Abstract
Exercise stress electrocardiography (ExECG) is underutilized as the initial test modality in patients with interpretable electrocardiograms able to exercise. Although, stress myocardial imaging techniques provide valuable diagnostic and prognostic information, variables derived from ExECG can yield substantial data for risk stratification, either supplementary to imaging variables, or without concurrent imaging. In addition to exercise-induced ischemic ST depression, such markers as ST segment elevation in lead AVR, abnormal heart rate recovery post-exercise, failure to achieve target heart rate, and poor exercise capacity improve risk stratification of ExECG. For example, patients achieving ≥10 METS on ExECG have a very low prevalence of inducible ischemia and an excellent prognosis. In contrast, cardiac imaging techniques add diagnostic and prognostic value in higher risk populations (e.g. poor functional capacity, diabetes, chronic kidney disease). Optimal test selection for symptomatic patients with suspected coronary artery disease requires a patient-centered approach factoring in the risk/benefit ratio and cost-effectiveness.
Introduction
Despite considerable improvements in the identification and treatment of coronary artery disease (CAD), this condition remains highly morbid and the most common cause of death in the Western world.(1) Concomitantly, the number of noninvasive radionuclide and echocardiographic imaging studies performed to identify ischemic heart disease has grown considerably. More than 9 million myocardial perfusion imaging (MPI) studies were performed in 2008, at a cost of greater than $1 billion, with the added risk of radiation exposure.(2,3)
The latest ACC-AHA guidelines on exercise testing, diagnosis and management of stable ischemic heart disease and ACC-AHA appropriate use criteria for cardiac radionuclide imaging recommend exercise stress electrocardiography (ExECG) as the initial diagnostic test in patients at intermediate pretest risk who are able to exercise and have an interpretable resting electrocardiogram.(4-6) Despite these recommendations, the majority of patients still undergo stress imaging as the initial testing strategy. In the Prospective Multicenter Imaging Study for Evaluation of Chest Pain (PROMISE) trial, examining the comparative effectiveness of an anatomical versus functional testing approach to evaluate symptoms concerning for ischemia at leading academic medical centers, 89.8% of those undergoing stress testing had imaging performed.(7) A broader administrative database found a similarly high 75% rate of imaging performed with stress testing.(8) This finding is stable over time and likely occurs due to a widespread perception that ExECG alone has insufficient diagnostic accuracy for CAD detection.(9)
As many as 60-70% of MPI studies ordered for CAD detection are normal.(10,11) Advanced imaging modalities, such as positron emission tomography (PET) and cardiovascular magnetic resonance (CMR) MPI, and improved SPECT hardware and protocols can increase the diagnostic accuracy of stress imaging with a reduction in radiation exposure. This shifts the pendulum toward the performance of stress MPI. On the other hand, the rationale for using ExECG alone as the initial test for CAD detection, particularly in patients with good exercise tolerance, has become more compelling with new data published in the literature.(12,13)
This review will summarize the latest advances in ExECG and explore its application in the era of advanced imaging technologies. We will show that this diagnostic testing modality should still play a major role in the noninvasive evaluation of patients with symptoms of suspected ischemic CAD.
Diagnostic and Prognostic Value of Exercise ST Changes
A meta-analysis of 24,047 patients in 147 studies found ExECG to have a pooled sensitivity of 68% and specificity of 77% for detection of CAD.(14) Restricting the analysis to the 3 studies free of workup bias lowered sensitivity considerably to 50% and increased the specificity to 90%.(6) ExECG has a positive likelihood ratio (LR) of 2.18 and a negative LR of 0.32 for CAD.(15) Confounders such as resting ST-depression, digoxin usage, and left ventricular hypertrophy with repolarization changes decrease specificity, while mild single vessel disease decreases sensitivity. Despite these confounders, ExECG is still considered diagnostic in most patients able to reach 85% of their maximum age-predicted heart rate.
Exercise-induced ST depression is also a powerful predictor of cardiac events. Two landmark studies of large, predominately male populations, found the presence and extent of ST depression to be a powerful prognostic marker. In the Duke study of 2,842 patients, the maximum ST deviation was the strongest predictor of both cardiac death and a composite of cardiac death and nonfatal MI.(16) A Coronary Artery Surgery Study (CASS) database analysis by Weiner et al. found the extent of ST depression to be one of the two most powerful prognostic markers on exercise testing, along with the duration of exercise.(17) Patients who could reach stage 3 of the Bruce protocol with <1mm ST depression had a yearly mortality rate of <1%. In contrast, patients with ≥1mm ST depression who were unable to complete stage 1 of the Bruce protocol had a 5% yearly mortality rate.
Additional Tools to Increase Diagnostic and Prognostic Utility
Exercise Capacity
There are many additional variables that supplement ST depression and add to the diagnostic and prognostic utility of ExECG. The most powerful of these is exercise capacity.
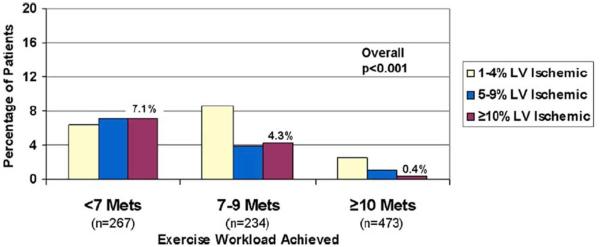
Multiple studies have correlated exercise workload to the likelihood of significant myocardial ischemia on MPI and subsequent events. Bourque et al. found that patients attaining <7 METS had an 18-fold higher prevalence of substantial (≥10%) left ventricular (LV) ischemia compared with those reaching ≥10 METS. The latter group with good exercise tolerance had a very low (0.4%) prevalence of ≥10% LV ischemia (Figure 1).(18) A follow-up analysis of the cohort reaching ≥10-METS revealed very low rates of cardiac death (0.1%/year) and nonfatal MI (0.7%/year).(19) The favorable diagnostic and prognostic impact of achieving a high exercise workload has been confirmed in multiple subsequent studies, including a Canadian cohort of 9,605 patients, and an analysis using patients who underwent exercise echocardiography.(20,21) In the Canadian population, there was a 1% risk of ≥10% LV ischemia and a death or MI rate <2% even in those with ≥5% LV ischemia. Some studies have shown exercise echocardiography and MPI to retain prognostic significance after accounting for workload.(22,23) The degree of benefit and whether improved diagnostic accuracy and prognostic assessment warrant routine imaging are key issues. Patients with prior MI might be one subgroup that benefits.(22)
Figure 1. Prevalence of increasing percentages of left ventricular ischemia stratified by exercise workload in patients undergoing exercise stress electrocardiography.
There was a significant decrease in prevalence of ischemia as the workload achieved increased. LV= left ventricular; METS=metabolic equivalents. With permission from Bourque et al.(18)
Prior studies have shown similar prognostic utility using exercise capacity as a continuous variable. The value of exercise capacity is consistent in both those with and without known CAD.(17,24) In a cohort of 3,400 patients who underwent thallium-201 MPI, exercise capacity was even a stronger predictor of mortality than the extent of perfusion defects. High exercise workload is also a marker of a decreased risk of cardiac events, including cardiac death, nonfatal MI, and coronary revascularization.(19,25-27) These associations remain even in the setting of ischemic ST depression.
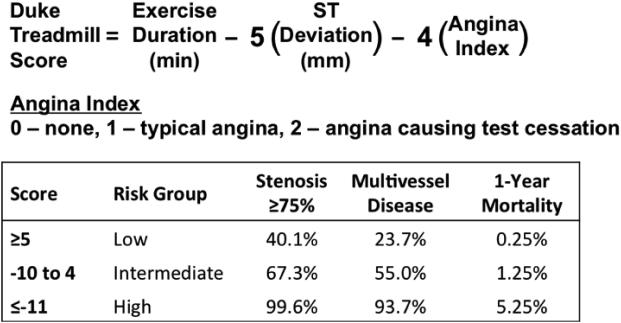
Other surrogates of exercise capacity have diagnostic and prognostic value, such as the rate-pressure product (RPP), which predicts the probability of three-vessel or left main CAD.(28) Composite clinical scores, such as the Duke Treadmill Score (DTS), provide added prognostic information by combining multiple predictors into one measure. The DTS incorporates exercise duration, exercise-induced ST changes, and stress-induced angina.(16) It is a strong indicator of any and multivessel obstructive disease and mortality (Figure 2). The DTS is most useful when it falls in the high- and low-risk groups. Myocardial perfusion imaging (MPI) is useful in risk stratifying patients with an intermediate DTS.(29)
Figure 2. Duke Treadmill Score calculation and utility.
Equation for calculation of the Duke Treadmill Score and division into low, intermediate, and high risk groups based on likelihood of having a stenosis ≥75%, multivessel disease, and 1-year all-cause mortality. Adapted from Mark et al.(16,100) and Shaw et al.(101).
Stress Electrocardiographic Findings Beyond ST Depression
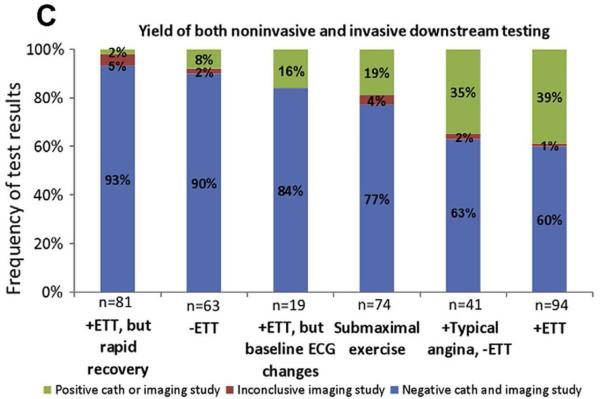
Markers other than exercise-induced ST depression have diagnostic and prognostic value, such as the rapidity of recovery of ST segment changes.(30,31) Christman et al. found a low 2% rate of positive imaging or findings of CAD on angiography and a 0.7% rate of a composite endpoint of cardiovascular death, nonfatal MI, or coronary revascularization in patients with a positive exercise treadmill test but rapid ST segment recovery (Figure 3).(30) Other potential enhancements to the standard dichotomous presence of ≥1mm ST depression include heart rate adjustment in the form of the ST/HR slope and ST/HR index.
Figure 3. Frequency of positive downstream imaging and invasive angiography by stress electrocardiographic findings.
ECG=electrocardiographic; ETT=exercise treadmill testing. With permission from Christman et al.(30)
Lead AVR is often neglected in ExECG interpretation but has unique vector positioning. This allows it to function as a “pseudo-intracavitary” lead that may identify anterior wall transmural ischemia.(32) Uthamalingam et al. found ≥1mm AVR elevation during ExECG to be the strongest predictor of an obstructive left main or ostial left anterior descending (LAD) artery stenosis with a diagnostic accuracy of 80% and a 2.6-fold increase in post-test probability.(33) A major limitation of the current AVR data is the absence of studies examining imaging findings and events in the general population not undergoing invasive angiography.
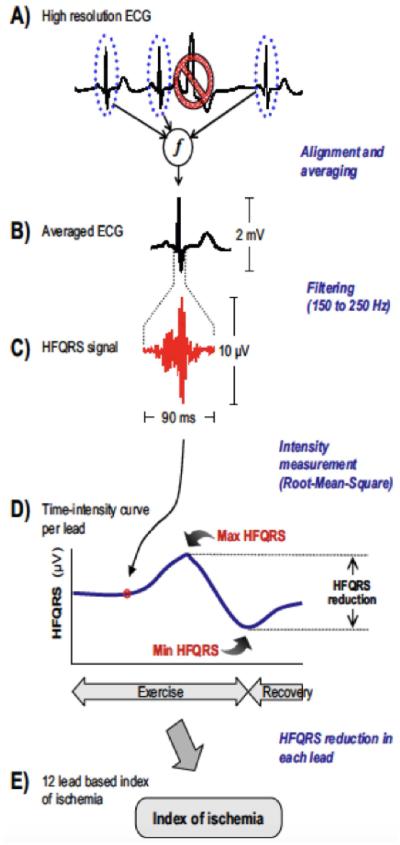
Other suggested measurements on standard ExECG that may have diagnostic value include changes in QRS duration, R-wave amplitude, and length of the rate adjusted QT-interval.(34) A novel marker not routinely available but with substantial potential as a useful adjunct to ST depression is high-frequency mid-QRS (HF-QRS) analysis, an interrogation of the signal in the 150-250 Hz frequency (Figure 4). An abnormal HF-QRS signal increases the sensitivity of ischemia detection from 39% to 69% (p<0.005) and the specificity from 82% to 86% (p<0.05).(35) This promising tool requires additional validation in independent cohorts and with assessment of cardiac events before it can be routinely applied in clinical practice.
Figure 4. High frequency QRS analysis (HF-QRS).
Multiple representative QRS complexes are aligned and averaged. The signal in the 150-250 Hz frequency band is measured in each of the standard 12 leads, and a time intensity curve is generated. A decrease in the HF-QRS signal that is both ≥50% and ≥1µV in 3 or more leads is concerning for ischemia. ECG=electrocardiogram.With permission from Sharir et al.(35)
Physiologic Markers and Symptoms
Several physiologic markers during stress testing can augment the diagnostic accuracy of ExECG and have prognostic importance. These includes the heart rate and blood pressure responses to exercise, and symptoms during testing.(36)
An impaired chronotropic response has been associated with a >2-fold increase in perfusion defects and a higher risk of CAD and cardiac events.(37,38) Heart rate recovery post-exercise also carries significant diagnostic and prognostic power. Cole et al. found a relative risk of death of 2.0 (1.5-2.7, p<0.001) for those with a <12 bpm heart rate drop 1 minute post-exercise after risk-factor adjustment in 2,428 patients.(39) A retrospective analysis of 2,193 men found a heart rate recovery <22bpm at 2 minutes to be predictive of mortality and the presence of CAD.(40) Heart rate recovery retains its prognostic influence in patients with known CAD.(41)
Changes in blood pressure response during ExECG are also potential indicators of CAD, although less validated than changes in heart rate. The systolic blood pressure (SBP) typically decreases at least 15% by 3 minutes post-exercise. An abnormal SBP recovery ratio of >0.9 (SBP at 3 minutes/SBP at peak exercise) has been found to have comparable diagnostic accuracy to ST depression and incremental value for the identification of CAD.(42) This same ratio correlates with the extent and severity of thallium-201 perfusion defects.(43) A >10mmHg SBP drop during exercise and a delayed decline in SBP after exercise have been associated with high-risk multi-vessel or left main disease in men with less specificity in women.(44-46)
Treadmill-induced typical angina increases the sensitivity for the diagnosis of CAD and indicates more extensive myocardial ischemia and higher event rates, particularly in the setting of ischemic ST depression.(47-49) The risk of events is substantially higher when symptoms are induced at a lower workload.(47)
Special Populations
Women
The pioneering work on ExECG was done in predominately male populations. Although the prevalence of CAD is less than in men, the mortality from CAD in women is higher.(49,50) There are unique challenges in the female population, as women often present with more atypical symptoms at an older age.(51) Moreover, the diagnostic accuracy of ExECG is lower in women. The pooled sensitivity and specificity in 3,721 women from 19 studies were 61% and 70%, respectively, compared with 68% and 77% in men.(14,52) The decreased specificity of ST depression in women is thought to be partially due to a digoxin-like estrogen effect, lower ECG voltage, and an increased prevalence of baseline ST-T changes.(50,51,53,54)
The lower prevalence of CAD in women decreases the positive predictive value of a positive test compared with men.(50) Barolsky et al. found the positive predictive value in women to be 47% compared with 77% in men; the negative predictive value was not significantly different (78% for women versus 81%).(55) Therefore, although an abnormal test result in women has a higher likelihood of being falsely positive, a negative study is equally effective at ruling out disease. Due to this high negative predictive value, existing guidelines recommend ExECG as the initial study of choice in women at intermediate risk for CAD who are able to exercise.(6,50)
As with men, ExECG has significant prognostic value in women, especially when combined with additional variables such as exercise capacity and heart rate recovery. Exercise capacity is more predictive of mortality in men (hazard ratio 2.89 in men, 0.99 in women). Nevertheless, the Duke Treadmill Score (DTS) has preserved diagnostic and prognostic power in women.(51) Chronotropic incompetence has a stronger relationship with MI in women (HR 2.79 versus 1.29).(56) In asymptomatic women, ST segment depression has not been found to be predictive of cardiac or all-cause mortality.(57)
Many clinicians refer women directly to MPI due to concern about ExECG accuracy. This argument was refuted by the WOMEN trial, in which 824 symptomatic women with good exercise tolerance were randomized to ExECG with or without MPI. The group without MPI had a 48% diagnostic cost savings with no difference in major adverse cardiac events over 2 years of follow-up.(58) This study supports a strategy of ExECG as the initial form of ischemia evaluation in women able to exercise with an interpretable ECG, as recommended for men.
Diabetes Mellitus
Patients with diabetes mellitus are an important subpopulation of those undergoing stress testing. The presence and severity of CAD are greater in patients with diabetes, and the prevalence of this comorbidity is rapidly increasing.(59) Diabetic patients are particularly challenging because they often present with atypical symptoms, and there is a high prevalence of silent ischemia in asymptomatic patients.(60) These factors have led to a high rate of stress testing in patients with diabetes.
ExECG appears to have similar diagnostic accuracy and prognostic significance in patients with and without diabetes.(59,61) However, imaging has typically been performed in this subpopulation, and historical rates of SPECT abnormalities as high as 47-59.5% have supported this practice.(62,63)
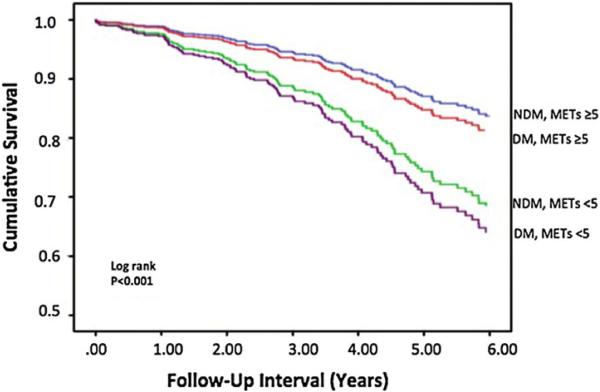
However, the prevalence of ischemia and risk of future cardiac events may be decreasing with contemporary aggressive medical therapy in diabetic patients. A recent study of predominately-symptomatic stable outpatient diabetic patients found a 21.8% risk of any ischemia, a lower than expected 5.0% prevalence of ≥10% LV ischemia, and a low rate of cardiac death/nonfatal MI (0.6%/year), especially given 40.3% had known CAD.(64) Diabetic patients who achieve higher workloads on ExECG (≥5 METS in Padala et al., ≥10 METS in Bourque et al.) in contemporary cohorts have a low risk of future cardiac events similar to non-diabetic patients (Figure 5).(18,65)
Figure 5. Survival by diabetes status and stress exercise workload achieved.
Patients able to achieve ≥5 METS of exercise workload have a similar survival irrespective of diabetes status. DM=diabetes mellitus; METS=metabolic equivalents; NDM=no diabetes mellitus. With permission from Padala et al.(65)
Advanced imaging modalities can provide additional risk stratification in the diabetic population. The presence of a non-zero computed tomographic (CT) coronary calcium score, unrecognized myocardial scar by CMR, and reduced flow reserve by PET MPI all identify an increased risk of cardiac events.(66-69) Whether these patients should have more aggressive treatment goals remains to be determined.
Given these complex factors, the decision of whether to image patients with diabetes must be individualized. Diabetic patients with good functional capacity who are deemed able to achieve a high exercise workload may receive sufficient risk stratification with ExECG alone. However, for higher-risk patients with diabetes, such as those with poor exercise tolerance, LV dysfunction, nephropathy, vascular disease, or an abnormal resting ECG, and in those in whom microvascular disease is suspected, implementation of cardiac imaging may significantly aid in risk stratification.
Elderly
Age is not a consideration in the recommendation for ExECG in the current guidelines on exercise testing.(6) However, functional limitations and comorbidities in the elderly lead to a higher rate of necessary conversion to pharmacologic stress imaging. Moreover, the elderly are not well represented in studies examining the diagnostic and prognostic value of ExECG. They are an important subgroup given their higher prevalence of CAD and cardiac events.
Studies that have been performed in the elderly confirm the prognostic importance of exercise capacity.(70,71) However, ST depression did not predict cardiac events in a cohort of 514 elderly patients ≥65 years of age; the DTS did not have prognostic benefit in another elderly cohort.(70,72) However, SPECT perfusion defects were associated with cardiac death in the same elderly cohort.(73) Abnormal SPECT imaging also predicts nonfatal MI and coronary revascularization in the elderly.(71,74,75) These findings suggest that imaging should be considered in the initial workup to detect CAD in the elderly.
Advances in imaging technology have a substantial impact in the elderly population. Given the lower lifetime attributable risk of cancer in older patients, reductions in radiation are less of an issue, though still beneficial.(76) The shortened protocols possible with PET imaging and newer SPECT cameras can be helpful in this cohort with a higher likelihood of musculoskeletal pain and other mechanical limitations that complicate long table times. Sufficient levels of stress are less likely in the elderly due to decreases in exercise capacity and a higher potential for chronotropic incompetence. Combined protocols can ameliorate this issue, facilitating the collection of exercise data followed by vasodilator administration to ensure a diagnostic test.
Baseline ECG Abnormalities
Several baseline ECG abnormalities affect the test characteristics of ExECG. In a cohort of 1,282 patients referred for chest pain evaluation, resting ST depression <1mm increased the sensitivity of ExECG from 45% to 77% but decreased specificity from 84% to 48% with no change in overall diagnostic accuracy (6,77)
Patients with left bundle-branch block (LBBB) require imaging due to a high false-positive rate.(78,79) The role of ExECG in the setting of right bundle-branch block (RBBB) is less clear. ST changes in the anterior precordial leads have a false-positive rate of 66%, but specificity is preserved in leads V5 and V6.(80) Yen et al. found a decreased sensitivity of 27% but a preserved specificity of 87% using leads V5 and V6 in 133 patients, the largest cohort studied with RBBB.(81) Susmano, on the other hand, showed an excellent sensitivity of 89%, though only 12 patients were analyzed.(82) The ACC-AHA exercise testing guidelines support the use of ExECG in RBBB.(6)
Importance of Reaching ≥85% Maximum Age-Predicted Heart Rate
Given the late occurrence of ECG changes in the ischemic cascade and known limited sensitivity of ExECG, reaching an adequate level of workload and heart rate is essential. Heller et al. found that reaching only 70% compared with ≥85% of maximum age-predicted heart rate (MAPHR), leads to a reduction in the incidence of stress defects from 100% to 47% and a reduction in angina from 84% to 26%.(83) Subsequent studies in patients not achieving target heart rates have found reductions in the degree and extent of ischemia on MPI, with decreases seen irrespective of the number of diseased vessels.(84,85) Combination protocols with vasodilator administration in those unable to achieve sufficient exercise workload substantially improve the diagnosis of ischemia and are safe and feasible.(86,87) In one study of symptomatic patients, receiving a combination protocol increased ischemic segments from 7 to 40.(88)
Patient-Centered Approach to Ischemia Evaluation
Advances in imaging technology have created a broad menu of options for the evaluation of ischemic heart disease that facilitate a patient-centered approach to ischemia evaluation.(89) Patient symptoms, comorbidities, and functional status are used to identify which array of appropriate tests are considered. Patients are referred for the test that provides the most clinically meaningful information with the least risk, cost, and inconvenience.
In many patients, adequate risk stratification is achieved with a simple strategy of ExECG without imaging. A study of the yield of downstream testing and subsequent cardiac events in 3,656 patients undergoing ExECG found low rates of referral to MPI (9.0%) and invasive angiography (2.3%).(30) Over a 2.5-year mean follow-up, the rate of cardiac death, nonfatal MI, and coronary revascularization was very low in those with negative (0.2%) and inconclusive (1.3%) stress studies. A large administrative database found a similarly low 6.9% rate of invasive angiography at one year. In the functional testing subgroup of the PROMISE trial, those who underwent stress electrocardiography had a similarly low rate of the combined endpoint of adverse cardiac outcomes as those who underwent imaging.(7) These data support an initial strategy of ExECG alone in appropriate patients.
Stress myocardial perfusion imaging (MPI) provides minimal incremental value in patients with a low-risk exercise stress test, a low-risk Duke Treadmill Score, or a high rate-pressure product without ST-segment depression.(18,28,90,91) In addition, the low cardiac event rates in stable patients treated medically in recent landmark studies such as COURAGE and BARI-2D have challenged the paradigm of selecting coronary revascularization as the initial therapeutic strategy, potentially reducing the need for identification of low-levels of ischemia.(92,93)
However, despite the low risk of events with negative ExECG, there continues to be widespread use of concurrent imaging.(9) Novel protocols that add additional testing in specific higher risk situations may encourage a strategy of ExECG alone.
Novel Protocols
Two novel protocols that refine ExECG and reduce the likelihood of missing significant ischemia are the use of provisional MPI in patients unable to achieve a high exercise workload and the incorporation of coronary calcium scoring. Given the low prevalence of significant ischemia and cardiac events in patients able to achieve 10-METS, Duvall et al. reviewed a hypothetical protocol where MPI would not be performed in patients <65 years of age with no known CAD and an interpretable rest ECG who achieved a diagnostic heart rate and ≥10-METS without significant exercise-induced ECG changes or symptoms.(94) In this low-risk subset, there was a very low rate of significant (≥10%) LV ischemia (0.6%/year), 5.9% abnormal studies, and a 98.9% 5-year survival, comparable to the favorable survival after negative MPI. Limitations of this provisional MPI testing approach include the low number of patients able to avoid imaging (29% in this study), test supervision issues, patient and physician support, and incompatibility with the current insurance payment models.(95) Further refinement of this concept is necessary before it will be ready for adoption.
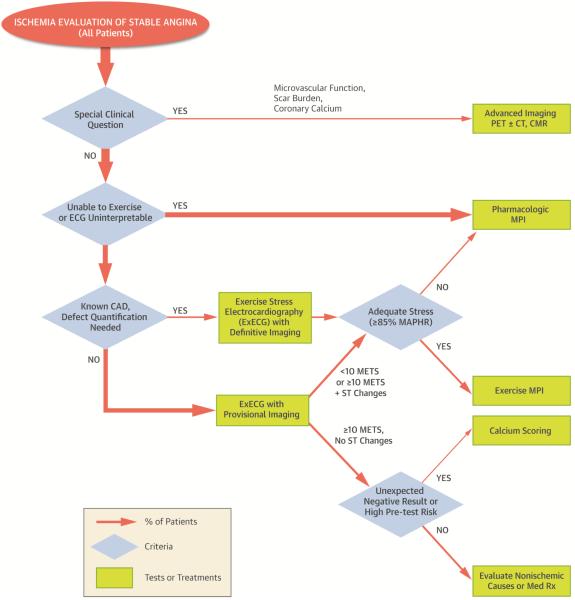
An additional method of improving the diagnostic accuracy of ExECG is to add coronary calcium scoring under certain higher risk circumstances, such as in patients with particularly high pretest risk, those with a suspected false positive ST segment response, or patients with typical angina and negative ExECG. A coronary calcium score >0 has 98% sensitivity for coronary atherosclerosis, reducing the likelihood of a false negative evaluation.(96) Identification of non-obstructive CAD can also guide the intensity of medical therapy.(97) Rozanski et al. showed a low risk of inducible ischemia in patients with low to intermediate pretest probability of disease and a coronary calcium score <400.(98) This approach may be especially useful if combined with exercise workload. The demonstration of a 0 coronary calcium score in higher risk patients able to achieve 10-METS with negative ExECG would provide further evidence of low posttest risk of significant CAD, thereby eliminating the need for more costly downstream imaging.(Central Illustration). The optimal protocols to most effectively integrate coronary calcium scoring have not been developed and would need additional study with outcomes assessment prior to widespread adoption.
Central Illustration. Stress test selection and performance algorithm.
Higher risk patients with unique clinical needs, such as assessment of microvascular function, can receive advanced ischemia imaging. In the remainder, those unable to exercise or with an uninterpretable ECG receive pharmacologic MPI. Patients able to exercise with known CAD in whom defect quantification is needed undergo exercise stress MPI. If they do not achieve 85% MAPHR, they receive pharmacologic MPI under a combined protocol. All others undergo ExECG with provisional imaging. If they achieve ≥10 METS and have no ST changes, they undergo evaluation for nonischemic chest pain or receive medical therapy. Unexpected negative results in patients with high pre-test risk can trigger calcium scoring for additional risk stratification. All others undergo exercise MPI or a combined protocol with pharmacologic stress if they are unable to achieve an adequate exercise heart rate (≥85% MAPHR). CAD=coronary artery disease; CMR=cardiac magnetic resonance; CT=computed tomography; ECG=electrocardiogram; ExECG=exercise stress electrocardiography; MAPHR=maximum age-predicted heart rate; METS=metabolic equivalents; MPI=myocardial perfusion imaging; PET=positron emission tomography; Rx=treatment.
Conclusions
The studies cited in this review strongly suggest that ExECG remains the recommended method of initial evaluation in intermediate-risk patients with symptoms consistent with CAD. This approach is supported by a large and growing number of supplementary variables, such as exercise workload achieved and ECG changes beyond ST depression, that improve diagnostic accuracy and provide incremental prognostic information. Moreover, there is increasing evidence in the literature suggesting that revascularization may only be beneficial in patients with high levels of ischemia.(92,99) At the same time, advances in imaging technology have improved diagnostic accuracy while decreasing test duration and radiation exposure, thereby shifting the risk/benefit ratio towards imaging in higher risk patients who cannot achieve a high workload or attain their target heart rate on ExECG.
A stress test selection and performance algorithm is provided in the Central Illustration that incorporates myocardial imaging in select patients with special clinical needs, provisional imaging in patients unable to achieve a high workload, calcium scoring as further evaluation after unexpected negative results, and adjunctive pharmacologic stress in those unable to achieve diagnostic heart rates. Novel imaging protocols and optimal patient selection may assist with appropriate utilization of cardiac imaging technologies, when necessary, to complement ExECG. Application of an algorithm such as this could result in substantial savings to the health care system without reducing the quality of care. Additional research with prospective outcome studies is needed to refine these approaches.
Acknowledgements
We would like to thank Christopher Kramer, MD for his assistance in the preparation of this manuscript.
Grant Support: NIH K-Award 5K23HL119620-02
Disclosure: Dr. Bourque receives research grant support from Astellas Global Development
Abbreviations
- CAD
coronary artery disease
- CT
computed tomography
- DTS
Duke Treadmill score
- ECG
electrocardiogram
- ExECG
exercise stress electrocardiography
- LV
left ventricular
- MAPHR
maximum age-predicted heart rate
- METS
metabolic equivalents
- MPI
myocardial perfusion imaging
- PET
positron emission tomography
- SPECT
single photon-emission computed tomography
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Murphy SL, Xu J, Kochanek KD. Deaths: final data for 2010. National vital statistics reports : from the Centers for Disease Control and Prevention, National Center for Health Statistics, National Vital Statistics System. 2013;61:1–117. [PubMed] [Google Scholar]
- 2.Lauer MS. Elements of danger--the case of medical imaging. The New England journal of medicine. 2009;361:841–3. doi: 10.1056/NEJMp0904735. [DOI] [PubMed] [Google Scholar]
- 3.2008 Nuclear Medicine. Des Plains, IL: 2008. Market Summary Report; pp. IV19–IV30. [Google Scholar]
- 4.Hendel RC, Berman DS, Di Carli MF, et al. ACCF/ASNC/ACR/AHA/ASE/SCCT/SCMR/SNM 2009 Appropriate Use Criteria for Cardiac Radionuclide Imaging: A Report of the American College of Cardiology Foundation Appropriate Use Criteria Task Force, the American Society of Nuclear Cardiology, the American College of Radiology, the American Heart Association, the American Society of Echocardiography, the Society of Cardiovascular Computed Tomography, the Society for Cardiovascular Magnetic Resonance, and the Society of Nuclear Medicine. Journal of the American College of Cardiology. 2009;53:2201–29. doi: 10.1016/j.jacc.2009.02.013. [DOI] [PubMed] [Google Scholar]
- 5.Fihn SD, Gardin JM, Abrams J, et al. 2012 ACCF/AHA/ACP/AATS/PCNA/SCAI/STS guideline for the diagnosis and management of patients with stable ischemic heart disease: executive summary: a report of the American College of Cardiology Foundation/American Heart Association task force on practice guidelines, and the American College of Physicians, American Association for Thoracic Surgery, Preventive Cardiovascular Nurses Association, Society for Cardiovascular Angiography and Interventions, and Society of Thoracic Surgeons. Circulation. 2012;126:3097–137. doi: 10.1161/CIR.0b013e3182776f83. [DOI] [PubMed] [Google Scholar]
- 6.Gibbons RJ, Balady GJ, Bricker JT, et al. ACC/AHA 2002 guideline update for exercise testing: summary article. A report of the American College of Cardiology/American Heart Association Task Force on Practice Guidelines (Committee to Update the 1997 Exercise Testing Guidelines) Journal of the American College of Cardiology. 2002;40:1531–40. doi: 10.1016/s0735-1097(02)02164-2. [DOI] [PubMed] [Google Scholar]
- 7.Douglas PS, Hoffmann U, Patel MR, et al. Outcomes of anatomical versus functional testing for coronary artery disease. The New England journal of medicine. 2015;372:1291–300. doi: 10.1056/NEJMoa1415516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Mudrick DW, Cowper PA, Shah BR, et al. Downstream procedures and outcomes after stress testing for chest pain without known coronary artery disease in the United States. American heart journal. 2012;163:454–61. doi: 10.1016/j.ahj.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Andrus BW, Welch HG. Medicare services provided by cardiologists in the United States: 1999-2008. Circulation Cardiovascular quality and outcomes. 2012;5:31–6. doi: 10.1161/CIRCOUTCOMES.111.961813. [DOI] [PubMed] [Google Scholar]
- 10.Shaw LJ, Hendel RC, Heller GV, Borges-Neto S, Cerqueira M, Berman DS. Prognostic estimation of coronary artery disease risk with resting perfusion abnormalities and stress ischemia on myocardial perfusion SPECT. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2008;15:762–73. doi: 10.1007/BF03007357. [DOI] [PubMed] [Google Scholar]
- 11.Berman DS, Kang X, Slomka PJ, et al. Underestimation of extent of ischemia by gated SPECT myocardial perfusion imaging in patients with left main coronary artery disease. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2007;14:521–8. doi: 10.1016/j.nuclcard.2007.05.008. [DOI] [PubMed] [Google Scholar]
- 12.DePuey EG. Advances in SPECT camera software and hardware: currently available and new on the horizon. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012;19:551–81. doi: 10.1007/s12350-012-9544-7. quiz 585. [DOI] [PubMed] [Google Scholar]
- 13.Fazel R, Gerber TC, Balter S, et al. Approaches to enhancing radiation safety in cardiovascular imaging: a scientific statement from the american heart association. Circulation. 2014;130:1730–48. doi: 10.1161/CIR.0000000000000048. [DOI] [PubMed] [Google Scholar]
- 14.Detrano R, Gianrossi R, Froelicher V. The diagnostic accuracy of the exercise electrocardiogram: a meta-analysis of 22 years of research. Progress in cardiovascular diseases. 1989;32:173–206. doi: 10.1016/0033-0620(89)90025-x. [DOI] [PubMed] [Google Scholar]
- 15.Banerjee A, Newman DR, Van den Bruel A, Heneghan C. Diagnostic accuracy of exercise stress testing for coronary artery disease: a systematic review and meta-analysis of prospective studies. International journal of clinical practice. 2012;66:477–92. doi: 10.1111/j.1742-1241.2012.02900.x. [DOI] [PubMed] [Google Scholar]
- 16.Mark DB, Hlatky MA, Harrell FE, Jr., Lee KL, Califf RM, Pryor DB. Exercise treadmill score for predicting prognosis in coronary artery disease. Annals of internal medicine. 1987;106:793–800. doi: 10.7326/0003-4819-106-6-793. [DOI] [PubMed] [Google Scholar]
- 17.Weiner DA, Ryan TJ, McCabe CH, et al. Prognostic importance of a clinical profile and exercise test in medically treated patients with coronary artery disease. Journal of the American College of Cardiology. 1984;3:772–9. doi: 10.1016/s0735-1097(84)80254-5. [DOI] [PubMed] [Google Scholar]
- 18.Bourque JM, Holland BH, Watson DD, Beller GA. Achieving an exercise workload of > or = 10 metabolic equivalents predicts a very low risk of inducible ischemia: does myocardial perfusion imaging have a role? Journal of the American College of Cardiology. 2009;54:538–45. doi: 10.1016/j.jacc.2009.04.042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bourque JM, Charlton GT, Holland BH, Belyea CM, Watson DD, Beller GA. Prognosis in patients achieving >/=10 METS on exercise stress testing: was SPECT imaging useful? Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2011;18:230–7. doi: 10.1007/s12350-010-9323-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Lee DS, Verocai F, Husain M, et al. Cardiovascular outcomes are predicted by exercise-stress myocardial perfusion imaging: Impact on death, myocardial infarction, and coronary revascularization procedures. American heart journal. 2011;161:900–7. doi: 10.1016/j.ahj.2011.01.019. [DOI] [PubMed] [Google Scholar]
- 21.Fine NM, Pellikka PA, Scott CG, Gharacholou SM, McCully RB. Characteristics and outcomes of patients who achieve high workload (>/=10 metabolic equivalents) during treadmill exercise echocardiography. Mayo Clinic proceedings. 2013;88:1408–19. doi: 10.1016/j.mayocp.2013.07.021. [DOI] [PubMed] [Google Scholar]
- 22.McCully RB, Ommen SR, Klarich KW, Burger KN, Mahoney DW, Pellikka PA. Prognosis of patients with good exercise capacity and mildly abnormal exercise echocardiography results: identification of an at-risk subgroup. J Am Soc Echocardiogr. 2005;18:644–8. doi: 10.1016/j.echo.2004.10.027. [DOI] [PubMed] [Google Scholar]
- 23.Diaz LA, Brunken RC, Blackstone EH, Snader CE, Lauer MS. Independent contribution of myocardial perfusion defects to exercise capacity and heart rate recovery for prediction of all-cause mortality in patients with known or suspected coronary heart disease. Journal of the American College of Cardiology. 2001;37:1558–64. doi: 10.1016/s0735-1097(01)01205-0. [DOI] [PubMed] [Google Scholar]
- 24.Myers J, Prakash M, Froelicher V, Do D, Partington S, Atwood JE. Exercise capacity and mortality among men referred for exercise testing. The New England journal of medicine. 2002;346:793–801. doi: 10.1056/NEJMoa011858. [DOI] [PubMed] [Google Scholar]
- 25.Snader CE, Marwick TH, Pashkow FJ, Harvey SA, Thomas JD, Lauer MS. Importance of estimated functional capacity as a predictor of all-cause mortality among patients referred for exercise thallium single-photon emission computed tomography: report of 3,400 patients from a single center. Journal of the American College of Cardiology. 1997;30:641–8. doi: 10.1016/s0735-1097(97)00217-9. [DOI] [PubMed] [Google Scholar]
- 26.Thompson CA, Jabbour S, Goldberg RJ, et al. Exercise performance-based outcomes of medically treated patients with coronary artery disease and profound ST segment depression. Journal of the American College of Cardiology. 2000;36:2140–5. doi: 10.1016/s0735-1097(00)01004-4. [DOI] [PubMed] [Google Scholar]
- 27.LaMonte MJ, Fitzgerald SJ, Levine BD, et al. Coronary artery calcium, exercise tolerance, and CHD events in asymptomatic men. Atherosclerosis. 2006;189:157–62. doi: 10.1016/j.atherosclerosis.2005.12.014. [DOI] [PubMed] [Google Scholar]
- 28.Christian TF, Miller TD, Bailey KR, Gibbons RJ. Exercise tomographic thallium-201 imaging in patients with severe coronary artery disease and normal electrocardiograms. Annals of internal medicine. 1994;121:825–32. doi: 10.7326/0003-4819-121-11-199412010-00001. [DOI] [PubMed] [Google Scholar]
- 29.Gibbons RJ, Hodge DO, Berman DS, et al. Long-term outcome of patients with intermediate-risk exercise electrocardiograms who do not have myocardial perfusion defects on radionuclide imaging. Circulation. 1999;100:2140–5. doi: 10.1161/01.cir.100.21.2140. [DOI] [PubMed] [Google Scholar]
- 30.Christman MP, Bittencourt MS, Hulten E, et al. Yield of downstream tests after exercise treadmill testing: a prospective cohort study. Journal of the American College of Cardiology. 2014;63:1264–74. doi: 10.1016/j.jacc.2013.11.052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Rich JD, Chen S, Ward RP. Comparison of high risk stress myocardial perfusion imaging findings in men with rapid versus prolonged recovery of ST-segment depression after exercise stress testing. The American journal of cardiology. 2010;105:1361–4. doi: 10.1016/j.amjcard.2009.12.061. [DOI] [PubMed] [Google Scholar]
- 32.Michaelides AP, Psomadaki ZD, Richter DJ, et al. Significance of exercise-induced simultaneous ST-segment changes in lead aVR and V5. International journal of cardiology. 1999;71:49–56. doi: 10.1016/s0167-5273(99)00115-1. [DOI] [PubMed] [Google Scholar]
- 33.Uthamalingam S, Zheng H, Leavitt M, et al. Exercise-induced ST-segment elevation in ECG lead aVR is a useful indicator of significant left main or ostial LAD coronary artery stenosis. JACC Cardiovascular imaging. 2011;4:176–86. doi: 10.1016/j.jcmg.2010.11.014. [DOI] [PubMed] [Google Scholar]
- 34.Kligfield P, Lauer MS. Exercise electrocardiogram testing: beyond the ST segment. Circulation. 2006;114:2070–82. doi: 10.1161/CIRCULATIONAHA.105.561944. [DOI] [PubMed] [Google Scholar]
- 35.Sharir T, Merzon K, Kruchin I, et al. Use of electrocardiographic depolarization abnormalities for detection of stress-induced ischemia as defined by myocardial perfusion imaging. The American journal of cardiology. 2012;109:642–50. doi: 10.1016/j.amjcard.2011.10.022. [DOI] [PubMed] [Google Scholar]
- 36.Ho PM, Maddox TM, Ross C, Rumsfeld JS, Magid DJ. Impaired chronotropic response to exercise stress testing in patients with diabetes predicts future cardiovascular events. Diabetes care. 2008;31:1531–3. doi: 10.2337/dc08-0616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Lauer MS, Okin PM, Larson MG, Evans JC, Levy D. Impaired heart rate response to graded exercise. Prognostic implications of chronotropic incompetence in the Framingham Heart Study. Circulation. 1996;93:1520–6. doi: 10.1161/01.cir.93.8.1520. [DOI] [PubMed] [Google Scholar]
- 38.Dresing TJ, Blackstone EH, Pashkow FJ, Snader CE, Marwick TH, Lauer MS. Usefulness of impaired chronotropic response to exercise as a predictor of mortality, independent of the severity of coronary artery disease. The American journal of cardiology. 2000;86:602–9. doi: 10.1016/s0002-9149(00)01036-5. [DOI] [PubMed] [Google Scholar]
- 39.Cole CR, Blackstone EH, Pashkow FJ, Snader CE, Lauer MS. Heart-rate recovery immediately after exercise as a predictor of mortality. The New England journal of medicine. 1999;341:1351–7. doi: 10.1056/NEJM199910283411804. [DOI] [PubMed] [Google Scholar]
- 40.Lipinski MJ, Vetrovec GW, Froelicher VF. Importance of the first two minutes of heart rate recovery after exercise treadmill testing in predicting mortality and the presence of coronary artery disease in men. The American journal of cardiology. 2004;93:445–9. doi: 10.1016/j.amjcard.2003.10.039. [DOI] [PubMed] [Google Scholar]
- 41.Gayda M, Bourassa MG, Tardif JC, Fortier A, Juneau M, Nigam A. Heart rate recovery after exercise and long-term prognosis in patients with coronary artery disease. The Canadian journal of cardiology. 2012;28:201–7. doi: 10.1016/j.cjca.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 42.Tsuda M, Hatano K, Hayashi H, Yokota M, Hirai M, Saito H. Diagnostic value of postexercise systolic blood pressure response for detecting coronary artery disease in patients with or without hypertension. American heart journal. 1993;125:718–25. doi: 10.1016/0002-8703(93)90163-4. [DOI] [PubMed] [Google Scholar]
- 43.Taylor AJ, Beller GA. Postexercise systolic blood pressure response: association with the presence and extent of perfusion abnormalities on thallium-201 scintigraphy. American heart journal. 1995;129:227–34. doi: 10.1016/0002-8703(95)90002-0. [DOI] [PubMed] [Google Scholar]
- 44.McHam SA, Marwick TH, Pashkow FJ, Lauer MS. Delayed systolic blood pressure recovery after graded exercise: an independent correlate of angiographic coronary disease. Journal of the American College of Cardiology. 1999;34:754–9. doi: 10.1016/s0735-1097(99)00269-7. [DOI] [PubMed] [Google Scholar]
- 45.Morris SN, Phillips JF, Jordan JW, McHenry PL. Incidence and significance of decreases in systolic blood pressure during graded treadmill exercise testing. The American journal of cardiology. 1978;41:221–6. doi: 10.1016/0002-9149(78)90160-1. [DOI] [PubMed] [Google Scholar]
- 46.Levites R, Baker T, Anderson GJ. The significance of hypotension developing during treadmill exercise testing. American heart journal. 1978;95:747–53. doi: 10.1016/0002-8703(78)90505-7. [DOI] [PubMed] [Google Scholar]
- 47.Cole JP, Ellestad MH. Significance of chest pain during treadmill exercise: correlation with coronary events. The American journal of cardiology. 1978;41:227–32. doi: 10.1016/0002-9149(78)90161-3. [DOI] [PubMed] [Google Scholar]
- 48.Tavel ME, Shaar C. Relation between the electrocardiographic stress test and degree and location of myocardial ischemia. The American journal of cardiology. 1999;84:119–24. doi: 10.1016/s0002-9149(99)00219-2. [DOI] [PubMed] [Google Scholar]
- 49.Lloyd-Jones D, Adams RJ, Brown TM, et al. Executive summary: heart disease and stroke statistics--2010 update: a report from the American Heart Association. Circulation. 2010;121:948–54. doi: 10.1161/CIRCULATIONAHA.109.192666. [DOI] [PubMed] [Google Scholar]
- 50.Mieres JH, Shaw LJ, Arai A, et al. Role of noninvasive testing in the clinical evaluation of women with suspected coronary artery disease: Consensus statement from the Cardiac Imaging Committee, Council on Clinical Cardiology, and the Cardiovascular Imaging and Intervention Committee, Council on Cardiovascular Radiology and Intervention, American Heart Association. Circulation. 2005;111:682–96. doi: 10.1161/01.CIR.0000155233.67287.60. [DOI] [PubMed] [Google Scholar]
- 51.Kohli P, Gulati M. Exercise stress testing in women: going back to the basics. Circulation. 2010;122:2570–80. doi: 10.1161/CIRCULATIONAHA.109.914754. [DOI] [PubMed] [Google Scholar]
- 52.Kwok Y, Kim C, Grady D, Segal M, Redberg R. Meta-analysis of exercise testing to detect coronary artery disease in women. The American journal of cardiology. 1999;83:660–6. doi: 10.1016/s0002-9149(98)00963-1. [DOI] [PubMed] [Google Scholar]
- 53.Cumming GR, Dufresne C, Kich L, Samm J. Exercise electrocardiogram patterns in normal women. British heart journal. 1973;35:1055–61. doi: 10.1136/hrt.35.10.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Morise AP, Beto R. The specificity of exercise electrocardiography in women grouped by estrogen status. International journal of cardiology. 1997;60:55–65. doi: 10.1016/s0167-5273(97)02953-7. [DOI] [PubMed] [Google Scholar]
- 55.Barolsky SM, Gilbert CA, Faruqui A, Nutter DO, Schlant RC. Differences in electrocardiographic response to exercise of women and men: a non-Bayesian factor. Circulation. 1979;60:1021–7. doi: 10.1161/01.cir.60.5.1021. [DOI] [PubMed] [Google Scholar]
- 56.Daugherty SL, Magid DJ, Kikla JR, et al. Gender differences in the prognostic value of exercise treadmill test characteristics. American heart journal. 2011;161:908–14. doi: 10.1016/j.ahj.2011.01.021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Gulati M, Pandey DK, Arnsdorf MF, et al. Exercise capacity and the risk of death in women: the St James Women Take Heart Project. Circulation. 2003;108:1554–9. doi: 10.1161/01.CIR.0000091080.57509.E9. [DOI] [PubMed] [Google Scholar]
- 58.Shaw LJ, Mieres JH, Hendel RH, et al. Comparative effectiveness of exercise electrocardiography with or without myocardial perfusion single photon emission computed tomography in women with suspected coronary artery disease: results from the What Is the Optimal Method for Ischemia Evaluation in Women (WOMEN) trial. Circulation. 2011;124:1239–49. doi: 10.1161/CIRCULATIONAHA.111.029660. [DOI] [PubMed] [Google Scholar]
- 59.Albers AR, Krichavsky MZ, Balady GJ. Stress testing in patients with diabetes mellitus: diagnostic and prognostic value. Circulation. 2006;113:583–92. doi: 10.1161/CIRCULATIONAHA.105.584524. [DOI] [PubMed] [Google Scholar]
- 60.Wackers FJ, Young LH, Inzucchi SE, et al. Detection of silent myocardial ischemia in asymptomatic diabetic subjects: the DIAD study. Diabetes care. 2004;27:1954–61. doi: 10.2337/diacare.27.8.1954. [DOI] [PubMed] [Google Scholar]
- 61.Lee DP, Fearon WF, Froelicher VF. Clinical utility of the exercise ECG in patients with diabetes and chest pain. Chest. 2001;119:1576–81. doi: 10.1378/chest.119.5.1576. [DOI] [PubMed] [Google Scholar]
- 62.Wiersma JJ, Verberne HJ, Trip MD, et al. Prevalence of myocardial ischaemia as assessed with myocardial perfusion scintigraphy in patients with diabetes mellitus type 2 and mild anginal symptoms. European journal of nuclear medicine and molecular imaging. 2006;33:1468–76. doi: 10.1007/s00259-006-0165-8. [DOI] [PubMed] [Google Scholar]
- 63.Miller TD, Rajagopalan N, Hodge DO, Frye RL, Gibbons RJ. Yield of stress single-photon emission computed tomography in asymptomatic patients with diabetes. American heart journal. 2004;147:890–6. doi: 10.1016/j.ahj.2003.08.015. [DOI] [PubMed] [Google Scholar]
- 64.Bourque JM, Patel CA, Ali MM, Perez M, Watson DD, Beller GA. Prevalence and predictors of ischemia and outcomes in outpatients with diabetes mellitus referred for single-photon emission computed tomography myocardial perfusion imaging. Circulation Cardiovascular imaging. 2013;6:466–77. doi: 10.1161/CIRCIMAGING.112.000259. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Padala SK, Ghatak A, Padala S, Katten DM, Polk DM, Heller GV. Cardiovascular risk stratification in diabetic patients following stress single-photon emission-computed tomography myocardial perfusion imaging: the impact of achieved exercise level. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2014;21:1132–43. doi: 10.1007/s12350-014-9986-1. [DOI] [PubMed] [Google Scholar]
- 66.Raggi P, Shaw LJ, Berman DS, Callister TQ. Prognostic value of coronary artery calcium screening in subjects with and without diabetes. Journal of the American College of Cardiology. 2004;43:1663–9. doi: 10.1016/j.jacc.2003.09.068. [DOI] [PubMed] [Google Scholar]
- 67.Agarwal S, Cox AJ, Herrington DM, et al. Coronary calcium score predicts cardiovascular mortality in diabetes: diabetes heart study. Diabetes care. 2013;36:972–7. doi: 10.2337/dc12-1548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kwong RY, Sattar H, Wu H, et al. Incidence and prognostic implication of unrecognized myocardial scar characterized by cardiac magnetic resonance in diabetic patients without clinical evidence of myocardial infarction. Circulation. 2008;118:1011–20. doi: 10.1161/CIRCULATIONAHA.107.727826. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Murthy VL, Naya M, Foster CR, et al. Association between coronary vascular dysfunction and cardiac mortality in patients with and without diabetes mellitus. Circulation. 2012;126:1858–68. doi: 10.1161/CIRCULATIONAHA.112.120402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Goraya TY, Jacobsen SJ, Pellikka PA, et al. Prognostic value of treadmill exercise testing in elderly persons. Annals of internal medicine. 2000;132:862–70. doi: 10.7326/0003-4819-132-11-200006060-00003. [DOI] [PubMed] [Google Scholar]
- 71.Steingart RM, Hodnett P, Musso J, Feuerman M. Exercise myocardial perfusion imaging in elderly patients. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2002;9:573–80. doi: 10.1067/mnc.2002.127013. [DOI] [PubMed] [Google Scholar]
- 72.Kwok JM, Miller TD, Hodge DO, Gibbons RJ. Prognostic value of the Duke treadmill score in the elderly. Journal of the American College of Cardiology. 2002;39:1475–81. doi: 10.1016/s0735-1097(02)01769-2. [DOI] [PubMed] [Google Scholar]
- 73.Valeti US, Miller TD, Hodge DO, Gibbons RJ. Exercise single-photon emission computed tomography provides effective risk stratification of elderly men and elderly women. Circulation. 2005;111:1771–6. doi: 10.1161/01.CIR.0000160862.36124.8E. [DOI] [PubMed] [Google Scholar]
- 74.Iskandrian AS, Heo J, Decoskey D, Askenase A, Segal BL. Use of exercise thallium-201 imaging for risk stratification of elderly patients with coronary artery disease. The American journal of cardiology. 1988;61:269–72. doi: 10.1016/0002-9149(88)90929-0. [DOI] [PubMed] [Google Scholar]
- 75.De Winter O, Velghe A, Van de Veire N, et al. Incremental prognostic value of combined perfusion and function assessment during myocardial gated SPECT in patients aged 75 years or older. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2005;12:662–70. doi: 10.1016/j.nuclcard.2005.08.002. [DOI] [PubMed] [Google Scholar]
- 76.Smith-Bindman R, Lipson J, Marcus R, et al. Radiation dose associated with common computed tomography examinations and the associated lifetime attributable risk of cancer. Archives of internal medicine. 2009;169:2078–86. doi: 10.1001/archinternmed.2009.427. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.Fearon WF, Lee DP, Froelicher VF. The effect of resting ST segment depression on the diagnostic characteristics of the exercise treadmill test. Journal of the American College of Cardiology. 2000;35:1206–11. doi: 10.1016/s0735-1097(00)00518-0. [DOI] [PubMed] [Google Scholar]
- 78.Orzan F, Garcia E, Mathur VS, Hall RJ. Is the treadmill exercise test useful for evaluating coronary artery disease in patients with complete left bundle branch block? The American journal of cardiology. 1978;42:36–40. doi: 10.1016/0002-9149(78)90981-5. [DOI] [PubMed] [Google Scholar]
- 79.Meyers DG, Bendon KA, Hankins JH, Stratbucker RA. The effect of baseline electrocardiographic abnormalities on the diagnostic accuracy of exercise-induced ST segment changes. American heart journal. 1990;119:272–6. doi: 10.1016/s0002-8703(05)80016-x. [DOI] [PubMed] [Google Scholar]
- 80.Tanaka T, Friedman MJ, Okada RD, Buckels LJ, Marcus FI. Diagnostic value of exercise-induced S-T segment depression in patients with right bundle branch block. The American journal of cardiology. 1978;41:670–3. doi: 10.1016/0002-9149(78)90815-9. [DOI] [PubMed] [Google Scholar]
- 81.Yen RS, Miranda C, Froelicher VF. Diagnostic and prognostic accuracy of the exercise electrocardiogram in patients with preexisting right bundle branch block. American heart journal. 1994;127:1521–5. doi: 10.1016/0002-8703(94)90380-8. [DOI] [PubMed] [Google Scholar]
- 82.Susmano A, Teran JC. Value of treadmill exercise testing in patients with complete bundle branch block. Angiology. 1979;30:395–406. doi: 10.1177/000331977903000603. [DOI] [PubMed] [Google Scholar]
- 83.Heller GV, Ahmed I, Tilkemeier PL, Barbour MM, Garber CE. Comparison of chest pain, electrocardiographic changes and thallium-201 scintigraphy during varying exercise intensities in men with stable angina pectoris. The American journal of cardiology. 1991;68:569–74. doi: 10.1016/0002-9149(91)90345-l. [DOI] [PubMed] [Google Scholar]
- 84.Heller GV, Ahmed I, Tilkemeier PL, Barbour MM, Garber CE. Influence of exercise intensity on the presence, distribution, and size of thallium-201 defects. American heart journal. 1992;123:909–16. doi: 10.1016/0002-8703(92)90695-r. [DOI] [PubMed] [Google Scholar]
- 85.Iskandrian AS, Heo J, Kong B, Lyons E. Effect of exercise level on the ability of thallium-201 tomographic imaging in detecting coronary artery disease: analysis of 461 patients. Journal of the American College of Cardiology. 1989;14:1477–86. doi: 10.1016/0735-1097(89)90385-9. [DOI] [PubMed] [Google Scholar]
- 86.Partington SL, Lanka V, Hainer J, et al. Safety and feasibility of regadenoson use for suboptimal heart rate response during symptom-limited standard Bruce exercise stress test. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012;19:970–8. doi: 10.1007/s12350-012-9562-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Ross MI, Wu E, Wilkins JT, et al. Safety and feasibility of adjunctive regadenoson injection at peak exercise during exercise myocardial perfusion imaging: The Both Exercise and Regadenoson Stress Test (BERST) trial. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2013;20:197–204. doi: 10.1007/s12350-013-9679-1. [DOI] [PubMed] [Google Scholar]
- 88.Verzijlbergen JF, Vermeersch PH, Laarman GJ, Ascoop CA. Inadequate exercise leads to suboptimal imaging. Thallium-201 myocardial perfusion imaging after dipyridamole combined with low-level exercise unmasks ischemia in symptomatic patients with non-diagnostic thallium-201 scans who exercise submaximally. J Nucl Med. 1991;32:2071–8. [PubMed] [Google Scholar]
- 89.Depuey EG, Mahmarian JJ, Miller TD, et al. Patient-centered imaging. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2012;19:185–215. doi: 10.1007/s12350-012-9523-z. [DOI] [PubMed] [Google Scholar]
- 90.Hachamovitch R, Berman DS, Kiat H, Cohen I, Friedman JD, Shaw LJ. Value of stress myocardial perfusion single photon emission computed tomography in patients with normal resting electrocardiograms: an evaluation of incremental prognostic value and cost-effectiveness. Circulation. 2002;105:823–9. doi: 10.1161/hc0702.103973. [DOI] [PubMed] [Google Scholar]
- 91.Poornima IG, Miller TD, Christian TF, Hodge DO, Bailey KR, Gibbons RJ. Utility of myocardial perfusion imaging in patients with low-risk treadmill scores. Journal of the American College of Cardiology. 2004;43:194–9. doi: 10.1016/j.jacc.2003.09.029. [DOI] [PubMed] [Google Scholar]
- 92.Boden WE, O'Rourke RA, Teo KK, et al. Optimal medical therapy with or without PCI for stable coronary disease. The New England journal of medicine. 2007;356:1503–16. doi: 10.1056/NEJMoa070829. [DOI] [PubMed] [Google Scholar]
- 93.Group BDS, Frye RL, August P, et al. A randomized trial of therapies for type 2 diabetes and coronary artery disease. The New England journal of medicine. 2009;360:2503–15. doi: 10.1056/NEJMoa0805796. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Duvall WL, Levine EJ, Moonthungal S, Fardanesh M, Croft LB, Henzlova MJ. A hypothetical protocol for the provisional use of perfusion imaging with exercise stress testing. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2013;20:739–47. doi: 10.1007/s12350-013-9710-6. [DOI] [PubMed] [Google Scholar]
- 95.Beller GA, Bateman TM. Provisional use of myocardial perfusion imaging in patients undergoing exercise stress testing: a worthy concept fraught with challenges. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2013;20:711–4. doi: 10.1007/s12350-013-9742-y. [DOI] [PubMed] [Google Scholar]
- 96.Lamont DH, Budoff MJ, Shavelle DM, Shavelle R, Brundage BH, Hagar JM. Coronary calcium scanning adds incremental value to patients with positive stress tests. American heart journal. 2002;143:861–7. doi: 10.1067/mhj.2002.120972. [DOI] [PubMed] [Google Scholar]
- 97.Thompson RC, McGhie AI, Moser KW, et al. Clinical utility of coronary calcium scoring after nonischemic myocardial perfusion imaging. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2005;12:392–400. doi: 10.1016/j.nuclcard.2005.04.006. [DOI] [PubMed] [Google Scholar]
- 98.Rozanski A, Gransar H, Wong ND, et al. Use of coronary calcium scanning for predicting inducible myocardial ischemia: Influence of patients' clinical presentation. Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2007;14:669–79. doi: 10.1016/j.nuclcard.2007.07.005. [DOI] [PubMed] [Google Scholar]
- 99.Hachamovitch R, Rozanski A, Hayes SW, et al. Predicting therapeutic benefit from myocardial revascularization procedures: are measurements of both resting left ventricular ejection fraction and stress-induced myocardial ischemia necessary? Journal of nuclear cardiology : official publication of the American Society of Nuclear Cardiology. 2006;13:768–78. doi: 10.1016/j.nuclcard.2006.08.017. [DOI] [PubMed] [Google Scholar]
- 100.Mark DB, Shaw L, Harrell FE, et al. Prognostic value of a treadmill exercise score in outpatients with suspected coronary artery disease. New England Journal of Medicine. 1991;325(12):849–53. doi: 10.1056/NEJM199109193251204. [DOI] [PubMed] [Google Scholar]
- 101.Shaw LJ, Peterson ED, Shaw LK, et al. Use of a prognostic treadmill score in identifying diagnostic coronary disease subgroups. Circulation. 1998;98:1622–30. doi: 10.1161/01.cir.98.16.1622. [DOI] [PubMed] [Google Scholar]