Abstract
Previous studies examining blood pressure change over time have modelled an “average” population trajectory. Recent research among older adults suggests there may be subgroups with different blood pressure trajectories. Identifying subgroups at risk of developing adult hypertension early in life can inform effective risk reduction efforts. We sought to identify different systolic blood pressure trajectories from childhood, their correlated risk factors and early midlife cardiovascular outcomes. Blood pressure data at ages 7, 11, 18, 26, 32 and 38 years from a longitudinal, representative birth cohort study (n=975) were used to identify four distinct trajectory groups via group-based trajectory modeling: ‘normal’ (21.8%), ‘high-normal’ (43.3%), ‘prehypertensive’ (31.6%), and ‘hypertensive’ (4.2%). The categories refer to blood pressure beginning at age 7 and most recently measured at age 38. Family history of high blood pressure (OR=43.23, 95% CI 5.27, 354.65), male gender (OR=109.48, 95% CI=26.82, 446.96), being first born (OR=2.5 95% CI=1.00, 8.69) and low birthweight (OR=2.79, 95% CI 2.49, 3.09) were associated with hypertensive group membership (compared to the normal group). Higher body mass index and cigarette smoking resulted in increasing blood pressure across trajectories, particularly for the higher blood pressure groups. Prehypertensive and hypertensive trajectory groups had worse cardiovascular outcomes by early midlife. Harmful blood pressure trajectories are identifiable in childhood, associated with both antecedent and modifiable risk factors over time, and predict adult cardiovascular disease risk. Early detection, subsequent targeted prevention and/or intervention may reduce the lifecourse burden associated with higher blood pressure.
Keywords: blood pressure, follow-up studies, hypertension, pediatrics, risk factor, high blood pressure
High blood pressure in adulthood is a leading cause of morbidity and mortality, a major modifiable risk factor for cardiovascular disease, and associated with other cardiovascular risk factors including impaired glucose tolerance, obesity and dyslipidemia1. The standard approach of treating high blood pressure in middle and old-age can help mitigate these risks, but considerable burden remains. An approach that identifies those at greatest risk of developing high blood pressure much earlier in life, could permit more effective risk reduction via earlier, age-appropriate prevention and intervention strategies.
In this context, universal routine screening to identify children at risk of developing high blood pressure in adulthood has been recommended by some organizations2, 3. However, in 2013 the U.S. Preventive Services Task Force concluded that current evidence was insufficient to assess the balance of benefits and harms of screening for primary hypertension in children and adolescents4. Proponents of universal screening note that blood pressure levels track from childhood to adulthood and that children with elevated blood pressure are more likely to develop hypertension in adulthood compared to children with low blood pressure5, 6. Importantly, hypertension is often asymptomatic and elevated blood pressure is associated with organ damage and changes in cardiac structure in children7. However, critics of universal screening argue that (a) the correlations between child and adult blood pressure levels are too weak8 (with blood pressure percentiles for children and adolescents used to define normative values not based on later cardiovascular risk)9 and (b) that further research is needed examining the longitudinal association between childhood blood pressure, adult hypertension and cardiovascular disease4.
Due to conflicting recommendations, clinicians need better information for medical decision-making around risk assessment and potential intervention options. Prospective-epidemiologic studies can provide such information. Recent longitudinal research suggests that there may be subgroups with differential blood pressure trajectories among older populations5. If there is developmental heterogeneity in blood pressure trajectories over time then (i) it should be theoretically possible to identify early in life, groups of people at greatest risk of developing high or clinically significant blood pressure in adulthood (ii) the investigation of risk factors that differentiate between healthy and unhealthy trajectories is an important objective.
A burgeoning body of evidence points to a range of early life predictors of high blood pressure in adulthood. These include intrauterine (e.g. low birthweight10, being first born11, maternal pregnancy hypertension12), postnatal (e.g. not being breastfed)13, familial (e.g. family history of high blood pressure)14, and psychosocial factors (e.g. childhood low socioeconomic status (SES))15. Males are significantly more likely to have higher blood pressure than females from childhood to midlife16.
Identification of factors that modify high blood pressure development from childhood to adulthood may help clinicians to stage ‘age-appropriate interventions’. Excess body weight is an established risk factor for high blood pressure, and increases in body mass index (BMI) have been associated with higher blood pressure tracking from childhood to adulthood17. Other potentially modifiable risk factors include excess alcohol consumption18 and cigarette smoking19; however, studies have mostly been cross-sectional, or among older cohorts.
To date researchers have commonly resorted to using assignment rules based on subjective categorization criteria (e.g. 95th percentile) to test taxonomical theories regarding normal versus pathological development. In this study, advanced longitudinal methods were applied to test the hypothesis that blood pressure changes are best understood via the investigation of subgroups within the population that differ in their rate of blood pressure increase over time. Although relatively new to health research, advanced longitudinal statistical modeling techniques have been used to examine developmental trajectories for other health states20. We sought to identify early midlife cardiovascular correlates of these developmental trajectories to help demonstrate broader clinical utility. To the best of our knowledge, this is the first time that this type of modeling has been used to identify blood pressure trajectory groups from childhood to adulthood in a single longitudinal birth cohort study.
The aims of the present research were to (i) identify latent groups of individuals sharing systolic blood pressure developmental trajectories from 7 to 38 years, (ii) identify early life predictors and potential modifiers of these trajectories, and (iii) describe the association between trajectories and early midlife cardiovascular risk indicators.
Methods
Participants are members of the Dunedin Multidisciplinary Health and Development Study, which has tracked the development of 1,037 individuals born in 1972–1973 in Dunedin, New Zealand. The study design and inclusion criteria have been described elsewhere21 (for details, see the online-only Data Supplement).
The present study used systolic blood pressure data measured at ages 7, 11, 18, 26, 32 and 38 years (all ages at which more than one measure of blood pressure was taken). Blood pressure was measured in a quiet room, using a cuff of appropriate size, with the Study member in a seated position, by medically trained assessors. Up to 18 years, a London School of Hygiene and Tropical Medicine blind mercury sphygmomanometer (Cinetronics Ltd., Mildenhall, United Kingdom) was used. Up to 38 years, a Hawksley random-zero sphygmomanometer (Hawksley and Sons Ltd., Sussex, United Kingdom) with a constant deflation valve was used. Systolic blood pressure was assessed as the first Korotkoff sound and based on the mean of either two or three measures taken at five minute intervals according to a standard protocol. Antihypertensive medication use information was collected at ages 26 (n=6), 32 (n=12) and 38 years (n=26). Participants were coded as having a systolic blood pressure of 140 mmHg for the assessment age they were on medication. Data were included if participants had blood pressure information at three or more ages (n=975), with information at a specific age subsequently excluded if a participant was pregnant (n=30 at age 26; n=26 at age 32; n=8 at age 38) (for details on the inclusion criteria based on blood pressure data, see the online-only Data Supplement).
Early life factors
Birthweight (grams) and maternal pregnancy hypertension (DBP ≥ 90 mmHg) data were taken from antenatal and perinatal hospital records. Birth order data (first born, second born, third born and higher) were obtained from parent report at age 3. Breastfeeding information was collected at the age 3 assessment and checked against validated health records22.
The collection of family history data about high blood pressure among study members’ biological siblings, parents and grandparents was conducted in 2003–2006 and is described elsewhere23 (for details, see the online-only Data Supplement).
Childhood SES from birth to 5 years was based on parents’ self-reported occupational status24 (for details, see the online-only Data Supplement).
Effect modifiers from 7 to 38 years
Height was recorded to the nearest millimeter using a portable stadiometer (Harpenden; Holtain, Ltd). Weight was measured to the nearest 0.1 kg using calibrated scales. Participants were weighed in light clothing. BMI was calculated in kilograms per square meter (weight (kg)/height (m)2)25.
Alcohol consumption at 7 and 11 years was coded as zero for all participants. At age 18, participants were asked how often they usually drank alcohol. At ages 26, 32 and 38 years, participants reported how many drinks (standard units) on average they consumed weekly.
No participants smoked daily at 7 or 11 years, so daily smoking was coded as zero. The current number of cigarettes smoked per day (for at least one month in the previous year) was recorded at ages 18, 26, 32 and 38.
Age 38 cardiovascular risk indicators
Biomarkers were obtained for measurements of nonfasting total cholesterol (mmol/L), HDL cholesterol level (mmol/L), triglyceride level (mmol/L) and glycated hemoglobin at age 38. Venipuncture was conducted at the same time each day (4:15–4:45 pm). Ninety five percent of the sample consented to phlebotomy, with pregnant women at this age excluded from the analyses (for further details on biomarker assessment, see the online-only Data Supplement).
Waist and hip girth were measured by averaging two measurements taken using a steel tape calibrated in centimeters with millimeter gradations. Waist girth was taken as the perimeter at the level of the noticeable waist and hip girth at the widest point of the hips.
A composite measure of metabolic abnormalities (excluding blood pressure) based on the United States National Cholesterol Education Program Adult Treatment Panel III guidelines was created (see the online-only Data Supplement)26. In brief, participants were considered to have met the criteria for the presence of metabolic abnormalities if they had three of four abnormalities.
Statistical analysis
Group based trajectory modelling (GBTM) was used to identify distinctive groups of individual trajectories in the population from ages 7 to 38 years. We used Proc Traj, a Statistical Analysis Software macro to estimate the model parameters27 using a censored normal model appropriate for continuous normally distributed data28.
A number of criteria20, 28 were used to determine the number of blood pressure trajectory groups and the trajectory shapes (e.g. cubic in age) (for details, see the online-only Data Supplement). To determine the best model, 12 models were fitted.
We examined two types of covariates, early life factors and effect modifiers (time varying covariates). To examine the association between cardiovascular disease risk indicators at 38 years and blood pressure trajectories, we used analysis of variance for continuous measures and χ2 statistics for categorical measures.
Results
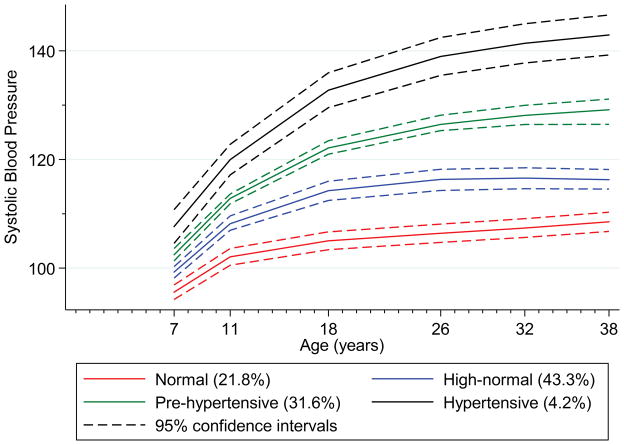
A four trajectory group model with cubic specifications for all groups was identified. Figure 1 shows the plotted predicted trajectory lines for each of the four groups designated as “normal”, “high-normal”, “prehypertensive” and “hypertensive”, with 95% confidence intervals based on predicted trajectory means. The means for all four groups significantly differed from each other at all ages, beginning at age 7 years. The normal group (21.8% of the sample population) and high-normal group (43.3%) had mean blood pressures in adulthood in the normal systolic blood pressure range (90–120 mmHg). The prehypertensive group (31.6%) had mean systolic blood pressures within the prehypertensive range (120–139 mmHg) throughout adulthood. The hypertensive group (4.2%) had the highest mean blood pressure at 7 years and displayed the steepest rise in blood pressure with mean blood pressure in the hypertensive range at 38 years (≥140 mmHg). Table S1 provides data on predictive values for prehypertension (≥120 mmHg) and hypertension (≥140 mmHg) at age 38 based on prehypertensive and hypertensive group membership and blood pressure measured at each assessment age.
Figure 1.
Plot of predicted trajectory lines with 95% confidence intervals for the four blood pressure trajectory groups identified in a general population longitudinal birth cohort
Both early life factors and effect modifiers were examined in relation to blood pressure trajectories (Tables 1 and 2). Table 1 also provides data on the characteristics of participants for early life factors based on trajectory group membership. Information on the physical characteristics of participants and effect modifiers over time based on group membership is presented in Table S2. Analyses were limited to 913 study members in the multivariable trajectory model due to some missing data among the covariates. There were a number of individuals assigned to different trajectory groups as a consequence of adding all covariates (early life factors and effect modifiers), resulting in a 4% improvement in model fit (Bayesian Information Criterion = −17577.86 vs. −18172.22).
Table 1.
Risk factors for blood pressure trajectory group membership: univariate and multivariable analyses, age 7 to 38 years
| Univariate | Multivariable* | |||||
|---|---|---|---|---|---|---|
| Risk factor | N (%)† | Odds ratio | 95% (CI) | N (%)† | Odds ratio | 95% CI |
| Maternal Hypertension (vs. no) | ||||||
| Normal group | 9 (4.9) | 1.0 | 6 (3.9) | 1.0 | ||
| High-normal group | 32 (7.9) | 1.43 | 0.54, 3.78 | 32 (8.0) | 1.89 | 0.64, 5.47 |
| Prehypertensive group | 32 (11.1) | 2.57 | 1.11, 5.95 | 38 (11.9) | 3.59 | 1.24, 10.34 |
| Hypertensive group | 5 (14.3) | 2.50 | 0.62, 10.06 | 2 (4.9) | 0.92 | 0.15, 5.51 |
| First born (vs. others) | ||||||
| Normal group | 61 (33.3) | 1.0 | 53 (34.9) | 1.0 | ||
| High-normal group | 113 (27.8) | 0.78 | 0.47, 1.30 | 113 (28.2) | 0.98 | 0.53, 1.82 |
| Prehypertensive group | 100 (34.7) | 1.11 | 0.70, 1.76 | 103 (32.3) | 1.04 | 0.54, 2.00 |
| Hypertensive group | 14 (40.0) | 1.41 | 0.61, 3.26 | 19 (46.3) | 2.95 | 1.00, 8.69 |
| Male (vs. female) | ||||||
| Normal group | 32 (17.5) | 1.0 | 10 (6.6) | 1.0 | ||
| High-normal group | 189 (46.4) | 7.83 | 3.70, 16.55 | 163 (40.6) | 7.76 | 3.24, 18.58 |
| Prehypertensive group | 216 (75.0) | 35.33 | 16.56, 75.41 | 260 (81.5) | 47.66 | 19.73, 115.13 |
| Hypertensive group | 33 (94.3) | 113.34 | 22.09, 581.41 | 37 (90.2) | 109.48 | 26.82, 466.96 |
| Breastfeeding <4wks (vs 4wks) | ||||||
| Normal group | 99 (54.1) | 1.0 | 86 (56.6) | 1.0 | ||
| High-normal group | 199 (48.9) | 0.64 | 0.40, 1.02 | 186 (46.4) | 0.73 | 0.43, 1.23 |
| Prehypertensive group | 147 (51.0) | 0.84 | 0.54, 1.29 | 172 (53.9) | 0.96 | 0.55, 1.67 |
| Hypertensive group | 14 (40.0) | 0.47 | 0.20, 1.06 | 15 (36.6) | 0.49 | 0.20, 1.20 |
| Low SES (vs others) | ||||||
| Normal group | 22 (12.0) | 1.0 | 18 (11.8) | 1.0 | ||
| High-normal group | 55 (13.5) | 1.37 | 0.65, 2.90 | 58 (14.5) | 1.03 | 0.42, 2.52 |
| Prehypertensive group | 32 (11.1) | 1.08 | 0.53, 2.19 | 34 (10.7) | 0.98 | 0.36, 2.65 |
| Hypertensive group | 6 (17.1) | 2.08 | 0.71, 6.12 | 5 (12.2) | 0.72 | 0.17, 3.12 |
|
| ||||||
| Risk factor | Mean (SD) | Odds ratio | 95% (CI) | Mean (SD) | Odds ratio | 95% CI |
|
| ||||||
| Birthweight (kgs) | ||||||
| Normal group | 3.37 (0.51) | 1.0 | 3.41 (0.47) | 1.0 | ||
| High-normal group | 3.39 (0.52) | 1.14 | 0.73, 1.78 | 3.39 (0.52) | 0.77 | 0.46, 1.30 |
| Prehypertensive group | 3.37 (0.52) | 1.02 | 0.68, 1.54 | 3.39 (0.54) | 0.62 | 0.36, 1.07 |
| Hypertensive group | 3.44 (0.58) | 1.13 | 0.51, 2.51 | 3.23 (0.48) | 0.36 | 0.16, 0.83 |
| Proportion of relatives with high blood pressure | ||||||
| Normal group | 0.22 (0.16) | 1.0 | 0.21 (0.16) | 1.0 | ||
| High-normal group | 0.24 (0.20) | 2.56 | 0.69, 9.47 | 0.24 (0.19) | 3.27 | 0.81, 13.24 |
| Prehypertensive group | 0.27 (0.20) | 6.38 | 1.91, 21.32 | 0.27 (0.20) | 6.15 | 1.39, 27.14 |
| Hypertensive group | 0.34 (0.18) | 31.79 | 4.41, 202.00 | 0.34 (0.18) | 43.23 | 5.27, 354.65 |
Model contains early life factors (maternal hypertension, birthweight, birth order, gender, family history of high blood pressure, breastfeeding, early childhood socioeconomic status) and effect modifiers (body mass index, alcohol consumption, cigarette smoking)
Number and percentage with risk factor in each trajectory group
Table 2.
Effect modifiers influencing blood pressure trajectory level within each group: univariate and multivariable analyses, age 7 to 38 years
| Univariate | Multivariable* | |||||
|---|---|---|---|---|---|---|
| Risk factor | Shift in trajectory per unit change in variable | 95% CI | p-value | Shift in trajectory per unit change in variable | 95% CI | p-value |
| Body mass index | ||||||
| Normal group | 0.53 | 0.41, 0.65 | <.001 | 0.50 | 0.36, 0.63 | <.001 |
| High-normal group | 0.92 | 0.78, 1.05 | <.001 | 0.79 | 0.67, 0.91 | <.001 |
| Prehypertensive group | 1.09 | 0.94, 1.24 | <.001 | 1.04 | 0.89, 1.18 | <.001 |
| Hypertensive group | 1.62 | 1.18, 2.07 | <.001 | 1.70 | 1.25, 2.15 | <.001 |
| Average weekly alcohol consumption | ||||||
| Normal group | 0.02 | −0.03, 0.07 | .49 | 0.01 | −0.06, 0.08 | .72 |
| High-normal group | 0.08 | 0.03, 0.13 | .002 | 0.02 | −0.03, 0.06 | .47 |
| Prehypertensive group | −0.04 | −0.10, 0.03 | .30 | −0.02 | −0.06, 0.03 | .49 |
| Hypertensive group | 0.00 | −0.11, 0.11 | .98 | 0.06 | −0.11, 0.23 | .47 |
| Number of cigarette per day (in last month) | ||||||
| Normal group | 0.05 | −0.05, 0.15 | .33 | 0.07 | −0.04, 0.18 | .19 |
| High-normal group | 0.11 | 0.03, 0.19 | .007 | 0.07 | 0.00, 0.15 | .05 |
| Prehypertensive group | 0.01 | −0.08, 0.11 | .83 | 0.09 | 0.01, 0.17 | .02 |
| Hypertensive group | 0.05 | −0.11, 0.22 | .52 | 0.23 | −0.07, 0.53 | .13 |
Model contains early life factors (maternal hypertension, birthweight, birth order, gender, family history of high blood pressure, breastfeeding, early childhood socioeconomic status) and effect modifiers (body mass index, alcohol consumption, cigarette smoking)
In the fully adjusted model (including all covariates), males had significantly greater odds of being in the high-normal, prehypertensive and hypertensive group compared to the normal group (Table 1). Participants who had a higher proportion of relatives with high blood pressure had greater odds of being in the prehypertensive (OR=6.15, 95% CI 1.39, 27.14) or the hypertensive group (OR=43.23, 95% CI 5.27, 354.65) compared to the normal group. Study members whose mothers had pregnancy hypertension had greater odds of being in the prehypertensive group (OR=3.59, 95% CI 1.24, 10.34) but not the hypertensive group (OR=0.92, 95% CI 0.15, 5.51) when compared to the normal group. Participants who were first born had higher odds of being in the hypertensive group (OR=2.95, 95% CI 1.00, 8.69) compared to the normal group, and study members with lower birth weights had increased odds of being in the hypertensive group (OR=2.79, 95% CI 2.49, 3.09). No significant associations were found for early SES or breastfeeding.
Increasing BMI was significantly associated with an upward shift in all four blood pressure trajectory groups (Table 2). Analyses were then undertaken to determine whether BMI had a greater effect on the trajectory levels of the high-normal, prehypertensive or hypertensive groups compared to those in the normal group (i.e. whether coefficients were significantly different by group). The effect of BMI over time was significantly different (i.e. stronger) for two groups (the prehypertensive, χ2=21.4, p<.001 and hypertensive group, χ2=9.0, p=.002) when compared to the normal group. Increasing number of daily cigarettes was also significantly associated with an upward shift in trajectories for the high-normal and prehypertensive groups. No significant associations were found between trajectory groups and alcohol consumption.
Mean values for cardiovascular risk outcomes at age 38 based on trajectory group membership are presented in Table 3. Significant mean differences were found between groups and cardiovascular risk indicators. After controlling for covariates, significant differences were found for waist/hip ratio (F(3, 868) = 42.59, p<.001), total cholesterol level (F(3, 849) = 11.08, p<.001), HDL cholesterol level (F(3, 849) = 4.00, p =.008), glycated hemoglobin (F(3, 839) = 3.35, p=.02) triglyceride level (F(3, 849) = 16.59, p<.001), and the presence of metabolic abnormality (χ2=8.22, p=.04). In general, the higher the risk for high blood pressure, the greater the risk for other cardiovascular risk factors.
Table 3.
Blood pressure trajectory group membership from ages 7 to 38 years and cardiovascular health related outcome measures and risk factors at 38 years
| Univariate | Multivariable* | |||
|---|---|---|---|---|
| Risk Factor | Mean | P-Value† | Mean | P-Value† |
| Waist hip ratio‡ (mm) | <.001 | <.001 | ||
| Normal group | .809 | .802 | ||
| High-normal group | .848 | .847 | ||
| Prehypertensive | .885 | .887 | ||
| Hypertensive group | .929 | .885 | ||
| Total cholesterol | <.001 | <.001 | ||
| Normal group | 4.90 | 5.03 | ||
| High-normal group | 5.14 | 5.07 | ||
| Prehypertensive group | 5.44 | 5.46 | ||
| Hypertensive group | 5.85 | 5.45 | ||
| High Density Lipoprotein cholesterol | <.001 | .008 | ||
| Normal group | 1.56 | 1.54 | ||
| High-normal group | 1.47 | 1.47 | ||
| Prehypertensive group | 1.38 | 1.40 | ||
| Hypertensive group | 1.26 | 1.39 | ||
| Glycated haemoglobin concentration | .06 | .019 | ||
| Normal group | 5.32 | 5.30 | ||
| High-normal group | 5.40 | 5.39 | ||
| Prehypertensive group | 5.43 | 5.45 | ||
| Hypertensive group | 5.52 | 5.36 | ||
| Triglyceride level | <.001 | <.001 | ||
| Normal group | 1.54 | 1.58 | ||
| High-normal group | 1.98 | 1.91 | ||
| Prehypertensive group | 2.41 | 2.49 | ||
| Hypertensive group | 3.05 | 2.24 | ||
| % | % | |||
| Composite index of metabolic abnormalities§ | <.001¶ | .04 | ||
| Normal group | 4.1 | 6.3 | ||
| High-normal group | 8.6 | 10.1 | ||
| Prehypertensive group | 14.9 | 13.5 | ||
| Hypertensive group | 24.2 | 2.6 | ||
Model contains early life factors (maternal hypertension, birthweight, birth order, gender, family history of high blood pressure, breastfeeding, early childhood socioeconomic status) and effect modifiers (body mass index, alcohol consumption, cigarette smoking)
Analysis of Variance
The same pattern and strength of association was found when only waist circumference was used (data not shown)
≥3 risk factors endorsed: (i) High waist circumference >880mm (women) >1020mm (men); (ii) High density lipoprotein cholesterol <40 mmol/L (men), <50 mmol/L (women), or cholesterol medication; (iii) Glycated hemoglobin >5.7%; (iv) Triglycerides >200/2.26 mmol/L, or cholesterol medication
Discussion
The earliest age at which medical practitioners can identify young healthy individuals who are likely to develop high blood pressure in adulthood is not known. The present paper addresses this question and reports four new findings: (i) those at greatest risk of developing high blood pressure in adulthood had elevated blood pressure at 7 years and were distinguishable from those with normal blood pressure trajectories from childhood into early midlife; (ii) several early life risk factors that antedated our first blood pressure measurement at age 7 were identified using trajectory modelling. These risk factors differentiated between groups of individuals on healthy and unhealthy trajectories; (iii) BMI was a major effect modifier for all blood pressure trajectories over time, with effects strongest among the highest blood pressure groups. Cigarette smoking also modified trajectories for the normal-high and prehypertensive groups; (iv) blood pressure trajectories leading to pre-hypertension and hypertension in adulthood, whilst of concern in and of themselves, also predicted a range of other cardiovascular risk indicators in early midlife, including a composite index of metabolic abnormalities, indicating even greater clinical salience of these high-risk trajectories.
Against a background of conflicting evidence regarding the pros and cons of population-based screening for blood pressure risk, new evidence aiding clinical risk assessments earlier in the lifecourse is timely. By implementing a trajectory approach, we found that individuals destined to become prehypertensive and hypertensive by early midlife comprised approximately one third of this general population sample. Moreover, by using data collected over time we were able to better predict those individuals who developed prehypertension and hypertension in early mid-life compared with measures of blood pressure taken at only one time point (e.g. 7 years) (Table S1) suggesting the importance of routine blood pressure measurements beginning in childhood.
Prior blood pressure research has linked risk factors measured early in life with high blood pressure at a single point in time later in life. This present research goes beyond that by using group based trajectory modelling to link risk factors to trajectories capturing the entire developmental course of blood pressure from ages 7 to 38 years. These included a number of established risk factors such as having a family history of high blood pressure, male gender, maternal pregnancy hypertension, and lower birthweight. Current data strongly supports male gender and a family history of hypertension as important early life risk factors and these are recommended as part of the US-Preventive Services Task Force risk assessment for primary hypertension in children. The odds ratios for male gender and having a family history of high blood pressure were high but the confidence intervals for the odds ratios were wide, possibly reflecting the small number of participants in these groups particularly in the hypertensive group. Identifying the effects of pregnancy and in-utero factors, such as low birthweight, can help to determine the development of cardiovascular risk indicators, including high blood pressure29. An association between maternal pregnancy hypertension and offspring’s blood pressure most likely results from both a genetic transmission from parent to child plus in-utero effects. A more recent finding was also replicated in our study; that is, first born children had higher odds of being in a trajectory group resulting in hypertension compared to the normotensive group. Although there are no obvious mechanisms underlying the link between being first born and having higher blood pressure, physical changes occur in the uterus during implantation and placentation in the first pregnancy. During later pregnancies these changes are more apparent with increased efficiency of placental invasion of the uterine wall and subsequent improved nutrient flow to the fetus30. Higher blood pressure in later life may therefore be associated with a degree of in-utero nutrient restriction of first borns compared to later borns31. Neither breastfeeding duration nor lower early childhood SES were significantly associated with blood pressure trajectory group membership.
Knowledge about modifiable risk factors that alter blood pressure trajectories can help determine intervention foci. This study showed that increasing BMI predicted an upward shift in blood pressure trajectory levels across the entire population. However, the impact of higher BMI was greatest among the higher blood pressure groups, suggesting excess body weight may be particularly problematic for individuals already on a trajectory toward developing higher blood pressure. Our results support recommendations for blood pressure screening of children and adolescents who are overweight and obese32 and population-based strategies that focus on maintaining a healthy weight. Similar to previous longitudinal studies19, we found that cigarette smoking was a significant risk factor and associated with an upward shift in trajectories for those in the high-normal and prehypertensive groups (approximately 75% of population). No statistically significant association was found for the hypertensive group; this may be due to small numbers in that group. Alcohol was not associated with shifts in trajectory levels in our study participants as they entered early mid-life. Future research is required to ascertain if this lack of association persists at older ages.
To determine the predictive validity of the developmental blood pressure trajectory groups, early midlife cardiovascular correlates of these trajectories were examined. Individuals in the higher blood pressure trajectory groups were more likely to have higher levels of known cardiovascular risk factors at age 38. These findings suggest that high systolic blood pressure in early life can serve as a generic risk indicator for poorer cardiovascular health more generally. These findings also support recommendations that routine screening for increased blood pressure in childhood may help to prevent subsequent cardiovascular disease in later life via age-appropriate intervention3, 33. Further follow-up is needed to determine whether this will translate into decreased life expectancy and cardiovascular disease endpoints (e.g. cardiac arrest, stroke).
There are several strengths and some limitations in the present study. Few prospective studies exist with the breadth of data to investigate the range of determinants of blood pressure risk factor trajectories in this way. This representative population study has experienced very low rates of attrition over almost four decades giving confidence in the observed associations. Early life information was validated using hospital records and measures were created via multiple sources (e.g. family history of high blood pressure). The limitations of the present study must also be considered. Firstly, despite having BMI information, we could not examine the association between physical activity and blood pressure because activity information was not collected at all time-points. Secondly, although we were able to examine the effect that alcohol consumption and cigarette smoking had over time, frequency of use data was restricted to information collected at ages 18 and older.
Perspectives
Millions die annually due to diseases directly associated with hypertension, and this number is rising. Intervening in early life to reduce high blood pressure may help increase life expectancy worldwide and reduce the associated burden of disease. The findings from this study suggest that groups of individuals who will develop high systolic blood pressure in early to mid-adulthood have elevated blood pressure in childhood, and that these individuals are more likely to be affected by cardiovascular disease-related comorbidity by age 38 years. In other words, estimating risk for future hypertension (and cardiovascular disease more generally) can be undertaken among children, long before systolic blood pressure rises to clinically detectable levels of pre-hypertension or hypertension in adulthood. Importantly, this research reinforces the importance of encouraging lifestyle changes including supporting the maintenance of a healthy body weight, particularly for those already on a high risk blood pressure trajectory into adulthood.
Supplementary Material
Novelty and Significance.
What is new?
In a longitudinal birth cohort study of 1000 participants we conducted trajectory modelling to identify four distinct developmental systolic blood pressure trajectory groups from ages 7 to 38 years. Groups at greatest risk of developing high blood pressure and related cardiovascular co-morbidities (e.g. higher total cholesterol) at age 38 years had higher blood pressure beginning at age 7 compared to individuals in normal blood pressure trajectory groups.
What is relevant?
Individuals in harmful developmental blood pressure trajectory groups were more likely to be male, have a family history of hypertension, be first born, and born lower birthweight; information that is useful for screening purposes. Higher body mass index and cigarette smoking over time resulted in increasing blood pressure levels, particularly for those individuals in higher blood pressure groups. Encouraging lifestyle changes (e.g. weight reduction, the maintenance of a healthy body weight and smoking cessation) may help to lower blood pressure levels over time, particularly for those individuals on a trajectory to developing hypertension.
Summary
Our findings suggest that it is possible to identify early in life, harmful blood pressure trajectories that are associated with both antecedent and modifiable risk factors over time, and predict cardiovascular disease risk in adulthood.
Acknowledgments
The authors are indebted to Phil Silva, the founder of the Dunedin Study, and to the Study members and their families for their long-term involvement.
Sources of Funding
The Dunedin Multidisciplinary Health and Development Study is supported by the New Zealand Health Research Council (HRC) and the New Zealand Ministry of Business, Innovation and Employment (MBIE). This research also received support from the United Kingdom Medical Research Council (Grant G0100527) and the National Institute of Aging (Grants R01AG032282 and R01AG048895). Reremoana Theodore was supported by a University of Otago Health Sciences Postdoctoral Fellowship and a HRC Erihapeti Rehu-Murchie fellowship (Grant 13/579).
References
- 1.Chobanian AV, Bakris GL, Black HR, Cushman WC, Green LA, Izzo JL, Jr, Jones DW, Materson BJ, Oparil S, Wright JT, Jr, Roccella EJ Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure, National Heart Lung, and Blood Institute, National High Blood Pressure Education Program Coordinating Committee. Seventh report of the Joint National Committee on Prevention, Detection, Evaluation, and Treatment of High Blood Pressure. Hypertension. 2003;42:1206–1252. doi: 10.1161/01.HYP.0000107251.49515.c2. [DOI] [PubMed] [Google Scholar]
- 2.Expert Panel of Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescents. Expert Panel on Integrated Guidelines for Cardiovascular Health and Risk Reduction in Children and Adolescence: Summary report. Pediatrics. 2011;128:S213–256. doi: 10.1542/peds.2009-2107C. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Pickering TG, Hall JE, Appel LJ, Falkner BE, Graves JW, Hill MN, Jones DH, Kurtz T, Sheps SG, Roccella EJ Council on High Blood Pressure Research Professional and Public Education Subcommittee American Heart Association. . Recommendations for blood pressure measurement in humans: An AHA scientific statement from the Council on High Blood Pressure Research Professional and Public Education Subcommittee. Journal of Clinical Hypertension. 2005;7:102–109. doi: 10.1111/j.1524-6175.2005.04377.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Moyer VA on behalf of the U. S. Preventive Services Task Force. Screening for primary hypertension in children and adolescents: U.S. Preventive Services Task Force recommendation statement. Annals of Internal Medicine. 2013;159:613–619. doi: 10.7326/0003-4819-159-9-201311050-00725. [DOI] [PubMed] [Google Scholar]
- 5.Wills AK, Lawlor DA, Muniz-Terrara G, Matthews F, Cooper R, Ghosh AK, Kuh D, Hardy R. Population heterogeneity in trajectories of midlife blood pressure. Epidemiology. 2012;23:203–211. doi: 10.1097/EDE.0b013e3182456567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chen X, Wang Y. Tracking of blood pressure from childhood to adulthood: A systematic review and meta-regression analysis. Circulation. 2008;117:3171–3180. doi: 10.1161/CIRCULATIONAHA.107.730366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Urbina EM, Khoury PR, McCoy C, Daniels SR, Kimball TR, Dolan LM. Cardiac and vascular consequences of pre-hypertension in youth. Journal of Clinical Hypertension. 2011;13:332–342. doi: 10.1111/j.1751-7176.2011.00471.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Lurbe E. Childhood blood pressure: A window to adult hypertension. J Hypertens. 2003;21:2001–2003. doi: 10.1097/00004872-200311000-00004. [DOI] [PubMed] [Google Scholar]
- 9.Chiolero A, Bovet P, Paradis G. Screening for elevated blood pressure in children and adolescents. JAMA Pediatrics. 2013;167:266–273. doi: 10.1001/jamapediatrics.2013.438. [DOI] [PubMed] [Google Scholar]
- 10.Huxley RR, Shiell AW, Law CM. The role of size at birth and postnatal catchup growth in determining systolic blood pressure: A systematic review of the literature. J Hypertens. 2000;18:815–831. doi: 10.1097/00004872-200018070-00002. [DOI] [PubMed] [Google Scholar]
- 11.Ayyavoo A, Savage T, Derraik JGB, Hofman PL, Cutfield WS. First-born children have reduced insulin sensitivity and higher daytime blood pressure compared to later-born children. Journal of Clinical Endocrinology and Metabolism. 2013;98:1248–1253. doi: 10.1210/jc.2012-3531. [DOI] [PubMed] [Google Scholar]
- 12.Law CM, Barker DJP, Bull AR, Osmond C. Maternal and fetal influences on blood pressure. Archives of Disease in Childhood. 1991;66:1291–1295. doi: 10.1136/adc.66.11.1291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Martin RM, Ness AR, Gunnell D, Emmett P, Smith GD. Does breast-feeding in infancy lower blood pressure in childhood? Circulation. 2004;109:1259–1266. doi: 10.1161/01.CIR.0000118468.76447.CE. [DOI] [PubMed] [Google Scholar]
- 14.Munger RG, Prineas RJ, Gomez-Marin O. Persistent elevation of blood pressure among children with a family history of hypertension: The Minneapolis Children’s Blood Pressure Study. J Hypertens. 1988;6:647–653. doi: 10.1097/00004872-198808000-00008. [DOI] [PubMed] [Google Scholar]
- 15.Hardy R, Kuh D, Langenberg C, Wadsworth ME. Birthweight, childhood social class, and change in adult blood pressure in the 1946 British Birth Cohort. Lancet. 2005;362:1178–1183. doi: 10.1016/S0140-6736(03)14539-4. [DOI] [PubMed] [Google Scholar]
- 16.Wang X, Poole JC, Treiber FA, Harshfield GA, Hanevold CD, Snieder H. Ethnic and gender differences in ambulatory blood pressure trajectories: Results from a 15-year longitudinal study in youth and young adults. Circulation. 2006;114:2780–2787. doi: 10.1161/CIRCULATIONAHA.106.643940. [DOI] [PubMed] [Google Scholar]
- 17.Burke V, Beilin LJ, Dunbar D. Tracking of blood pressure in Australian children. J Hypertens. 2001;19:1185–1192. doi: 10.1097/00004872-200107000-00002. [DOI] [PubMed] [Google Scholar]
- 18.DeFrank RS, Jenkins CD, Rose RM. A longitudinal investigation of the relationships among alcohol consumption, psychosocial factors, and blood pressure. Psychosom Med. 1987;49:236–249. doi: 10.1097/00006842-198705000-00003. [DOI] [PubMed] [Google Scholar]
- 19.Parikh NI, Pencina MJ, Wang TJ, Benjamin EJ, Lanier KJ, Levy D, D’Agostino RB, Sr, Kannel WB, Vasan RS. A risk score for predicting near-term incidence of hypertension: The Framingham Heart Study. Annals of Internal Medicine. 2008;148:102–110. doi: 10.7326/0003-4819-148-2-200801150-00005. [DOI] [PubMed] [Google Scholar]
- 20.Ostbye T, Malhotra R, Landerman LR. Body mass trajectories through adulthood: Results from the National Longitudinal Survey of Youth 1979 cohort (1981–2006) Int J Epidemiol. 2011;40:240–250. doi: 10.1093/ije/dyq142. [DOI] [PubMed] [Google Scholar]
- 21.Poulton R, Moffitt TE, Silva PA. The Dunedin Multidisciplinary Health and Development Study: Overview of the first 40 years, with an eye to the future. Social Psychiatry and Psychiatric Epidemiology. 2015;50:679–693. doi: 10.1007/s00127-015-1048-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Sears MR, Greene JM, Willan A, Taylor DR, Flannery EM, Cowan JO, Herbison GP, Poulton R. Long-term relation between breast-feeding and development of atopy and asthma in chidlren and young adults: A longitudinal study. Lancet. 2002;360:901–907. doi: 10.1016/S0140-6736(02)11025-7. [DOI] [PubMed] [Google Scholar]
- 23.Melchior M, Moffitt TE, Milne BJ, Poulton R, Caspi A. Why do children from socioeconomically disadvantaged families suffer from poor health when they reach adulthood? A life-course study. American Journal of Epidemiology. 2007;166:966–974. doi: 10.1093/aje/kwm155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Elley WB, Irving JC. Revised socio-economic index for New Zealand. New Zealand Journal of Educational Studies. 1976;11:25–36. [Google Scholar]
- 25.Belsky DW, Caspi A, Goldman-Mellor S, Meier MH, Ramrakha S, Poulton R, Moffitt TE. Is obesity associated with a decline in intelligence quotient during the first half of the life course? Am J Epidemiol. 2013;178:1461–1468. doi: 10.1093/aje/kwt135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Alberti KG, Eckel RH, Grundy SM, Zimmet PZ, Cleenman JI, Donato KA, Fruchart J-C, James PT, Loria CM, Smith SC. Harmonizing the metabolic syndrome: A joint interim statement of the International Diabetes Federation Task Force on Epidemiology and Prevention; National Heart, Lung, and Blood Institute; American Heart Association; World Heart Federation; International Atherosclerosis Society; and International Association for the Study of Obesity. Circulation. 2009;120:1640–1645. doi: 10.1161/CIRCULATIONAHA.109.192644. [DOI] [PubMed] [Google Scholar]
- 27.Jones BL, Nagin DS, Roeder K. A SAS procedure based on mixture models for estimating developmental trajectories. Sociological Research and Methods. 2001;29:374–393. [Google Scholar]
- 28.Nagin DS. Group-based modeling of development. Cambridge: Harvard University Press; 2005. [Google Scholar]
- 29.Huxley RR, Neil A, Collins R. Unravelling the fetal origins hypothesis: Is there really an inverse association between birthweight and subsequent blood pressure? Lancet. 2002;360:659–665. doi: 10.1016/S0140-6736(02)09834-3. [DOI] [PubMed] [Google Scholar]
- 30.Khong TY, Adema E, Erwich J. On an anatomical basis for the increase of birth weight in second and subsequent born children. Placenta. 2003;24:348–353. doi: 10.1053/plac.2002.0922. [DOI] [PubMed] [Google Scholar]
- 31.Gluckman PD, Hanson MA. Maternal constraint of fetal growth and its consequences. Seminar in Fetal and Neonatal Medicine. 2004:419–425. doi: 10.1016/j.siny.2004.03.001. [DOI] [PubMed] [Google Scholar]
- 32.Koebnick C, Black MH, Wu J, Martinez MP, Smith N, Kuizon B, Cuan D, Young DR, Lawrence JM, Jacobsen SJ. High blood pressure in overweight and obese youth: Implications for screening. Journal of Clinical Hypertension. 2013;15:793–805. doi: 10.1111/jch.12199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.National High Blood Pressure Education Program Working Group on High Blood Pressure in Children and Adolescents. The fourth report on the diagnosis, evaluation, and treatment of high blood pressure in children and adolescents. Pediatrics. 2004;114:555–576. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.