Abstract
Background
HIV acquisition in the female genital tract remains incompletely understood. Quantitative data on biological HIV risk factors, the influence of reproductive hormones, and infection risk are lacking. We evaluated vaginal epithelial thickness during the menstrual cycle in pigtail macaques (Macaca nemestrina). This model previously revealed increased susceptibility to vaginal infection during and following progesterone-dominated periods in the menstrual cycle.
Methods
Nucleated and non-nucleated (superficial) epithelial layers were quantitated throughout the menstrual cycle of 16 macaques. We examined the relationship with previously estimated vaginal SHIVSF162P3 acquisition time points in the cycle of 43 different animals repeatedly exposed to low virus doses.
Results
In the luteal phase (days 17 to cycle end), the mean vaginal epithelium thinned to 66% of mean follicular thickness (days 1-16; p=0.007, Mann-Whitney test). Analyzing four-day segments, the epithelium was thickest on days 9-12, and thinned to 31% thereof on days 29-32, with reductions of nucleated and non-nucleated layers to 36 and 15% of their previous thickness, respectively. The proportion of animals with estimated SHIV acquisition in each cycle segment correlated with non-nucleated layer thinning (Pearson’s r = 0.7, p<0.05, linear regression analysis), but not nucleated layer thinning (Pearson’s r = 0.6, p=0.15).
Conclusions
These data provide a detailed picture of dynamic cycle-related changes in the vaginal epithelium of pigtail macaques. Substantial thinning occurred in the superficial, non-nucleated layer, which maintains the vaginal microbiome. The findings support vaginal tissue architecture as susceptibility factor for infection and contribute to our understanding of innate resistance to SHIV infection.
Keywords: HIV, animal model, reproductive immunology, hormonal contraception, infection risk
Introduction
HIV acquisition and establishment of systemic infection in the female genital tract remain incompletely understood1. New insights could lead to new approaches for biomedical HIV prevention strategies. The vaginal epithelium has been implicated as site of first HIV transmission to infiltrating or underlying target cells. In macaques, Hu et al demonstrated that SIV rapidly traverses the vaginal epithelium after intra-vaginal exposure 2, and Carias et al reported efficient penetration of this barrier, suggesting a simple “diffusive percolation mechanism” 3. Thinning of vaginal epithelial tissues under hormonal influences is documented in rhesus4,5 and pigtail6-9 macaques. It occurs to a substantially lesser degree in humans during naturally progesterone-dominated periods of the menstrual cycle 10,11 , and also during progestin-based hormonal contraception in some studies10-12, but not others 13,14. However, extent and time course of thinning have not been established with great detail.
Epithelial thinning has been interpreted as a likely contributor to increased SHIV risk during the menstrual cycle of macaques5,6. Other biological factors have been discussed as putative HIV risk factors (reviewed in15,16), and they may influence SHIV risk as well (e.g., changes in the vaginal microbiome, mucosal inflammation, target cell infiltration of the epithelium, and others). Quantitative data are lacking to link SHIV or HIV acquisition to these biological factors. The factors may also be regulated during the increased HIV acquisition risk reported during hormonal contraception use in some observational studies17,18 in women. Careful studies of each of these putative biological SHIV or HIV risk factors are expected to contribute to a comprehensive scientific evaluation of the relationship of hormonal contraception and SHIV/ HIV risk.
We here measure the thickness of vaginal epithelium during the menstrual cycle of pigtail macaques. This tissue has many similarities with skin 19, including its barrier function, as demonstrated with regards to HIV 20. It is stratified squamous epithelium consisting of four variably distinguishable cell layers, i.e., the superficial stratum corneum (facing the vaginal canal), and underlying stratum granulosum, spinosum, and basale 3,19. HIV target cells, i.e., Langerhans and CD4 T cells can be found in the epithelium, but mainly reside in the underlying lamina propria. We quantitate the epithelial layer thicknesses in uninfected animals, and correlate the data to SHIV acquisition previously measured in a different group of animals. Our research group has used pigtail macaques for testing of anti-HIV prevention modalities targeted to women, using the repeat-low dose (RLD) virus exposure approach (e.g., 21). It models human sexual HIV exposure, as not every exposure leads to infection 22. Using our animal model, we previously noticed that vaginal SHIVSF162P3 acquisition did not occur randomly throughout the menstrual cycles in these animals, but rather happened preferentially during two weeks prior to and throughout menstruation 23,24. This was during the progesterone peak of the cycle, and in the following week when progesterone receded. This raised the question of whether hormone-induced epithelial thinning would correlate with estimated time of infection in this model, as would be expected if it indeed is a susceptibility factor for infection.
Methods
Ethics statement
The Institutional Animal Care and Use Committee (IACUC) of the Centers for Disease Control and Prevention (CDC) approved all macaque procedures. This study was carried out in strict accordance with the recommendations in the Guide for the Care and Use of Laboratory Animals 25. All procedures were performed under anesthesia using ketamine, often in combination with Telazol.
Animals, biopsy collection, menstrual cycle determination
Sixteen female pigtail macaques (Macaca nemestrina) were born elsewhere, purchased, and housed at CDC. Ages ranged from 4.5 to 14.2 years. Sexual and reproductive histories were unknown, except for macaque PHQ1 (IDs listed in Table 1) who had six live births. Vaginal biopsy was performed by taking 4.2 mmy × 4.2 mm punches with a rigid punch biopsy instrument (EuroMed, Tuttlingen, Germany) from three separate sites each time a biopsy was taken as previously described 8. Sites were recorded in radial degrees and distance to vaginal os, and repeat biopsy of the same location was avoided. Biopsies were scheduled with two or three week intervals. Menstrual cycle day of biopsies was retrospectively determined using a combination of plasma progesterone (previously described 23) measured once per week, and menstrual blood and sex skin (perineal tumescence) observation on each weekday 26, and recorded on a scale of 0/1 and 1-4, respectively. All 16 animals had menstrual cycles (data not shown). An example of menstrual cycle determination is shown in Fig 1. for animal BB0499. Day one of the cycle was defined by the first day of menstrual bleeding. Bleeding was not observed for two animals. In those, day 1 was defined when progesterone reached background levels after a steep drop 23. Follicular phase was defined as cycle days 1-16, while the luteal phase was days 17 until cycle end. Animal ages were evenly distributed across menstrual cycle time points at time of biopsy (data not shown). Estimated SHIVSF162P3 acquisition time points throughout the menstrual cycle from 43 other animals are summarized elsewhere 24. Age at infection was available for 19 of the 43 animals, and ranged from 2.7 to 18.5 years. All 43 infected animals had menstrual cycles 23,24.
Table 1.
Summary of vaginal epithelial measurements
| Animal ID |
Menstrual cycle day |
Age at biopsy (years) |
Epithelium (um) mean |
SD | Nucleated layer (um) mean |
SD | Non- nucleated layer (um) mean |
SD | Number of measurements |
|---|---|---|---|---|---|---|---|---|---|
| PHQ1 | 1 | 14.08 | 82 | 48 | 82 | 48 | 0 | 0 | 157 |
| BB405 | 3 | 6.42 | 276 | 128 | 276 | 128 | 0 | 0 | 83 |
| BB050 | 3 | 4.42 | 361 | 89 | 240 | 71 | 121 | 43 | 43 |
| BB108 | 8 | 6.58 | 319 | 84 | 224 | 84 | 95 | 31 | 92 |
| BB480 | 8 | 6.42 | 270 | 91 | 185 | 65 | 85 | 38 | 445 |
| BB552 | 8 | 4.58 | 389 | 98 | 338 | 88 | 51 | 34 | 135 |
| BB967 | 8 | 8.58 | 418 | 111 | 270 | 72 | 148 | 55 | 235 |
| BB049 | 8 | 6 | 260 | 141 | 190 | 68 | 70 | 87 | 141 |
| BB173 | 8 | 9.58 | 325 | 143 | 214 | 181 | 111 | 71 | 149 |
| BB981 | 8 | 12.58 | 535 | 104 | 358 | 103 | 176 | 39 | 149 |
| PPi2 | 8 | 7.58 | 273 | 72 | 254 | 78 | 19 | 30 | 213 |
| BB480 | 9 | 6.33 | 475 | 199 | 361 | 163 | 113 | 53 | 157 |
| BB537 | 9 | 6.67 | 392 | 158 | 292 | 124 | 100 | 63 | 178 |
| BB537 | 15 | 6.75 | 248 | 67 | 198 | 65 | 50 | 18 | 93 |
| BB770 | 15 | 9.17 | 532 | 109 | 407 | 140 | 125 | 50 | 124 |
| PHQ1 | 15 | 14.17 | 388 | 101 | 243 | 65 | 145 | 70 | 104 |
| BB405 | 17 | 6.5 | 445 | 111 | 272 | 79 | 173 | 64 | 103 |
| BB552 | 18 | 4.5 | 259 | 94 | 169 | 96 | 90 | 28 | 182 |
| BB981 | 22 | 12.58 | 394 | 160 | 304 | 110 | 89 | 75 | 140 |
| BB173 | 22 | 9.67 | 250 | 97 | 182 | 74 | 69 | 39 | 58 |
| BB480 | 23 | 6.42 | 265 | 92 | 162 | 64 | 104 | 48 | 177 |
| BB050 | 25 | 4.5 | 156 | 58 | 130 | 55 | 26 | 27 | 138 |
| BB269 | 25 | 6.33 | 216 | 81 | 169 | 81 | 47 | 10 | 11 |
| BB480 | 25 | 6.33 | 200 | 69 | 155 | 63 | 44 | 17 | 145 |
| BB770 | 25 | 9.25 | 284 | 52 | 178 | 44 | 107 | 25 | 125 |
| BB521 | 27 | 6.17 | 211 | 58 | 139 | 47 | 72 | 40 | 175 |
| BB108 | 29 | 6.5 | 84 | 48 | 84 | 48 | 0 | 0 | 224 |
| BB049 | 29 | 6.08 | 114 | 75 | 99 | 58 | 15 | 33 | 52 |
| BB967 | 29 | 8.67 | 224 | 88 | 189 | 81 | 35 | 31 | 185 |
| PPi2 | 29 | 7.5 | 112 | 113 | 99 | 94 | 13 | 21 | 82 |
Summary of vaginal epithelial measurements throughout the menstrual cycle of sixteen pigtail macaques, ordered by day of menstrual cycle. The table provides mean values from all measurements at each biopsy occasion, with standard deviations (SD). Thirty biopsy results are shown; two additional biopsies were not usable. Each line summarizes results from one animal (ID=identification number), at one time point. “Epithelium” refers to the sum of mean nucleated and non-nucleated layers, measured in micrometers (um), from 11 – 445 measurements (last column), depending on the size of the harvested tissues.
Fig. 1.
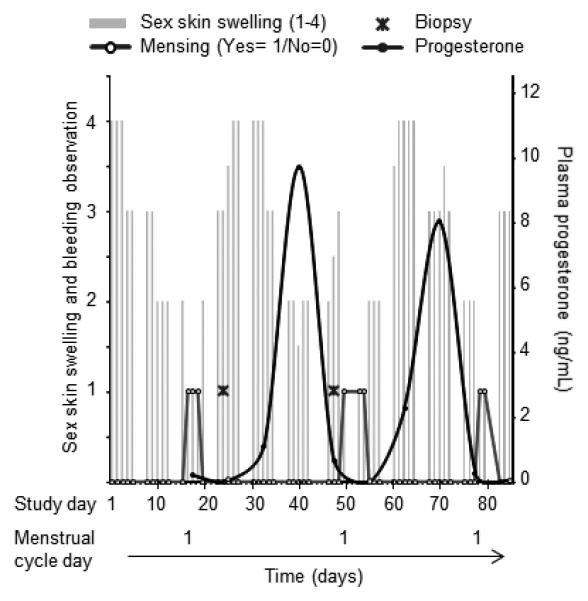
Menstrual cycle starting time determination in example macaque BB0499. Menstrual cycle start and duration were determined retrospectively using daily observation of bleeding (open circles), sex skin swelling (recorded on a scale of 1-4, left axis, grey bars), and plasma progesterone (right axis, filled circles). In this example, onset of menstrual bleeding defined day one of the cycle. Biopsies (cross symbol) were collected on days 8 and 29 of the cycle for this animal.
Epithelial thickness measurements
Biopsy tissues were fixed in 10% neutral buffered formalin for 72 hours, processed for routine paraffin histology, sectioned at four microns, mounted on slides, and stained with hematoxylin-eosin (H&E). Whole slide images were captured with the ScanScope system (Aperio, Vista, CA), and epithelial thickness was measured using the HALO image analysis software’s epithelial thickness algorithm (Indica Labs, Corrales, NM), with manual delineation of the non-nucleated and nucleated cell layers by a certified veterinary pathologist. The pathologist was aware of animal ID and calendar date of sample collection, but did not know corresponding time point within menstrual cycle. Both layer thicknesses were measured at 50 µm intervals along the entire length of appropriately oriented tissue biopsies, and total thickness was calculated by addition of these two measurements. Measurements from up to three tissue pieces per biopsy time point were recorded, and amounted to 11 – 445 measurements per biopsy (Table 1), with a mean of 142.
Statistics
Statistical computations (mean tissue depths, standard deviations, linear regressions, Mann-Whitney tests) were performed using GraphPad Prism software version 5.03 (San Diego, CA). To visualize patterns in the data, smoothed curves were fit to the data using a spline function (df=5)27. Loess smoothing provided similar results (data not shown). To obtain adequate smoothing of the curve in the lower and upper tails and by taking into account the cyclical characteristics of the menstrual cycle data, the last data point was replicated at time prior to the lower boundary of data and the observation from the first day of the cycle was replicated after the upper boundary.
Results
Dynamic changes in the vaginal epithelium throughout the menstrual cycle
Vaginal epithelial architecture was evaluated at two time points within the cycle of sixteen pigtail macaques. Fig. 2A shows cyclical variations in epithelial thickness and degree of keratinization from two animals. Biopsies from BB173 show the epithelium on days 8 and 22 of the cycle. BB405 is shown with thinned epithelium without keratinization on cycle day 3, and thicker epithelium with a prominent keratinized superficial layer (s. corneum) on cycle day 17. To quantitate changes, we measured thickness of the superficial non-nucleated layer separately from the underlying nucleated layers. Further discrimination of the nucleated epithelial layers (s. granulosum, spinosum, basale)3,19,28 was not attempted due to the lack of clear delineation between the layers. Fig. 2B shows examples of the output from image analysis software used for thickness measurements, after manual demarcation of the border between non-nucleated and nucleated epithelial layers. Tissues from animal BB770 is shown on cycle day 15 (left, top and bottom) and from animal BB0540 on cycle day 25 (right panel, bottom).
Fig. 2 A.
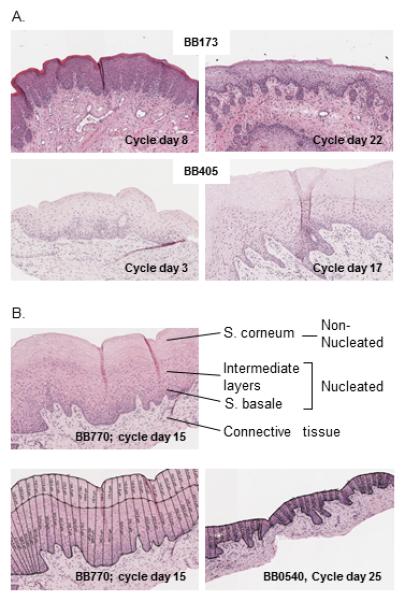
Example H&E stained vaginal biopsies from two pigtail macaques at two different time points each (10x magnification). Text inserts refer to macaque identification numbers and menstrual cycle day. B: Top: Illustration of non-nucleated and nucleated vaginal epithelial layers. Intermediate layers refer to s. granulosum and spinosum. Bottom: Analysis parameters applied using software as described in the text, measuring epithelial thickness at 50 micron intervals across all mounted parts of the biopsies. A mean 142 measurements were analyzed for each biopsy occasion.
Table 1 summarizes epithelial thickness measurements from individual macaques. The thinnest epithelium was measured in animal PHQ1 on cycle day 1, at 82 micrometers. BB981 and BB770 had the thickest epithelium at 535 and 532 μm on menstrual cycle days 8 and 15, respectively. Of note, three animals completely lacked the superficial non-nucleated epithelial layer on days 1, 3, or 29 (PHQ1, BB405, BB108). We compared the thickness of the entire epithelium between follicular and luteal phases, as others have previously done in pigtail and rhesus macaques 5,6. The mean thickness was 346 um in the follicular, and 230 um in the luteal phase, a reduction to 66%. This difference was statistically significant (p=0.007, Mann-Whitney test).
Mean thickness is shown in 4-day intervals throughout the cycle to visualize dynamic changes in greater detail in Fig. 3A. The epithelium was thickest on days 9-12 with a mean overall thickness of 434 μm, and thinnest at 134 μm on days 29-32, right before menstruation, a relative thinning to 31% from peak to nadir. At these times, large relative thickness changes occurred in the superficial, non-nucleated layer from peak mean of 106 μm to a minimum mean 16 μm, a relative reduction to 15%. For the nucleated layers, the corresponding mean thicknesses were 327 and 118 μm, respectively, a reduction to 36%. Fig. 3B depicts dynamic changes in the epithelium and its component layers during the cycle using a smoothing algorithm.
Fig. 3.
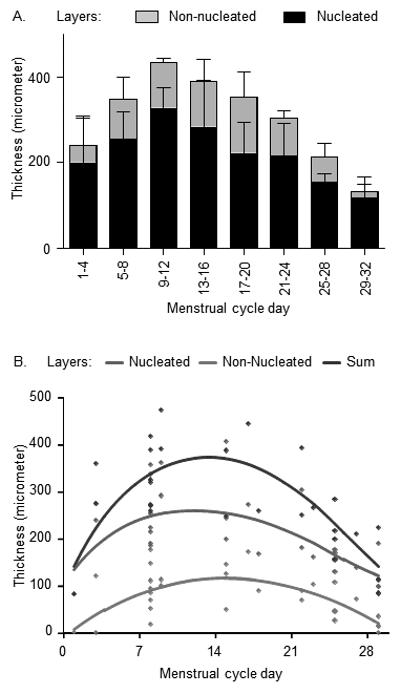
Dynamic changes in the vaginal epithelium during the menstrual cycle. A: Mean epithelial thickness and SD (standard deviation, error bars) of the layers in animals with measurements in the indicated 4-day cycle segments. B: The dynamic changes are graphed using smoothed curves for epithelial thickness layers as indicated.
The relationship between epithelial thickness and estimated SHIV acquisition
We previously reported estimated SHIV acquisition time points within the menstrual cycle of 43 different macaques 24. The macaques were repeatedly exposed to weekly or twice-weekly low dose virus throughout their cycles, with random exposure start times in relation to the cycle, resulting in infection after a median five exposures 24. Biopsy collection is invasive and likely increases susceptibility to infection by compromising the vaginal epithelium and creating virus entry points. We therefore used the previously collected data 24 and independently examined the relationship with vaginal epithelial thickness during corresponding time segments within the cycle of the sixteen uninfected macaques. Fig. 4A shows previously reported estimated SHIV acquisition time points, re-graphed in 4-day intervals for analysis purposes. As previously reported, most infections were estimated to occur on days 1-8 and 25-32 of the cycle 24. Estimated infections negatively correlated with thickness of the non-nucleated layer (Pearson’s r = −0.7, p<0.05, linear regression analysis), but less, and not statistically significant, with thickness of the nucleated layer (Pearson’s r=−0.6, p=0.15, Fig. 4B, C). The correlation of infection and overall epithelial thickness, i.e., sum of both layers was not statistically significant (Person’s r = −0.7, p=0.06, graphed results not shown). The negative correlation findings were further supported by the patterns in the smoothed data curves (Fig. 4D). This graph demonstrates an inverse relationship, with epithelial thickness lowest at times of highest transmission events and thickest at times of lowest transmission events, i.e., at the beginning and end of the menstrual cycle.
Fig. 4.
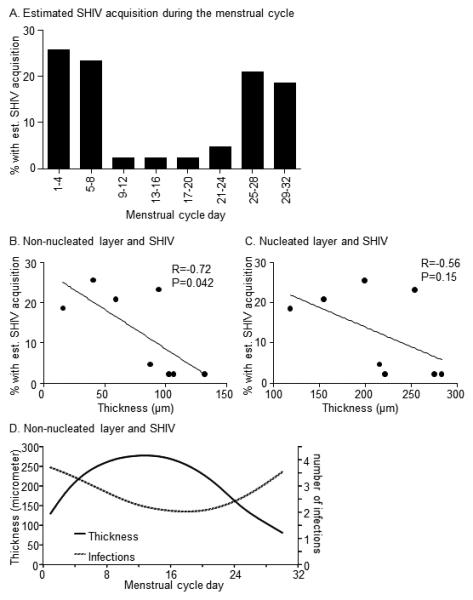
Relationship of epithelial thickness and susceptibility to SHIV infection A. The graph shows the percentage of 43 female pigtail macaques with est. (=estimated) SHIV acquisition time point at the indicated menstrual cycle segments of four days. The macaques became SHIVSF162P3 infected after repeated vaginal exposure at low virus dose, as published 24. Data were analyzed and plotted for 4-day intervals. B, C: Scatter plots of the thickness of mean vaginal epithelial layers during 4-day menstrual cycle segments, and the estimated SHIV acquisition in corresponding 4-day segments in 43 different macaques. The line represent linear regression analysis; R = Pearson co-efficient; p-value results from hypothesis test of non-zero slope. The right Y axis refers to the number of infections as reported in 24. D. Smoothed data were graphed to further examine the relationship of non-nucleated layer thickness and susceptibility to infection throughout the menstrual cycle.
The relationship between age, epithelial thickness, and susceptibility to infection
All biopsied animals were of reproductive age, ranging from 4.5 to 14.2 years (Table 1). We examined whether epithelial thickness varied with age using a stratified multivariable regression model that controlled for the changing depth of epithelium during the complete menstrual cycle. The model stratum with data points from time periods when the epithelium was thickest, i.e. not thinned as a likely result of hormonal influences, was of particular interest. We thus selected the half of the menstrual cycle when the mean epithelium was thickest, i.e., days 5 – 20 (Fig. 3A). At these times, thickness of the non-nucleated layer correlated with increasing age (p=0.002, Fig. 5A), but thickness of the nucleated layer did not (p=0.46 Fig. 5B), and neither did overall thickness (p=0.08, multivariable regression, graphed data not shown). We also examined age and thickness at times of the cycle when the tissues are thinned due to hormonal influences, i.e., days 1-5 and days 21 - 32. There was no association between age and thinning for nucleated or non-nucleated layers (data not shown). Finally, we inquired whether younger animals were more susceptible to vaginal infection in the RLD animal model, since their non-nucleated layer of the vaginal epithelium was thinner in mid-cycle compared to older animals. We were able to determine birth dates and age at infection for nineteen of the 43 previously examined, infected animals 24. In those, younger age was not associated with increased susceptibility to infection (Fig. 5C), as there was no significant correlation between age and the number of exposures it took to infect animals (Pearson’s r = 0.1, p>0.05, linear regression analysis, Fig. 5C), though this model did not control for potential confounding by time in menstrual cycle.
Fig. 5.
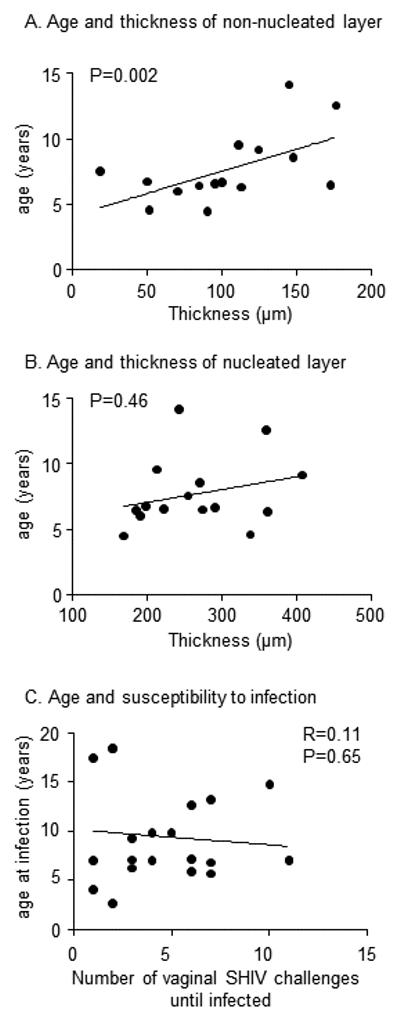
Age, vaginal epithelial thickness, and susceptibility to infection. A, B. Scatterplots show the distribution of mean vaginal layer thickness and age of 16 macaques, ranging from 4.5 to 14.2 years. Thickness was evaluated between days 5-20, i.e., when thickness was high, and not thinned by hormonal influences. The line represents the linear relationship; p-value tests the hypothesis of non-zero slope in a multivariable model that controlled for changing levels of thickness over the course of the menstrual cycle C. Scatterplot shows the age of 19 macaques when they became vaginally infected with repeated, low doses of SHIVSF162P3 as described in 24.
Discussion
The study objective was to describe extent and dynamics of vaginal epithelial changes during the menstrual cycle of pigtail macaques with greater detail in time than was previously studied. We found that the epithelium is thinned to 66% of its former thickness in the luteal compared to the follicular phase, similar to previous findings in macaques5,6. However, it is with more narrow time intervals that we see the continuously changing thickness of vaginal tissue in these animals. When comparing the thickest and thinnest four-day intervals of the cycle, a maximum thinning to 31% was observed. Remarkably, during this same timeframe, the non-nucleated layer underwent thinning to 15% of its maximum depth. The extent of epithelial changes are not fully realized when the menstrual cycle is analyzed as follicular or luteal cycle phases. Another study objective was to assess the relationship between times of vaginal infection and epithelial thinning. In using two distinct sets of study animals, we correlated time of thinnest non-nucleated epithelial layer with times of highest infection probability. Our study provides new, and more quantitative support for a barrier function of the vaginal epithelium and its long-suspected role 4 for SIV and SHIV infection risk in female macaques.
Thinning of the superficial epithelial vaginal layer (s. corneum) correlated significantly with SHIV infections during the cycle. The s. corneum may be particularly critical for host defense 16,28. This layer has only loose intercellular junctions, and can therefore easily be penetrated by invading pathogens 2,3. Its’ cells are terminally differentiated, have largely lost nuclei and protein production machinery, and can therefore not mount immune responses de novo. Nonetheless, the layer is thought to play an important role in anti-HIV defense because it is glycogen-rich, a feature essential for nourishing the vaginal microbiome which maintains the vaginal pH. The role of the microbiome in host defense is only partially elucidated, but it is clear that variations in the microbiome leading to bacterial vaginosis increase HIV risk 29,30. Of note, however, there are substantial differences between human and pigtail macaque genital microbial populations 31. The s. corneum also exfoliates invading pathogens by continuously shedding itself and any attached pathogens. It is thus possible that thinning promotes SHIV uptake not only by reducing physical barriers and distance to target cells, but also through the associated changes in bacterial microflora or other innate resistance mechanisms.
There are important differences between the human and pigtail vaginal epithelium. Human vaginal epithelium is non-keratinizing and is less subject to thinning during the cycle and in response to hormonal contraception than in macaques. Mauck et al found a small but statistically significant change in the mean epithelial height of the vaginal wall between follicular and luteal phase in twenty women with mean age of 35.4 years10. Calculated by us to match our analysis, the epithelium thinned during luteal phase to 83% or 73% of its follicular phase thickness (from 212 to 174.6 microns, and from 283.6 to 205.9 microns, respectively), for two independent pathologists’ readings. There was no further reduction in thickness during DMPA (depot-medroxyprogesterone acetate, brand name Depo-Provera®) use. A reduction during the cycle was also reported by Chandra et al 11, calculated by us as a reduction to 86%, although thinning was not statistically significant. DMPA use resulted in thinning in some studies 12, but not others 13,14. It is thus clear that hormone-induced fluctuations in vaginal epithelial thickness are more limited in humans compared to pigtail macaques. However, the dynamics and extent of human thinning may not have been examined in similar detail as recorded here, and may be underestimated.
Study limitations include the small number of animals, inability to biopsy more than twice per animal per menstrual cycle, and the potential inaccuracy of infection times due to an unknown eclipse period as previously discussed 23,24. The relationship between epithelial thickness and susceptibility to infection is based upon ecological inference, as aggregate data from distinct animals rather than a single population of animals were studied. This was not possible because biopsy collection is expected to promote SHIV acquisition. We thus cannot conclude that vaginal epithelial thickness is a “correlate of protection” from SHIV infection. However, our findings do not refute this possibility. Also, we did not collect information on infiltrating lymphocytes, i.e., target cells for HIV.
All of our animals were purchased after they reached sexual maturity, which occurs at age 2.5 to 3 years in this species. We are thus not able to examine thinning or susceptibility to infection in very young females. We also could not examine the relationship of thinning with other reproductive factors that may be associated with increasing age, e.g., increased sexual experience, or parity, as this information was not included in health records at animal purchase. It is important to point out that the relationship of increasing thickness with age is expected only for reproductive age animals, and not animals past their reproductive stage. In women, the vaginal epithelium thins after menopause, likely due to low estrogen. Menopause can occur in macaques 32, but is not expected before age 25 years, well past the age range of our pigtail macaque cohort.
In conclusion, we documented the extent and kinetics of vaginal epithelial thinning in macaques by quantitating distinct vaginal epithelial layer thicknesses throughout the menstrual cycle. Our analysis suggests greater thinning during menstruation than previously observed. We describe the relationship of vaginal thinning and susceptibility to infection during the menstrual cycle. These data contribute to our understanding of innate resistance mechanisms to SHIV infection.
Summary.
A study in an animal model for vaginal HIV infection reveals a quantitative, correlative relationship between infection risk during the menstrual cycle and thinning of the superficial vaginal epithelial layer.
Acknowledgements
The authors wish to thank members of the animal model team and of the Animal Resources Branch for contributions to animal procedures. We thank colleagues who gave samples and data from 43 infected control macaques for previously published analyses critical for the current study. We thank the Wisconsin National Primate Center for performing progesterone analyses. Data were presented as abstract 2084170 at CROI (Conference on Retroviruses and Opportunistic Infections), February 2015, Seattle, WA. Disclaimer: The findings and conclusions in this report are those of the authors and do not necessarily represent the official position of the Centers for Disease Control and Prevention.
Sources of funding: Funded by CDC, and by inter-agency agreement Y1-AI-0681-02 between NIH and CDC
Footnotes
Statement: The authors do not have a commercial or other association that might pose a conflict of interest
References
- 1.Shattock RJ, Moore JP. Inhibiting sexual transmission of HIV-1 infection. Nature reviews. Microbiology. 2003;2003(1):25–34. doi: 10.1038/nrmicro729. [DOI] [PubMed] [Google Scholar]
- 2.Hu J, Gardner MB, Miller CJ. Simian immunodeficiency virus rapidly penetrates the cervicovaginal mucosa after intravaginal inoculation and infects intraepithelial dendritic cells. Journal of virology. 2000;2000(13):6087–6095. doi: 10.1128/jvi.74.13.6087-6095.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Carias AM, McCoombe S, McRaven M, et al. Defining the interaction of HIV-1 with the mucosal barriers of the female reproductive tract. Journal of virology. 2013;2013(21):11388–11400. doi: 10.1128/JVI.01377-13. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Marx PA, Spira AI, Gettie A, et al. Progesterone implants enhance SIV vaginal transmission and early virus load. Nature medicine. 1996;1996(10):1084–1089. doi: 10.1038/nm1096-1084. [DOI] [PubMed] [Google Scholar]
- 5.Poonia B, Walter L, Dufour J, Harrison R, Marx PA, Veazey RS. Cyclic changes in the vaginal epithelium of normal rhesus macaques. J Endocrinol. 2006;2006(3):829–835. doi: 10.1677/joe.1.06873. [DOI] [PubMed] [Google Scholar]
- 6.Hadzic SV, Wang X, Dufour J, et al. Comparison of the vaginal environment of Macaca mulatta and Macaca nemestrina throughout the menstrual cycle. Am J Reprod Immunol. 2014;2014(4):322–329. doi: 10.1111/aji.12201. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Radzio J, Hanley K, Mitchell J, et al. Physiologic doses of depot-medroxyprogesterone acetate do not increase acute plasma simian HIV viremia or mucosal virus shedding in pigtail macaques. AIDS (London, England) 2014;2014(10):1431–1439. doi: 10.1097/QAD.0000000000000294. [DOI] [PubMed] [Google Scholar]
- 8.Dietz Ostergaard S, Butler K, Ritter JM, et al. A combined oral contraceptive affects mucosal SHIV susceptibility factors in a pigtail macaque (Macaca nemestrina) model. Journal of medical primatology. 2015;2015(2):97–107. doi: 10.1111/jmp.12157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Butler K, Ritter J, Ellis S, et al. Analysis of Putative Mucosal SHIV Susceptibility Factors during Repeated DMPA Treatments in Pigtail Macaques. Journal of medical primatology. 2015 doi: 10.1111/jmp.12188. in press. [DOI] [PubMed] [Google Scholar]
- 10.Mauck CK, Callahan MM, Baker J, et al. The effect of one injection of Depo-Provera on the human vaginal epithelium and cervical ectopy. Contraception. 1999;1999(1):15–24. doi: 10.1016/s0010-7824(99)00058-x. [DOI] [PubMed] [Google Scholar]
- 11.Chandra N, Thurman AR, Anderson S, et al. Depot medroxyprogesterone acetate increases immune cell numbers and activation markers in human vaginal mucosal tissues. AIDS research and human retroviruses. 2013;2013(3):592–601. doi: 10.1089/aid.2012.0271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Miller L, Patton DT, Meier A, Thwin SS, Hooton TM, Eschenbach DA. Depomedroxyprogesterone-induced hypoestrogenism and changes in vaginal flora and epithelium. Obstet Gynecol. 2000;2000(3):431–439. doi: 10.1016/s0029-7844(00)00906-6. [DOI] [PubMed] [Google Scholar]
- 13.Bahamondes MV, Castro S, Marchi NM, et al. Human vaginal histology in long-term users of the injectable contraceptive depot-medroxyprogesterone acetate. Contraception. 2014;2014(2):117–122. doi: 10.1016/j.contraception.2014.01.024. [DOI] [PubMed] [Google Scholar]
- 14.Mitchell CM, McLemore L, Westerberg K, et al. Long-term effect of depot medroxyprogesterone acetate on vaginal microbiota, epithelial thickness and HIV target cells. The Journal of infectious diseases. 2014;2014(4):651–655. doi: 10.1093/infdis/jiu176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Murphy K, Irvin SC, Herold BC. Research gaps in defining the biological link between HIV risk and hormonal contraception. Am J Reprod Immunol. 2014;2014(2):228–235. doi: 10.1111/aji.12209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Achilles SL, Hillier SL. The complexity of contraceptives: understanding their impact on genital immune cells and vaginal microbiota. AIDS (London, England) 2013;27(Suppl 1):S5–15. doi: 10.1097/QAD.0000000000000058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Polis CB, Curtis KM. Use of hormonal contraceptives and HIV acquisition in women: a systematic review of the epidemiological evidence. Lancet Infect Dis. 2013 doi: 10.1016/S1473-3099(13)70155-5. [DOI] [PubMed] [Google Scholar]
- 18.Morrison CS, Chen PL, Kwok C, et al. Hormonal contraception and the risk of HIV acquisition: an individual participant data meta-analysis. PLoS medicine. 2015;12(1):e1001778. doi: 10.1371/journal.pmed.1001778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Baroni A, Buommino E, De Gregorio V, Ruocco E, Ruocco V, Wolf R. Structure and function of the epidermis related to barrier properties. Clinics in dermatology. 2012;2012(3):257–262. doi: 10.1016/j.clindermatol.2011.08.007. [DOI] [PubMed] [Google Scholar]
- 20.Miller CJ, Shattock RJ. Target cells in vaginal HIV transmission. Microbes and infection / Institut Pasteur. 2003;2003(1):59–67. doi: 10.1016/s1286-4579(02)00056-4. [DOI] [PubMed] [Google Scholar]
- 21.Parikh UM, Dobard C, Sharma S, et al. Complete protection from repeated vaginal simian-human immunodeficiency virus exposures in macaques by a topical gel containing tenofovir alone or with emtricitabine. Journal of virology. 2009;2009(20):10358–10365. doi: 10.1128/JVI.01073-09. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Patel P, Borkowf CB, Brooks JT, Lasry A, Lansky A, Mermin J. Estimating per-act HIV transmission risk: a systematic review. AIDS (London, England) 2014;2014(10):1509–1519. doi: 10.1097/QAD.0000000000000298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Vishwanathan SA, Guenthner PC, Lin CY, et al. High susceptibility to repeated, low-dose, vaginal SHIV exposure late in the luteal phase of the menstrual cycle of pigtail macaques. Journal of acquired immune deficiency syndromes (1999) 2011;2011(4):261–264. doi: 10.1097/QAI.0b013e318220ebd3. [DOI] [PubMed] [Google Scholar]
- 24.Kersh EN, Henning T, Vishwanathan SA, et al. SHIV susceptibility changes during the menstrual cycle of pigtail macaques. Journal of medical primatology. 2014;2014(5):310–316. doi: 10.1111/jmp.12124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.National Research Council (U.S.) Guide for the care and use of laboratory animals. 8th National Academies Press; Washington, D.C.: 2011. Committee for the Update of the Guide for the Care and Use of Laboratory Animals., Institute for Laboratory Animal Research (U.S.), National Academies Press (U.S.) [Google Scholar]
- 26.Livingston L, Sweeney E, Mitchell J, et al. Hormonal synchronization of the menstrual cycles of pigtail macaques to facilitate biomedical research including modeling HIV susceptibility. Journal of medical primatology. 2011;2011(3):164–170. doi: 10.1111/j.1600-0684.2010.00465.x. [DOI] [PubMed] [Google Scholar]
- 27.Hastie TJ, Tibshirani RJ. Generalized Additive Models. Chapman & Hall/CRC Monographs on Statistics & Applied Probability. 1990 [Google Scholar]
- 28.Anderson DJ, Marathe J, Pudney J. The structure of the human vaginal stratum corneum and its role in immune defense. Am J Reprod Immunol. 2014;2014(6):618–623. doi: 10.1111/aji.12230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Martin HL, Richardson BA, Nyange PM, et al. Vaginal lactobacilli, microbial flora, and risk of human immunodeficiency virus type 1 and sexually transmitted disease acquisition. The Journal of infectious diseases. 1999;1999(6):1863–1868. doi: 10.1086/315127. [DOI] [PubMed] [Google Scholar]
- 30.Taha TE, Gray RH, Kumwenda NI, et al. HIV infection and disturbances of vaginal flora during pregnancy. Journal of acquired immune deficiency syndromes and human retrovirology : official publication of the International Retrovirology Association. 1999;1999(1):52–59. doi: 10.1097/00042560-199901010-00008. [DOI] [PubMed] [Google Scholar]
- 31.Spear GT, Kersh E, Guenthner P, et al. Longitudinal assessment of pigtailed macaque lower genital tract microbiota by pyrosequencing reveals dissimilarity to the genital microbiota of healthy humans. AIDS research and human retroviruses. 2012;2012(10):1244–1249. doi: 10.1089/aid.2011.0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Walker ML, Herndon JG. Menopause in nonhuman primates? Biology of reproduction. 2008;2008(3):398–406. doi: 10.1095/biolreprod.108.068536. [DOI] [PMC free article] [PubMed] [Google Scholar]


