meaning “normal (healthy) cartilage”, to 6 representing “highly degenerated cartilage with ≥ 75% of areas with full thickness cartilage loss”.*
Results were pooled to derive an overall cartilage lesion score for the whole knee joint..
| WORMS Cartilage | Subjects
|
P-value | |
|---|---|---|---|
| African American n = 100 |
Caucasian American n = 100 |
||
| WORMS Cartilage lesion scoreˆ | |||
| Global knee joint | 3.61 [3.03–4.17] | 3.62 [3.14–4.10] | 0.970 |
| Lateral Femur | 0.32 [0.17–0.46] | 0.29 [0.16–0.42] | 0.804 |
| Lateral Tibia | 0.57 [0.39–0.77] | 0.62 [0.45–0.76] | 0.686 |
| Medial Femur | 0.58 [0.36–0.79] | 0.45 [0.28–0.61] | 0.306 |
| Medial Tibia | 0.09 [0.00–0.17] | 0.02 [−0.01–0.05] | 0.150 |
| Patella | 2.05 [1.76–2.34] | 2.26 [1.97–2.55] | 0.337 |
| WORMS Grade = 0 | |||
| Lateral Femur (n %) | 83 (100) | 80 (100) | 0.578 |
| Lateral Tibia (n %) | 64 (100) | 54 (100) | 0.157 |
| Medial Femur (n %) | 72 (100) | 74 (100) | 0.732 |
| Medial Tibia (n %) | 95 (100) | 98 (100) | 0.257 |
| Patella (n %) | 14 (100) | 9 (100) | 0.297 |
| WORMS Grade = 0 and 1 | |||
| Lateral Femur (n %) | 88 (100) | 93(100) | 0.251 |
| Lateral Tibia (n %) | 85 (100) | 89(100) | 0.394 |
| Medial Femur (n %) | 82(100) | 85(100) | 0.549 |
| Medial Tibia (n %) | 98(100) | 100 (100) | 0.157 |
| Patella (n %) | 45(100) | 39(100) | 0.396 |
| WORMS Grade ≥ 2 | |||
| Lateral Femur (n %) | 12(100) | 7(100) | 0.251 |
| Lateral Tibia (n %) | 15(100) | 11(100) | 0.394 |
| Medial Femur (n %) | 18(100) | 15(100) | 0.549 |
| Medial Tibia (n %) | 2(100) | 0(0) | 0.157 |
| Patella (n %) | 55(100) | 61(100) | 0.396 |
Data are given as mean values [95% Confidence Intervals ]
A detailed outline of the WORMS cartilage lesion scoring system is given in Table 1.
T2 measurements
African-American women exhibited significantly lower mean T2 values than Caucasian-American women in the pooled analysis of global knee cartilage (p<0.001) as well as in each compartmental analysis (lateral femur p=0.001, medial femur p<0.001, lateral tibia p=0.019, medial tibia p=0.001), except for the patella (p=0.147) (Table 4). Figure 3 shows representative color-coded sagittal T2 maps of the medial femur of an African-American women (Figure 3A) and the correspondent Caucasian-American women (Figure 3B). The sub-analysis which included only those matched African-American and Caucasian pairs without focal cartilage lesions (WORMS=0 or 1), showed similar results: mean T2 values in African-Americans were generally lower than those in Caucasians. Differences were most pronounced in the lateral femur (p=0.001), the medial femur (p=0.012), and the medial tibia (p=0.029).
Table 4.
Cartilage T2 values (in ms) and mean gray level co-occurence matrix (GLCM) parameters
| Parameter | Subjects
|
p-value | |
|---|---|---|---|
| African American n = 100 |
Caucasian American n = 100 |
||
| Mean cartilage T2* | 32.03 [31.71–32.35] | 32.86 [32.49–33.22] | <0.001 |
| LF T2 | 33.61 [33.16–34.06] | 34.54 [34.10 –34.98] | 0.001 |
| LT T2 | 27.77 [27.32–28.21] | 28.52 [27.99–29.05] | 0.019 |
| MF T2 | 36.92 [36.44–37.40] | 38.02 [37.49–38.54] | <0.001 |
| MT T2 | 28.92 [28.55–29.28] | 29.77 [29.35–30.19] | 0.001 |
| PAT T2 | 32.94 [32.44–33.44] | 33.43 [32.92–33.94] | 0.147 |
| Mean Variance* | 203.49 [196.80–210.18] | 215.78 [208.40–223.37] | 0. 001 |
| LF Variance | 191.60 [184.13–199.07] | 200.39 [192.07–208.70] | 0.051 |
| LT Variance | 144.51 [137.87–151.17] | 161.55 [152.75–170.34] | 0.001 |
| MF Variance | 257.22 [246.56–267.88] | 281.09 [269.41–292.77] | <0.001 |
| MT Variance | 197.92 [187.84–208.01] | 205.21 [195.03–215.39] | 0.259 |
| PAT Variance | 226.21 [214.91–237.50] | 230.65 [219.74–241.57] | 0.533 |
LF Lateral Femur; LT Lateral Tibia; MF Medial Tibia; MT Medial Tibia; PAT Patella
Global knee joint
p-values < 0.05 are in bold.
Data are given as means and [95% confidence intervals ]
Fig. 3.
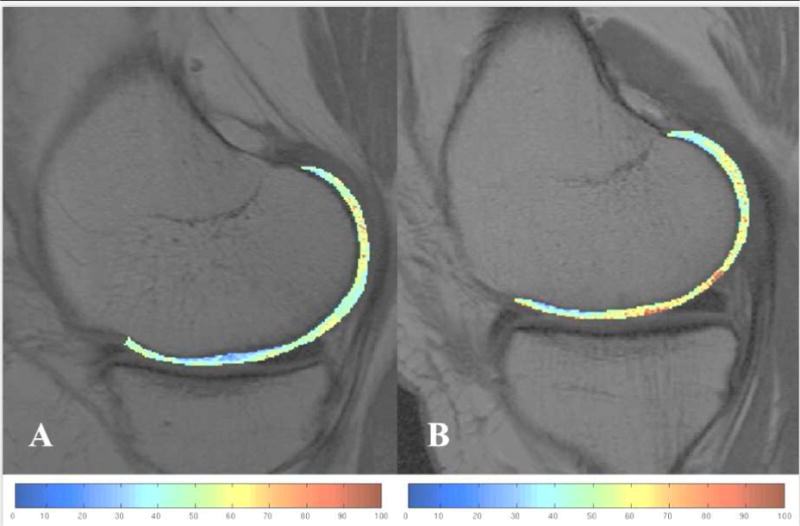
Representative color-coded sagittal T2 maps showing the segmented medial femur of an African-American subject (A) and a matched Caucasian-American subject (B). Cartilage of the African-American subject (A) shows lower T2 values.
Laminar cartilage analysis
Results of laminar superficial and deep layer analysis are displayed in Table 5. Mean superficial cartilage layer T2 values in African-American women were significantly lower than mean superficial T2 values in Caucasian-American women in each compartment and global knee joint except in the patella (lateral and medial femur, medial tibia and global knee joint: p<0.001; lateral tibia: p=0.003). The deep layer global T2 values were also significantly lower in the African-American women compared to Caucasian-American women (p=0.031). Differences were most pronounced at the deep T2 layers of the lateral femur, medial femur, and patella (lateral femur: p=0.019, medial femur: p=0.038, patella: p=0.018), while deep layer T2 values of the tibial compartments were not significantly different among both races (lateral tibia: p=0.529, medial tibia: p=0.160).
Table 5.
Cartilage T2 values (in ms) and mean gray level co-occurrence matrix (GLCM) parameters
| Parameter | Subjects
|
P-value | |
|---|---|---|---|
| African American n = 100 |
Caucasian American n = 100 |
||
| Laminar analysis | |||
| Mean Superficial Layer T2* | 34.67 [34.27–35.06] | 35.91 [35.46–36.35] | <0.001 |
| LF Superficial Layer T2 | 35.62 [35.09–36.14] | 36.94 [36.38–37.50] | <0.001 |
| LT Superficial T2 | 31.08 [30.50–31.66] | 32.35 [31.67–33.03] | 0.003 |
| MF Superficial T2 | 38.42 [37.89–38.94] | 39.93 [39.32–40.54] | <0.001 |
| MT Superficial T2 | 31.51 [30.96–32.05] | 33.48 [32.90–34.07] | <0.001 |
| PAT Superficial T2 | 36.71 [36.08–37.34] | 36.82 [36.23–37.42] | 0.776 |
| Mean Deep Layer T2* | 29.53 [29.23–29.82] | 29.93 [29.61–30.26] | 0.031 |
| LF Deep Layer T2 | 31.51 [31.07–31.95] | 32.16 [31.73–32.59] | 0.019 |
| LT Deep Layer T2 | 24.49 [24.13–24.85] | 24.63 [24.20–25.07] | 0.579 |
| MF Deep Layer T2 | 35.54 [35.00–36.07] | 36.22 [35.65–36.78] | 0.038 |
| MT Deep Layer T2 | 26.73 [26.41–27.06] | 26.41 [26.04–26.78] | 0.160 |
| PAT Deep Layer T2 | 29.37 [28.90–29.85] | 30.22 [29.72–30.73] | 0.018 |
| Texture analysis | |||
| Mean Contrast* | 281.83 [271.31–292.35] | 295.01 [283.40–306.84] | 0.018 |
| LF Contrast | 265.62 [254.67–276.58] | 274.60 [262.48–286.72] | 0.202 |
| LT Contrast | 184.02 [174.75–193.28] | 197.93 [185.18–210.69] | 0.038 |
| MF Contrast | 372.66 [354.97–390.35] | 405.57 [385.91–425.23] | <0.001 |
| MT Contrast | 291.95 [275.44–308.45] | 296.02 [279.19–312.84] | 0.703 |
| PAT Contrast | 294.90 [278.83–310.97] | 300.95 [284.16–317.73] | 0.536 |
| Mean Entropy* | 6.20 [6.17–6.23] | 6.24 [6.20–6.28] | 0.090 |
| LF Entropy | 6.59 [6.54–6.65] | 6.65 [6.60–6.70] | 0.119 |
| LT Entropy | 5.68 [5.63–5.74] | 5.77 [5.71–5.83] | 0.035 |
| MF Entropy | 6.83 [6.79–6.88] | 6.88 [6.83–6.93] | 0.113 |
| MT Entropy | 5.83 [5.79–5.88] | 5.91 [5.87–5.96] | 0.009 |
| PAT Entropy | 6.06 [6.00–6.12] | 6.00 [5.93–6.06] | 0.157 |
LF Lateral Femur; LT Lateral Tibia; MF Medial Tibia; MT Medial Tibia; PAT Patella
Global knee joint
P values < 0.05 are in bold.
Data are given as mean values [95% Confidence Intervals ]
GLCM texture analysis
With respect to GLCM texture parameters, articular knee cartilage of African-American women exhibited a more homogenous spatial distribution of T2 values than the knee cartilage of Caucasian-American women (Table 4 and 5). All three GLCM-features – contrast, variance and entropy – showed lower values in the whole joint cartilage and in all five compartments of the African-American group, except the patella, but did not always reach statistical significance. In African-American women pixel pairs appeared more regular, and therefore less entrop, in particular in the medial tibia (p=0.009) and in the lateral tibia (p=0.035). In addition, mean variance and contrast of the total joint cartilage in African-American women were also significantly lower (variance: p=0.001, contrast: p=0.018). This was in agreement with the compartimental analysis, which revealed for both features differences that were most pronounced in the medial femur (variance MF: p < 0.001; contrast MF: p < 0.001), and the lateral tibia (variance LT p=0.001; contrast LT: p=0.038).
DISCUSSION
In this study we investigated the biochemical composition of knee cartilage in African-American women and Caucasian-American women using 3 Tesla MRI T2 relaxation time measurements. Both groups were closely matched and exhibited either no or a very low and similar prevalence and severity of cartilage lesion on 3T MRI evaluation.
Our most important finding was that African-American women had significantly lower and more homogeneous mean T2 values in all compartments and the whole joint cartilage except for the patella compared to the matched Caucasian-American women. At first glance, this is somewhat surprising. We would have expected their T2 values to be higher and more heterogeneous relative to Caucasian-American women since African-American women are at increased risk for OA and previous studies have reported that elevations in the mean and heterogeneity of cartilage T2 values are indicative of early cartilage degeneration [20, 37, 40, 41].
Why T2 values in African-American women are lower and more homogenous and how these findings relate to a higher prevalence of OA in African-American women remains to be determined. Knee cartilage consists of a relatively small amount of chondrocytes that are embedded in an extracellular matrix (ECM) composed primarily of water (about 70%), collagen type II (about 25 %) and proteoglycans and underlies a regular turnover [42]. T2 relaxation time measures in the contrary are known to correlate strongly with cartilage water content [17] and to show inverse correlations with cartilage collagen content [18]. Based on these facts, lower mean T2 values in standard and laminar analysis may be the result of a higher cartilage collagen content, a lower cartilage water content or a combination of both. Supporting evidence for a higher collagen content in African-American women knee cartilage comes from the Johnston County study in which higher serum levels of cartilage-oligomeric matrix protein (COMP) were reported for African-Americans women than Caucasians[13]. As COMP is a glycoprotein that accelerates type 2 collagen fibril formation and stabilizes the collagen network[43], higher COMP serum levels in AA might therefore be reflective of higher cartilage collagen content. In this context it seems noteworthy that African-Americans have in general a higher tendency towards excessive scarring and keloid formation than Caucasians, which is mainly ascribed to an excessive collagen deposition in the dermis [44]. Further histological studies are needed to verify if collagen content in African-American knee cartilage is indeed shifted towards higher levels relative to Caucasians.
Besides from a potential higher collagen content, lower T2 values could also arise from a diminished cartilage water content. Potential mechanisms involve either a decreased water binding capacity of African-American cartilage or an increased cartilage water loss or a reduced water entry into the cartilage due to more compact collagen bundles. To date, no studies exist on the hydration status of cartilage by race. So far, lower cartilage water content has been regarded as beneficial, as increased water content and increased water mobility were linked to OA [21]. However, as cartilage is an avascular and alympathic tissue, in which nutrition and elimination of waste products are diffusion-dependent [42], one might speculate that a too dense and too water-impermeable cartilage matrix might hamper proper nutrition of chondrocytes and promote accelerated cartilage degeneration. This could potentially explain the apparent paradoxon between low and homogeneous T2 values and a high prevalence of OA in African-American women.
Besides its sensitivity to hydration and collagen content, T2 relaxation time measurements have been also attributed a strong dependence of collagen fiber orientation [16]. Unfortunately our current texture analysis generates spatially invariant results and therefore is not suited to interprete our findings in correspondence to the natural collagen fibril organization of cartilage. However, taking into account that cartilage in African-American women has lower T2 and less heterogeneity suggests the possibility that their collagen structure is more organized. In the future, more advanced texture techniques including cartilage flattening algorithms[45] should be applied to compare directional differences in texture GLCM parameters between races.
In summary, our observed differences in T2 relaxations time measurements and in texture could be explained by a higher concentration of collagen type 2 in African-American cartilage, a lower water content or differences in collagen fiber orientation. So far, it has been difficult to demonstrate differences in biochemical knee cartilage composition using histopathological studies. In fact, when we performed a literature research, we did not find any histologic study nor any other study looking at cartilage composition or cartilage collagen content in different races. To the best of our knowledge this is the first study that indicates racial differences in biochemical knee cartilage composition.
Although novel for cartilage, racial differences have been recently described for the skin as well as for musculoskeletal tissues such as bone. Girardeau and coworkers found that skin types differed morphologically and functionally in their dermal component between Caucasian and African-American individuals [46]. Also, differences in bone microarchitecture and bone mineral density were observed in African-American women compared to Caucasian women [47]. Those studies indirectly provide support for our results of T2 relaxation time measurement of knee cartilage.
While all other compartments showed significant results, differences at the patella were not demonstrated in this study. These might have been eliminated by the presence of advanced focal patellar osteoarthritic damage as demonstrated by a mean cartilage lesion score of higher than 2 in this compartment for both groups. A previous study showed an inverse correlation of longitudinal T2 changes versus baseline T2 values and morphological cartilage abnormalities, which suggests that once morphological cartilage defects occur, T2 values may be limited for evaluating further cartilage degradation [28]. This could have impacted the patella T2 values in our study.
Because compartmental T2 values do not account for the spatial distribution of T2 values within the compartments, our study also investigated laminar and texture pattern of cartilage spatial distribution of T2 values within the cartilage. When separating the cartilage into a superficial layer adjacent to the joint space and a deep layer adjacent to the bone-cartilage interface, we found in agreement with our global T2 measurements results, that superficial cartilage layer T2 values in African-American women were significantly lower than T2 values in Caucasian-Americans in each compartment and the whole knee joint cartilage except for the patella. In keeping with our global T2 findings, we also observed significantly lower T2 values in African-American women than in Caucasian-Americans in the deep layers of medial and lateral femur, patella, and whole joint cartilage, but not in the tibia. The lack of deep layer T2 differences among races in the tibia is most likely attributable to the impact of chemical shift artifacts from the bone/cartilage interface. These artifacts are most pronounced in the tibia, in particular in the T2 sequence that was analyzed here, which used a frequency encoding direction from head to foot.
Of note, African-American women experienced a greater percentage of knee symptoms compared to Caucasian-American women. This seems at first glance unexpected, as the cartilage was in both cohorts predominantly and in similar percentages lesion free and both cohorts consisted merely of fairly young and healthy subjects with no or doubtful signs of OA (KL grade ≤ 1). However, previous studies have demonstrated in line with our finding that African-Americans seem to exhibit in general a greater sensitivity to pain, a lower pain threshold and also a lower tolerance for pain compared to Caucasians[48–50]. This racial disparity in pain sensitivity was lately confirmed even in racial cohorts with present OA [51] and might be due to genetic variants in the pain μ-opioid receptor in African Americans, which alter the function and changed responsiveness to known μ-opioid receptor ligands [52].
Although African-Americans are known to have a higher prevalence of OA, we surprisingly observed similar rates of total knee replacements (TKR) in the family histories of African-American and Caucasian-American women. A decreased propensity of African-Americans to undergo TKR has long been described[53] and is still persistent[54]. Explanations include their belief systems [12], less structural and functional social support [55] and higher postoperative infection and complication rates[56]. Due to these reasons, the OA-ridden African-American ancestors of our study subjects might not have undergone TKR as frequently as ancestors from their Caucasian-American counterparts.
Our study has several limitations: First, to investigate the effect of race on knee cartilage composition, study subjects from a normal cohort are the best choice, however, the OAI normal cohort does not contain any African-American women. Second, although our rigorous matching policy allowed us to control for most of the OA-related confounders, we could not account for all factors that have been linked to the development of OA. Particularly, we lacked information on medication intake [57], bone mineral density[58], occupational history[9] or the degree of muscle weakness[59]. Third, the differences in mean T2 that we detected between the two races, appear quite subtle, but are indeed meaningful. Lastly, due to the study design it was not feasible to obtain histological correlation to prove our results. However, the detected disparities in cartilage mean T2 values between groups that were paralleled by a lack of differences in WORMS macrostructural cartilage scores make it seem worthwhile to assess the biochemical properties of cartilage in-vitro. In addition, other quantitative MRI techniques such as delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) and T1rho have been shown to be associated with the biomechanical properties of cartilage in vivo and proteoglycan content [60–64] and could be used in future studies to collect more information on racial differences of cartilage. Also, longitudinal studies may provide further insight on the effect of race on changes in knee cartilage composition. Correlation of elevated knee cartilage T2 measurements with clinical findings should be investigated and interpreted further.
In conclusion, using MRI T2 relaxation time measurements we found significant racial differences in biochemical knee cartilage composition between strictly matched African-American and Caucasian women with a similar degree of degeneration as well as in matched pairs that had no cartilage lesions. Our findings may imply the need to conduct separate analyses by race when studying prediction of outcomes by T2 relaxation time measurements and should spur further research on the evolution of OA by race over time. Moreover, once histologically confirmed, our results may have potential implications in later patient care: African-American women might need special OA therapies and also special preventive strategies that are tailored their specific cartilage needs.
Acknowledgments
Role of funding source: The study was supported by the Osteoarthritis Initiative, a public–private partnership comprising 5 NIH contracts (National Institute of Arthritis and Musculoskeletal and Skin Diseases contracts N01-AR-2-2258, N01-AR-2-2259, N01-AR-2-2260, N01-AR-2-2261, and N01-AR-2-2262) with research conducted by the Osteoarthritis Initiative Study Investigators. Private funding partners include Merck Research, Novartis Pharmaceuticals, GlaxoSmithKline, and Pfizer; the private sector funding for the Osteoarthritis Initiative is managed by the Foundation for the National Institutes of Health.
The analyses in this study were funded through the National Institute of Arthritis and Musculoskeletal and Skin Diseases grants U01-AR059507 and P50-AR060752.
The study was also funded by a Grant from Beijing High Levels of Health Technical Talent Team of Construction Project (no. 2013-3-033).
This manuscript has received the approval of the OAI Publication Committee based on a review of its scientific content and data interpretation.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Authors’ contributions
AY is the primary author and contributed to the manuscript in the following ways: conception and design, patient selection, cartilage segmentation, analysis and interpretation of data, statistical modeling, manuscript drafting and revision. UH and AY contributed equally. UH contributed to the manuscript in the conception and design, analysis and interpretation of data, statistical modeling, manuscript drafting and revision. The following authors contributed as described: MK (patient selection, cartilage segmentation, statistical analysis, interpretation of data, and revision), GBJ (statistical analysis and revision), FL and HL(patient selection), CM and MN(statistical expertise and revision), NEL(revision) and TML (study supervisor, conception and design, data analysis and interpretation, manuscript revision).
Conflict of interest
The authors declare that they have no conflict of interest.
Contributor Information
A. YU, Email: Aihong.Yu@ucsf.edu.
U. Heilmeier, Email: Ursula.Heilmeier@ucsf.edu.
M. Kretzschmar, Email: Martin.Kretzschmar@ucsf.edu.
G.B. Joseph, Email: gabby.joseph@ucsf.edu.
F. Liu, Email: fliu@psg.ucsf.edu.
H. Liebl, Email: Hans.Liebl@ucsf.edu.
C.E. McCulloch, Email: cmcculloch@epi.ucsf.edu.
M.C. Nevitt, Email: mnevitt@psg.ucsf.edu.
Nancy E. Lane, Email: nancy.lane@ucdmc.ucdavis.edu.
T.M. Link, Email: thomas.link@ucsf.edu.
References
- 1.Loeser RF, Goldring SR, Scanzello CR, Goldring MB. Osteoarthritis: a disease of the joint as an organ. Arthritis Rheum. 2012;64:1697–1707. doi: 10.1002/art.34453. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cross M, Smith E, Hoy D, Nolte S, Ackerman I, Fransen M, et al. The global burden of hip and knee osteoarthritis: estimates from the global burden of disease 2010 study. Ann Rheum Dis. 2014;73:1323–1330. doi: 10.1136/annrheumdis-2013-204763. [DOI] [PubMed] [Google Scholar]
- 3.Lawrence RC, Helmick CG, Arnett FC, Deyo RA, Felson DT, Giannini EH, et al. Estimates of the prevalence of arthritis and selected musculoskeletal disorders in the United States. Arthritis Rheum. 1998;41:778–799. doi: 10.1002/1529-0131(199805)41:5<778::AID-ART4>3.0.CO;2-V. [DOI] [PubMed] [Google Scholar]
- 4.Lawrence RC, Felson DT, Helmick CG, Arnold LM, Choi H, Deyo RA, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Woolf AD, Pfleger B. Burden of major musculoskeletal conditions. Bull World Health Organ. 2003;81:646–656. [PMC free article] [PubMed] [Google Scholar]
- 6.Hunter DJ, McDougall JJ, Keefe FJ. The symptoms of osteoarthritis and the genesis of pain. Rheum Dis Clin North Am. 2008;34:623–643. doi: 10.1016/j.rdc.2008.05.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Neogi T, Zhang Y. Epidemiology of osteoarthritis. Rheum Dis Clin North Am. 2013;39:1–19. doi: 10.1016/j.rdc.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Jordan JM, Helmick CG, Renner JB, Luta G, Dragomir AD, Woodard J, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–180. [PubMed] [Google Scholar]
- 9.Anderson JJ, Felson DT. Factors associated with osteoarthritis of the knee in the first national Health and Nutrition Examination Survey (HANES I). Evidence for an association with overweight, race, and physical demands of work. Am J Epidemiol. 1988;128:179–189. doi: 10.1093/oxfordjournals.aje.a114939. [DOI] [PubMed] [Google Scholar]
- 10.Sowers M, Lachance L, Hochberg M, Jamadar D. Radiographically defined osteoarthritis of the hand and knee in young and middle-aged African American and Caucasian women. Osteoarthritis Cartilage. 2000;8:69–77. doi: 10.1053/joca.1999.0273. [DOI] [PubMed] [Google Scholar]
- 11.Jones AC, Kwoh CK, Groeneveld PW, Mor M, Geng M, Ibrahim SA. Investigating racial differences in coping with chronic osteoarthritis pain. J Cross Cult Gerontol. 2008;23:339–347. doi: 10.1007/s10823-008-9071-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Forster FJ. Older African-Americans with osteoarthritis of the knee preferred to avoid total knee replacement surgery. Evid Based Nurs. 2005;8:32. doi: 10.1136/ebn.8.1.32. [DOI] [PubMed] [Google Scholar]
- 13.Jordan JM, Luta G, Stabler T, Renner JB, Dragomir AD, Vilim V, et al. Ethnic and sex differences in serum levels of cartilage oligomeric matrix protein: the Johnston County Osteoarthritis Project. Arthritis Rheum. 2003;48:675–681. doi: 10.1002/art.10822. [DOI] [PubMed] [Google Scholar]
- 14.Muller G, Michel A, Altenburg E. COMP (cartilage oligomeric matrix protein) is synthesized in ligament, tendon, meniscus, and articular cartilage. Connect Tissue Res. 1998;39:233–244. doi: 10.3109/03008209809021499. [DOI] [PubMed] [Google Scholar]
- 15.Elliott AL, Kraus VB, Luta G, Stabler T, Renner JB, Woodard J, et al. Serum hyaluronan levels and radiographic knee and hip osteoarthritis in African Americans and Caucasians in the Johnston County Osteoarthritis Project. Arthritis Rheum. 2005;52:105–111. doi: 10.1002/art.20724. [DOI] [PubMed] [Google Scholar]
- 16.Mosher TJ, Dardzinski BJ. Cartilage MRI T2 relaxation time mapping: overview and applications. Semin Musculoskelet Radiol. 2004;8:355–368. doi: 10.1055/s-2004-861764. [DOI] [PubMed] [Google Scholar]
- 17.Lusse S, Knauss R, Werner A, Grunder W, Arnold K. Action of compression and cations on the proton and deuterium relaxation in cartilage. Magn Reson Med. 1995;33:483–489. doi: 10.1002/mrm.1910330405. [DOI] [PubMed] [Google Scholar]
- 18.Nieminen MT, Toyras J, Rieppo J, Hakumaki JM, Silvennoinen J, Helminen HJ, et al. Quantitative MR microscopy of enzymatically degraded articular cartilage. Magn Reson Med. 2000;43:676–681. doi: 10.1002/(sici)1522-2594(200005)43:5<676::aid-mrm9>3.0.co;2-x. [DOI] [PubMed] [Google Scholar]
- 19.Meyer FA. The use of enzyme-modified tissues to study selected aspects of tissue structure and function. In: Maroudas A, Kuettner K, editors. Methods in Cartilage Research. New York: Academic Press; 1990. pp. 222–227. [Google Scholar]
- 20.Mosher TJ, Dardzinski BJ, Smith MB. Human articular cartilage: influence of aging and early symptomatic degeneration on the spatial variation of T2–preliminary findings at 3 T. Radiology. 2000;214:259–266. doi: 10.1148/radiology.214.1.r00ja15259. [DOI] [PubMed] [Google Scholar]
- 21.Armstrong CG, Mow VC. Variations in the intrinsic mechanical properties of human articular cartilage with age, degeneration, and water content. J Bone Joint Surg Am. 1982;64:88–94. [PubMed] [Google Scholar]
- 22.Carballido-Gamio J, Blumenkrantz G, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T(2) knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative. Magn Reson Med. 2010;63:465–472. doi: 10.1002/mrm.22201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Haralick R, Shanmugam K, Dinstein I. Textural features for image classification. IEEE Trans Syst Man Cybern. 1973;SMC-1:610–618. [Google Scholar]
- 24.Haralick R. Statistical and structured approaches to texture. Proceedings of the IEEE. 1979;61:786–803. [Google Scholar]
- 25.Carballido-Gamio J, Joseph GB, Lynch JA, Link TM, Majumdar S. Longitudinal analysis of MRI T2 knee cartilage laminar organization in a subset of patients from the osteoarthritis initiative: a texture approach. Magn Reson Med. 2011;65:1184–1194. doi: 10.1002/mrm.22693. [DOI] [PubMed] [Google Scholar]
- 26.Wise BL, Niu J, Yang M, Lane NE, Harvey W, Felson DT, et al. Patterns of compartment involvement in tibiofemoral osteoarthritis in men and women and in whites and African Americans. Arthritis Care Res (Hoboken) 2012;64:847–852. doi: 10.1002/acr.21606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Felson DT, Nevitt MC. Epidemiologic studies for osteoarthritis: new versus conventional study design approaches. Rheum Dis Clin North Am. 2004;30:783–797. doi: 10.1016/j.rdc.2004.07.005. vii. [DOI] [PubMed] [Google Scholar]
- 28.Jungmann PM, Kraus MS, Nardo L, Liebl H, Alizai H, Joseph GB, et al. T2 relaxation time measurements are limited in monitoring progression, once advanced cartilage defects at the knee occur: Longitudinal data from the osteoarthritis initiative. J Magn Reson Imaging. 2013;38:1415–1424. doi: 10.1002/jmri.24137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Kellgren JH, Lawrence JS. Radiological assessment of osteoarthrosis. Ann Rheum Dis. 1957;16:494–502. doi: 10.1136/ard.16.4.494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Felson DT, Niu J, Guermazi A, Sack B, Aliabadi P. Defining radiographic incidence and progression of knee osteoarthritis: suggested modifications of the Kellgren and Lawrence scale. Ann Rheum Dis. 2011;70:1884–1886. doi: 10.1136/ard.2011.155119. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Peterfy CG, Schneider E, Nevitt M. The osteoarthritis initiative: report on the design rationale for the magnetic resonance imaging protocol for the knee. Osteoarthritis Cartilage. 2008;16:1433–1441. doi: 10.1016/j.joca.2008.06.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Peterfy CG, Guermazi A, Zaim S, Tirman PF, Miaux Y, White D, et al. Whole-Organ Magnetic Resonance Imaging Score (WORMS) of the knee in osteoarthritis. Osteoarthritis Cartilage. 2004;12:177–190. doi: 10.1016/j.joca.2003.11.003. [DOI] [PubMed] [Google Scholar]
- 33.Carballido-Gamio J, Bauer JS, Stahl R, Lee KY, Krause S, Link TM, et al. Inter-subject comparison of MRI knee cartilage thickness. Med Image Anal. 2008;12:120–135. doi: 10.1016/j.media.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Stehling C, Baum T, Mueller-Hoecker C, Liebl H, Carballido-Gamio J, Joseph GB, et al. A novel fast knee cartilage segmentation technique for T2 measurements at MR imaging–data from the Osteoarthritis Initiative. Osteoarthritis Cartilage. 2011;19:984–989. doi: 10.1016/j.joca.2011.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Maier CF, Tan SG, Hariharan H, Potter HG. T2 quantitation of articular cartilage at 1.5 T. J Magn Reson Imaging. 2003;17:358–364. doi: 10.1002/jmri.10263. [DOI] [PubMed] [Google Scholar]
- 36.Smith HE, Mosher TJ, Dardzinski BJ, Collins BG, Collins CM, Yang QX, et al. Spatial variation in cartilage T2 of the knee. J Magn Reson Imaging. 2001;14:50–55. doi: 10.1002/jmri.1150. [DOI] [PubMed] [Google Scholar]
- 37.Joseph GB, Baum T, Carballido-Gamio J, Nardo L, Virayavanich W, Alizai H, et al. Texture analysis of cartilage T2 maps: individuals with risk factors for OA have higher and more heterogeneous knee cartilage MR T2 compared to normal controls–data from the osteoarthritis initiative. Arthritis Res Ther. 2011;13:R153. doi: 10.1186/ar3469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Hall-Beyer M. The GLCM Tutorial Current Version: 2.10. 2007 [Google Scholar]
- 39.Laberge MA, Baum T, Virayavanich W, Nardo L, Nevitt MC, Lynch J, et al. Obesity increases the prevalence and severity of focal knee abnormalities diagnosed using 3T MRI in middle-aged subjects–data from the Osteoarthritis Initiative. Skeletal Radiol. 2012;41:633–641. doi: 10.1007/s00256-011-1259-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Blumenkrantz G, Stahl R, Carballido-Gamio J, Zhao S, Lu Y, Munoz T, et al. The feasibility of characterizing the spatial distribution of cartilage T(2) using texture analysis. Osteoarthritis Cartilage. 2008;16:584–590. doi: 10.1016/j.joca.2007.10.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Dunn TC, Lu Y, Jin H, Ries MD, Majumdar S. T2 relaxation time of cartilage at MR imaging: comparison with severity of knee osteoarthritis. Radiology. 2004;232:592–598. doi: 10.1148/radiol.2322030976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Dijkgraaf LC, de Bont LG, Boering G, Liem RS. Normal cartilage structure, biochemistry, and metabolism: a review of the literature. J Oral Maxillofac Surg. 1995;53:924–929. doi: 10.1016/0278-2391(95)90283-x. [DOI] [PubMed] [Google Scholar]
- 43.Halasz K, Kassner A, Morgelin M, Heinegard D. COMP acts as a catalyst in collagen fibrillogenesis. J Biol Chem. 2007;282:31166–31173. doi: 10.1074/jbc.M705735200. [DOI] [PubMed] [Google Scholar]
- 44.Kelly AP. Update on the management of keloids. Semin Cutan Med Surg. 2009;28:71–76. doi: 10.1016/j.sder.2009.04.002. [DOI] [PubMed] [Google Scholar]
- 45.Schooler J, Kumar D, Nardo L, McCulloch C, Li X, Link TM, et al. Longitudinal evaluation of T1rho and T2 spatial distribution in osteoarthritic and healthy medial knee cartilage. Osteoarthritis Cartilage. 2014;22:51–62. doi: 10.1016/j.joca.2013.10.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Girardeau S, Mine S, Pageon H, Asselineau D. The Caucasian and African skin types differ morphologically and functionally in their dermal component. Exp Dermatol. 2009;18:704–711. doi: 10.1111/j.1600-0625.2009.00843.x. [DOI] [PubMed] [Google Scholar]
- 47.Putman MS, Yu EW, Lee H, Neer RM, Schindler E, Taylor AP, et al. Differences in skeletal microarchitecture and strength in African-American and white women. J Bone Miner Res. 2013;28:2177–2185. doi: 10.1002/jbmr.1953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Rahim-Williams B, Riley JL, 3rd, Williams AK, Fillingim RB. A quantitative review of ethnic group differences in experimental pain response: do biology, psychology, and culture matter? Pain Med. 2012;13:522–540. doi: 10.1111/j.1526-4637.2012.01336.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Campbell CM, Edwards RR, Fillingim RB. Ethnic differences in responses to multiple experimental pain stimuli. Pain. 2005;113:20–26. doi: 10.1016/j.pain.2004.08.013. [DOI] [PubMed] [Google Scholar]
- 50.Edwards RR, Fillingim RB. Ethnic differences in thermal pain responses. Psychosom Med. 1999;61:346–354. doi: 10.1097/00006842-199905000-00014. [DOI] [PubMed] [Google Scholar]
- 51.Cruz-Almeida Y, Sibille KT, Goodin BR, Petrov ME, Bartley EJ, Riley JL, 3rd, et al. Racial and ethnic differences in older adults with knee osteoarthritis. Arthritis Rheumatol. 2014;66:1800–1810. doi: 10.1002/art.38620. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Fortin JP, Ci L, Schroeder J, Goldstein C, Montefusco MC, Peter I, et al. The mu-opioid receptor variant N190K is unresponsive to peptide agonists yet can be rescued by small-molecule drugs. Mol Pharmacol. 2010;78:837–845. doi: 10.1124/mol.110.064188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Jordan JM. Impact of Race/Ethnicity in OA Treatment. HSS J. 2012;8:39–41. doi: 10.1007/s11420-011-9256-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Singh JA, Lu X, Rosenthal GE, Ibrahim S, Cram P. Racial disparities in knee and hip total joint arthroplasty: an 18-year analysis of national Medicare data. Ann Rheum Dis. 2014;73:2107–2115. doi: 10.1136/annrheumdis-2013-203494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Lavernia CJ, Alcerro JC, Contreras JS, Rossi MD. Ethnic and racial factors influencing well-being, perceived pain, and physical function after primary total joint arthroplasty. Clin Orthop Relat Res. 2011;469:1838–1845. doi: 10.1007/s11999-011-1841-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Ibrahim SA, Stone RA, Han X, Cohen P, Fine MJ, Henderson WG, et al. Racial/ethnic differences in surgical outcomes in veterans following knee or hip arthroplasty. Arthritis Rheum. 2005;52:3143–3151. doi: 10.1002/art.21304. [DOI] [PubMed] [Google Scholar]
- 57.Karsdal MA, Byrjalsen I, Leeming DJ, Christiansen C. Tibolone inhibits bone resorption without secondary positive effects on cartilage degradation. BMC Musculoskelet Disord. 2008;9:153. doi: 10.1186/1471-2474-9-153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Zhang Y, Hannan MT, Chaisson CE, McAlindon TE, Evans SR, Aliabadi P, et al. Bone mineral density and risk of incident and progressive radiographic knee osteoarthritis in women: the Framingham Study. J Rheumatol. 2000;27:1032–1037. [PubMed] [Google Scholar]
- 59.Thorstensson CA, Petersson IF, Jacobsson LT, Boegard TL, Roos EM. Reduced functional performance in the lower extremity predicted radiographic knee osteoarthritis five years later. Ann Rheum Dis. 2004;63:402–407. doi: 10.1136/ard.2003.007583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Kumahashi N, Tadenuma T, Kuwata S, Fukuba E, Uchio Y. A longitudinal study of the quantitative evaluation of patella cartilage after total knee replacement by delayed gadolinium-enhanced magnetic resonance imaging of cartilage (dGEMRIC) and T2 mapping at 3.0 T: preliminary results. Osteoarthritis Cartilage. 2013;21:126–135. doi: 10.1016/j.joca.2012.09.004. [DOI] [PubMed] [Google Scholar]
- 61.Buchbender C, Scherer A, Kropil P, Korbl B, Quentin M, Reichelt D, et al. Cartilage quality in rheumatoid arthritis: comparison of T2* mapping, native T1 mapping, dGEMRIC, DeltaR1 and value of pre-contrast imaging. Skeletal Radiol. 2012;41:685–692. doi: 10.1007/s00256-011-1276-2. [DOI] [PubMed] [Google Scholar]
- 62.Crema MD, Hunter DJ, Burstein D, Roemer FW, Li L, Eckstein F, et al. Association of changes in delayed gadolinium-enhanced MRI of cartilage (dGEMRIC) with changes in cartilage thickness in the medial tibiofemoral compartment of the knee: a 2 year follow-up study using 3.0 T MRI. Ann Rheum Dis. 2013 doi: 10.1136/annrheumdis-2012-203083. [DOI] [PubMed] [Google Scholar]
- 63.Nishioka H, Hirose J, Nakamura E, Oniki Y, Takada K, Yamashita Y, et al. T1rho and T2 mapping reveal the in vivo extracellular matrix of articular cartilage. J Magn Reson Imaging. 2012;35:147–155. doi: 10.1002/jmri.22811. [DOI] [PubMed] [Google Scholar]
- 64.Matzat SJ, van Tiel J, Gold GE, Oei EH. Quantitative MRI techniques of cartilage composition. Quant Imaging Med Surg. 2013;3:162–174. doi: 10.3978/j.issn.2223-4292.2013.06.04. [DOI] [PMC free article] [PubMed] [Google Scholar]


