Abstract
Classical conditioning can profoundly modify subsequent pain responses, but the mechanisms driving this effect are unresolved. Pain-conditioning studies typically condition cues to primary aversive reinforcers; hence, subsequent pain modulation could reflect learned pre-cognitive associations and/or expectancies that are conceptual in nature. We isolated conceptual contributions using a thermal pain-conditioning procedure in which different cues (CShigh and CSlow) were repeatedly paired with symbolic representations of high and low noxious heat, respectively. In a subsequent test phase, identical noxious stimuli evoked larger skin-conductance responses (SCRs) and pain ratings when preceded by CShigh than CSlow cues. These effects were mediated by participants’ self-reported expectancies. CShigh cues also evoked larger anticipatory SCRs, but larger anticipatory SCRs predicted smaller subsequent heat-evoked SCRs. These results provide novel evidence that conditioned modulation of pain physiology can be acquired through purely conceptual processes, and that self-reported expectancies and physiological threat responses have opposing effects on pain.
Keywords: classical conditioning, pain modulation, expectancy, skin conductance response, multilevel-mediation analysis
Introduction
Our prior experiences strongly affect how we perceive and respond to stimuli and events (e.g.,(Greene, Botros, Beck, & Fei-Fei, 2015)). Among the many varieties of percepts and judgments influenced by past experience, effects on pain have particularly important clinical and translational implications. Predicting and avoiding pain shapes our behavior in profound ways, and pain is the major reason people seek medical care across disorders. Ratings of pain experience are a primary outcome in clinical trials, and they form the basis for medical diagnosis and treatment decisions (Dworkin et al., 2003; Levy, 1996).
One of the most powerful ways to influence pain is classical conditioning. A vast animal literature on conditioning induces learning by pairing cues (conditioned stimuli, CSs) with primary aversive or appetitive outcomes (unconditioned stimuli, UCSs; e.g., shocks or food). Whereas many studies are interested primarily in behavioral and physiological responses to the cues themselves, pain-conditioning studies have demonstrated robust effects of cues on responses to noxious stimuli in both animals and humans (for reviews, see (Atlas & Wager, 2013; Fanselow, 1986)). However, the nature of the learning that underlies pain modulation—and, specifically, the degree to which conceptual thoughts and expectations are involved—is not well understood.
Whether one is studying conditioned responses to cues or their effects on subsequent pain, the learning induced by conditioning is often assumed to be pre-cognitive, i.e., to involveplasticity in neural circuits independent of expectations and other forms of conceptual thought, and not open to introspection. Consistent with this idea, some types of conditioned autonomic and behavioral responses, including analgesia, can be acquired in decerebrate animals and humans (Berntson & Micco, 1976; Berntson, Tuber, Ronca, & Bachman, 1983) or in the isolated spinal cord (Grau, Salinas, Illich, & Meagher, 1990), suggesting that they rely on associative learning in subcortical systems. In addition, conditioned responses are often mediated by synaptic plasticity in specific pathways (Antonov, Antonova, Kandel, & Hawkins, 2001; Glanzman, 1995; Hawkins, Abrams, Carew, & Kandel, 1983; Johansen, Cain, Ostroff, & LeDoux, 2011).
Human pain modulation also appears to be highly dependent on the pairing of cues and reinforcers, suggesting that pre-cognitive learning may be critical. The paradigm that produces the most reliable effects on human pain involves two phases: In the learning phase, high- and low-pain cues are repeatedly reinforced with high- and low-intensity noxious stimuli, respectively. In the subsequent test phase, high- and low-pain cues are followed by identical noxious stimuli, allowing a test of cue effects on pain. Typically, pain responses in the test phase are larger following high- than low-pain cues (Atlas, Bolger, Lindquist, & Wager, 2010; Atlas & Wager, 2012; Colloca, Petrovic, Wager, Ingvar, & Benedetti, 2010; Voudouris, Peck, & Coleman, 1990). In contrast, verbal suggestion alone does typically not produce robust pain-modulation effects (Colloca et al., 2010; Colloca et al., 2008; de Jong, van Baast, Arntz, & Merckelbach, 1996; Montgomery & Kirsch, 1997; Voudouris et al., 1990), suggesting that pain modulation may require experience-based, pre-cognitive learning.
However, there is another part of the story on conditioned pain modulation that suggests the opposite: That pre-cognitive associations are not enough, and cognitive expectations are required. Formal mediation analyses have found that conditioned-cue effects on reported pain are mediated by participants’ self-reported expectancies (Montgomery & Kirsch, 1997). In addition, conditioning does not typically produce pain modulation when people are not aware of the cue-pain associations (Carlino et al., 2015; Montgomery & Kirsch, 1997; Watson, El-Deredy, Bentley, Vogt, & Jones, 2006). These findings suggest that conditioned modulation of pain is mediated by conceptual representations, i.e., by conscious, reportable pain expectancies.
One way these apparently discrepant findings may be reconciled is that both conditioning and conceptual processes are required, but neither alone is sufficient to modulate pain. Previous studies have not provided clear tests of this hypothesis: Because virtually all pain-conditioning studies use primary aversive stimuli as UCSs, pre-cognitive and conceptual effects are inherently confounded. Conditioning may induce both pre-cognitive synaptic plasticity and conceptual expectancies in parallel (Kirsch, 2004; Montgomery & Kirsch, 1997). A few paradigms do not appear to involve primary reinforcers, such as ‘social-conditioning’ studies that use representations of other individuals’ pain as UCSs (Colloca & Benedetti, 2009; Vogtle, Barke, & Kroner-Herwig, 2013). However, pain expressions in others may serve as primary reinforcers, as they are affective, arousing stimuli in their own right; hence, the degree to which they are purely conceptual reinforcers is unclear. Thus, what is actually learned during pain conditioning, and which types of learning processes (i.e., pre-cognitive vs. conceptual) mediate conditioned modulation of pain, are critical open questions.
In this study, we used two approaches to dissociate the roles of pre-cognitive associations and conceptual representations in conditioned modulation of pain. First, we asked if conditioned-cue effects on heat-pain responses could be obtained without any differential primary reinforcement of high- and low-pain cues. Such pain modulation would necessarily be conceptual in nature. To this end, we developed a ‘symbolic-conditioning’ paradigm, in which the reinforcers that served as UCSs during learning are only linked with pain via conceptual processing. Visual cues (CShigh and CSlow) were repeatedly paired with pictures of thermometers displaying high or low temperatures, respectively. During a subsequent test phase, the CShigh and CSlow were followed by identical noxious heat stimuli (i.e., the cues were never differentially reinforced with actual pain), and we examined the cues’ effects on physiological pain responses (heat-evoked skin conductance responses; SCRs) and reported pain. If conditioned modulation of pain is driven by pre-cognitive associations, symbolic conditioning should not affect pain responses during the test phase. If it depends on conceptual representations, however, symbolic conditioning may affect subsequent pain responses. Our second approach was analytic: We used multilevel-mediation analyses to examine whether self-reported expectancies formally mediate conditioned-cue effects on heat-evoked SCRs. If so, this would show that expectancies not only mediate conditioning effects on reported pain (Montgomery & Kirsch, 1997), but also on pain physiology, which is less likely to be affected by demand characteristics (Orne, 1962) and decision biases.
A second aim of our study was to compare the effects of self-reported expectancy with the effects of an autonomic preparatory response, namely anticipatory (cue-evoked) SCR. Dissociating the effects of these two types of anticipatory responses on subsequent pain may shed light on a seemingly inconsistent finding in human and animal pain-conditioning studies. As mentioned above, human participants typically show stronger pain responses following high- than low-pain cues. In contrast, in rodent studies, pain-predictive conditioned cues typically reduce the animal's pain response (conditioned analgesia; (Chance, White, Krynock, & Rosecrans, 1977)), which has been attributed to fear- or threat-induced analgesia (Fanselow, 1986). One possible explanation for this apparent discrepancy is that physiological threat responses suppress pain through an automatic compensatory process, whereas expectancies have the opposite effect (i.e., increased pain with increasing expected pain). These two effects may dominate in animal and human studies, respectively, leading to the apparent discrepancy. To examine this hypothesis, we measured trial-to-trial variation in participants’ self-reported pain expectancy and anticipatory SCR amplitude, and tested whether—and if so, in which direction—each of these anticipatory responses mediated conditioned-cue effects on heat-evoked SCRs and pain ratings.
Methods
Participants
We tested 30 healthy participants with no history of psychiatric, neurological, or pain disorders, and no current pain. All participants gave written informed consent and received $12 per hour for their participation. The experiment was approved by the Institutional Review Board of the University of Colorado Boulder. We chose the sample size (N=30) based on large to very large effect sizes in previous heat-pain conditioning studies with primary reinforcers conducted in our lab (e.g., (Atlas et al., 2010; Schafer, Colloca, & Wager, 2015)). Sample sizes of ~30 provide approximately 80% power to detect an effect size of Cohen's d = 0.54 or larger, an effect size near the lower bound of what we would expect under the hypothesis that conditioning effects on pain are mediated by conceptual representations.
One participant decided to stop before the end of the test phase because she found the heat stimuli too painful. In addition, one participant had to be excluded because of thermode failure. Finally, we excluded the data from two other participants who did not show SCRs to the heat stimuli (mean SCR amplitude < .05 μS). Thus, the final data set was based on twenty-six participants (mean age = 25, range = 18-55 years; 18 males).
General procedure
Participants first completed a calibration procedure to ensure that they were not abnormally sensitive or insensitive to thermal stimuli. During the calibration, we delivered three thermal stimuli (peak temperature = 45-50°C; ramp rate = 40°C/s; 1.0 s at peak temperature) to each of five sites on the volar surface of participants’ left inner forearm, using a Contact Heat-Evoked Potential Stimulator (CHEPS; 27-mm diameter Peltier thermode; Medoc Ltd., Israel). Following each stimulus, participants rated their experienced pain on a 100-unit visual analog scale (VAS) with anchors of “no pain” and “worst-imaginable pain”, respectively, using a mouse. Participants then performed the conditioning phase followed by the test phase, which are described in detail below.
Conditioning phase
At the beginning of the conditioning phase, we instructed participants that they would learn associations between specific shapes and specific heat levels. We also told them that the heat levels would be displayed on a thermometer during the first part of the experiment (the conditioning phase), and applied to their forearm during the second part of the experiment (the test phase).
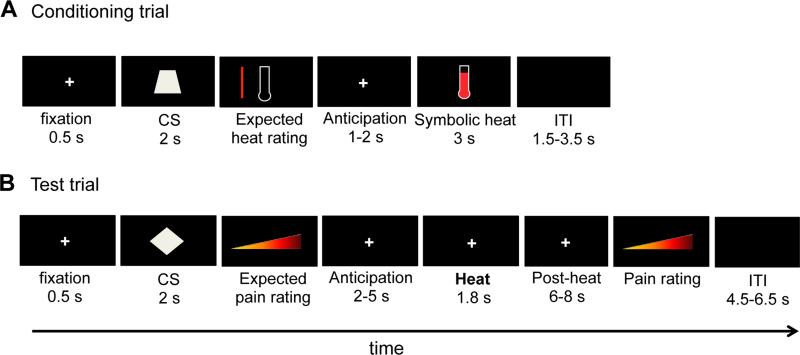
Each conditioning trial started with a 500-ms fixation screen, after which one of six geometric shapes (the CS; a square, parallelogram, diamond, triangle, trapezoid, or pentagon) appeared at the center of the screen for 2 seconds. Following the CS, participants indicated which heat level they expected on a 100-unit vertical VAS with lower and upper anchors of “baseline skin temperature” and “extremely hot”, respectively, which was displayed alongside a picture of a blank thermometer. One or 2 seconds later, the thermometer indicated a specific heat level (the UCS) for 3 s (Fig. 1A). Three of the CSs (the CShigh) were always followed by a thermometer indicating a high heat level, and the other three CSs (the CSlow) were always followed by a thermometer indicating a low heat level. Within both the ‘high’ and the ‘low’ heat category we used five different heat levels, in random order; hence the specific heat outcomes varied across trials (the average heat levels following each CSlow and CShigh were 37.0% and 81.6% of the thermometer scale, respectively). Then, after an inter-trial interval ranging from 1.5 to 3.5 s, during which an empty screen was displayed, the next trial started. Participants completed 120 conditioning trials (20 trials per CS, in random order), and could take a short break after every 30 trials.
Figure 1.
Experimental design. A. One conditioning trial. Note that a 1-second instruction screen (“HIGH” or “LOW) preceded the CS in one third of the conditioning trials (see Methods and Supplementary Material). B. One test trial.
To examine potential interactions between the effects of conceptual conditioning and the level of explicit information about cue meaning, we combined each CShigh and CSlow cue with either (i) no instructions; (ii) one-time valid instructions, provided before the start of the conditioning phase, about the level associated with the CS; or (iii) valid instructions about the upcoming heat level during each conditioning trial, through the presentation of the word “LOW” or “HIGH” for 1 s immediately before CS onset. Each participant completed 40 trials (20 CShigh and 20 CSlow trials) of each of the three instruction conditions, in random order. The allocation of the geometric shapes (CSs) to the different conditions was counterbalanced across participants. To foreshadow the results, this instruction manipulation did not substantially affect the effects of CS type (CShigh vs. CSlow) on any of the dependent variables in the test phase. Therefore, we will report test-phase results based on data pooled across the three instruction conditions, and report the slight variations that were found as a function of instruction condition in the Supplementary Material.
Test phase
Each test trial started with a 500-ms fixation screen. Then, one of the CSs from the preceding conditioning phase, or the word LOW or HIGH, was presented for 2 seconds. After this, participants indicated how much pain they expected on that trial, on a horizontal 100-unit VAS with anchors of “no pain” and “worst-imaginable pain”. Two to 5 seconds later, a CHEPS stimulus (peak temperature = 47 or 48°C; ramp rate = 40°C/s; 1.0 s at peak temperature) was delivered to the volar surface of the left inner forearm. Between stimuli, the thermode maintained a baseline temperature of 32°C. Six to 8 seconds after heat offset, the visual analog scale re-appeared on the screen and participants rated how much pain they had experienced on that trial. Then, after an inter-trial interval ranging from 4.5 to 6.5 s, during which an empty screen was displayed, the next trial started (Fig. 1B).
Unbeknownst to the participants, the temperature of all heat stimuli was either 47 or 48°C, on half of the trials each, and did not differ as a function of the preceding cue. Participants completed 10 trials for each of the CSs/words, in random order (i.e., 80 test trials in total). After every 16 trials, we moved the thermode to a new site on the participant's forearm. To reduce the impact of site-specific habituation effects (Jepma, Jones, & Wager, 2014), we administered one initial 48°C stimulus to each skin site before starting the first trial on that site.
Skin conductance response (SCR)
We recorded participants’ electrodermal activity throughout the experiment using 11 mm Ag/AgCl electrodes (Biopac Systems, Goleta CA, USA) attached to the medial phalanges of the left index and middle finger. The hand with the SCR electrodes attached rested on a table and participants were instructed to sit still and not to move their left hand during the task. Data were sampled at 500 Hz.
In offline analyses, we applied a 5-Hz low-pass filter to the skin-conductance signal, and down-sampled the signal to 50 Hz. We calculated the CS-evoked, thermometer-evoked and heat-evoked SCRs by subtracting the mean signal during the 2 s before stimulus onset from the time course of the signal during the 8 or 10 s following stimulus onset (10 s for the contact heat stimuli, 8 s for the visual stimuli). We defined trial-specific peak SCR amplitudes as the maximum value of the SCR signal during the 0-8 s interval following CS or thermometer onset, and as the maximum value during the 4-10 s interval following contact-heat onset.
In the main analyses we report, we included the data from all trials in the analyses, to prevent introducing a sampling bias by selectively removing the most extreme values. However, to check for extreme values, we examined the distributions of each participant's single-trial SCR amplitudes, and defined potential “outliers” as values that fall more than 3 standard deviations from the mean (on average 1.7 CS-evoked SCR and 1.1 heat-evoked SCR). To examine whether any observed effects may have been driven by these extreme SCR values, we repeated our mediation analyses while excluding these trials (see Mediation results section).
Statistical analyses
We conducted multi-level regression and mediation analyses on the single-trial behavioral data and SCR amplitudes, using the Multilevel Mediation toolbox (http://wagerlab.colorado.edu/tools (Atlas et al., 2010)).
Regression analyses conditioning phase
To examine explicit learning of the CS-heat associations during the conditioning phase, we modeled the effects of CS type (CShigh vs. CSlow) and the CS type × trial interactions (using both the linear and quadratic effects of CS-specific trial) on expected-heat ratings. We also contrasted the CS type × trial interaction effects for the no-instruction condition vs. the two instruction conditions, and for the one-time instruction vs. the per-trial instruction condition. To test for the acquisition of conditioned SCRs to the CSs, we repeated the above-described analysis on CS-evoked SCR amplitudes. In this analysis, we also accounted for potential adaptation of SCRs over time by modeling the linear and quadratic effects of trial, and we tested whether was a main effect of instruction condition. Finally, we tested the effects of symbolic heat level (high or low), the linear and quadratic effects of trial, and the heat level × trial interactions on participants’ thermometer-evoked SCR amplitudes.
Regression analyses test phase
To test for conditioning effects on heat-evoked SCRs and pain ratings during the test phase, we modeled the effects of stimulus temperature, CS type (CShigh vs. CSlow), the temperature × CS type interaction, and the CS type × instruction interactions. To test whether CS effects changed over time (e.g., due to extinction learning), we also modeled the CS type x trial interactions. To account for potential site-specific and/or site-nonspecific pain adaptation effects (Jepma et al., 2014), we also modeled the linear and quadratic effects of both site-specific and site-nonspecific repetition.
After running the regression models including all above-mentioned regressors, we excluded the regressors that did not predict the dependent variable (p's > 0.10). We report the results from these reduced models; these are very similar to those from the initial full models.
Multilevel-mediation analyses test phase
We next tested whether CS effects on pain responses in the test phase were formally mediated by participants’ CS-evoked SCRs and/or pain-expectancy ratings, using multilevel-mediation analyses. Our mediation analyses tested whether (i) there was an effect of CS type (CShigh vs. CSlow) on CS-evoked SCR amplitude and pain-expectancy ratings (paths a); (ii) CS-evoked SCRs and pain-expectancy ratings were predictive of heat-evoked SCRs and pain ratings, when controlling for CS type (paths b); and (iii) the relationship between CS type and heat-evoked SCRs/pain ratings (path c) decreased when controlling for CS-evoked SCR or pain-expectancy ratings (c-c’, equivalent to a*b + cov(a,b)). This last test is a test of mediation. We controlled for stimulus temperature and for the linear and quadratic effects of site-specific and site-nonspecific repetition, by including these effects as covariates in the mediation models. We tested the significance of all effects using a bootstrap procedure (100,000 bootstrap samples). Bootstrapping does not require the assumptions of normality or equality of variance for valid inference, which is a primary motivation for its widespread use in mediation tests.
Results
Conditioning phase
Participants gradually learned the heat levels predicted by each CShigh and CSlow cue during the first ~7 trials of each cue, as expected (Figure S1A in the Supplementary Material). When explicit instructions about cue meaning were provided in addition to the conditioning process, participants immediately tracked the heat levels predicted by CShigh and CSlow cues, also as expected (Figure S1A in the Supplementary Material). These effects were reflected in significant effects of CS type, CS type × trial interactions, and CS type × trial × [No-instruction vs. Instruction] interactions (all p's < .001; see Table S1 in the Supplementary Material for the statistics and effect sizes of all significant predictors).
The CSs evoked small SCRs (~5-10× smaller than those evoked by noxious heat stimuli during the subsequent test phase) that did not differ across CShigh and CSlow cues (p = .228). The CSs that had been paired with explicit instructions about their associated heat levels evoked smaller SCRs than the CSs for which no prior information had been provided (p = .015; Figure S1B and Table S1 in the Supplementary Material), possibly reflecting enhanced cognitive processing of the CSs whose associated heat levels could only be learnt from experience. There were no CS type × Trial (Figure S2 in the Supplementary Material) or CS type x Instruction interactions (all p's > .250), indicating that SCRs to the CShigh and CSlow cues did not differ either early or late in learning, in any of the instruction conditions.
The thermometer pictures also evoked small SCRs that did not differ as a function of displayed heat level (p > .250; Figure S3 in the Supplementary Material). There were no Heat level × Trial interactions on thermometer-evoked SCR amplitude either (all p's > .250).
To summarize the conditioning results, participants did not show a differential SCR to the symbolic UCSs (high vs. low heat levels displayed on thermometers) and, although they successfully learned the heat-predictive values of the CSs, participants did not acquire increased SCRs to CShigh vs. CSlow cues during the conditioning phase either. These findings confirm that the symbolic representations of high vs. low heat were not primary aversive UCSs, nor were they spontaneously acquired CSs to heat pain, which would be expected to elicit differential SCRs. Thus, any CS effects on subsequent pain responses unlikely reflect second-order conditioning of the CSs to previously acquired thermometer-heat pain associations (Gewirtz & Davis, 2000), but instead must be driven by the CSs’ conceptual representation of high vs. low heat acquired during the symbolic-conditioning procedure.
Test phase
Our main results concern the effects of symbolic conditioning on participants’ responses to painful heat in the test phase. The effects of CS type (CShigh vs. CSlow) on both heat-evoked SCRs and reported pain were highly significant in all instruction conditions. Therefore, we report results pooled across instruction conditions, and report the slight variations that were found as a function of instruction condition in the Additional Results in the Supplementary Material.
Conditioning effects on heat-evoked SCRs
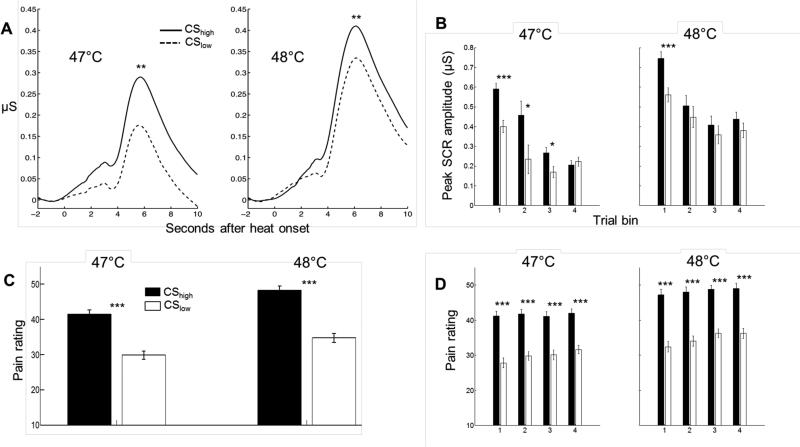
Heat-evoked SCRs were larger on CShigh than CSlow trials (p = .007; Fig. 2A; Table 1). Thus, the symbolically conditioned cues affected participants’ autonomic response to the noxious heat stimuli. As expected, heat-evoked SCRs were larger following 48°C than 47°C heat stimuli as well (p < .001; Table 1), but there was no temperature × CS type interaction (p > .250). The CS effect on heat-evoked SCR amplitude decreased over the course of the test phase (CS type × trial interaction, p = .005), and was absent during the last part of the test phase (Fig. 2B). This may have resulted from the habituation of heat-evoked SCRs overall (strong negative repetition effects, Table 1), producing a floor effect.
Figure 2.
Conditioned modulation of pain responses in the test phase. A. Grand average heat-evoked SCR, as a function of stimulus temperature and CS type. B. Mean heat-evoked SCR amplitude as a function of stimulus temperature and CS type, in each of the four quartiles of the test phase. Error bars are within-subject standard errors. C. Mean pain ratings as a function of stimulus temperature and CS type. D. Mean pain ratings as a function of stimulus temperature and CS type, in each of the four quartiles of the test phase. *** p < .001, ** p < .01, * p < .05
Table 1.
Predictors of heat-evoked SCR amplitude and pain rating and in the test phase
| Coefficient | STE | Cohen's d | t | p | |
|---|---|---|---|---|---|
| Heat-evoked SCR | |||||
| Intercept | 0.39 | 0.09 | 0.85 | 4.62 | < 0.001 |
| Temperature (48°C > 47°C) | 0.06 | 0.01 | 1.18 | 4.12 | < 0.001 |
| CS (CShigh > CSlow) | 0.03 | 0.01 | 0.59 | 2.94 | 0.007 |
| CS × Trial L | −0.0008 | 0.0003 | 0.52 | 3.21 | 0.005 |
| Site-nonspecific repetition L | −0.07 | 0.02 | 0.69 | 4.27 | < 0.001 |
| Site-nonspecific repetition Q | 0.02 | 0.01 | 0.39 | 2.72 | 0.01 |
| Site-specific repetition L | −0.01 | 0.002 | 0.98 | 4.15 | < 0.001 |
| Site-specific repetition Q | 0.001 | 0.0004 | 0.49 | 2.87 | 0.01 |
| Pain rating | |||||
| Intercept | 38.6 | 3.14 | 2.41 | 12.3 | < 0.001 |
| Temperature (48°C > 47°C) | 2.77 | 0.28 | 1.94 | 9.85 | < 0.001 |
| CS (CShigh > CSlow) | 7.05 | 0.95 | 1.46 | 7.39 | < 0.001 |
| Temperature × CS | 0.29 | 0.1 | 0.57 | 2.87 | 0.009 |
| CS × Trial L | −0.02 | 0.01 | 0.39 | 1.83 | 0.08 |
| CS × [No-instruction > average] | −1.18 | 0.46 | 0.50 | 2.56 | 0.02 |
| CS × [Per-trial instruction > average] | −1.49 | 0.86 | 0.34 | 1.74 | 0.09 |
| Site-nonspecific repetition L | 0.64 | 0.38 | 0.33 | 1.7 | 0.10 |
| Site-specific repetition Q | 0.02 | 0.01 | 0.39 | 1.99 | 0.06 |
Note. L = linear effect; Q = quadratic effect; STE = standard error of the mean
Conditioning effects on heat-evoked pain ratings
As expected, participants reported higher pain following 48°C than 47°C heat stimuli (p < .001; see Table 1 for the statistics and effect sizes of all significant predictors). In addition, pain ratings were higher on CShigh than CSlow trials (p < .001; Figure 2C; Table 1). There was a CS type × temperature effect as well (p = .009), reflecting that the effect of CS type was larger for the 48°C than 47°C stimuli. In addition, there was a trend for a negative CS type x trial interaction (p = .080), reflecting a slight decrease of the CS effect on pain ratings over the course of the test phase. However, the CS effect on pain rating was still highly significant at the end of the test phase (Figure 2D).
Mediation results
We next used multi-level mediation analyses to test whether the CS effects on autonomic and self-reported pain responses were formally mediated by trial-to-trial variation in anticipatory (CS-evoked) SCR amplitude and/or pain-expectancy ratings.
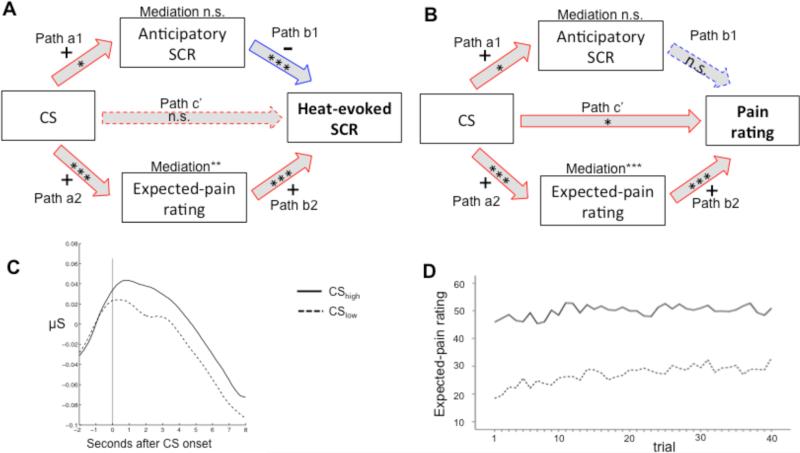
We first examined whether anticipatory SCR amplitude and/or pain-expectancy ratings mediated the CS effect on heat-evoked SCR amplitude (Fig. 3A). Both anticipatory SCRs and pain-expectancy ratings were higher following CShigh than CSlow cues (paths a1 and a2, p = .038 and p < .001), respectively. Interestingly, anticipatory SCR amplitude negatively predicted heat-evoked SCR amplitude when controlling for CS type (path b1, p < .001), but did not significantly mediate the CS effect on heat-evoked SCR (a*b1, p > .250). In contrast, pain-expectancy ratings positively predicted heat-evoked SCR amplitude when controlling for CS type (path b2, p < .001), and fully mediated the CS effect on heat-evoked SCRs (a*b2, p = .006; path c’, p > .250).
Figure 3.
Mediation results. A. Mediation of the CS effect on heat-evoked SCR. Red and blue arrows indicate positive and negative paths, respectively. Note that the anticipatory SCR and expected-pain rating mediators were tested in separate mediation models. Including both mediators in a single model produced highly similar results. B. Mediation of the CS effect on pain rating. *** p < .001, ** p < .01, * p < .05 C. Grand-average SCR time-locked to CShigh and CSlow onset in the test phase. Note that skin-conductance levels started to rise before CS onset, likely reflecting the anticipation of the CS. This makes it hard to define SCR onset but, since the different cues were presented in a random and unpredictable order, does not preclude a comparison of SCR amplitude across CS types. D. Mean expected-pain rating on each CShigh and CSlow trial in the test phase.
In two other mediation models, we asked whether pain-expectancy ratings and anticipatory SCR mediated the CS effects on pain ratings (Fig. 3B). CS effects on anticipatory SCR (path a1) and expected pain (path a2) are as described above. Anticipatory SCR amplitude did not predict pain rating when controlling for CS type (path b1; there was a non-significant negative relationship, p > .250) and did not mediate the CS effect on pain ratings (p = .192). Pain-expectancy ratings positively predicted pain ratings when controlling for CS type (path b2, p < .001), and formally mediated the CS effect on pain ratings (a*b2, p < .001). When controlling for pain-expectancy ratings, the CS effect on pain ratings remained marginally significant (path c’, p = .045), implying a partial rather than a full mediation.
The negative relationship between anticipatory and heat-evoked SCR amplitude could potentially be explained by an amplitude dependent refractory period, such that a larger SCR to the CS leads to a larger suppression of the SCR to the subsequent heat stimulus. If this is the case, the negative relationship between anticipatory and heat-evoked SCRs should decrease when the interval between them increases. To test this hypothesis, we added the duration of the interval between CS onset and heat onset—which varied across trials due to the jittered heat-anticipation delay and variation in pain-expectancy rating times—as a covariate to the first mediation analysis. This did not change the results: if anything, path b (the negative effect of anticipatory SCR on heat-evoked SCR) became even stronger when controlling for the CS-heat interval (p < .00001).
The results reported above were based on all trials, to prevent the introduction of a sampling bias by selectively removing extreme values. To examine whether any of the observed effects may have been driven by extreme SCR values on specific trials, we repeated the mediation analyses after excluding trials on which SCR amplitudes were more than 3 standard deviations from the within-person mean (on average 1.7 trials for anticipatory SCRs and 1.1 trials for heat-evoked SCRs). This yielded the same pattern of significant results for all tests, with the exception of the CS effect on anticipatory SCR amplitude (path a1 in Figure 3), which was just under significance after exclusion of extreme values (p = .13). Exclusion of extreme values did not change the negative relationship between anticipatory SCR and heat-evoked SCR, nor the effects of CS and expected-pain rating on heat-evoked SCR. The same pattern of results was found when excluding trials on which SCR amplitudes were more than 2 standard deviations from the mean (on average 4.9 trials for anticipatory SCRs and 4.3 trials for heat-evoked SCRs).
Finally, we also examined the correlations between single-trial SCR and rating values while controlling only for temperature and site-specific and site-nonspecific repetition. We did this separately for each participant, and tested whether the correlation coefficients differed from
0 using one-sample t-tests. Expected-pain ratings correlated positively with both pain ratings (mean R = 0.71, 95%-confidence interval (CI) = 0.64 to 0.79; t(25) = 20.3, p < .001) and heat-evoked SCR amplitude (mean R = 0.16, CI = 0.09 to 0.23; t(25) = 4.7, p < .001). Anticipatory SCR amplitude correlated negatively with heat-evoked SCR amplitude (mean R = −0.11, CI = −0.17 to -0.04; t(25) = 3.5, p = .002), but did not correlate with pain rating (mean R = 0.01, 95%-confidence interval = −0.04 to 0.06; t(25) = 0.55, p > .25). These correlations corroborate the Path b relationships revealed by our mediation analyses. We note that the SCR measures yielded relatively low correlations, due to the low signal-to-noise ratio inherent in single-trial physiological data, but are reliable across participants. Low R values mean simply that we cannot predict outcomes very accurately for individual trials, but here we are concerned with the reliability of the effects tested in the population. Finally, there was no correlation between expected-pain rating and anticipatory SCR amplitude (mean R = 0.004, CI = −0.05 to 0.05; t(25) = 0.18, p > .25), suggesting that these measures reflect two independent anticipatory processes.
Discussion
Expectations and learning together shape pain processing based on environmental cues (Rescorla, 1988; Stewart-Williams & Podd, 2004), but the mechanisms driving these effects are largely unresolved. Our results demonstrate robust modulation of self-reported and physiological pain responses following a purely conceptual conditioning procedure. Furthermore, conditioned-cue effects on heat-evoked SCRs were fully mediated by reported expectations. These findings indicate that conceptual representations, rather than pre-cognitive associations, are critical in conditioned pain modulation in humans.
Conditioning to conceptual reinforcers provides a unique way to disentangle pre-cognitive and conceptual influences. An interesting question concerns the relationship of our findings with recent demonstrations of pain modulation following ‘social-conditioning’ procedures in which UCSs were observations of other people's pain responses (Colloca & Benedetti, 2009; Vogtle et al., 2013). Whereas pain expressions in others may serve as primary reinforcers, due to their affective nature, the thermometer images that served as UCSs in our paradigm were not affectively arousing and were only linked with pain itself via conceptual processing. In addition, pain assessments in social-conditioning studies have focused on self-reported pain, which is sensitive to decision biases. For example, in the only ‘social conditioning’ study to our knowledge to examine autonomic physiology, Colloca and Benedetti (2009) found heart-rate deceleration during anticipation with high-pain vs. low-pain cues. This finding is likely related to anticipatory orienting rather than pain-evoked autonomic responses. Our study significantly extends this work by showing modulation of pain physiology following purely conceptual conditioning.
If conceptual representations are a crucial ingredient in pain modulation, why are verbal suggestions alone typically much less effective than suggestion combined with conditioning? The weak effects of verbal suggestion alone on pain is a recurring theme (Colloca et al., 2010; Colloca et al., 2008; de Jong et al., 1996; Voudouris et al., 1990). One possibility is that expectancies created by one-time information are too weak. Repeatedly experiencing cue-outcome associations may strengthen people's trust in the cues’ predictive value, and thereby their capacity to modulate pain. Another interesting possibility is that conditioning results in more precise, and therefore more powerful, expectancies about cue-outcome associations. More precise expectancies would be expected to yield greater pain modulation in a Bayesian framework, as precise prior information is more influential than diffuse information when integrating expectations with incoming sensory data (Buchel, Geuter, Sprenger, & Eippert, 2014). Verbal suggestions of ‘low’ vs. ‘high’ pain are rather nonspecific, and therefore may lead to diffuse, uncertain expectancies. According to this account, conditioning to certain conceptual representations—such as visually displayed heat levels—may counter-intuitively produce stronger pain-modulation effects than conditioning to primary noxious stimuli, which perceived intensity is more ambiguous. Future studies that directly compare effects of primary and conceptual conditioning could test this hypothesis.
A unique feature of our study is that we measured trial-to-trial variation in physiological pain-anticipation (anticipatory SCRs) and pain (heat-evoked SCRs) responses, and the relationship between them. Anticipatory SCRs in the test phase were larger following CShigh than CSlow cues, consistent with anticipatory threat. Anticipatory SCRs and self-reported expectancies oppositely affected pain responses, suggesting that anticipatory SCRs were not open to introspection. The suppressing effect of anticipatory SCRs on heat-evoked SCRs was unlikely due to an amplitude-dependent refractory period, as anticipatory SCRs were much smaller than heat-evoked SCRs, controlling for the CS-heat delay did not weaken the effect, and a previous study has found a positive rather than negative relationship between SCR amplitudes evoked by two successive stimuli (Bach, Flandin, Friston, & Dolan, 2010). Instead, the opposing effects of self-reported expectancy and anticipatory SCR may reflect two distinct pain-modulatory effects: (i) an expectancy-driven effect that causes assimilation of the pain response towards the expected pain, and (ii) a pre-cognitive compensatory mechanism, through which an autonomic threat response has analgesic effects. The second effect may reflect ‘fear’-conditioned analgesia prominent in the animal literature (Chance et al., 1977; Fanselow, 1986; Helmstetter & Fanselow, 1987) but not in human pain-conditioning studies (see (Flor & Grusser, 1999) for an exception). Which of the two processes we identified predominates may depend on the pain-related outcome measured (e.g., self-reports vs. autonomic responses), threat intensity (Rhudy & Meagher, 2000), and species (Kirsch, 2004). The expectancy effect dominated in our study, resulting in robust net assimilation of pain responses toward the predictive values of the cues.
In conclusion, the present study provides novel evidence that conceptual representations can influence pain physiology in a way that is surprisingly robust. Investigation of the neural mechanisms underlying the acquisition and expression of conceptually conditioned pain modulation is an important objective for future research. For example, it is still an open question whether the expression of conceptually conditioned pain modulation is mediated by brain systems that also mediate nociceptive pain, or by cognitive and/or valuation systems that do not interact with the nociceptive system (Woo, Roy, Buhle, & Wager, 2015). Finally, it will be interesting to directly compare the effects of conceptual and primary pain conditioning. We did not include the latter condition, which has been studied extensively in previous studies, as our aim was to examine whether conceptual conditioning can modify pain responses at all. Having demonstrated this, further examination of the similarities and differences between conceptual and primary conditioning is an important next step.
Supplementary Material
Acknowledgements
This research was made possible with the support of National Institutes of Health grants NIMH 2R01MH076136 and R01DA027794 (to T.D.W.).
References
- Antonov I, Antonova I, Kandel ER, Hawkins RD. The contribution of activity-dependent synaptic plasticity to classical conditioning in Aplysia. The Journal of neuroscience: the official journal of the Society for Neuroscience. 2001;21(16):6413–6422. doi: 10.1523/JNEUROSCI.21-16-06413.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Bolger N, Lindquist MA, Wager TD. Brain mediators of predictive cue effects on perceived pain. The Journal of neuroscience : the official journal of the Society for Neuroscience. 2010;30(39):12964–12977. doi: 10.1523/JNEUROSCI.0057-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atlas LY, Wager TD. How expectations shape pain. Neuroscience letters. 2012;520(2):140–148. doi: 10.1016/j.neulet.2012.03.039. [DOI] [PubMed] [Google Scholar]
- Atlas LY, Wager TD. Expectations and Beliefs: Insights from Cognitive Neuroscience. In: Ochsner KNK, S.M., editors. The Oxford Handbook of Cognitive Neuroscience Volume 2: The Cutting Edges. Oxford University Press; 2013. [Google Scholar]
- Bach DR, Flandin G, Friston KJ, Dolan RJ. Modelling event-related skin conductance responses. International journal of psychophysiology: official journal of the International Organization of Psychophysiology. 2010;75(3):349–356. doi: 10.1016/j.ijpsycho.2010.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Berntson GG, Micco DJ. Organization of brainstem behavioral systems. Brain research bulletin. 1976;1(5):471–483. doi: 10.1016/0361-9230(76)90117-9. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Tuber DS, Ronca AE, Bachman DS. The decerebrate human: associative learning. Experimental neurology. 1983;81(1):77–88. doi: 10.1016/0014-4886(83)90158-9. [DOI] [PubMed] [Google Scholar]
- Buchel C, Geuter S, Sprenger C, Eippert F. Placebo analgesia: a predictive coding perspective. Neuron. 2014;81(6):1223–1239. doi: 10.1016/j.neuron.2014.02.042. [DOI] [PubMed] [Google Scholar]
- Carlino E, Torta DM, Piedimonte A, Frisaldi E, Vighetti S, Benedetti F. Role of explicit verbal information in conditioned analgesia. European journal of pain. 2015;19(4):546–553. doi: 10.1002/ejp.579. [DOI] [PubMed] [Google Scholar]
- Chance WT, White AC, Krynock GM, Rosecrans JA. Autoanalgesia: behaviorally activated antinociception. European journal of pharmacology. 1977;44(3):283–284. doi: 10.1016/0014-2999(77)90076-0. [DOI] [PubMed] [Google Scholar]
- Colloca L, Benedetti F. Placebo analgesia induced by social observational learning. Pain. 2009;144(1-2):28–34. doi: 10.1016/j.pain.2009.01.033. [DOI] [PubMed] [Google Scholar]
- Colloca L, Petrovic P, Wager TD, Ingvar M, Benedetti F. How the number of learning trials affects placebo and nocebo responses. Pain. 2010;151(2):430–439. doi: 10.1016/j.pain.2010.08.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colloca L, Tinazzi M, Recchia S, Le Pera D, Fiaschi A, Benedetti F, et al. Learning potentiates neurophysiological and behavioral placebo analgesic responses. Pain. 2008;139(2):306–314. doi: 10.1016/j.pain.2008.04.021. [DOI] [PubMed] [Google Scholar]
- de Jong PJ, van Baast R, Arntz A, Merckelbach H. The placebo effect in pain reduction: the influence of conditioning experiences and response expectancies. International journal of behavioral medicine. 1996;3(1):14–29. doi: 10.1207/s15327558ijbm0301_2. [DOI] [PubMed] [Google Scholar]
- Dworkin RH, Backonja M, Rowbotham MC, Allen RR, Argoff CR, Bennett GJ, et al. Advances in neuropathic pain: diagnosis, mechanisms, and treatment recommendations. Archives of neurology. 2003;60(11):1524–1534. doi: 10.1001/archneur.60.11.1524. [DOI] [PubMed] [Google Scholar]
- Fanselow MS. Conditioned fear-induced opiate analgesia: a competing motivational state theory of stress analgesia. Annals of the New York Academy of Sciences. 1986;467:40–54. doi: 10.1111/j.1749-6632.1986.tb14617.x. [DOI] [PubMed] [Google Scholar]
- Flor H, Grusser SM. Conditioned stress-induced analgesia in humans. European journal of pain. 1999;3(4):317–324. doi: 10.1053/eujp.1999.0137. [DOI] [PubMed] [Google Scholar]
- Gewirtz JC, Davis M. Using pavlovian higher-order conditioning paradigms to investigate the neural substrates of emotional learning and memory. Learn Mem. 2000;7(5):257–266. doi: 10.1101/lm.35200. [DOI] [PubMed] [Google Scholar]
- Glanzman DL. The cellular basis of classical conditioning in Aplysia californica--it's less simple than you think. Trends in neurosciences. 1995;18(1):30–36. doi: 10.1016/0166-2236(95)93947-v. [DOI] [PubMed] [Google Scholar]
- Grau JW, Salinas JA, Illich PA, Meagher MW. Associative learning and memory for an antinociceptive response in the spinalized rat. Behavioral neuroscience. 1990;104(3):489–494. doi: 10.1037//0735-7044.104.3.489. [DOI] [PubMed] [Google Scholar]
- Greene MR, Botros AP, Beck DM, Fei-Fei L. What you see is what you expect: rapid scene understanding benefits from prior experience. Attention, perception & psychophysics. 2015;77(4):1239–1251. doi: 10.3758/s13414-015-0859-8. [DOI] [PubMed] [Google Scholar]
- Hawkins RD, Abrams TW, Carew TJ, Kandel ER. A cellular mechanism of classical conditioning in Aplysia: activity-dependent amplification of presynaptic facilitation. Science. 1983;219(4583):400–405. doi: 10.1126/science.6294833. [DOI] [PubMed] [Google Scholar]
- Helmstetter FJ, Fanselow MS. Effects of naltrexone on learning and performance of conditional fear-induced freezing and opioid analgesia. Physiology & behavior. 1987;39(4):501–505. doi: 10.1016/0031-9384(87)90380-5. [DOI] [PubMed] [Google Scholar]
- Jepma M, Jones M, Wager TD. The dynamics of pain: evidence for simultaneous site-specific habituation and site-nonspecific sensitization in thermal pain. The journal of pain : official journal of the American Pain Society. 2014;15(7):734746. doi: 10.1016/j.jpain.2014.02.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johansen JP, Cain CK, Ostroff LE, LeDoux JE. Molecular mechanisms of fear learning and memory. Cell. 2011;147(3):509–524. doi: 10.1016/j.cell.2011.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirsch I. Conditioning, expectancy, and the placebo effect: comment on Stewart-Williams and Podd (2004). Psychological bulletin. 2004;130(2):341–343. doi: 10.1037/0033-2909.130.2.341. discussion 344345. [DOI] [PubMed] [Google Scholar]
- Levy MH. Pharmacologic treatment of cancer pain. The New England journal of medicine. 1996;335(15):1124–1132. doi: 10.1056/NEJM199610103351507. [DOI] [PubMed] [Google Scholar]
- Montgomery GH, Kirsch I. Classical conditioning and the placebo effect. Pain. 1997;72(1-2):107–113. doi: 10.1016/s0304-3959(97)00016-x. [DOI] [PubMed] [Google Scholar]
- Orne MT. On the Social-Psychology of the Psychological Experiment - with Particular Reference to Demand Characteristics and Their Implications. American Psychologist. 1962;17(11):776–783. [Google Scholar]
- Rescorla RA. Pavlovian conditioning. It's not what you think it is. The American psychologist. 1988;43(3):151–160. doi: 10.1037//0003-066x.43.3.151. [DOI] [PubMed] [Google Scholar]
- Rhudy JL, Meagher MW. Fear and anxiety: divergent effects on human pain thresholds. Pain. 2000;84(1):65–75. doi: 10.1016/S0304-3959(99)00183-9. [DOI] [PubMed] [Google Scholar]
- Schafer SM, Colloca L, Wager TD. Conditioned placebo analgesia persists when subjects know they are receiving a placebo. The journal of pain : official journal of the American Pain Society. 2015;16(5):412–420. doi: 10.1016/j.jpain.2014.12.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stewart-Williams S, Podd J. The placebo effect: dissolving the expectancy versus conditioning debate. Psychological bulletin. 2004;130(2):324–340. doi: 10.1037/0033-2909.130.2.324. [DOI] [PubMed] [Google Scholar]
- Vogtle E, Barke A, Kroner-Herwig B. Nocebo hyperalgesia induced by social observational learning. Pain. 2013;154(8):1427–1433. doi: 10.1016/j.pain.2013.04.041. [DOI] [PubMed] [Google Scholar]
- Voudouris NJ, Peck CL, Coleman G. The role of conditioning and verbal expectancy in the placebo response. Pain. 1990;43(1):121–128. doi: 10.1016/0304-3959(90)90057-K. [DOI] [PubMed] [Google Scholar]
- Watson A, El-Deredy W, Bentley DE, Vogt BA, Jones AK. Categories of placebo response in the absence of site-specific expectation of analgesia. Pain. 2006;126(1-3):115–122. doi: 10.1016/j.pain.2006.06.021. [DOI] [PubMed] [Google Scholar]
- Woo CW, Roy M, Buhle JT, Wager TD. Distinct brain systems mediate the effects of nociceptive input and self-regulation on pain. PLoS biology. 2015;13(1):e1002036. doi: 10.1371/journal.pbio.1002036. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.