Abstract
Objective
To examine whether there were differential rates of hepatitis C virus (HCV) incidence in injecting drug-using youths who did and did not report involvement in survival sex work.
Design
Data were derived from 2 prospective cohort studies of injecting drug users (May 1, 1996, to July 31, 2007). Analyses were restricted to HCV antibody-negative youths who completed baseline and at least 1 follow-up assessment.
Setting
Vancouver, British Columbia, Canada.
Participants
Of 3074 injecting drug users, 364 (11.8%) were youths (aged 14-24 years) with a median age of 21.3 years and a duration of injecting drug use of 3 years.
Main Exposure
Survival sex work involvement.
Main Outcome Measure
The Kaplan-Meier method and Cox proportional hazards regression were used to compare HCV incidence among youths who did and did not report survival sex work.
Results
Baseline HCV prevalence was 51%, with youths involved in survival sex work significantly more likely to be HCV antibody positive (60% vs 44%; P = .002). In baseline HCV antibody-negative youths, the cumulative HCV incidence at 36 months was significantly higher in those involved in survival sex work (68.4% vs 38.8%; P < .001). The HCV incidence density was 36.8 (95% confidence interval [CI], 24.2-53.5) per 100 person-years in youths reporting survival sex work involvement at baseline compared with 14.1 (9.4-20.3) per 100 person-years in youths not reporting survival sex work. In multivariate Cox proportional hazards analyses, survival sex work was the strongest predictor of elevated HCV incidence (adjusted relative hazard, 2.30; 95% CI, 1.27-4.15).
Conclusion
This study calls attention to the critical need for evidence-based social and structural HCV prevention efforts that target youths engaged in survival sex work.
According to recent World Health Organization estimates, more than 170 million people are infected with hepatitis C virus (HCV) worldwide,1 with injecting drug use contributing to more than 90% of new infections. The HCV itself is associated with significant morbidity and mortality1,2 and has also recently been shown to be an important biomarker for evolving human immunodeficiency virus (HIV) epidemics in injecting drug users (IDUs).3 Sharing of used syringes remains the primary mode of HCV transmission.1,2 There is also some evidence to suggest that sharing of other injection and noninjection paraphernalia, such as filters and cookers, may increase the risk of transmission.4-7
It is estimated that most HCV infections occur early in an IDU’s career due to high transmissibility of the HCV and increased risky drug use practices in young IDUs.8-11 Among street-involved youths who inject drugs, the HCV incidence ranges from 10 to 36 per 100 person-years.8,12 Although youths who inject drugs represent a crucial window for targeted prevention efforts, the epidemiologic features of HCV among this population remain poorly defined.
As a highly marginalized population, street youths experience heightened rates of homelessness, mental illness, drug-related harms, violence, sexual exploitation, and premature mortality.13,14 Recent evidence15-17 suggests significant potential for increased drug-related harm in street youths who exchange sex for survival due to heightened risk environments, poorer access to services, and increased concurrency of sex and drug use partners. Research in female and transgender sex workers in Vancouver, British Columbia, Canada, has demonstrated that enforcement of criminalized prostitution legislation, including prohibitions on soliciting in public spaces, displaces younger sex workers to more isolated spaces away from health services and syringe exchange programs,18 reduces sex workers’ ability to negotiate condom use,19 and increases the odds of physical and sexual violence.20 Given the rapid acquisition of HCV and the urgent need to identify highly susceptible subgroups of youths and new injecting initiates,8,11,12 coupled with growing concern of harms in young IDUs who exchange sex, we sought to prospectively examine the HCV incidence during a 10-year period in youths who did and did not report involvement in survival sex work.
METHODS
Data were derived through a collaboration between 2 prospective cohort studies of IDUs in Vancouver: (1) the Vancouver Injection Drug Users Study (VIDUS), an ongoing open prospective cohort study initiated in 1996 through snowball sampling methods and targeted outreach at local services (eg, syringe exchange programs),21 and (2) the Scientific Evaluation of Supervised Injecting (SEOSI) cohort, an ongoing open prospective cohort study initiated in 2003 through random sampling methods from Vancouver’s supervised injecting facility.22 Both studies involved HCV antibody testing and interviewer-administered questionnaires at baseline and semiannual follow-up. As previously reported,23,24 the follow-up procedures and questionnaire items were identical in both studies to allow for merging of the data sets. Both studies received ethical approval from the University of British Columbia/Providence Healthcare Research Ethics Board. Analyses were restricted to youths (aged 14-24 years) recruited between May 1, 1996, and July 31, 2007. The definition of youth was based on Centers for Disease Control and Prevention guidelines for HCV prevention.25 For these analyses, and consistent with previous work,23,24 youths who were recruited into both cohorts were retained only in the cohort in which they were first enrolled. Because these 2 cohorts share research office space and outreach staff, there were no cases in which youths were lost to follow-up in one study but maintained in the other.
The HCV incidence rates were compared in youths who did and did not report involvement in survival sex work. Survival sex work was defined as exchanging sex for money, drugs, shelter, or other commodities in the previous 6 months.8 Analyses were restricted to youths who were HCV antibody negative at baseline and who had at least 1 follow-up visit. Variables considered based on an a priori-defined statistical protocol for HCV incidence were age; sex; ethnicity; unstable housing; residency in the IDU epicenter (Vancouver’s Downtown Eastside); daily heroin, cocaine, and crystal methamphetamine injection; daily crack cocaine smoking; receptive syringe sharing; and unprotected sex (inconsistent condom use for vaginal or anal sex). Given evidence of an elevated burden of HIV infection in individuals of Aboriginal ancestry,23 ethnicity was categorized as self-identified as Aboriginal vs non-Aboriginal. All variable definitions were consistent with those used in earlier studies21,23 and refer to the 6 months before the interview.
Kaplan-Meier Analyses
Cumulative HCV incidence was calculated for youths who did and did not report survival sex work at baseline. As previously noted,8 the date of HCV seroconversion was estimated to be the midpoint between the last negative and the first positive antibody test results. Participants who remained persistently HCV seronegative were right censored at the time of their most recent available HCV antibody test result before July 31, 2007. Time zero for all prospective analyses was the date of recruitment into the respective cohorts.
Cox Proportional Hazards Regression Analyses
Unadjusted and adjusted relative hazards (RHs) of HCV seroconversion were calculated using Cox proportional hazards regression. All variables, including survival sex work, were treated as time-updated covariates on the basis of semiannual follow-up data. Kuyper et al26,27 demonstrated that less than 5% of IDUs engaged in sex work in this cohort had ceased sex work at a subsequent follow-up visit, suggesting that sex work is a persistent resource acquisition strategy for this population. For the multivariate model, a fixed model was built that adjusted for all variables that retained statistical significance at P < .05 in unadjusted analyses and for cohort of recruitment. Given the concern of differences in risk for HCV acquisition by age, we conducted stratified models by age group (14-19 years and 20-24 years) to calculate RHs of HCV seroconversion using Cox proportional hazards regression.
RESULTS
During the study, 3074 IDUs were recruited into either the VIDUS or the SEOSI, of whom 364 (11.8%) met the Centers for Disease Control and Prevention definition of youth (14-24 years old).25 No differences were noted in the demographic characteristics or risk factors between youths included in the analyses and those lost to follow-up. Baseline HCV prevalence was 51%. Youths reporting survival sex work at baseline were significantly more likely to have positive HCV test results compared with those not reporting survival sex work (60% vs 44%, P = .002). Median participant age was 21.3 years (interquartile range, 19.5-22.7 years), with no differences in age by survival sex work involvement (P = .35). No differences in survival sex work were observed by sex, consistent with earlier work26,27 demonstrating close to identical risk factors for survival sex work in male and female IDUs in this cohort.
Of the 179 youths who were HCV negative at baseline, 127 (70.9%) had at least 1 follow-up visit. As of July 31, 2007, 56 HCV seroconversions were observed, yielding an incidence density of 20.1 (95% confidence interval [CI], 15.2-26.1) per 100 person-years. There was no difference in duration of IDU by HCV seroconversion (P = .19).
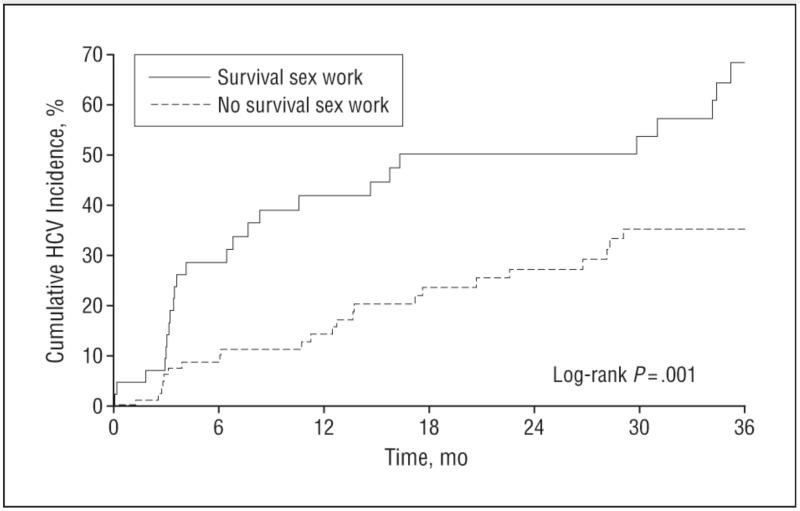
The Kaplan-Meier cumulative HCV incidence after 36 months of follow-up was 68.4% in those who reported survival sex work at baseline compared with 38.8% in those who did not (log-rank P < .001) (Figure). As of July 31, 2007, the HCV incidence density in youths engaged in survival sex work at baseline was 36.8 (95% CI, 24.2-53.5) per 100 person-years compared with 14.1 (9.4-20.3) per 100 person-years in those not engaged in survival sex work.
Figure 1. Cumulative hepatitis C virus (HCV) incidence in young injecting drug users (14-24 years old) by survival sex work involvement at baseline in Vancouver, British Columbia, Canada, 1996-2007.
In unadjusted Cox regression analyses, the RH of HCV seroconversion for youths engaged in survival sex work was 3.04 (95% CI, 1.73-5.32) (Table 1). In analyses stratified by age group, the RH of HCV seroconversion for youths aged 14 to 19 years engaged in survival sex work was 2.94 (95% CI, 1.23-7.05) and for youths aged 20 to 24 years was 2.94 (1.28-6.74) (Table 2). In the final model, after adjustment for all variables significant at P < .05 in univariate analyses and for cohort of recruitment, survival sex work (RH = 2.30; 95% CI, 1.27-4.15) and receptive syringe sharing (1.80; 1.00-3.24) remained significantly associated with time to HCV infection (Table 1).
Table 1. Cox Proportional Hazards Regression Analysis of Time to HCV Infection in Young IDUs (Aged 14-24 Years) in Vancouver, British Columbia, Canada.
| Relative Hazard (95% Confidence Interval) |
||
|---|---|---|
| Unadjusted | Adjusteda | |
| Age | 0.94 (0.84-1.05) | NA |
| Female sex | 1.39 (0.79-2.45) | NA |
| Aboriginal ethnicity | 0.91 (0.45-1.82) | NA |
| Survival sex workb | 3.04 (1.73-5.32) | 2.30 (1.27-4.15) |
| Unstable housingb | 1.99 (1.10-3.59) | 1.34 (0.72-2.51) |
| Residing in IDU epicenterb | 2.53 (1.42-4.49) | 1.75 (0.93-3.26) |
| Unprotected sex (inconsistent condom use)b |
0.98 (0.56-1.70) | NA |
| Receptive syringe sharingb | 2.02 (1.14-3.57) | 1.80 (1.00-3.24) |
| Daily heroin injectionb | 1.99 (1.12-3.55) | 1.34 (0.73-2.45) |
| Daily cocaine injectionb | 2.18 (1.18-4.02) | 1.52 (0.80-2.86) |
| Daily crystal methamphetamine injectionb |
0.45 (0.06-3.26) | NA |
| Daily crack cocaine smokingb | 1.28 (0.71-2.33) | NA |
Abbreviations: HCV, hepatitis C virus; IDU, injecting drug user; NA, not applicable.
Adjusted for cohort of recruitment and variables significanPt at .05 (ie, unstable housing, residing in an IDU epicenter, daily heroin injection, and daily cocaine injection).
Refers to behaviors and patterns in the past 6 months. All analyses were restricted to HCV-negative youths (n = 179) who had at least 1 follow-up visit (n = 127).
Table 2. Relative Hazards of Time to HCV Infection in Young IDUs Stratified by Age Group.
| Unadjusted Relative Hazard (95% Confidence Interval) |
||
|---|---|---|
| Youths Aged 14 - 19 y |
Youths Aged 20 - 24 y |
|
| Age | 1.17 (0.78-1.75) | 0.91 (0.65-1.28) |
| Female sex | 0.63 (0.26-1.57) | 2.11 (0.96-4.67) |
| Aboriginal ethnicity | 0.44 (0.10-1.87) | 1.61 (0.69-3.74) |
| Survival sex worka | 2.94 (1.23-7.05) | 2.94 (1.28-6.74) |
| Unstable housinga | 2.14 (0.88-5.21) | 1.46 (0.63-3.36) |
| Residing in IDU epicentera | 2.09 (0.86-5.04) | 4.06 (1.59-10.36) |
| Unprotected sex (inconsistent condom use)a |
1.67 (0.70-3.98) | 0.80 (0.36-1.79) |
| Receptive syringe sharinga | 2.75 (1.07-7.10) | 1.75 (0.76-4.02) |
| Daily heroin injectiona | 1.32 (0.52-3.37) | 1.90 (0.85-4.25) |
| Daily cocaine injectiona | 1.23 (0.47-3.20) | 3.00 (1.19-7.40) |
| Daily crystal methamphetamine injectiona |
1.21 (0.16-9.19) | 0.38 (0.05-2.80) |
| Daily crack cocaine smokinga | 0.85 (0.29-2.54) | 1.52 (0.67-3.47) |
Abbreviations: HCV, hepatitis C virus; IDU, injecting drug user
Refers to behaviors and patterns in the past 6 months. All analyses were restricted to HCV-negative youths (n= 179) who had at least 1 follow-up visit (n = 127).
COMMENT
This study demonstrates a drastically elevated HCV incidence in youths engaged in survival sex work in a large Canadian city. Of particular concern, the cumulative HCV incidence reached 50% at 18 months of follow-up and close to 70% after 36 months of follow-up in HCV antibody-negative youths involved in survival sex work. Although there is conflicting evidence regarding the potential for sexual HCV transmission, it remains a relatively ineffective mode of transmission.28 Instead, the elevated HCV incidence in youths engaged in survival sex work herein likely reflects differing social networks or risk environment of injection whereby barriers to accessing clean syringes or HCV prevalence in those lending syringes is higher. For example, ubiquitous interpersonal relationships among IDUs, including the formation of drug-using sexual partnerships and the collusion of money for drug procurement with sex partners, have been shown to elevate the HCV incidence.12 Evidence suggests that drug-using youths involved in survival sex work have even greater overlap in their sexual and drug use networks and are more likely to have older intimate partners who control access to and preparation of drugs, thereby reducing their ability to negotiate safer injecting practices.29-34 These findings warrant consideration of HCV interventions that target sexual partnerships and transactions as key sites of drug risk management in youths.31
This study offers the first longitudinal analysis demonstrating survival sex as a primary risk factor for HCV acquisition in drug-using youths. Of concern given the established synergistic link between HIV and HCV epidemics,3 the Bush administration’s legal ban on access to global HIV research and intervention funding targeting individuals engaged in sex work, known as the “antiprostitution pledge,” was retained in Congress’ renewal of the President’s Emergency Plan for AIDS Relief in August 2008.31 The failure to base public health policy on scientific evidence continues to challenge prevention and resource capacity targeting blood-borne transmission in vulnerable youths.35,36 Furthermore, although growing evidence demonstrates the critical role of social and structural interventions in promoting risk reduction in sex workers in developing country settings and indoor sex work establishments,15,37,38 there continues to be limited evaluation of these strategies in street-involved sex workers and those in developed country settings. Evidence-based strategies to mitigate harms among sex workers, supported by World Health Organization best practices,15,37,39 include the removal of criminal sanctions that target sex workers and clients, structural support for peer sex work networks that regulate safer industry practices (eg, occupational health and safety standards), and safer-environment interventions, such as managed indoor spaces and regulated non-harassment zones close to health and harm reduction services.
The present longitudinal evaluation of HCV incidence extends earlier trends observed in younger adult IDUs (18-30 years old) between 2000 and 200112 and in older, longer-term IDUs.40 A preliminary HCV incidence study8 (1996-2000) in young IDUs in the VIDUS cohort found no relationship with engagement in survival sex work in this setting. Similarly, although survival sex work predicted HIV acquisition in earlier unadjusted analyses, we found no significant relationship in the final model.41 Subsequent longitudinal follow-up during an additional 7 years supported by the merging of 2 IDU cohorts has enabled further prospective analyses of factors that predict HCV seroconversion in street-involved youths who inject drugs.
Several limitations need to be considered. First, self-reported practices, particularly stigmatized behaviors, may be subject to social desirability bias. However, any underestimation of survival sex work would likely have attenuated the effect size toward the null. Second, although there are always concerns of generalizability because of the inability to recruit a random sample of IDUs, sample demographics between the cohorts were consistent with the general IDU population in Vancouver.23,24 Third, differing recruitment methods and times of initiation were used to derive the VIDUS and SEOSI cohorts,21,22 and there is a possibility that risk factors for HCV changed across time. Consistent with earlier work,23,24 we sought to address this by adjusting for cohort of recruitment. Fourth, our reliance on HCV antibody testing may have overestimated rates of chronic HCV infection. Fifth, it is possible that in some cases, HCV RNA was present at the visit before antibody seroconversion due to the 60-day delay in detectable HCV RNA antibody. If this were the case, time to HCV incidence would have been shorter than we reported. However, there is no reason to suggest that either of these cases would be different for youths who do and do not exchange sex.
In summary, this study demonstrates markedly elevated baseline HCV prevalence and subsequent HCV incidence in youths involved in survival sex work. Given the brief window of opportunity for HCV prevention in highly susceptible youths, this study calls attention to a critical need for evidence-based social and structural prevention, including evaluation of legal policy reforms, that targets youths engaged in survival sex work and suggests that legal barriers to intervention be reconsidered.31
Acknowledgments
Funding/Support:
The SEOSI was supported by the Canadian Institutes of Health Research (CIHR) grant HPR-85526, Vancouver Coastal Health, and Health Canada. The VIDUS is supported by National Institutes of Health (NIH) grant R01 DA011591 and CIHR grant HHP-67262. Dr Kerr was supported by a CIHR New Investigator Award and a Michael Smith Foundation for Health Research (MSFHR) Scholar Award. Mr Marshall was supported by a CIHR Canada Graduate Research Award and an MSFHR Senior Graduate Trainee Award. Dr Tyndall was supported by an MSFHR Senior Scholar Award. Dr Montaner was supported by an Avant-Garde Award from the National Institute on Drug Abuse.
Role of the Sponsor:
The various sponsors had no role in the design and conduct of the study; in the collection, analysis, and interpretation of the data; or in the preparation, review, or approval of the manuscript.
Footnotes
Author Contributions:
Dr Wood had full access to all the data in the study and takes responsibility for the integrity of the data and the accuracy of the data analysis. Study concept and design: Shannon, Kerr, and Wood. Acquisition of data: Kerr and Wood. Analysis and interpretation of data: Shannon, Kerr, Marshall, Li, Zhang, Strathdee, Tyndall, Montaner, and Wood. Drafting of the manuscript: Shannon.Critical revision of the manuscript for important intellectual content: Shannon, Kerr, Marshall, Li, Zhang, Strathdee, Tyndall, Montaner, and Wood. Statistical analysis: Marshall, Li, and Zhang. Obtained funding: Kerr, Tyndall, Montaner, and Wood. Study supervision: Kerr, Strathdee, Tyndall, Montaner, and Wood.
Financial Disclosure:
Dr Montaner has received educational grants from, has served as an ad hoc advisor to, or has spoken at various events sponsored by Abbott Laboratories, Agouron Pharmaceuticals Inc, Boehringer Ingelheim Pharmaceuticals Inc, Borean Pharma AS, Bristol-Myers Squibb, DuPont Pharma, Gilead Sciences, GlaxoSmithKline, Hoffmann-La Roche, Immune Response Corp, Incyte, Janssen-Ortho Inc, Kucera Pharmaceutical Co, Merck Frosst Laboratories, Pfizer Canada Inc, Sanofi Pasteur, Shire Biochem Inc, Tibotec Pharmaceuticals Ltd, and Trimeris Inc.
Disclaimer:
The views expressed herein do not represent the official policies of Health Canada.
Additional Contributions: We thank the VIDUS and SEOSI participants for their willingness to be included in this study and current and past VIDUS and SEOSI investigators and staff; Deborah Graham, LLM; Tricia Collingham, BSc; Caitlin Johnston, BA; Steve Kain, RN; and Calvin Lai, MMath, PStat, for their research and administrative assistance; and the staff of Insite, the Portland Hotel Society, and Vancouver Coastal Health (Chris Buchner, MHA, David Marsh, MD, CCSAM, and Heather Hay, RN, MScA).
REFERENCES
- 1.World Health Organization [Accessed January 18, 2009];Hepatitis C fact sheet. http://www.who.int/mediacentre/factsheets/fs164/en.
- 2.Shepard CW, Finelli L, Alter MJ. Global epidemiology of hepatitis C virus infection. Lancet Infect Dis. 2005;5(9):558–567. doi: 10.1016/S1473-3099(05)70216-4. [DOI] [PubMed] [Google Scholar]
- 3.Des Jarlais DC, Arasteh K, McKnight C, Hagan H, Perlman D, Friedman SR. Using hepatitis C virus and herpes simplex virus-2 to track HIV among injecting drug users in New York City [published online ahead of print December 23, 2008] Drug Alcohol Depend. 2009;101(1-2):88–91. doi: 10.1016/j.drugalcdep.2008.11.007. [DOI] [PubMed] [Google Scholar]
- 4.Thiede H, Hagan H, Campbell JV, et al. DUIT Study Team Prevalence and correlates of indirect sharing practices among young adult injection drug users in five U.S. cities. Drug Alcohol Depend. 2007;91(suppl 1):S39–S47. doi: 10.1016/j.drugalcdep.2007.03.001. [DOI] [PubMed] [Google Scholar]
- 5.Tortu S, McMahon JM, Pouget ER, Hamid R. Sharing of noninjection drug-use implements as a risk factor for hepatitis C. Subst Use Misuse. 2004;39(2):211–224. doi: 10.1081/ja-120028488. [DOI] [PubMed] [Google Scholar]
- 6.Hagan H, Thiede H, Weiss NS, Hopkins SG, Duchin JS, Alexander ER. Sharing of drug preparation equipment as a risk factor for hepatitis C. Am J Public Health. 2001;91(1):42–46. doi: 10.2105/ajph.91.1.42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.De P, Roy E, Boivin J, Cox C, Morissette C. Risk of hepatitis C virus transmission through drug preparation equipment: a systematic and methodological review. J Viral Hepat. 2008;15(4):279–292. doi: 10.1111/j.1365-2893.2007.00942.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Miller CL, Johnston C, Spittal PM, et al. Opportunities for prevention: hepatitis C prevalence and incidence in a cohort of young injection drug users. Hepatology. 2002;36(3):737–742. doi: 10.1053/jhep.2002.35065. [DOI] [PubMed] [Google Scholar]
- 9.Hagan H, Thiede H, Des Jarlais DC. Hepatitis C virus infection among injection drug users: survival analysis of time to seroconversion. Epidemiology. 2004;15(5):543–549. doi: 10.1097/01.ede.0000135170.54913.9d. [DOI] [PubMed] [Google Scholar]
- 10.Maher L, Jalaludin B, Chant K, et al. Incidence and risk factors for hepatitis C seroconversion in injecting drug users in Australia. Addiction. 2006;101(10):1499–1508. doi: 10.1111/j.1360-0443.2006.01543.x. [DOI] [PubMed] [Google Scholar]
- 11.Judd A, Hickman M, Jones S, et al. Incidence of hepatitis C virus and HIV among new injecting drug users in London: prospective cohort study. BMJ. 2005;330(7481):24–25. doi: 10.1136/bmj.38286.841227.7C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Hahn JA, Page-Shafer K, Lum PJ, et al. Hepatitis C virus seroconversion among young injection drug users: relationships and risks. J Infect Dis. 2002;186(11):1558–1564. doi: 10.1086/345554. [DOI] [PubMed] [Google Scholar]
- 13.Miller CL, Spittal PM, LaLiberte N, et al. Females experiencing sexual and drug vulnerabilities are at elevated risk for HIV infection among youth who use injection drugs. J Acquir Immune Defic Syndr. 2002;30(3):335–341. doi: 10.1097/00126334-200207010-00010. [DOI] [PubMed] [Google Scholar]
- 14.Roy E, Haley N, Leclerc P, Cédras L, Blais L, Boivin JF. Drug injection among street youths in Montreal: predictors of initiation. J Urban Health. 2003;80(1):92–105. doi: 10.1093/jurban/jtg092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rekart ML. Sex-work harm reduction. Lancet. 2005;366(9503):2123–2134. doi: 10.1016/S0140-6736(05)67732-X. [DOI] [PubMed] [Google Scholar]
- 16.Strathdee SA, Philbin MM, Semple SJ, et al. Correlates of injection drug use among female sex workers in two Mexico-U.S. border cities. Drug Alcohol Depend. 2008;92(1-3):132–140. doi: 10.1016/j.drugalcdep.2007.07.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Goodyear MD, Cusick L. Protection of sex workers. BMJ. 2007;334(7584):52–53. doi: 10.1136/bmj.39087.642801.BE. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shannon K, Rusch M, Shoveller J, Alexson D, Gibson K, Tyndall MW, Maka Project Partnership Mapping violence and policing as an environmental-structural barrier to health service and syringe availability among substance-using women in street-level sex work. Int J Drug Policy. 2008;19(2):140–147. doi: 10.1016/j.drugpo.2007.11.024. [DOI] [PubMed] [Google Scholar]
- 19.Shannon K, Strathdee SA, Shoveller J, Rusch M, Kerr T, Tyndall MW. Structural and environmental barriers to condom use negotiation with clients among female sex workers: implications for HIV-prevention strategies and policy. Am J Public Health. 2009;99(4):659–665. doi: 10.2105/AJPH.2007.129858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Shannon K, Kerr T, Strathdee SA, Shoveller J, Montaner JS, Tyndall MW. Prevalence and structural correlates of gender based violence among a prospective cohort of female sex workers. BMJ. 2009;339:b2939. doi: 10.1136/bmj.b2939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Strathdee SA, Patrick DM, Currie SL, et al. Needle exchange is not enough: lessons from the Vancouver injecting drug use study. AIDS. 1997;11(8):F59–F65. doi: 10.1097/00002030-199708000-00001. [DOI] [PubMed] [Google Scholar]
- 22.Wood E, Kerr T, Lloyd-Smith E, et al. Methodology for evaluating Insite: Canada’s first medically supervised safer injection facility for injection drug users. Harm Reduct J. 2004;1(1):9. doi: 10.1186/1477-7517-1-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Kerr T, Tyndall MW, Li K, Montaner JS, Wood E. Safer injection facility use and syringe sharing in injection drug users. Lancet. 2005;366(9482):316–318. doi: 10.1016/S0140-6736(05)66475-6. [DOI] [PubMed] [Google Scholar]
- 24.Wood E, Montaner JS, Li K, et al. Burden of HIV infection among aboriginal injection drug users in Vancouver, British Columbia. Am J Public Health. 2008;98(3):515–519. doi: 10.2105/AJPH.2007.114595. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Centers for Disease Control and Prevention [Accessed January 2009];National prevention strategy: a comprehensive strategy for the prevention and control of hepatitis C virus infection and its consequences. http://www.cdc.gov/hepatitis/HCV/Strategy/NatHepCPrevStrategy.htm. Published Summer 2001.
- 26.Kuyper LM, Lampinen TM, Li K, et al. Factors associated with sex trade involvement among male participants in a prospective study of injection drug users. Sex Transm Infect. 2004;80(6):531–535. doi: 10.1136/sti.2004.011106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Kuyper LM, Palepu A, Kerr T, et al. Factors associated with sex-trade involvement among female injection drug users in a Canadian setting. Addict Res Theory. 2005;13(2):193–199. [Google Scholar]
- 28.NIH Consensus Statement on Management of Hepatitis C NIH Consens State Sci Statements. 2002;19(3):1–46. 2002. [PubMed] [Google Scholar]
- 29.Grund JP, Friedman SR, Stern LS, et al. Syringe-mediated drug sharing among injecting drug users: patterns, social context and implications for transmission of blood-borne pathogens. Soc Sci Med. 1996;42(5):691–703. doi: 10.1016/0277-9536(95)00193-x. [DOI] [PubMed] [Google Scholar]
- 30.Sherman SG, Latkin CA, Gielen AC. Social factors related to syringe sharing among injecting partners: a focus on gender. Subst Use Misuse. 2001;36(14):2113–2136. doi: 10.1081/ja-100108439. [DOI] [PubMed] [Google Scholar]
- 31.Rhodes T, Quirk A. Drug users’ sexual relationships and the social organisation of risk: the sexual relationship as a site of risk management. Soc Sci Med. 1998;46(2):157–169. doi: 10.1016/s0277-9536(97)00156-1. [DOI] [PubMed] [Google Scholar]
- 32.De P, Cox J, Boivin JF, Platt RW, Jolly AM. The importance of social networks in their association to drug equipment sharing among injection drug users: a review. Addiction. 2007;102(11):1730–1739. doi: 10.1111/j.1360-0443.2007.01936.x. [DOI] [PubMed] [Google Scholar]
- 33.Bourgois P, Prince B, Moss A. The everyday violence of hepatitis C among young women who inject drugs in San Francisco. Hum Organ. 2004;63(3):253–264. doi: 10.17730/humo.63.3.h1phxbhrb7m4mlv0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Shannon K, Kerr T, Allinott S, Chettiar J, Shoveller JS, Tyndall MW. Social and structural violence and power relations in mitigating HIV risk of drug-using women in survival sex work. Soc Sci Med. 2008;66(4):911–921. doi: 10.1016/j.socscimed.2007.11.008. [DOI] [PubMed] [Google Scholar]
- 35.Bristol N. US Senate passes new PEPFAR bill. Lancet. 2008;372(9635):277–278. doi: 10.1016/s0140-6736(08)61093-4. [DOI] [PubMed] [Google Scholar]
- 36.Masenior NF, Beyrer C. The US anti-prostitution pledge: First Amendment challenges and public health priorities. PLoS Med. 2007;4(7):e207. doi: 10.1371/journal.pmed.0040207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Shahmanesh M, Patel V, Mabey D, Cowan F. Effectiveness of interventions for the prevention of HIV and other sexually transmitted infections in female sex workers in resource poor setting: a systematic review. Trop Med Int Health. 2008;13(5):659–679. doi: 10.1111/j.1365-3156.2008.02040.x. [DOI] [PubMed] [Google Scholar]
- 38.Sweat MD, Denison JA. Reducing HIV incidence in developing countries with structural and environmental interventions. AIDS. 1995;9(suppl A):S251–S257. [PubMed] [Google Scholar]
- 39.World Health Organization . Toolkit for Targeted HIV Prevention and Care in Sex Work Settings. Department of HIV/AIDS, World Health Organization; Geneva, Switzerland: 2005. [Google Scholar]
- 40.Roy E, Alary M, Morissette C, et al. SurvUDI Working Group High hepatitis C virus prevalence and incidence among Canadian intravenous drug users. Int J STD AIDS. 2007;18(1):23–27. doi: 10.1258/095646207779949880. [DOI] [PubMed] [Google Scholar]
- 41.Wood E, Schachar J, Li K, et al. Sex trade involvement is associated with elevated HIV incidence among injection drug users in Vancouver. Addict Res Theory. 2007;15(3):321–325. [Google Scholar]