Abstract
The objective of this work was to test the hypotheses that a) more frequent cognitive activity in late life is associated with higher brain diffusion anisotropy and lower trace of the diffusion tensor, and b) brain diffusion characteristics partially mediate the association of late life cognitive activity with cognition. As part of a longitudinal cohort study, 379 older people without dementia rated their frequency of participation in cognitive activities, completed a battery of cognitive function tests, and underwent diffusion tensor imaging. We used tract-based spatial statistics to test the association between late life cognitive activity and brain diffusion characteristics. Clusters with statistically significant findings defined regions of interest in which we tested the hypothesis that diffusion characteristics partially mediate the association of late life cognitive activity with cognition. More frequent cognitive activity in late life was associated with higher level of global cognition after adjustment for age, sex, education, and indicators of early life cognitive enrichment (p=0.001). More frequent cognitive activity was also related to higher fractional anisotropy in the left superior and inferior longitudinal fasciculi, left fornix, and corpus callosum, and lower trace in the thalamus (p<0.05, FWE-corrected). After controlling for fractional anisotropy or trace from these regions, the regression coefficient for the association of late life cognitive activity with cognition was reduced by as much as 26 %. These findings suggest that the association of late life cognitive activity with cognition may be partially mediated by brain diffusion characteristics.
Keywords: Cognitive activity, Cognition, Magnetic resonance imaging, MRI, Diffusion tensor imaging, DTI
Introduction
More frequent cognitive activity in late life is associated with a slower rate of cognitive decline (Ghisletta et al. 2006; Hultsch et al. 1999; Schooler and Mulatu 2001; Wilson et al. 2003, 2012), even after controlling for the effects of early life experiences and neuropathologies typically linked to late life dementia and cognitive impairment (Wilson et al. 2013a). This suggests that late life cognitive activity influences cognition through an association with cognitive or neural reserve (Hall et al. 2009; Stern 2012; Wilson et al. 2007). The neurobiological basis of this association is not well understood, but is thought to include in part neuronal density (Katzman et al. 1988; Wilson et al. 2013b) and synaptic proteins (Honer et al. 2012).
Neuroimaging research suggests that cognitive activity can lead to changes in brain characteristics including brain tissue structure as assessed by diffusion-weighted magnetic resonance imaging (MRI). More specifically, cross-sectional studies have shown that occupations (Imfeld et al. 2009; Roberts et al. 2010) or experiences (Lee et al. 2010) challenging particular cognitive functions are associated with differences in brain diffusion characteristics, and longitudinal neuroimaging studies have consistently shown changes in brain diffusion of persons after cognitively demanding training (Chapman et al. 2015; Engvig et al. 2012; Lövdén et al. 2010; Sagi et al. 2012; Schlegel et al. 2012). These changes in brain diffusion characteristics were attributed to alterations in brain tissue structure due to cognitive activity. Therefore, one plausible mechanism for the influence of late life cognitive activity on cognition may be that frequent participation in cognitively stimulating pursuits in late life may enhance brain tissue structural integrity, increasing efficiency of related cognitive systems and enhancing cognitive reserve. However, a recent study reported lack of an association between late life cognitive activity and brain diffusion characteristics (Gow et al. 2012).
In the present study, we examined associations among late life cognitive activity, cognition, and brain diffusion characteristics as assessed by diffusion tensor magnetic resonance imaging (DTI) (Le Bihan et al. 2001). Older persons without dementia participating in the Rush Memory and Aging Project (Bennett et al. 2012) rated their frequency of participation in cognitively stimulating activities, completed a battery of cognitive function tests, and underwent brain DTI. In analyses, we established the association of late life cognitive activity with cognition, and tested the hypotheses: a) that more frequent cognitive activity in late life is associated with higher brain diffusion anisotropy and lower trace of the diffusion tensor (typically representing higher brain tissue structural integrity), and b) that brain diffusion characteristics partially mediate the association of late life cognitive activity with cognition.
Methods
Participants
All participants are from the Rush Memory and Aging Project (Bennett et al. 2012), an ongoing longitudinal clinical-pathologic cohort study of aging and Alzheimer’s disease that began in 1997. Eligibility requires agreement to annual clinical evaluations and brain donation at death. Persons included in the present study also agreed to biannual brain MRI. All participants provided written informed consent. The study was approved by the institutional review board of Rush University Medical Center.
Participants underwent annual uniform structured clinical evaluations, including medical history, neurological examination, and cognitive function testing (Bennett et al. 2006a, b). A diagnosis of dementia and Alzheimer’s disease was determined in accordance with the criteria of the National Institute of Neurological and Communicative Disorders and Stroke and the Alzheimer Disease and Related Disorders Association (McKhann et al. 1984). Mild cognitive impairment (MCI) was classified in persons who had cognitive impairment but did not meet dementia criteria (Boyle et al. 2006). MR images were reviewed by a neuroradiologist. Participants with dementia, brain surgery, large infarcts, or structural brain abnormalities (e.g., tumors) not typical of aging and visible with MRI, were excluded. Persons with white matter lesions appearing hyper-intense in T2-weighted images (often referred to as white matter hyperintensities, WMHs), small infarcts, or microbleeds were not excluded.
At the time of these analyses, 1556 participants had completed the baseline clinical evaluation. Of these, 362 died and 59 withdrew from the study before the MRI sub-study began in February of 2009. Of the remaining 1135 persons, 444 had at least one brain MRI. Of those, 48 were excluded due to dementia and 17 due to other exclusion criteria or image artifacts discovered during quality checks. Analyses are based on the remaining 379 participants. They had a mean age of 82 years (SD=7), a mean of 15 years of education (SD=3), a mean Mini-Mental State Examination score of 28.5 (SD= 1.5) at the time of MRI, and 77 % were women.
Assessment of cognitive activity and cognitive resources
Participants rated their early life (at baseline only) and current (annually) frequency of participation in cognitively stimulating activities on a scale from 1 to 5 (1: indicated participation in the activity once a year or less; 2: several times a year; 3: several times a month; 4: several times a week; 5: every day or almost every day) using a structured questionnaire. There were 30 items on early life cognitive activity (11 about childhood, 10 about young adulthood, and 9 about middle age) and 7 items on current activity (Wilson et al. 2005). Activities included reading a newspaper, writing letters, visiting a library, attending a play, and playing games such as chess or checkers. Composite measures of early life and current cognitive activity were constructed by averaging item scores from each period (Wilson et al. 2005) (possible range of values for each composite measure was 1 to 5). A higher score on this late life cognitive activity scale has been associated with less rapid cognitive decline (Wilson et al. 2007, 2012, 2013a) and lower risk of developing MCI and Alzheimer’s disease (Wilson et al. 2007).
Participants were also asked about the availability of cognitive resources (e.g., atlas, encyclopedia, globe) in their home at ages 12 and 40 (Wilson et al. 2005). The number of resources (out of 8) at each age were added to yield an early life cognitive resource score (possible range of values was 0 to 8).
Assessment of cognition
A battery of 21 cognitive tests was administered annually (Bennett et al. 2006a, 2012) including the Mini-Mental State Examination, the Complex Ideational Material, 7 episodic memory tests, 3 semantic memory tests, 3 working memory tests, 4 perceptual speed tests, and 2 visuospatial ability tests. The Mini-Mental State Examination was used only for descriptive purposes and the Complex Ideational Material was only used for diagnostic classification. Raw scores on the remaining 19 tests were converted to z-scores. A person’s z-scores across all 19 tests were averaged to yield a single composite global cognitive score. Composite scores for each of the five cognitive domains (episodic memory, semantic memory, working memory, perceptual speed, visuospatial ability) were constructed by averaging the z-scores from the corresponding individual tests (Bennett et al. 2006a, 2012; Wilson et al. 2005).
Image acquisition
Brain MR imaging was conducted on all participants using a 1.5 Tesla General Electric MRI scanner (Waukesha, WI). High-resolution T1-weighted anatomical data was obtained using a 3D magnetization-prepared rapid acquisition gradient-echo (MPRAGE) sequence with: echo-time (TE)= 2.8 msec, repetition time (TR)=6.3 msec, preparation time= 1000 msec, flip-angle=8°, field-of-view (FOV)=24 cm×24 cm, 160 sagittal slices, slice thickness=1 mm, 224×192 acquisition matrix, and two repetitions. T2-weighted fluid attenuated inversion recovery (FLAIR) data was collected on all participants using a 2D fast spin-echo sequence with: TE= 120 msec, TR=8 s, inversion time=2 s, FOV=24 cm×24 cm, 42 oblique axial slices, slice thickness=3 mm, 256×224 acquisition matrix. Finally, spin-echo echo-planar DTI data was obtained for all participants using: TE=84.6 msec, TR=5.4 s, FOV=24 cm×24 cm, 36 axial slices, slice thickness=3 mm, 128×128 acquisition matrix, b=900 s/mm2 for 12 diffusion directions, and two b=0 s/mm2 volumes. The DTI data acquisition was repeated 6 times for a total of 72 diffusion-weighted and 12 b=0 s/mm2 image volumes.
Image processing
WMHs were automatically segmented for each participant using a support vector machine classifier based on both MPRAGE and FLAIR information. For the DTI data, corrections for bulk motion and distortions due to eddy-currents and magnetic field non-uniformities, B-matrix reorientation, and diffusion tensor calculation were conducted using TORTOISE (www.tortoisedti.org). Maps of the fractional anisotropy (FA), trace of the diffusion tensor, axial and radial diffusivity were produced (Le Bihan et al. 2001). Next, the WMH mask of each participant was transformed to the space of the corresponding processed DTI data based on the transformation of the FLAIR image volume to the preprocessed b=0 s/mm2 volume. A detailed description of the image processing steps can be found in Arfanakis et al. (2013).
Statistical analysis
We first used Spearman correlation to assess associations of late life cognitive activity with global cognition, age, level of education, and early life cognitive activity and resources, and Student’s t test to assess the association of late life cognitive activity with sex. We then used multiple linear regression to test the association of global cognition (dependent variable) with late life cognitive activity, controlling for age, sex, level of education, and early life cognitive activity and resources.
We tested the hypothesis that more frequent cognitive activity in late life is associated with higher brain diffusion anisotropy and lower trace of the diffusion tensor, in voxel-wise analyses using Tract-Based Spatial Statistics (Smith et al. 2006). For that purpose, the FA volumes from all participants were non-linearly spatially transformed to the mean FA template of the IIT Human Brain Atlas (v.3.2) (www.iit.edu/~mri) (Varentsova et al. 2014). The local FA maxima from each participant’s spatially transformed FA volume were then projected onto the white matter skeleton (Smith et al. 2006) of the IIT Human Brain Atlas (v.3.2). The same projection parameters were used to project the trace, axial diffusivity, radial diffusivity, and WMH mask values onto the white matter skeleton. Multiple linear regression was then used to test the association of FA along the white matter skeleton (dependent variable) with late life cognitive activity, while controlling for age, sex, level of education, early life cognitive activity and resources, and presence of WMHs voxel-wise. Separate multiple linear regression models were used to test the association of trace, axial and radial diffusivity (dependent variables) with late life cognitive activity, controlling for the same covariates mentioned above. The null distribution was built using the “randomise” tool in FSL (FMRIB, University of Oxford, UK) and 5000 permutations of the data. Differences were considered significant at p<0.05, Family Wise Error corrected. The Threshold-Free Cluster Enhancement method was used to define clusters with significant differences (Smith and Nichols 2009).
We then tested the hypothesis that diffusion characteristics in clusters derived from the voxel-wise analyses partially mediate the association of late life cognitive activity with cognition. Mediation analysis included a sequence of three linear regression models testing a) if late life cognitive activity was a significant predictor of a cluster’s FA, b) if the FA of a cluster was related to cognition, and c) if the FA contribution reduced the effect of late life cognitive activity by entering both variables as predictors of cognition. First, for each cluster that showed a significant association of FA with late life cognitive activity in the voxel-wise analysis, we extracted the mean FA of that cluster for each participant. The multiple linear regression conducted in the voxel-wise analysis was repeated per cluster, using the mean FA of the cluster as the dependent variable, and replacing the parameter controlling for the presence of WMH in a voxel with the percent of the cluster occupied by WMHs. Multiple linear regression was then used in each cluster to test the association of global cognition (dependent variable), as well as the performance in each of the five cognitive domains separately, with mean FA in the cluster, controlling for the same covariates as above. For these domains that showed a significant link to both mean FA of a cluster and late life cognitive activity, multiple linear regression was conducted including both the mean FA of the cluster and late life cognitive activity as independent variables, and controlling for the same covariates as above. We then calculated the indirect effect of late life cognitive activity on cognition through FA using bootstrap and bias corrected confidence intervals. The percent change in the regression coefficient of the late life cognitive activity term after adding the mean FA of a cluster in the model was also calculated. The same mediation analysis was repeated for clusters exhibiting significant links between the trace of the diffusion tensor and late life cognitive activity in the voxel-wise analysis (using mean trace of the cluster in the mediation analysis instead of mean FA). Throughout this section correlations were considered significant at p<0.05.
Results
Cognitive activity and cognition
The measure of late life cognitive activity had a mean of 3.3 (SD=0.6; range 1.1 to 4.6). More frequent late life cognitive activity was associated with higher levels of education (Table 1), early life cognitive activity and resources, but was not related to age (Table 1) or sex (t[377]=−0.66, p=0.51). The mean global cognition score was 0.3 (SD=0.5; range −1.8 to 1.5). Linear regression showed that higher level of late life cognitive activity was associated with higher level of global cognition (estimate=0.15, standard error [SE]=0.04, p=0.001) controlling for age, sex, education, and early life cognitive activity and resources.
Table 1.
Spearman correlations between late life cognitive activity, age, level of education, early life cognitive activity and resources, and global cognitiona
| Age | Education | Early life cognitive activity | Early life cognitive resources | Global cognition | |
|---|---|---|---|---|---|
| Late life cognitive activity | 0.03 | 0.25 | 0.41 | 0.27 | 0.28 |
| p=0.55 | p<0.0001 | p<0.0001 | p<0.0001 | p<0.0001 | |
| Age | 1 | −0.10 | −0.03 | −0.06 | −0.30 |
| p=0.052 | p=0.53 | p=0.23 | p<0.0001 | ||
| Education | 1 | 0.40 | 0.31 | 0.34 | |
| p<0.0001 | p<0.0001 | p<0.0001 | |||
| Early life cognitive activity | 1 | 0.56 | 0.31 | ||
| p<0.0001 | p<0.0001 | ||||
| Early life cognitive resources | 1 | 0.24 | |||
| p<0.0001 |
Significant correlations are shown in bold letters
Cognitive activity and brain diffusion characteristics
We tested the hypothesis that more frequent cognitive activity in late life is associated with higher FA and lower trace of the diffusion tensor in the brain. As hypothesized, more frequent late life cognitive activity was related to higher FA values in a number of white matter regions, controlling for age, sex, education, early life cognitive activity and resources, and presence of WMHs voxel-wise (p<0.05) (Fig. 1). These regions included the left superior and inferior longitudinal fasciculi, left fornix, and the genu and body of the corpus callosum (Fig. 1). Higher level of late life cognitive activity was also related to lower trace, axial, and radial diffusivity in the thalamus, controlling for the same covariates (p<0.05) (Fig. 2).
Fig. 1.
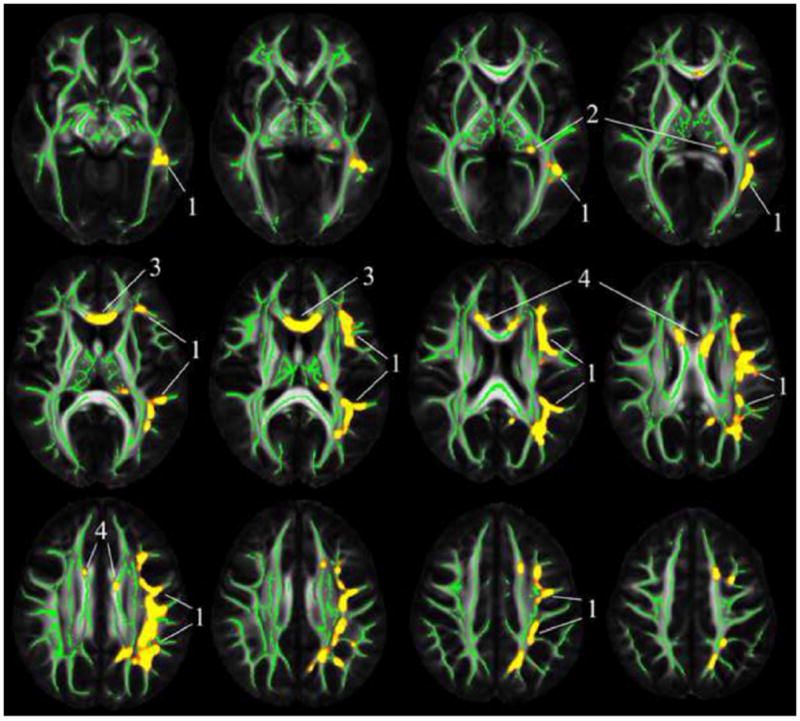
Regions of the white matter skeleton in which more frequent late life cognitive activity was related to higher FA are shown in yellow (controlling for age, sex, level of education, early life cognitive activity and resources, and presence of WMHs voxel-wise) (p<0.05, corrected for multiple comparisons). The location of clusters 1–4 used in the region of interest analyses is shown. Mean FA maps of the IIT Human Brain Atlas (v.3.2) (grayscale) and the corresponding white matter skeleton (green color) are shown in the background
Fig. 2.
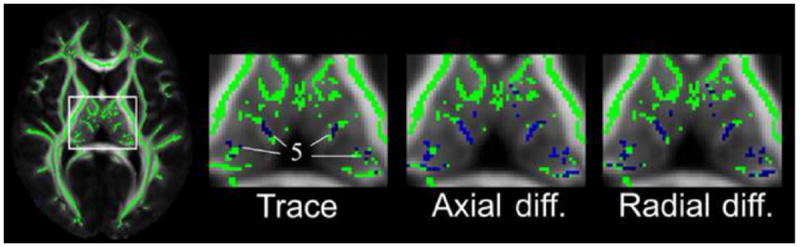
Regions of the thalamus in which more frequent late life cognitive activity was related to lower trace, axial and radial diffusivity are shown in blue (controlling for age, sex, level of education, early life cognitive activity and resources, and presence of WMHs voxel-wise) (p<0.05, corrected for multiple comparisons). The location of cluster 5 used in the region of interest analyses is shown. Mean FA maps of the IIT Human Brain Atlas (v.3.2) (grayscale) and the corresponding white matter skeleton (green color) are shown in the background
Cognitive activity, cognition, and brain diffusion characteristics
To test the hypothesis that brain diffusion characteristics partially mediate the association of late life cognitive activity with cognition, we first grouped voxels with significant correlations between diffusion measures and late life cognitive activity into 5 clusters, and extracted average diffusion characteristics from each cluster. Cluster 1 was the largest and included the voxels with significant findings in the left superior and inferior longitudinal fasciculi (Fig. 1); cluster 2 included the voxels in the left fornix (Fig. 1); cluster 3 included the voxels in and near the genu (Fig. 1); cluster 4 included the voxels in the body of the corpus callosum (Fig. 1); and cluster 5 included the voxels in the thalamus (Fig. 2). In each cluster, linear regression using average diffusion characteristics showed similar associations between diffusion measures and late life cognitive activity as in the voxel-wise analyses. Higher mean FA values in clusters 1–4 and lower mean trace values in cluster 5 were associated with higher levels of global cognition (Table 2, Model A). The partial mediation effect of mean FA from cluster 1 and cluster 2 on the association of late life cognitive activity with global cognition was 0.04 (95 % CI 0.01 to 0.08) and 0.03 (95 % CI 0.01 to 0.07), respectively, corresponding to a decrease in the regression coefficient of late life cognitive activity by 26 and 22 %, respectively (Table 2, Model B) (Fig. 3).
Table 2.
Association of late life cognitive activity and diffusion measures from five clusters with global cognitiona
| Model A
|
Model B
|
Δβllca (%) | |||||
|---|---|---|---|---|---|---|---|
| β (SE) | p | βdiff (SEdiff) | p | βllca (SEllca) | p | ||
| Late life cognitive activity | 0.15 (0.04) | 0.001 | |||||
| Cluster 1 FA | 2.78 (0.71) | <0.001 | 2.31 (0.73) | 0.002 | 0.11 (0.04) | 0.01 | −26 |
| Cluster 2 FA | 1.53 (0.38) | <0.001 | 1.31 (0.38) | 0.001 | 0.11 (0.04) | 0.01 | −22 |
| Cluster 3 FA | 0.80 (0.38) | 0.04 | 0.61 (0.38) | 0.11 | 0.13 (0.04) | 0.002 | |
| Cluster 4 FA | 0.81 (0.33) | 0.01 | 0.64 (0.33) | 0.054 | 0.13 (0.04) | 0.002 | |
| Cluster 5 trace | −0.05 (0.03) | 0.04 | −0.03 (0.03) | 0.17 | 0.13 (0.04) | 0.003 | |
β estimate, SE standard error, (βdiff, SEdiff) estimate and standard error for diffusion measure, (βllca, SEllca) estimate and standard error for late life cognitive activity measure, Δβllca change in the estimate for the late life cognitive activity measure from Model A to Model B
Separate linear regression models with global cognition as the dependent variable. Model A included either the late life cognitive activity measure or a diffusion measure from clusters 1–5 separately (the diffusion measure was FA for clusters 1–4, and trace for cluster 5). Model B included the late life cognitive activity measure and a diffusion measure. All models controlled for age, sex, level of education, early life cognitive activity and resources, and percent of cluster occupied by WMHs. Significant associations are in bold letters. The percent change in βllca from Model A to Model B (Δβllca) is shown when partial mediation is present
Fig. 3.
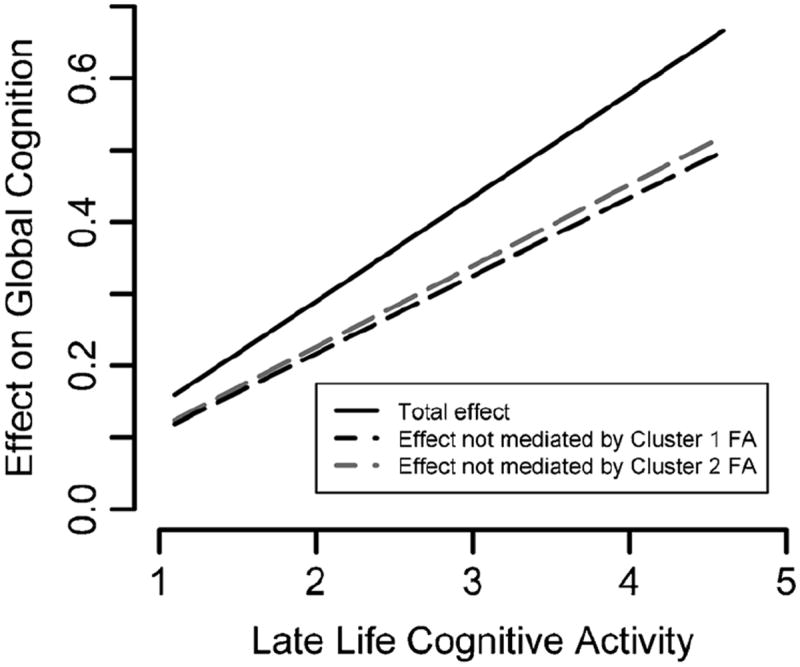
Plot of the total (solid line) versus direct (dashed lines) effect of late life cognitive activity on global cognition. The black and gray dashed lines represent the effect that is not mediated by the mean FA of cluster 1 and 2, respectively. The difference in slope between the solid and dashed lines is due to partial mediation of mean FA from clusters 1 and 2 on the association of late life cognitive activity with global cognition
We repeated the analyses separately for each of the individual cognitive domains. Higher mean FA values in clusters 1–4 were associated with higher levels of semantic memory (Table 3, Model A) and perceptual speed (Table 4, Model A), in clusters 1 and 2 with higher levels of episodic memory (Table 5), and in clusters 3 and 4 with higher levels of visuo-spatial ability (Table 5). Higher mean trace values in cluster 5 were associated with lower levels of perceptual speed (Table 4, Model A). The partial mediation effect of mean FA from cluster 1 and cluster 2 on the association of late life cognitive activity with semantic memory was 0.04 (95 % CI 0.02 to 0.08) for both clusters, corresponding to a decrease in the regression coefficient of late life cognitive activity by 23 and 20 %, respectively (Table 3, Model B). The partial mediation effect of mean FA from clusters 1–4, and mean trace from cluster 5, on the association of late life cognitive activity with perceptual speed was: [cluster 1 FA: 0.06 (95 % CI 0.03 to 0.11)], [cluster 2 FA: 0.05 (95 % CI 0.02 to 0.09)], [cluster 3 FA: 0.03 (95 % CI 0.01 to 0.07)], [cluster 4 FA: 0.03 (95 % CI 0.01 to 0.07)], [cluster 5 trace: 0.05 (95 % CI 0.02 to 0.09)], corresponding to a decrease in the regression coefficient of late life cognitive activity by 21, 18, 12, 10 and 16 %, respectively (Table 4, Model B).
Table 3.
Association of late life cognitive activity and diffusion measures from five clusters with semantic memorya
| Model A
|
Model B
|
Δβllca (%) | |||||
|---|---|---|---|---|---|---|---|
| β (SE) | p | βdiff (SEdiff) | p | βllca (SEllca) | p | ||
| Late life cognitive activity | 0.19 (0.05) | <0.001 | |||||
| Cluster 1 FA | 3.37 (0.85) | <0.001 | 2.74 (0.87) | 0.002 | 0.15 (0.05) | 0.006 | −23 |
| Cluster 2 FA | 1.88 (0.46) | <0.001 | 1.59 (0.46) | 0.001 | 0.15 (0.05) | 0.004 | −20 |
| Cluster 3 FA | 1.05 (0.45) | 0.02 | 0.80 (0.45) | 0.08 | 0.18 (0.05) | 0.001 | |
| Cluster 4 FA | 0.91 (0.40) | 0.02 | 0.68 (0.40) | 0.09 | 0.18 (0.05) | 0.001 | |
| Cluster 5 trace | −0.06 (0.03) | 0.07 | |||||
β estimate, SE standard error, (βdiff, SEdiff) estimate and standard error for diffusion measure, (βllca, SEllca) estimate and standard error for late life cognitive activity measure, Δβllca change in the estimate for the late life cognitive activity measure from Model A to Model B
Separate linear regression models with semantic memory as the dependent variable. Model A included either the late life cognitive activity measure or a diffusion measure from clusters 1–5 separately (the diffusion measure was FA for clusters 1–4, and trace for cluster 5). Model B included the late life cognitive activity measure and a diffusion measure. All models controlled for age, sex, level of education, early life cognitive activity and resources, and percent of cluster occupied by WMHs. Significant associations are in bold letters. The percent change in βllca from Model A to Model B (Δβllca) is shown when partial mediation is present
Table 4.
Association of late life cognitive activity and diffusion measures from five clusters with perceptual speeda
| Model A
|
Model B
|
Δβllca (%) | |||||
|---|---|---|---|---|---|---|---|
| β (SE) | p | βdiff (SEdiff) | p | βllca (SEllca) | p | ||
| Late life cognitive activity | 0.29 (0.06) | <0.001 | |||||
| Cluster 1 FA | 4.76 (0.97) | <0.001 | 3.80 (0.99) | <0.001 | 0.22 (0.06) | <0.001 | −21 |
| Cluster 2 FA | 2.53 (0.52) | <0.001 | 2.09 (0.52) | <0.001 | 0.23 (0.06) | <0.001 | −18 |
| Cluster 3 FA | 2.14 (0.51) | <0.001 | 1.79 (0.51) | <0.001 | 0.25 (0.06) | <0.001 | −12 |
| Cluster 4 FA | 1.63 (0.45) | <0.001 | 1.29 (0.45) | 0.004 | 0.27 (0.06) | <0.001 | −10 |
| Cluster 5 trace | −0.15 (0.03) | <0.001 | −0.12 (0.03) | 0.001 | 0.24 (0.06) | <0.001 | −16 |
β estimate, SE standard error, (βdiff, SEdiff) estimate and standard error for diffusion measure, (βllca, SEllca) estimate and standard error for late life cognitive activity measure, Δβllca change in the estimate for the late life cognitive activity measure from Model A to Model B
Separate linear regression models with perceptual speed as the dependent variable. Model A included either the late life cognitive activity measure or a diffusion measure from clusters 1–5 separately (the diffusion measure was FA for clusters 1–4, and trace for cluster 5). Model B included the late life cognitive activity measure and a diffusion measure. All models controlled for age, sex, level of education, early life cognitive activity and resources, and percent of cluster occupied by WMHs. Significant associations are in bold letters. The percent change in βllca from Model A to Model B (Δβllca) is shown when partial mediation is present
Table 5.
Association of late life cognitive activity and diffusion measures from five clusters with episodic memory, visuospatial ability, and working memorya
| Episodic memory
|
Visuospatial ability
|
Working memory
|
||||
|---|---|---|---|---|---|---|
| β (SE) | p | β (SE) | p | β (SE) | p | |
| Late life cognitive activity | 0.10 (0.06) | 0.14 | −0.03 (0.06) | 0.70 | 0.14 (0.07) | 0.03 |
| Cluster 1 FA | 2.16 (1.05) | 0.04 | 1.61 (1.06) | 0.13 | 1.76 (1.11) | 0.11 |
| Cluster 2 FA | 1.31 (0.56) | 0.02 | 0.63 (0.57) | 0.27 | 0.97 (0.59) | 0.10 |
| Cluster 3 FA | −0.09 (0.56) | 0.87 | 1.29 (0.56) | 0.02 | 0.50 (0.59) | 0.39 |
| Cluster 4 FA | 0.25 (0.49) | 0.60 | 1.30 (0.49) | 0.008 | 0.61 (0.51) | 0.23 |
| Cluster 5 trace | −0.002 (0.036) | 0.95 | −0.07 (0.04) | 0.07 | −0.02 (0.04) | 0.62 |
β estimate, SE standard error
Separate linear regression models with episodic memory, visuospatial ability, or working memory as the dependent variable. Each model included either the late life cognitive activity measure or a diffusion measure from clusters 1–5 (the diffusion measure was FA for clusters 1–4, and trace for cluster 5). All models controlled for age, sex, level of education, early life cognitive activity and resources, and percent of cluster occupied by WMHs. Significant associations are in bold letters. Mediation was not investigated since performance in each of the three cognitive domains was not associated with either the late life cognitive activity measure or the diffusion measures
Discussion
Older persons without dementia participating in the Rush Memory and Aging Project (Bennett et al. 2012) rated their frequency of participation in cognitively stimulating activities, completed a battery of cognitive function tests, and underwent brain DTI. More frequent cognitive activity in late life was associated with higher level of global cognition after adjustment for age, sex, education, and indicators of early life cognitive enrichment. More frequent late life cognitive activity was also related to higher FA in the left superior and inferior longitudinal fasciculi, left fornix, and corpus callosum (genu and body), and lower trace of the diffusion tensor in the thalamus. After controlling for FA or trace from these regions, the regression coefficient for the association of late life cognitive activity with cognition was reduced by as much as 26 %.
The finding that more frequent cognitive activity in late life was associated with higher level of global cognition is consistent with previous work (Ghisletta et al. 2006; Hultsch et al. 1999; Schooler and Mulatu 2001; Wilson et al. 2003, 2012, 2013a). The important new finding was that brain diffusion characteristics were shown to partially mediate this association. Recent work demonstrated that the link between late life cognitive activity and cognition is independent of neuropathologic burden (Wilson et al. 2013a), supporting the cognitive reserve hypothesis. This suggests that more frequent late life cognitive activity enhances cognition by enhancing one or more brain properties, and this set of brain properties contributes to cognitive reserve. Consequently, our finding that the association of late life cognitive activity with cognition was partially mediated by brain diffusion characteristics suggests that these may be some of the brain properties supporting the link between late life cognitive activity and cognition. Diffusion characteristics may therefore constitute components of neural reserve, counterbalancing cognitive loss associated with neuropathology.
Although brain diffusion characteristics are closely linked to structural tissue properties such as axonal density, axonal diameter, degree of myelination, and intravoxel coherence of axonal orientation (Sen and Basser 2005), DTI measures are non-specific and, therefore, the precise tissue properties involved in the observed associations of diffusion characteristics with late life cognitive activity and cognition are uncertain. Nevertheless, animal studies have shown that neuroplasticity induced by cognitive training or different experiences may include neurogenesis, synaptogenesis, dendrogenesis, axonal or synaptic sprouting, changes in morphology of astrocytes, and increased myelination, and that these changes may be detectable by DTI (Blumenfeld-Katzir et al. 2011; Buschkuehl et al. 2012; Markham and Greenough 2004; Sagi et al. 2012). The higher FA and lower trace, axial and radial diffusivity detected for higher levels of late life cognitive activity, are consistent with the neuroplastic mechanisms observed in animal studies. Histological investigation of brain structural properties as a function of late life cognitive activity is warranted.
The significant role of clusters 1–5 in the association of late life cognitive activity with cognition as demonstrated in this work is in general agreement with existing knowledge about the functional significance of the underlying brain regions. Cluster 1 included the left inferior longitudinal fasciculus, which has been linked to thought disorders, visual emotion and cognitive impairment (Chanraud et al. 2010), the arcuate fasciculus, heavily implicated in language (Makris et al. 2005), the superior longitudinal fasciculus II, involved in the perception of the visual space and focusing of attention in different parts of space (Makris et al. 2005), and the superior longitudinal fasciculus III, involved in the articulatory component of language (Makris et al. 2005). Cluster 2 included the fornix, which is important in formation of memory and global cognitive function (Rudebeck et al. 2009). Clusters 3 and 4 included the genu and anterior body of the corpus callosum, structures that are important to the efficiency of inter-hemispheric communication, and are linked to perceptual speed (Bucur et al. 2008) and cognitive decline in aging (Lövdén et al. 2010). Finally, cluster 5 included portions of the thalamus, a major relay of information not only between the periphery and the cortex, but also between cortical areas (Sherman 2007). The cognitive activities and functions assessed in this study are linked to the brain functions supported by tissue in clusters 1–5. For example, language, focusing attention in different parts of space, formation of memory, communication between hemispheres and cortical areas, play a central role in the cognitive activities and functions assessed. This suggests that frequent participation in cognitive activities in late life may enhance the structural properties of brain tissue supporting the function involved in these activities, thereby strengthening related cognitive systems.
A recent study reported lack of an association between late life cognitive activity and brain diffusion characteristics (Gow et al. 2012). A number of factors may have contributed to this discrepancy. First, the previous work investigated selected white matter fiber bundles, some of which we also do not report significant findings in. Second, due to substantial normal variation of diffusion characteristics within a fiber bundle, averaging diffusion characteristics over all voxels in a bundle may have reduced sensitivity in the previously published work. Furthermore, the previous study did not control for white matter hyperintensities in regression models testing the association of diffusion characteristics with cognitive activity. Finally, differences in demographic characteristics as well as the activities assessed may have also contributed to the lack of an association between late life cognitive activity and brain diffusion characteristics in the study by Gow et al. 2012.
Strengths and limitations of our study should be noted. A large number of older persons without dementia participated in this research. Cognitive activity and cognition were assessed with psychometrically established scales, minimizing measurement error. Thorough image processing and voxel-wise statistical analysis provided high data quality, minimized errors due to inter-subject mis-registration, and controlled for white matter hyperintensities. One limitation is that assessment of early life cognitive activity was based on retrospective report, which may have limited accuracy. In addition, due to the cross-sectional nature of this study, the directionality of the presented relations cannot be established. Finally, the cohort is selected and, therefore, the generalizability of the results will need to be demonstrated.
In conclusion, more frequent cognitive activity in late life was shown to be associated with higher level of cognition, in agreement with previous work. Since prevention of cognitive decline is currently the best strategy for reducing the burden of cognitive impairment, more frequent participation in cognitively stimulating activities in late life may be an important behavior modification for enhancing cognition. Furthermore, it was demonstrated that brain diffusion characteristics partially mediated the association of late life cognitive activity with cognition. Brain diffusion characteristics may therefore constitute components of neural reserve, influencing the brain’s capacity to tolerate pathology. Additional work is warranted to establish causality in the presented relations involving brain diffusion characteristics, to identify additional pathways that may enhance diffusion characteristics, and to ascertain the different components of neural reserve.
Acknowledgments
This research was supported by the National Institute on Aging (R01AG017917, P30AG010161), National Institute on Minority Health and Health Disparities (P20MD006886), National Institute of Neurological Disorders and Stroke (R21NS076827), National Institute of Biomedical Imaging and Bioengineering (R21EB006525), and the Illinois Department of Public Health.
Footnotes
Conflict of Interest Konstantinos Arfanakis, Robert S. Wilson, Christopher M. Barth, Ana W. Capuano, Anil Vasireddi, Shengwei Zhang, Debra A. Fleischman, and David A. Bennett declare that they have no conflicts of interest.
Informed Consent All procedures followed were in accordance with the ethical standards of the responsible committee on human experimentation (institutional and national) and with the Helsinki Declaration of 1975, and the applicable revisions at the time of the investigation. Informed consent was obtained from all patients for being included in the study.
References
- Arfanakis K, Fleischman DA, Grisot G, Barth CM, Varentsova A, Morris MC, et al. Systemic inflammation in non-demented elderly human subjects: brain microstructure and cognition. PLoS ONE. 2013;8:e73107. doi: 10.1371/journal.pone.0073107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Aggarwal NT, Arvanitakis Z, Shah RC, Kelly JF, et al. Decision rules guiding the clinical diagnosis of Alzheimer’s disease in two community-based cohort studies compared to standard practice in a clinic-based cohort study. Neuroepidemiology. 2006a;27:169–176. doi: 10.1159/000096129. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Arvanitakis Z, Kelly JF, Aggarwal NT, Shah RC, et al. Neuropathology of older persons without cognitive impairment from two community-based studies. Neurology. 2006b;66:1837–1844. doi: 10.1212/01.wnl.0000219668.47116.e6. [DOI] [PubMed] [Google Scholar]
- Bennett DA, Schneider JA, Buchman AS, Barnes LL, Boyle PA, Wilson RS. Overview and findings from the rush memory and aging project. Current Alzheimer Research. 2012;9:646–663. doi: 10.2174/156720512801322663. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blumenfeld-Katzir T, Pasternak O, Dagan M, Assaf Y. Diffusion MRI of structural brain plasticity induced by a learning and memory task. PLoS ONE. 2011;6:e20678. doi: 10.1371/journal.pone.0020678. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boyle PA, Wilson RS, Aggarwal NT, Tang Y, Bennett DA. Mild cognitive impairment: risk of Alzheimer’s disease and rate of cognitive decline. Neurology. 2006;67:441–445. doi: 10.1212/01.wnl.0000228244.10416.20. [DOI] [PubMed] [Google Scholar]
- Bucur B, Madden DJ, Spaniol J, Provenzale JM, Cabeza R, White LE, et al. Age-related slowing of memory retrieval: contributions of perceptual speed and cerebral white matter integrity. Neurobiology of Aging. 2008;29:1070–1079. doi: 10.1016/j.neurobiolaging.2007.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buschkuehl M, Jaeggi SM, Jonides J. Neuronal effects following working memory training. Developmental Cognitive Neuroscience. 2012;2(Suppl 1):S167–179. doi: 10.1016/j.dcn.2011.10.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chanraud S, Zahr N, Sullivan EV, Pfefferbaum A. MR diffusion tensor imaging: a window into white matter integrity of the working brain. Neuropsychology Review. 2010;20:209–225. doi: 10.1007/s11065-010-9129-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chapman SB, Aslan S, Spence JS, Hart JJ, Jr, Bartz EK, Didehbani N, et al. Neural mechanisms of brain plasticity with complex cognitive training in healthy seniors. Cerebral Cortex. 2015;25:396–405. doi: 10.1093/cercor/bht234. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engvig A, Fjell AM, Westlye LT, Moberget T, Sundseth Ø, Larsen VA, et al. Memory training impacts short-term changes in aging white matter: a longitudinal diffusion tensor imaging study. Human Brain Mapping. 2012;33:2390–2406. doi: 10.1002/hbm.21370. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghisletta P, Bickel JF, Lövdén M. Does activity engagement protect against cognitive decline in old age? Methodological and analytical considerations. Journals of Gerontology Series B, Psychological Sciences and Social Sciences. 2006;61:253–261. doi: 10.1093/geronb/61.5.p253. [DOI] [PubMed] [Google Scholar]
- Gow AJ, Bastin ME, Muñoz Maniega S, Valdés Hernández MC, Morris Z, Murray C, et al. Neuroprotective lifestyles and the aging brain: activity, atrophy, and white matter integrity. Neurology. 2012;79:1802–1808. doi: 10.1212/WNL.0b013e3182703fd2. [DOI] [PubMed] [Google Scholar]
- Hall CB, Lipton RB, Sliwinski M, Katz MJ, Derby CA, Verghese J. Cognitive activities delay onset of memory decline in persons who develop dementia. Neurology. 2009;73:356–361. doi: 10.1212/WNL.0b013e3181b04ae3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Honer WG, Barr AM, Sawada K, Thornton AE, Morris MC, Leurgans SE, et al. Cognitive reserve, presynaptic proteins and dementia in the elderly. Translational Psychiatry. 2012;2:e114. doi: 10.1038/tp.2012.38. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hultsch DF, Hertzog C, Small BJ, Dixon RA. Use it or lose it: engaged lifestyle as a buffer of cognitive decline in aging? Psychology and Aging. 1999;14:245–263. doi: 10.1037//0882-7974.14.2.245. [DOI] [PubMed] [Google Scholar]
- Imfeld A, Oechslin MS, Meyer M, Loenneker T, Jancke L. White matter plasticity in the corticospinal tract of musicians: a diffusion tensor imaging study. NeuroImage. 2009;46:600–607. doi: 10.1016/j.neuroimage.2009.02.025. [DOI] [PubMed] [Google Scholar]
- Katzman R, Terry R, DeTeresa R, Brown T, Davies P, Fuld P, et al. Clinical, pathological, and neurochemical changes in dementia: a subgroup with preserved mental status and numerous neocortical plaques. Annals of Neurology. 1988;23:138–144. doi: 10.1002/ana.410230206. [DOI] [PubMed] [Google Scholar]
- Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. Journal of Magnetic Resonance Imaging. 2001;13:534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- Lee B, Park JY, Jung WH, Kim HS, Oh JS, Choi CH, et al. White matter neuroplastic changes in long-term trained players of the game of “Baduk” (GO): a voxel-based diffusion-tensor imaging study. NeuroImage. 2010;52:9–19. doi: 10.1016/j.neuroimage.2010.04.014. [DOI] [PubMed] [Google Scholar]
- Lövdén M, Bodammer NC, Kühn S, Kaufmann J, Schütze H, Tempelmann C, et al. Experience-dependent plasticity of white-matter microstructure extends into old age. Neuropsychologia. 2010;48:3878–3883. doi: 10.1016/j.neuropsychologia.2010.08.026. [DOI] [PubMed] [Google Scholar]
- Makris N, Kennedy DN, McInerney S, Sorensen AG, Wang R, Caviness VS, Jr, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cerebral Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- Markham JA, Greenough WT. Experience-driven brain plasticity: beyond the synapse. Neuron Glia Biology. 2004;1:351–363. doi: 10.1017/s1740925x05000219. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McKhann G, Drachman D, Folstein M, Katzman R, Price D, Stadlan EM. Clinical diagnosis of Alzheimer’s disease: report of the NINCDS-ADRDA work group under the auspices of department of health and human services task force on Alzheimer’s disease. Neurology. 1984;34:939–944. doi: 10.1212/wnl.34.7.939. [DOI] [PubMed] [Google Scholar]
- Roberts RE, Anderson EJ, Husain M. Expert cognitive control and individual differences associated with frontal and parietal white matter microstructure. Journal of Neuroscience. 2010;30:17063–17067. doi: 10.1523/JNEUROSCI.4879-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rudebeck SR, Scholz J, Millington R, Rohenkohl G, Johansen-Berg H, Lee AC. Fornix microstructure correlates with recollection but not familiarity memory. Journal of Neuroscience. 2009;29:14987–14992. doi: 10.1523/JNEUROSCI.4707-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi Y, Tavor I, Hofstetter S, Tzur-Moryosef S, Blumenfeld-Katzir T, Assaf Y. Learning in the fast lane: new insights into neuroplasticity. Neuron. 2012;73:1195–1203. doi: 10.1016/j.neuron.2012.01.025. [DOI] [PubMed] [Google Scholar]
- Schlegel AA, Rudelson JJ, Tse PU. White matter structure changes as adults learn a second language. Journal of Cognitive Neuroscience. 2012;24:1664–1670. doi: 10.1162/jocn_a_00240. [DOI] [PubMed] [Google Scholar]
- Schooler C, Mulatu MS. The reciprocal effects of leisure time activities and intellectual functioning in older people: a longitudinal analysis. Psychology and Aging. 2001;16:466–482. doi: 10.1037//0882-7974.16.3.466. [DOI] [PubMed] [Google Scholar]
- Sen PN, Basser PJ. A model for diffusion in white matter in the brain. Biophysical Journal. 2005;89:2927–2938. doi: 10.1529/biophysj.105.063016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherman SM. The thalamus is more than just a relay. Current Opinion in Neurobiology. 2007;17:417–422. doi: 10.1016/j.conb.2007.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SM, Nichols TE. Threshold-free cluster enhancement: addressing problems of smoothing, threshold dependence and localization in cluster inference. NeuroImage. 2009;44:83–98. doi: 10.1016/j.neuroimage.2008.03.061. [DOI] [PubMed] [Google Scholar]
- Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, et al. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. NeuroImage. 2006;31:1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- Stern Y. Cognitive reserve in ageing and Alzheimer’s disease. Lancet Neurology. 2012;11:1006–1012. doi: 10.1016/S1474-4422(12)70191-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Varentsova A, Zhang S, Arfanakis K. Development of a high angular resolution diffusion imaging human brain template. NeuroImage. 2014;91:177–186. doi: 10.1016/j.neuroimage.2014.01.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Bennett DA, Bienias JL, Mendes de Leon CF, Morris MC, Evans DA. Cognitive activity and cognitive decline in a biracial community population. Neurology. 2003;61:812–816. doi: 10.1212/01.wnl.0000083989.44027.05. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Barnes LL, Krueger KR, Hoganson G, Bienias JL, Bennett DA. Early and late life cognitive activity and cognitive systems in old age. Journal of the International Neuropsychological Society. 2005;11:400–407. [PubMed] [Google Scholar]
- Wilson RS, Scherr PA, Schneider JA, Tang Y, Bennett DA. Relation of cognitive activity to risk of developing Alzheimer disease. Neurology. 2007;69:1911–1920. doi: 10.1212/01.wnl.0000271087.67782.cb. [DOI] [PubMed] [Google Scholar]
- Wilson RS, Segawa E, Boyle PA, Bennett DA. Influence of late-life cognitive activity on cognitive health. Neurology. 2012;78:1123–1129. doi: 10.1212/WNL.0b013e31824f8c03. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Boyle PA, Yu L, Barnes LL, Schneider JA, Bennett DA. Life-span cognitive activity, neuropathologic burden, and cognitive aging. Neurology. 2013a;81:314–321. doi: 10.1212/WNL.0b013e31829c5e8a. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wilson RS, Nag S, Boyle PA, Hizel LP, Yu L, Buchman AS, et al. Neural reserve, neuronal density in the locus ceruleus, and cognitive decline. Neurology. 2013b;80:1202–1208. doi: 10.1212/WNL.0b013e3182897103. [DOI] [PMC free article] [PubMed] [Google Scholar]


