Decisions that reduce insect diversity and decrease the network strength in insect communities result in higher pest abundance.
Keywords: Crop science, Ecology, entomology
Abstract
Recent shifts in agricultural practices have resulted in altered pesticide use patterns, land use intensification, and landscape simplification, all of which threaten biodiversity in and near farms. Pests are major challenges to food security, and responses to pests can represent unintended socioeconomic and environmental costs. Characteristics of the ecological community influence pest populations, but the nature of these interactions remains poorly understood within realistic community complexities and on operating farms. We examine how species diversity and the topology of linkages in species’ abundances affect pest abundance on maize farms across the Northern Great Plains. Our results show that increased species diversity, community evenness, and linkage strength and network centrality within a biological network all correlate with significantly reduced pest populations. This supports the assertion that reduced biological complexity on farms is associated with increased pest populations and provides a further justification for diversification of agroecosystems to improve the profitability, safety, and sustainability of food production systems. Bioinventories as comprehensive as the one conducted here are conspicuously absent for most agroecosystems but provide an important baseline for community and ecosystem ecology and the effects of food production on local biodiversity and ecosystem function. Network analyses of abundance correlations of entire communities (rather than focal interactions, for example, trophic interactions) can reveal key network characteristics, especially the importance and nature of network centrality, which aid in understanding how these communities function.
INTRODUCTION
The complexity of interactions that form a biological community produces a suite of community attributes that may be correlated with ecosystem function. The number or abundance of taxa and the relative taxonomic composition of communities are important bases for understanding the role of biodiversity in ecosystem function (1–4). In addition to identifying which constituents compose a community, the species interactions within a community dictate the types and magnitude of potential ecosystem goods and services, and network analysis can be a powerful tool in describing these interactions (5–7). Disproportionately large strides have been made in the social sciences in applying network analysis to solve problems (8), and ecological network analysis is arguably still comparably in its infancy. Efforts to apply network analyses to ecological systems largely focus on simplified interaction matrices representing antagonistic relationships (food webs) (5, 6, 9, 10) with a few exceptions that focus on mutualisms (for example, plant-pollinator interactions) (11). Although conceptually simple in their execution, ecological networks based on abundance correlations simultaneously account for predicted and unexpected interactions to establish how species interaction networks result in a particular ecosystem function, such as reducing pest abundance.
Agroecosystems represent the dominant biome on Earth [25 to 40% of land is devoted to agriculture (12, 13)], and the importance of decisions made within farmland to both food security and health of the environment is well documented (14–16). Recent shifts in agricultural practices have resulted in altered pesticide use patterns and increases in prophylactic pesticide applications in several prominent crops (17–20), land use intensification, and landscape simplification (21–23), all of which threaten biodiversity in and near farms (24–26). Pests are major challenges to food security (27), and responses to pests can represent unintended socioeconomic and environmental costs (28, 29). Despite interest in key species within particular crops (for example, pests and their natural enemies), it is arguable that there remains a poor understanding of biological communities within agroecosystems and how community complexity contributes to ecosystem functions. For example, one plant species (Zea mays) currently occupies nearly 5% of the land surface of the contiguous United States (and 95% of certain counties) (30), and comprehensive bioinventories of the arthropod species that occur within this habitat throughout an extended region are scarce. This is despite the tremendous economic investments (we estimate that $3.2 billion was spent to manage maize pests in the United States during 2013) that are input into maize fields to manage insect communities. With few exceptions, agroecosystems have escaped study by formal network analyses (31); as a case in point, none of the 313 food webs compiled into two comprehensive databases (32, 33) focused on agroecosystems. The prevalence and importance of maize and its pest complex to agriculture make this a good agroecosystem to focus on for understanding what components of biodiversity affect pest proliferation.
RESULTS
Here, we explore how the structure of a diverse arthropod community [37,185 arthropod specimens segregated into 106 operational taxonomic units (OTUs)] affects pest abundance on actual maize farms (53 operating farms were inventoried). Species diversity within the arthropod community is negatively correlated with pest abundance on maize farms. Specifically, pests declined as species diversity (Shannon H: F1,51 = 8.18, N = 53, P = 0.006) and community evenness (J: F1,51 = 9.03, N = 53, P = 0.004) within a community increased (Fig. 1). Species richness (F1,52 = 0.38, N = 53, P = 0.54) and total non–pest abundance (F1,52 = 0.29, N = 53, P = 0.59) were not correlated with pest abundance on these farms. This research suggests that it is not the number or abundance of species within a community, but rather the balance of species within these communities that contributes to pest suppression in maize fields. This confirms the importance of community evenness in pest suppression (2) and suggests that species diversity of the entire community [not just higher trophic levels (34)] may contribute to pest suppression within realistic arthropod communities. To be clear, richness and abundance are integral components of diversity and have been correlated to specific mechanisms that contribute to pest management, for example, predation (35, 36). Nevertheless, more balance in the relative abundances of species within communities clearly influences pest proliferation.
Fig. 1. High biodiversity is correlated with low pest abundance in maize.
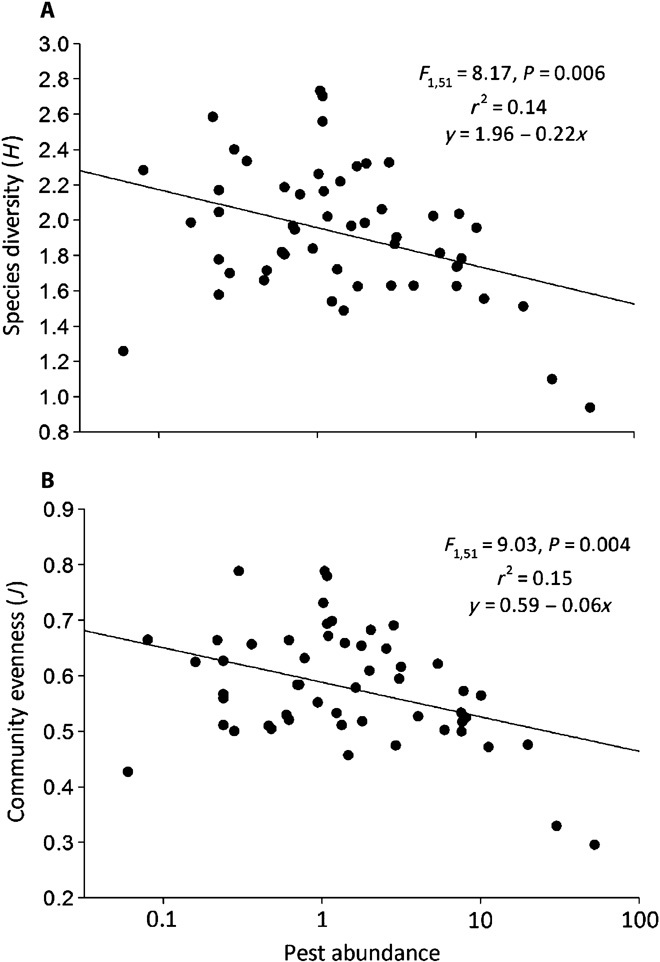
(A and B) Here, biodiversity is measured as the Shannon Index (A) and community evenness (B). Each data point represents a single farm; the complete foliar arthropod community was identified at each field, and the pest abundance (Diabrotica spp., lepidopteran pests, and aphids) per plant (log-transformed) was enumerated.
There were strong trends in network characteristics that contribute to pest reduction within a community (fig. S1). On its most basic level, the total number of linkages among species [total adjacency index (m): F1,9 = 9.71, N = 10, P = 0.01; r2 = 0.55; y = 184.73 − 63.68x], the average number of linkages per species [connectivity (C): F1,9 = 8.77, N = 10, P = 0.01], and the average proportion of linkages per species relative to the number of linkages possible in a network [average degree (d): F1,9 = 5.72, N = 10, P = 0.04] were negatively correlated with pest abundance (Fig. 2 displays connectivity and average degree). The proportion of taxa abundances not linked with other members of the community was positively correlated with pest abundance (F1,9 = 4.73, N = 10, P = 0.06; r2 = 0.37; y = 0.17 + 0.07x). We interpret this as another metric of network strength; unconnected species within the network weaken the overall degree and connectivity of the network. Species diversity or evenness within a community was not correlated with average degree (H: F1,9 = 0.87, N = 10, P = 0.37; J: F1,9 = 1.13, P = 0.32), indicating that although networks are inherently reliant on aspects of species diversity and evenness, network characteristics operate independently of species diversity in affecting pest populations (9, 31). The results demonstrate that more cohesive species networks, for example, those that have more significant correlations between species’ abundances, have fewer pests. Moreover, networks that have more taxa that do not respond numerically to other taxa in the community have greater pest populations.
Fig. 2. Increasing network linkage strength and reduced pest populations in maize.
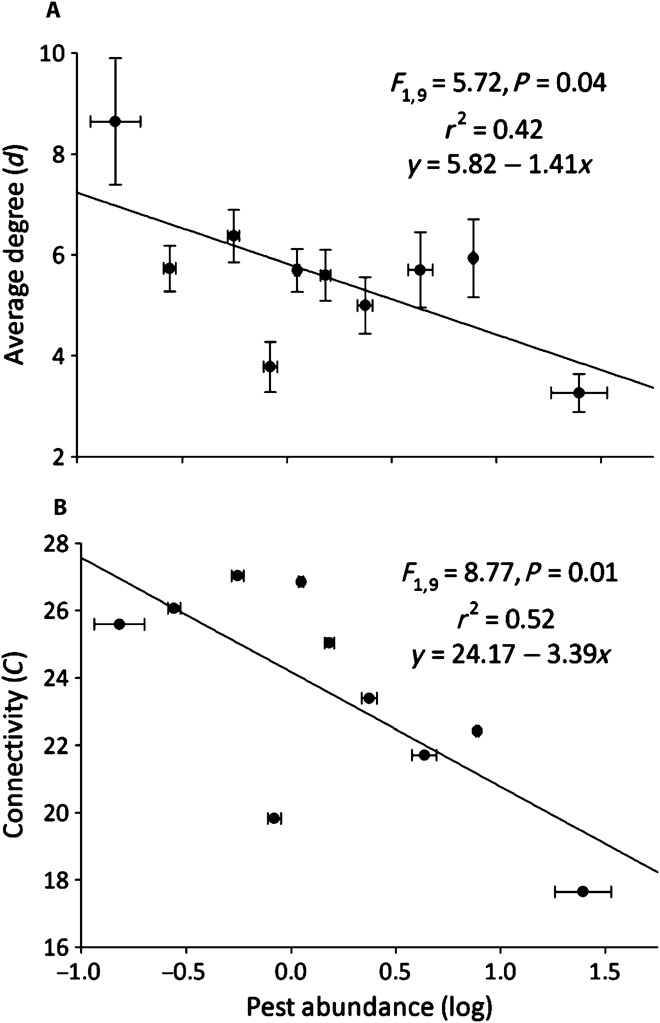
(A and B) Here, network strength is represented as the average proportion of linkages per species relative to those possible within the network (degree; A) and the average number of linkages per species within the network (connectivity; B). The entire foliar arthropod community was described on 53 maize farms, and pest abundance (Diabrotica spp., lepidopteran pests, and aphids) per plant was recorded. Farms were assigned to 1 of 10 groups based on their pest abundance (n = 5 to 6 farms each), and pairwise Spearman correlation tests were conducted for all taxa within the community; linkages were assigned to significant coefficients. Error bars represent SEM. Connectivity (Randić connectivity index) is a network-wide metric, so variance is not applicable.
A deeper exploration of the network topology reveals that the distribution of these linkages within the network also influences pest abundance. Specifically, networks with high variation in the number of linkages per species, divided by the maximum variation that a network can have (degree centralization; F1,9 = 4.98, N = 10, P = 0.05; Fig. 3), are associated with higher pest abundance. This was also true for networks that had an asymmetrical distribution of linkages that resulted in a few highly connected central species (F1,9 = 4.98, N = 10, P = 0.05; r2 = 0.39; y = 6.00 − 1.34x). Three other metrics of centrality were also measured: closeness centrality that accounts for the number of linkages required for a species within the network to reach another species in the network; eigenvector centrality measures not only how connected a focal taxon is but also how connected its nearest neighbors are; and betweenness centrality, which measures how many distinct groups of taxa (components) are created when linkages are randomly removed from the network. Low-average eigenvector centrality (F1,9 = 3.67, N = 10, P = 0.09; r2 = 0.31; y = 0.053 − 0.0064x) and low closeness centrality (F1,9 = 7.18, N = 10, P = 0.03; r2 = 0.47; y = 0.07 − 0.012x) were correlated with higher pest abundance in a community. Given the importance of closeness centrality, it was surprising to find that average path length (or the number of links required for a species to reach another in the network) [mean (SEM), 1.65 ± 0.24 links; F1,9 = 0.78, N = 10, P = 0.40] and maximum path length (4.60 ± 0.67 links; F1,9 = 1.03, N = 10, P = 0.34) within a network were uncorrelated with pest abundance. Betweenness centrality of a network (0.014 ± 0.007; F1,9 = 1.24, P = 0.30), the number of distinct components of species (F1,9 = 1.83, N = 10, P = 0.21), and the size of the largest component (the component within the network with the greatest set of connected species) (F1,9 = 1.64, N = 10, P = 0.24) were not tied to pest populations. There were a few large components and many small components of species within each network; the mean (SEM) number of components was 21.40 ± 1.56, and the mean largest of these per network contained 18.84 ± 2.61% taxa. This characteristic has previously been shown to increase the stability of ecological networks based on trophic interactions (37). The lower centrality we observed in these networks is in contrast to ecological networks based on other interaction types, which often display strong asymmetry in the degree magnitudes among species (38). In sum, ecological networks with compact subnetworks containing more centrally connected species with highly connected neighbors have fewer pests (fig. S1).
Fig. 3. Network centralization is associated with fewer pests in maize.
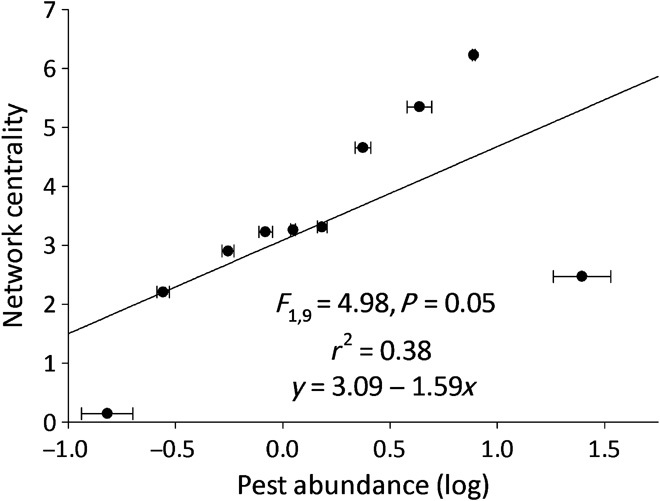
The entire foliar arthropod community was described on 53 maize farms, and pest abundance (Diabrotica spp., lepidopteran pests, and aphids) per plant was recorded. Farms were assigned to 1 of 10 groups based on their pest abundance (n = 5 to 6 farms each), and pairwise Spearman correlation tests were conducted for all taxa within the community; linkages were assigned to significant coefficients. Network centralization is defined as a sum of the ratio of the variation in d for a species to the maximum variation in d in the network. Error bars represent SEM.
DISCUSSION
Realizing that the number and topology of abundance correlations within the community network are associated with fewer pests allows the deeper exploration of specific antagonistic and mutualistic mechanisms that promote pest suppression and species conservation. Examples of diversity-dependent mechanisms that facilitate pest suppression include competition and predation (39), and shared physiological requirements for habitat and abiotic conditions also likely influence these abundance correlations (40). Predator diversity, species identity, species richness, community evenness, and niche compartmentalization all influence pest populations (1, 34, 35, 41). Direct or indirect herbivore competition for shared resources may also restrict the performance of a focal pest (42, 43). The resulting positive or negative connections between relative abundances of species are likely reflected in our network analysis. The reality is that interaction networks are complex, and unforeseen drivers of correlations in species abundances in scale [for example, microbial symbioses (44, 45), landscapes (24)] and time (legacy effects of previous interactions) are likely overlooked when focusing on just one subset of interactions (for example, trophic interactions, pollination webs, a snapshot of abundance correlations). A central question that remains is why or how the number of linkages among species is inherently tied to pest performance.
The importance of the association of biodiversity and ecological network structure with low pest populations provides goals that can be targeted with sustainable cropping systems that require minimal inputs for pest management. Our research suggests that agronomic practices that promote high levels of arthropod diversity fundamentally require fewer agronomic inputs. For example, reducing tillage (46, 47), increasing vegetation diversity on farms [for example, lengthening crop rotations, including cover crops in rotations, intercropping, managing field margins (48)], and developing minimal-till organic agriculture (49) should help increase biodiversity. Thus, the level of diversity and network strength and centrality necessary to reduce pest populations under varying crop production scenarios merits additional attention. Many factors influence regional and local pest populations in focal fields, including producer decisions [for example, Bt crop planting (50)], cropping behaviors, and climates. These research results also provide mechanistic support to the notion that using pest management practices that reduce biodiversity and species interactions will create systems where pests will continually pose problems [that is, the pesticide treadmill (51)]. A scenario worth further attention is the effects of Bt maize on these interactions; Bt maize eliminates or reduces target pest species, and due to its specificity, this pest management practice seldom reduces nontarget arthropod abundances (52). Thus, a pest management strategy that reduces a particular pest while preserving the local arthropod community could feasibly reinforce management benefits of other pests in the system provided by insect community structure and network strength. The effects of agronomic practices on attributes of network structure that are influential in reducing pest populations and the effects of species abundance correlations on other ecosystem goods and services remain important areas of future research. Finally, network and community structure change substantially over time, and other study systems and geographic regions may experience drivers different from those present in the maize agroecosystem of the Northern Great Plains. Thus, it might be expected that the spatiotemporal dynamics of community structure (how these communities change) could provide further insight to pest outbreaks.
METHODS
Experimental design
Maize fields (N = 53, >1.6 km apart) were selected throughout a ≈95,000 km2 area in eastern South Dakota over two study years (40). Conditions in the fields represent a continuum of precipitation (from 400 to 660 mm annually), elevation (305 to 550 m above sea level), mean annual temperatures (4.4° to 11.1°C), Bt corn adoption rates (11 of 13 respondents from this study planted Bt maize elsewhere on their farms), crop rotation patterns (96% of 48 respondents from this study practiced a 2- to 5-year crop rotation), crop diversity in the surrounding landscape matrix (3 to 10 crop species planted within a 3000-m radius), proportion of the landscape devoted to cropland (4 to 79%; 3000-m radius), proportion of the surrounding landscape planted with maize (2 to 48%; 3000-m radius), and heterogeneity of habitats within the landscape (measured as Shannon H; 0.56 to 2.07; 3000-m radius). Thus, the extensive number of sites examined for this study was strategized to produce a range of distinct arthropod communities, although specific site histories were not always recorded at each site, and not every contingency or landscape characteristic was considered when selecting experimental sites. Non-Bt maize fields were at least 4 ha in size and received no insecticides or 0.25 mg of active ingredient per kernel of thiamethoxam or clothianidin. Non-Bt maize was specifically selected to allow the examination of undisturbed arthropod communities with intact pest communities. Recent high levels of Bt corn adoption reduce or eliminate some of the focal pests from the system (50), and the effects of pest and farm management practices on community dynamics are worthy of further study. Arthropods were sampled within 10 days of maize anthesis. Over 15 years of field experience, the authors have observed that the insect community is most robust during this period of the season [the size of the plants is greatest at this point, maize pollen is attractive to a broad suite of taxa, and basal species in the food web (for example, aphids, thrips) become apparent], and this is the only time during the season that most serious maize pests co-occur in the plant foliage. Maize plants (n = 50 per site) were collected at least 8 m from the field margin and dissected in situ. All arthropods on or in the plant were identified to the lowest taxonomic unit possible under field conditions. The numbers of each taxon and numbers of predators and herbivores per plant were subsequently estimated. The total number of pests (aphids, Diabrotica spp. adults, Ostrinia nubilalis and Loxagrotis albicosta preimaginal life stages, and Trichoplusia ni and Helicoverpa zea larvae) per plant was calculated for each farm. Population estimates showed that our site selection produced a range of pest populations at these sites (as examples, Diabrotica spp. ranged from 0 to 7.6 beetles per site, O. nubilalis ranged from 0 to 0.6 larvae, and aphids ranged from 0 to 52 per plant) (40).
Community characteristics
Species diversity (Shannon H), evenness (J), species richness, and total arthropod abundance were compared to pest densities on each farm using linear regression analysis. The 53 farms were divided into 10 groups (each with 5 to 6 farms) and a separate network was generated for each group. The farms were stratified according to pest abundance, where the first network had the five farms with the lowest pest density (fig. S1A) and the final network was composed of the five farms with the highest pest density (fig. S1B). Each network was constructed from significant correlations (Spearman rank) that were identified among the abundances of OTUs per species in each group of farms, and the linkage density (L; number of significant linkages per taxon) was determined. Adjacency index (m) of a network is the total number of linkages between species. Average degree (d) of a network is defined as 2L/S (where S is the number of taxa in the network). Connectivity (C) of the network was measured using the Randić connectivity index, or the sum of 1/(didj)1/2, where di and dj are the degrees of two linked species. Components (or compartments) of each network were defined as distinct, unconnected clusters of species. Four metrics of network centralization were calculated. Degree centralization of a network is calculated as the variation in the degrees divided by the maximum variation in degrees that a network can have. Under this metric, a network with greater discrepancies in its range of d is considered more centralized. Closeness centrality of a specific taxon is the number of species within a network divided by the sum of all linkages (path length) between the focal taxon and all others in the network. We calculated the average closeness centrality for each network. Eigenvector centrality ranks centrality on the basis of how many linkages a taxon has, but also considers how connected its most adjacent neighbors are. The eigenvector centrality of a vertex is a measure of the extent to which it is linked to vertices with high eigenvector centrality. Finally, betweenness centrality (also known as robustness) considers centrality as how many components are created by deleting certain taxa from the network.
Statistical analysis
All network parameters were generated with algorithms used in Pajek64 (version 3.14, http://pajek.imfm.si/). Mean m, d, C, and centrality metrics per group were regressed with the mean pest abundance (log-transformed) in each group using least squares linear regression analysis. The mean species diversity (H) and evenness (J) per group were contrasted with d using linear regression analysis. Values are considered significantly different when α < 0.10, and all statistics were conducted using Systat 13 (Systat Software Inc.).
Acknowledgments
J. Fergen, E. Opuku, T. McDonald, M. Bredeson, C. Kruse, M. Longfellow, R. Schmid, and A. Walter helped gather and collate the arthropod collections. Mention of trade names or commercial products in this publication is solely for the purpose of providing specific information and does not imply recommendation or endorsement by the U.S. Department of Agriculture (USDA). Funding: This research was funded by USDA-AFRI (Agriculture and Food Research Initiative) award 2010-85605-20552 and USDA–National Institute of Food and Agriculture–Biotechnology Risk Assessment Research Grants project SDW-2012-01639. Author contributions: J.G.L. and S.W.F. contributed equally to all aspects of this research project and manuscript preparation. Competing interests: The authors declare that they have no competing interests.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/6/e1500558/DC1
Fig. S1. Species networks representing the maize arthropod communities with lowest (A) and highest (B) pest abundances.
REFERENCES AND NOTES
- 1.Finke D. L., Snyder W. E., Niche partitioning increases resource exploitation by diverse communities. Science 321, 1488–1490 (2008). [DOI] [PubMed] [Google Scholar]
- 2.Crowder D. W., Northfield T. D., Strand M. R., Snyder W. E., Organic agriculture promotes evenness and natural pest control. Nature 466, 109–112 (2010). [DOI] [PubMed] [Google Scholar]
- 3.Hooper D. U., Chapin F. S. III, Ewel J. J., Hector A., Inchausti P., Lavorel S., Lawton J. H., Lodge D. M., Loreau M., Naeem S., Schmid B., Setälä H., Symstad A. J., Vandermeer J., Wardle D. A., Effects of biodiversity on ecosystem functioning: A consensus of current knowledge. Ecol. Monogr. 75, 3–35 (2005). [Google Scholar]
- 4.Cardinale B. J., Srivastava D. S., Duffy J. E., Wright J. P., Downing A. L., Sankaran M., Jouseau C., Effects of biodiversity on the functioning of trophic groups and ecosystems. Nature 443, 989–992 (2006). [DOI] [PubMed] [Google Scholar]
- 5.Dunne J. A., Williams R. J., Martinez N. D., Food-web structure and network theory: The role of connectance and size. Proc. Natl. Acad. Sci. U.S.A. 99, 12917–12922 (2002). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Montoya J. M., Pimm S. L., Solé R. V., Ecological networks and their fragility. Nature 442, 259–264 (2006). [DOI] [PubMed] [Google Scholar]
- 7.Solé R. V., Montoya J. M., Complexity and fragility in ecological networks. Proc. Biol. Sci. 268, 2039–2045 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.J. Scott, P. J. Carrington, Eds., The SAGE Handbook of Social Network Analysis (SAGE, Los Angeles, CA, 2012). [Google Scholar]
- 9.Tylianakis J. M., Laliberté E., Nielsen A., Bascompte J., Conservation of species interaction networks. Biol. Conserv. 143, 2270–2279 (2010). [Google Scholar]
- 10.G. A. Polis, K. O. Winemiller, Eds., Food Webs: Integration of Patterns and Dynamics (Chapman and Hall, New York, 1996). [Google Scholar]
- 11.Hagen M., Kissling W. D., Rasmussen C., De Aguiar M. A. M., Brown L. E., Carstensen D. W., Alves-Dos-Santos I., Dupont Y. L., Edwards F. K., Genini J., Guimarães P. R. Jr, Jenkins G. B., Jordano P., Kaiser-Bunbury C. N., Ledger M. E., Maia K. P., Marquitti F. M. D., Mclaughlin Ó., Morellato L. P. C., O’Gorman E. J., Trøjelsgaard K., Tylianakis J. M., Vidal M. M., Woodward G., Olesen J. M., Biodiversity, species interactions, and ecological networks in a fragmented world. Adv. Ecol. Res. 46, 89–210 (2012). [Google Scholar]
- 12.Food and Agriculture Organization (FAO), The State of Food and Agriculture: Paying Farmers for Environmental Services 38 (FAO Food and Agricultural Series, Food and Agriculture Organization of the United Nations, Rome, Italy, 2007). [Google Scholar]
- 13.Millenium Ecosystem Assessment (MEA), Ecosystems and Human Well-being: Biodiversity Synthesis (World Resources Institute, Washington, DC, 2005). [Google Scholar]
- 14.Donner S. D., Kucharik C. J., Corn-based ethanol production compromises goal of reducing nitrogen export by the Mississippi River. Proc. Natl. Acad. Sci. U.S.A. 105, 4513–4518 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Tscharntke T., Clough Y., Wanger T. C., Jackson L., Motzke I., Perfecto I., Vandermeer J., Whitbread A., Global food security, biodiversity conservation and the future of agricultural intensification. Biol. Conserv. 151, 53–59 (2012). [Google Scholar]
- 16.Godfray H. C. J., Garnett T., Food security and sustainable intensification. Philos. Trans. R Soc. Lond. B Biol. Sci. 369, 20120273 (2014). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Fausti S. W., McDonald T. M., Lundgren J. G., Li J., Keating A. R., Catangui M., Insecticide use and crop selection in regions with high GM adoption rates. Renew. Agr. Food Syst. 27, 295–304 (2012). [Google Scholar]
- 18.Douglas M. R., Tooker J. F., Large-scale deployment of seed treatments has driven rapid increase in use of neonicotinoid insecticides and preemptive pest management in U.S. field crops. Environ. Sci. Technol. 49, 5088–5097 (2015). [DOI] [PubMed] [Google Scholar]
- 19.Gray M. E., Relevance of traditional integrated pest management (IPM) strategies for commercial corn producers in a transgenic agroecosystem: A bygone era? J. Agr. Food Chem. 59, 5852–5858 (2011). [DOI] [PubMed] [Google Scholar]
- 20.Meehan T. D., Werling B. P., Landis D. A., Gratton C., Agricultural landscape simplification and insecticide use in the Midwestern United States. Proc. Natl. Acad. Sci. U.S.A. 108, 11500–11505 (2011). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.S. Wallander, R. Claassen, C. Nickerson, The Ethanol Decade: An Expansion of U.S. Corn Production, 2000–2009 (Economic Information Bulletin Number 79, U.S. Department of Agriculture, Economic Research Service, 2011). [Google Scholar]
- 22.Fausti S. W., Lundgren J. G., The causes and unintended consequences of a paradigm shift in corn production practices. Environ. Sci. Pol. 52, 41–50 (2015). [Google Scholar]
- 23.Johnston C. A., Agricultural expansion: Land use shell game in the U.S. Northern Plains. Landscape Ecol. 29, 81–95 (2014). [Google Scholar]
- 24.Tscharntke T., Tylianakis J. M., Rand T. A., Didham R. K., Fahrig L., Batáry P., Bengtsson J., Clough Y., Crist T. O., Dormann C. F., Ewers R. M., Fründ J., Holt R. D., Holzschuh A., Klein A. M., Kleijn D., Kremen C., Landis D. A., Laurance W., Lindenmayer D., Scherber C., Sodhi N., Steffan-Dewenter I., Thies C., van der Putten W. H., Westphal C., Landscape moderation of biodiversity patterns and processes—Eight hypotheses. Biol. Rev. Camb. Philos. Soc. 87, 661–685 (2012). [DOI] [PubMed] [Google Scholar]
- 25.Tscharntke T., Klein A.-M., Kruess A., Steffan-Dewenter I., Thies C., Landscape perspectives on agricultural intensification and biodiversity—Ecosystem service management. Ecol. Letters 8, 857–874 (2005). [Google Scholar]
- 26.Wright C. K., Wimberly M. C., Recent land use change in the Western Corn Belt threatens grasslands and wetlands. Proc. Natl. Acad. Sci. U.S.A. 110, 4134–4139 (2013). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oerke E. C., Crop losses to pests. J. Agr. Sci. 144, 31–43 (2006). [Google Scholar]
- 28.Pimentel D., Environmental and economic costs of the application of pesticides primarily in the United States. Environ. Dev. Sust. 7, 229–252 (2005). [Google Scholar]
- 29.van der Werf H. M. G., Assessing the impact of pesticides on the environment. Agr. Ecosyst. Environ. 60, 81–96 (1996). [Google Scholar]
- 30.National Agricultural Statistics Service (NASS); www.nass.usda.gov [accessed 28 May 2015].
- 31.D. A. Bohan, G. Woodward, in Advances in Ecological Research: Ecological Networks in an Agricultural World, G. Woodward, D. A. Bohan, Eds. (Academic Press, Amsterdam, Netherlands, 2013), pp. 1–66. [Google Scholar]
- 32.J. E. Cohen, F. Briand, C. M. Newman, Community Food Webs: Data and Theory (Springer-Verlag, Berlin, 1990). [Google Scholar]
- 33.Eklöf A., Jacob U., Kopp J., Bosch J., Castro-Urgal R., Chacoff N. P., Dalsgaard B., de Sassi C., Galetti M., Guimarães P. R., Lomáscolo S. B., González A. M. M., Pizo M. A., Rader R., Rodrigo A., Tylianakis J. M., Vázquez D. P., Allesina S., The dimensionality of ecological networks. Ecol. Letters 16, 577–583 (2013). [DOI] [PubMed] [Google Scholar]
- 34.Letourneau D. K., Jedlicka J. A., Bothwell S. G., Moreno C. R., Effects of natural enemy biodiversity on the suppression of arthropod herbivores in terrestrial ecosystems. Annu. Rev. Ecol. Evol. Syst. 40, 573–592 (2009). [Google Scholar]
- 35.Lundgren J. G., Fergen J. K., Predator community structure and trophic linkage strength to a focal prey: The influence of the prey’s anti-predator defense. Mol. Ecol. 23, 3790–3798 (2014). [DOI] [PubMed] [Google Scholar]
- 36.Griffiths G. J. K., Wilby A., Crawley M. J., Thomas M. B., Density-dependent effects of predator species-richness in diversity–function studies. Ecology 89, 2986–2993 (2008). [DOI] [PubMed] [Google Scholar]
- 37.Krause A. E., Frank K. A., Mason D. M., Ulanowicz R. E., Taylor W. W., Compartments revealed in food-web structure. Nature 426, 282–285 (2003). [DOI] [PubMed] [Google Scholar]
- 38.Bascompte J., Jordano P., Plant-animal mutualistic networks: The architecture of biodiversity. Annu. Rev. Ecol. Evol. Syst. 38, 567–593 (2007). [Google Scholar]
- 39.Root R. B., Organization of a plant-arthropod assocation in simple and diverse habitats: The fauna of collards (Brassica oleracea). Ecol. Monogr. 43, 95–124 (1973). [Google Scholar]
- 40.Lundgren J. G., McDonald T. M., Rand T. A., Fausti S. W., Spatial and numerical relationships of arthropod communities associated with key pests of maize. J. Appl. Entomol. 139, 446–456 (2015). [Google Scholar]
- 41.Straub C. S., Snyder W. E., Species identity dominates the relationship between predator biodiversity and herbivore suppression. Ecology 87, 277–282 (2006). [DOI] [PubMed] [Google Scholar]
- 42.Chilcutt C. F., Cannibalism of Helicoverpa zea (Lepidoptera: Noctuidae) from Bacillus thuringiensis (Bt) transgenic corn versus non-Bt corn. J. Econ. Entomol. 99, 728–732 (2006). [DOI] [PubMed] [Google Scholar]
- 43.Woodson W. D., Interspecific and intraspecific larval competition between Diabrotica virgifera virgifera and Diabrotica barberi (Coleoptera: Chrysomelidae). Environ. Entomol. 23, 612–616 (1994). [Google Scholar]
- 44.Dillon R. J., Dillon V. M., The gut bacteria of insects: Nonpathogenic interactions. Annu. Rev. Entomol. 49, 71–92 (2004). [DOI] [PubMed] [Google Scholar]
- 45.Clay K., Schardl C., Evolutionary origins and ecological consequences of endophyte symbiosis with grasses. Am. Nat. 160, S99–S127 (2002). [DOI] [PubMed] [Google Scholar]
- 46.Lehman R. M., Cambardella C. A., Stott D. E., Acosta-Martinez V., Manter D. K., Buyer J. S., Maul J. E., Smith J. L., Collins H. P., Halvorson J. J., Kremer R. J., Lundgren J. G., Ducey T. F., Jin V. L., Karlen D. L., Understanding and enhancing soil biological health: The solution for reversing soil degradation. Sustainability 7, 988–1027 (2015). [Google Scholar]
- 47.Kladivko E. J., Tillage systems and soil ecology. Soil Tillage Res. 61, 61–76 (2001). [Google Scholar]
- 48.Letourneau D. K., Armbrecht I., Rivera B. S., Lerma J. M., Carmona E. J., Daza M. C., Escobar S., Galindo V., Gutiérrez C., López S. D., Mejía J. L., Rangel A. M., Rangel J. H., Rivera L., Saavedra C. A., Torres A. M., Trujillo A. R., Does plant diversity benefit agroecosystems? A synthetic review. Ecol. Appl. 21, 9–21 (2011). [DOI] [PubMed] [Google Scholar]
- 49.Bengtsson J., Ahnström J., Weibull A.-C., The effects of organic agriculture on biodiversity and abundance: A meta-analysis. J. Appl. Ecol. 42, 261–269 (2005). [Google Scholar]
- 50.Hutchison W. D., Burkness E. C., Mitchell P. D., Moon R. D., Leslie T. W., Fleischer S. J., Abrahamson M., Hamilton K. L., Steffey K. L., Gray M. E., Hellmich R. L., Kaster L. V., Hunt T. E., Wright R. J., Pecinovsky K., Rabaey T. L., Flood B. R., Raun E. S., Areawide suppression of European corn borer with Bt maize reaps savings to non-Bt maize growers. Science 330, 222–225 (2010). [DOI] [PubMed] [Google Scholar]
- 51.R. Van den Bosch, The Pesticide Conspiracy (University of California Press, Berkeley, CA, 1989), pp. 226. [Google Scholar]
- 52.Wolfenbarger L. L., Naranjo S. E., Lundgren J. G., Bitzer R. J., Watrud L. S., Bt crop effects on functional guilds of non-target arthropods: A meta-analysis. PLOS One 3, e2118 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/6/e1500558/DC1
Fig. S1. Species networks representing the maize arthropod communities with lowest (A) and highest (B) pest abundances.


