Abstract
A recent study demonstrated a significant role for leukotriene B4 (LTB4) causing pulmonary vascular remodeling in pulmonary arterial hypertension (PAH). LTB4 was found to directly injure luminal endothelial cells and promote growth of the smooth muscle cell layer of pulmonary arterioles. The purpose of the current study was to determine the effects of LTB4 on the pulmonary adventitial layer, largely composed of fibroblasts. Here, we demonstrate that LTB4 enhanced human pulmonary artery adventitial fibroblast (HPAAF) proliferation, migration and differentiation in a dose-dependent manner through its cognate G-protein coupled receptor, BLT1. LTB4 activated HPAAF by up-regulating p38 MAPK as well as Nox4 signaling pathways. In an autoimmune model of PH, inhibition of these pathways blocked perivascular inflammation, decreased Nox4 expression, reduced reactive oxygen species production, reversed arteriolar adventitial fibroblast activation and attenuated PH development. This study uncovers a novel mechanism by which LTB4 further promotes PAH pathogenesis, beyond its established effects on endothelial and smooth muscle cells, by activating adventitial fibroblasts.
Keywords: LTB4, inflammation, fibroblast, vascular remodeling, p38 MAPK, NADPH oxidase 4, pulmonary arterial hypertension
Introduction
PAH is a life threatening disease associated with a wide variety of disorders.1–5 Current concepts of disease pathogenesis invoke a variety of factors including vasoconstriction, metabolic derangement, BMPR2 dysregulation, and inflammation that work in concert to produce serious pulmonary vasculopathy.5–8 The majority of patients with Group I PAH exhibit evidence of systemic inflammation polarized towards Th1/Th17 or Th2 immunity depending on the underlying cause.7 Emerging evidence suggests that innate immunity contributes to disease development in certain forms of the condition including PAH associated with connective tissue diseases; the degree of perivascular macrophage infiltration has been shown to correlate directly with vascular pathology and deranged hemodynamics.7, 9, 10
In PAH, remodeling of small-to-medium sized pulmonary arterioles is characterized by changes in all three layers of the vascular wall, including the intimal endothelial cells, medial smooth muscle cells and adventitial fibroblasts.11 An “inside-out” response is a widely-accepted concept of pulmonary vascular remodeling, in which infiltration of various inflammatory cells induce endothelial apoptosis and promote growth of the smooth muscle cell layer.12 However, recent studies strongly support an “outside-in” mechanism in which adventitial fibroblasts serve as a source of pathologic stimuli permeating the vascular wall.12–15 Vascular adventitial fibroblasts in this outer layer are activated by a variety of pathways that result in heightened proliferative, migratory, fibrotic, and inflammatory activity, including p38 mitogen-activated protein kinases (MAPK) and NADPH oxidase 4 (Nox4).6, 16–23
The p38 MAPK pathway plays a pivotal role in numerous cellular functions.24, 25 Elevated p38 MAPK activity contributes to hypoxia-induced pulmonary artery fibroblast proliferation.17–19, 26 Similarly, increased expression of Nox4, in the absence of other stimuli, is sufficient to induce HPAAF proliferation and migration.20 Nox is one of the major sources of cellular reactive oxygen species (ROS) known to be pathogenic in PAH.2, 27–29 Interaction between the p38 MAPK and Nox4 signaling pathways has been proposed for other diseases.30 However, whether activation of p38 MAPK and Nox4 pathways in fibroblasts are related to perivascular inflammation observed in the pulmonary adventitial transformation of PAH remains unknown. Our recent study demonstrated that the inflammatory mediator, LTB4, directly mediated some of the pathologic changes observed in the inner and medial layers of pulmonary arterioles and raised the possibility that this molecule could affect pulmonary arteriolar adventitial cells as well.
LTB4 is one of a group of a leukotrienes, lipid mediators produced from arachidonic acid (AA) metabolism through the 5-lipoxygenase (5-LO) pathway.31 5-LO, in concert with 5-LO-activating protein (FLAP), converts AA to LTA4. LTA4 is then either hydrolyzed by LTA4 hydrolase to form LTB4, or is conjugated with reduced glutathione by LTC4 synthase to yield LTC4. Leukotrienes, especially LTB4, are implicated in a number of inflammatory diseases including asthma,32 atherosclerosis,33 stroke,34 and myocardial infarction.35 In an animal model of autoimmune PAH, we discovered that LTB4 was significantly elevated in the bronchoalveolar lavage fluid (BALF) of pulmonary hypertensive animals. We previously found that LTB4 blood levels were elevated significantly in PAH patients, especially in those with connective tissue disorders; these latter individuals exhibited mean LTB4 levels about fivefold higher than those in healthy controls.10 By distinction, six of eight iPAH patients in that study exhibited normal LTB4 levels. (A large scale study of LTB4 serum levels is currently being undertaken to more definitively assess this leukotriene in all Group I PAH conditions.) 10 We demonstrated that LTB4 induced endothelial cell apoptosis and promoted smooth muscle cell proliferation in vitro. Blocking LTB4 production or LTB4-mediated signaling reversed established severe PH in addition to restoring remodeled pulmonary vasculature to patency.10 Given the strong role of LTB4 in this model of severe PAH, we investigated whether LTB4 also play a role in the phenotypic adventitial changes observed in PAH.
Material and Methods
Animal Model
All in vivo experimental studies were approved by the Veterans Affairs Palo Alto Animal Care and Use Committee. Six to eight week old athymic nude rats (rnu/rnu; Charles River Laboratories) were injected subcutaneously (s.c.) with a single dose of either SU5416 (SU, 10mg/kg), dissolved in DMSO or DMSO (vehicle) alone. SU is a small molecule inhibitor of the cytoplasmic tyrosine kinase segment of the VEGF receptors flt and KDR (VEGFR1 and VEGFR2) and alone is sufficient to induce pulmonary hypertension (PH) in athymic rats.36 (By convention, animal models of PAH are referred to as PH). All animals were maintained in normoxic conditions. Bestatin, an LTA4 hydrolase inhibitor, was given orally at a dose of 1mg/kg daily starting at the time of SU administration. The p38 MAPK inhibitor (SB203580) was injected at a dose of 4mg/kg i.p. three times per week starting at the time of SU injection. The dose was based on similar dosing regimens in prior in vivo studies.37, 38
Human plasma LTB4 measurements
The study was approved by the Institutional Review Board at Stanford University with appropriate informed consent. Serum from de-identified healthy controls or patients with systemic sclerosis (SSc)-PAH was obtained from the IRB-approved Stanford Pulmonary Hypertension Biobank. LTB4 concentration was then determined by using the LTB4 enzyme immunoassay (EIA) kit (Cayman Chemical) according to the manufacturer's protocol.
Human NTproBNP measurements
Blood samples were collected at study entry by venipuncture in tubes containing EDTA. Blood samples were centrifuged at 3500g for 10 min at 4 °C immediately after collection, and plasma samples were stored at −70 °C. NT-proBNP (ECLIA Elecsys 2010 analyzer, Roche Diagnostics) were measured by commercially available assays in plasma samples never thawed before. The intra-assay coefficient of variation was 2.9%, and the interassay coefficient of variation was 3.6%.
Immunohistochemistry of human lung tissue
Paraffin-embedded, formalin-fixed human lung tissues from two healthy control subjects and two SSc-PAH patients were obtained from the Pulmonary Hypertension Breakthrough Initiative Tissue Bank at Stanford. Antigen retrieval was performed by steaming the slides for 45 min using the IHC-TekTM Epitope Retrieval Steamer system and then blocked with 1% donkey serum for 1 hr. The slides were incubated with anti–5-LO (Cell Signaling Technology), anti-CD68 (Dako), or anti-S100A4 (LifeSpan Biosciences Inc.) in 1% donkey serum overnight at 4°C, followed by anti-rabbit Alexa Fluor 488 (Invitrogen) and anti-mouse Alexa Fluor 594 (Invitrogen) for 1 hr at room temperature. Images were acquired using a Zeiss 700 confocal microscope and analyzed with ImageJ.
Immunohistochemistry of rat lung tissue
Lung samples were snap-frozen in OCT solution and were stored at −80°C. The following antibodies were used for immunohistochemistry: anti–5-LO (1:50, Cell Signaling Technology); anti-Nox4 (1:25, Abcam); anti-vimentin (1:20, Abcam) and anti-CD90 (1:200, Abcam). Images were analyzed with ImageJ blindly by two evaluators (X.J, W.T.). The adventitial compartment of the pulmonary vessels was determined by S100A4, vimentin or CD90 staining. 5-LO positive cells within this margin were defined as “around the adventitia”, while cells that were positive for 5-LO staining but resided outside the yellow double dash line were categorized as “outside the adventitia” in Figure S1. Images were acquired by Zeiss 710 confocal microscope.
Cell culture
HPAAFs were purchased from ScienCell, and were grown in Fibroblast Medium (ScienCell); this media consisted of 2% fetal bovine serum (FBS), 1% fibroblast growth supplement and 1% of penicillin/streptomycin solution. Normal human lung fibroblasts (HLFs) were purchased from ATCC (LL24, ATCC® CCL-15™), and were grown in Dulbecco's Modified Eagle Medium (DMEM, Life Technologies) containing 10% FBS and 1% penicillin/streptomycin solution. Cells were grown at 37°C in 5% CO2 incubator and used between passages 3–8. LTB4 (Cayman Chemical, 200,400,800nM), U75302 (Cayman Chemical, 1μM), SB203580 (Cell signaling, 10μM) and apocynin (Sigma, 300μM) were used for treatment of cells.
Amplex Red assay
Hydrogen peroxide (H2O2) measurements in cells were made using the horseradish peroxidase-linked Amplex Red fluorescence assay.39 Cells were incubated with 50 μM Amplex Red (Invitrogen) and 0.125 U/ml horseradish peroxidase (Sigma) at 37°C for 10 min. Fluorescence readings were made at an excitation wavelength of 544 nm and an emission detection wavelength of 590 nm. Relative fluorescent units were calculated after subtraction of control groups (with catalase).
Western blots
Cells were washed with ice-cold phosphate-buffered saline and lysed in RIPA lysis and extraction buffer (Thermo Scientific) with a Halt protease inhibitor and phosphatase inhibitor cocktail (Thermo Scientific). Supernatants were collected and protein concentration was determined by the BCA assay (Thermo Scientific). Equal amounts of protein were size-fractionated by 10% SDS–polyacrylamide gel and immunoblotted with corresponding antibodies.
MTT assay
Cell proliferation was evaluated by the 3-(4,5-dimethylthiazol-2-yl)-2,5-diphenyl tetrazolium bromide (MTT) assay (Sigma). After synchronization for 24 hrs by serum starvation, cells were treated with LTB4 and inhibitors for 72hrs. 5 mg/ml of MTT were added and incubated for 4 hrs. Solubilization solution was added to dissolve the MTT formazan crystals. Absorbance was spectrophotometrically measured at a wavelength of 570 nm with background subtraction at 690 nm.
BrdU assay
BrdU Cell Proliferation Assay kit (Cell signaling) was used to quantify cell proliferation. BrdU was added and last 12 hrs. Read absorbance at 450nm.
Migration assay
Cell migration was performed using a Boyden Chamber assay with 24 well, 8μm pore size membrane invasion chambers (Fisher). Cells were synchronized with serum starvation, and 2 × 104 cells were seeded into the upper chamber of the transwell. LTB4 and inhibitors were added to the lower chamber. Membranes with migrated cells were fixed with methanol and stained with hematoxylin and eosin for 12 hrs. The mean number of cells in five 10× random fields was used for quantification analysis.
Measurements of Hemodynamics
Rats were anesthetized with ketamine hydrochloride (70 mg/kg) and xylazine (10 mg/kg). Right ventricular systolic pressure (RVSP) measurements were obtained through the jugular vein into the right ventricle (RV) using Micro Tip pressure transducer (model SPR-671, 1.4F) (Millar Instruments). Signals were recorded continuously with a TC-510 pressure control unit 236927/R17 (Millar Instruments) coupled to a Bridge Amp (AD Instruments). Data was collected with the Powerlab7 data acquisition system (AD Instruments) and analyzed with Chart Pro software (AD Instruments). The RV was dissected from the left ventricle (LV) and septum (S). The Fulton index of RV/(LV+S) was calculated using the weight of RV, LV and S to determine the degree of right ventricular hypertrophy (RVH).
Echocardiography (Echo)
Echo evaluation of RV dimensions and pulmonary hemodynamics were performed using the Vivid 7 Dimension Cardiovascular Imaging System (GE), equipped with a 14 MHz transducer. Rats were lightly sedated with isoflurane for the duration of the procedure. The chests were depilated and the rats were laid supine on a warming handling platform. Pulmonary artery doppler tracings were obtained from the pulmonary artery parasternal short-axis view. The RV free wall was imaged from a modified parasternal long-axis view. All measurements were made in the expiratory phase of the respiratory cycle.
Statistical Analysis
GraphPad Prism® version 5.0c was used for statistical analysis. With normally distributed data, unpaired t-tests were applied for comparison of 2 groups. One-way analysis of variance (ANOVA) analyses were used to compare multi-groups. Differences between various groups at multiple time points were compared using two-way ANOVA with Bonferroni multiple comparisons test for post hoc analyses. For comparisons between multiple experimental groups at a single time point, the Kruskall-Wallis test followed by Dunn's multiple comparisons test for post hoc analyses was used. All data were represented as means ± SEM (standard error of the mean), and a p value of < 0.05 was considered significant.
Results
5-LO/LTB4 signaling was increased around the pulmonary vascular adventitia in PAH
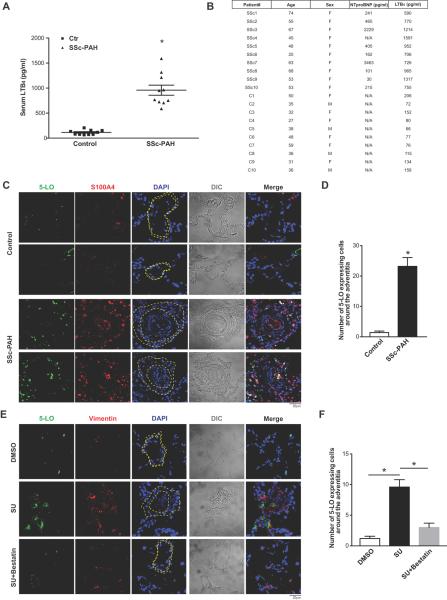
We previously evaluated the action of LTB4 on cells within the intima and media10 and now sought to assess its effects on the adventitia, where vascular inflammation is most prominent in PAH. The pulmonary arteriolar adventitial space is chiefly composed of fibroblasts and has recently been shown to induce a distinct proinflammatory/profibrotic macrophage phenotype in PH.40 Because SSc patients are prone to developing PAH and are known to have persistently activated fibroblasts,1, 41–43 we assessed LTB4 production in SSc-PAH blood and tissue. LTB4 levels were significantly elevated in SSc-PAH patients. These individuals exhibited mean LTB4 levels approximately 10 fold higher than that of the control group (Figure 1A, B). There was no significant correlation between serum LTB4 levels and NTproBNP levels. 5-LO positive cells were noted in close proximity to the adventitial fibroblasts, stained with S100A4 (also called fibroblast specific protein 1, FSP1), in SSc-PAH compared to controls (Figure 1C, D). ~70% of 5-LO+ cells observed in the thickened adventitia were CD68+ macrophages (Figure S1A, B). In diseased PH lungs, co-staining of 5-LO and S100A4 was also observed in the intimal cells of occluded vessels suggesting an activation of LTB4 biosynthetic machinery in these cells.
Figure 1.
5-LO/LTB4 signaling is increased around pulmonary vascular adventitial fibroblasts in PAH. A, Plasma LTB4 concentration in 10 healthy controls and 10 SSc-PAH patients. B, Demography table. C, Representative immunofluorescence images of human lung sections stained with 5-LO (green) and S100A4 (fibroblasts, red) from healthy individuals and SSc-PAH patients. D, Morphometric analysis of images in C. Number of 5-LO positive cells within 5 μm of the pulmonary adventitia. E, Representative immunofluorescence images of lung sections stained with 5-LO (green) and Vimentin (fibroblasts, red) from DMSO (negative control), SU (PH) or SU+bestatin (bestatin-treated) animals. F, Morphometric analysis of E. In A, data are presented in the scatter plots showing minimal to maximal values and all data points. In C, E, DAPI (blue) stains nuclei; differential interference contrast (DIC) highlights alveolar and vascular structures; n=5. Yellow dashed lines indicate the adventitia area. In A, D, F, data are presented as mean ± SEM. (*: p<0.05)
In a model of autoimmune PAH,36 enhanced 5-LO expression was observed either adjacent to, or co-localized with adventitial fibroblasts, stained with vimentin, from SU-treated athymic rat lungs (Figure 1E, F). LTB4 synthesis inhibition with bestatin, an intervention that both prevents and reverses experimental PH, prevented adventitial remodeling and also mitigated 5-LO expression.10 These cumulative results suggest that increased LTB4 biosynthesis is related to the expansion of vascular adventitial fibroblasts in PH.
LTB4 promoted proliferation, migration and differentiation of HPAAFs
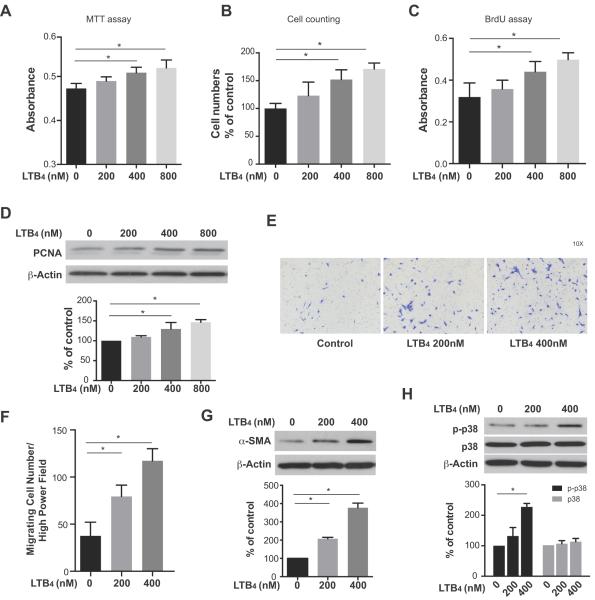
During the pathological remodeling process of PH, PAAFs show enhanced proliferation, migration and differentiation.12–14 To determine whether LTB4 itself promotes HPAAF proliferation in the absence of other stimuli, we first monitored HPAAF growth in response to various physiologically-relevant10 concentrations of LTB4 using the MTT assay, cell counting and BrdU assay (Figure 2A–C,). LTB4 promoted HPAAF proliferation in a dose-dependent manner. By contrast, when lung parenchymal fibroblasts were cultured in the same LTB4 conditions, no significant changes in cell proliferation were detected (Figure S2), a finding suggesting that the response to LTB4 is cell type- dependent. Western blot analysis of HPAAFs demonstrated increased expression of the proliferating cell nuclear antigen (PCNA) in LTB4-exposed HPAAFs consistent with cell growth (Figure 2D).
Figure 2.
LTB4 promotes proliferation, migration and differentiation in HPAAF. Proliferation of HPAAF with increasing doses of LTB4 treatment for 72hrs was measured by A, the MTT assay, B, cell counting, C, BrdU assay. Data are presented as mean ± SEM. (*: p<0.05) D, PCNA expression was measured by western blot after treatment with LTB4 for 24hr. E, F, Migration of HPAAF after LTB4 exposure were determined and quantified by Boyden Chamber assay. Data are presented as mean ± SEM. (*: p<0.05) G, H α-SMA and p-p38 MAPK expression after LTB4 treatment for 24hr as determined by western blot. β-actin was used as a loading control. The experiments were repeated three times.
To further assess LTB4 effects on HPAAFs, in vitro migration was evaluated using a Boyden Chamber assay, which showed that the promotion of in vitro migration was concentration-dependent (Figure 2E, F). Because the differentiation of HPAAFs into collagen-producing, α-SM-actin-expressing myofibroblasts is critical for vascular stiffness, contractility, and angiogenesis in PAH,14, 44 we investigated effects of LTB4 on myofibroblast differentiation. LTB4 promoted myofibroblast differentiation in a concentration-dependent manner (Figure 2G). Next, because p38 MAPK also mediates the proliferation and migration of PAAFs, but not systemic artery fibroblasts,26 we tested whether LTB4 promoted HPAAF activation through p38 MAPK signaling. Western blots show increased phosphorylation of p38 MAPK in the presence of LTB4, consistent with LTB4 activation of p38 MAPK signaling in HPAAFs (Figure 2H). These data collectively demonstrate, for the first time, that LTB4 mediates HPAAF proliferation, migration and differentiation and concomitantly activates signaling via p38 MAPK.
LTB4-mediated HPAAF proliferation, migration and differentiation required BLT1 receptor signaling and p38MAPK pathway activation
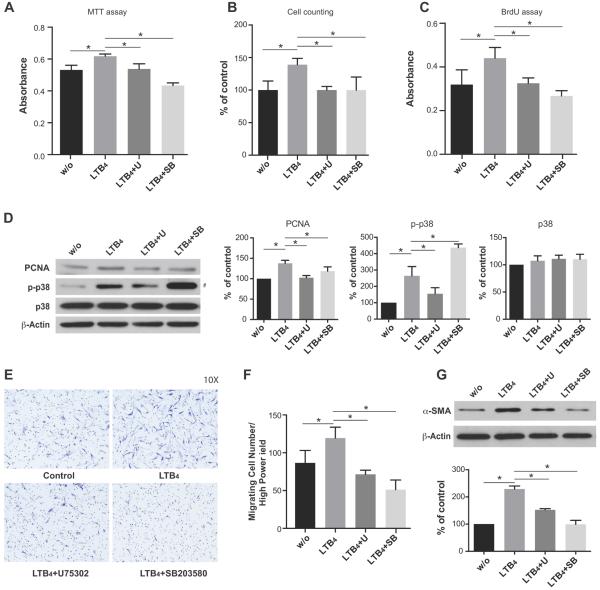
To further investigate the molecular mechanism by which LTB4 induces HPAAF activation, we used the BLT1 antagonist (U75302) and p38 MAPK inhibitor (SB203580) in HPAAF cultures. U75302 (1μM) reversed the pro-proliferative HPAAF activity of LTB4 as determined by the MTT assay, cell counting, BrdU assay and by PCNA expression (Figure 3A–D). Similarly, treatment with SB203580 (10μM) inhibited LTB4 induced cell proliferation (Figure 3A–C, Figure S3). Western blot analysis of phosphorylated p38 MAPK protein levels showed that LTB4-mediated p38 MAPK activation was dampened by BLT1 blockade (Figure 3D). (Increased p38 phosphorylation in the SB203580-treated group (Figure 3D) is likely attributable to the fact that this agent inhibits p38 catalytic activity by binding to the ATP binding pocket without affecting phosphorylation of p38 by upstream kinases).45 Collectively, these data suggests that LTB4 controls HPAAF growth through engagement of its high affinity receptor, BLT1, and through activation of p38 MAPK. Inhibition of p38 MAPK signaling by pre-treating cells with SB203580 limited LTB4-mediated fibroblast migration and fibroblast to myofibroblast transformation (Figure 3E–G).
Figure 3.
LTB4 induced HPAAF proliferation, migration and differentiation were inhibited by pretreatment with BLT1 blockade (U75302) or p38 MAPK inhibition (SB203580). A, HPAAF proliferation, B, cell counting, C, BrdU assay, D, PCNA expression E, F, migration and G, differentiation were determined after pretreatment of LTB4 receptor antagonist U75302 (1μM) or p38 MAPK inhibitor SB203580 (10μM) in the presence of LTB4. (#: According to the manufacturer, increased p38 phosphorylation in the SB203580-treated group in Figure 3D is likely attributable to the fact that this agent inhibits p38 catalytic activity by binding to the ATP binding pocket without affecting phosphorylation of p38 by upstream kinases).45 Data are presented as mean ± SEM (*: p<0.05). The experiments were repeated three times.
Blocking p38 MAPK signaling attenuated experimental autoimmune PH
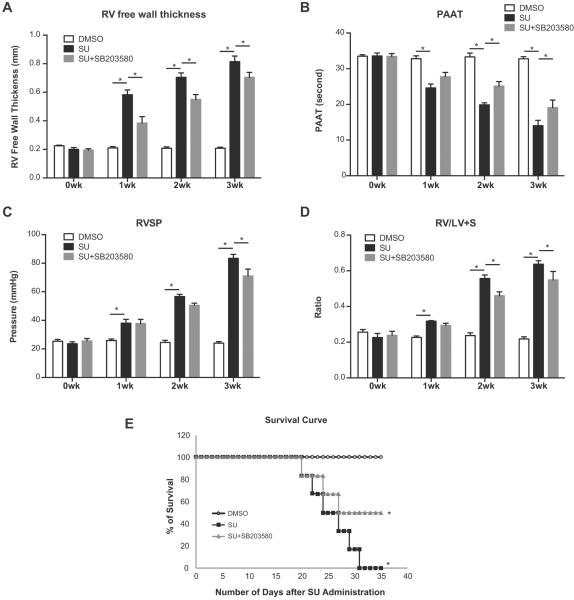
Because LTB4-dependent activation of p38 MAPK pathway was strongly implicated as a critical mediator of PAAFs in autoimmune PH and because p38 MAPK phosphorylates 5-LO in polymorphonuclear leukocytes and causes a 4-fold increase in 5-LO activity,46 we hypothesized that inhibiting p38 MAPK signaling could have protective effects in this model of autoimmune PH. SB203580 dosing was initiated at the time of SU administration in athymic rats. Post-SU administration, echocardiographic evidence of PH was first detected between week 1–2 and severe PH was evident by week 3. Invasive hemodynamic measures were taken at 3 weeks after SU administration. In the SU-athymic PH rats treated with SB203580, serial echocardiography showed that pulmonary hypertension was attenuated. Interval improvement is manifested by decreasing RV wall thickness and longer pulmonary artery acceleration times (PAAT) (Figure 4A, B). Accordingly, reduced RVSP and RVH were observed with SB203580 treatment (Figure 4C, D). Improvement in mortality was found 3 weeks after SB203580 treatment, indicating that p38 MAPK inhibition treatment is effective in attenuating PH progression and mortality in this model of autoimmune PAH (Figure 4E). With p38i, the inflammatory markers (CCR2, TNFα and CX3CR1) in lung tissue were also decreased (Figure S4).
Figure 4.
Blocking p38 MAPK signaling attenuates experimental PH. Rats were treated with the p38 MAPK inhibition (SB203580) starting at the time of SU administration. A, B, Animals were monitored by echocardiography weekly. C, D, Hemodynamic measurements were done at week 3. RVSP measurements in DMSO, SU, and p38 MAPK inhibition treatment groups were assessed at week 3 post-SU. RV hypertrophy (RVH) measurements as assessed by the RV/LV+S weight ratios. E, Survival of rats after treatment was compared with DMSO and SU rats. (n=6 per group). Data are expressed as means ± SEM. (*: p<0.05) The experiments were repeated three times.
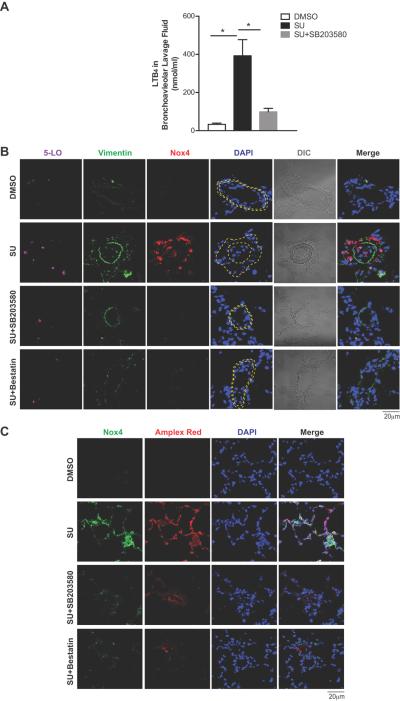
Blocking LTB4 synthesis and inhibiting p38 MAPK signaling decreased fibroblast expansion and Nox4 expression in experimental PH
Globally inhibiting p38 MAPK signaling with SB203580 treatment also decreased LTB4 levels in bronchoalveolar lavage fluid (BALF) and reduced 5-LO expression associated with the thickening of adventitial fibroblast layer in PH (Figure 5A, B). Oxidative stress is widely associated with vascular injury because of an excessive production of ROS, which can act through p38 MAPK to effect cellular changes.47 Elevated ROS lead to vascular remodeling and increased PA pressures in PH.29, 43, 48, 49 Nox is one of the major sources of cellular ROS known to be pathogenic in PAH, 2, 27–29 and Nox4 is the most important isoform of Nox in adventitial fibroblasts. Nox4 positive fibroblasts were increased in PH lungs (Figure 5B) as were Nox4/5-LO double positive fibroblasts. Both bestatin and SB203580 treatment significantly reduced Nox4 expression in PAAF, and decreased the thickening of pulmonary arterioles. (Figure 5B, S5)
Figure 5.
Blocking LTB4 synthesis and inhibiting p38 MAPK signaling decreases fibroblast activation and Nox4 in experimental PAH. A, LTB4 concentrations in the bronchoalveolar lavage fluid (BALF) of DMSO, SU or SU+SB203580 animals. B, Immunofluorescence images of rat lung tissues stained with 5-LO (magenta), Vimentin (green) and Nox4 (red). Yellow dash lines approximate the adventitial zone. C, Representative immunofluorescence images of lung sections stained with Nox4 (green). Amplex Red (red) indicates tissue H2O2 level. DAPI (blue) stains nuclei; DIC highlights alveolar and vascular structures; n= 5. Data are expressed as means ± SEM. (*: p<0.05)
We next investigated whether blocking LTB4 biosynthesis with bestatin or inhibiting p38 MAPK signaling with SB203580 could also reduce tissue H2O2 levels in PH lungs. Tissue H2O2 production was measured using horseradish peroxidase-linked Amplex Red. Increased Amplex Red fluorescence correlates with elevated Nox4 expression in the lung. Both bestatin and SB203580 treatment effectively reduced Nox4-related H2O2 production (Figure 5C).
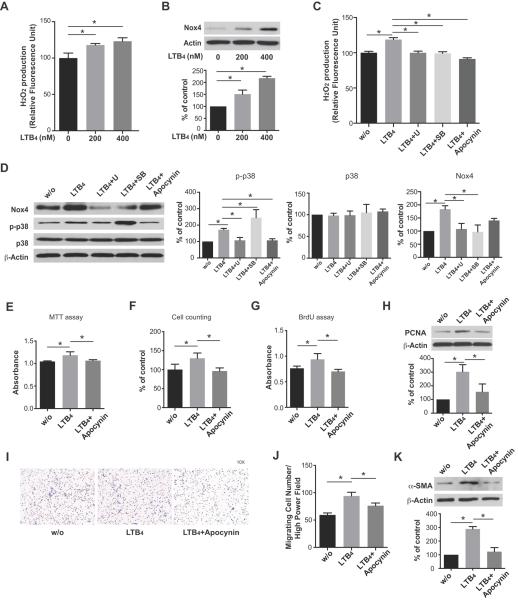
LTB4 induced Nox4 expression and hydrogen peroxide (H2O2) production and activation of HPAAFs
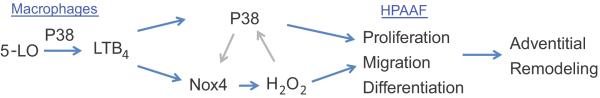
Because up-regulation of Nox4 and H2O2 in proliferating pulmonary adventitial fibroblasts has been noted in developing PH, we next investigated whether Nox4 and H2O2 production in fibroblasts were increased by LTB4. HPAAFs treated with LTB4 lead to an elevated Nox4 expression with a corresponding increased H2O2 production in a concentration-dependent manner (Figure 6A, B). H2O2 production was inhibited by blocking BLT1, p38 MAPK, and Nox4 (Figure 6C). Note again in Figure 6C, increased p38 phosphorylation in the SB203580-treated group likely due to this agent inhibiting p38 catalytic activity without affecting phosphorylation of p38 as described in Figure 3B. Inhibition of Nox4 decreased activation of p38 MAPK and inhibition of p38 MAPK also inhibited Nox4 expression (Figure 6D). These results suggest that a feed-forward system in which ROS can activate p38 MAPK, and that p38 MAPK can subsequently up-regulate Nox4 expression and ROS production in HPAAFs. Furthermore, proliferation, migration and differentiation of HPAAF, induced by LTB4, were reversed by Nox4 inhibition (Figure 6E–K, Figure S3). Cumulatively, these results strongly support an important role for LTB4 and its receptor BLT1 in the activation of HPAAFs via the p38 MAPK pathway and acting in concert with the ROS molecule, Nox4. (Figure 7)
Figure 6.
LTB4 induced Nox4 expression, hydrogen peroxide (H2O2) production and HPAAF activation. A, HPAAFs were treated with LTB4 at concentrations of 200 and 400nM; H2O2 production was measured by Amplex assay and B, Nox4 expression was determined by western blot. C, After pretreatment with U75302 (1μM), SB203580 (10μM) or the Nox4 inhibitor apocynin (300μM), the effects of exogenous LTB4 on HPAAFs were assayed. H2O2 production was measured by the Amplex assay. D, Western blots were used to determine the expression of Nox4, p-p38 MAPK and total p38 MAPK; β-actin was used as a loading control. E, MTT assay, F, cell counting, G, BrdU assay, H, PCNA expression, I, J, migration and K, differentiation were determined with Nox inhibition (apocynin) in the presence of LTB4. (#: Note again in Figure 6D, increased p38 phosphorylation in the SB203580-treated group likely due to this agent inhibiting p38 catalytic activity without affecting phosphorylation of p38 as described in Figure 3D).45 (*: p<0.05) The experiments were repeated three times.
Figure 7.
Schematic of LTB4 induce p38 MAPK and Nox4 in HPAAF.
Discussion
We recently described an important role for the increased expression of macrophage-derived LTB4 in PH, showing how this eicosanoid specifically induces PA endothelial apoptosis and smooth muscle cell proliferation;10 two pathological events strongly implicated in the pathogenesis of PAH. Unexplored in this prior study was a role for LTB4 in the third outer layer of the affected arterioles, the adventitia. This outer vascular zone is where abundant LTB4-secreting macrophages are chiefly observed.40, 50 In the current study, we uncovered a novel function of LTB4, specifically the activation of HPAAFs. Interestingly, this effect was not observed in non-vascular lung fibroblasts. The possibility that other fibroblast populations would not proliferate in response to LTB4 was suggested by a previous study in which a leukotriene-blocking FLAP inhibitor had no effect on serum-induced growth on the NIH/3T3 cell line (mouse embryonic fibroblast cell line).51 We demonstrated that LTB4 induced HPAAF proliferation, migration and differentiation through p38 MAPK activation and up-regulation of Nox4. Blocking LTB4 signaling through its cognate high-affinity heterotrimeric G protein–coupled receptor, BLT1, inhibited LTB4-mediated proliferation, migration and differentiation of HPAAF. Blocking p38 or Nox4 did the same, demonstrating a linked pathway. In the context of our prior study,10 LTB4 appears to be an important inflammatory mediator, which is highly expressed at the site of disease activity in PAH which is uniquely capable of modulating activation of a variety of cell types culminating in remodeling of the entire vascular wall.
We confirmed that diseased pulmonary arterioles are surrounded by a large population of 5-LO+ cells, which are mostly CD68+ macrophages that are in close proximity to adventitial fibroblasts. Some adventitial fibroblasts and cells within the occluded vascular lumen of the PAH lung tissue were 5-LO+, suggesting that there is an aberrantly active LTB4 biosynthetic machinery localized in these cells. Previous studies suggest that S100A4+ endothelial cells are involved in tumor angiogenesis.52 The co-expression of S100A4 and 5-LO in the intimal lumen also suggests an association between LTB4 signaling and the occlusive intimal remodeling of PAH.
PAH, especially SSc-PAH, is characterized by robust fibroproliferative changes in pulmonary arteries.53, 54 PAAFs undergo significant phenotypic changes characterized by increased proliferative, migratory, fibrotic, and inflammatory activity.12 These phenotypic changes have been demonstrated to mediate macrophage-associated inflammation that influence blood vessel tone and cause vascular remodeling.16, 55 The sequence of events leading to PAAF activation is not fully understood, but evidence suggests that activation of p38 MAPK and increased expression of Nox4 in the adventitia may contribute to the altered fibroblast behavior.
As a member of the MAPK family, p38 MAPK is a critically important signaling pathway affecting inflammation, shear stress, and hypoxia.24 Pharmacological inhibition of p38 MAPK increases nitric oxide generation, reduces superoxide anion burden, and restores hypoxia-induced endothelial dysfunction in rats with hypoxia-induced PH.26 Here we demonstrated that the LTB4-induced activation of HPAAF is p38 MAPK-dependent. Because p38 MAPK catalyzes the phosphorylation of 5-LO at the Ser271 site, p38 MAPK regulates LTB4 production in leukocytes at the enzymatic posttranslational level.56 Our data support the concept of a positive feedback loop between LTB4 and p38 MAPK in HPAAFs. Additionally, in our rat model of autoimmune PH, inhibition of p38 MAPK with SB203580 attenuated LTB4-associated perivascular inflammation, decreased the expression of Nox4, prevented structural changes in the arteriolar adventitia, and limited the development of PH. However, compared with the previously demonstrated effects of LTB4 antagonism,10 the impact of p38 MAPK inhibition in vivo was relatively less effective. Importantly, LTB4 inhibitors, not only prevent PAH development (in contrast to the mild attenuation observed with the p38 MAPK inhibitor used in the current study) but they also reverse established pulmonary vascular disease.10
The modest effect of p38 inhibition in this autoimmune animal model likely reflects the pleiotropic nature of p38 MAPK activity in autoimmune PH. For example IL-6, a cytokine implicated in PAH development and autoimmune disease, also inhibited p38 signaling in the development of PH in a mouse model suggesting that dampening this signaling cascade is associated with deleterious effects.57,58 Beyond its myriad effects on inflammation, p38 inhibition also appeared to exert an effect on RV remodeling even before an effect on RVSP and PAAT was detected. When considered together with the improved survival observed in rats receiving this therapy, this finding suggested that this drug exerted a direct salubrious effect on the myocardium. Several studies have documented the cardioprotective properties of p38 inhibition during myocardial infarction and cardiac ischemia-reperfusion injury.59–62
In addition to the pleiotropic nature of p38 signaling, another factor possibly mitigating p38 inhibitor effects is that LTB4 may be activating fibroblasts through additional previously-implicated pathways such as JNK and PKC.22, 23 However, given that p38 MAPK inhibitors have now been demonstrated to have at least some effect in at least three pre-clinical models of PH, 63 there is at least the possibility selective p38 inhibition may play some clinical role as an adjunctive treatment for certain PH conditions. While early clinical studies of p38 MAPK inhibitors in autoimmune disease demonstrated poor efficacy and unacceptable side effects, there has been cautious optimism about the use of other p38 MAPK inhibitor compounds in chronic obstructive pulmonary disease and atherosclerosis.25
Given the role of p38 MAPK in governing hypoxic stress in fibroblasts, we sought to evaluate the key source of ROS in PAH. Nox4, a member of the NADPH oxidase family, is a major intracellular source of reactive oxygen species (ROS).28, 29, 64 Nox4 is key for mediating numerous cellular function including cellular proliferation, differentiation, migration, and apoptosis.65 Of the 5 Nox isoforms encoded by the human genome, four (Nox1, Nox2, Nox4, and Nox5) are expressed in vascular cells. In mice, genetic deletion of Nox2 has been shown to reverse hypoxia-initiated PH; and Nox1 has been shown to be important for systemic hypertension. However, it was recently discovered that only expression of the Nox4 isoform increases in rat models of PH and in human PH. Increased expression of Nox4 induces fibroblast proliferation and migration. Similarly, Nox4 inhibition reduces the proliferation of fibroblasts that are isolated from the pulmonary arteries of monocrotaline-treated rats. Nox4 inhibitors effectively prevent monocrotaline-induced PH but are not therapeutically sufficient to halt the disease progression.20 Of relevance to the current study, LTB4 has been shown to induce Nox activation and ROS production in mammalian cells.66, 67 Here, we confirmed that Nox4 expression is present mainly in pulmonary adventitial fibroblasts in the athymic rat model of experimental autoimmune PH. In addition, we documented that LTB4 induces the protein expression of Nox4 in HPAAFs in a concentration-dependent manner, and that this expression correlates with increased p38 MAPK activity. In the current study, inhibiting p38 MAPK decreased Nox4 expression. Given previous studies that showed Nox4 overexpression causes p38 MAPK phosphorylation,67, 68 p38 MAPK and Nox4 pathways are likely synergistic in the development of PAH.
In conclusion, LTB4, which causes PA endothelial cell apoptosis and PA smooth muscle cell growth, also causes BLT1-dependent PAAF activation via p38 MAPK signaling in concert with Nox4 generation. Collectively these findings emphasize the role of LTB4 in the pathobiology of autoimmune PAH.
Perspectives
A microvasculopathy with an essential inflammatory component underlies PAH. The poor prognosis of patients afflicted by this disease despite treatment with the currently available vasodilator drugs makes the development of new treatment strategies imperative. LTB4 can induce vascular inflammation in all three layers of pulmonary arterioles causing endothelial cell apoptosis, vascular smooth muscle cell and fibroblast proliferation. LTB4-directed therapeutic strategies appear to be justified and should be evaluated in autoimmune forms of severe PAH.
Supplementary Material
Novelty and Significance.
What is New?
Our studies demonstrate that the eicosanoid, LTB4, activates pulmonary artery fibroblasts, a finding of potential relevance in the pathogenesis of PAH.
We also show that LTB4 stimulates these cells by binding to its high affinity receptor, BLT1, on fibroblasts and through activation of p38 MAPK and Nox4.
What is Relevant?
LTB4 is an important mediator of vascular inflammation in PAH, acting on pulmonary artery endothelial, smooth muscle cells and adventitial fibroblasts.
Activated macrophages, which are actively secreting LTB4, are concentrated in the outer adventitial layer of affected arterioles and may help explain the link between inflammation and disease.
Summary
Results from this study show a novel mechanism by which LTB4 facilitates PAH, beyond its established effects on endothelial and smooth muscle cells, by activating adventitial fibroblasts.
Acknowledgements
The authors would like to acknowledge Dr. Lajos Gera for the synthesis of SU5416 and Dr. Lingli Wang for preparing the human tissue sections for histology.
Sources of Funding This work was supported by NIH grants HL125739, HL082662 (M.R.N.), NHLBIHV-10-05 (M.R., M.R.N)
Footnotes
Disclosures Authors W.T. and M.R.N. co-founded Eiccose, LLC, a company which is currently investigating the role of LTB4 antagonism in clinical PAH.
References
- 1.Sung YK, Chung L. Connective tissue disease-associated pulmonary arterial hypertension. Rheum Dis Clin North Am. 2015;41:295–313. doi: 10.1016/j.rdc.2015.01.003. [DOI] [PubMed] [Google Scholar]
- 2.Lai YC, Potoka KC, Champion HC, Mora AL, Gladwin MT. Pulmonary arterial hypertension: The clinical syndrome. Circulation research. 2014;115:115–130. doi: 10.1161/CIRCRESAHA.115.301146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Zamanian RT, Kudelko KT, Sung YK, de Jesus Perez V, Liu J, Spiekerkoetter E. Current clinical management of pulmonary arterial hypertension. Circulation research. 2014;115:131–147. doi: 10.1161/CIRCRESAHA.115.303827. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.McLaughlin VV, Shah SJ, Souza R, Humbert M. Management of pulmonary arterial hypertension. Journal of the American College of Cardiology. 2015;65:1976–1997. doi: 10.1016/j.jacc.2015.03.540. [DOI] [PubMed] [Google Scholar]
- 5.Humbert M, Lau EM, Montani D, Jais X, Sitbon O, Simonneau G. Advances in therapeutic interventions for patients with pulmonary arterial hypertension. Circulation. 2014;130:2189–2208. doi: 10.1161/CIRCULATIONAHA.114.006974. [DOI] [PubMed] [Google Scholar]
- 6.Burke DL, Frid MG, Kunrath CL, Karoor V, Anwar A, Wagner BD, Strassheim D, Stenmark KR. Sustained hypoxia promotes the development of a pulmonary artery-specific chronic inflammatory microenvironment. Am J Physiol Lung Cell Mol Physiol. 2009;297:L238–250. doi: 10.1152/ajplung.90591.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rabinovitch M, Guignabert C, Humbert M, Nicolls MR. Inflammation and immunity in the pathogenesis of pulmonary arterial hypertension. Circulation research. 2014;115:165–175. doi: 10.1161/CIRCRESAHA.113.301141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Guignabert C, Tu L, Girerd B, Ricard N, Huertas A, Montani D, Humbert M. New molecular targets of pulmonary vascular remodeling in pulmonary arterial hypertension: Importance of endothelial communication. Chest. 2015;147:529–537. doi: 10.1378/chest.14-0862. [DOI] [PubMed] [Google Scholar]
- 9.Archer SL, Weir EK, Wilkins MR. Basic science of pulmonary arterial hypertension for clinicians: New concepts and experimental therapies. Circulation. 2010;121:2045–2066. doi: 10.1161/CIRCULATIONAHA.108.847707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tian W, Jiang X, Tamosiuniene R, Sung YK, Qian J, Dhillon G, Gera L, Farkas L, Rabinovitch M, Zamanian RT, Inayathullah M, Fridlib M, Rajadas J, Peters-Golden M, Voelkel NF, Nicolls MR. Blocking macrophage leukotriene b4 prevents endothelial injury and reverses pulmonary hypertension. Sci Transl Med. 2013;5:200ra117. doi: 10.1126/scitranslmed.3006674. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Tuder RM, Marecki JC, Richter A, Fijalkowska I, Flores S. Pathology of pulmonary hypertension. Clin Chest Med. 2007;28:23–42. vii. doi: 10.1016/j.ccm.2006.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Stenmark KR, Frid MG, Yeager M, Li M, Riddle S, McKinsey T, El Kasmi KC. Targeting the adventitial microenvironment in pulmonary hypertension: A potential approach to therapy that considers epigenetic change. Pulmonary circulation. 2012;2:3–14. doi: 10.4103/2045-8932.94817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Stenmark KR, Davie N, Frid M, Gerasimovskaya E, Das M. Role of the adventitia in pulmonary vascular remodeling. Physiology. 2006;21:134–145. doi: 10.1152/physiol.00053.2005. [DOI] [PubMed] [Google Scholar]
- 14.Stenmark KR, Nozik-Grayck E, Gerasimovskaya E, Anwar A, Li M, Riddle S, Frid M. The adventitia: Essential role in pulmonary vascular remodeling. Comprehensive Physiology. 2011;1:141–161. doi: 10.1002/cphy.c090017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Stenmark KR, Yeager ME, El Kasmi KC, Nozik-Grayck E, Gerasimovskaya EV, Li M, Riddle SR, Frid MG. The adventitia: Essential regulator of vascular wall structure and function. Annual review of physiology. 2013;75:23–47. doi: 10.1146/annurev-physiol-030212-183802. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Stenmark KR, Fagan KA, Frid MG. Hypoxia-induced pulmonary vascular remodeling: Cellular and molecular mechanisms. Circ Res. 2006;99:675–691. doi: 10.1161/01.RES.0000243584.45145.3f. [DOI] [PubMed] [Google Scholar]
- 17.Carlin CM, Peacock AJ, Welsh DJ. Fluvastatin inhibits hypoxic proliferation and p38 mapk activity in pulmonary artery fibroblasts. Am J Resp Cell Mol. 2007;37:447–456. doi: 10.1165/rcmb.2007-0012OC. [DOI] [PubMed] [Google Scholar]
- 18.Welsh DJ, Peacock AJ, MacLean M, Harnett M. Chronic hypoxia induces constitutive p38 mitogen-activated protein kinase activity that correlates with enhanced cellular proliferation in fibroblasts from rat pulmonary but not systemic arteries. Am J Respir Crit Care Med. 2001;164:282–289. doi: 10.1164/ajrccm.164.2.2008054. [DOI] [PubMed] [Google Scholar]
- 19.Welsh DJ, Scott PH, Peacock AJ. P38 map kinase isoform activity and cell cycle regulators in the proliferative response of pulmonary and systemic artery fibroblasts to acute hypoxia. Pulm Pharmacol Ther. 2006;19:128–138. doi: 10.1016/j.pupt.2005.04.008. [DOI] [PubMed] [Google Scholar]
- 20.Barman SA, Chen F, Su Y, Dimitropoulou C, Wang Y, Catravas JD, Han W, Orfi L, Szantai-Kis C, Keri G, Szabadkai I, Barabutis N, Rafikova O, Rafikov R, Black SM, Jonigk D, Giannis A, Asmis R, Stepp DW, Ramesh G, Fulton DJ. Nadph oxidase 4 is expressed in pulmonary artery adventitia and contributes to hypertensive vascular remodeling. Arteriosclerosis, thrombosis, and vascular biology. 2014;34:1704–1715. doi: 10.1161/ATVBAHA.114.303848. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Li M, Riddle SR, Frid MG, El Kasmi KC, McKinsey TA, Sokol RJ, Strassheim D, Meyrick B, Yeager ME, Flockton AR, McKeon BA, Lemon DD, Horn TR, Anwar A, Barajas C, Stenmark KR. Emergence of fibroblasts with a proinflammatory epigenetically altered phenotype in severe hypoxic pulmonary hypertension. J Immunol. 2011;187:2711–2722. doi: 10.4049/jimmunol.1100479. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Panzhinskiy E, Zawada WM, Stenmark KR, Das M. Hypoxia induces unique proliferative response in adventitial fibroblasts by activating pdgfbeta receptor-jnk1 signalling. Cardiovasc Res. 2012;95:356–365. doi: 10.1093/cvr/cvs194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Das M, Burns N, Wilson SJ, Zawada WM, Stenmark KR. Hypoxia exposure induces the emergence of fibroblasts lacking replication repressor signals of pkczeta in the pulmonary artery adventitia. Cardiovasc Res. 2008;78:440–448. doi: 10.1093/cvr/cvn014. [DOI] [PubMed] [Google Scholar]
- 24.Zarubin T, Han J. Activation and signaling of the p38 map kinase pathway. Cell Res. 2005;15:11–18. doi: 10.1038/sj.cr.7290257. [DOI] [PubMed] [Google Scholar]
- 25.Fisk M, Gajendragadkar PR, Maki-Petaja KM, Wilkinson IB, Cheriyan J. Therapeutic potential of p38 map kinase inhibition in the management of cardiovascular disease. American journal of cardiovascular drugs : drugs, devices, and other interventions. 2014;14:155–165. doi: 10.1007/s40256-014-0063-6. [DOI] [PubMed] [Google Scholar]
- 26.Weerackody RP, Welsh DJ, Wadsworth RM, Peacock AJ. Inhibition of p38 mapk reverses hypoxia-induced pulmonary artery endothelial dysfunction. Am J Physiol Heart Circ Physiol. 2009;296:H1312–1320. doi: 10.1152/ajpheart.00977.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Brown DI, Griendling KK. Nox proteins in signal transduction. Free radical biology & medicine. 2009;47:1239–1253. doi: 10.1016/j.freeradbiomed.2009.07.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Taverne YJ, Bogers AJ, Duncker DJ, Merkus D. Reactive oxygen species and the cardiovascular system. Oxidative medicine and cellular longevity. 2013;2013:862423. doi: 10.1155/2013/862423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Aggarwal S, Gross CM, Sharma S, Fineman JR, Black SM. Reactive oxygen species in pulmonary vascular remodeling. Comprehensive Physiology. 2013;3:1011–1034. doi: 10.1002/cphy.c120024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Park S, Ahn JY, Lim MJ, Kim MH, Yun YS, Jeong G, Song JY. Sustained expression of nadph oxidase 4 by p38 mapk-akt signaling potentiates radiation-induced differentiation of lung fibroblasts. Journal of molecular medicine. 2010;88:807–816. doi: 10.1007/s00109-010-0622-5. [DOI] [PubMed] [Google Scholar]
- 31.Peters-Golden M, Brock TG. 5-lipoxygenase and flap. Prostaglandins, leukotrienes, and essential fatty acids. 2003;69:99–109. doi: 10.1016/s0952-3278(03)00070-x. [DOI] [PubMed] [Google Scholar]
- 32.Israel E, Rubin P, Kemp JP, Grossman J, Pierson W, Siegel SC, Tinkelman D, Murray JJ, Busse W, Segal AT, Fish J, Kaiser HB, Ledford D, Wenzel S, Rosenthal R, Cohn J, Lanni C, Pearlman H, Karahalios P, Drazen JM. The effect of inhibition of 5-lipoxygenase by zileuton in mild-to-moderate asthma. Annals of internal medicine. 1993;119:1059–1066. doi: 10.7326/0003-4819-119-11-199312010-00001. [DOI] [PubMed] [Google Scholar]
- 33.Spanbroek R, Grabner R, Lotzer K, Hildner M, Urbach A, Ruhling K, Moos MP, Kaiser B, Cohnert TU, Wahlers T, Zieske A, Plenz G, Robenek H, Salbach P, Kuhn H, Radmark O, Samuelsson B, Habenicht AJ. Expanding expression of the 5-lipoxygenase pathway within the arterial wall during human atherogenesis. P Natl Acad Sci USA. 2003;100:1238–1243. doi: 10.1073/pnas.242716099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Helgadottir A, Manolescu A, Thorleifsson G, Gretarsdottir S, Jonsdottir H, Thorsteinsdottir U, Samani NJ, Gudmundsson G, Grant SF, Thorgeirsson G, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Johannsson H, Gudmundsdottir O, Gurney ME, Sainz J, Thorhallsdottir M, Andresdottir M, Frigge ML, Topol EJ, Kong A, Gudnason V, Hakonarson H, Gulcher JR, Stefansson K. The gene encoding 5-lipoxygenase activating protein confers risk of myocardial infarction and stroke. Nature genetics. 2004;36:233–239. doi: 10.1038/ng1311. [DOI] [PubMed] [Google Scholar]
- 35.Helgadottir A, Manolescu A, Helgason A, Thorleifsson G, Thorsteinsdottir U, Gudbjartsson DF, Gretarsdottir S, Magnusson KP, Gudmundsson G, Hicks A, Jonsson T, Grant SF, Sainz J, O'Brien SJ, Sveinbjornsdottir S, Valdimarsson EM, Matthiasson SE, Levey AI, Abramson JL, Reilly MP, Vaccarino V, Wolfe ML, Gudnason V, Quyyumi AA, Topol EJ, Rader DJ, Thorgeirsson G, Gulcher JR, Hakonarson H, Kong A, Stefansson K. A variant of the gene encoding leukotriene a4 hydrolase confers ethnicity-specific risk of myocardial infarction. Nature genetics. 2006;38:68–74. doi: 10.1038/ng1692. [DOI] [PubMed] [Google Scholar]
- 36.Tamosiuniene R, Tian W, Dhillon G, Wang L, Sung YK, Gera L, Patterson AJ, Agrawal R, Rabinovitch M, Ambler K, Long CS, Voelkel NF, Nicolls MR. Regulatory t cells limit vascular endothelial injury and prevent pulmonary hypertension. Circ Res. 2011;109:867–879. doi: 10.1161/CIRCRESAHA.110.236927. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Prasadam I, Mao X, Wang Y, Shi W, Crawford R, Xiao Y. Inhibition of p38 pathway leads to oa-like changes in a rat animal model. Rheumatology (Oxford) 2012;51:813–823. doi: 10.1093/rheumatology/ker360. [DOI] [PubMed] [Google Scholar]
- 38.Chen XL, Xia ZF, Yu YX, Wei D, Wang CR, Ben DF. P38 mitogen-activated protein kinase inhibition attenuates burn-induced liver injury in rats. Burns : journal of the International Society for Burn Injuries. 2005;31:320–330. doi: 10.1016/j.burns.2004.10.015. [DOI] [PubMed] [Google Scholar]
- 39.Qian J, Chen F, Kovalenkov Y, Pandey D, Moseley MA, Foster MW, Black SM, Venema RC, Stepp DW, Fulton DJ. Nitric oxide reduces nadph oxidase 5 (nox5) activity by reversible s-nitrosylation. Free radical biology & medicine. 2012;52:1806–1819. doi: 10.1016/j.freeradbiomed.2012.02.029. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, Fini MA, Graham BB, Tuder RM, Friedman JE, Eltzschig HK, Sokol RJ, Stenmark KR. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. J Immunol. 2014;193:597–609. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Pannu J, Asano Y, Nakerakanti S, Smith E, Jablonska S, Blaszczyk M, ten Dijke P, Trojanowska M. Smad1 pathway is activated in systemic sclerosis fibroblasts and is targeted by imatinib mesylate. Arthritis Rheum. 2008;58:2528–2537. doi: 10.1002/art.23698. [DOI] [PubMed] [Google Scholar]
- 42.Badesch DB, Raskob GE, Elliott CG, Krichman AM, Farber HW, Frost AE, Barst RJ, Benza RL, Liou TG, Turner M, Giles S, Feldkircher K, Miller DP, McGoon MD. Pulmonary arterial hypertension: Baseline characteristics from the reveal registry. Chest. 2010;137:376–387. doi: 10.1378/chest.09-1140. [DOI] [PubMed] [Google Scholar]
- 43.Denton CP, Black CM. Pulmonary hypertension in systemic sclerosis. Rheum Dis Clin North Am. 2003;29:335–349. vii. doi: 10.1016/s0889-857x(03)00024-3. [DOI] [PubMed] [Google Scholar]
- 44.Forte A, Della Corte A, De Feo M, Cerasuolo F, Cipollaro M. Role of myofibroblasts in vascular remodelling: Focus on restenosis and aneurysm. Cardiovascular research. 2010;88:395–405. doi: 10.1093/cvr/cvq224. [DOI] [PubMed] [Google Scholar]
- 45.Kumar S, Jiang MS, Adams JL, Lee JC. Pyridinylimidazole compound sb 203580 inhibits the activity but not the activation of p38 mitogen-activated protein kinase. Biochemical and biophysical research communications. 1999;263:825–831. doi: 10.1006/bbrc.1999.1454. [DOI] [PubMed] [Google Scholar]
- 46.Werz O, Klemm J, Samuelsson B, Radmark O. 5-lipoxygenase is phosphorylated by p38 kinase-dependent mapkap kinases. Proc Natl Acad Sci U S A. 2000;97:5261–5266. doi: 10.1073/pnas.050588997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Ito K, Hirao A, Arai F, Takubo K, Matsuoka S, Miyamoto K, Ohmura M, Naka K, Hosokawa K, Ikeda Y, Suda T. Reactive oxygen species act through p38 mapk to limit the lifespan of hematopoietic stem cells. Nat Med. 2006;12:446–451. doi: 10.1038/nm1388. [DOI] [PubMed] [Google Scholar]
- 48.Zuo L, Rose BA, Roberts WJ, He F, Banes-Berceli AK. Molecular characterization of reactive oxygen species in systemic and pulmonary hypertension. Am J Hypertens. 2014;27:643–650. doi: 10.1093/ajh/hpt292. [DOI] [PubMed] [Google Scholar]
- 49.Nozik-Grayck E, Stenmark KR. Role of reactive oxygen species in chronic hypoxia-induced pulmonary hypertension and vascular remodeling. Advances in experimental medicine and biology. 2007;618:101–112. doi: 10.1007/978-0-387-75434-5_8. [DOI] [PubMed] [Google Scholar]
- 50.Stenmark KR, Frid MG, Yeager M, Li M, Riddle S, McKinsey T, El Kasmi KC. Targeting the adventitial microenvironment in pulmonary hypertension: A potential approach to therapy that considers epigenetic change. Pulm Circ. 2012;2:3–14. doi: 10.4103/2045-8932.94817. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Brock TG, Lee YJ, Maydanski E, Marburger TL, Luo M, Paine R, 3rd, Peters-Golden M. Nuclear localization of leukotriene a4 hydrolase in type ii alveolar epithelial cells in normal and fibrotic lung. American journal of physiology. Lung cellular and molecular physiology. 2005;289:L224–232. doi: 10.1152/ajplung.00423.2004. [DOI] [PubMed] [Google Scholar]
- 52.Ochiya T, Takenaga K, Endo H. Silencing of s100a4, a metastasis-associated protein, in endothelial cells inhibits tumor angiogenesis and growth. Angiogenesis. 2014;17:17–26. doi: 10.1007/s10456-013-9372-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Varga J, Abraham D. Systemic sclerosis: A prototypic multisystem fibrotic disorder. J Clin Invest. 2007;117:557–567. doi: 10.1172/JCI31139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Yaqub A, Chung L. Epidemiology and risk factors for pulmonary hypertension in systemic sclerosis. Current rheumatology reports. 2013;15:302. doi: 10.1007/s11926-012-0302-2. [DOI] [PubMed] [Google Scholar]
- 55.El Kasmi KC, Pugliese SC, Riddle SR, Poth JM, Anderson AL, Frid MG, Li M, Pullamsetti SS, Savai R, Nagel MA, Fini MA, Graham BB, Tuder RM, Friedman JE, Eltzschig HK, Sokol RJ, Stenmark KR. Adventitial fibroblasts induce a distinct proinflammatory/profibrotic macrophage phenotype in pulmonary hypertension. Journal of immunology. 2014;193:597–609. doi: 10.4049/jimmunol.1303048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Radmark O, Samuelsson B. Regulation of the activity of 5-lipoxygenase, a key enzyme in leukotriene biosynthesis. Biochem Biophys Res Commun. 2010;396:105–110. doi: 10.1016/j.bbrc.2010.02.173. [DOI] [PubMed] [Google Scholar]
- 57.Steiner MK, Syrkina OL, Kolliputi N, Mark EJ, Hales CA, Waxman AB. Interleukin-6 overexpression induces pulmonary hypertension. Circ Res. 2009;104:236–244. doi: 10.1161/CIRCRESAHA.108.182014. 228p following 244. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ishihara K, Hirano T. Il-6 in autoimmune disease and chronic inflammatory proliferative disease. Cytokine & growth factor reviews. 2002;13:357–368. doi: 10.1016/s1359-6101(02)00027-8. [DOI] [PubMed] [Google Scholar]
- 59.Kaiser RA, Lyons JM, Duffy JY, Wagner CJ, McLean KM, O'Neill TP, Pearl JM, Molkentin JD. Inhibition of p38 reduces myocardial infarction injury in the mouse but not pig after ischemia-reperfusion. Am J Physiol Heart Circ Physiol. 2005;289:H2747–2751. doi: 10.1152/ajpheart.01280.2004. [DOI] [PubMed] [Google Scholar]
- 60.Ma XL, Kumar S, Gao F, Louden CS, Lopez BL, Christopher TA, Wang C, Lee JC, Feuerstein GZ, Yue TL. Inhibition of p38 mitogen-activated protein kinase decreases cardiomyocyte apoptosis and improves cardiac function after myocardial ischemia and reperfusion. Circulation. 1999;99:1685–1691. doi: 10.1161/01.cir.99.13.1685. [DOI] [PubMed] [Google Scholar]
- 61.Liu YH, Wang D, Rhaleb NE, Yang XP, Xu J, Sankey SS, Rudolph AE, Carretero OA. Inhibition of p38 mitogen-activated protein kinase protects the heart against cardiac remodeling in mice with heart failure resulting from myocardial infarction. Journal of cardiac failure. 2005;11:74–81. doi: 10.1016/j.cardfail.2004.04.004. [DOI] [PubMed] [Google Scholar]
- 62.Newby LK, Marber MS, Melloni C, Sarov-Blat L, Aberle LH, Aylward PE, Cai G, de Winter RJ, Hamm CW, Heitner JF, Kim R, Lerman A, Patel MR, Tanguay JF, Lepore JJ, Al-Khalidi HR, Sprecher DL, Granger CB, Investigators S. Losmapimod, a novel p38 mitogen-activated protein kinase inhibitor, in non-st-segment elevation myocardial infarction: A randomised phase 2 trial. Lancet. 2014;384:1187–1195. doi: 10.1016/S0140-6736(14)60417-7. [DOI] [PubMed] [Google Scholar]
- 63.Church AC, Martin DH, Wadsworth RM, Bryson G, Fisher AJ, Welsh DJ, Peacock AJ. The reversal of pulmonary vascular remodelling through inhibition of p38mapk-alpha : A potential novel anti-inflammatory strategy in pulmonary hypertension. Am J Physiol Lung Cell Mol Physiol. 2015 doi: 10.1152/ajplung.00038.2015. ajplung 00038 02015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Brieger K, Schiavone S, Miller FJ, Jr., Krause KH. Reactive oxygen species: From health to disease. Swiss Med Wkly. 2012;142:w13659. doi: 10.4414/smw.2012.13659. [DOI] [PubMed] [Google Scholar]
- 65.Chen F, Haigh S, Barman S, Fulton DJ. From form to function: The role of nox4 in the cardiovascular system. Frontiers in physiology. 2012;3:412. doi: 10.3389/fphys.2012.00412. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Cho KJ, Seo JM, Kim JH. Bioactive lipoxygenase metabolites stimulation of nadph oxidases and reactive oxygen species. Molecules and cells. 2011;32:1–5. doi: 10.1007/s10059-011-1021-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Goettsch C, Goettsch W, Muller G, Seebach J, Schnittler HJ, Morawietz H. Nox4 overexpression activates reactive oxygen species and p38 mapk in human endothelial cells. Biochem Biophys Res Commun. 2009;380:355–360. doi: 10.1016/j.bbrc.2009.01.107. [DOI] [PubMed] [Google Scholar]
- 68.Li J, Stouffs M, Serrander L, Banfi B, Bettiol E, Charnay Y, Steger K, Krause KH, Jaconi ME. The nadph oxidase nox4 drives cardiac differentiation: Role in regulating cardiac transcription factors and map kinase activation. Molecular biology of the cell. 2006;17:3978–3988. doi: 10.1091/mbc.E05-06-0532. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.