Structures of gold clusters resemble the benzene and DNA molecules and reveal a “supermolecule” origin of the magic-sized clusters.
Keywords: Gold cluster, Structure, nanoparticle, anisotropic growth
Abstract
Magic-sized clusters, as the intermediate state between molecules and nanoparticles, exhibit critical transitions of structures and material properties. We report two unique structures of gold clusters solved by x-ray crystallography, including Au40 and Au52 protected by thiolates. The Au40 and Au52 clusters exhibit a high level of complexity, with the gold atoms in the cluster first segregated into four-atom tetrahedral units—which then coil up into a Kekulé-like ring in the Au40 cluster and a DNA-like double helix in Au52. The solved structures imply a new “supermolecule” origin for revealing the stability of certain magic-sized gold clusters. The formation of supermolecular structures originates in the surface ligand bonding–induced stress and its propagation through the face-centered cubic (FCC) lattice. Moreover, the two structures reveal anisotropic growth of the FCC lattice in the cluster regime, which provides implications for the important roles of ligands at the atomic level. The rich structural information encoded in the Au40 and Au52 clusters provides atomic-scale insight into some important issues in cluster, nanoscale, and surface sciences.
INTRODUCTION
Metal clusters containing tens to hundreds of metal atoms constitute an important regime that bridges molecular materials (typically <1 nm) and nanoparticle materials (typically >3 nm). Because of their intermediate state, metal clusters often exhibit distinct properties in catalysis, optics, electronics, and magnetism, and thus hold great potential as functional materials (1–4). On the other hand, clusters might share some features with molecules and nanoparticles: they can be viewed as the miniature of nanoparticles, or the “maxiature” of molecules. Important molecular and nanoscale information may be encoded inside the cluster, and many fundamental issues (for example, the origin of magic sizes and the structural evolution pattern) and real-world applications require the knowledge of atomic structures of clusters.
The thiolate-protected gold clusters referred to as Aun(SR)m have emerged as a new frontier in cluster research, not only because of their high stability, atomic precision, and wide size tunability (5) but also for the rich gold thiolate chemistry—which is broadly used in surface functionalization of nanoparticles and two-dimensional (2D) films (6). It is these merits of Aun(SR)m clusters that bestow on them the potential to shed light on mysterious issues in the cluster, nanoscale, and surface sciences. The recent research efforts in structural characterization of ultrasmall Aun(SR)m clusters with n ≤ 38 and n ≥ 102 have revealed some common features: (i) the inner cores of clusters are constructed from various polyhedra and their derivatives; (ii) the surfaces of clusters are protected by the Au-SR oligomeric staple or ring motifs (5, 7); and (iii) their stability is often interpreted by the shell-closing “superatom” model with stable electronic structure [for example, Au25(SR)18− as an 8e superatom and Au102(SR)44 as a 58e superatom] (8, 9), which is similar to other ligand-protected metal clusters and gas-phase clusters (10–15). However, certain Aun(SR)m clusters do not conform to the superatom category, such as Au38(SR)24 (16). The polyhedron-based kernels, Au-SR surface oligomeric motifs, and superatom model have been serving as the basis for analysis and prediction of Aun(SR)m cluster structures (7, 17–19).
However, despite the advances in Aun(SR)m cluster research, several basic and critical issues remain to be addressed. First, the origin of the stability of magic sizes in solution-phase clusters is still unclear. This issue lies in the center of cluster research. Although the well-known superatom model can explain the stability of a few magic sizes (8–14), it cannot accommodate the newly discovered magic sizes. Second, the crystal structural information of medium-sized gold clusters (between Au38 and Au102) is still missing (8, 16) except for some theoretical work (18–21), and most of the solved structures are concentrated on the smaller end (5). This knowledge gap precludes the understanding of the growth pattern of clusters. Third, the thiolate bonding and patterning structures on the gold crystalline facets remain largely unknown. We have recently revealed the formation of aesthetic “helical-stripe” patterns of -S-Au-S- motifs on the curved surface of Au133(SR)52 nanoparticles (22); however, it remains to be seen whether the same protecting mode exists on gold crystalline surfaces.
In an effort to gain insight into the above issues, we herein report two unique structures of Aun(SR)m clusters, that is, Au40(SR)24 and Au52(SR)32. The rich structural information encoded in these two clusters provides atomic-scale insight into some major issues, including the origin of magic sizes in clusters, the shape control of nanocrystals, and the self-assembled monolayers of thiolates on gold crystalline facets. Significantly, unlike the previously reported superatom or close-shell clusters (8–14), Au40 and Au52 exhibit a high level of complexity, with the gold atoms in the cluster first segregated into four-atom tetrahedral units, which then coil up into a Kekulé-like ring in the Au40 cluster and a DNA-like double helix in Au52. These structures are better viewed as “supermolecules” rather than superatoms. Also, the Au40 and Au52 structures reveal the construction of clusters in a manner similar to the anisotropic growth of nanocrystals, reflecting the early stage of “shape control” in the cluster regime. Moreover, the anisotropic growth patterns of Au40 and Au52 lead to the exposure of the extended Au(111) and Au(100) facets on clusters, which provide valuable crystallographic information on thiolate bonding and patterning on gold crystalline facets.
RESULTS AND DISCUSSION
The Au40 and Au52 clusters were synthesized by a two-step “size-focusing” method (1). The key aspects involve careful control of reaction kinetics and a proper selection of protecting thiolates (SR); see Materials and Methods for details. The Au40 was synthesized with 2-methylbenzenethiolate (o-MBT), formulated as Au40(o-MBT)24 (23), whereas the Au52 was synthesized with 4-tert-butylbenzenethiolate (TBBT), formulated as Au52(TBBT)32. Both Au40 and Au52 are highly stable because they were thermodynamically selected through harsh size-focusing processes (1, 23). Their structures were determined by single-crystal x-ray crystallography (tables S1 to S4). Both clusters are chiral, and the unit cells of Au40 and Au52 single crystals comprise a pair of enantiomers for each cluster (Figs. 1, A and B, and 2, A and B).
Fig. 1. Total structure of the Au40(o-MBT)24 cluster.
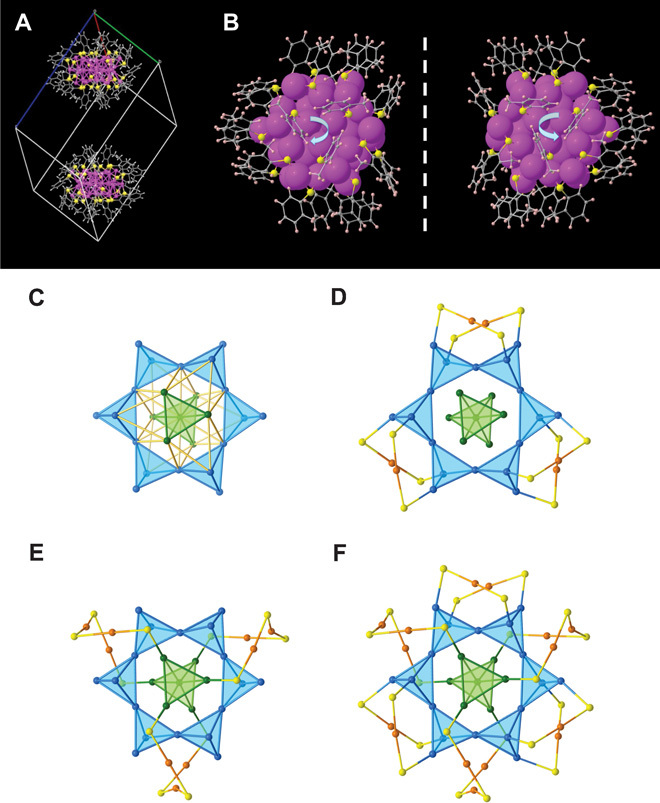
(A) Unit cell comprising two enantiomers. (B) Mirror symmetry of the enantiomers. (C) Snowflake-like Au25 kernel with tetrahedral units coiled up into a Kekulé-like superstructure. (D) Six monomeric staples protecting the Kekulé ring. (E) Three trimeric staples protecting the central Au7 bi-tetrahedron. (F) Overall Au40S24 framework. Blue/green, Au atoms in the kernel; orange, Au atoms in the staples; yellow, sulfur; gray, carbon; pink, hydrogen.
Fig. 2. Total structure of the Au52(TBBT)32 cluster.
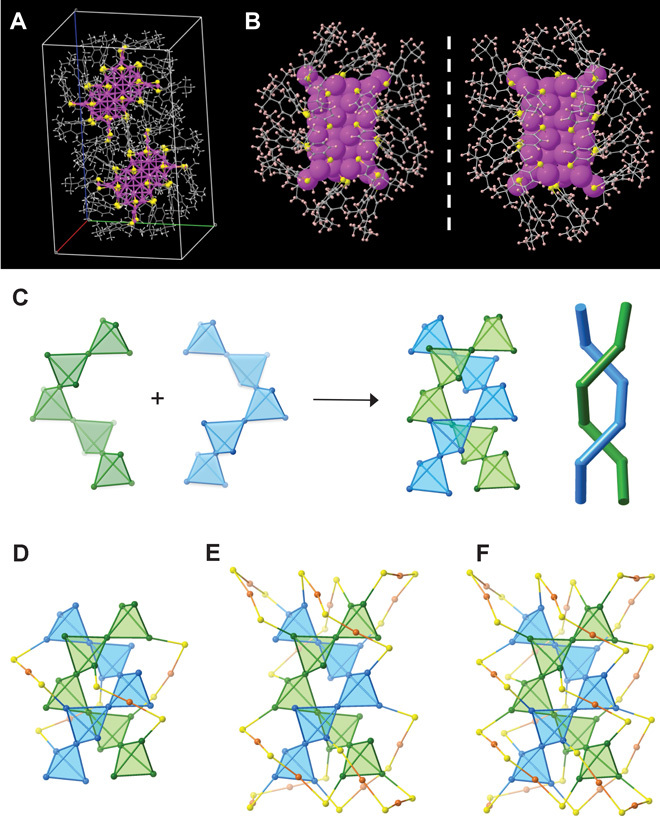
(A) Unit cell comprising two enantiomers. (B) Mirror symmetry of the enantiomers. (C) Two helical pentatetrahedral strands forming the double helical kernel. (D) Four monomeric staples protecting the waist of the kernel. (E) Four dimeric staples protecting the top and another four protecting the bottom of the kernel. (F) Overall Au52S32 framework. Blue/green, Au atoms in the kernel; orange, Au atoms in the staples; yellow, sulfur; gray, carbon; pink, hydrogen.
Supermolecular view of Au40(SR)24 and Au52(SR)32 clusters
The structure of Au40(SR)24 has long been a puzzle since the first isolation of this magic size from a mixture with the Au38(SR)24 cluster (24). Density functional theory (DFT) predicted bi-icosahedron–based core structures for Au40(SR)24 (18, 19), which are similar to the structure of Au38(SR)24. Here, we found that the overall structure of Au40(o-MBT)24 is a unique oblate shape (that is, a hexagonal prism; Fig. 1, A and B), which is drastically different from the previous theoretical prediction. An anatomy of the structure reveals a 25–gold atom kernel resembling a snowflake (Fig. 1C) and nine surface-protecting staples (Fig. 1, D and E). Significantly, we found that the gold atoms in the kernel are segregated into eight tetrahedral Au4 units, evidenced by the Au-Au bond length differences (vide infra). Two of the tetrahedral units form the central bi-tetrahedral antiprism (Fig. 1C, green), and the remaining six tetrahedra form a Kekulé-like external ring with alternatively facing-up-and-down arrangement of the tetrahedra (Fig. 1C, blue). The Kekulé ring is protected by six Au(SR)2 monomer staples (Fig. 1D), whereas the central bi-tetrahedron is protected by three Au3(SR)4 trimer staples (Fig. 1E). The kernel adopts achiral D3d symmetry, but the overall Au40S24 framework has chiral D3 symmetry (Fig. 1F), which is due to the rotative arrangement of the surface staple motifs. The discovery of the Kekulé-like Au40 structure indicates molecular complexity in this cluster, as opposed to a simple superatom-dimer as predicted on the basis of the Au38(SR)24 structure (18, 19).
The segregation of tetrahedral Au4 units and the formation of an elegant superstructure are also observed in another magic-sized cluster, Au52(TBBT)32 (Fig. 2). This indicates certain generality of the supermolecular complexity in clusters. The Au52 cluster has a 32–gold atom kernel, which is segregated into 10 tetrahedral units (Fig. 2C). The tetrahedra are assembled into a double helical superstructure resembling the double-stranded DNA. Within each helix, five tetrahedra are connected by vertex sharing (Fig. 2C). This double helix is protected by four Au(SR)2 monomer staples at the waist (Fig. 2D) and eight Au2(SR)3 dimer staples at the top and bottom (Fig. 2E). Both the helical kernel and the overall Au52S32 framework (Fig. 2F) adopt the same D2 symmetry, hence a chiral cluster. The left- and right-handed helical kernels are shown in fig. S1.
The segregation of tetrahedral Au4 units in both Au40 and Au52 clusters is manifested in the Au-Au bond length differences. A plot of Au-Au bond lengths according to the different Au positions shows four “steps” (Fig. 3). In the Au40(o-MBT)24, the Au-Au bonds within the eight tetrahedral units are the shortest (average, 2.78 and 2.76 Å, respectively; Fig. 3A, group I), whereas the Au-Au bonds connecting the central bi-tetrahedron and external tetrahedra are longer, with an average of 2.90 Å (Fig. 3A, group II). The Au-Au bonds between the Kekulé-like kernel and the surface staple motifs are among the longest, with an average length of 3.01 Å for trimeric staples and 3.16 Å for the monomeric staples (Fig. 3A, groups III and IV). The case of Au52(TBBT)32 is similar, with Au-Au bonds within each tetrahedron-coiled helix being shorter than those between the two helices (Fig. 3B). It is this inhomogeneous distribution of Au-Au bond lengths that distinguishes the Au4 units in the clusters.
Fig. 3. Au-Au bond length distributions in the clusters.
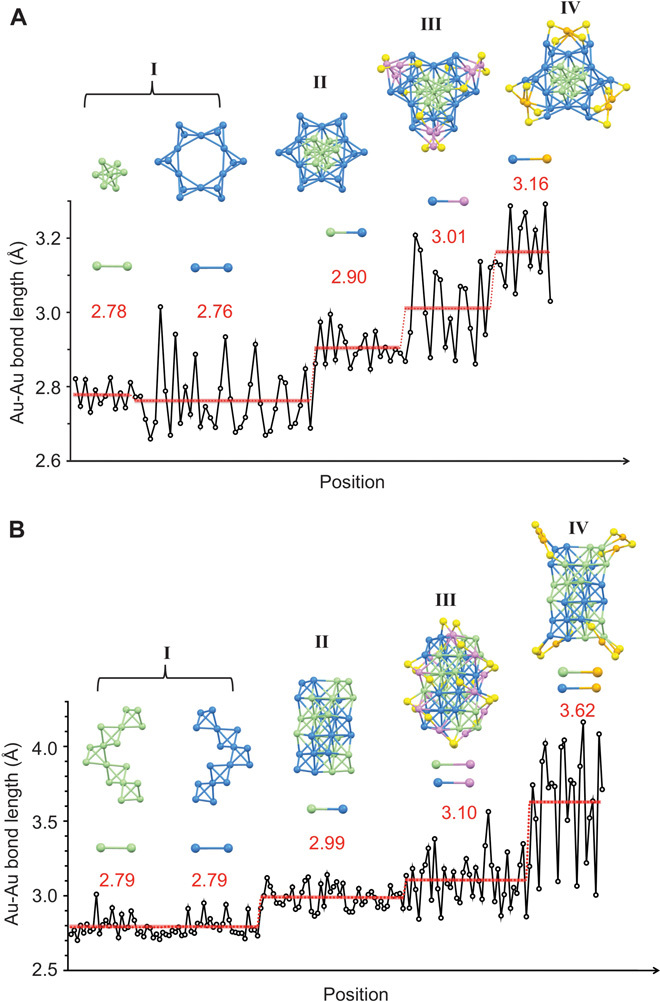
(A) Au40(o-MBT)24. (B) Au52(TBBT)32. Green/blue/magenta/orange, gold; yellow, sulfur.
The electronic structures of both Au40(o-MBT)24 and Au52(TBBT)32 clusters were analyzed by DFT to provide some insights into the magic stability of both clusters. The entire ligands (that is, without simplifying as –SCH3) were included in the calculations considering their important roles in stabilizing the two structures. For the Au40(o-MBT)24 cluster, we indeed found that the distribution of the highest-occupied molecular orbital (HOMO) follows a six-lobe hexagonal pattern (fig. S2, green circles), that is, a “supermolecular” picture. Each lobe is located in the corresponding Au4 tetrahedron. The phase of the HOMO alternates (fig. S2, red and blue lobes) in line with the facing up/down of Au4 units in the Kekulé ring. Furthermore, the molecular orbitals of the Au4 units connect to the π orbitals of the ligands in the six monomer staples, exhibiting an external “blade”-like configuration (fig. S2). For the Au52(TBBT)32 cluster, the HOMO exhibits some features of the segregated Au4 units and a helical pattern (fig. S3), but these are less prominent than the features in planar Au40(o-MBT)24. Further analysis of the atomic charges of Au40(o-MBT)24 indicates that the Au-S bond has an ionic bond character (table S5) and the core Au atoms stabilize the ionic bond. Similar features are also found in Au52(TBBT)32. Overall, the symmetric Au4 patterns plus interactions with the surface ligands stabilize the multi-tetrahedron superstructures.
From the valence electron count perspective, previous work showed the Au4 cluster complexes [for example, Au4(PR3)42+ and Au4I2(PR3)4] having two electrons (25). Thiolate is monovalent and consumes one Au 6s valence electron in bonding, so the remaining free 6s electrons in Au40(SR)24 are 40 − 24 = 16e, in agreement with the eight tetrahedral units in Au40(SR)24. The Au52(SR)32 cluster has 10 tetrahedra, hence 20e (that is, 52 − 32 = 20). The Au4 tetrahedron can be taken as a 2e superatom, but the Au4-based Kekulé-like and helical patterns call for a supermolecular picture, rather than the simple superatomic model (9). The elegant patterns observed in Au40 and Au52 are not existent in smaller clusters such as Au28 and Au36 (26–28), although the Au4 unit was identified in these small clusters. Also, the previous theoretical work proposed an Au4 network picture for thiolate-protected Au18, Au20, and Au24 clusters (29), but no regular pattern was involved. Notably, the tetrahedral configuration is also favored in gas-phase gold clusters, for example, the Au20 tetrahedral cluster (15). This supermolecular picture from the Au40 and Au52 clusters provides a more complex origin of stable magic sizes in gold clusters than the superatom picture, and it reveals the assembly of “atoms” into “molecules” in the cluster regime. The supermolecular picture is expected to accommodate more magic sizes in the future.
Anisotropic growth of the clusters and origin of supermolecular structures
An intriguing question is what drives the formation of the supermolecular patterns of tetrahedra in the Au40 and Au52 clusters. To answer this question, we first need to have the overall pictures of the two structures without considering the differences in the Au-Au bond lengths. Specifically, when viewed as an entity, the 40 gold atoms in the Au40(o-MBT)24 can all be fit into a face-centered cubic (FCC) lattice with some distortions (Fig. 4, A to D). Three layers of gold atoms are stacked along the [111] direction in an a-b-c manner, forming a hexagonal prism. For the Au52(TBBT)32, 48 of the 52 gold atoms can also be fit into the FCC lattice, with the atoms assembled in the [100] direction, forming a tetragonal rod enclosed by {100} facets (Fig. 4, E to H). Correspondingly, the surface-protecting Aux(SR)x + 1 staples are decomposed into simple bridging thiolates (fig. S4). This alternative view of the Au40 and Au52 clusters reveals the anisotropic layer-by-layer construction mode of magic-sized gold clusters, similar to the anisotropic growth of 2D nanoprisms and 1D nanorods in shape-controlled nanocrystals (30, 31). It also provides atomic-scale insight into the effect of selective surface passivation in tailoring the particle shape (32).
Fig. 4. Anisotropic growth of the gold FCC lattice into a hexagonal prism in Au40(o-MBT)24 and a tetragonal rod in Au52(TBBT)32.
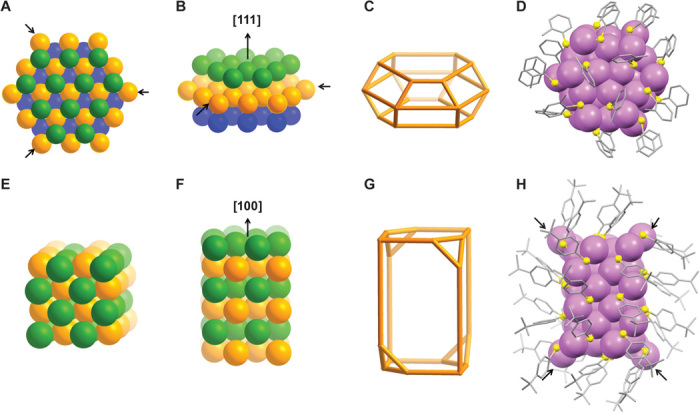
(A to C) Model of a 43–gold atom hexagonal prism composed of three layers (green, orange, and blue) stacked along the [111] direction in an a-b-c manner (the three arrows indicate the three missing gold atoms in the real Au40 cluster). (D) Au40(o-MBT)24 as a hexagonal prism. (E to G) Model of a 48–gold atom tetragonal rod composed of six layers stacked along the [100] direction. (H) Au52(TBBT)32 as a tetragonal rod. The four gold atoms not included in the FCC lattice are indicated by arrows.
The segregation of Au4 tetrahedral units in the FCC lattice is due to the surface-protecting thiolates or, more specifically, the directional covalent bonds between thiolates and gold atoms. The bonding of the sulfur atom in the thiolate adopts the tetragonal configuration, with two orbitals bonded to two gold atoms, one bonded to the carbon tail, and one for the lone-pair electrons (Fig. 5, A and B). The three atoms in the Au-S-Au bridge form a triangle, with an Au-S bond length of ~2.33 Å and an average Au-S-Au angle of 87.4 ± 3.6° in Au40 and 92.6 ± 7.3° in Au52. Because of the geometric restriction of the Au-S-Au triangle, the two Au atoms underneath the sulfur bridge are pushed apart, with an average Au-Au bond length of 3.21 Å in Au40 and 3.35 Å in Au52, longer than the bulk Au-Au bond length of 2.88 Å (Fig. 5, A and B).
Fig. 5. Thiolate bonding and patterning on the crystalline facets of Au40 and Au52.
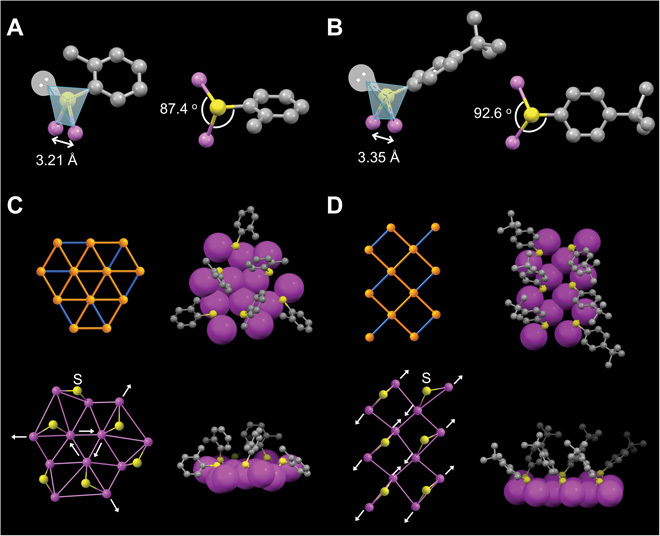
(A) Tetragonal configuration of sulfur atom of o-MBT in the Au40 cluster. (B) Tetragonal configuration of sulfur atom of TBBT in Au52. (C) Twelve–gold atom {111} facets on the Au40 cluster. (D) Twelve–gold atom {100} facets on the Au52 cluster. Orange/magenta, Au; yellow, S; gray, C.
This thiolate bonding effect can be easily viewed on the 2D surface of the clusters, as reflected in the inhomogeneous distribution of Au-Au bond lengths (Fig. 5, C and D). As discussed above, the overall anisotropic growth of the Au40 and Au52 clusters leads to the exposure of the extended gold crystalline facets, that is, the 12-atom {111} facets in the Au40 hexagonal prism (Fig. 5C) and the 12-atom {100} facets in the Au52 tetragonal rod (Fig. 5D). Every two gold atoms on the facet are bridged by one thiolate ligand (Fig. 5, C and D). With the thiolates bridging onto the gold crystalline facets, the Au-Au distance underneath the bridging S is expanded to meet the coordination requirements of the S bridge. On the other hand, the Au-Au pairs adjacent to the bridging thiolates are squeezed together by the forces from different directions, resulting in shorter Au-Au distances (Fig. 5, C and D, bottom). Such a demonstration of inhomogeneous distribution of Au-Au distances in 2D surface can be applied in the 3D nanoclusters because the thiolate bonding force can further penetrate into a few layers of gold atoms (fig. S5). Together, the surface thiolate bonding causes a stress, and this stress propagates into the anisotropic-shaped FCC cluster to induce segregation of Au4 tetrahedra and their further coiling up into hierarchical patterns.
CONCLUSION
Here, we have presented two novel structures of thiolate-protected gold clusters. The implications of the Au40 and Au52 structures are manifold. First, the two clusters illustrate the supermolecular complexity, and such a view can explain more magic-sizes of clusters than the early superatom model. The supermolecular picture is reminiscent of the fact that an unlimited number of stable molecules can be assembled from a limited number of atoms. Second, they reveal an anisotropic growth of FCC lattice at the atomic level, as reflected in the 2D hexagonal prism of the Au40 and the 1D tetragonal rod of the Au52 structure. The two structures imply the important roles of ligands in the anisotropic growth at the atomic level. Third, the new structures add a new dimension in constructing highly stable clusters in an anisotropic fashion other than the isotropic shell-by-shell growth (22) or polyhedron-fusion mode (24, 33, 34). The new supermolecular picture is expected to advance further understanding of the cluster structure and stability, and the Au40 and Au52 cluster materials will provide more insights into shape and surface-dependent properties and applications.
MATERIALS AND METHODS
Chemicals
The following chemicals were used: tetrachloroauric(III) acid (HAuCl4·3H2O, 99.99% metals basis, Sigma-Aldrich), tetraoctylammonium bromide (TOAB, 98%, Fluka), o-MBT (97%, TCI), TBBT (97%, Alfa Aesar), sodium borohydride (NaBH4, 99.9%, Sigma-Aldrich); methanol [high-performance liquid chromatography (HPLC) grade, 99.9%, Sigma-Aldrich], pentane (HPLC grade, 99.9%, Sigma-Aldrich), dichloromethane (HPLC grade, 99.9%, Sigma-Aldrich), toluene (HPLC grade, 99.9%, Sigma-Aldrich), and tetrahydrofuran (HPLC grade, 99.9%, Sigma-Aldrich). All chemicals were used as received.
Synthesis
The Au40(o-MBT)24 cluster was synthesized by a two-step size-focusing method (23). In the first step, 0.25 mmol of HAuCl4 was reduced by 1.27 mmol of o-MBT to form Au(I)-o-MBT polymers in a toluene solution containing 0.29 mmol of TOAB. The Au(I)-oMBT polymers were further reduced to size-mixed Aux(o-MBT)y clusters by 2.5 mmol of NaBH4 (dissolved in 5 ml of water). In the second step, the polydispersed Aux(o-MBT)y clusters were reacted with excess of o-MBT thiol at 90°C for 48 hours. The Au52(TBBT)32 cluster was synthesized by a similar process. In the first step, 0.125 mmol of HAuCl4 was reacted with 0.625 mmol of TBBT in 10 ml of tetrahydrofuran, followed by reduction to size-mixed Aux(TBBT)y clusters by 1.25 mmol of NaBH4 (in 5 ml of water). In the second step, the Aux(TBBT)y mixture was reacted with excess TBBT thiol at 80°C for 24 hours. Both clusters were separated from the reaction mixture by precipitation with methanol and crystallized in the pentane/CH2Cl2 solvents.
X-ray crystallography
Data of both Au40(o-MBT)24 and Au52(TBBT)32 were collected on a Bruker X8 Prospector Ultra equipped with an Apex II charge-coupled device detector and an IμS microfocus CuKα x-ray source (λ = 1.54178 Å) under cold N2 flow at 150 K.
For Au40(o-MBT)24, a piece of brown crystal with dimensions of 0.16 × 0.10 × 0.01 mm was mounted onto a MiTeGen MicroMeshes with fluorolube. A triclinic unit cell with dimensions a = 18.9983(5) Å, b = 19.0751(5) Å, c = 35.6255(9) Å, α = 81.5670(17)°, β = 81.4980(16)°, and γ = 60.9600(15)° was derived from the least-squares refinement of 9930 reflections in the range of 2.660 < θ < 55.122. Centrosymmetric space group P-1 was determined on the basis of intensity statistics and the lack of systematic absences. The data were collected to 0.94 Å. After integration of the data by the Bruker SAINT program, empirical absorption correction was applied using the program SADABS. The maximum and minimum transmittance (Tmax and Tmin) values were 0.6319 and 0.0455, respectively. The structure was solved with a direct method using the Bruker SHELXTL program. All the Au and S atoms were located, and all the C atoms were generated through subsequent difference Fourier syntheses. Idealized atom positions were calculated for all hydrogen atoms [with d-(Cmethyl-H) = 0.979 Å and d-(Cphenyl-H) = 0.95 Å]. All the Au, S, and C atoms were refined anisotropically, and all the H atoms were refined isotropically.
For Au52(TBBT)32, a piece of black crystal with dimensions of 0.20 × 0.08 × 0.02 mm was used for data collection. A triclinic unit cell with dimensions a = 24.3807(9) Å, b = 24.8559(10) Å, c = 39.973(2) Å, α = 97.027(4)°, β = 99.189(4)°, and γ = 117.673(2)° was derived from the least-squares refinement of 9938 reflections in the range of 2.365 < θ < 51.159. Centrosymmetric space group P-1 was determined on the basis of intensity statistics and the lack of systematic absences. The data were collected to 0.99 Å. The Tmax and Tmin values were 0.5367 and 0.0531, respectively. The structure was solved with a direct method using Bruker SHELXTL. All the Au and S atoms were located; all phenyl C and most t-butyl C atoms were generated through subsequent difference Fourier syntheses. However, some of the t-Bu C atoms were difficult to locate because of disordering of t-Bu groups as well as interference of surrounding solvent electron density, so a rigid TBBT fragment was used in these cases. Idealized atom positions were calculated for all hydrogen atoms [with d-(Cmethyl-H) = 0.979 Å and d-(Cphenyl-H) = 0.95 Å]. All the Au, S, and phenyl C atoms were refined anisotropically. The t-Bu C and all H atoms were refined isotropically.
DFT simulations
To gain insight into the electronic structures of the newly observed Au40(o-MBT)24 and Au52(TBBT)32 nanoclusters, we performed DFT calculations with the RI (resolution of the identity) approximation. In the calculations, the structures of the clusters are exactly the same ones observed by the single crystal x-ray crystallography, taking full account of each ligand, o-MBT, or TBBT. The TURBOMOLE version 6.6 package of ab initio quantum chemistry programs was used in all the calculations. The double-ζ valence quality plus polarization basis in the TURBOMOLE basis set library was adopted in the calculations along with a 60-electron, relativistic, effective core potential for the gold atom.
Funding
R.J. received financial support from the Air Force Office of Scientific Research (AFOSR) under AFOSR Award no. FA9550-15-1-9999 (FA9550-15-1-0154) and the Camille Dreyfus Teacher-Scholar Awards Program. K.N. received financial support from Elements Strategy Initiative for Catalysts and Batteries and a Grant-in-Aid for Scientific Research (no. 25288012) from the Ministry of Education, Culture, Sports, Science, and Technology of Japan. Author contributions: C.Z., Y.C., and R.J. were responsible for synthesis and crystallization and for the design of the project. C.L. and N.L.R. conducted the x-ray crystallographic analysis. K.N. carried out DFT calculations. All authors contributed to the writing of the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: All data is available in the manuscript and the Supplementary Materials.
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/9/e1500425/DC1
Materials and Methods
Fig. S1. The left- and right-handed isomers of the chiral double helical Au32 kernel in Au52(TBBT)32.
Fig. S2. DFT-simulated HOMO distribution of Au40(o-MBT)24.
Fig. S3. DFT-simulated HOMO distribution of Au52(TBBT)32.
Fig. S4. Arrangement of thiolates on the flat surface of Au40(o-MBT)24 and Au52(TBBT)32 clusters.
Fig. S5. Penetration of surface bridging forces into the kernel, leading to the segregation of tetrahedral units.
Table S1. Crystal data and structure refinement for Au40(o-MBT)24.
Table S2. Crystal data and structure refinement for Au52(TBBT)32.
Table S3. Atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2 ×103) for Au40(o-MBT)24.
Table S4. Atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2 × 103) for Au52(TBBT)32.
Table S5. Calculated atomic charges.
REFERENCES AND NOTES
- 1.Qian H., Zhu M., Wu Z., Jin R., Quantum sized gold nanoclusters with atomic precision. Acc. Chem. Res. 45, 1470–1479 (2012). [DOI] [PubMed] [Google Scholar]
- 2.Yamazoe S., Koyasu K., Tsukuda T., Nonscalable oxidation catalysis of gold clusters. Acc. Chem. Res. 47, 816–824 (2014). [DOI] [PubMed] [Google Scholar]
- 3.Barrabés N., Zhang B., Bürgi T., Racemization of chiral Pd2Au36(SC2H4Ph)24: Doping increases the flexibility of the cluster surface. J. Am. Chem. Soc. 136, 14361–14364 (2014). [DOI] [PubMed] [Google Scholar]
- 4.Yau S. H., Varnavski O., Goodson T. III, An ultrafast look at Au nanoclusters. Acc. Chem. Res. 46, 1506–1516 (2013). [DOI] [PubMed] [Google Scholar]
- 5.Jin R., Atomically precise metal nanoclusters: Stable sizes and optical properties. Nanoscale 7, 1549–1565 (2015). [DOI] [PubMed] [Google Scholar]
- 6.Mirkin C. A., Letsinger R. L., Mucic R. C., Storhoff J. J., A DNA-based method for rationally assembling nanoparticles into macroscopic materials. Nature 382, 607–609 (1996). [DOI] [PubMed] [Google Scholar]
- 7.Pei Y., Zeng X. C., Investigating the structural evolution of thiolate protected gold clusters from first-principles. Nanoscale 4, 4054–4072 (2012). [DOI] [PubMed] [Google Scholar]
- 8.Jadzinsky P. D., Calero G., Ackerson C. J., Bushnell D. A., Kornberg R. D., Structure of a thiol monolayer-protected gold nanoparticle at 1.1 Å resolution. Science 318, 430–433 (2007). [DOI] [PubMed] [Google Scholar]
- 9.Walter M., Akola J., Lopez-Acevedo O., Jadzinsky P. D., Calero G., Ackerson C. J., Whetten R. L., Grönbeck H., Häkkinen H., A unified view of ligand-protected gold clusters as superatom complexes. Proc. Natl. Acad. Sci. U.S.A. 105, 9157–9162 (2008). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Desireddy A., Conn B. E., Guo J., Yoon B., Barnett R. N., Monahan B. M., Kirschbaum K., Griffith W. P., Whetten R. L., Landman U., Bigioni T. P., Ultrastable silver nanoparticles. Nature 501, 399–402 (2013). [DOI] [PubMed] [Google Scholar]
- 11.Yang H., Wang Y., Huang H., Gell L., Lehtovaara L., Malola S., Häkkinen H., Zheng N., All-thiol-stabilized Ag44 and Au12Ag32 nanoparticles with single-crystal structures. Nat. Commun. 4, 2422 (2013). [DOI] [PubMed] [Google Scholar]
- 12.Wan X.-K., Tang Q., Yuan S.-F., Jiang D.-., Wang Q.-M., Au19 nanocluster featuring a V-shaped alkynyl–gold motif. J. Am. Chem. Soc. 137, 652–655 (2015). [DOI] [PubMed] [Google Scholar]
- 13.Knight W. D., Clemenger K., de Heer W. A., Saunders W. A., Chou M. Y., Cohen M. L., Electronic shell structure and abundances of sodium clusters. Phys. Rev. Lett. 52, 2141–2143 (1984). [Google Scholar]
- 14.Lin Z., Kanters R. P. F., Mingos D. M. P., Closed-shell electronic requirements for condensed clusters of the group 11 elements. Inorg. Chem. 30, 91–95 (1991). [Google Scholar]
- 15.Li J., Li X., Zhai H.-J., Wang L.-S., Au20: A tetrahedral cluster. Science 299, 864–867 (2003). [DOI] [PubMed] [Google Scholar]
- 16.Qian H., Eckenhoff W. T., Zhu Y., Pintauer T., Jin R., Total structure determination of thiolate-protected Au38 nanoparticles. J. Am. Chem. Soc. 132, 8280–8281 (2010). [DOI] [PubMed] [Google Scholar]
- 17.Li H., Li L., Pedersen A., Gao Y., Khetrapal N., Jónsson H., Zeng X. C., Magic-number gold nanoclusters with diameters from 1 to 3.5 nm: Relative stability and catalytic activity for CO oxidation. Nano Lett. 15, 682–688 (2015). [DOI] [PubMed] [Google Scholar]
- 18.Malola S., Lehtovaara L., Knoppe S., Hu K.-J., Palmer R. E., Bürgi T., Häkkinen H., Au40(SR)24 cluster as a chiral dimer of 8-electron superatoms: Structure and optical properties. J. Am. Chem. Soc. 134, 19560–19563 (2012). [DOI] [PubMed] [Google Scholar]
- 19.Jiang D.-e., The expanding universe of thiolated gold nanoclusters and beyond. Nanoscale 5, 7149–7160 (2013). [DOI] [PubMed] [Google Scholar]
- 20.Nimmala P. R., Yoon B., Whetten R. L., Landman U., Dass A., Au67(SR)35 nanomolecules: Characteristic size-specific optical, electrochemical, structural properties and first-principles theoretical analysis. J. Phys. Chem. A 117, 504–517 (2013). [DOI] [PubMed] [Google Scholar]
- 21.Xu W. W., Gao Y., Zeng X. C., Unraveling structures of protection ligands on gold nanoparticle Au68(SH)32. Sci. Adv. 1, e1400211 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Zeng C., Chen Y., Kirschbaum K., Appavoo K., Sfeir M. Y., Jin R., Structural patterns at all scales in a nonmetallic chiral Au133(SR)52 nanoparticle. Sci. Adv. 1, e1500045 (2015). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Chen Y., Zeng C., Kauffman D. R., Jin R., Tuning the magic size of atomically Precise gold nanoclusters via isomeric methylbenzenethiols. Nano Lett. 15, 3603–3609 (2015). [DOI] [PubMed] [Google Scholar]
- 24.Qian H., Zhu Y., Jin R., Isolation of ubiquitous Au40(SR)24 clusters from the 8 kDa gold clusters. J. Am. Chem. Soc. 132, 4583–4585 (2010). [DOI] [PubMed] [Google Scholar]
- 25.D. M. P. Mingos, in Gold Clusters, Colloids and Nanoparticles II, D. M. P. Mingos, Ed. (Springer, Switzerland, 2014), pp. 1–65. [Google Scholar]
- 26.Zeng C., Qian H., Li T., Li G., Rosi N. L., Yoon B., Barnett R. N., Whetten R. L., Landman U., Jin R., Total structure and electronic properties of the gold nanocrystal Au36(SR)24. Angew. Chem. Int. Ed. 51, 13114–13118 (2012). [DOI] [PubMed] [Google Scholar]
- 27.Chevrier D. M., Chatt A., Zhang P., Zeng C., Jin R., Unique bonding properties of the Au36(SR)24 nanocluster with FCC-like core. J. Phys. Chem. Lett. 4, 3186–3191 (2013). [DOI] [PubMed] [Google Scholar]
- 28.Zeng C., Liu C., Chen Y., Rosi N. L., Jin R., Gold-thiolate ring as a protecting motif in the Au20(SR)16 nanocluster and implications. J. Am. Chem. Soc. 136, 11922–11925 (2014). [DOI] [PubMed] [Google Scholar]
- 29.Cheng L., Yuan Y., Zhang X., Yang J., Superatom networks in thiolate-protected gold nanoparticles. Angew. Chem. Int. Ed. 52, 9035–9039 (2013). [DOI] [PubMed] [Google Scholar]
- 30.Jin R., Cao Y., Mirkin C. A., Kelly K. L., Schatz G. C., Zheng J. G., Photoinduced conversion of silver nanospheres to nanoprisms. Science 294, 1901–1903 (2001). [DOI] [PubMed] [Google Scholar]
- 31.Nikoobakht B., El-Sayed M. A., Preparation and growth mechanism of gold nanorods (NRs) using seed-mediated growth method. Chem. Mater. 15, 1957–1962 (2003). [Google Scholar]
- 32.Personick M. L., Mirkin C. A., Making sense of the mayhem behind shape control in the synthesis of gold nanoparticles. J. Am. Chem. Soc. 135, 18238–18247 (2013). [DOI] [PubMed] [Google Scholar]
- 33.Teo B. K., Zhang H., Clusters of clusters: Self-organization and self-similarity in the intermediate stages of cluster growth of Au–Ag supraclusters. Proc. Natl. Acad. Sci. U.S.A. 88, 5067–5071 (1991). [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Mednikov E. G., Dahl L. F., Nanosized Pd37(CO)28{P(p-Tolyl)3}12 containing geometrically unprecedented central 23-atom interpenetrating tri-icosahedral palladium kernel of double icosahedral units: Its postulated metal-core evolution and resulting stereochemical implications. J. Am. Chem. Soc. 130, 14813–14821 (2008). [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/9/e1500425/DC1
Materials and Methods
Fig. S1. The left- and right-handed isomers of the chiral double helical Au32 kernel in Au52(TBBT)32.
Fig. S2. DFT-simulated HOMO distribution of Au40(o-MBT)24.
Fig. S3. DFT-simulated HOMO distribution of Au52(TBBT)32.
Fig. S4. Arrangement of thiolates on the flat surface of Au40(o-MBT)24 and Au52(TBBT)32 clusters.
Fig. S5. Penetration of surface bridging forces into the kernel, leading to the segregation of tetrahedral units.
Table S1. Crystal data and structure refinement for Au40(o-MBT)24.
Table S2. Crystal data and structure refinement for Au52(TBBT)32.
Table S3. Atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2 ×103) for Au40(o-MBT)24.
Table S4. Atomic coordinates (×104) and equivalent isotropic displacement parameters (Å2 × 103) for Au52(TBBT)32.
Table S5. Calculated atomic charges.


