Introduction
Attachment describes the tendency in a child to seek protective proximity in relationship to a caregiver [1]. Attachment theory states that maternal sensitivity to a child's needs – emotional attunement – likely influences organization of the child's brain [2]. Accordingly, evidence indicates that early maternal deprivation adversely affects the anatomy of White Matter (WM), specifically in reduced WM diffusivity in children with a history of orphanage rearing compared with controls [3]. In this way, neural changes attributable to early postnatal experience may have a legacy into later stages of life, extending or limiting the functional capacity of the brain in the balance of the lifespan [4]. By contrast, exposure to a socially rich environment is associated with changes in WM microstructure and improved social cognition [5]–[7] .
WM is composed of fibers (axons) which carry information between the neurons [8]. One important component of the WM microstructure is myelin, a dielectric membrane surrounding the axon that increases the velocity of transmitted information down the axon body [8]. Longitudinal studies point to increased myelination from childhood to adolescence [9], a period in the human lifespan during which the immature brain is sensitive to environmental stimulation. It follows that maternal stimulation could affect myelination of the child's developing brain and thereby facilitate neural connections among brain structures key to emotional and social information processing such as connection within structures in the link limbic system and between limbic system and distant brain regions. Accordingly. evidences indicates that decreased functional and structural connectivity of in the amygdala is linked with attachment avoidance [7], and increased WM integrity in multiple fiber pathways including cingulum and hypothalamic paths is associated with social network diversity in humans [6]. Moreover, a biobehavioural model proposed by Porges & Furman, (2011) suggest that increased myelination in vagal fibers improve neuro regulation of the autonomic nervous system supporting the infants to express appropriate social engagement behavior, in turn facilitating development of social skills. On this argument, a recent study by De Pisapia et al. (2014) reported that higher Fractional Anisotropy (FA), a product of myelination, was associated with greater social competence. FA is an index of directionality of diffusion, whose values change according to the structure of the axonal cell membranes and myelin sheath [11]. These findings informed the present study in that, according to attachment theory, social abilities are predicted by quality of attachment [1] which shape construction of the individual's subsequent relationships from childhood to adulthood [1]. However, no studies have yet explored associations between security in the maternal relationship and WM connectivity in healthy young adults. Except for Whittle et al. (2009) on adolescents’ changing gray matter volume in association with maternal behavior, our knowledge of effects of parenting in the normal range and neural development in the child remains limited.
Based on the extant literature, showing positive association between FA and social skills driven by increased myelination [5], [6] as well as decreased WM connectivity associated in with attachment avoidance [7] we hypothesized that efficient WM connectivity within and between limbic regionswould be associated with security in the maternal relationship experienced during childhood. We therefore expected to find positive associations between FA and attachment security in WM tracts previously associated with interpersonal competence by De Pisapia and colleagues (2014), namely: Uncinate Fasciculus (UF), Cingulum (CM), the Forceps Minor (FM), Infero-Fronto Occipital Fasciculus (IFOF), Inferior Longitudinal Fasciculus (ILF), and Superior Longitudinal Fasciculus (SLF). These same tracts have been reported to be associated with adverse childhood experiences [13], emotional processing and attachment avoidance [7], [14], and so they are likely also influenced by the quality of the person's attachment relationship history from childhood.
Method
Participants
Fifty-three healthy right handed young adults (31 males; M age = 23.56 years, SD = 2.68) took part to the study. No participant had any major internal, psychiatric or neurological disorder (including mood disorders), as assessed by a medical screening before the scanning sections. All the participant had at least a high school diploma. The study was approved by the University of Trento Ethical Committee, and all participants gave written informed consent. The procedure consisted in two parts. First participants underwent Diffusion Tensor Imaging (DTI) and then completed the Kerns Security Scale (SS) on the basis of the relationship they had with their mother during childhood.
Security Measure
The SS is a self-reported measure of perceived childhood attachment security. It is a well-established psychometrically valid measure used to evaluate attachment status after childhood [15]. Here, we asked participants to rate their maternal relationship by thinking back to when they were children. The test is structured as a 15-item questionnaire; the participants have to rate whether one of two statements is sort of true for them or really true for them. The two statements are presented as one item in a forced-choice format to minimize response bias due to perceptions of social desirability (e.g., “Some kids find it easy to trust their mom BUT other kids are not sure if they can trust their mom”). Items are averaged (α = .87), with higher scores indicating greater security with mothers. In initial testing performed by Kerns and colleagues (1996), scores on the SS showed adequate range (1.62-4.00) and internal consistency (α = .84). The sample mean was 3.24 (SD = 0.57). SS scores demonstrate high test-retest stability (r = .75, Median duration = 14 days). The validity of the SS as a measure of perceived attachment security in adolescence was assessed by Van Ryzin and Leve (2012). For the present study, we conducted a confirmatory factor analysis with Mplus (Muthén & Muthén, 2009) and modeled a single latent factor to obtain an SS score on a continuous dimension of security. Then, we used the FSCORE command within Mplus to obtain latent factor scores for subsequent analysis. The latent factor approach is superior to the calculation of mean scores as it adjusts for measurement error, increasing both power and measurement reliability (Kline, 2011).
Image Acquisition and DTI processing
DTI was used to acquire images of WM structures with a 4T Bruker Medspec scanner (Bruker Medical, Ettlingen, Germany) using a birdcage-transmit, eight-channel receive head coil (USA Instruments, Inc., Ohio, USA). The image acquisition protocol followed exactly De Pisapia et al. (2014). All diffusion weighted images were processed using tools from the FMRIB software library (FSL, version 4.1.5; http://www.fmrib.ox.ac.uk/fsl) running on a Linux operating system. First, DICOM files were converted to the nifti format, using the DICOM-to-nifti converter (by Chris Rorden, Delphi: http://www.mccauslandcenter.sc.edu/mricro/mricron/index.html). Then, each dataset was corrected for head movement and eddy current distortions using an affine transformation of each diffusion weighted image and b0 image to the first b0 image, used as reference. Next, a binary brain mask was generated from the non-diffusion weighted image using the BET brain extraction tool [16]. Diffusion tensor model was fitted independently for each voxel within the brain mask, and maps of Fractional Anisotropy (FA), Mean Diffusivity (MD), and Axial Diffusivity (AD) were generated for each participant. A Radial Diffusivity (RD) map was also generated averaging the second and the third eigenvalues (RD = (l2 + l3)/2). FA describes the degree of anisotropy of the water diffusion within a voxel and is a reliable index of micro-structural integrity of WM and so a measure of how strongly directional the local tract structure is. Its value ranges from 0 (minimum coherence in the WM structures) to 1 (maximum coherence in WM structures). MD is expected mainly to reflect tissue damage in pathological conditions. RD and AD are sub-components of FA and are related to myelination and axonal integrity, respectively [17].
DTI analysis
To inspect regional associations with the SS, we conducted a Region Of Interest (ROI) analysis based on the results in De Pisapia et al. (2014). We used Tract-Based Spatial Statistics (TBSS; Smith et al., 2006) to acquire the group level WM structure from which FA, MD, RD, and AD images were extracted. TBSS is a technique that aims to improve the sensitivity, objectivity, and interpretability of multi-subject diffusion imaging studies and has been proposed to reduce the problems related to possible misalignment of different subjects’ co-registered data through the use of an optimized non-linear registration followed by projection onto an alignment-invariant tract representation. TBSS allows for valid conclusions to be drawn from the subsequent voxelwise analysis (for details on the TBSS steps, see Andersson, Jenkinson, & Smith, 2007). Bilateral tracts ROIs were defined using the probabilistic Johns Hopkins University White Matter Atlas [20] provided by “fslview”. A threshold of 10 was applied at each tract, and the results binarized to obtain masks that were used to crop the group-wise WM skeleton generated by the TBSS. Finally, for each ROI mean FA, MD, RD, and AD was calculated and, as FA was not normally distributed, correlated using Spearman correlation coefficient against the SS scores. (Fig. 1) shows a graphical representation of the tracts included in the ROI.
Fig. 1.
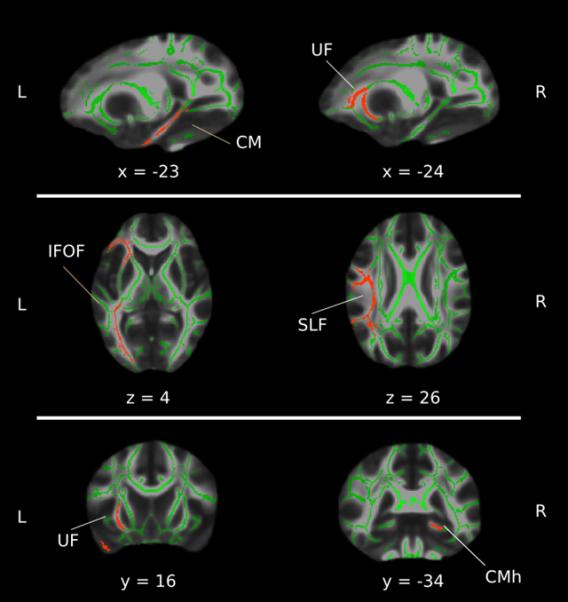
Graphical representation of the tracts included in the ROI analysis. From top to bottom: coronal, axial and sagittal views of the ROI, including CMh, UF, IFOF, SLF. The background image is the mean FA mask of the sample fitted in the MNI template. Shown in red are the tract included in the ROIs cropped on the FA skeleton map (in green). The image is reported in the neurological orientation, x, y, and z, coordinates are based on the MNI atlas.
Results
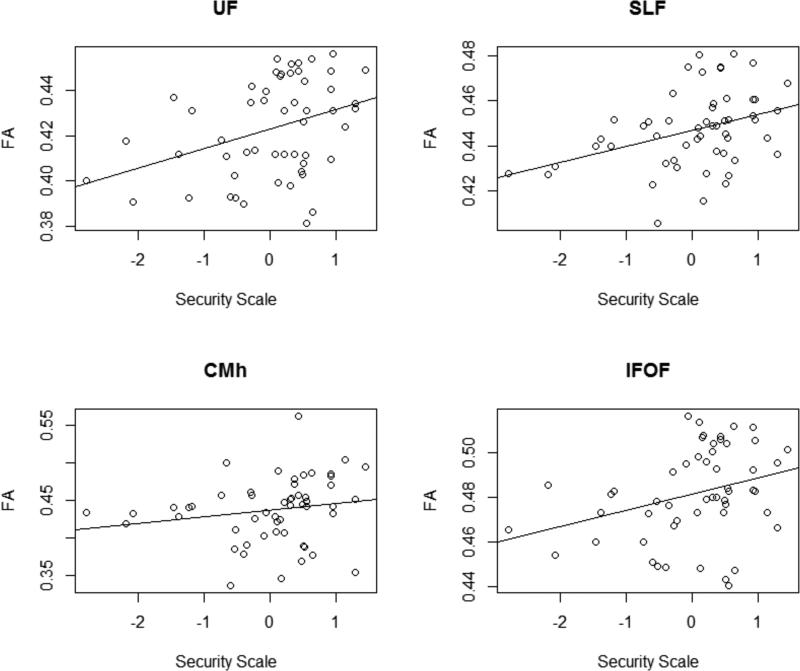
We found positive relations between FA and SS in four WM tracts of the left hemisphere: UF (ρ = .31 p = .02), IFOF (ρ = .30 p = .03), SLF (ρ = .37 p = .005), and the hippocampal region of Cingulum: CMh (ρ = .29 p = .03); (Fig. 2). In no tracts was MD correlated with SS. Additional analyses established the relative associations of AD and RD with FA. Negative correlations emerged between RD and SS in all tracts: UF (ρ = −.29 p = .03), IFOF (ρ = −.32 p = .02), SLF (ρ = −.30 p = .03), and CMh (ρ = −.30 p = .02). In no tracts was AD correlated with SS.
Fig. 2.
From left to right: scatter plot of the correlation between Fractional Anisotropy (FA) and the Security Scale in (a) Uncinate Fasciculus (UF) (ρ = .31 p = .02), Superior Longitudinal Fasciculus (SLF) (ρ = .37 p = .005), Cingulum (CMh) (ρ = .29 p = .03) and Inferior Fronto Occipital Fasciculus (IFOF) (ρ = .30 p = .03). All the tracts belong to the left hemisphere.
Discussion
The present study was designed to investigate associations between security in the young adult's maternal attachment relationship and WM structural connectivity in the young adult's brain. We found correlations between WM integrity and security in the maternal relationship in four WM association fibers: CM, UF, IFOF, and SLF.
CM and UF are components of the limbic system. CM conveys communication between structures in the limbic system and facilitates prefrontal, parietal, and temporal interactions [8] and is known for its function in human emotion processing, memory, and language. The UF connects components of the limbic system (amygdala, hippocampus) with frontal regions. UF fibers have been associated with processes related to emotional and social cognition, and their disruption has been linked with specific pathologies. In particular, the CM is related to apathy [14] and cognitive control [21]. Underconnectivity in the UF is associated with autism spectrum disorder [22]. Notably, a recent meta-analysis revealed a significant FA reduction in the left UF in autistic compared to typically developing individuals [22]. IFOF and SLF are large bundles of fibers interconnecting brain regions involved in high-level cognitive functions [8]. The SLF connects frontal, parietal, and temporal lobes [8], and its disruption is also linked to autism [22]. The IFOF connects the frontal lobe with parietal and temporal lobes, it intermingles with UF (Mori et al., 2008), and its functions include integration of visual and auditory cortex with prefrontal cortex.
Our findings linking these specific WM fibers to maternal security extend those of De Pisapia et al. (2014), who reported an association between the same tracts and interpersonal competence in young adults. Moreover, our findings are consistent with the results of Govndan and colleagues (2010), who observed reduced integrity of UF and SLF in children with a history of early social deprivation. Together, these findings support a tenet of attachment theory which states that the quality of the mother-infant relationship affects the construction of children's socioemotional abilities and future adult relationships [1].
In the present work, the positive correlation between FA and maternal security was driven by a negative correlation between RD and the SS. Like FA, RD is generally linked to myelination and axonal packing, whereas AD can vary with fiber diameter and axon coherence [16]. This result is consistent with previous longitudinal studies in which increases of FA, paired with reductions of RD and AD remaining constant, have been interpreted in terms of an increase in myelination from childhood into adolescence and young adulthood [9].
In contrast with De Pisapia and colleagues (2014) and with Schore's (2000) hypothesis regarding the role of the right hemisphere (RH) in affect regulation, we found that no tract in the RH correlated with attachment security. The RH has previously been associated with emotional and social cognition [23] and likely plays a role in attachment formation. However, findings regarding a right-left hemisphere distinction in emotional cognition are actually mixed in the literature, bringing this strict dichotomy into question (see Abbott, Cumming, Fidler, & Lindell, 2013). Moreover, attachment measured in childhood, as well as interpersonal competence measured in young adults, refer to the ongoing situation of the individual; in contrast, here we asked young adults to rate the relationship they had with their mother during childhood. This task likely involves cognitive processes of self-referential thinking and retrieval of autobiographical memory, both of which typically recruit left hemisphere involvement [25]. However, evidence indicates that the left hemisphere might also be involved in the formation of internal working models. For example, Benetti et al. (2010) found that high attachment-related anxiety in healthy adults was associated with decreased gray matter volume in the right anterior temporal pole and increased gray matter in the left lateral orbital gyrus [13]. This result supports the idea that the attachment relationship might affect the development of brain structures involved in social and emotional processing and that this influence is not limited to the right hemisphere.
The cross-sectional nature of the present study only indicates a correlation between a psychological measure (attachment) and a biological measure (structural connectivity of the brain) and thus does not establish any causal relation. Given the complexity of the parent-child relationship, and the number of different factors that play important roles in relationship development and expression, further assessment of the impact of attachment on brain development requires additional study. That said, this study constitutes an important first step in attempts to find experimental evidence for regulatory theories of attachment.
This research also contributes to the neuroscience of mother-child interaction. Our aim was to investigate possible relations between attachment relationships and brain connectivity in young adults, demonstrating the feasibility of applying neuroimaging methods – in this case DTI - to the field of social developmental neuroscience. This approach affords clinicians novel tools to couple clinical investigations with neuroanatomical information and thereby overcome limitations of sole reliance on self-reports.
Acknowledgements
This research was supported by the University of Trento and the Intramural Research Program of the NIH, NICHD.
Footnotes
The authors whose names are listed above certify that they have NO affiliations with or involvement in any organization or entity with any financial interest (such as honoraria; educational grants; participation in speakers’ bureaus; membership, employment, consultancies, stock ownership, or other equity interest; and expert testimony or patent-licensing arrangements), or non-financial interest (such as personal or professional relationships, affiliations, knowledge or beliefs) in the subject matter or materials discussed in this manuscript.
References
- 1.Bowlby J. Attachment and Loss. New York: Basic Book. 1969 [Google Scholar]
- 2.Bornstein MH. The infant mind: Origins of the social brain. Guilford Press; 2013. Mother-infant attunement: A multilevel approach via body, brain, and behavior. pp. 266–298. [Google Scholar]
- 3.Govindan RM, Behen ME, Helder E, Makki MI, Chugani HT. Altered water diffusivity in cortical association tracts in children with early deprivation identified with tract-based spatial statistics (TBSS) Cereb. Cortex. 2010;20(3):561–569. doi: 10.1093/cercor/bhp122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Bornstein MH. Fiske S, editor. Human Infancy ... and the Rest of the Lifespan. annual review of psychology. 2014;65:121–158. doi: 10.1146/annurev-psych-120710-100359. 65. ANNUAL REVIEWS. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.De Pisapia N, Serra M, Rigo P, Jager J, Papinutto N, Esposito G, Venuti P, Bornstein MH. Interpersonal competence in young adulthood and right laterality in white matter. J. Cogn. Neurosci. 2014 Jun;26(6):1257–65. doi: 10.1162/jocn_a_00534. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Molesworth T, Sheu L, Cohen S, Gianaros P, Verstynen T. Social network diversity predicts white matter microstructural integrity in humans. Psychosom. Med. 76(3):A–9. 2014. doi: 10.1093/scan/nsv001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Rigon A, Duff MC, Voss MW. Structural and functional neural correlates of self-reported attachment in healthy adults: evidence for an amygdalar involvement. Brain Imaging Behav. 2015 Sep; doi: 10.1007/s11682-015-9446-9. [DOI] [PubMed] [Google Scholar]
- 8.Mori S, Oishi K, Jiang H, Jiang L, Li X, Akhter K, Hua K, Faria AV, Mahmood A, Woods R, Toga AW, Pike GB, Neto PR, Evans A, Zhang J, Huang H, Miller MI, van Zijl P, Mazziotta J. Stereotaxic white matter atlas based on diffusion tensor imaging in an ICBM template. Neuroimage. 2008;40(2):570–582. doi: 10.1016/j.neuroimage.2007.12.035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Lebel C, Beaulieu C. Longitudinal development of human brain wiring continues from childhood into adulthood. J. Neurosci. 2011;31(30):10937–10947. doi: 10.1523/JNEUROSCI.5302-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Porges SW, Furman SA. The early development of the autonomic nervous system provides a neural platform for social behaviour: A polyvagal perspective. Infant Child Dev. 2011;20(1):106–118. doi: 10.1002/icd.688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Pierpaoli C, Basser PJ. Toward a quantitative assessment of diffusion anisotropy. Magn Reson Med. 1996;36(6):893–906. doi: 10.1002/mrm.1910360612. [DOI] [PubMed] [Google Scholar]
- 12.Whittle S, Yap MBH, Yucel M, Sheeber L, Simmons JG, Pantelis C, Allen NB. Maternal responses to adolescent positive affect are associated with adolescents’ reward neuroanatomy. Soc. Cogn. Affect. Neurosci. 2009;4(3):247–256. doi: 10.1093/scan/nsp012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Benedetti F, Bollettini I, Radaelli D, Poletti S, Locatelli C, Falini A, Smeraldi E, Colombo C. Adverse childhood experiences influence white matter microstructure in patients with bipolar disorder. Psychol. Med. 2014:1–14. doi: 10.1017/S0033291714000506. [DOI] [PubMed] [Google Scholar]
- 14.Woolley SC, Zhang Y, Schuff N, Weiner MW, Katz JS. Neuroanatomical correlates of apathy in ALS using 4 Tesla diffusion tensor MRI. Amyotroph. Lateral Scler. 2011;12(1):52–58. doi: 10.3109/17482968.2010.521842. [DOI] [PubMed] [Google Scholar]
- 15.Main M, Kaplan N, Cassidy J. Security in infancy; childhood, and adulthood: A move to the level of representation. Monographs of the Society for Research in Child Development. 1985;50(1–2):66–104. [Google Scholar]
- 16.Song SK, Ramsbottom SSW,MJ, Chang C, Russell J, Cross AH. Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. Neuroimage. 2002;17(3):1429–1436. doi: 10.1006/nimg.2002.1267. [DOI] [PubMed] [Google Scholar]
- 17.Beaulieu C. The basis of anisotropic water diffusion in the nervous system - a technical review. NMR Biomed. 2002;15(7–8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 18.Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TEJ. Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage. 2006 Jul;31(4):1487–1505. doi: 10.1016/j.neuroimage.2006.02.024. [DOI] [PubMed] [Google Scholar]
- 19.Andersson JL, Jenkinson M, Smith S. Non-linear registration aka Spatial normalisation FMRIB Technial Report TR07JA2. 2007 [Google Scholar]
- 20.Hua K, Zhang J, Wakana S, Jiang H, Li X, Reich DS, Calabresi PA, Pekar JJ, van Zijl PCM, Mori S. Tract probability maps in stereotaxic spaces: Analyses of white matter anatomy and tract-specific quantification. Neuroimage. 2008;39(1):336–347. doi: 10.1016/j.neuroimage.2007.07.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Metzler-Baddeley C, Jones D, Steventon J, Westacott L, Aggleton JP, O’Sullivan MJ. Cingulum microstructure predicts cognitive control in older age and mild cognitive impairment. J. Neurosci. 2012 Dec;32(49):17612–9. doi: 10.1523/JNEUROSCI.3299-12.2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Aoki Y, Abe O, Nippashi Y, Yamasue H. Comparison of white matter integrity between autism spectrum disorder subjects and typically developing individuals: a meta-analysis of diffusion tensor imaging tractography studies. Mol. Autism. 2013;4(1):25. doi: 10.1186/2040-2392-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Spence S, Shapiro D, Zaidel E. The role of the right hemisphere in the physological and cognitive components of emotional processing. Psychophysiology. 1996;33(2):112–122. doi: 10.1111/j.1469-8986.1996.tb02115.x. [DOI] [PubMed] [Google Scholar]
- 24.Abbott JD, Cumming G, Fidler F, Lindell AK. The perception of positive and negative facial expressions in unilateral brain-damaged patients: A meta-analysis. Laterality. 2013;18(4):437–59. doi: 10.1080/1357650X.2012.703206. [DOI] [PubMed] [Google Scholar]
- 25.Maguire EA. Neuroimaging studies of autobiographical event memory. Philos. Trans. R. Soc. Lond. B. Biol. Sci. 2001;356(1413):1441–1451. doi: 10.1098/rstb.2001.0944. [DOI] [PMC free article] [PubMed] [Google Scholar]