First detections of ethyl alcohol and glycolaldehyde in a comet provide new constraints on solar system chemical complexity.
Keywords: space science, astronomy, comets, complex organic molecules
Abstract
The presence of numerous complex organic molecules (COMs; defined as those containing six or more atoms) around protostars shows that star formation is accompanied by an increase of molecular complexity. These COMs may be part of the material from which planetesimals and, ultimately, planets formed. Comets represent some of the oldest and most primitive material in the solar system, including ices, and are thus our best window into the volatile composition of the solar protoplanetary disk. Molecules identified to be present in cometary ices include water, simple hydrocarbons, oxygen, sulfur, and nitrogen-bearing species, as well as a few COMs, such as ethylene glycol and glycine. We report the detection of 21 molecules in comet C/2014 Q2 (Lovejoy), including the first identification of ethyl alcohol (ethanol, C2H5OH) and the simplest monosaccharide sugar glycolaldehyde (CH2OHCHO) in a comet. The abundances of ethanol and glycolaldehyde, respectively 5 and 0.8% relative to methanol (0.12 and 0.02% relative to water), are somewhat higher than the values measured in solar-type protostars. Overall, the high abundance of COMs in cometary ices supports the formation through grain-surface reactions in the solar system protoplanetary disk.
INTRODUCTION
Comet C/2014 Q2 (Lovejoy) is a long-period comet originating from the Oort cloud, which passed its perihelion on 30 January 2015, at 1.290 astronomical units (AU) from the Sun. This comet was a naked-eye object in January and February 2015. At perihelion, its water production rate exceeded 20 metric tons/s. It was thus one of the most active comets in the neighborhood of Earth’s orbit since the passage in 1997 of the extraordinarily active comet C/1995 O1 (Hale-Bopp). Bright comets offer the opportunity to detect numerous species in their atmospheres and to search for new molecules outgassing from the nuclear ices.
Using the 30-m telescope of the Institut de Radioastronomie Millimétrique (IRAM), located on Pico Veleta in the Sierra Nevada (Spain), we observed the atmosphere of comet Lovejoy between 13–16 and 23–26 January 2015, when the comet was the brightest and the most productive, at a distance of 0.6 AU from Earth and of 1.3 AU from the Sun. Because of the versatility of the receivers and spectrometers at the IRAM 30m telescope (1), we were able to survey most of the 210–272 GHz (λ ~1 mm) spectral domain with high spectral resolution and sensitivity. The angular resolution is ~9″ to 12″ at these frequencies, corresponding to ~3300 to 5400 km at the distance of the comet. The targeted spectral range covers many molecular rotational lines and has been successfully used to identify complex organic molecules (COMs) in comets (2–8) and in star-forming regions, for example, in the studies of Belloche et al. (9) and Caux et al. (10).
RESULTS
In the spectral survey of comet Lovejoy, we detected lines of 21 molecules (Table 1), two of which, ethyl alcohol (ethanol, C2H5OH) and glycolaldehyde (CH2OHCHO), were detected for the first time in a comet. We secured the detection of ethanol and glycolaldehyde by averaging the strongest lines present in the observed spectral domain. Ethanol is detected through lines of both trans and gauche conformers. Most other detected COMs and simple organic molecules, such as ethylene glycol [(CH2OH)2], methyl formate (HCOOCH3), formamide (NH2CHO), formic acid (HCOOH), and acetaldehyde (CH3CHO), were first detected in comet Hale-Bopp and later confirmed in other comets (6–8). Figure 1 shows the spectra of eight organic species detected in comet Lovejoy.
Table 1. Abundance of molecules detected in comet Lovejoy.
Abundances relative to water of the 21 molecules detected in comet Lovejoy are based on the average of production rate ratios for the 13–16 and 23–25 January periods. The uncertainty on the abundances, taking into account all sources of errors, including uncertainty on the water production rate, is below 20%, except for NS for which it is 30% (4σ detection).
| Abundances relative to water (%) | |||||
| CHO molecules | Nitrogenous molecules | Sulfurated molecules | |||
| CO | 1.8 | HCN | 0.09 | H2S | 0.5 |
| H2CO | 0.3 | HNC | 0.004 | OCS | 0.034 |
| CH3OH | 2.4 | HNCO | 0.009 | H2CS | 0.013 |
| HCOOH | 0.028 | CH3CN | 0.015 | CS | 0.043 |
| (CH2OH)2 | 0.07 | HC3N | 0.002 | SO | 0.038 |
| HCOOCH3 | 0.08 | NH2CHO | 0.008 | NS | 0.006 |
| CH3CHO | 0.047 | ||||
| CH2OHCHO | 0.016 | ||||
| C2H5OH | 0.12 | ||||
Fig. 1. Spectra of organics in comet C/2014 Q2 (Lovejoy).
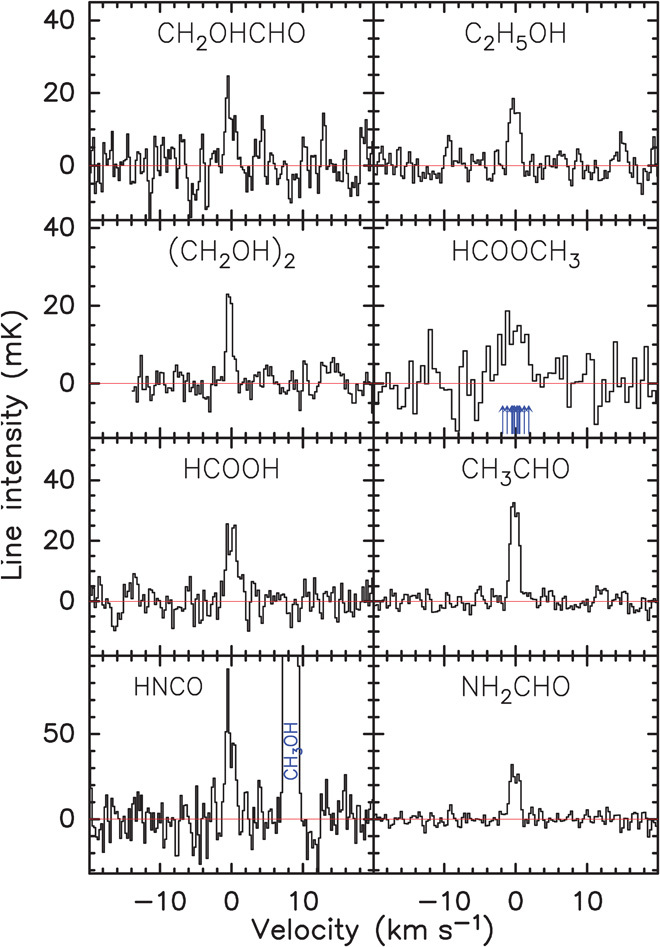
The observations were obtained with the IRAM 30m radio telescope in the 211–272 GHz band between 13 and 25 January 2015. The velocity scale is in the nucleus rest frame. Intensity is given in the main beam temperature scale. Spectra, from top left, are glycolaldehyde (CH2OHCHO, average of two lines), ethyl alcohol (ethanol, C2H5OH, average of 13 lines), aGg′ ethylene glycol [(CH2OH)2, average of 14 lines], methyl formate (HCOOCH3, average of two groups of blends of several lines, whose positions are marked by blue arrows), formic acid (HCOOH, average of six lines), acetaldehyde (CH3CHO, average of 40 lines), isocyanic acid [HNCO(110,11–100,10) line at 241.774 GHz], and formamide (NH2CHO, average of 10 lines). The signal-to-noise ratio is 6 for glycolaldehyde, 10 for ethanol, and higher than 7 for the other molecules.
Relative abundances can be estimated by deriving molecular production rates from the observed line intensities. In this active comet, most molecules within the field of view are close to local thermal equilibrium (LTE). To take into account departures from LTE in the less dense parts of the coma due to radiative decay, the line intensities were analyzed using an excitation model (11, 12). From the numerous detected methanol lines, we derived a rotational temperature of 68.0 ± 0.7 K and a gas temperature of 73 K. Assuming isotropic outgassing, and using the gas velocity of 0.8 km/s derived from the spectrally resolved strong lines, we determined the production rate for all observed molecules (1). Table 1 provides the abundances relative to water, using water production rates of QH2O = 5 × 1029 and 6 × 1029 molecules s−1 for the periods 13–16 and 23–26 January 2015, respectively. These values were deduced from the observations of the OH radical and H2O performed with the Nançay radio telescope and Odin space observatory, respectively, in January 2015. The conformers of ethanol were found to be in the relative proportion trans/gauche of 1.5 ± 0.3. Because of the low energy barrier between the two ethanol conformers (~57 K), trans-to-gauche interconversion was rapid in the collisional region of the comet (less than 1 ps at 73 K), whereas interconversion through radiative decay was expected to be slow (13). The trans/gauche ratio was close to the equilibrium value of 1.1 expected at the temperature of the sampled gas (73 K).
DISCUSSION
Production rates relative to water measured in comet Lovejoy are lower by a factor of 2 to 3 than those in comet Hale-Bopp, except for CH3OH and HCOOCH3, which show similar abundances, and for cyanoacetylene (HC3N), which is depleted in Lovejoy by a factor of 10 (6). Another notable exception is CO, which is a factor of >10 less abundant in Lovejoy, but Hale-Bopp belongs to the small family of CO-rich comets (14). The depletion remains when comparing with abundances measured in comets other than Hale-Bopp (table S2). Composition diversity is often observed in the comet population, but this is the first time that depletion is observed for such a large number of molecules in a comet.
Ethanol and glycolaldehyde, as well as the other COMs observed in comets, are found in warm (≥100 K) regions surrounding newly born stars, at the time when the collapsing envelope feeding the star has not yet dissipated (2). These regions, called hot corinos or cores for low- or high-mass protostars, exhibit a rich molecular inventory dominated by saturated species. COMs may have been synthesized in the cold prestellar core period by grain-surface reactions, when all heavy element–bearing molecules were frozen onto grains (15, 16). The efficiency of surface chemistry is increased during the warm-up phase because of the increased mobility of atoms and radicals on warm grains, producing large molecules (17). It is not clear to what extent these compounds are preserved in the protoplanetary disk. However, chemical models show that COMs are also efficiently formed on grain surfaces in the disk owing to the intense UV illumination from the newly born star (17). The presence of interstellar-like organic compounds in comets suggests that they are preserved materials synthesized in the outskirts of the solar nebula or at earlier stages of solar system formation.
In Fig. 2, the abundances (normalized to methanol) of nine organic molecules measured in comets Lovejoy and Hale-Bopp are compared to those measured in two interstellar sources. Orion-KL is the best-studied hot-core source because of its proximity and molecular wealth. Quantitative comparison with comets is restricted to methanol, ethanol (C2H5OH), ethylene glycol [(CH2OH)2], and glycolaldehyde (CH2OHCHO) measured at the same position in Orion-KL (18). The ethylene glycol abundance in this source is much lower than those in the four comets, where it has been detected, and this conclusion also applies to the hot-core Sgr B2(N) (8, 18). Glycolaldehyde has not been detected in Orion-KL (18), but the derived abundance upper limit (not shown in the figure) is also much lower than the value measured in comet Lovejoy. IRAS 162293-2422 is the best-studied hot corino. The two components of this solar-type binary protostar exhibit large compositional differences, with sources A and B being richer in nitrogen- and oxygen-bearing molecules, respectively (10). There is an overall agreement between source B and comet organic composition (Fig. 2), although comets are much richer in ethylene glycol (by two orders of magnitude) and ethanol (by a factor of 6), whereas they are depleted in ketene (CH2CO). Contrarily to Orion-KL, IRAS 162293-2422(B) (19) is moderately underabundant (by a factor of 2.5) in glycolaldehyde compared to comet Lovejoy. The same properties are observed for other hot corinos (20).
Fig. 2. Abundances of complex organics in comets and protostars.
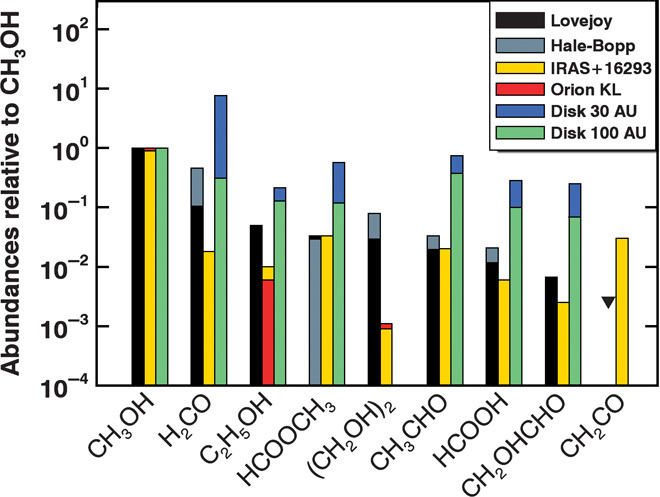
The abundances measured in comets Lovejoy (Table 1) and Hale-Bopp (table S2) are compared with those measured in the low-mass protostar IRAS 16293-2422(B) (19, 33) and in the high-mass protostar Orion-KL (18) and with results from chemical modeling in a protoplanetary disk (25). Abundances are given relative to CH3OH, the most abundant organic molecule in these sources. H2CO values may be less relevant because, for comets, a significant fraction is released from grains (34), whereas for IRAS 16293-2422, the plotted value pertains to the sum of the contributions to the two components A and B of the binary source. For CH2CO, the black triangle is a 3σ upper limit obtained in comet Lovejoy.
Comparison with protoplanetary disks is more difficult. The molecules observed in protoplanetary disks are mainly small species and associated isotopologs. The most complex identified species are H2CO, HC3N, CH3CN, and c-C3H2 (21–24). The HCN/HC3N/CH3CN abundances are estimated to be in the 1:0.05–0.2:0.05–0.2 proportion in the MCW 480 disk, at radial distances corresponding to the comet formation zone (24). This compares well with ratios measured in comets, for example, 1:0.02:0.16 and 1:0.08:0.08 in comets Lovejoy and Hale-Bopp, respectively. A comparison with COM abundances in protoplanetary disks expected from chemical models (25) is shown in Fig. 2. In addition to grain-surface reactions, reactive desorption and irradiation of ice mantle material in the mid-plane of the disk are included in the chemical model, and enhance the abundances of the more complex molecules in the disk grains (25). However, ethylene glycol is produced with a negligible abundance in this model, which implies that efficient formation routes must be found to explain its large abundance in comets (25). Whereas ethylene glycol formation is investigated through the addition of two CH2OH radicals (25), an alternative grain-surface formation route in protoplanetary disks involves sequential atom-atom additions (26), for which reaction rates and reaction barriers need to be measured for implementation in chemical models. Altogether, the high COM abundances in cometary ices, often higher than the hot-corino abundances (Fig. 2), are in line with their synthesis through grain-surface reactions and ice irradiation in the early solar nebula.
Although the possible role of comets in the delivery of terrestrial water has recently been questioned in favor of an asteroidal source (27), the COMs found in comets, which have their origin in the volatile phase of the solar nebula, are likely related to those present in asteroids (28). The identification of biologically important COMs in cometary ices is thus an important step toward our understanding of the origin of life on Earth.
MATERIALS AND METHODS
Observations
Comet C/2014 Q2 (Lovejoy) was observed with the IRAM 30m radio telescope during two periods: on 13.8, 15.8, and 16.8 January 2015 (geocentric distance Δ = 0.496 to 0.528 AU) and on 23.7, 24.7, 25.7, and 26.7 January 2015 (Δ = 0.624 to 0.675 AU) with very good weather conditions. The heliocentric distances were 1.31 and 1.29 AU, respectively. Perihelion was on 30.07 January UT, at 1.290 AU from the Sun. The 13–25 and 26 January observations were obtained with the EMIR 1- and 3-mm receivers, respectively. The main backend we used was a Fourier transform spectrometer, which covers a frequency range of 2 × 8 GHz (two side bands separated by 8 GHz, each in two linear polarizations) in a single setup, with a high spectral resolution of 200 kHz. The spectral resolution (0.3 to 0.2 km s−1 when converted into Doppler velocity) allows the determination of the velocity profile of the narrow cometary lines (~2 km s−1). Using three different tunings, we covered the 210–218, 225–233, and 240–272 GHz frequency ranges. Given the excitation conditions encountered in cometary comae at 1 to 1.5 AU from the Sun, most of the molecules have their strongest lines in this frequency domain. Complex cometary molecules have many lines expected to have similar intensities in this domain, and we obtained secure detections when averaging the signal of the lines expected to be the strongest according to our models (8). The number of lines detected with a signal-to-noise ratio higher than 3 was respectively 2 for CH2OHCHO, 8 for C2H5OH, 2 for HCOOCH3, 37 for CH3CHO, 7 for (CH2OH)2, 9 for HCOOH, 10 for NH2CHO, and 4 for HNCO. Seven lines were used for the upper limit on ketene (CH2CO).
The half-power beamwidth of IRAM 30m at these frequencies ranged from 9.1″ to 11.6″, which corresponded to 3300 to 5400 km at the comet distance. The pointing accuracy, which was regularly checked on reference pointing sources and also on the comet with coarse maps of the strongest lines, was better than 2″.
The time variation of the activity of the comet could be monitored because of the presence of several strong CH3OH lines in each setup. We did not see any variation greater than ±20% during the observations [QCH3OH = 1.2 ± 0.2 × 1028 molecules s−1 (13–16 January) and 1.4 ± 0.2 × 1028 molecules s−1 (23–25 January)]. This justifies the averaging of several days of observations to improve the signal-to-noise ratio and to derive production rates that are more precise. Table S1 provides a list of the strongest ethanol and glycolaldehyde lines identified in the spectra and the production rates derived for each line. Several other lines are individually present only at the 1σ to 3σ level but yield a detection when combined together and were used to compute production rates.
Excitation models and production rate determination
For all molecules, we used the latest spectroscopic data available in the JPL (29) or CDMS (30) database, both for line identification and computation of line strengths. In our model, the population distribution of the ground vibrational state of molecule slowly evolves from LTE at the temperature Tgas maintained by collisions with neutrals and electrons close to the nucleus [(11), (12 ), and references therein] to fluorescence equilibrium in less dense parts of the coma. Collisional rates and electron density are based on a water production rate of 5 × 1029 molecules s−1. We derived Tgas = 73 K from the relative line intensities of the strong methanol lines (figs. S1 and S2). For the other molecules, the line intensity ratios (or rotational temperature) agree with the model [for example, Trot(CH3CHO) = 67 ± 15 K for 68 K predicted]. Except for a few molecules such as methanol or linear molecules, we did not take into account the pumping of the rotational levels by the fluorescence of the infrared vibrational bands, but this process is not expected to play a major role within ~5000 km of the coma sampled here.
The local gas density is described with the Haser model, which assumes isotropic radial outgassing at a constant velocity. The mean gas expansion velocity of 0.80 km s−1 has been derived from the shape of the lines with the highest signal-to-noise ratio: for example, the strong CH3OH(5+2–4+1E) line at 266.838 GHz, observed both with the Fourier transform spectrometer and the VESPA autocorrelator (resolution of 40 kHz), has a linewidth of 1.61 ± 0.08 km s−1, suggesting a mean velocity of 0.80 ± 0.04 km s−1. Given the expansion velocity and beam size, the observations sampled relatively “fresh” molecules, which left the nucleus less than 1.8 hours before the observation. We took the molecular lifetimes from previous research (4, 6, 7, 31), but at this heliocentric distance (1.3 AU), the derived production rates are not very sensitive to the photodissociation rate of the molecule, provided that its lifetime at 1 AU is more than 4000 s. The line intensities are computed with our radiative transfer code for a Gaussian beam. Lines are optically thin, implying that the production rates are proportional to the line intensities. When several lines of a molecule are observed, the production rate is computed using a weighted mean of the production rates computed for each individual line because not all the lines have the same noise level. All lines with an expected intensity of ≥1σ and that are within the observed spectral range are taken into account, even if not detected, to avoid biasing the production rate estimate. The spectra shown in Fig. 1 were obtained by averaging only the stronger lines, weighted according to the noise of each individual spectrum.
For ketene, we obtain a marginal 3σ value of 4.3 ± 1.4 × 1025 molecules s−1 (0.008% relative to water), but the detection of this molecule needs to be confirmed in a brighter comet. Ethanol is expected to be either in the trans or gauche states, which are treated separately in our model. Trans and gauche lines yield similar production rates for both conformers (QE-trans = 4.6 ± 0.6 × 1026 molecules s−1, QE-gauche = 3.0 ± 0.4 × 1026 molecules s−1). The measured ethanol trans/gauche ratio of 1.5 ± 0.3 (1.0 ± 0.2 if we take only the 14 lines with signal-to-noise ratio ≥2 from table S1) corresponds to a conformeric equilibrium temperature of 51+11−6 K, slightly lower but close to the Tgas derived above. Using a cooler temperature for ethanol will not significantly change the derived abundance. The intensity and derived total production rates for the strongest lines of glycolaldehyde and ethanol (assuming 50% in each state for ethanol) are provided in table S1. In the case of the NS radical (Table 1), we have computed the production rate and abundance, assuming a release from close to the nucleus.
Water production rate
The reference water production rate was primarily derived from observations of the OH radical maser lines at 18 cm carried out with the Nançay radio telescope at the same time. During the 12–17 January period, observations centered on the comet and at 3.0′ offset positions (fig. S3) were used to constrain the quenching of the maser inversion (32) yielding an average water production rate QH2O = 5.0 ± 0.2 × 1029 molecules s−1. OH observations performed between 18 and 25 January were all centered on the nucleus so the water production rates are less constrained for this period. Observations of H2O were performed with the Odin satellite on 30 January to 3 February (fig. S3). Analysis of both centered and offset positions up to 3′ yields an average production rate QH2O = 7.5 ± 0.3 × 1029 molecules s−1. Because several methanol lines were detected in each observing setup with the IRAM 30m, we also used the methanol production rate as a proxy for the evolution of water outgassing, correcting possible biases due to larger pointing uncertainties during the period 23–25 January (no coarse mapping was available). From the interpolation of the Nançay and Odin measurements, we estimate that during the period of 23–25 January, QH2O ≈ 6.0 × 1029 molecules s−1. The uncertainty on the water outgassing rate should be less than 10%, but we cannot fully exclude that part of the water vapor observed in the 2′ to 8′ Odin and Nançay beams comes from the sublimation of icy grains conducting to an overestimate of the water outgassing from the nucleus surface. Nevertheless, we do not find clear evidence for a distributed source of gases in the coma of comet Lovejoy: coarse maps of HCN and CH3OH up to 20″ from the nucleus show no evidence for a distributed source, and the interpretation of the Odin water maps does not require considering a distributed source of H2O beyond 1′.
Acknowledgments
The observations were conducted under the target of opportunity proposal D04-14 and regular proposal 128-14. We acknowledge the support from the IRAM director for awarding us discretionary time, and the IRAM staff for its support and for scheduling the observations on short notice. Funding: IRAM is supported by INSU/CNRS (France), MPG (Germany), and IGN (Spain). S.N.M. is supported for this work through the NASA Planetary Astronomy Program. Author contributions: N.B. coordinated the observation program. N.B. and R.M. conducted the IRAM observations. J.C. and A.S. were responsible for the Nançay and Odin comet observations, respectively. P.C. reduced the Nançay OH observations. N.B. carried out the data reduction and modeling of the IRAM and Odin data. D.B.-M. provided the comparative study on complex organics in comet and protostars and other galactic sources of interest. All authors contributed to the observation planning and commented on the manuscript. Competing interests: The authors declare that they have no competing interests. Data and materials availability: The spectral data set is available at the CDS via anonymous ftp to http://cdsarc.u-strasbg.fr (ftp://130.79.128.5).
SUPPLEMENTARY MATERIALS
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/9/e1500863/DC1
Table S1. Observed strongest lines and production rates.
Table S2. Abundances relative to water.
Fig. S1. Rotational diagram of the methanol lines observed between 248 and 256 GHz.
Fig. S2. Rotational diagram of the methanol lines observed around 242 GHz.
Fig. S3. Water production rate: OH and H216O observations.
REFERENCES AND NOTES
- 1.Materials and methods are available as supplementary materials on Science online.
- 2.Herbst E., van Dishoeck E. F., Complex organic interstellar molecules. Ann. Rev. Astron. Astrophys. 47, 427–480 (2009). [Google Scholar]
- 3.D. Bockelée-Morvan, J. Crovisier, J. M. Mumma, H. A. Weaver, in Comets II, M. C. Festou, H. U. Keller, H. A. Weaver Eds. (Univ. of Arizona Press, Tucson, AZ, 2004), pp. 391–423. [Google Scholar]
- 4.Crovisier J., Bockelée-Morvan D., Colom P., Biver N., Despois D., Lis D. C.; Team for target-of-opportunity radio observations of comets , The composition of ices in comet C/1995 O1 (Hale-Bopp) from radio spectroscopy. Astron. Astrophys. 418, 1141–1157 (2004). [Google Scholar]
- 5.Elsila J. E., Glavin D. P., Dworkin J. P., Cometary glycine detected in samples returned by Stardust. Meteoritics Plan. Sci. 44, 1323–1330 (2009). [Google Scholar]
- 6.Bockelée-Morvan D., Lis D. C., Wink J. E., Despois D., Crovisier J., Bachiller R., Benford D. J., Biver N., Colom P., Davies J. K., Gérard E., Germain B., Houde M., Mehringer D., Moreno R., Paubert G., Phillips T. G., Rauer H., New molecules found in comet C/1995 O1 (Hale-Bopp). Investigating the link between cometary and interstellar material. Astron. Astrophys., 353, 1101–1114 (2000). [Google Scholar]
- 7.Crovisier J., Bockelée-Morvan D., Biver N., Colom P., Despois D., Lis D. C., Ethylene glycol in comet C/1995 O1 (Hale-Bopp). Astron. Astrophys. 418, L35–L38 (2004). [Google Scholar]
- 8.Biver N., Bockelée-Morvan D., Debout V., Crovisier J., Boissier J., Lis D. C., Dello Russo N., Moreno R., Colom P., Paubert G., Vervack R., Weaver H. A., Complex organic molecules in comets C/2012 F6 (Lemmon) and C/2013 R1 (Lovejoy): Detection of ethylene glycol and formamide. Astron. Astrophys. 566, L5 (2014). [Google Scholar]
- 9.Belloche A., Müller H. S. P., Menten K. M., Schilke P., Comito C., Complex organic molecules in the interstellar medium: IRAM 30 m line survey of Sagittarius B2(N) and (M). Astron. Astrophys. 559, A47 (2013). [Google Scholar]
- 10.Caux E., Kahane C., Castets A., Coutens A., Ceccarelli C., Bacmann A., Bisschop S., Bottinelli S., Comito C., Helmich F. P., Lefloch B., Parise B., Schilke P., Tielens A. G. G. M., van Dishoeck E., Vastel C., Wakelam V., Walters A., TIMASSS: The IRAS 16293-2422 millimeter and submillimeter spectral survey. I. Observations, calibration, and analysis of the line kinematics. Astron. Astrophys. 532, A23 (2011). [Google Scholar]
- 11.Biver N., Bockelée-Morvan D., Crovisier J., Henry F., Davies J. K., Matthews H. E., Colom P., Gérard E., Lis D. C., Phillips T. G., Rantakyrö F., Haikala L., Weaver H. A., Spectroscopic Observations of Comet C/1999 H1 (Lee) with the SEST, JCMT, CSO, IRAM, and NanÇay Radio Telescopes. Astron. J. 120, 1554–1570 (2000). [Google Scholar]
- 12.Biver N., Bockelée-Morvan D., Crovisier J., Lis D. C., Moreno R., Colom P., Henry F., Herpin F., Paubert G., Womack M., Radio wavelength molecular observations of comets C/1999 T1 (McNaught-Hartley), C/2001 A2 (LINEAR), C/2000 WM1 (LINEAR) and 153P/Ikeya-Zhang. Astron. Astrophys. 449, 1255–1270 (2006). [Google Scholar]
- 13.Pearson J. C., Brauer C. S., Drouin B. J., The asymmetric top–asymmetric frame internal rotation spectrum of ethyl alcohol. J. Mol. Spec. 251, 394–409 (2008). [Google Scholar]
- 14.Paganini L., Mumma M. J., Villanueva G. L., Keane J. V., Blake G. A., Bonev B. P., DiSanti M. A., Gibb E. L., Meech K. J., C/2013 R1 (LOVEJOY) at IR wavelengths and the variability of co abundances among Oort cloud comets. Astrophys. J. 791, 122 (2014). [Google Scholar]
- 15.Vastel C., Ceccarelli C., Lefloch B., Bachiller R., The origin of complex organic molecules in prestellar cores. Astrophys. J. 795, L2 (2015). [Google Scholar]
- 16.Charnley S. B., Chemical models of interstellar gas-grain processes—III. Molecular depletion in NGC 2024. Mon. Not. Roy. Astron. Soc. 291, 455–460 (1997). [Google Scholar]
- 17.Garrod R. T., Weaver S. L. W., Herbst E., Complex chemistry in star-forming regions: An expanded gas-grain warm-up chemical model. Astrophys. J. 682, 283–302 (2008). [Google Scholar]
- 18.Brouillet N., Despois D., Lu X.-H., Baudry A., Cernicharo J., Bockelée-Morvan D., Crovisier J., Biver N., Antifreeze in the hot core of Orion First detection of ethylene glycol in Orion-KL. Astron. Astrophys. 576, A129 (2015). [Google Scholar]
- 19.Jørgensen J. K., Favre C., Bisschop S. E., Bourke T. L., van Dishoeck E. F., Schmalzl M., Detection of the simplest sugar, glycolaldehyde, in a solar-type protostar with ALMA. Astrophys. J. 757, L4 (2012). [Google Scholar]
- 20.Taquet V., López-Sepulcre A., Ceccarelli C., Neri R., Kahane C., Charnley S. B., Constraining the abundances of complex organics in the inner regions of solar-type protostars. Astrophys. J. 804, 81 (2015). [Google Scholar]
- 21.Dutrey A., Guilloteau S., Guélin M., Chemistry of protosolar-like nebulae: The molecular content of the DM Tau and GG Tau disks. Astron. Astrophys. 317, L55–L58 (1997). [Google Scholar]
- 22.Chapillon E., Dutrey A., Guilloteau S., Piétu V., Wakelam V., Hersant F., Gueth F., Henning T., Launhardt R., Schreyer K., Chemistry in disks. VII. First detection of HC3N in protoplanetary disks. Astrophys. J. 756, 58 (2012). [Google Scholar]
- 23.Qi C., Öberg K. I., Wilner D. J., Rosenfeld K. A., First detection of c-C3H2 in a circumstellar disk. Astrophys. J. 765, L14 (2013). [Google Scholar]
- 24.Öberg K. I., Guzmán V. V., Furuya K., Qi C., Aikawa Y., Andrews S. M., Loomis R., Wilner D. J., The comet-like composition of a protoplanetary disk as revealed by complex cyanides. Nature 520, 198–201 (2015). [DOI] [PubMed] [Google Scholar]
- 25.Walsh C., Millar T. J., Nomura H., Herbst E., Weaver S. W., Aikawa Y., Laas J. C., Vasyunin A. I., Complex organic molecules in protoplanetary disks. Astron. Astrophys. 563, A33 (2014). [Google Scholar]
- 26.S. B. Charnley, in Astronomical and Biochemical Origins and the Search for Life in the Universe, IAU Colloquium 161, C. B. Cosmovici, S. Bowyer, D. Werthimer Eds. (Bologna, 1997), pp. 89–96. [Google Scholar]
- 27.Alexander C. M., Bowden R., Fogel M. L., Howard K. T., Herd C. D., Nittler L. R., The provenances of asteroids, and their contributions to the volatile inventories of the terrestrial planets. Science 337, 721–723 (2012). [DOI] [PubMed] [Google Scholar]
- 28.C. M. O’D. Alexander, in The Molecular Universe, Proceedings of the International Astronomical Union, IAU Symposium, Volume 280 (International Astronomical Union, Washington, 2011), pp. 288–301. [Google Scholar]
- 29.Pickett H. M., Poynter R. L., Cohen E. A., Delitsky M. L., Pearson J. C., Müller H. S. P., Submillimeter, millimeter, and microwave spectral line catalog. J. Quant. Spectrosc. Rad. Transfer 60, 883–890 (1998). [Google Scholar]
- 30.Müller H. S. P., Schlöder F., Stutzki J., Winnewisser G., The Cologne Database for Molecular Spectroscopy, CDMS: A useful tool for astronomers and spectroscopists. J. Mol. Struct. 742, 215–227 (2005). [Google Scholar]
- 31.Crovisier J., Photodestruction rates for cometary parent molecules. J. Geophys. Res. 99-E2, 3777–3781 (1994). [Google Scholar]
- 32.Gérard E., Crovisier J., Colom P., Biver N., Bockelée-Morvan D., Rauer H., Observations of the OH radical in comet C/1996 B2 (Hyakutake) with the Nançay radio telescope. Planet. Space Sci. 46, 569–577 (1998). [Google Scholar]
- 33.Bisschop S. E., Jørgensen J. K., Bourke T. L., Bottinelli S., van Dishoeck E. F., An interferometric study of the low-mass protostar IRAS 16293-2422: Small scale organic chemistry. Astron. Astrophys. 488, 959–968 (2008). [Google Scholar]
- 34.Fray N., Bénilan Y., Cottin H., Gazeau M.-C., New experimental results on the degradation of polyoxymethylene: Application to the origin of the formaldehyde extended source in comets. J. Geophys. Res. 109-E7, E07S12 (2004). [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary material for this article is available at http://advances.sciencemag.org/cgi/content/full/1/9/e1500863/DC1
Table S1. Observed strongest lines and production rates.
Table S2. Abundances relative to water.
Fig. S1. Rotational diagram of the methanol lines observed between 248 and 256 GHz.
Fig. S2. Rotational diagram of the methanol lines observed around 242 GHz.
Fig. S3. Water production rate: OH and H216O observations.


