Abstract
We have previously demonstrated that noxious stimulation of craniofacial tissues including the frontal dura reflexly evokes significant increases in neck muscle electromyographic (EMG) activity. The primary aim of this study was to determine whether purinergic receptor mechanisms may be involved in these EMG effects, and whether N-methyl-d-aspartate (NMDA) receptor processes modulate the purinergic mechanisms. Application of the P2×1, P2×3 and P2×2/3 receptor agonist α,β-methylene ATP (but not vehicle) to the dural surface evoked a significant (P<0.05) increase in ipsilateral neck EMG activity that could be suppressed by dural or intrathecal application of the selective P2×1, P2×3 and P2×2/3 receptor antagonist 2′,3′-O-(2,4,6-trinitrophenyl) ATP (TNPATP) but not by vehicle; the intrathecal application of 2-amino-5-phosphonopentanoic acid, an NMDA receptor antagonist, also significantly reduced the neck EMG activity evoked by dural application of α,β-methylene ATP. These data suggest that purinergic receptor mechanisms contribute to the increased neck activity that can be reflexly evoked by noxious stimulation of the frontal dura, and that NMDA as well as purinergic receptor mechanisms in the medulla may modulate these purinergic-related effects.
Keywords: animal pain model, dura, glutamatergic, headache, pain, purinergic
Introduction
We have previously demonstrated that application to the frontal dura of the inflammatory irritant mustard oil (MO), a small-fibre excitant and inflammatory irritant that acts through transient receptor potential cation channel, member A1 receptors, reflexly evokes significant increases in neck muscle electromyographic (EMG) activity [1]. Dural nociceptive afferent inputs to the brainstem have been implicated in mechanisms involved in migraine headache, and processes involving ATP and glutamate have been shown to be associated with some headache conditions [2–4]. It is well-documented that glutamate is critically involved in nociceptive transmission through its release from nociceptive primary afferents and its action on N-methyl-d-aspartate (NMDA) receptors in the spinal dorsal horn and in the medullary dorsal horn (MDH, which is also known as trigeminal brainstem subnucleus caudalis) [5–9]. ATP has also been implicated in nociceptive transmission through an action that may involve ionotropic (P2X) or metabotropic (P2Y) purinergic receptors. The P2X receptors are a family of ligand-gated ion channels of which there are several subtypes that form homomeric or heteromeric channels [3], and the P2 × 3 and P2 × 2/3 subtypes have especially been implicated in nociceptive mechanisms in the spinal dorsal horn and MDH [3,8–12]. Furthermore, there is evidence that ATP may regulate glutamate release via an action involving both P2X and NMDA receptor mechanisms in the spinal dorsal horn and MDH [9,11]. Both NMDA and P2X receptor mechanisms have also been shown to contribute to modulating trigeminal nociceptive afferent inputs to the MDH in the brainstem and contribute to processes underlying brainstem sensorimotor circuits [5,6,10,13 –15]. Therefore, it is possible that P2X and NMDA receptor mechanisms contribute to the reflex effects of dural stimulation on neck muscle activity. Thus, an objective of this study was to determine whether purinergic receptor mechanisms may be involved in these EMG effects by testing whether application of the P2 × 1, P2 × 3 and P2 × 2/3 receptor agonist α,β-methylene ATP (α,β-meATP) to the frontal dura evokes neck muscle EMG activity that can be blocked by dural or intrathecal (i.t.) application to the medulla of the selective P2 × 1, P2 × 3 and P2 × 2/3 receptor antagonist 2′,3′-O-(2,4,6-trinitrophenyl) ATP (TNP-ATP). We also tested whether i. t. application of 2-amino-5-phosphonopentanoic acid (APV), an NMDA receptor antagonist, can also block any neck muscle EMG activity evoked by the dural application of α,β-meATP.
Methods
Animal preparation
A total of 30 adult male Sprague–Dawley rats (Charles River, Senneville, Quebec, Canada) were used in the present study. They were housed in individual cages in a room under 12 : 12 h light/dark cycle and were allowed 1–2 weeks of adaptation (23 ± 1°C) after arrival. Water and food were available ad libitum. On the day of the experiment, the weight of the animals was between 225 and 350 g. All surgical and experimental procedures were approved by the University of Toronto Animal Care Committee, and were in accordance with the regulations of the Ontario Animal Research Act (Canada). The procedures were generally similar to those previously described in detail [1,5,15], and thus are only briefly outlined here.
Under general anaesthesia (O2:1 l/min, halothane, 1.5–2%), a tracheotomy was performed in each rat. As craniofacial noxious stimulation may evoke reflex effects in jaw, facial or tongue muscles as well as neck muscles [1,5,15], bipolar EMG electrodes were placed into neck (semispinalis), periorbital (orbicularis oculi) and jaw (masseter, anterior digastric) muscles bilaterally, as well as into the tongue (genioglossus) musculature. The right frontal dura was then exposed by using a dental drill to remove part of the skull (1–2mm from the central suture and bregma), and attention was paid to avoid damage to the superior sagittal sinus. A 27 G dental needle (45° bevel) covered with a plastic sleeve (except for the distal 10 mm, which typically was the distance between the skin at the posterior edge of the skull and surface of the caudal brainstem) was inserted into the subarachnoid space overlying the medullary component of the caudal brainstem. The needle was connected with polyethylene-10 tubing to a syringe and a 10 µl of solution of either PBS (as i.t. vehicle control), TNP-ATP or APV was applied over a 10 s period through a delivery pump; the needle was encased in a fixed length of tubing to prevent its penetration into the medulla. This procedure was similar to that described by Tsuboi et al. [16]. The injection procedure usually took less than 5 min and aspiration of cerebrospinal fluid was used to confirm the i. t. placement of the needle. At the end of each experiment, the tip of the needle was also confirmed to be located above the medullary surface by postmortem examination. Any data from rats with a misplaced needle were discarded. To confirm further the target location of the i.t. injections, 10 µl of Evans Blue dye was injected i. t. in two intact rats that were killed 5 min later. At autopsy, the dye distribution was visualized on the surface of the medulla, with the highest concentration of dye near the obex region. Each animal was artificially ventilated throughout the whole surgical and experimental period, and the heart rate, oxygen saturation and rectal temperature were continuously monitored.
Drugs
The chemicals TNP-ATP (a selective antagonist of P2 × 1, P2× 3, and P2× 2/3 receptor subtypes; catalogue number: SML0740), α,β-meATP (a selective agonist of P2 × 1, P2× 3, and P2× 2/3 receptor subtypes; catalogue number: M6517) [17] and APV (an NMDA receptor antagonist; catalogue number: A8054) [15] were purchased from Sigma-Aldrich (St Louis, Missouri, USA). α, β-meATP was freshly dissolved in normal saline (NS), and TNP-ATP and APV were freshly dissolved in PBS (catalogue number: P4417) at pH 7.4 (Sigma-Aldrich).
Experimental paradigm
Under light anaesthesia (halothane, 0.6%), the EMG activities were first recorded for 30 min and then for another 30 min after the application of the vehicle for α,β-meATP (NS, 0.2 µl, soaked in a segment of dental paper point) to the right frontal dura. Then, to test the effect of i.t. application of TNP-ATP or APV, 10 µl of TNP-ATP (0.1 mM; α,β-meATP/TNP-ATP i.t. group, n= 9) or APV (0.05 mM; meATP/APV i.t. group, n =8) or PBS (as vehicle control; α,β-meATP/PBS i.t. group, n= 6) was administered i.t., and 5 min later a segment of dental paper point soaked with α,β-meATP (0.2 µl, 100 mM) was topically applied to the frontal dura and EMG activities recorded for another 30 min. To test the effects of dural application of TNP-ATP on the EMG activities evoked by α,β-meATP, the EMG activities were first recorded for 30 min and then for another 30 min after the application of NS (0.2 µl) to the right frontal dura, and then for 30 min following the coadministration to the dura of TNP-ATP (0.2 µl, 0.1 mM, soaked in a segment of dental paper point) and α,β-meATP (0.2 µl, 100 mM, soaked in a segment of dental paper point; α,β-meATP/ TNP-ATP dural group, n= 5). The doses for these drugs were based on our earlier studies, which included dose-response studies, showing the effectiveness of these doses at behavioural, EMG and neuronal levels [5,6,10, 13–15].
Data analysis
The area under the curve (AUC) of the EMG activities of each muscle was calculated and normalized (mV × min). The normalized AUC of EMG activity before (baseline) or after NS application to the dura was the AUC of the EMG activity during the 60 s periods immediately before or after NS application. The normalized AUC of EMG activity before or after α,β-meATP application to the dura was the AUC of the EMG activity during the period of 60 s immediately before α,β-meATP application or during the period of any evoked EMG activity increase above the baseline activity plus 2 SD, respectively. The normalized AUC of evoked EMG activity of each muscle after the NS or α,β-meATP application to the dura was compared with the baseline EMG activity level before the NS or α,β-meATP application with or without i.t. TNP-ATP, APV or PBS application were analysed with paired t-tests, and P less than 0.05 was considered to reflect statistical significance. All data were expressed as mean ± SD.
Results
Effects of α,β-meATP and TNP-ATP application to the frontal dura
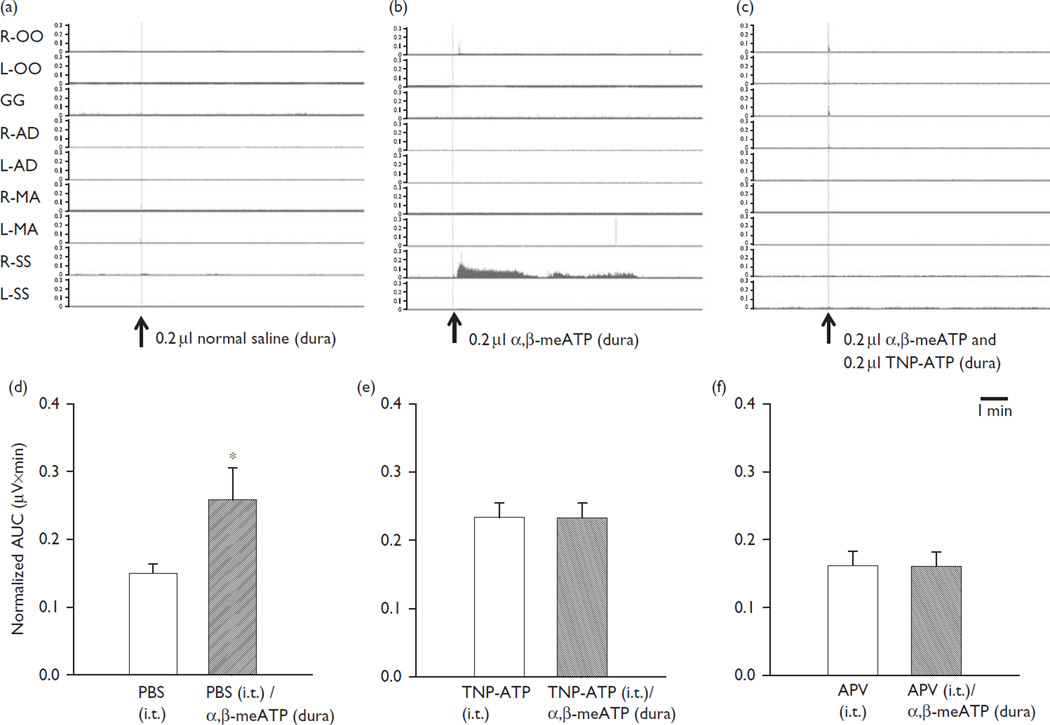
Application of vehicle (NS) to the frontal dura did not induce any significant changes (paired t-test, P > 0.05) in EMG activity in neck or any other muscle [orbicularis oculi: 0.183 ± 0.00642 (left) 0.174 ± 0.0715 (right); genioglossus: 0.106 ± 0.0355; masseter: 0.290 ± 0.0283 (left), 0.276 ± 0.0862 (right); anterior digastric: 0.360 ± 0.00931 (left); 0.223 ± 0.0563 (right); semispinalis: 0.226 ± 0.0252 (left), 0.206 ± 0.0666 mV× min (right), n =6] compared with the baseline EMG activity before NS application [orbicularis oculi: 0.184 ± 0.00744 (left); 0.198 ± 0.0855 (right); genioglossus: 0.110 ± 0.0381; masseter: 0.290 ± 0.0279 (left), 0.276 ± 0.0848 (right); anterior digastric: 0.364 ± 0.0144 (left); 0.265 ± 0.118 (right); semispinalis: 0.222 ± 0.0185 (left), 0.182 ± 0.0489 mV× min (right), n= 6] (Fig. 1a). Application of α,β-meATP to the frontal dura also did not produce any significant changes in periorbital, tongue, jaw and contralateral neck muscle EMG activities [orbicularis oculi: 0.184 ± 0.0161 (left); 0.239 ± 0.0632 (right); genioglossus: 0.110 ± 0.0340; masseter: 0.279 ± 0.0431 (left), 0.269 ± 0.0764 (right); anterior digastric: 0.357 ± 0.00555 (left); 0.214 ± 0.0676 (right); semispinalis: 0.223 ± 0.0174 mV× min (left); n= 6] compared with after application of α,β-meATP [orbicularis oculi: 0.183 ± 0.0101 (left); 0.225 ± 0.0823 (right); genioglossus: 0.112 ± 0.0291; masseter: 0.296 ± 0.0323 (left), 0.275 ± 0.0864 (right); anterior digastric: 0.359 ± 0.00443 (left); 0.268 ± 0.150 (right); semispinalis: 0.230 ± 0.0201 mV× min (left), n =6], but did evoke a significant increase (P <0.05) in ipsilateral neck muscle EMG activity [0.177 ± 0.0425 (before), 0.252 ± 0.0628 mV× min (after); n =6] at a mean (± SD) latency of 45.92 ± 25.10 s and mean duration of 423.30 ± 196.22 s, compared with the baseline EMG activity immediately before α,β-meATP application (Fig. 1b).
Fig. 1.
The effects of dural topical application of NS (0.2 µl) (a), α,β-meATP (0.2 µl) (b), α,β-meATP (0.2 µl) plus TNP-ATP (0.2 µl) (c) and i.t. PBS, TNP-ATP and APV applications on dural topical application of α,β-meATP-evoked ipsilateral neck EMG activities (d–f). (a, b and c) ‘Raw’ data before and after NS, α,β-meATP or α,β-meATP plus TNP-ATP application to the frontal dura. (d) The normalized AUC after i.t. PBS application (but before α,β-meATP application) and also after α,β-meATP application to the frontal dura. (e) The normalized AUC after i.t. TNP-ATP application (but before α,β-meATP application) and also after α,β-meATP application to the frontal dura. (f) The normalized AUC after i.t. APV application (but before α,β-meATP application) and also after α,β-meATP application to the frontal dura. Note that application of α,β-meATP to the frontal dura after i.t. PBS application evoked a significant increase in ipsilateral neck muscle EMG activity, compared with that immediately before α,β-meATP application. However, application of α,β-meATP to the frontal dura after i.t. TNP-ATP or APV application did not evoke a significant increase in ipsilateral neck muscle EMG activity, compared with that immediately before α,β-meATP application. APV, 2-amino-5-phosphonopentanoic acid; AUC, area under the curve; EMG, electromyography; GG, genioglossus muscle; i.t., intrathecal; L-AD, left anterior digastric; L-MA, left masseter; L-OO, left orbicularis oculi; L-SS, left semispinalis; α,β-meATP, α,β-methylene ATP; NS, normal saline; R-AD, right anterior digastric; R-MA, right masseter; R-OO, right orbicularis oculi; R-SS, right semispinalis; TNP-ATP, 2′,3′-O-(2,4,6-trinitrophenyl) ATP. Values reflect mean ±SD. *P<0.05.
Whereas NS application did not prevent the α,β-meATP-induced increase in ipsilateral neck EMG activity, this effect of α,β-meATP could be blocked by local application to the dura of TNP-ATP. Simultaneous application of α,β-meATP and TNP-ATP to the frontal dura (α,β- meATP/TNP-ATP dural group) did not evoke any significant EMG change (paired t-test, P > 0.05) in ipsilateral neck EMG activity (0.155 ± 0.077 mV× min, n= 5) compared with the baseline EMG activity immediately before the α,β-meATP and TNP-ATP application (0.156 ± 0.075 mV× min) (Fig. 1c).
Effects of i.t. administration of TNP-ATP or APV on EMG changes evoked by α,β-meATP application to the frontal dura
Unlike the significant increase in ipsilateral neck muscle EMG activity evoked by α,β-meATP when preceded by i.t. PBS (α,β-meATP/PBS i.t. group, n= 6) (Fig. 1d), the application of α,β-meATP to the frontal dura in the rats receiving i.t. TNP-ATP (α,β-meATP/TNP-ATP i.t. group, n =9) did not cause any significant change (P> 0.05) in ipsilateral neck EMG activity (0.233 ± 0.0214 mV× min) compared with the baseline EMG activity immediately before the α,β-meATP application (mean 0.233 ± 0.0216 mV× min) (Fig. 1e). In addition, the application of α,β-MeATP to the frontal dura in the rats receiving i.t. APV (α,β-MeATP/APV i.t. group, n= 8) was not associated with any significant change (P > 0.05) in ipsilateral neck EMG activity (0.161 ± 0.0210 mV× min) compared with the baseline EMG activity immediately before the α,β-meATP application (mean 0.162 ± 0.0212 mV× min) (Fig. 1f).
Discussion
This study has provided the first documentation that application of the P2 × 1, P2× 3 and P2 × 2/3 receptor agonist α,β-meATP to the frontal dura can evoke an increase in neck muscle EMG activity that can be blocked by the simultaneous dural application of TNPATP, a selective P2 × 1, P2× 3 and P2 × 2/3 receptor antagonist. The i.t. application to the medulla of TNPATP or the NMDA receptor antagonist APV can also block the increase in neck muscle EMG activity evoked by α,β-meATP application to the dura. Since ATP and glutamate are associated with some headache conditions [2–4] and neck muscle stiffness has been reported to occur with dura inflammation in migraine headache [18], tension-type headache [19] and meningitis [20], these novel findings suggest that some headache conditions may involve afferent inputs into the central nervous system that utilize dural purinergic processes and that are modulated by medullary purinergic and NMDA receptor mechanisms.
Involvement of P2X receptors in dura-evoked neck muscle activity
Purinergic receptors have been implicated in central neurotransmission from nociceptive primary afferent neurons, and ATP-mediated currents in sensory neurons have been shown to be mediated by both P2 × 3 and P2 × 2/3 receptors [3,17]. Their involvement in craniofacial nociceptive mechanisms is evident from several rodent studies. For example, dural and other craniofacial afferents express P2 × 3 and P2 × 2/3 receptors [13,21], and P2 × 3 receptors in particular are involved in the initiation and maintenance of central sensitization induced in MDH nociceptive neurons by MO application to the rat tooth pulp [10]. In addition, application specifically of α, β-meATP to the pulp induces nociceptive behavioural responses and MDH neuronal activity and central sensitization that can be attenuated by pulp application of TNP-ATP and also by medullary application of TNPATP [13,14]. Furthermore, i.t. superfusion over the medulla of apyrase (an ATP degrading enzyme) alone, or a combination of 1,3-dipropyl-8-cyclopentylxanthine (an adenosine A1 receptor antagonist) with apyrase, or TNPATP alone, can significantly reduce the extracellular release of glutamate induced in the MDH by application of MO to the pulp [22]. α,β-meATP also causes an increase in excitatory neurotransmission in vitro in MDH neurons that can be inhibited by the P2X receptor antagonist TNP-ATP [11]. In addition, application of α,β-meATP to the temporomandibular joint region produces a significant increase in jaw muscle EMG activity in a dose-dependent manner, and also produces nociceptive behaviour that can be significantly reduced by the coapplication of the broad spectrum P2X receptor antagonist pyridoxal-phosphate-6-azophenyl-20,40-disulphonic acid or the more selective antagonist TNP-ATP [15]. P2 × 3 receptor overexpression in the trigeminal ganglion has also been shown to be associated with ectopic mechanical allodynia in the whisker pad skin induced by complete Freund’s adjuvant injection into the lower lip [12].
These various findings collectively suggest that activation of P2 × (1,2/3,3) receptors in craniofacial tissues plays a critical role in the mediation of nociceptive behaviour and in producing central sensitization in MDH nociceptive neurons. However, until the present study, there were no physiological studies using experimental activation of dural purinergic receptors to test the possible role of purinergic receptors in dural nociceptive mechanisms, although the existence of P2X-positive trigeminal ganglion neurons innervating the dura mater has been documented [21]. It has previously been shown that activation of trigeminal afferents can evoke reflex responses in neck muscles that involve the trigeminal brainstem sensory nuclear complex including the MDH [1]. The evoked neck EMG activity documented in the present study is consistent with these earlier observations, but the present study has further shown that P2X-related afferent inputs from the dura can evoke these effects and that purinergic receptor mechanisms in the medulla can modulate these effects. This latter finding is consistent with previous evidence that MDH central sensitization and EMG activity induced by noxious craniofacial stimuli can be modulated by purinergic-related processes involving agents such as apyrase and TNP-ATP acting at P2X receptors [10,13–15].
Involvement of medullary NMDA receptor mechanisms in neck muscle activity evoked by P2X-related dural afferent inputs
NMDA receptor mechanisms have been implicated as crucial processes in the central sensitization that can be induced in the MDH and spinal dorsal horn following peripheral injury or inflammation (for review, see Coderre and colleagues [7,8]). Our finding that APV (but not PBS) application to the medulla can inhibit the α,β-meATP-evoked EMG activity also suggests the involvement of medullary NMDA receptors in the dura-evoked neck muscle activity. This finding is consistent with those of previous studies showing that noxious stimulation of tooth pulp, temporomandibular joint or masticatory muscle evokes glutamate release in the rat MDH [22] as well as jaw muscle EMG activity [5] and MDH central sensitization [5], all of which can be blocked by application of glutamatergic antagonists to the MDH. There is also evidence that P2X receptors and NMDA receptors functionally interact with each other in MDH neurons [11], that α,β-meATP acts on presynaptic afferent terminals in the MDH and spinal dorsal horn to increase glutamatergic neurotransmission [9,11], and that ATP is coreleased with neurotransmitters and gliotransmitters such as glutamate, and contributes to the development of hyperalgesia through activation of P2X receptors [3,8,13].
Possible role of P2X receptors in headache mechanisms
Purinergic receptors have been postulated to be associated with migraine and other headaches [3,4]. For example, ATP and its breakdown products adenosine 5′-monophosphate and adenosine have been suggested to mediate the vasodilatation that occurs in migraine following the initial vasospasm and subsequent hypoxia [2–4]. Furthermore, the levels of ATP increase greatly in the extracellular fluid in response to hyperexcitable cortical activity that precedes the cortical spreading depression implicated in migraine mechanisms [23]. Elevated levels of ATP have been reported to play an important role in nociceptive processes by either sensitization or activation of nociceptive afferents, including those that innervate the meningeal blood vessels and that are involved in migraine nociceptive processes [3]. As many trigeminal afferent neurons, including those that innervate the dura, express P2 × 3 receptors and also release calcitonin gene-related peptide (CGRP) [21,24], it is possible that P2 × 3 receptors are involved in the migraine mechanisms by facilitating the release of CGRP and promoting an inflammatory response. In addition, endogenous substances released during a migraine attack such as CGRP and nerve growth factor may sensitize trigeminal afferent neurons via enhanced expression and function of ATP-gated P2 × 3 receptors through diverse intracellular signalling pathways [4]. It is also noteworthy that the current study has shown the involvement of purinergic receptor mechanisms specifically in duraevoked neck muscle activity, consistent with previous findings of P2X receptors in the dura and MDH [10,11, 21], and, as noted above, of the involvement of MDH as an interneuronal site in trigeminally evoked neck muscle reflex pathways [1]. Thus, it is also possible that P2X receptor mechanisms in the dura and MDH may play an important role in neck stiffness, which is a common symptom of migraine and other types of headaches [18–20].
Technical considerations
In the present study, α,β-meATP and TNP-ATP were applied to the frontal dura by a dental point soaked in the chemical(s). The placement of the paper point could itself conceivably have caused mechanical stimulation of dural afferents, but this is unlikely as (i) there were no changes noted in any tongue, jaw or neck EMG activities following placement of a NS-dipped paper point; (ii) application of α,β-meATP evoked only an increase in ipsilateral neck EMG activity; and (iii) simultaneous application of the P2X receptor antagonist TNP-ATP blocked the α,β-meATP-evoked EMG activity. In addition, considerable care was taken to ensure there was no damage to the dura and thereby avoid the possibility that the applied drugs could have acted on the underlying cerebral cortex directly, given that there are P2X receptors in the rat cortex [25]. Furthermore, there is evidence of P2X receptors in the dura itself (see below), and thus the dura was very likely the site of action of the purinergic agents. We cannot be certain of the P2X receptor subtype(s) acted upon by α,β-meATP or TNP-ATP, both of which are selective for P2 × 1, P2× 3 and P2 × 2/3, although at much higher concentrations than used here they may also act on P2 × 2, P2× 4, or P2× 5–7 receptors [17]. Previous studies of purinergic receptor mechanisms involving dural afferents or MDH neurones have implicated especially P2 × 3 or P2 × 2/3 receptor subtypes [10, 11,21]. Another technical consideration is that drugs applied i.t. may have spread beyond the caudal medulla, but postmortem inspection confirmed the medullary location of the injection site and the distribution of Evans Blue dye on the surface of the medulla near the obex region, consistent with previous findings using the technique adopted in the present study for i.t. application of drugs to the caudal medulla [16].
Conclusion
The present findings suggest that purinergic receptor mechanisms may contribute to the increased neck activity that can be reflexly evoked by noxious stimulation of the frontal dura, and that NMDA receptor as well as purinergic mechanisms in the medulla may modulate the effects.
Acknowledgements
The authors are thankful to Susan Carter and Dr Jason Lee for their technical assistance.
This research was supported by CIHR grant MOP-4918, NIH grant DE-04786, and the Canada Research Chair program.
Footnotes
Conflicts of interest
There are no conflicts of interest.
References
- 1.Hu JW, Vernon H, Tatourian I. Changes in neck electromyography associated with meningeal noxious stimulation. J Manipulative Physiol Ther. 1995;18:577–581. [PubMed] [Google Scholar]
- 2.Baad-Hansen L, Cairns B, Ernberg M, Svensson P. Effect of systemic monosodium glutamate (MSG) on headache and pericranial muscle sensitivity. Cephalalgia. 2010;30:68–76. doi: 10.1111/j.1468-2982.2009.01881.x. [DOI] [PubMed] [Google Scholar]
- 3.Burnstock G. Purinergic mechanisms and pain – an update. Eur J Pharmacol. 2013;716:24–40. doi: 10.1016/j.ejphar.2013.01.078. [DOI] [PubMed] [Google Scholar]
- 4.Giniatullin R, Nistri A, Fabbretti E. Molecular mechanisms of sensitization of pain-transducing P2 × 3 receptors by the migraine mediators CGRP and NGF. Mol Neurobiol. 2008;37:83–90. doi: 10.1007/s12035-008-8020-5. [DOI] [PubMed] [Google Scholar]
- 5.Cairns BE, Sessle BJ, Hu JW. Temporomandibular-evoked jaw muscle reflex: role of brain stem NMDA and non-NMDA receptors. Neuroreport. 2001;12:1875–1878. doi: 10.1097/00001756-200107030-00022. [DOI] [PubMed] [Google Scholar]
- 6.Chiang CY, Park SJ, Kwan CL, Hu JW, Sessle BJ. NMDA receptor mechanisms contribute to neuroplasticity induced in caudalis nociceptive neurons by tooth pulp stimulation. J Neurophysiol. 1998;80:2621–2631. doi: 10.1152/jn.1998.80.5.2621. [DOI] [PubMed] [Google Scholar]
- 7.Coderre TJ. Spinal cord mechanisms of hyperalgesia and allodynia. In: Bushnell MC, Basbaum AI, editors. The Senses: A Comprehensive Reference. San Diego, CA: Academic Press; 2006. pp. 339–380. [Google Scholar]
- 8.Sessle BJ. Peripheral and central mechanisms of orofacial inflammatory pain. Int Rev Neurobiol. 2011;97:179–206. doi: 10.1016/B978-0-12-385198-7.00007-2. [DOI] [PubMed] [Google Scholar]
- 9.Gu JG, MacDermott AB. Activation of ATP P2X receptors elicits glutamate release from sensory neuron synapses. Nature. 1997;389:749–753. doi: 10.1038/39639. [DOI] [PubMed] [Google Scholar]
- 10.Chiang CY, Zhang S, Xie YF, Hu JW, Dostrovsky JO, Salter MW, Sessle BJ. Endogenous ATP involvement in mustard-oil-induced central sensitization in trigeminal subnucleus caudalis (medullary dorsal horn) J Neurophysiol. 2005;94:1751–1760. doi: 10.1152/jn.00223.2005. [DOI] [PubMed] [Google Scholar]
- 11.Jennings EA, Christie MJ, Sessle BJ. ATP potentiates neurotransmission in the rat trigeminal subnucleus caudalis. Neuroreport. 2006;17:1507–1510. doi: 10.1097/01.wnr.0000234740.97076.95. [DOI] [PubMed] [Google Scholar]
- 12.Yasuda M, Shinoda M, Kiyomoto M, Honda K, Suzuki A, Tamagawa T, et al. P2 × 3 receptor mediates ectopic mechanical allodynia with inflamed lower lip in mice. Neurosci Lett. 2012;528:67–72. doi: 10.1016/j.neulet.2012.08.067. [DOI] [PubMed] [Google Scholar]
- 13.Adachi K, Shimizu K, Hu JW, Suzuki I, Sakagami H, Koshikawa N, et al. Purinergic receptors are involved in tooth-pulp evoked nocifensive behavior and brainstem neuronal activity. Mol Pain. 2010;6:59. doi: 10.1186/1744-8069-6-59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cherkas PS, Dostrovsky JO, Sessle BJ. Activation of peripheral P2X receptors is sufficient to induce central sensitization in rat medullary dorsal horn nociceptive neurons. Neurosci Lett. 2012;526:160–163. doi: 10.1016/j.neulet.2012.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Watanabe T, Tsuboi Y, Sessle BJ, Iwata K, Hu JW. P2X and NMDA receptor involvement in temporomandibular joint-evoked reflex activity in rat jaw muscles. Brain Res. 2010;1346:83–91. doi: 10.1016/j.brainres.2010.05.055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Tsuboi Y, Iwata K, Dostrovsky JO, Chiang CY, Sessle BJ, Hu JW. Modulation of astroglial glutamine synthetase activity affects nociceptive behaviour and central sensitization of medullary dorsal horn nociceptive neurons in a rat model of chronic pulpitis. Eur J Neurosci. 2011;34:292–302. doi: 10.1111/j.1460-9568.2011.07747.x. [DOI] [PubMed] [Google Scholar]
- 17.Coddou C, Yan Z, Obsil T, Huidobro-Toro JP, Stojilkovic SS. Activation and regulation of purinergic P2X receptor channels. Pharmacol Rev. 2011;63:641–683. doi: 10.1124/pr.110.003129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Okuma H, Kitagawa Y, Takagi S. Clinical efficacy of rizatriptan for patients with migraine: efficacy of drug therapy for migraine accompanied by tension headache-like symptoms, focusing on neck stiffness. J Headache Pain. 2005;6:455–458. doi: 10.1007/s10194-005-0249-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Fernández-Mayoralas DM, Fernández-de-las-Peñas C, Palacios-Ceña D, Cantarero-Villanueva I, Fernández-Lao C, Pareja JA. Restricted neck mobility in children with chronic tension type headache: a blinded, controlled study. J Headache Pain. 2010;11:399–404. doi: 10.1007/s10194-010-0224-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.van de Beek D, de Gans J, Spanjaard L, Weisfelt M, Reitsma JB, Vermeulen M. Clinical features and prognostic factors in adults with bacterial meningitis. N Engl J Med. 2004;35:1849–1859. doi: 10.1056/NEJMoa040845. [DOI] [PubMed] [Google Scholar]
- 21.Staikopoulos V, Sessle BJ, Furness JB, Jennings EA. Localization of P2 × 2 and P2 × 3 receptors in rat trigeminal ganglion neurons. Neuroscience. 2007;144:208–216. doi: 10.1016/j.neuroscience.2006.09.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Kumar N, Cherkas PS, Chiang CY, Dostrovsky JO, Sessle BJ, Coderre TJ. Involvement of ATP in noxious stimulus-evoked release of glutamate in rat medullary dorsal horn: a microdialysis study. Neurochem Int. 2012;61:1276–1279. doi: 10.1016/j.neuint.2012.10.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Schock SC, Munyao N, Yakubchyk Y, Sabourin LA, Hakim AM, Ventureyra EC, Thompson CS. Cortical spreading depression releases ATP into the extracellular space and purinergic receptor activation contributes to the induction of ischemic tolerance. Brain Res. 2007;1168:129–138. doi: 10.1016/j.brainres.2007.06.070. [DOI] [PubMed] [Google Scholar]
- 24.Fabbretti E, D’Arco M, Fabbro A, Simonetti M, Nistri A, Giniatullin R. Delayed upregulation of ATP P2 × 3 receptors of trigeminal sensory neurons by calcitonin gene-related peptide. J Neurosci. 2006;26:6163–6171. doi: 10.1523/JNEUROSCI.0647-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bo X, Burnstock G. Distribution of [3H]alpha,beta-methylene ATP binding sites in rat brain and spinal cord. Neuroreport. 1994;5:1601–1604. doi: 10.1097/00001756-199408150-00015. [DOI] [PubMed] [Google Scholar]