Abstract
Despite significant improvements in treatment, cure rates for many cancers remain suboptimal. The rise of cytotoxic chemotherapy has led to curative therapy for a subset of cancers, though intrinsic treatment resistance is difficult to predict for individual patients. The recent wave of molecularly targeted therapies has focused on druggable activating mutations, and is thus limited to specific subsets of patients. The lessons learned from these two disparate approaches suggest the need for therapies that borrow aspects of both, targeting biological properties of cancer that are at once distinct from normal cells and yet common enough to make the drugs widely applicable across a range of cancer subtypes. The intrinsic mitochondrial pathway of apoptosis represents one such promising target for new therapies, and successfully targeting this pathway has the potential to alter the therapeutic landscape of therapy for a variety of cancers. Here, we discuss the biology of the intrinsic pathway of apoptosis, an assay known as BH3 profiling that can interrogate this pathway, early attempts to target BCL-2 clinically, and the recent promising results with the BCL-2 antagonist venetoclax (ABT-199) in clinical trials in hematologic malignancies.
Introduction
Apoptosis
Most chemotherapeutic and targeted cancer therapies kill tumor cells through the generation of pro-death signaling that initiates the intrinsic apoptotic pathway of programmed cell death (the two other major operative mechanisms of tumor cell killing, the extrinsic cell death pathway and autophagy, are discussed in detail elsewhere in this issue (1,2). The point of no return in the apoptotic cascade is mitochondrial outer membrane permeabilization (MOMP); once it has occurred, mitochondrial permabilization leads to the formation of an apoptosome, which facilitates caspase activation and subsequently triggers the other hallmarks of apoptotic cell death. The cellular decision to initiate MOMP is controlled by a delicate balance between the pro- and anti-apoptotic molecules of the B cell leukemia/lymphoma-2 (BCL-2) family. This review discusses the clinical use of agents designed to inhibit BCL-2 and related molecules; strategies for targeting other anti-apoptotic mechanisms, in particular the IAP family of proteins that inhibit caspase activation, are discussed elsewhere in this issue (3).
BCL-2
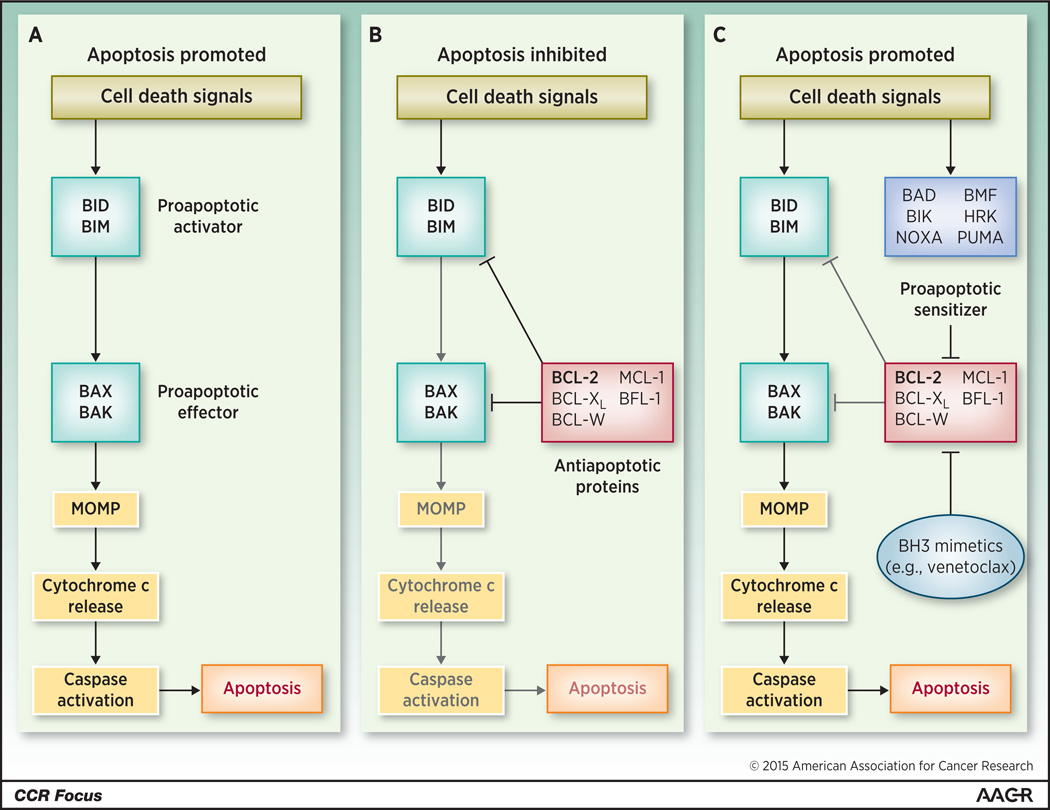
BCL-2, first identified as one of the genes involved in the t(14;18) found in follicular lymphoma (4), is one of the primary anti-apoptotic proteins (5), along with BCL-XL(6), BCL-w (7), MCL-1(8), and BFL-1(9). Anti-apoptotic proteins act by binding pro-apoptotic activators such as BID and BIM (Figure 1). When sequestered by anti-apoptotic proteins, BID and BIM are unable to interact with the direct effector proapoptotic molecules BAX and BAK, preventing their oligomerization and therefore MOMP (10). Anti-apoptotic proteins can also bind BAX and BAK directly, preventing their homo-oligomerization, which is required for MOMP. BCL-2 and its anti-apoptotic cousins bind pro-apoptotic molecules at a shared domain known as BCL-2 Homology 3 (BH3).
Figure 1. Overview of pro- and anti-apoptotic molecules.
(A) Cell death signals trigger BID and BIM to activate BAX and BAK, which in turn initiate mitochondrial outer membrane permeabilization (MOMP) and lead to apoptosis. (B) Anti-apoptotic molecules, including BCL-2, antagonize both activator and effector molecules and block the apoptotic cascade. (C) Cell death signals also activate sensitizer molecules, which antagonize anti-apoptotic molecules and release the block on apoptosis. This physiologic role is pharmacologically recapitulated by BH3-mimetic drugs such as venetoclax.
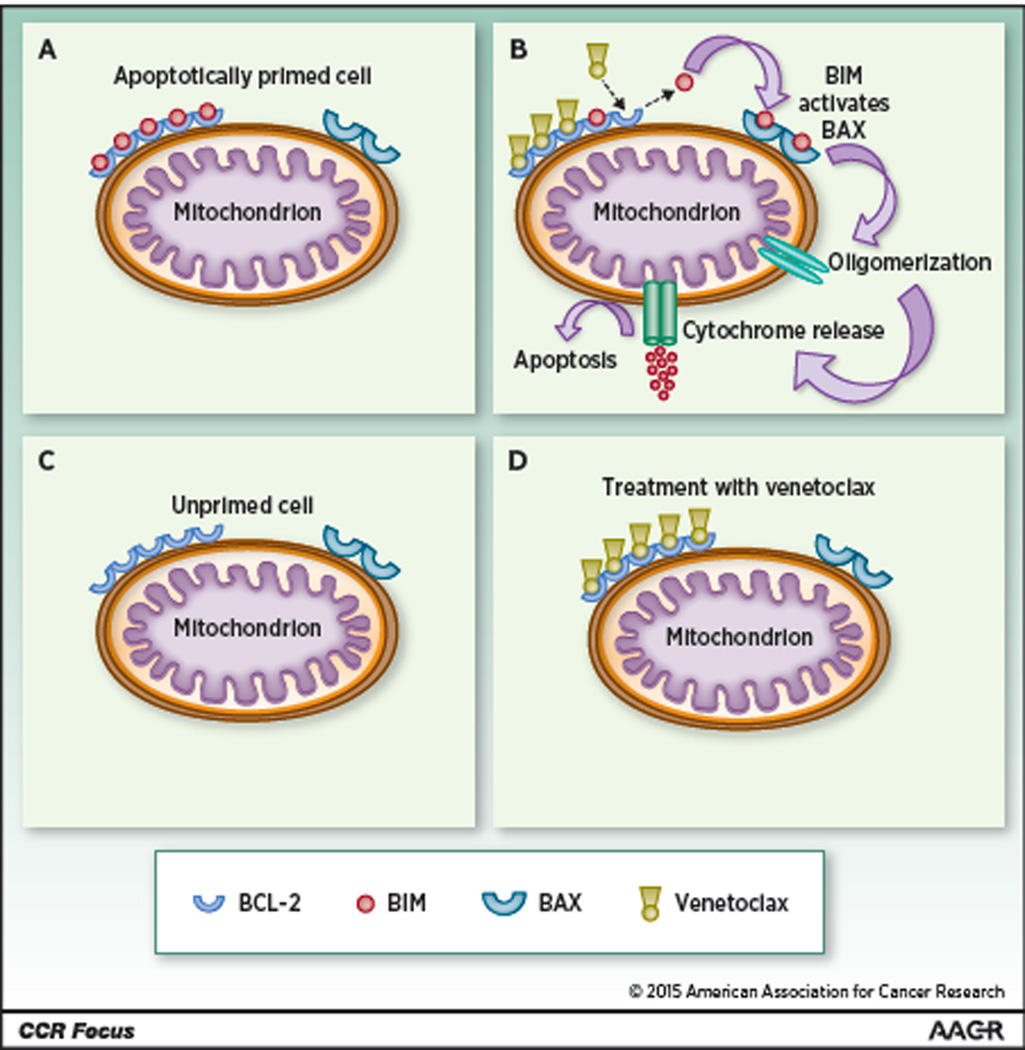
Early studies of the relationships between these molecules suggested that many cancers have a dependence on BCL-2 and other anti-apoptotic molecules for their survival (11). For example, relative to other peripheral blood mononuclear cells, chronic lymphocytic leukemia (CLL) cells express high levels of BCL-2 protein(12). Bcl-2 protein expression level alone, however, cannot account for the propensity of a cell to undergo apoptosis, as this intricate system also depends on the interactions of Bcl-2 with other anti- and pro-apoptotic proteins. A more physiological assessment of these interactions can be obtained through BH3 profiling, a functional assay that measures the ability of a range of BH3-only domain containing peptides to trigger cytochrome c release from mitochondria in a cell of interest (see Text Text Box 1). The pattern of response can then be compared to known molecular interactions between pro- and anti-apoptotic BCL-2 family members to determine the specific anti-apoptotic protein dependencies of the given cell (12,13). BH3 profiling can also assess the proximity of a cell to the apoptotic threshold (a property known as ‘mitochondrial priming’, Figure 2). The potential clinical relevance of BH3 profiling is discussed in additional detail below; a summary of the agents described in this article can be found in Table 1.
Text Box 1. Mitochondrial priming and BH3 profiling.
The observation that different tumors have differing sensitivities to cytotoxic chemotherapy led to the concept of “mitochondrial priming,” which refers to a cell’s proximity to the apoptotic threshold. At a molecular level, primed cells have a high fraction of their anti-apoptotic proteins such as BCL-2 bound to pro-apoptotic BH3-only proteins such as BIM and BID, whereas unprimed cells have a low fraction of anti-apoptotic proteins bound to pro-apoptotic BH3-only proteins (see Figure 2). Primed cells are more sensitive than unprimed cells to chemotherapy and to BH3-mimetic drugs such as venetoclax, which binds with high affinity to the anti-apoptotic protein Bcl-2. BH3 profiling is a functional assay in which cells from a specific tumor sample are interrogated with a range of BH3-domain containing peptides that simulate the actions of their corresponding full length BH3-only pro-apoptotic proteins. The degree of cytochrome c release (a proxy for mitochondrial outer membrane permeabilization) is then compared for different peptides at different concentrations to assess the degree of mitochondrial priming of the cell. BH3 profiling can also predict the specific anti-apoptotic proteins on which a particular cell depends for survival.
Figure 2. Apoptotic priming and venetoclax (VCX) method of action.
(A) In an apoptotically primed cell, BCL-2 or other anti-apoptotic molecules sequester BIM (or BID) and prevent interaction with effector molecules such as BAK or BAX. (B) Binding of VCX to BCL-2 displaces BIM, allowing it to interact with BAX (or BAK), which then oligomerizes and allows efflux of cytochrome C from the mitochondrion. (C) A cell with a low degree of apoptotic priming has relatively little BIM or BID. In this case, treatment with VCX (D) has little effect in and of itself, though this might not preclude synergy with additional chemotherapy.
Table 1.
Agents targeting the BCL-2 family
| Drug | Mechanism | Diseases Tested (Phase of Trial) |
Comments |
|---|---|---|---|
| Oblimersen | Antisense DNA targeting BCL2 | Myeloma (III) | Potency probably overestimated by preclinical studies |
| Obatoclax | Pan-inhibitor of BH3-domain containing proteins | CLL, MDS, AML, NSCLC, Hodgkin lymphoma (I) SCLC (II) |
Formulation and off-target effects led to poor tolerability. |
| ABT-737 | Similar to navitoclax | None | Not used clinically due to lack of oral bioavailability |
| Navitoclax (ABT-263) | BCL-2 and BCL-XL inhibitor | CLL, NHL (I) SCLC (I) |
Use limited by significant thrombocytopenia due to concomitant BCL-XL inhibition |
| Venetoclax (ABT-199) | Specific BCL-2 inhibitor | CLL (I-III) NHL (I–III) AML (I, II) Myeloma (I) |
Promising efficacy in hematologic malignancies, starting to be explored in solid tumors |
Early Attempts to Target BCL-2 in the Clinic
Oblimersen
Oblimersen is a single-stranded 18-mer DNA molecule complimentary to BCL-2 mRNA (14). In cell lines, oblimersen had been shown to inhibit BCL-2 protein expression, presumably by hybridizing with BCL-2 mRNA(15). Despite some evidence of benefit in phase I studies of chronic lymphocytic leukemia (CLL)(16), myeloma (17), and melanoma (18), oblimersen was not effective in a phase III study in myeloma (19), and only modestly beneficial when added to fludarabine in a phase III study of CLL (20). Given these results, it did not receive FDA approval and further development of the drug was not pursued. Subsequent studies in both primary tumor cells and mononuclear blood cells from patients treated with oblimersen suggested that its inhibition of BCL-2 protein expression was significantly less potent than had been predicted by the first in vitro studies in cell lines (21), highlighting the limitations of cell lines and the importance of assessing drug activity in primary tumor cells in preclinical development.
Obatoclax
Another anti-BCL-2 agent tested in trials was obatoclax (GX-15-070), a small molecule which is thought to bind the BH3 domain of BCL-2 (as well as those of BCL-XL and MCL-1), thus preventing the anti-apoptotic proteins from sequestering pro-apoptotic BH3-only proteins (22). Obatoclax was only modestly efficacious in the clinic. For example, a phase I trial adding it to fludarabine and rituximab in relapsed/refractory CLL showed a partial response rate of 54% with no complete responses (23), and a phase II trial in small-cell lung cancer (SCLC) showed no benefit when it was added to the standard regimen of carboplatin and etoposide (24). As with oblimersen, subsequent analyses suggested that obatoclax may behave differently in vivo compared to the original in vitro studies (25). For instance, significant thrombocytopenia, a well-known on-target effect of BCL-XL inhibition, was never observed in patients treated with obatoclax. Due to its formulation, obatoclax also had neurological side effects such as mental status changes, which further limited its clinical development (26). Furthermore, additional in vitro studies showed that obatoclax can trigger apoptosis in cells lacking BAX and BAK, suggesting an alternative mechanism of action.(27) It is important to remember that although the results of these early experiences with both oblimersen and obatoclax were disappointing, these results reflect the inadequacy of these individual molecules rather than that of the overall strategy targeting BCL-2 in cancer.
Navitoclax (ABT-263)
The most potent and selective BCL-2 antagonists engineered to date are those developed by Abbott Laboratories (now AbbVie), beginning with ABT-737(28) and its orally-bioavailable counterpart navitoclax (ABT-263)(29,30). These “BH3-mimetic” molecules mimic the pro-apoptotic action of BH3-only proteins by binding directly to the BH3-binding domains of anti-apoptotic molecules, thereby displacing native BH3-only proteins (e.g. BIM, BAD, Figure 2). ABT-737 and navitoclax have binding affinities for BCL-2 family proteins on the order of 10–10,000 times greater than other molecules, including obatoclax (31). ABT-737, whose BH3-binding profile directly mirrors that of BAD BH3 protein, has poor oral bioavailability and has been limited to in vitro and animal studies.
Navitoclax (formerly ABT-263) is an orally bioavailable, relatively non-selective BCL-2 family inhibitor with high affinity for BCL-2, BCL-XL, and BCL-w, and substantially less affinity for MCL-1 (31). Early-phase clinical trials, particularly in hematologic malignancies, were promising. For example, in a phase I trial that included 29 patients with relapsed or refractory CLL, nine (35%) had a partial remission with navitoclax alone and 7 others had stable disease for at least six months, with overall progression-free survival in the cohort of 25 months (32). The activity of ABT-263 monotherapy in solid tumors was less promising. For example, in a phase II study in 39 patients with relapsed small cell lung cancer (SCLC), only one patient (2.6%) had a partial response, and 9 patients (23%) had stable disease, with a median progression-free survival of only 1.5 months (33). Subsequent studies have shown that, at least in SCLC, high expression of BIM without concomitant MCL-1 expression predicts navitoclax efficacy, suggesting a potential opportunity to retarget the molecule in a more selected cohort (34).
The major limitation of navitoclax in clinical use, however, has been the frequent development of thrombocytopenia, which can be severe. This toxicity is a predicted consequence of the drug’s strong inhibition of BCL-XL, a primary barrier to apoptosis in aging platelets (35); platelet production, on the other hand, appears to be spared or even increased. Specific pharmacokinetic strategies, such as gradual dose increases and daily rather than pulsed administration, have been able to mitigate the thrombocytopenia to a certain extent (32), and navitoclax remains under clinical exploration in a number of cancers. Although clinically relevant bleeding has not been reported in the studies described above, this on-target toxicity of navitoclax has nevertheless limited its development, particularly in many hematologic malignancies, in which baseline thrombocytopenia is often prominent.
Venetoclax (ABT-199)
The elucidation of the mechanism by which navitoclax causes thrombocytopenia suggested that a more selective BCL-2 inhibitor might avoid this toxicity and allow for higher dosing to maximize clinical efficacy. This led to the rational reverse engineering of navitoclax to yield venetoclax, an orally bioavailable BCL-2-specific inhibitor originally known as ABT-199/GDC-0199 (also manufactured by AbbVie) (36). Side-by-side pharmacodynamic comparison of venetoclax to navitoclax showed that venetoclax has a slightly higher avidity for BCL-2, and three orders of magnitude less avidity for BCL-XL (30). Initial in vitro studies confirmed that venetoclax rapidly kills malignant cells through the intrinsic mitochondrial apoptosis pathway and is selective for cells dependent on BCL-2, but not those dependent on BCL-XL. In preclinical models, the drug exhibited efficacy against a wide variety of tumor types, including leukemias, non-Hodgkin lymphoma, and myeloma, with no significant thrombocytopenia observed in in vivo models.
Clinical Uses of Venetoclax
Chronic lymphocytic leukemia
Several results from preclinical studies suggested that CLL would be the logical disease in which to first study venetoclax in the clinic. CLL is known to express high levels of Bcl-2 and pro-apoptotic BH3-only proteins, and in vitro BH3 profiling of CLL patient samples has demonstrated on a functional level that CLL cells from most patients are dependent on Bcl-2 for survival (13), which may be due in part to interactions between CLL cells and the surrounding bone marrow stroma (37). Moreover, in a small cohort of CLL patients for whom baseline samples were collected prior to starting first-line therapy, the degree of mitochondrial priming appeared to correlate with treatment responsiveness (37). Primary CLL cells were among those shown to be most sensitive to ABT-199 ex vivo, with substantial induction of apoptosis observed within just one hour of treatment.
The first-in-human study of venetoclax (M12-175) is a large, ongoing multicenter dose-escalation study of venetoclax monotherapy in relapsed/refractory CLL (38) and non-Hodgkin lymphoma (NHL) (39). An interim analysis of the CLL arm of this study found that the majority of the 105 patients had clinically high-risk disease (75% were immunoglobulin heavy chain variable region (IGHV) unmutated, and 22% harbored 17p deletions or TP53 mutations), and were heavily pre-treated, with a median of four prior lines of therapy. Venetoclax was well-tolerated by most patients. Mild gastrointestinal toxicity was seen, with diarrhea (40%) and nausea (35%) being most common, generally grade 1/2, and manageable with supportive care. One-third of patients developed grade 3/4 neutropenia, but only 7% had febrile neutropenia. This neutropenia was not entirely unexpected given prior ex vivo studies showing that Bcl-2 blockade accelerates FasL-mediated apoptosis in neutrophils (40) and other work showing a specific sensitivity to BCL-2 inhibition in myeloid precursors that did not extend to inhibition of other pro-apoptotic molecules like BCL-XL (34). Moreover, neutropenia was generally responsive to growth factor support. Consistent with predictions and preclinical models, grade 3/4 thrombocytopenia was uncommon (7% of patients). Other adverse effects included anemia (10%), hyperglycemia (7%), tumor lysis syndrome (TLS, 7%), and hypokalemia (5%). Only 7 serious adverse events were felt to be related to venetoclax (4 episodes of febrile neutropenia and three episodes of TLS). The most serious toxicity observed with venetoclax was tumor lysis syndrome, which resulted in acute kidney injury leading to the need for dialysis in one patient and presumed sudden cardiac death in a patient treated at 1,200 mg daily dosing. Based on these events, the protocol was revised to include a lower initial dose, a slower stepwise intra-patient dose escalation, and intensive TLS prophylaxis and monitoring. Utilizing this new strategy, no additional clinical TLS was observed, and the recommended phase 2 dose was determined to be 400 mg qd.
With regard to efficacy, deep responses were observed in the peripheral blood, lymph nodes, and bone marrow of the majority of patients. Of 78 evaluable subjects, 60 (77%) had an objective response by 2008 IW-CLL criteria (41), with 18 (23%) complete responses and 42 (54%) partial responses. Equivalent response rates were seen in all high-risk groups, including del(17p) (15 of 19 patients responded (79%), including 5 CRs), fludarabine-refractory patients (31 of 41 patients responded (76%), 9 CRs) and IGHV unmutated patients (18 of 24 patients responded (75%), 7 CRs). Six of the 18 patients with CRs were found to have no evidence of minimal residual disease (MRD) by four-color flow cytometry, although this MRD analysis was not pre-planned and was assessed by heterogeneous local methodologies. At this interim analysis, 37 patients had discontinued: 22 with progressive disease, 12 for adverse events, and three for other reasons (one patient needed to start warfarin and two proceeded to allogeneic stem cell transplant in CR). The median PFS for the entire cohort was estimated at 18 months, but this included many patients treated at lower doses in the early dose escalation phase of the trial. For those patients treated at or above a dose of 400 mg daily, median progression-free survival had not been reached.
While these results were impressive for a single agent, preclinical studies showed that venetoclax sensitizes CLL cells to monoclonal antibodies and cytotoxic agents (30), suggesting that it might be even more effective as a component of a multidrug regimen. Based on these preclinical data, a phase Ib study of venetoclax plus rituximab (M13-982) was opened to assess the safety and efficacy of this combination (42). The most recent presentation of interim data reported results for 49 patients with relapsed/refractory CLL, 20% of whom had 17p deletions and 57% of whom had progressed after fludarabine. Compared to the monotherapy study, this cohort was somewhat less heavily pretreated, having received a median of only two prior therapies.
The initial results from the M13-982 study show that venetoclax plus rituximab has generally been a safe and tolerable regimen for most patients. Neutropenia was again the most frequent grade 3/4 adverse event (47% of patients), but febrile neutropenia remained rare (6%). Grade 3/4 thrombocytopenia and anemia were somewhat more frequent than in the monotherapy setting (16% and 14%, respectively). Serious adverse events attributed to the study drugs were rare, and included febrile neutropenia (4%), infusion reactions (4%), and tumor lysis syndrome (4%). During the initial venetoclax monotherapy lead-in period of this study, there was another case of fatal TLS in a patient with extremely bulky lymphadenopathy. This death, in conjunction with the death on the M12-175 study, led to a revamping of the study design (as discussed above) after which no additional clinical TLS events were observed in the next 32 patients. The recommended phase two dose for venetoclax in combination with rituximab was the same as for monotherapy at 400 mg daily. At the time of interim analysis, 10 of 49 patients had discontinued: six for progressive disease, two for adverse events, and two withdrew consent.
With regard to the preliminary efficacy the ORR was 88% (43 of 49 patients), with 22 partial responses (45%) and 15 complete responses (31%). An additional six patients with PRs were not yet confirmed. MRD analysis by high-sensitivity flow cytometry showed that 9 of the 15 patients with CRs were MRD negative. Interestingly, 8 of 22 patients achieving PR were MRD negative in the marrow or blood; several of these patients had lymph nodes that just barely met criteria for enlargement, raising the question of whether this residual mass potentially represented scar tissue rather than residual CLL. Interestingly, five patients who achieved CR with MRD negativity have since opted to discontinue the venetoclax. Although one has since had slight progression and has technically moved back into the PR category, the other four continue to have no evidence of disease, with three now off venetoclax for longer than they were on it (median 12 months) in continued MRD-negative CR, suggesting an impressive durability to these deep responses (42).
Non-Hodgkin lymphoma
Preclinical studies showed that venetoclax also has significant activity against a number of NHL cell lines, including diffuse large B cell lymphoma (DLBCL), follicular lymphoma (FL), and mantle cell lymphoma (MCL)(30). Its in vitro activity is best in lymphomas with BCL-2 activation or translocations involving the BCL-2 locus, such as t(14;18), the hallmark of follicular lymphoma, and in double-hit lymphoma (DHL), which harbors translocations of both BCL-2 and MYC. As in CLL, venetoclax also appears to enhance the efficacy of chemotherapy in NHL xenograft models.
At an interim analysis, the NHL arm of the phase I first-in-human M12-175 study included 62 patients with a range of NHL subtypes, including MCL (20 patients), DLBCL (19), FL (14), Waldenstrom Macroglobulinemia (WM, 4), marginal zone lymphoma (MZL, 3), multiple myeloma (1), and primary mediastinal B-cell lymphoma (PMBCL, 1). Similar to the CLL cohort, the NHL cohort was heavily pretreated, having received a median of three prior lines of therapy.
As in the CLL arm, Grade 1/2 nausea, diarrhea, anemia, and fatigue were the most common adverse events observed. The most common grade 3–4 adverse event was anemia (12 patients, 19%). Neutropenia was somewhat less common than in the CLL cohorts (6 patients, 10%). Two dose-limiting toxicities were observed at the 600 mg range, including one episode of grade 4 neutropenia and one episode of grade 3 febrile neutropenia. Laboratory tumor lysis syndrome was observed in some MCL patients, but was without clinical sequelae.
Among 59 evaluable patients, the overall response rate was 48%, but as would be expected in such a heterogeneous cohort, the range was variable and depended on lymphoma subtype. The best activity was seen in MCL (13/19 patients (68%), including 1 CR). The ORR in DLBCL was 28% (5/18 patients, 1 CR) and in FL was 31% (4/13 patients, 1 CR). The relatively low response rate in FL is somewhat surprising given the high levels of BCL-2 expression created by the hallmark t(14;18), but illustrates that BCL-2 expression alone is not sufficient to predict BCL-2 dependence. Notably, most of the responses in DLBCL and FL were observed in higher dosing cohorts, suggesting that a higher drug exposure may be required to achieve response in patients with these histologic subtypes (39).
Acute myeloid leukemia
Despite prior work showing high Bcl-2 expression in myeloblasts (43), it was somewhat surprising that in vitro treatment with venetoclax efficiently killed myeloblasts from a variety of sources, including cell lines, primary patient samples, and murine primary xenografts (44), as BCL-2 was previously thought to be more important as a survival factor in lymphoid, rather than myeloid lineages. BH3 profiling, however, showed that a significant proportion of myeloblasts are indeed BCL-2 dependent, and that differing degrees of apoptotic priming in patient-derived samples appear to correlate with the variance in chemoresponsiveness seen in the clinical setting (45). As referenced above, subsequent studies have gone on to show that this BCL-2 dependence may extend to normal myeloid precursors as well (34). These data provided a strong rationale for studying venetoclax as a treatment for patients with AML.
An interim analysis of a phase 2, multicenter study to evaluate the efficacy of single-agent venetoclax in patients with relapsed or refractory AML, or as first-line therapy in patients deemed unfit to receive intensive chemotherapy was recently presented (46). At this interim analysis, 32 patients had enrolled on study (30 with relapsed/refractory disease). Most patients had high-risk features, including pre-existing myelodysplastic syndrome (MDS, 37.5%), FLT3-ITD mutations (19%), and older age (median 71 years). Venetoclax appeared to be safe and well-tolerated, with a similar range of adverse events seen in other studies, though perhaps a slightly higher rate of grade 3/4 febrile neutropenia (25%). No clinically significant TLS was observed.
Only 1/28 evaluable patients in this study achieved a CR (3%), with 4 achieving CR with incomplete blood count recovery (CRi, 12%), for an ORR of 15%. Interestingly, three of these 5 patients had activating IDH1 or IDH2 mutations, and another 3 patients with IDH2 mutations demonstrated anti-leukemic activity that did not meet formal criteria for response due to a lack of hematologic recovery. Overall, six of 11 patients with IDH mutations had evidence of anti-tumor activity with venetoclax; of the five who did not, two had concomitant FLT3-ITDs. Although two of the five patients with CRs became MRD-negative by flow cytometry, though the majority of responses were not durable. In particular, all three CR/CRi patients with IDH mutations had relapsed by 12 weeks on study, whereas the two CR/CRi without IDH mutations remained in remission. The preliminary results of this study demonstrate anti-tumor activity of venetoclax as a single agent in AML, with good tolerability. They support the development of additional clinical trials in AML looking at venetoclax in combination with chemotherapy and other targeted therapies; some of these, including a phase 1/2 study of venetoclax in combination with low-dose cytarabine (NCT02287233) and a phase 1b study combining venetoclax with one of the two hypomethylating agents decitabine or azacitidine (NCT02203773), are already enrolling patients.
Other Malignancies
Acute lymphoblastic leukemia
Early T-cell progenitor acute lymphoblastic leukemia (ETP-ALL) is a high-risk subgroup of T-cell ALL with a particularly poor prognosis. BH3 profiling of primary patient samples from patients with ETP-ALL revealed significant dependence on BCL-2 (47). This was distinct even when comparing to primary samples derived from patients with other subtypes of T-ALL, some of which were dependent on BCL-XL. As might be expected, ETP-ALL samples displayed increased sensitivity to in vitro treatment with venetoclax. In a related study, venetoclax and cytarabine had a synergistic effect against the T-ALL cell line LOUCY, which approximates the ETP phenotype, but not against more differentiated T-ALL cell lines (48). These preclinical data provide a strong rationale for pursuing a clinical trial of venetoclax specifically in ETP-ALL.
Waldenstrom’s macroglobulinemia
The NHL arm of the M12-175 study discussed above had 4 patients with Waldenstrom Macroglobulinemia (WM), all of whom responded to treatment with venetoclax. Preclinical studies give reason to hope that this efficacy will carry forward in larger cohorts of WM patients treated either with venetoclax alone or with combination therapy. In particular, recent studies in CXCR4WHIM WM cells, which carry a mutation conferring resistance to the BTK inhibitor ibrutinib, showed that treatment with venetoclax can restore ibrutinib sensitivity (49). Venetoclax can also directly induce apoptosis in CXCR4WT WM cells and also appears to sensitize WM cells to the PI3k-δ isoform inhibitor idelalisib.
Multiple myeloma
BCL-2 is expressed in many cases of myeloma, and in those cases appears to be important for survival (50). Preclinical studies using ABT-737 showed that the drug efficiently killed a number of myeloma cell lines, all of which were distinguished by the presence of translocations involving CCND1, the gene encoding cyclin D1 (51). These studies have since been replicated using venetoclax (52) and a recent phase I trial of 24 patients with relapsed or refractory myeloma showed responses in 3 of 7 patients with CCND1/IGH translocation, with 2 of these patients achieving CR (53). Interestingly, this translocation is also the pathophysiologic hallmark of mantle cell lymphoma, a NHL subtype against which venetoclax appears to be particularly effective. Venetoclax is now in clinical trials in patients with relapsed or refractory myeloma as part of multidrug regimens that include bortezomib and dexamethasone (NCT01794507).
Breast cancer
BCL-2 expression appears to have variable prognostic significance in breast cancer. It has been best studied in luminal cancers, where it is expressed in 85% of cases and appears to correlate with a favorable prognosis and response to chemotherapy (54). Although neither ABT-263 nor ABT-199 induce breast cancer xenograft regression when used as single agents, more recent studies have shown that the combinations of ABT-199 with tamoxifen (55) and ABT-737 with docetaxel (56) are more effective at inducing xenograft breast tumor regression than either tamoxifen or docetaxel alone. So far, these studies have only been performed in estrogen receptor (ER)+ breast cancers, and responses appear to be limited to those with positive BCL-2 expression by immunohistochemistry. These intriguing findings are worthy of further exploration.
Future Directions
A number of questions about the biology of BCL-2 in cancer and its therapeutic targeting remain unanswered or unexplored. First, is the optimal therapeutic role for venetoclax as monotherapy or in combination? Although initially tested as monotherapy in early clinical studies, in vitro studies suggest that venetoclax may be most effective as a chemosensitizing agent, in effect removing cancer cells’ major lines of defense against the pro-apoptotic effects of chemotherapy. A number of ongoing or upcoming studies will investigate these possibilities, including in CLL, where trials have either recently opened or are in development to combine venetoclax with newer anti-CD20 monoclonal antibodies and kinase inhibitors (NCT02427451), both in the relapsed/refractory and eventually in the frontline setting, and in AML, where a recently opened trial combines venetoclax with the hypomethylating agents azacitidine or decitabine (NCT02203773) and future studies will combine venetoclax with chemotherapy.
Second, are there molecular tools that can predict clinical response to venetoclax? Thus far, BH3 profiling has been largely reserved to preclinical studies or descriptive studies incorporated into trials; however, the technique is also appealing as a clinically applicable assay, in which patient samples could be profiled in real time to assess potential sensitivity to small molecules such as venetoclax, and clinical decisions could be influenced by the results. Preliminary data with BH3 profiling suggest that the level of mitochondrial priming in pre-treatment samples from patients on the M12-175 trial may be associated with the depth of response to venetoclax in CLL (57). BH3 profiling of pretreatment samples from twelve patients treated with venetoclax for relapsed/refractory AML showed similar utility in predicting treatment response (58). Additionally, genetic profiling may uncover important mutations that predict either sensitivity or resistance to BCL-2 blockade. For example, IDH1 and IDH2 mutations have recently been shown to predict BCL-2 dependence in vitro (59), a fact that appears to be supported by the initial experience using venetoclax in AML patients.
Third, what are the mechanisms that contribute to the development of venetoclax resistance? In some cancers, this may occur via upregulation of other anti-apoptotic molecules like BCL-XL or MCL-1, though this has been difficult to conclusively show in the clinic. Recent preclinical studies have shown potent pro-apoptotic effects of specific MCL-1 inhibitors, both alone and in combination with ABT-263 (60). A number of other drugs, all thought to be acting via MCL-1 inhibition, are being explored for utility in overcoming venetoclax resistance, including CDK9 inhibitors (61), MEK inhibitors (62), sorafenib (63), and novel agents such as the pan-BCL2 family inhibitor (−)BI97D6 (64).
In other cancers, resistance may be due to acquired mutations in BCL-2 or other related proteins. For example, one recent study showed that prolonged exposure of lymphoma cell lines to venetoclax selected for missense mutations in BCL-2 that disrupt the drug’s binding to the BH3 domain, thereby inhibiting apoptosis, whereas other venetoclax-resistant lymphomas were found to harbor inactivating mutations in BAX that prevent the molecule from anchoring to the outer mitochondrial membrane (65). An improved understanding of these resistance mechanisms may ultimately allow the development of new strategies that subvert these resistance mechanisms; for example, if BCL-2 missense mutations were found to occur in patients on chronic dosing of venetoclax, trials of bolus, pulsatile dosing of venetoclax, could explore whether abrogating the selective pressure for the development of resistance mutations would result in more durable responses. Given that the killing of malignant cells by venetoclax appears to depend more on achieving Cmax compared to area under the curve (AUC), it seems likely that a strategy of high doses of venetoclax given less frequently would be particularly effective, though this hypothesis will need to be explored in clinical trials.
Some of the observations made in studying the evolution of resistance in cancers previously sensitive to BCL-2 inhibition lead to a final question: can strategies targeting addiction to anti-apoptotic molecules be extended to other cancers? Despite promising results in the range of malignancies detailed above, the agents described in this review have shown little efficacy in many other cancers, including many solid tumors (66–68). Recent studies have shown that there are at least two reasons for this. One is that some tumors are dependent on anti-apoptotic molecules other than BCL-2 for survival; for example, a recent study showed that some non-small cell lung cancer cell lines appear to be more dependent on BCL-XL than on BCL-2, and selective BCL-XL inhibition significantly increased the anti-tumor effect of docetaxel in vitro (34). A second is that some cancers, although reliant to a certain extent on BCL-2, upregulate additional anti-apoptotic molecules as well. Recently, for example, it was found that certain small cell lung cancer lines can be sensitized to BCL-2 inhibition with ABT-263 by inhibition of TORC1/2, which leads to reduced MCL-1 protein levels (69). These new insights suggest additional avenues of investigation that may significantly expand the role of venetoclax and other agents that similarly inhibit anti-apoptotic proteins.
Conclusions
Our improving understanding of the fundamental protection afforded to cancer cells by the anti-apoptotic protein BCL-2 has opened a new therapeutic avenue in cancer treatment. Although early efforts at therapeutically targeting BCL-2 were only modestly successful, the highly selective oral BCL-2 antagonist venetoclax has shown promise in a range of malignancies as both monotherapy and in combination with existing regimens. The FDA recently granted venetoclax a breakthrough designation for relapsed/refractory CLL with 17p deletion, and it appears likely that the drug will receive approval in the near future. Moving forward, biomarkers such as BH3 profiling may help us to further refine our understanding of BCL-2 biology and have the potential to become clinically relevant tools to predict sensitivity to venetoclax and other drugs targeting the BCL-2 family. Finally, a better understanding of the mechanisms by which resistance to BCL-2 inhibition develops will allow us to develop strategies to subvert these mechanisms, thereby optimizing the therapeutic potential of this powerful new approach. Although the early clinical studies have focused primarily on hematologic malignancies, the fundamental role of the mitochondrial pathway of apoptosis in cancer more broadly suggests that the lessons learned from these initial clinical studies have the potential to make a major impact on the broader world of cancer therapeutics.
Acknowledgments
Grant Support
C.J. Gibson is supported by the NIH under award number T32HL116324. M.S. Davids is supported by an ASCO Career Development Award.
Footnotes
Disclosure of Potential Conflicts of Interest
M.S. Davids is a consultant/advisory board member for AbbVie, Genentech, Gilead, Infinity Pharmaceuticals, and Janssen. No potential conflicts of interest were disclosed by the other author.
References
- 1.White E, Mehnert J, Chan CS. Autophagy, metabolism, and cancer. Clin Cancer Res. 2015;21 doi: 10.1158/1078-0432.CCR-15-0490. xxx-xxx. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Martinez-Lostao L, Anel A, Pardo J. How do cytotoxic lymphocytes kill cancer cells? Clin Cancer Res. 2015;21 doi: 10.1158/1078-0432.CCR-15-0685. xxx-xxx. [DOI] [PubMed] [Google Scholar]
- 3.Fulda S. Promises and challenges of Smac mimetics as cancer therapeutics. Clin Cancer Res. 2015;21 doi: 10.1158/1078-0432.CCR-15-0365. xxx-xxx. [DOI] [PubMed] [Google Scholar]
- 4.Tsujimoto Y, Finger LR, Yunis J, Nowell PC, Croce CM. Cloning of the chromosome breakpoint of neoplastic B cells with the t(14;18) chromosome translocation. Science. 1984;226:1097–1099. doi: 10.1126/science.6093263. [DOI] [PubMed] [Google Scholar]
- 5.Hockenbery D, Nuñez G, Milliman C, Schreiber RD, Korsmeyer SJ. Bcl-2 is an inner mitochondrial membrane protein that blocks programmed cell death. Nature. 1990;348:334–336. doi: 10.1038/348334a0. [DOI] [PubMed] [Google Scholar]
- 6.Boise LH, González-García M, Postema CE, Ding L, Lindsten T, Turka LA, et al. bcl-x, a bcl-2-related gene that functions as a dominant regulator of apoptotic cell death. Cell. 1993;74:597–608. doi: 10.1016/0092-8674(93)90508-n. [DOI] [PubMed] [Google Scholar]
- 7.Gibson L, Holmgreen SP, Huang DC, Bernard O, Copeland NG, Jenkins NA, et al. bcl-w, a novel member of the bcl-2 family, promotes cell survival. Oncogene. 1996;13:665–675. [PubMed] [Google Scholar]
- 8.Kozopas KM, Yang T, Buchan HL, Zhou P, Craig RW. MCL1, a gene expressed in programmed myeloid cell differentiation, has sequence similarity to BCL2. Proc Natl Acad Sci U S A. 1993;90:3516–3520. doi: 10.1073/pnas.90.8.3516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Choi SS, Park IC, Yun JW, Sung YC, Hong SI, Shin HS. A novel Bcl-2 related gene, Bfl-1, is overexpressed in stomach cancer and preferentially expressed in bone marrow. Oncogene. 1995;11:1693–1698. [PubMed] [Google Scholar]
- 10.Oltvai ZN, Milliman CL, Korsmeyer SJ. Bcl-2 heterodimerizes in vivo with a conserved homolog, Bax, that accelerates programmed cell death. Cell. 1993;74:609–619. doi: 10.1016/0092-8674(93)90509-o. [DOI] [PubMed] [Google Scholar]
- 11.Letai AG. Diagnosing and exploiting cancer’s addiction to blocks in apoptosis. Nat Rev Cancer. 2008;8:121–132. doi: 10.1038/nrc2297. [DOI] [PubMed] [Google Scholar]
- 12.Deng J, Carlson N, Takeyama K, Dal Cin P, Shipp M, Letai A. BH3 profiling identifies three distinct classes of apoptotic blocks to predict response to ABT-737 and conventional chemotherapeutic agents. Cancer Cell. 2007;12:171–185. doi: 10.1016/j.ccr.2007.07.001. [DOI] [PubMed] [Google Scholar]
- 13.Del Gaizo Moore V, Brown JR, Certo M, Love TM, Novina CD, Letai A. Chronic lymphocytic leukemia requires BCL2 to sequester prodeath BIM, explaining sensitivity to BCL2 antagonist ABT-737. J Clin Invest. 2007;117:112–121. doi: 10.1172/JCI28281. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Schlagbauer-Wadl H, Klosner G, Heere-Ress E, Waltering S, Moll I, Wolff K, et al. Bcl-2 antisense oligonucleotides (G3139) inhibit Merkel cell carcinoma growth in SCID mice. J Invest Dermatol. 2000;114:725–730. doi: 10.1046/j.1523-1747.2000.00937.x. [DOI] [PubMed] [Google Scholar]
- 15.Ramanarayanan J, Hernandez-Ilizaliturri FJ, Chanan-Khan A, Czuczman MS. Pro-apoptotic therapy with the oligonucleotide Genasense (oblimersen sodium) targeting Bcl-2 protein expression enhances the biological anti-tumour activity of rituximab. Br J Haematol. 2004;127:519–530. doi: 10.1111/j.1365-2141.2004.05239.x. [DOI] [PubMed] [Google Scholar]
- 16.O’Brien SM, Cunningham CC, Golenkov AK, Turkina AG, Novick SC, Rai KR. Phase I to II multicenter study of oblimersen sodium, a Bcl-2 antisense oligonucleotide, in patients with advanced chronic lymphocytic leukemia. J Clin Oncol. 2005;23:7697–7702. doi: 10.1200/JCO.2005.02.4364. [DOI] [PubMed] [Google Scholar]
- 17.Badros AZ, Goloubeva O, Rapoport AP, Ratterree B, Gahres N, Meisenberg B, et al. Phase II study of G3139, a Bcl-2 antisense oligonucleotide, in combination with dexamethasone and thalidomide in relapsed multiple myeloma patients. J Clin Oncol. 2005;23:4089–4099. doi: 10.1200/JCO.2005.14.381. [DOI] [PubMed] [Google Scholar]
- 18.Bedikian AY, Millward M, Pehamberger H, Conry R, Gore M, Trefzer U, et al. Bcl-2 antisense (oblimersen sodium) plus dacarbazine in patients with advanced melanoma: the Oblimersen Melanoma Study Group. J Clin Oncol. 2006;24:4738–4745. doi: 10.1200/JCO.2006.06.0483. [DOI] [PubMed] [Google Scholar]
- 19.Chanan-Khan AA, Niesvizky R, Hohl RJ, Zimmerman TM, Christiansen NP, Schiller GJ, et al. Phase III randomised study of dexamethasone with or without oblimersen sodium for patients with advanced multiple myeloma. Leuk Lymphoma. 2009;50:559–565. doi: 10.1080/10428190902748971. [DOI] [PubMed] [Google Scholar]
- 20.O’Brien S, Moore JO, Boyd TE, Larratt LM, Skotnicki AB, Koziner B, et al. 5-year survival in patients with relapsed or refractory chronic lymphocytic leukemia in a randomized, phase III trial of fludarabine plus cyclophosphamide with or without oblimersen. J Clin Oncol. 2009;27:5208–5212. doi: 10.1200/JCO.2009.22.5748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Dai G, Chan KK, Liu S, Hoyt D, Whitman S, Klisovic M, et al. Cellular uptake and intracellular levels of the bcl-2 antisense g3139 in cultured cells and treated patients with acute myeloid leukemia. Clin Cancer Res. 2005;11:2998–3008. doi: 10.1158/1078-0432.CCR-04-1505. [DOI] [PubMed] [Google Scholar]
- 22.Nguyen M, Marcellus RC, Roulston A, Watson M, Serfass L, Murthy Madiraju SR, et al. Small molecule obatoclax (GX15-070) antagonizes MCL-1 and overcomes MCL-1-mediated resistance to apoptosis. Proc Natl Acad Sci U S A. 2007;104:19512–19517. doi: 10.1073/pnas.0709443104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Brown JR, Tesar B, Werner L, Mikler E, Reynolds H, Thompson C, et al. Obatoclax in combination with fludarabine and rituximab (FR) is well-tolerated and shows promising clinical activity in relapsed CLL/SLL [abstract]. Proceedings of the 53rd ASH Annual Meeting and Exposition; 2011 Dec 10–13; San Diego, CA. Washington (DC): American Society of Hematology; 2011. Abstract nr 2865. [Google Scholar]
- 24.Langer CJ, Albert I, Ross HJ, Kovacs P, Blakely LJ, Pajkos G, et al. Randomized phase II study of carboplatin and etoposide with or without obatoclax mesylate in extensive-stage small cell lung cancer. Lung Cancer Amst Neth. 2014;85:420–428. doi: 10.1016/j.lungcan.2014.05.003. [DOI] [PubMed] [Google Scholar]
- 25.Konopleva M, Watt J, Contractor R, Tsao T, Harris D, Estrov Z, et al. Mechanisms of antileukemic activity of the novel Bcl-2 homology domain-3 mimetic GX15-070 (obatoclax) Cancer Res. 2008;68:3413–3420. doi: 10.1158/0008-5472.CAN-07-1919. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Schimmer AD, O’Brien S, Kantarjian H, Brandwein J, Cheson BD, Minden MD, et al. A phase I study of the pan bcl-2 family inhibitor obatoclax mesylate in patients with advanced hematologic malignancies. Clin Cancer Res. 2008;14:8295–8301. doi: 10.1158/1078-0432.CCR-08-0999. [DOI] [PubMed] [Google Scholar]
- 27.McCoy F, Hurwitz J, McTavish N, Paul I, Barnes C, O’Hagan B, et al. Obatoclax induces Atg7-dependent autophagy independent of beclin-1 and BAX/BAK. Cell Death Dis. 2010;1:e108. doi: 10.1038/cddis.2010.86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Oltersdorf T, Elmore SW, Shoemaker AR, Armstrong RC, Augeri DJ, Belli BA, et al. An inhibitor of Bcl-2 family proteins induces regression of solid tumours. Nature. 2005;435:677–681. doi: 10.1038/nature03579. [DOI] [PubMed] [Google Scholar]
- 29.Tse C, Shoemaker AR, Adickes J, Anderson MG, Chen J, Jin S, et al. ABT-263: a potent and orally bioavailable Bcl-2 family inhibitor. Cancer Res. 2008;68:3421–3428. doi: 10.1158/0008-5472.CAN-07-5836. [DOI] [PubMed] [Google Scholar]
- 30.Souers AJ, Leverson JD, Boghaert ER, Ackler SL, Catron ND, Chen J, et al. ABT-199, a potent and selective BCL-2 inhibitor, achieves antitumor activity while sparing platelets. Nat Med. 2013;19:202–208. doi: 10.1038/nm.3048. [DOI] [PubMed] [Google Scholar]
- 31.Vogler M, Weber K, Dinsdale D, Schmitz I, Schulze-Osthoff K, Dyer MJS, et al. Different forms of cell death induced by putative BCL2 inhibitors. Cell Death Differ. 2009;16:1030–1039. doi: 10.1038/cdd.2009.48. [DOI] [PubMed] [Google Scholar]
- 32.Roberts AW, Seymour JF, Brown JR, Wierda WG, Kipps TJ, Khaw SL, et al. Substantial susceptibility of chronic lymphocytic leukemia to BCL2 inhibition: results of a phase I study of navitoclax in patients with relapsed or refractory disease. J Clin Oncol. 2012;30:488–496. doi: 10.1200/JCO.2011.34.7898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Rudin CM, Hann CL, Garon EB, Ribeiro de Oliveira M, Bonomi PD, Camidge DR, et al. Phase II study of single-agent navitoclax (ABT-263) and biomarker correlates in patients with relapsed small cell lung cancer. Clin Cancer Res. 2012;18:3163–3169. doi: 10.1158/1078-0432.CCR-11-3090. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Leverson JD, Phillips DC, Mitten MJ, Boghaert ER, Diaz D, Tahir SK, et al. Exploiting selective BCL-2 family inhibitors to dissect cell survival dependencies and define improved strategies for cancer therapy. Sci Transl Med. 2015;7:279ra40–279ra40. doi: 10.1126/scitranslmed.aaa4642. [DOI] [PubMed] [Google Scholar]
- 35.Schoenwaelder SM, Jarman KE, Gardiner EE, Hua M, Qiao J, White MJ, et al. Bcl-xL-inhibitory BH3 mimetics can induce a transient thrombocytopathy that undermines the hemostatic function of platelets. Blood. 2011;118:1663–1674. doi: 10.1182/blood-2011-04-347849. [DOI] [PubMed] [Google Scholar]
- 36.Davids MS, Letai A. ABT-199: taking dead aim at BCL-2. Cancer Cell. 2013;23:139–141. doi: 10.1016/j.ccr.2013.01.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Davids MS, Deng J, Wiestner A, Lannutti BJ, Wang L, Wu CJ, et al. Decreased mitochondrial apoptotic priming underlies stroma-mediated treatment resistance in chronic lymphocytic leukemia. Blood. 2012;120:3501–3509. doi: 10.1182/blood-2012-02-414060. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Seymour JF, Davids M, Pagel JM, Kahl BS, Weirda WG, Puvvada S, et al. ABT-199 (GDC-0199) in relapsed/refractory (R/R) chronic lymphocytic leukemia (CLL) and small lymphocytic lymphoma (SLL): High complete- response rate and durable disease control. J Clin Oncol. 2014;32:5s. (suppl abstr 7015). [Google Scholar]
- 39.Davids MS, Seymour JF, Gerecitano JF, Kahl BS, Pagel JM, Weirda WG, et al. Phase I study of ABT-199 (GDC-0199) in patients with relapsed/refractory (R/R) non-Hodgkin lymphoma (NHL): responses observed in diffuse large B-cell (DLBCL) and follicular lymphoma (FL) at higher cohort doses. J Clin Oncol. 2014;32:5s. (suppl; abstr 8522). [PubMed] [Google Scholar]
- 40.Croker BA, O’Donnell JA, Nowell CJ, Metcalf D, Dewson G, Campbell KJ, et al. Fas-mediated neutrophil apoptosis is accelerated by Bid, Bak, and Bax and inhibited by Bcl-2 and Mcl-1. Proc Natl Acad Sci U S A. 2011;108:13135–13140. doi: 10.1073/pnas.1110358108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Hallek M, Cheson BD, Catovsky D, Caligaris-Cappio F, Dighiero G, Döhner H, et al. Guidelines for the diagnosis and treatment of chronic lymphocytic leukemia: a report from the International Workshop on Chronic Lymphocytic Leukemia updating the National Cancer Institute-Working Group 1996 guidelines. Blood. 2008;111:5446–5456. doi: 10.1182/blood-2007-06-093906. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Roberts A, Ma S, Brander D, Kipps T, Barrientos J, Davids M, et al. Determination of recommended phase 2 dose of ABT-199 (GDC-0199) combined with rituximab (R) in patients with relapsed / refractory (R/R) chronic lymphocytic leukemia (CLL) [abstract]. Proceedings of the 56th ASH Annual Meeting and Exposition; 2014 Dec 6–9; San Francisco CA. Washington (DC): American Society of Hematology; 2014. Abstract nr 325. [Google Scholar]
- 43.Konopleva M, Contractor R, Tsao T, Samudio I, Ruvolo PP, Kitada S, et al. Mechanisms of apoptosis sensitivity and resistance to the BH3 mimetic ABT-737 in acute myeloid leukemia. Cancer Cell. 2006;10:375–388. doi: 10.1016/j.ccr.2006.10.006. [DOI] [PubMed] [Google Scholar]
- 44.Pan R, Hogdal LJ, Benito JM, Bucci D, Han L, Borthakur G, et al. Selective BCL-2 inhibition by ABT-199 causes on-target cell death in acute myeloid leukemia. Cancer Discov. 2014;4:362–375. doi: 10.1158/2159-8290.CD-13-0609. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Vo T-T, Ryan J, Carrasco R, Neuberg D, Rossi DJ, Stone RM, et al. Relative mitochondrial priming of myeloblasts and normal HSCs determines chemotherapeutic success in AML. Cell. 2012;151:344–355. doi: 10.1016/j.cell.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Konopleva M, Pollyea DA, Potluri J. Paper: A Phase 2 Study of ABT-199 (GDC-0199) in Patients with Acute Myelogenous Leukemia (AML) [abstract]. Proceedings of the 56th ASH Annual Meeting and Exposition; 2014 Dec 6–9; San Francisco, CA; Washington (DC): American Society of Hematology; 2014. Abstract nr 118. [Google Scholar]
- 47.Chonghaile TN, Roderick JE, Glenfield C, Ryan J, Sallan SE, Silverman LB, et al. Maturation stage of T-cell acute lymphoblastic leukemia determines BCL-2 versus BCL-XL dependence and sensitivity to ABT-199. Cancer Discov. 2014;4:1074–1087. doi: 10.1158/2159-8290.CD-14-0353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Anderson NM, Harrold I, Mansour MR, Sanda T, McKeown M, Nagykary N, et al. BCL2-specific inhibitor ABT-199 synergizes strongly with cytarabine against the early immature LOUCY cell line but not more-differentiated T-ALL cell lines. Leukemia. 2014;28:1145–1148. doi: 10.1038/leu.2013.377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Cao Y, Yang G, Hunter ZR, Liu X, Xu L, Chen J, et al. The BCL2 antagonist ABT-199 triggers apoptosis, and augments ibrutinib and idelalisib mediated cytotoxicity in CXCR4 Wild-type and CXCR4 WHIM mutated Waldenstrom macroglobulinaemia cells. Br J Haematol. 2015;170:134–138. doi: 10.1111/bjh.13278. [DOI] [PubMed] [Google Scholar]
- 50.Bodet L, Ménoret E, Descamps G, Pellat-Deceunynck C, Bataille R, Le Gouill S, et al. BH3-only protein Bik is involved in both apoptosis induction and sensitivity to oxidative stress in multiple myeloma. Br J Cancer. 2010;103:1808–1814. doi: 10.1038/sj.bjc.6605981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bodet L, Gomez-Bougie P, Touzeau C, Dousset C, Descamps G, Maïga S, et al. ABT-737 is highly effective against molecular subgroups of multiple myeloma. Blood. 2011;118:3901–3910. doi: 10.1182/blood-2010-11-317438. [DOI] [PubMed] [Google Scholar]
- 52.Touzeau C, Dousset C, Le Gouill S, Sampath D, Leverson JD, Souers AJ, et al. The Bcl-2 specific BH3 mimetic ABT-199: a promising targeted therapy for t(11;14) multiple myeloma. Leukemia. 2014;28:210–212. doi: 10.1038/leu.2013.216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Kumar SK, Vij R, Kaufman J, Mikhael J, Facon T, Moreau P, et al. Venetoclax (ABT-199/GDC-0199) monotherapy for relapsed/refractory multiple myeloma: phase I safety and efficacy [abstract]. Proceedings of the 21st Congress of the European Hematology Association; 2015 Jun 11–14; Vienna, Austria. The Hague, The Netherlands: European Hematology Association; 2015. Abstract nr P658. [Google Scholar]
- 54.Dawson S-J, Makretsov N, Blows FM, Driver KE, Provenzano E, Le Quesne J, et al. BCL2 in breast cancer: a favourable prognostic marker across molecular subtypes and independent of adjuvant therapy received. Br J Cancer. 2010;103:668–675. doi: 10.1038/sj.bjc.6605736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Vaillant F, Merino D, Lee L, Breslin K, Pal B, Ritchie ME, et al. Targeting BCL-2 with the BH3 mimetic ABT-199 in estrogen receptor-positive breast cancer. Cancer Cell. 2013;24:120–129. doi: 10.1016/j.ccr.2013.06.002. [DOI] [PubMed] [Google Scholar]
- 56.Oakes SR, Vaillant F, Lim E, Lee L, Breslin K, Feleppa F, et al. Sensitization of BCL-2-expressing breast tumors to chemotherapy by the BH3 mimetic ABT-737. Proc Natl Acad Sci U S A. 2012;109:2766–2771. doi: 10.1073/pnas.1104778108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Davids MS, Ding J, Ryan J, Fernandes S, Brown JR, Letai AG. Mitochondrial apoptotic priming is associated with clinical response to the Bcl-2 antagonist ABT-199 in chronic lymphocytic leukemia [abstract]. Proceedings of the 56th ASH Annual Meeting and Exposition; 2014 Dec 6–9; San Francisco, CA. Washington (DC): American Society of Hematology; 2014. Abstract nr 1940. [Google Scholar]
- 58.Hogdal LJ, Chyla BJ, McKeegan E, Leverson J, Potluri J, Falotico N, et al. BH3 profiling predicts clinical response in a phase II clinical trial of ABT-199 (GDC-0199) in acute myeloid leukemia [abstract]. Proceedings of the 106th Annual Meeting of the American Association for Cancer Research; 2015 Apr 18–22; Philadelphia, PA. Philadelphia (PA): AACR; 2015. Abstract nr 2834. [Google Scholar]
- 59.Chan SM, Thomas D, Corces-Zimmerman MR, Xavy S, Rastogi S, Hong W-J, et al. Isocitrate dehydrogenase 1 and 2 mutations induce BCL-2 dependence in acute myeloid leukemia. Nat Med. 2015;21:178–184. doi: 10.1038/nm.3788. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Leverson JD, Zhang H, Chen J, Tahir SK, Phillips DC, Xue J, et al. Potent and selective small-molecule MCL-1 inhibitors demonstrate on-target cancer cell killing activity as single agents and in combination with ABT-263 (navitoclax) Cell Death Dis. 2015;6:e1590. doi: 10.1038/cddis.2014.561. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Chen J, Jin S, Tapang P, Tahir SK, Smith M, Xue J, et al. CDK9 inhibition reverses resistance to ABT-199 (GDC-0199) by down-regulating MCL-1 [abstract]. Proceedings of the 56th ASH Annual Meeting and Exposition; 2014 Dec 6–9; San Francisco, CA. Washington (DC): American Society of Hematology; 2014. Abstract nr 2161. [Google Scholar]
- 62.Konopleva M, Milella M, Ruvolo P, Watts JC, Ricciardi MR, Korchin B, et al. MEK inhibition enhances ABT-737-induced leukemia cell apoptosis via prevention of ERK-activated MCL-1 induction and modulation of MCL-1/BIM complex. Leukemia. 2012;26:778–787. doi: 10.1038/leu.2011.287. [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 63.Rahmani M, Aust MM, Attkisson E, Williams DC, Ferreira-Gonzalez A, Grant S. Inhibition of Bcl-2 antiapoptotic members by obatoclax potently enhances sorafenib-induced apoptosis in human myeloid leukemia cells through a Bim-dependent process. Blood. 2012;119:6089–6098. doi: 10.1182/blood-2011-09-378141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Pan R, Ruvolo VR, Wei J, Konopleva M, Reed JC, Pellecchia M, et al. Inhibition of Mcl-1 with the pan–Bcl-2 family inhibitor (−)BI97D6 overcomes ABT-737 resistance in acute myeloid leukemia. Blood. 2015;126:363–372. doi: 10.1182/blood-2014-10-604975. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Fresquet V, Rieger M, Carolis C, García-Barchino MJ, Martinez-Climent JA. Acquired mutations in BCL2 family proteins conferring resistance to the BH3 mimetic ABT-199 in lymphoma. Blood. 2014;123:4111–4119. doi: 10.1182/blood-2014-03-560284. [DOI] [PubMed] [Google Scholar]
- 66.Sonpavde G, Matveev V, Burke JM, Caton JR, Fleming MT, Hutson TE, et al. Randomized phase II trial of docetaxel plus prednisone in combination with placebo or AT-101, an oral small molecule Bcl-2 family antagonist, as first-line therapy for metastatic castration-resistant prostate cancer. Ann Oncol. 2012;23:1803–1808. doi: 10.1093/annonc/mdr555. [DOI] [PubMed] [Google Scholar]
- 67.Bedikian AY, Garbe C, Conry R, Lebbe C, Grob JJ Genasense Melanoma Study Group. Dacarbazine with or without oblimersen (a Bcl-2 antisense oligonucleotide) in chemotherapy-naive patients with advanced melanoma and low-normal serum lactate dehydrogenase: “The AGENDA trial”. Melanoma Res. 2014;24:237–243. doi: 10.1097/CMR.0000000000000056. [DOI] [PubMed] [Google Scholar]
- 68.Chiappori A, Williams C, Northfelt DW, Adams JW, Malik S, Edelman MJ, et al. Obatoclax mesylate, a pan-bcl-2 inhibitor, in combination with docetaxel in a phase 1/2 trial in relapsed non-small-cell lung cancer. J Thorac Oncol. 2014;9:121–125. doi: 10.1097/JTO.0000000000000027. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Faber AC, Farago AF, Costa C, Dastur A, Gomez-Caraballo M, Robbins R, et al. Assessment of ABT-263 activity across a cancer cell line collection leads to a potent combination therapy for small-cell lung cancer. Proc Natl Acad Sci U S A. 2015;112:E1288–E1296. doi: 10.1073/pnas.1411848112. [DOI] [PMC free article] [PubMed] [Google Scholar]