SUMMARY
A patient with refractory multiple myeloma received an infusion of CTL019 cells, a cellular therapy consisting of autologous T cells transduced with an anti-CD19 chimeric antigen receptor, after myeloablative chemotherapy (melphalan, 140 mg per square meter of body-surface area) and autologous stem-cell transplantation. Four years earlier, autologous transplantation with a higher melphalan dose (200 mg per square meter) had induced only a partial, transient response. Autologous transplantation followed by treatment with CTL019 cells led to a complete response with no evidence of progression and no measurable serum or urine monoclonal protein at the most recent evaluation, 12 months after treatment. This response was achieved despite the absence of CD19 expression in 99.95% of the patient’s neoplastic plasma cells. (Funded by Novartis and others; ClinicalTrials.gov number, NCT02135406.)
Transduction of autologous t cells to express cd19-specific chimeric antigen receptors is a promising immunotherapeutic approach for the treatment of B-cell cancers.1 We previously reported sustained regression of refractory chronic lymphocytic leukemia and B-cell acute lymphoblastic leukemia2–5 after infusion of CTL019 cells, which consist of autologous T cells expressing a CD3-zeta/CD137–based anti-CD19 chimeric antigen receptor from a lentiviral vector. Multiple myeloma is a B-lineage cancer that is reported to express CD19 infrequently6; hence, CD19 is not generally considered a valid immunotherapeutic target in multiple myeloma. Several reports, however, have suggested that a minor component of the multiple myeloma clone with drug-resistant, disease-propagating properties has a B-cell (i.e., CD19-positive) phenotype.7 In addition, our unpublished observations suggest that neoplastic plasma cells express low levels of CD19 more frequently than has previously been reported and that, in vitro, CTL019 cells are cytotoxic for cells with extremely low levels of CD19 expression. On the basis of these observations, we hypothesized that CTL019 cells would have efficacy in multiple myeloma. Since only a minor subset of cells in the multiple myeloma clone may express CD19, we further hypothesized that CTL019 cells might be effective only in conjunction with a therapy that depletes CD19-negative plasma cells, which make up the majority of neoplastic plasma cells in most cases. We therefore initiated a pilot clinical trial for patients with refractory multiple myeloma to test the safety and feasibility and preliminarily assess the efficacy of an infusion of CTL019 cells in conjunction with standard treatment for multiple myeloma, consisting of high-dose melphalan and autologous stem-cell transplantation. Here, we report the results for the first patient treated according to this protocol.
CASE REPORT
The patient received a diagnosis of IgA kappa multiple myeloma in 2009, at the age of 43 years, after presenting with vertebral compression fractures. She had an initial response to treatment with lenalidomide, bortezomib, and dexamethasone, but the disease progressed when therapy was stopped briefly to collect hematopoietic stem cells for autologous transplantation. She then received a 96-hour infusion of cisplatin, doxorubicin, cyclophosphamide, and etoposide. Hematopoietic stem cells were subsequently mobilized with filgrastim and collected. On May 14, 2010, the patient received high-dose melphalan (200 mg per square meter of body-surface area) and underwent autologous stem-cell transplantation. According to International Myeloma Working Group (IMWG) response criteria,8 the initial autologous transplantation resulted in a partial response (Fig. 1A). Maintenance lenalidomide was started approximately 1 month after transplantation. Approximately 2 months later, the patient’s serum IgA concentration began to rise, prompting the addition of bortezomib (Fig. 1A). IMWG criteria for post-transplantation progression were met 181 days after transplantation. Subsequent therapies included regimens incorporating lenalidomide, bortezomib, carfilzomib, pomalidomide, vorinostat, clarithromycin, and elotuzumab. In June 2014, after receiving nine prior lines of therapy, the patient enrolled in a clinical trial of treatment with CTL019 cells in conjunction with autologous stem-cell transplantation. Before the second autologous transplantation, two cycles of cyclophosphamide were administered (each at a dose of 1200 mg per square meter given over a 96-hour period) to control the myeloma during the screening evaluation and CTL019 manufacturing. At the time of the second transplantation (Fig. 1A), the patient’s serum IgA concentration was 6310 mg per deciliter, and the serum monoclonal protein concentration (M spike) was 6.2 g per deciliter. A bone marrow biopsy showed more than 95% involvement by multiple myeloma (Fig. 2A), a complex karyotype, and interphase fluorescence in situ hybridization (FISH) findings of t(4;14), monosomy 17 (i.e., deletion of TP53), and gain of 1q21, all of which are associated with a poor prognosis for patients with multiple myeloma.
Figure 1. Measures of Multiple Myeloma Disease Burden after Autologous Stem-Cell Transplantations (ASCTs), and Measures of CTL019 Frequency and Activity after the Second Transplantation.
Panel A shows the trend in IgA concentrations after the initial ASCT (left), with melphalan conditioning at a dose of 200 mg per square meter of body-surface area (MEL200), and after the second ASCT, with melphalan conditioning at a dose of 140 mg per square meter (MEL140) and CTL019 infusion (right). Additional therapy in the 6 months after the first ASCT included lenalidomide (LEN), bortezomib (BTZ), dexamethasone (DEX), and clarithromycin (CLR). The second ASCT was preceded by two cycles of continuous-infusion cyclophosphamide (CY). The serum monoclonal protein concentration (M spike) is also shown for the period before and after the second ASCT. Panel B shows CTL019 engraftment after the second ASCT, measured by means of flow cytometry as the number of cells per cubic millimeter (in peripheral blood only) and measured by means of a quantitative polymerase-chain-reaction assay (qPCR) as the number of copies of lentiviral vector sequence per microgram of genomic DNA (in peripheral blood and bone marrow), as well as the corresponding B-cell frequencies, measured as the number of cells per cubic millimeter. Panel C shows the serum ferritin, interferon-γ, and interleukin-6 concentrations.
Figure 2. Bone Marrow Core Biopsy Samples Obtained 2 Days before the Second ASCT and on Day 100 after the Second ASCT.
The bone marrow sample obtained before the second ASCT (Panel A) shows more than 95% involvement by multiple myeloma on hematoxylin and eosin staining (left) and CD138 immunostaining (right). The sample obtained 100 days after the ASCT (Panel B) shows 1 to 2% overall cellularity and no plasma cells on hematoxylin and eosin staining (left) and CD138 immunostaining (right).
METHODS
STUDY DESIGN
The trial was approved by the University of Pennsylvania institutional review board and was conducted in accordance with the protocol, which is available with the full text of this article at NEJM.org. All the authors participated in data collection and analysis, contributed to the writing and editing of the manuscript, and vouch for the completeness and accuracy of the data. The penultimate author made the decision to submit the manuscript for publication. Novartis, the funding sponsor of the study, approved the manuscript. Eligibility criteria included prior treatment with autologous transplantation, disease progression according to IMWG criteria within 1 year after the initial transplantation, and adequate cardiopulmonary function and cryopreserved autologous hematopoietic stem cells to undergo a second autologous transplantation. CTL019 cells are manufactured from an autologous leukapheresis product as previously described.4 The target CTL019 dose was 1×107 to 5×107 chimeric antigen receptor–expressing T cells. Study therapy consisted of high-dose melphalan (140 to 200 mg per square meter), reinfusion of autologous stem cells (≥2.1×106 cells per kilogram of body weight), and CTL019 infusion 12 to 14 days later. As specified by the protocol, maintenance therapy with lenalidomide beginning 100 days after transplantation was optional.
STUDY EVALUATIONS
Serum and urine protein electrophoresis, quantitative immunoglobulin measurements, serum free light-chain measurements, and bone marrow biopsy were performed at baseline, at days 42 and 100 after transplantation, and at additional time points as clinically indicated. Bone marrow and peripheral blood were assessed by means of flow cytometry and a quantitative polymerase-chain-reaction (PCR) assay for engraftment of CTL019. Flow cytometry was used on bone marrow samples obtained before and after transplantation in order to assess CD19 expression on multiple myeloma plasma cells and to evaluate the patient for post-transplantation minimal residual disease. CD19 expression was further characterized by means of fluorescence-activated cell sorting (FACS) of multiple myeloma plasma cells, followed by a quantitative reverse-transcriptase–PCR (RT-PCR) assay. Serum cytokine concentrations were assessed with the use of a Luminex assay as previously described.4 Finally, deep sequencing of IGH (the immunoglobulin heavy-chain gene) was performed to identify the myeloma-specific IGH sequence in the baseline bone marrow sample and to determine whether it persisted in the sample obtained on day 100, as a molecular measure of minimal residual disease.
RESULTS
FEASIBILITY AND TOXIC EFFECTS
The patient underwent autologous transplantation after receiving melphalan at a dose of 140 mg per square meter (reduced from the standard dose of 200 mg per square meter to minimize toxic effects). Transplantation-related toxic effects included grade 4 neutropenia and thrombocytopenia, grade 3 mucositis, grade 2 nausea and anorexia, neutropenic fever, and Staphylococcus aureus bacteremia. On the day of the CTL019 infusion (day 12 after transplantation), the absolute neutrophil count had risen to 200 per cubic millimeter, and the patient had been afebrile with sterile blood cultures for more than 48 hours. The CTL019 dose was 5×107 chimeric antigen receptor–expressing T cells. The absolute lymphocyte count was 840 per cubic millimeter (with T cells accounting for 49%) on the day of CTL019 infusion, as compared with 390 per cubic millimeter before the administration of melphalan. After CTL019 infusion, there were no fevers or other signs of the cytokine release syndrome, an inflammatory reaction that has been observed in other clinical studies of CTL019.2–5,9 The patient had hypogammaglobulinemia before transplantation, a common finding in patients with multiple myeloma; it persisted at day 100 after transplantation and was attributed to the effects of CTL019 on normal B cells and plasma cells. No other adverse events attributable to CTL019 were observed; any adverse events not specified earlier were grade 1 or 2. By day 100 after transplantation, all transplantation-related toxic effects had resolved. Lenalidomide, at a dose of 5 mg daily, was started on day 130 after transplantation; the dose was subsequently reduced to 5 mg twice weekly because of gastrointestinal toxic effects.
CLINICAL RESPONSE
The monoclonal IgA concentration (as determined by means of serum protein electrophoresis) and the total serum IgA concentration began to decline after transplantation and declined further after CTL019 infusion (Fig. 1A). The nadir IgA concentration was below the limit of quantitation (7 mg per deciliter). On day 100, a bone marrow biopsy showed 1 to 2% overall cellularity and no plasma cells (Fig. 2B). By this time, all criteria for “stringent complete response,” the best response category in the IMWG classification,8 were met except for the presence of a faint kappa light-chain band on urinary protein electrophoresis, which was not detected on repeat testing 1 month later. Flow-cytometric testing and IGH deep sequencing of the bone marrow sample obtained on day 100 for minimal residual disease were negative; IGH deep sequencing indicated a frequency of fewer than 1 neoplastic plasma cell in 3.11×106 bone marrow cells. As compared with the tumor burden in the patient’s bone marrow cells at baseline, which consisted almost entirely of malignant plasma cells (>95% as estimated morphologically) (Fig. 2A), there was more than a 5-log10 reduction in the tumor burden at day 100. Twelve months after transplantation, the patient had no evidence of monoclonal immunoglobulin on serum and urine immunofixation and no clinical signs or symptoms of multiple myeloma. Transplantation with CTL019 cells led to a more complete and more durable reduction in multiple myeloma burden than was obtained with the first transplantation and all other prior therapies.
CTL019 ENGRAFTMENT, SYSTEMIC INFLAMMATORY MARKERS, AND B-CELL APLASIA
CTL019 cells were detected in the peripheral blood from day 2 until day 47 after infusion (day 61 after transplantation) by means of both flow cytometry (at a frequency of 0.3 to 1.7 cells per cubic millimeter, or 0.1 to 0.2% of total peripheral-blood T cells) and quantitative PCR; CTL019 cells were also detected in the bone marrow on day 30 after infusion (day 42 after transplantation) by means of both flow cytometry (0.1% of T cells) and quantitative PCR but not on day 88 after infusion (day 100 after transplantation) (Fig. 1B). Infusion of CTL019 was followed by a rise in the serum interferon gamma and ferritin concentrations; the serum interleukin-6 concentration was elevated before CTL019 infusion and declined as CTL019 levels decreased (Fig. 1C). These inflammatory markers did not rise to the extent that has previously been associated with CTL019-mediated cytokine release syndrome.2–5 As expected, reconstitution of B cells was associated with loss of detectable CTL019 (Fig. 1B). Since B cells are typically detectable on day 14 in patients with multiple myeloma who undergo autologous transplantation and autologous T-cell infusion,10 the early B-cell aplasia in this patient was probably due to the combined effects of CTL019 and melphalan rather than the effect of high-dose melphalan alone.
CD19 EXPRESSION IN MULTIPLE MYELOMA CELLS
Bone marrow aspirate obtained at baseline, just before the patient underwent autologous transplantation, was analyzed by means of flow cytometry to assess CD19 expression on multiple myeloma plasma cells. The dominant population of neoplastic plasma cells was identified by its CD38+CD45− immunophenotype and kappa light-chain restriction (Fig. 3). CD19 was detected on merely 0.05% of the dominant multiple myeloma plasma-cell population (Fig. 3A). The minor CD19+ subset expressed intracellular kappa light chain (Fig. 3B) and B-cell maturation antigen (BCMA), a plasma-cell–specific marker (not shown), confirming its identity as a component of the neoplastic plasma-cell population. In addition, a small population of kappa-restricted plasma cells was identified among CD45+ cells, a minor subset of which was CD19+ (Fig. 4, shaded inset). Additional CD19+ populations in the baseline bone marrow sample included polyclonal CD19+ CD20+ B cells and a small population of CD45+ CD38(dim)+CD20−CD19+ light-chain–negative cells, probably representing pro-B or early pre-B cells (Fig. 4). For a more sensitive assessment of CD19 expression, we used FACS to sort the dominant neoplastic plasma-cell population, with two thresholds for CD19 expression (Fig. 3A), and we analyzed the sorted subsets of plasma cells for the presence of CD19 messenger RNA (mRNA) by means of an RT-PCR assay. No CD19 mRNA was detected in the 99.95% of myeloma plasma cells that were CD19-negative on flow cytometry (Fig. 3C).
Figure 3. CD19 Expression on Neoplastic Plasma Cells.
Panel A shows surface CD19 expression (right) on multiple myeloma plasma cells (CD38+CD45− gate, left) obtained from the pre-ASCT bone marrow aspirate. Brackets (right) indicate fluorescence thresholds that were used for fluorescence-activated cell sorting (FACS) of multiple myeloma plasma cells according to surface CD19 expression for analysis of CD19 messenger RNA levels. Panel B shows immunoglobulin light-chain expression in the overall population of malignant plasma cells (left) and the CD19+ subset of malignant plasma cells (right) from the pre-ASCT bone marrow aspirate. Panel C shows the results of reverse-transcriptase–polymerase-chain-reaction analysis of CD19 expression in FACS subsets of peripheral-blood mononuclear cells (PBMCs) from healthy donors and malignant plasma cells from the pre-ASCT bone marrow aspirate; results are normalized to expression in the CD19+ healthy-donor PBMC sample and plotted on a semi-logarithmic scale. ND denotes not detectable. I bars indicate 95% confidence intervals.
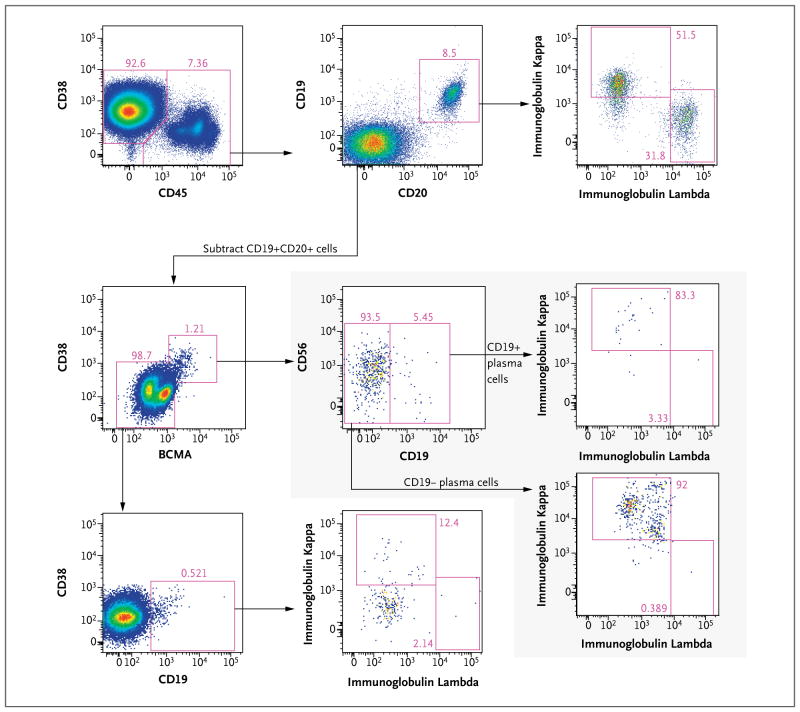
Figure 4. Characterization of CD19+ Cells in Bone Marrow Aspirate.
Flow-cytometric analysis of bone marrow aspirate obtained before transplantation shows CD19+ cells outside the dominant CD38+CD45− multiple myeloma plasma-cell population characterized in Figure 3. Before this analysis, gating was performed to exclude doublets, debris, T cells, monocytes, and dead cells (not shown). CD45+ cells (top row, left plot) were found to contain the following CD19+ populations: polyclonal CD19+CD20+ B cells (top row, middle and right plots); a minor population of plasma cells (BCMA+CD38+), a subset of which is CD19+ and all of which are kappa-restricted (shaded inset), indicating that they are a component of the multiple myeloma clone; and a small population of CD45+CD38(dim)+CD20−CD19+ cells without immunoglobulin light-chain expression, most likely representing pro-B cells or early pre-B cells (third row, left and middle plots). BCMA denotes B-cell maturation antigen.
DISCUSSION
We report a sustained complete response to an infusion of CTL019 cells in conjunction with autologous transplantation in a patient with advanced multiple myeloma. The superior reduction in disease burden and durability of this response in comparison with the response to the previous transplantation, despite the extensive intervening therapy and lower dose of melphalan, suggest that the favorable response is attributable to the combination of CTL019 and melphalan rather than melphalan alone. Reconstitution of normal CD19+ B cells and loss of detectable CTL019 indicate that sustained CTL019 activity was not required for this response.
Multiple myeloma in most cases consists predominantly of terminally differentiated CD19-negative plasma cells, though minor subsets, such as the small CD19+ plasma-cell population observed in the patient described here, may be identified with less differentiated phenotypes along the spectrum between B lymphocytes and plasma cells.7 Myeloma cells with these less differentiated phenotypes may make up a drug-resistant, clonogenic disease reservoir maintained by components of the bone marrow microenvironment11–14 and enriched by therapy with proteasome inhibitors.15 Therapies targeting these subsets may therefore have a synergistic effect with conventional myeloma therapies, and such synergy may explain the favorable response reported here. Alternatively, the clinically relevant target of CTL019 in this case may have been non-neoplastic CD19+ cells, which have been implicated in immune evasion and resistance to therapy in solid tumors.16–18
The patient described here benefited from CTL019 without the development of the cytokine release syndrome. We previously treated a patient who had multiple myeloma with a CTL019 dose of 5×108 cells on day 2 after autologous stem-cell transplantation, according to a single-patient, compassionate-use protocol. The patient had a very good partial response complicated by severe cytokine release syndrome and neurotoxic effects that were attributed to CTL019; these toxic effects were associated with a robust in vivo CTL019 expansion. As a result of these toxic effects and our previous observation of treatment-schedule–dependent effects on in vivo expansion of adoptively transferred T cells after autologous transplantation,19 the protocol for our current, ongoing clinical trial specified a lower CTL019 dose, with infusion scheduled for day 12, 13, or 14, rather than day 2, after transplantation.
Ten patients, including the patient described in detail here, have been treated so far in this trial (for details, see Table S1 in the Supplementary Appendix, available at NEJM.org). Six of the 10 patients remain progression-free. The only additional CTL019-attributable toxic effects observed have been one instance of grade 1 cytokine release syndrome and one instance of grade 3 enterocolitis due to autologous graft-versus-host disease.
In summary, we report a case of advanced, refractory multiple myeloma in which a durable complete response has been attained with CTL019 infusion after treatment with high-dose melphalan and autologous transplantation, despite the absence of CD19 expression in the vast majority of neoplastic cells.
Supplementary Material
Acknowledgments
Supported by Novartis; grants from the National Institutes of Health (K12CA076931 and T32CA009615, to Dr. Garfall; K08CA166039, to Dr. Maus; and 5R01CA165206, to Dr. June), the International Society for Advancement of Cytometry (to Dr. Mahnke), and the University of Pennsylvania Institute for Translational Medicine and Therapeutics (to Dr. Garfall); and a Conquer Cancer Foundation Young Investigator Award (to Dr. Maus).
We thank Jeff Finklestein, Farzana Nazimuddin, Harit Parakandi, and Marina Bogush for sample processing and flow-cytometric analysis; Irina Kulikovskaya and Minnal Gupta for the quantitative PCR assay; David Ambrose for assistance with FACS; Fang Chen for the Luminex assay; Alexey Bersenev, Anne Lamontagne, Alexander Malykhin, Neel Manvar, Matthew O’Rourke, Megan Suhoski-Davis, and members of the Clinical Cell and Vaccine Production Facility for cell manufacturing and testing; Colleen Callahan, Christine Strait, Brittany Girard, Margaret Tartaglione, Trish Hankins, Brandon Loudon, Christopher Grupp, Maya Mudambi, Laura Motley, Elizabeth Veloso, Amy Marshall, Lester Lledo, Joan Gilmore, Holly McConville, and James Capobianchi for clinical research assistance; Patricia Mangan, Kathleen Cunningham, and Mary Sell for assistance in performing autologous stem-cell transplantation; Ewelina Morawa at Novartis for discussions and protocol support; Frasier Wright and Katherine High at Children’s Hospital of Philadelphia Vector Core for clinical-grade vector production; Bipulendu Jena and Laurence Cooper for provision of the chimeric antigen receptor anti-idiotype detection reagent; Kenneth Anderson and Robert Green for referral of their patient for this protocol; David Porter for thoughtful advice; and Ravi Amaravadi for service as the medical monitor.
Footnotes
Disclosure forms provided by the authors are available with the full text of this article at NEJM.org.
References
- 1.Maus MV, Grupp SA, Porter DL, June CH. Antibody-modified T cells: CARs take the front seat for hematologic malignancies. Blood. 2014;123:2625–35. doi: 10.1182/blood-2013-11-492231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Porter DL, Levine BL, Kalos M, Bagg A, June CH. Chimeric antigen receptor–modified T cells in chronic lymphoid leukemia. N Engl J Med. 2011;365:725–33. doi: 10.1056/NEJMoa1103849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Kalos M, Levine BL, Porter DL, et al. T cells with chimeric antigen receptors have potent antitumor effects and can establish memory in patients with advanced leukemia. Sci Transl Med. 2011;3:95ra73. doi: 10.1126/scitranslmed.3002842. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med. 2014;371:1507–17. doi: 10.1056/NEJMoa1407222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Grupp SA, Kalos M, Barrett D, et al. Chimeric antigen receptor-modified T cells for acute lymphoid leukemia. N Engl J Med. 2013;368:1509–18. doi: 10.1056/NEJMoa1215134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Tembhare PR, Yuan CM, Venzon D, et al. Flow cytometric differentiation of abnormal and normal plasma cells in the bone marrow in patients with multiple myeloma and its precursor diseases. Leuk Res. 2014;38:371–6. doi: 10.1016/j.leukres.2013.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Hajek R, Okubote SA, Svachova H. Myeloma stem cell concepts, heterogeneity and plasticity of multiple myeloma. Br J Haematol. 2013;163:551–64. doi: 10.1111/bjh.12563. [DOI] [PubMed] [Google Scholar]
- 8.Durie BGM, Harousseau JL, Miguel JS, et al. International uniform response criteria for multiple myeloma. Leukemia. 2006;20:1467–73. doi: 10.1038/sj.leu.2404284. [DOI] [PubMed] [Google Scholar]
- 9.Lee DW, Gardner R, Porter DL, et al. Current concepts in the diagnosis and management of cytokine release syndrome. Blood. 2014;124:188–95. doi: 10.1182/blood-2014-05-552729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Stadtmauer EA, Vogl DT, Luning Prak E, et al. Transfer of influenza vaccine-primed costimulated autologous T cells after stem cell transplantation for multiple myeloma leads to reconstitution of influenza immunity: results of a randomized clinical trial. Blood. 2011;117:63–71. doi: 10.1182/blood-2010-07-296822. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Yaccoby S. The phenotypic plasticity of myeloma plasma cells as expressed by dedifferentiation into an immature, resilient, and apoptosis-resistant phenotype. Clin Cancer Res. 2005;11:7599–606. doi: 10.1158/1078-0432.CCR-05-0523. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Kukreja A, Hutchinson A, Dhodapkar K, et al. Enhancement of clonogenicity of human multiple myeloma by dendritic cells. J Exp Med. 2006;203:1859–65. doi: 10.1084/jem.20052136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Fuhler GM, Baanstra M, Chesik D, et al. Bone marrow stromal cell interaction reduces syndecan-1 expression and induces kinomic changes in myeloma cells. Exp Cell Res. 2010;316:1816–28. doi: 10.1016/j.yexcr.2010.03.013. [DOI] [PubMed] [Google Scholar]
- 14.Hideshima T, Mitsiades C, Ikeda H, et al. A proto-oncogene BCL6 is up-regulated in the bone marrow microenvironment in multiple myeloma cells. Blood. 2010;115:3772–5. doi: 10.1182/blood-2010-02-270082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Leung-Hagesteijn C, Erdmann N, Cheung G, et al. Xbp1s-negative tumor B cells and pre-plasmablasts mediate therapeutic proteasome inhibitor resistance in multiple myeloma. Cancer Cell. 2013;24:289–304. doi: 10.1016/j.ccr.2013.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Gunderson AJ, Coussens LM. B cells and their mediators as targets for therapy in solid tumors. Exp Cell Res. 2013;319:1644–9. doi: 10.1016/j.yexcr.2013.03.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Affara NI, Ruffell B, Medler TR, et al. B cells regulate macrophage phenotype and response to chemotherapy in squamous carcinomas. Cancer Cell. 2014;25:809–21. doi: 10.1016/j.ccr.2014.04.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Shalapour S, Font-Burgada J, Di Caro G, et al. Immunosuppressive plasma cells impede T-cell-dependent immunogenic chemotherapy. Nature. 2015;521:94–8. doi: 10.1038/nature14395. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Rapoport AP, Stadtmauer EA, Aqui N, et al. Rapid immune recovery and graft-versus-host disease-like engraftment syndrome following adoptive transfer of co-stimulated autologous T cells. Clin Cancer Res. 2009;15:4499–507. doi: 10.1158/1078-0432.CCR-09-0418. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.