Early evolution of the basal animal phylum illuminated by sclerotized and armored ctenophore species from early Cambrian period.
Keywords: Cambrian, Chengjiang biota, Ctenophora, skeleton
Abstract
Ctenophores are traditionally regarded as “lower” metazoans, sharing with cnidarians a diploblastic grade of organization. Unlike cnidarians, where skeletonization (biomineralization and sclerotization) evolved repeatedly among ecologically important taxa (for example, scleractinians and octocorals), living ctenophores are characteristically soft-bodied animals. We report six sclerotized and armored ctenophores from the early Cambrian period. They have diagnostic ctenophore features (for example, an octamerous symmetry, oral-aboral axis, aboral sense organ, and octaradially arranged ctene rows). Unlike most modern counterparts, however, they lack tentacles, have a sclerotized framework, and have eight pairs of ctene rows. They are resolved as a monophyletic group (Scleroctenophora new class) within the ctenophores. This clade reveals a cryptic history and sheds new light on the early evolution of this basal animal phylum. Skeletonization also occurs in some other Cambrian animal groups whose extant members are exclusively soft-bodied, suggesting the ecological importance of skeletonization in the Cambrian explosion.
INTRODUCTION
With some 150 described species, modern ctenophores (comb jellies) represent a relatively small animal phylum, dominated by soft-bodied, holopelagic, marine predators. Like cnidarians, they are diploblastic animals with an oral-aboral body axis, but they have distinctive characters including eight rows of comb-like ciliary plates (ctenes) for locomotion, an aboral sense organ, a biradial symmetry, and specialized adhesive cells (colloblasts) for feeding. Their exact phylogenetic position in the animal tree is controversial (1–3), with competing hypotheses positing them as the basalmost metazoans (4–7), a sister group to the cnidarians (8), or a sister group to the bilaterians (9). Within the Ctenophora, it is uncertain whether tentacleless members are evolutionarily primitive or derived (10–12). These various hypotheses make different predictions about the early evolutionary history of ctenophores, which can be tested against the fossil record. However, ctenophores have a generally poor fossil record. Here, we report several sclerotized and armored ctenophore species, based on new material and reinterpretation of previously published material (13–15) from the early Cambrian Chengjiang biota (ca. 520 Ma). Along with armored Cambrian entoprocts (16), phoronids (17), lobopods (18), and scalidophorans (19), the new fossils suggest a vanished Cambrian history of skeletonization in multiple animal groups, imply the ecological importance of skeletonization in the Cambrian explosion, and highlight the remarkable morphological disparity in certain Cambrian animal clades relative to their modern survivors (20).
RESULTS
The sclerotized ctenophores include three new species (Gemmactena actinala gen. et sp. nov., Thaumactena ensis gen. et sp. nov., and Galeactena hemispherica gen. et sp. nov.) and three previously described but herein amended species (Batofasciculus ramificans Hou et al., 1999; Maotianoascus octonarius Chen et Zhou, 1997; and Trigoides aclis Luo et Hu, 1999) (see the Supplementary Materials for systematic description of paleontology). These fossils are largely preserved as compressions and replicated by clays and carbonaceous films, with the surface generally covered by rusty iron oxides (after diagenetic pyrite). They share a basic body plan characterized by a tentacleless and octaradial body with an oral-aboral axis, eight rigid struts (termed here “spokes”) radiating from the aboral end and arched to converge to the oral end, eight soft-bodied flaps or lobes supported by the spokes, eight pairs of ctene rows, a conspicuous apical (or aboral) organ walled by eight rigid plates and housing a spheroidal or ellipsoidal statolith, and an oral region surrounded by eight apiculate lappets.
Gemmactena actinala gen. et sp. nov. is characterized by an apical (aboral) organ, a globose central body with eight flaps, and a peri-oral structure with eight lappets. The apical organ is conical to domical in shape and houses an ellipsoidal structure preserved as an oval carbonaceous film (Fig. 1 and fig. S1), most likely representing a prominent statolith (or statocyst) rich in organic material. The structural framework of the apical organ consists of eight aborally tapering plates (here termed “apical plates”), each with a medial groove on the inner surface (Fig. 1H). In one of the paratypes (Fig. 1G), apical plates are separate from each other but maintain their integrity, indicating considerable rigidity. The structural framework of the central body consists of eight rigid spokes, each corresponding to an apical plate and extending along the oral-aboral axis. A medial groove occurs on the inner surface of the spoke (Fig. 1E), and oblique striations are present on the outer surface (Fig. 1B). Each spoke is divided into a longer upper (aboral) part and a shorter lower (adoral) part, separated by a sharp kink. Spokes support eight flaps, which can be buried in different layers of sediments (Fig. 1, A and D). Each side of the flap is equipped with an arcuate band of closely spaced transverse bars (Fig. 1, A, C, and D). The band only occurs on the axial part of the flap, and the transverse bars do not cross the spoke. Thus, there are a total of eight pairs of such bands. These bands are interpreted as ctene rows and the transverse bars as ctenes. Dark strands that radiate from the base of the apical organ (Fig. 1K) are considered remains of meridional canals. The lower spokes converge to define a circumferential constriction that marks the boundary between the central body and the peri-oral structure with eight sharp lappets. Each oral lappet is supported by a rigid ridge presumably representing the adoral extension of a spoke. The apical plates, spokes, and oral ridges are preserved with positive relief and structural integrity, and interpreted as sclerotized structures.
Fig. 1.
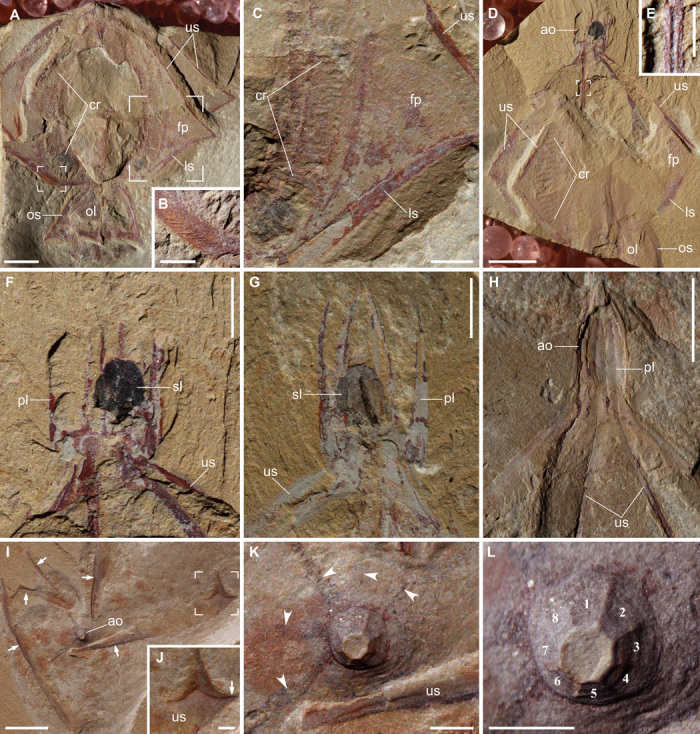
Gemmactena actinala gen. et sp. nov. (A) Holotype (ELEL-SJ100756A) showing radiating flap-like structures outlined by spokes, comb rows, and oral lappets. Apical organ not preserved. (B) Close-up of small focus area in (A) showing fine striae on spoke surface. (C) Close-up of large focus area in (A) showing remains of comb rows, as well as upper and lower spokes that frame a flap. (D) Paratype (ELEL-SJ081292A). (E) Close-up of focus area in (D) showing rigidity of a spoke with a medial groove (internal view). (F) Close-up of aboral region in (D) showing an ellipsoidal statolith preserved as dark remains surrounded by apical plates. (G) Counterpart of (F) showing complete apical plates detached from each other; remains of organic carbon are partially preserved as a dark band on the statolith. (H) Paratype (ELEL-SJ081366A) showing pointed dome-like apical organ walled by rigid plates (each with a medial groove continuous with a spoke). (I) Aborally compacted specimen ELEL-SJ120375A showing an apical organ and partially dislocated upper spokes (arrows). The apex of the apical organ was truncated and retained in counterpart during splitting. (J) Close-up of focus area in (I) showing distal end of upper spoke, with the kink marked by arrow. (K) Close-up of apical organ in (I); dark, equally spaced bands (arrowheads) radiating from the base of apical organ may represent remains of underlying meridional canals. (L) Close-up of apical organ showing considerable positive relief, presumably due to its rigidity imparted by apical plates (numbered). ao, apical organ; cr, comb row; fp, flap-like structure; ls, lower spoke; ol, oral lappet; os, oral skirt; pl, apical plate; sl, statolith; us, upper spoke. Scale bars, 5 mm (A, D, H, and I); 2 mm (C, F, and G); 1 mm (B, E, and J to L).
Thaumactena ensis gen. et sp. nov. (Fig. 2, A to D, and fig. S2) has a length/width ratio of about 10:1 in both adult (holotype) and juvenile (paratype) specimens. The elongate cone-like apical organ contains organic carbon remains (Fig. 2D), indicative of an apical organ housing a statolith. The wall of the apical organ branches adorally into a radial set of eight rigid, slender, gently curved spokes, preserved as bands of dark rusty iron oxides. Transverse bars arranged in longitudinal rows, interpreted as comb rows, occur along both sides of a spoke (Fig. 2, B and D). The bars have positive relief but are otherwise featureless, with no preserved evidence of fused cilia characteristic of ctenes in extant ctenophores. The spokes taper orally, extending to the terminus of the oral region. Around the oral region, the spokes and intervening membrane slightly diverge to form an “oral skirt.”
Fig. 2.
Thaumactena ensis gen. et sp. nov. (A to D), Galeactena hemispherica gen. et sp. nov. (E to I), and Batofasciculus ramificans (J to N). (A) Holotype (ELEL-SJ081427A) showing apical organ, body with rigid spokes (arrows), and oral skirt. (B) Close-up of aboral region in (A) showing arcuate spokes (arrows) and remains of ctenes (arrowheads); dark residues may represent gastric system. (C) Oral region in (A) (counterpart) showing flared oral skirt and membranous tissue supported by spokes (arrows). (D) Paratype (ELEL-SJ081563), a juvenile, showing spokes (arrows) and remains of ctene rows. (E) Holotype (ELI-JSCT0001). (F) Close-up of apical organ in (E) showing internal aboral canal and statolith remains. (G) Close-up of focus area in (E) showing remains of ctenes. (H) Paratype (ELI-JSCT0002). (I) Close-up of apical organ in (H). (J) Paratype (ELEL-SJ101932A) showing apical organ, body with spinose spokes, and oral region. (K) Close-up of oral region in (J) showing lappet-like structures. (L) Close-up of a spine (arrowed in J). (M) Close-up of aboral part in (J) showing apical plates, statolith, and radiating spokes (arrowheads). (N) Close-up of focus area in (M) showing a spine sheathed by membrane (arrows). ac, aboral canal; ao, apical organ; cr, ctene row; lp, lappet; or, oral region; os, oral skirt; pl, apical plates; sl, statolith. Arrowheads in (E), (G), and (H) indicate medial ridges of radiating lobes mantled by membranous fins. Scale bars, 5 mm (A to E, H, J, and M); 1 mm (F, G, I, K, L, and N).
Galeactena hemispherica gen. et sp. nov. (Fig. 2, E to I, and fig. S3, A to F) is characterized by a consistently vaulted hemispherical shape in all specimens, with little evidence of being twisted or distorted. Its preservation is different from the generally distorted morphologies of fully soft-bodied ctenophores from the Burgess Shale (11), indicating that it had a degree of structural integrity. Although no spokes or apical plates are preserved with strong relief, we infer that the structural integrity of Galeactena hemispherica was provided by lightly sclerotized hard parts. An elongate apical organ gradually tapers aborally to a blunt end. A spheroidal statolith preserved as carbonaceous remains resides within the distal end of the apical organ. A prominent central tube with preserved positive relief, interpreted as the aboral canal, tapers aborally and ends beneath the statolith (Fig. 2, F and I, and fig. S3, A, B, D, and E), although no anal canals or pores can be unambiguously identified. Eight meridional lobes extend along almost the entire body length, each gently thinning centrifugally into a medial ridge, which might represent a lightly sclerotized spoke (Fig. 2H). An arcuate, fin-like structure composed of membranous or cuticular tissues projects centrifugally along each medial ridge. A longitudinal row of transverse bars, preserved as organic remains with positive relief and interpreted as a comb row, occurs on both sides of a lobe (Fig. 2, E and G, and fig. S3, A to D). Thus, there are eight pairs of comb rows. The comb rows gradually widen adorally. A circumferential constriction separates the central body from a short oral skirt.
Three previously published Chengjiang taxa are here interpreted as sclerotized ctenophores. Batofasciculus ramificans was originally described with uncertain phylogenetic assignment (15, 21) but later tentatively considered as a ctenophore on the basis of an “aboral dome-like structure” and “eight lobes” (22, 23). New material herein (Fig. 2, J to N) reveals a statolith in the apical organ and an oral region, thus ascertaining a ctenophoran affinity. The statolith is preserved with carbonaceous residues, as revealed by energy-dispersive x-ray spectroscopic (EDS) analysis (fig. S4) and consistent with the carbonaceous preservation of statoliths in other Chengjiang ctenophores (for example, fig. S1). The eight arcuate spokes bear robust spines (Fig. 2, L to N, and figs. S4 to S6) and retain their structural integrity even when disarticulated (24), suggesting a remarkable degree of sclerotization. Maotianoascus octonarius (fig. S3G), which has been assigned to the Ctenophora (13, 15, 21–23), shares with Galeactena a constant, saclike body shape, an apical organ with a statolith, eight radiating lobes with medial membranous fins, well-developed comb rows, and an oral skirt, but the latter has a more elongate apical organ, a more distally located statolith, and a less rounded body form. Trigoides aclis (figs. S7 and S8), first described as an arthropod (14) and subsequently assigned to the Ctenophora (22), resembles Gemmactena in having apical plates, kinked spokes, an apical organ with statolith, comb rows, and oral lappets. However, it differs from Gemmactena in its wider body with nearly horizontal upper spokes that are shorter than lower spokes. In addition, an unnamed Chengjiang ctenophore (fig. S9) also shows rigid apical plates and radiating spokes, suggesting some degree of sclerotization.
DISCUSSION
We suggest that the suite of features (for example, oral-aboral body axis, apical organ, and ctenes arranged in octaradial rows) seen in the Chengjiang fossils are characteristic of ctenophores. The apical organ is considered an aboral sense organ, which in extant ctenophores consists of an ellipsoidal statolith housed in a transparent ciliary dome and serves as a gravitational receptor (25). However, it is much larger than its extant counterparts, with its dome constructed by rigid plates rather than cilia. The organic-rich spheroidal structure in the plated dome is interpreted as a statolith, consistent with the location and composition of extant ctenophore statoliths, which are composed of densely packed organic matter (precisely a ball of lithocytes) and microscopic carbonate granules (26, 27). Conceivably, there might exist balancers supporting the statolith, as well as ciliary grooves radiating from the base of the apical organ and then joining ctene rows, but such details were not preserved in current specimens. The presence of an apical organ with a statolith implies the evolution of a primitive nervous system (7).
The transverse bars arranged in eight pairs of longitudinal bands in the Chengjiang fossils are best interpreted as comb plates, which in living ctenophores consist of fused macrocilia, are arrayed in eight rows, and serve as propulsive “paddles”. They are typically preserved as organic remains with positive relief, and are likely equivalent to the cytoskeletal polster (cushion) of ctenes in living forms (28, 29) or remains of ctene muscles (7). The lack of cilia on the transverse bars is probably due to their detachment from polsters soon after death, as observed in extant ctenophores (10). The apical sense organ and octaradial comb rows with underlying meridional canals (Fig. 1K) suggest that the Chengjiang ctenophores most likely had a nektonic life mode, with their locomotion powered by ciliary ctenes. The radiating flaps (vane-like structures) in some taxa might have hydrodynamic significance to facilitate locomotion (and perhaps feeding). Closely coupled with the well developed ctene rows, the flaps (and perhaps the oral skirt and oral lappets) might have supplemented the comb rows to provide power strokes to propel the animals. The pencillate Thaumactena has a streamlined body similar to chaetognaths (arrow worms) and might have been an agile swimmer.
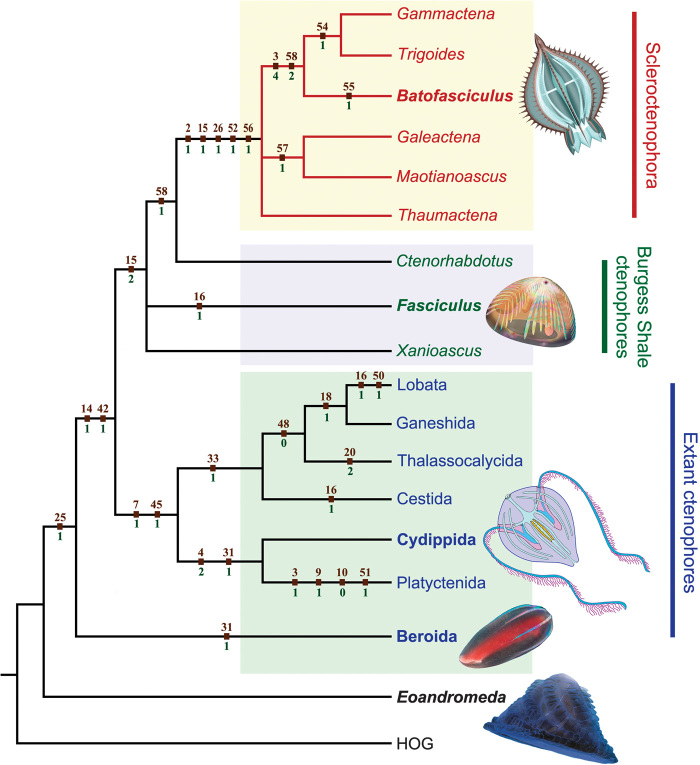
Despite their characteristic ctenophore features, the Chengjiang fossils are different from extant ctenophores in their sclerotization, eight pairs of ctene rows, and apparently radial (rather than biradial) symmetry because of the lack of tentacles (Fig. 3). A cladistic analysis based on 58 ecomorphological characters of both fossil and extant taxa shows that the skeletonized ctenophores from the Chengjiang biota form a monophyletic group that is subtended by Burgess Shale ctenophore fossils (Fig. 4); they are here described under the new class Scleroctenophora (see the Supplementary Materials). Additionally, the tentacleless Beroida is resolved as the most basal branch within crown-group ctenophore, and all tentaculate ctenophores form a monophyletic group. These cladistic results offer intriguing evidence that the earliest ctenophores were tentacleless (10, 11), contrary to molecular phylogenetic analyses indicating that the beroids secondarily lost the tentacles (12). Considering that all Cambrian and potentially Ediacaran ctenophore fossils are tentacleless (30–32), it is possible that the earliest ctenophores were octaradial and tentacleless, regardless of the phylogenetic position of the beroids.
Fig. 3.
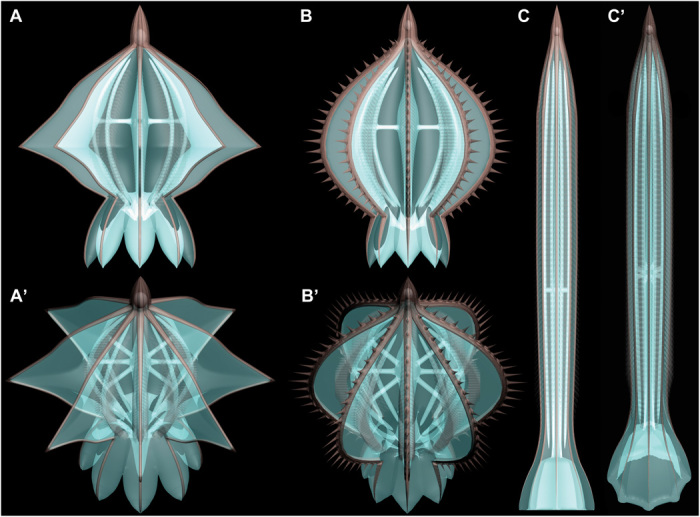
Idealized three-dimensional models of Cambrian skeletonized ctenophores. (A to C) Side views of Gemmactena actinala gen. et sp. nov, Batofasciculus ramificans, and Thaumactena ensis gen. et sp. nov., respectively. (A′ to C′) Oblique aboral views corresponding to (A) to (C).
Fig. 4.
Phylogenetic relationship of fossil and extant ctenophores based on a comprehensive cladistic analysis (tables S2 and S3). The skeletonized ctenophores from the Chengjiang biota form a clade here described as the new class Scleroctenophora. The cladogram is a strict consensus of the three most parsimonious trees. Apomorphies (character number and state above and below nodes, respectively) are mapped on the cladogram. Tree length = 53; consistency index = 0.9231; retention index = 0.9394; rescaled consistency index = 0.8671. Illustrated taxa are marked in bold.
The Chengjiang ctenophores described here are unique in their sclerotized skeletons, whose function, composition, and developmental origin are open to discussion. The spokes and apical plates of scleroctenophores may have provided both mechanical support for soft tissues and ecological defense against predators or adverse environments while not significantly compromising neutral buoyancy for nektonic organisms such as ctenophores. The spokes and apical plates of scleroctenophores were likely cuticular or chitinous in composition, but the presence of minerals in their skeletons cannot be completely ruled out, given that the epidermis of extant ctenophores can produce Mg-Ca carbonates that partially form the statolith (26) and that biominerals can be taphonomically lost in biomineralized Chengjiang fossils such as linguliformean branchiopods (33). The skeletons were probably ectodermally derived, given the topological relation between the apical plates and statolith, which is ectodermal in developmental origin among modern ctenophores. If so, the outermost organic covering (Figs. 1B and 2, E and N, and figs. S1A and S3, A, B, D, and G) may represent the epidermis, and the skeleton was produced within or underneath the epidermis, as in living hydrocorals (for example, Millepora) (25). Alternatively, the skeleton could have resulted from thickening and sclerotization of epidermal excretion, in a way analogous to the chitinous periderm (or perisarc) covering of living cnidarians (25); in this scenario, the outermost membrane may represent a recalcitrant integument.
The occurrence of sclerotized and armored skeletons in Cambrian representatives of several animal groups—including entoprocts (16), phoronids (17), lobopods (18), scalidophorans (19), and now ctenophores that are exclusively soft-bodied among modern survivors—is a remarkable phenomenon. The independent skeletonization among these diverse Cambrian animals provides indirect evidence for an intensified level of ecological interactions (for example, arms race) and also highlights the importance of paleontological data in illuminating the evolutionary legacy that would be otherwise inaccessible by studying living animals alone. The widespread occurrence of skeletonization echoes Stephen Jay Gould’s view of the striking morphological disparity of many animal phyla during their Cambrian debut (20), and the contrasting evolutionary trajectories of skeletonized cnidarians and ctenophores also elucidate the contingent fate of evolutionary innovations such as skeletonization.
MATERIALS AND METHODS
Fossil material referred to in this research includes a total of 37 specimens (table S1) collected from the lower Cambrian (Series 2) Yu’anshan Member mudstones of the Heilinpu (previously Chiungchussi) Formation, Kunming, Yunnan, South China. The fossiliferous mudstones belong to the Eoredlichia-Wutingaspis trilobite Zone and host the ~520 Ma Chengjiang biota. They were deposited in a restricted shallow-marine environment on the continental shelf of the Yangtze Platform, which was located at low paleolatitudes during the early Cambrian. Fossil localities include the Maotianshan and Xiaolantian sections in Chengjiang, the Sanjiezi section in Jinning, the Jianshan and Ercaicun sections in Haikou, and the Haoyicun section in Anning, all located within 50 km south of Kunming City. The specimens are assigned to three new taxa (Gemmactena actinala gen. et sp. nov., Thaumactena ensis gen. et sp. nov., and Galeactena hemispherica gen. et sp. nov.), three previously described species (Batofasciculus ramificans Hou et al., 1999; Maotianoascus octonarius Chen et Zhou, 1997; and Trigoides aclis Luo et Hu, 1999), and an unnamed form. Specimens are reposited in the Early Life Evolutionary Laboratory at China University of Geosciences (prefix ELEL-), Early Life Institute at Northwest University (prefix ELI-), Nanjing Institute of Geology and Palaeontology, Chinese Academy of Sciences (prefixes NIGP- and ELRC-), Research Center for Chengjiang Biota at Yunnan University (prefix RCCBYU-), and Yunnan Institute of Geological Science (prefix YDKS-).
Specimens were examined and mechanically prepared under a Zeiss Stemi-2000C stereomicroscope. Photographs were taken using a Canon EOS 5D Mark II optical camera with an EF 100mm f/2.8 USM lens. Three-dimensional reconstructions (Fig. 3) were conducted using the software Autodesk 3ds Max (9.0). Backscattered electron microscopy (BSE) and EDS were conducted on a Hitachi S-3400N field emission scanning electron microscope with an EDS system.
Acknowledgments
We thank S. Tamm, T. Shiganova, R. Scott, G. Mayer, S. Conway Morris, and J.-B. Caron for insightful comments; J. Chen, X. Hou, D. Siveter, S. Hu, F. Tang, A. Semenov, D. Erwin, and J.-B. Caron for providing fossil images; and J. Liu, M. Ju, H. Zhang, M. Cheng, Q. Lei, M. Wang, H. Yu, and S. Cheng for assistance in laboratory or field work. Funding: This work was supported by the Program for New Century Excellent Talents in University (NCET-13-1008), National Natural Science Foundation of China (41102012, 41272011, and Shaanxi-2012JZ5002), Fundamental Research Funds for the Central Universities (2010ZY07, 2011YXL013, and 2012097), Research Fund for Doctoral Program of Higher Education (20116101130002), and U.S. National Science Foundation (EAR-1250800). Author contributions: Q.O. designed the research. Q.O. and S.X. developed the interpretation, prepared figures, and wrote the manuscript, with input from other authors. Q.O., J.H., D.S., G.S., and Z.Z. contributed to the collection and analysis of fossil specimens. F.Z. reconstructed three-dimensional models of Chengjiang ctenophores. All authors participated in discussion of conclusions. Competing interests: The authors declare that they have no competing financial interests. Data and materials availability: Correspondence and requests for materials should be addressed to Q.O. (ouqiang@cugb.edu.cn).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/6/e1500092/DC1
Systematic Paleontology
Taphonomy and Preservation
Cladistic Analysis
Table S1. Preservation of ctenophore specimens from the early Cambrian Chengjiang biota.
Table S2. List of characters and coding comments on ctenophore features used in cladistic analysis.
Table S3. Data matrix used in cladistic analysis of ctenophores.
Fig. S1. BSE and EDS analyses of Gemmactena actinala gen. et sp. nov.
Fig. S2. Additional material of Thaumactena ensis gen. et sp. nov.
Fig. S3. Additional material of Galeactena hemispherica gen. et sp. nov. and type specimen of Maotianoascus octonarius Chen et Zhou, 1997.
Fig. S4. BSE and EDS analyses of Batofasciculus ramificans Hou et al., 1999.
Fig. S5. Additional material of Batofasciculus ramificans Hou et al., 1999.
Fig. S6. New material of Batofasciculus ramificans Hou et al., 1999.
Fig. S7. New material of Trigoides aclis Luo et Hu, 1999.
Fig. S8. New material of Trigoides aclis Luo et Hu, 1999.
Fig. S9. A diminutive, unnamed ctenophore from the Chengjiang Biota.
REFERENCES AND NOTES
- 1.Wallberg A., Thollesson M., Farris J. S., Jondelius U., The phylogenetic position of the comb jellies (Ctenophora) and the importance of taxonomic sampling. Cladistics 20, 558–578 (2004). [DOI] [PubMed] [Google Scholar]
- 2.Nosenko T., Schreiber F., Adamska M., Adamski M., Eitel M., Hammel J., Maldonado M., Müller W. E., Nickel M., Schierwater B., Vacelet J., Wiens M., Wörheide G., Deep metazoan phylogeny: When different genes tell different stories. Mol. Phylogenet. Evol. 67, 223–233 (2013). [DOI] [PubMed] [Google Scholar]
- 3.Tang F., Bengtson S., Wang Y., Wang X. L., Yin C. Y., Eoandromeda and the origin of Ctenophora. Evol. Dev. 13, 408–414 (2011). [DOI] [PubMed] [Google Scholar]
- 4.Dunn C. W., Hejnol A., Matus D. Q., Pang K., Browne W. E., Smith S. A., Seaver E., Rouse G. W., Obst M., Edgecombe G. D., Sørensen M. V., Haddock S. H., Schmidt-Rhaesa A., Okusu A., Kristensen R. M., Wheeler W. C., Martindale M. Q., Giribet G., Broad phylogenomic sampling improves resolution of the animal tree of life. Nature 452, 745–749 (2008). [DOI] [PubMed] [Google Scholar]
- 5.Hejnol A., Obst M., Stamatakis A., Ott M., Rouse G. W., Edgecombe G. D., Martinez P., Baguñà J., Bailly X., Jondelius U., Wiens M., Müller W. E., Seaver E., Wheeler W. C., Martindale M. Q., Giribet G., Dunn C. W., Assessing the root of bilaterian animals with scalable phylogenomic methods. Proc. R. Soc. B 276, 4315–4322 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ryan J. F., Pang K., Schnitzler C. E., Nguyen A. D., Moreland R. T., Simmons D. K., Koch B. J., Francis W. R., Havlak P.; NISC Comparative Sequencing Program, Smith S. A., Putnam N. H., Haddock S. H., Dunn C. W., Wolfsberg T. G., Mullikin J. C., Martindale M. Q., Baxevanis A. D., The genome of the ctenophore Mnemiopsis leidyi and its implications for cell type evolution. Science 342, 1242592 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Moroz L. L., Kocot K. M., Citarella M. R., Dosung S., Norekian T. P., Povolotskaya I. S., Grigorenko A. P., Dailey C., Berezikov E., Buckley K. M., Ptitsyn A., Reshetov D. O., Mukherjee K., Moroz T. P., Bobkova Y., Yu F., Kapitonov V. V., Jurka J., Bobkov Y. V., Swore J. J., Girardo D. O., Fodor A., Gusev F., Sanford R., Bruders R., Kittler E., Mills C. E., Rast J. P., Derelle R., Solovyev V. V., Kondrashov F. A., Swalla B. J., Sweedler J. V., Rogaev E. I., Halanych K. M., Kohn A. B., The ctenophore genome and the evolutionary origins of neural systems. Nature 510, 109–114 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Philippe H., Phylogenomics revives traditional views on deep animal relationships. Curr. Biol. 19, 706–712 (2009). [DOI] [PubMed] [Google Scholar]
- 9.Schierwater B., Eitel M., Jakob W., Osigus H. J., Hadrys H., Dellaporta S. L., Kolokotronis S. O., Desalle R., Concatenated analysis sheds light on early metazoan evolution and fuels a modern “urmetazoon” hypothesis. PLOS Biol. 7, e20 (2009). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.G. R. Harbison, in The Origins and Relationships of Lower Invertebrates, S. Conway Morris, J. D. George, R. Gibson, H. M. Platt, Eds. (Oxford Univ. Press, Oxford, 1985), pp. 78–100. [Google Scholar]
- 11.Conway Morris S., Collins D. H., Middle Cambrian ctenophores from the Stephen Formation, British Columbia, Canada, Philos. Trans. R. Soc. Lond. B 351, 279–308 (1996). [Google Scholar]
- 12.Podar M., Haddock S. H., Sogin M. L., Harbison G. R., A molecular phylogenetic framework for the phylum Ctenophora using 18S rRNA genes. Mol. Phylogenet. Evol. 21, 218–230 (2001). [DOI] [PubMed] [Google Scholar]
- 13.Chen J. Y., Zhou G. Q., Biology of the Chengjiang fauna. Bull. Natl. Mus. Nat. Sci. 10, 11–106 (1997). [Google Scholar]
- 14.H. L. Luo, S. Hu, L. Chen, S. Zhang, Y. Tao, Early Cambrian Chengjiang Fauna from Kunming Region, China (Yunnan Science and Technology Press, Kunming, 1999). [Google Scholar]
- 15.X. G. Hou, J. Bergstöm, H. F. Wang, X. H. Feng, A. L. Chen, The Chengjiang Fauna: Exceptionally Well-Preserved Animals from 530 Million Years Ago (Yunnan Science and Technology Press, Kunming, 1999). [Google Scholar]
- 16.Zhang Z. F., Li G. X., Holmer L. E., Brock G. A., Balthasar U., Skovsted C. B., Fu D. J., Zhang X. L., Wang H. Z., Butler A., Zhang Z. L., Cao C. Q., Han J., Liu J. N., Shu D. G., An early Cambrian agglutinated tubular lophophorate with brachiopod characters. Sci. Rep. 4, 4682 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Skovsted C. B., G. A. Brock, J. R. Paterson, L. E. Holmer, G. E. Budd, The scleritome of Eccentrotheca from the Lower Cambrian of South Australia: Lophophorate affinities and implications for tommotiid phylogeny. Geology 36, 171–174 (2008). [Google Scholar]
- 18.Liu J., Steiner M., Dunlop J. A., Keupp H., Shu D., Ou Q., Han J., Zhang Z., Zhang X., An armoured Cambrian lobopodian from China with arthropod-like appendages. Nature 470, 526–530 (2011). [DOI] [PubMed] [Google Scholar]
- 19.Han J., X. Zhang, Z. Zhang, D. Shu, A new platy-armored worm from the Early Cambrian Chengjiang Lagerstätte, South China, Acta Geol. Sin. 77, 1–6 (2003). [Google Scholar]
- 20.S. J. Gould, Wonderful Life (Norton, New York, 1989). [Google Scholar]
- 21.X. G. Hou, R. Aldridge, J. Bergstrom, D. J. Siveter, D. Siveter, X.-H. Feng, The Cambrian Fossils of Chengjiang, China: The Flowering of Early Animal Life (Blackwell, Oxford, 2004). [Google Scholar]
- 22.Hu S. X., Taphonomy and palaeoecology of the Early Cambrian Chengjiang Biota from eastern Yunnan, China, Berl. Paläobiol. Abh. 7, 1–197 (2005). [Google Scholar]
- 23.Hu S. X., M. Steiner, M. Zhu, B.-D. Erdtmann, H. Luo, L. Chen, B. Weber, Diverse pelagic predators from the Chengjiang Lagerstätte and the establishment of modern-style pelagic ecosystems in the early Cambrian. Palaeogeogr. Palaeoclimatol. Palaeoecol. 254, 307–316 (2007). [Google Scholar]
- 24.Han J., D. Shu, Z. Zhang, J. Liu, X. Zhang, Y. Yao, Preliminary notes on soft-bodied fossil concentrations from the Early Cambrian Chengjiang deposits. Chin. Sci. Bull. 51, 2482–2492 (2006). [Google Scholar]
- 25.E. E. Ruppert, R. S. Fox, R. D. Barnes, Invertebrate Zoology: A Functional Evolutionary Approach (Brooks/Cole, Belmont, 2004). [Google Scholar]
- 26.Aronova M. Z., Structural models of “simple” sense organs by the example of the first Metazoa. J. Evol. Biochem. Physiol. 45, 179–196 (2009). [PubMed] [Google Scholar]
- 27.H. Ehrlich, Biological Materials of Marine Origin: Invertebrates (Biologically-Inspired Systems) (Springer, New York, 2010). [Google Scholar]
- 28.M. L. Hernandez-Nicaise, in Microscopic Anatomy of Invertebrates, F. W. Harrison, Ed. (Wiley-Liss, New York, 1991), pp. 359–418. [Google Scholar]
- 29.Tamm S. L., Regeneration of ciliary comb plates in the ctenophore Mnemiopsis leidyi. i. morphology. J. Morphol. 273, 109–120 (2012). [DOI] [PubMed] [Google Scholar]
- 30.Shu D. G., Conway Morris S., Han J., Li Y., Zhang X. L., Hua H., Zhang Z. F., Liu J. N., Guo J. F., Yao Y., Yasui K., Lower Cambrian vendobionts from China and early diploblast evolution. Science 312, 731–734 (2006). [DOI] [PubMed] [Google Scholar]
- 31.Dzik J., Possible ctenophoran affinities of the Precambrian “sea-pen” Rangea. J. Morphol. 252, 315–334 (2002). [DOI] [PubMed] [Google Scholar]
- 32.Tang F., Eoandromeda octobrachiata and the evolution of early animals. Acta Geosci. Sin. 33, 721–729 (2012). [Google Scholar]
- 33.Forchielli A., M. Steiner, S. Hu, C. Lüter, H. Keupp, Taphonomy of the earliest Cambrian linguliform brachiopods. Acta Palaeontol. Pol. 59, 185–207 (2014). [Google Scholar]
- 34.Botting J. P., Reassessment of the problematic Burgess Shale sponge Takakkawia lineata Walcott, 1920. Can. J. Earth Sci. 49, 1087–1095 (2012). [Google Scholar]
- 35.Rigby J. K., Sponges of the Burgess shale (Middle Cambrian), British Columbia, Palaeontogr. Can. 2, 1–105 (1986). [Google Scholar]
- 36.Rigby J. K., Collins D., Sponges of the Middle Cambrian Burgess Shale and Stephen formations, British Columbia, R. Ontario Mus. Contrib. Sci. 1, 1–155 (2004). [Google Scholar]
- 37.J. Y. Chen, G. Q. Zhou, M. Y. Zhu, K. Y. Yeh, The Chengjiang Biota: A Unique Window of the Cambrian Explosion (National Museum of Natural Science, Taichung, 1996). [Google Scholar]
- 38.L. Z. Chen, H. L. Luo, S. X. Hu, G. Y. Yin, Z. W. Jiang, Z. L. Wu, F. Li, A. L. Chen, Early Cambrian Chengjiang Fauna in Eastern Yunnan, China (Yunnan Science and Technology Press, Kunming, 2002). [Google Scholar]
- 39.J. Y. Chen, The Dawn of Animal World (Jiangsu Science and Technology Press, Nanjing, 2004). [Google Scholar]
- 40.Chen J. Y., Schopf J. W., Bottjer D. J., Zhang C. Y., Kudryavtsev A. B., Tripathi A. B., Wang X. Q., Yang Y. H., Gao X., Yang Y., Raman spectra of a Lower Cambrian ctenophore embryo from SW Shaanxi, China, Proc. Natl. Acad. Sci. U.S.A. 104, 6289–6292 (2007). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.J. W. Schopf, A. B. Kudryavtsev, in Quantifying the Evolution of Early Life, M. Laflamme, J. D. Schiffbauer, Eds. (Springer, New York, 2011), pp. 241–270. [Google Scholar]
- 42.Anderson E., Schiffbauer J. D., Xiao S. H., Taphonomic study of Ediacaran organic-walled fossils confirms the importance of clay minerals and pyrite in Burgess Shale–type preservation. Geology 39, 643–646 (2011). [Google Scholar]
- 43.Cai Y. P., Schiffbauer J. D., Hua H., Xiao S. H., Preservational modes in the Ediacaran Gaojiashan Lagerstätte: Pyritization, aluminosilicification, and carbonaceous compression. Palaeogeogr. Palaeoclimatol. Palaeoecol. 326–328, 109–117 (2012). [Google Scholar]
- 44.Schiffbauer JD, Xiao S, Cai Y, Wallace AF, Hua H, Hunter J, Xu H, Peng Y, Kaufman AJ., A unifying model for Neoproterozoic–Palaeozoic exceptional fossil preservation through pyritization and carbonaceous compression. Nat. Commun. 5, e5754 (2014). [DOI] [PubMed] [Google Scholar]
- 45.R. C. Brusca, G. J. Brusca, Invertebrates (Sinauer Associates, Sunderland, MA, 2003). [Google Scholar]
- 46.G. R. Harbison, L. P. Madin, in Synopsis and Classification of Living Organisms (Vol. 1), S. P. Parker, Ed. (McGraw-Hill, New York, 1982), pp. 707–715. [Google Scholar]
- 47.Goloboff P. A., J. S. Farris, K. C. Nixon, TNT, a free program for phylogenetic analysis. Cladistics 24, 774–786 (2008). [Google Scholar]
- 48.Tang F., C. Yin, S. Bengtson, P. Liu, Z. Wang, L. Gao, Octoradiate spiral organisms in the Ediacaran of South China. Acta Geol. Sin. 82, 27–34 (2008). [Google Scholar]
- 49.Zhu M. Y., J. G. Gehling, S. Xiao, Y. Zhao, M. L. Droser, Eight-armed Ediacara fossil preserved in contrasting taphonomic windows from China and Australia. Geology 36, 867–870 (2008). [Google Scholar]
- 50.Swift H. F., Feeding behavior of the ctenophore Thalassocalyce inconstans: Revision of anatomy of the order Thalassocalycida. Mar. Biol. 156, 1049–1056 (2009). [Google Scholar]
- 51.L. H. Hyman, The Invertebrates: Protozoa Through Ctenophora (McGraw-Hill Publications, New York, 1940). [Google Scholar]
- 52.Harbison G. R., Miller R. L., Not all ctenophores are hermaphrodites. Studies on the systematics, distribution, sexuality and development of two species of Ocyropsis. Mar. Biol. 90, 413–424 (1986). [Google Scholar]
- 53.Tamm S. L., Calcium activation of macrocilia in the ctenophore Beroë. J. Comp. Physiol. A, 163, 23–31 (1988). [DOI] [PubMed] [Google Scholar]
- 54.Haddock S. H. D., Comparative feeding behavior of planktonic ctenophores. Integr. Comp. Biol. 47, 847–853 (2007). [DOI] [PubMed] [Google Scholar]
- 55.Tamm S. L., Formation of the statolith in the ctenophore Mnemiopsis leidyi. Biol. Bull. 227, 7–18 (2014). [DOI] [PubMed] [Google Scholar]
- 56.Lowe B., The role of Ca2+ in deflection-induced excitation of motile, mechanoresponsive balancer cilia in the ctenophore statocyst. J. Exp. Biol. 200, 1593–1606 (1997). [DOI] [PubMed] [Google Scholar]
- 57.Jager M, Dayraud C, Mialot A, Quéinnec E, le Guyader H, Manuel M., Evidence for involvement of Wnt signalling in body polarities, cell proliferation, and the neuro-sensory system in an adult ctenophore. PLOS One 8, e84363 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/6/e1500092/DC1
Systematic Paleontology
Taphonomy and Preservation
Cladistic Analysis
Table S1. Preservation of ctenophore specimens from the early Cambrian Chengjiang biota.
Table S2. List of characters and coding comments on ctenophore features used in cladistic analysis.
Table S3. Data matrix used in cladistic analysis of ctenophores.
Fig. S1. BSE and EDS analyses of Gemmactena actinala gen. et sp. nov.
Fig. S2. Additional material of Thaumactena ensis gen. et sp. nov.
Fig. S3. Additional material of Galeactena hemispherica gen. et sp. nov. and type specimen of Maotianoascus octonarius Chen et Zhou, 1997.
Fig. S4. BSE and EDS analyses of Batofasciculus ramificans Hou et al., 1999.
Fig. S5. Additional material of Batofasciculus ramificans Hou et al., 1999.
Fig. S6. New material of Batofasciculus ramificans Hou et al., 1999.
Fig. S7. New material of Trigoides aclis Luo et Hu, 1999.
Fig. S8. New material of Trigoides aclis Luo et Hu, 1999.
Fig. S9. A diminutive, unnamed ctenophore from the Chengjiang Biota.