Summary
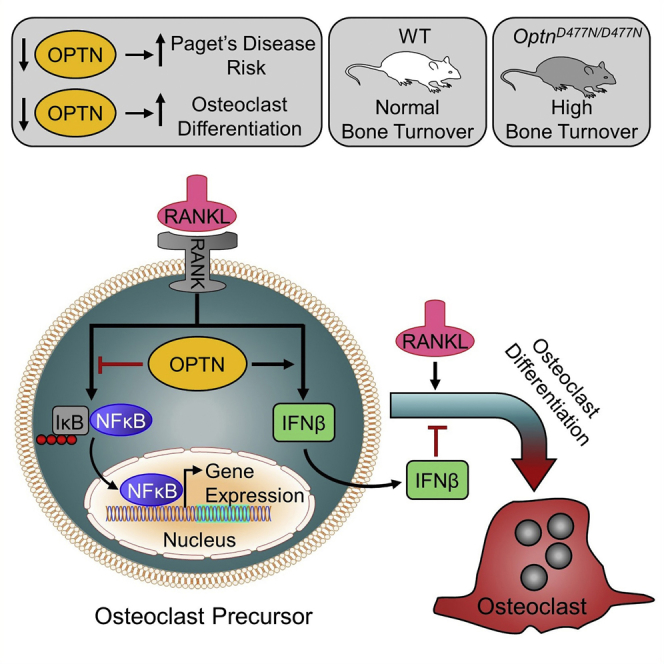
Paget’s disease of bone (PDB) is a common disease characterized by osteoclast activation that leads to various skeletal complications. Susceptibility to PDB is mediated by a common variant at the optineurin (OPTN) locus, which is associated with reduced levels of mRNA. However, it is unclear how this leads to the development of PDB. Here, we show that OPTN acts as a negative regulator of osteoclast differentiation in vitro and that mice with a loss-of-function mutation in Optn have increased osteoclast activity and bone turnover. Osteoclasts derived from Optn mutant mice have an increase in NF-κB activation and a reduction in interferon beta expression in response to RANKL when compared to wild-type mice. These studies identify OPTN as a regulator of bone resorption and are consistent with a model whereby genetically determined reductions in OPTN expression predispose to PDB by enhancing osteoclast differentiation.
Graphical Abstract
Highlights
-
•
Susceptibility to Paget's disease is associated with reduced optineurin expression
-
•
Optineurin knockdown enhances osteoclast differentiation
-
•
Loss of optineurin function increases bone turnover in vivo
-
•
Optineurin inhibits osteoclast formation by modulating NF-κB and IFN-β signaling
Using mouse models, Obaid et al. identify a role of optineurin in bone metabolism as a negative regulator of osteoclast differentiation. Loss of optineurin function leads to increased bone turnover in mice, suggesting a mechanism by which genetic variants in optineurin predispose to Paget’s disease of bone.
Introduction
Paget’s disease of bone (PDB) is a common skeletal disorder characterized by osteoclast activation, which provokes increased but disorganized bone turnover, leading to pathological fractures, bone deformity, and bone pain. The disease has a strong genetic component, but the genes responsible have not been fully characterized. The most important predisposing gene is SQSTM1, which is mutated in ∼10% of patients with the disease. The SQSTM1 causal mutations increase osteoclastogenesis by enhancing receptor activator of nuclear factor kappa B (NF-κB) (RANK) signaling in osteoclasts and their precursors (Cavey et al., 2006, Daroszewska et al., 2011, Hiruma et al., 2008). Linkage studies in families (Lucas et al., 2008) coupled with genome-wide association studies (GWAS) (Albagha et al., 2010, Albagha et al., 2011) have identified a strong susceptibility locus for PDB at the OPTN locus on chromosome 10p13. The OPTN gene encodes optineurin, a ubiquitously expressed cytoplasmic protein involved in many cellular processes including regulation of NF-κB signaling (Zhu et al., 2007), autophagy, and innate immunity (Wild et al., 2011), but the role of optineurin in bone metabolism is unknown. Here, we investigated the role of OPTN in regulating bone turnover and evaluated the molecular mechanisms by which variants at the OPTN locus predispose to PDB.
Results and Discussion
Reduced Expression of OPTN Predisposes to PDB
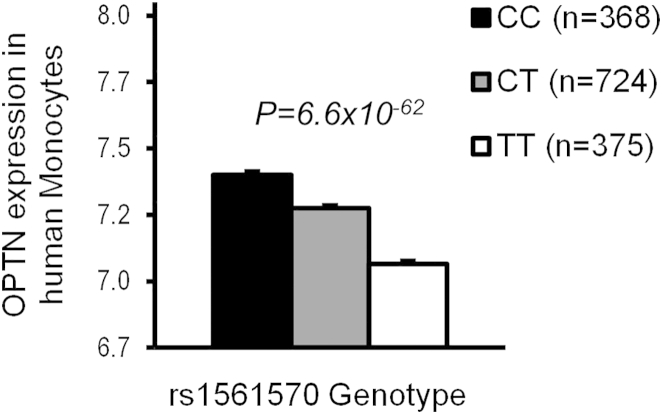
In order to identify disease-causing mutations in the OPTN locus, we conducted mutation screening of the coding exons of OPTN in 200 PDB patients, but results showed no mutations in the protein-coding region (data not shown). However, the top GWAS hit (rs1561570) was found to be a strong expression quantitative trait locus (eQTL) in human monocytes (Zeller et al., 2010) and in peripheral blood mononuclear cells (Westra et al., 2013) with substantially reduced levels of OPTN mRNA expression in carriers of the PDB-predisposing “T” allele with an evidence of an allele-dose effect (Figure 1). These observations indicate that susceptibility to PDB is associated with reduced expression of OPTN and raise the possibility that OPTN might act as a negative regulator of osteoclast function.
Figure 1.
The Paget’s-Disease-Associated SNP rs1561570 Is a Strong eQTL in Human Monocytes
OPTN gene expression levels are shown in relation to rs1561570 genotype in human monocytes. The Paget’s disease risk allele “T” is associated with reduced OPTN gene expression. Data are shown as mean ± SEM; n indicates the number of subjects in each genotype group. Data were extracted from (Zeller et al., 2010).
Optn Knockdown Enhances Osteoclast Differentiation
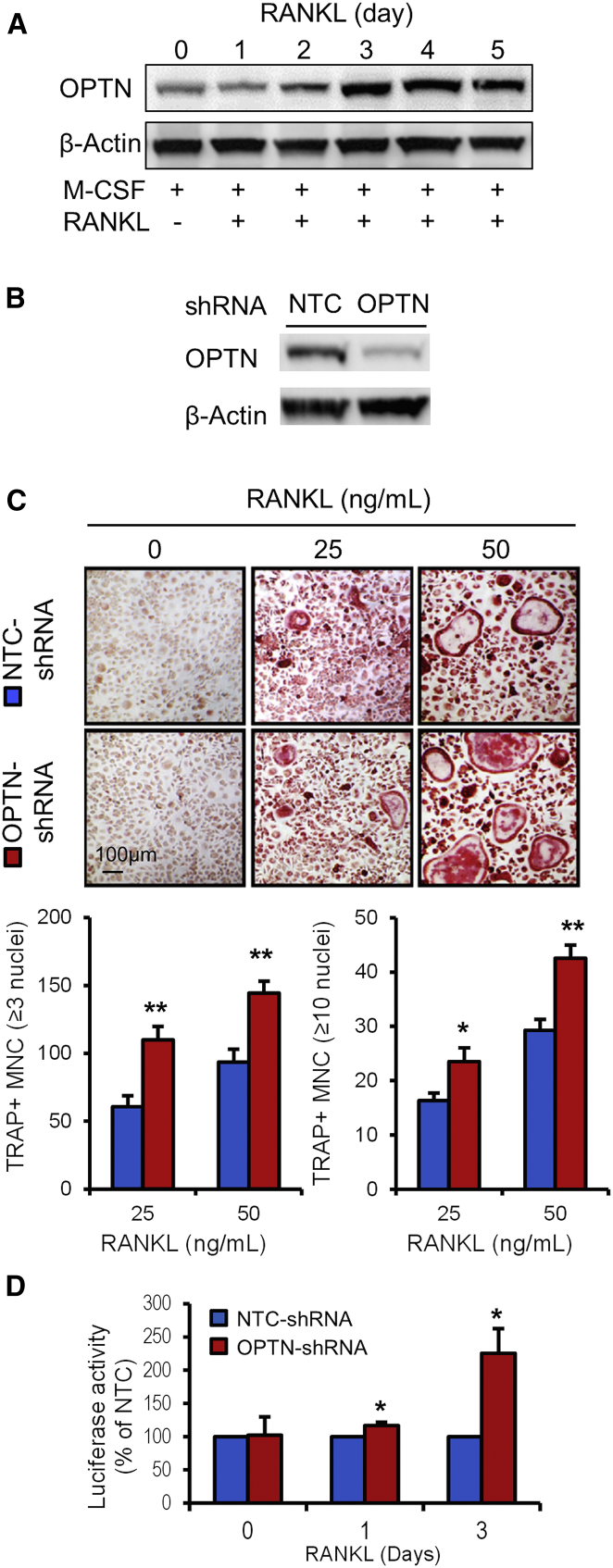
Here, we studied the expression of optineurin during osteoclast differentiation and investigated the effects of Optn knockdown in primary bone-marrow-derived macrophage (BMDM) cultures. Expression of optineurin increased considerably during osteoclast differentiation, following stimulation of the cultures with macrophage colony-stimulating factor (M-CSF) and receptor activator of NF-κB ligand (RANKL) (Figure 2A). Knockdown of optineurin by small hairpin RNA (shRNA) in M-CSF- and RANKL-stimulated BMDMs significantly increased osteoclast numbers and size when compared with a non-targeting shRNA control (Figures 2B and 2C). Overexpression of Optn in RAW 264 cells resulted in a substantial reduction in the number and size of multinucleated TRAP+ cells formed upon stimulation with RANKL (Figure S1). These experiments illustrate that optineurin acts as a negative regulator of osteoclast differentiation in vitro.
Figure 2.
Optn Knockdown in Mouse Bone-Marrow-Derived Macrophages Enhances Osteoclast Differentiation
(A) Immunoblot showing the expression of Optn during osteoclast differentiation. BMDMs were stimulated with M-CSF (25 ng/ml) and RANKL (100 ng/ml), and Optn expression was examined in cell lysate at the indicated time points. Anti-β-actin was used as loading control.
(B) Immunoblot showing Optn knockdown in BMDMs. Cells were transduced with lentiviral particles expressing shRNA targeting the Optn gene or non-targeting control (NTC).
(C) Enhanced osteoclast differentiation in Optn knockdown BMDM. Optn-depleted or NTC cells were stimulated with M-CSF (25 ng/ml) and RANKL (indicated concentrations), and TRAP+ multinucleated cells (MNC) were counted and shown as mean ± SEM from three independent experiments.
(D) RANKL-induced NF-κB activation during osteoclast differentiation. Optn-depleted or NTC cells were transduced with lentiviral particles expressing an NF-κB luciferase reporter followed by stimulation with M-CSF and RANKL, and reporter activity was measured at the indicated time points. Values are mean ± SEM from two independent experiments presented as % of NTC-shRNA. Blots and pictures are representative of three independent experiments. ∗p < 0.05; ∗∗p < 0.01 compared to NTC.
See also Figure S1.
Loss of Optn Function Induces Bone Turnover In Vivo
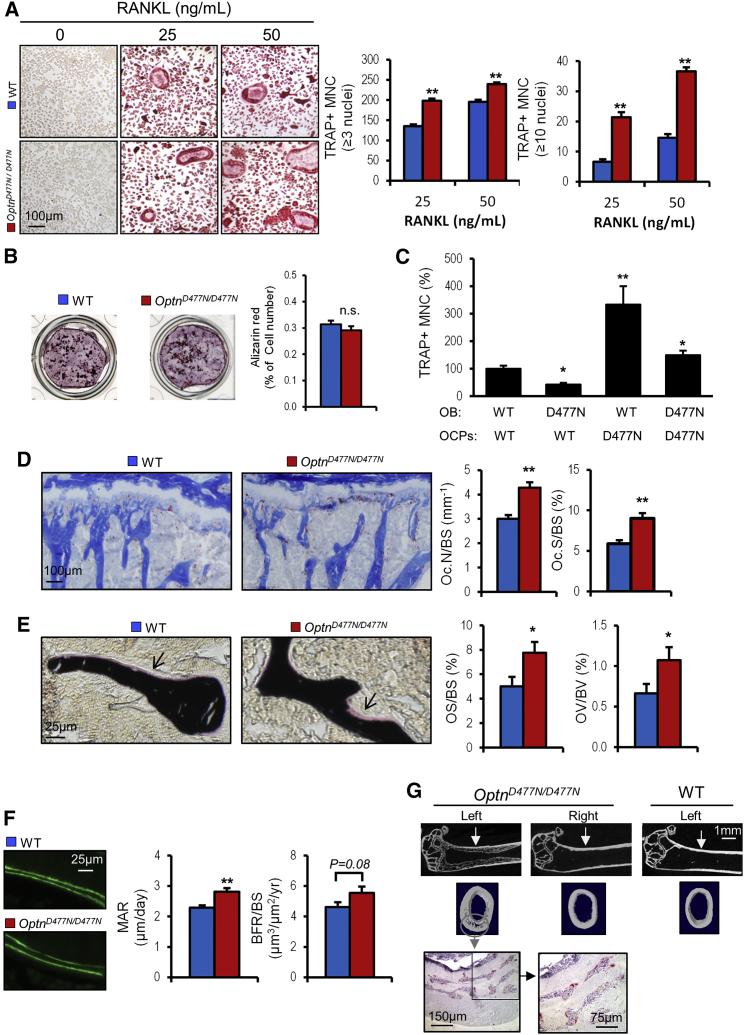
To investigate the effects of Optn on osteoclast activity and bone remodelling in vivo, we conducted skeletal phenotyping of mice homozygous for a D-to-N amino acid substitution at codon 477 of the optineurin protein (OptnD477N/D477N). This is a loss-of-function mutation that encodes a protein that is unable to bind to Lys63-linked ubiquitin chains (Gleason et al., 2011). Bone marrow macrophage cultures from OptnD477N/D477N mice formed significantly more osteoclasts than those from wild-type (WT) littermates and the number of hypernucleated osteoclasts was significantly increased (Figure 3A), replicating exactly the observations in WT cultures subjected to shRNA Optn knockdown (Figure 2C). To investigate if Optn affects osteoblast differentiation and/or function, we performed an in vitro bone nodule assay and osteoblast-osteoclast co-culture assays. There was no difference between OptnD477N/D477N and WT mice in the ability of calvarial osteoblasts to form bone nodules in vitro (Figure 3B). However, osteoblast-osteoclast co-culture assays showed that osteoblasts from OptnD477N/D477N mice had reduced ability to support osteoclast differentiation when compared to WT osteoblast cultures (Figure 3C). In contrast, and consistent with bone marrow culture data presented in Figure 3A, osteoclast precursor cells derived from OptnD477N/D477N mice showed enhanced sensitivity to osteoblast stimulation that appears to compensate for the reduction in the ability of mutant osteoblast to support osteoclast differentiation (Figure 3C).
Figure 3.
Bone Phenotype of OptnD477N/D477N Mice
(A) Enhanced osteoclast differentiation in OptnD477N/D477N bone-marrow-derived macrophages (BMDMs). WT and mutant BMDMs were stimulated with M-CSF and RANKL, and the number of TRAP + multinucleated cells (MNC) was counted and shown as mean ± SEM from three independent experiments.
(B) Bone nodule formation assessed by alizarin red staining in neonatal calvarial osteoblasts from WT and OptnD477N/D477N mice. Alizarin red values were corrected for viable cell count, and values represent mean ± SEM from three independent experiments.
(C) Osteoblast-osteoclast co-culture assay. Calvarial osteoblasts (OBs) were isolated from WT and OptnD477N/D477N mice and co-cultured with bone marrow osteoclast precursor cells (OCPs), and the number of TRAP+ MNC were counted. Values are mean ± SEM from two independent experiments presented as percentage of WT/WT combination.
(D–F) Histomorphometrical analysis of trabecular bone showing enhanced bone turnover in OptnD477N/D477N mice. Representative images of proximal tibial metaphysis stained with TRAP (D), Von Kossa (E), or calcein double labeling (F). Graphs on the right represent comparison of bone resorption indices (osteoclast number per bone surface [Oc.N/BS] and osteoclast surface per bone surface [Oc.S/BS]), bone formation indices (osteoid surface per bone surface [OS/BS] and osteoid volume per bone volume [OV/BV]), or dynamic bone formation indices (mineral apposition rate [MAR] and bone formation rate per bone surface [BFR/BS]) between WT and mutant mice. Data are shown as mean ± SEM from seven to eight animals per group.
(G) PDB-like lesions observed in the left femur of a 15-month-old OptnD477N/D477N mouse. Micro-CT images showing osteolytic bone lesion within the cortex and histological analysis showing enhanced osteoclastogenesis in the affected region. ∗p < 0.05, ∗∗p < 0.01; n.s., not significant compared to WT.
Quantitative bone histomorphometry showed that OptnD477N/D477N mice had significantly increased number of osteoclasts per bone surface (Oc.N/BS) and resorption surfaces (Oc.S/BS) as compared with WT (Figure 3D). Indices of bone formation (osteoid surface per bone surface [OS/BS], osteoid volume per bone volume [OV/BV], and mineral apposition rate [MAR]) were also significantly higher in OptnD477N/D477N mice (Figures 3E and 3F), and there was a non-significant trend (p = 0.08) for an increase in bone formation rate (BFR/BS). These observations illustrate that optineurin negatively regulates osteoclast activity both in vitro and in vivo. The lack of an effect on osteoblast differentiation in vitro suggests that the increased bone formation we observed in OptnD477N/D477N in vivo was most probably secondary to the increase in bone resorption, as occurs in humans with PDB (Ralston et al., 2008). Since PDB is also characterized by the development of focal osteolytic lesions that predominantly affect older people, we performed further skeletal phenotyping of OptnD477N/D477N mice to investigate bone mass and bone structure and to look for evidence of focal osteolytic lesions in aged mice using micro-computed tomography (micro-CT) scanning of lower limbs. There was no difference between genotypes in BV/TV, trabecular number, or structure in young mice (Figures S2A and S2B). However, analysis of older mice aged between 8 and 18 months revealed evidence of a focal osteolytic lesion in the left femur in one OptnD477N/D477N mutant mouse aged 15 months (Figure 3G; Table S1). We then investigated the presence of bone lesions in another loss-of-function mouse model in which the C-terminal polyubiquitin-binding domain of Optn has been deleted, resulting in a truncated protein with low expression (OptnΔEx12/ΔEx12). Analysis of the hindlimbs of OptnΔEx12/ΔEx12 mice (n = 8) by micro-CT showed no Paget’s-disease-like lesions (Table S1). These observations illustrate that while the D477N loss-of-function mutation in Optn increases bone turnover, this does not result in net bone loss, presumably because the increase in bone resorption is coupled with that of bone formation. The development of a focal osteolytic lesion in one OptnD477N/D477N mutant mouse (∼10% of mice aged ≥15 months), but not in OptnΔEx12/ΔEx12, supports the hypothesis that loss of function in optineurin can lead to a PDB-like phenotype while illustrating that additional factors must also be present for focal osteolytic lesions to become fully penetrant.
Optn Regulates Osteoclast Differentiation by Modulating NF-κB and Interferon Signaling
Controlled RANKL-induced NF-κB activation is essential for osteoclast differentiation and function and for the maintenance of normal bone turnover. Previous studies have suggested OPTN as a negative regulator of TNF-α-induced NF-κB activation in immune cells (Maruyama et al., 2010, Nagabhushana et al., 2011, Sudhakar et al., 2009, Zhu et al., 2007), but its role in RANKL-induced NF-κB activation is yet unknown. In order to investigate the effects of Optn on intracellular signaling in osteoclasts, we studied RANKL-induced NF-κB activation during osteoclast differentiation in cultures from OptnD477N/D477N mice as well as in Optn knockdown cultures. We found no difference in RANKL-induced NFκB activation, as measured by phosphorylation of IκBα, in BMDMs from D477N mutant mice compared with WT (Figure 4A). However, following RANKL stimulation, there was a progressive increase in NF-κB activation that was significantly greater in cultures from OptnD477N/D477N as compared with WT mice, with a maximal effect at 3 days (Figure 4B). Similar findings were observed in Optn knockdown cultures in which reduced expression of Optn by knockdown resulted in enhanced NF-κB activity after RANKL stimulation (Figure 2D). These findings indicate that the inhibitory effects of Optn become most apparent as osteoclast differentiation proceeds and is consistent with the observation that Optn levels increase substantially during osteoclast differentiation (Figure 2A). A similar mechanism has previously been reported in Cyld null mice, which show no abnormalities of RANKL-induced NF-κB activation in BMDMs but show enhanced NF-κB activation as osteoclast differentiation proceeds (Jin et al., 2008).
Figure 4.
RANKL-Induced NF-κB Activation and IFN-β Induction in OptnD477N/D477N Mice
(A) BMDMs from WT or mutant mice were stimulated with RANKL (100 ng/ml) and NF-κB activation was assessed by immunoblotting of pIκBα at the indicated time points.
(B) RANKL-induced NF-κB activation during osteoclast differentiation from WT and mutant mice. BMDMs were transduced with lentiviral particles expressing an NF-κB luciferase reporter followed by stimulation with M-CSF and RANKL, and reporter activity was measured at the indicated time points.
(C) Reduced binding of mutant OptnD477N protein to Cyld in osteoclasts. BMDMs from WT and mutant mice were stimulated with M-CSF and RANKL for 5 days and Optn was immunoprecipitated (IP) from cell lysate, and the presence of Cyld and Optn in the immunoprecipitates was analyzed by immunoblotting (IB). The graph to the right represents band quantification by densitometry of the amount of Cyld in the immunoprecipitates corrected for Optn.
(D) IFN-β mRNA expression in response to RANKL stimulation of WT and OptnD477N/D477N osteoclast precursors. Cells were stimulated with RANKL (100 ng/ml), and total RNA was extracted at the indicated time points and analyzed by quantitative real time PCR for IFN-β mRNA levels.
(E) Optn and c-Fos expression during osteoclast differentiation in WT and OptnD477N/D477N mice. BMDMs were stimulated with M-CSF (25 ng/ml) and RANKL (100 ng/ml) and expression was assessed by immunoblotting at the indicated time points; lane 1 (WT-0) was rearranged to ease comparison.
(F and G) Expression of TNFSF11 (F) and IL6 (G) during osteoblast differentiation in WT and OptnD477N/D477N mice. Calvarial osteoblasts were isolated and cultured in osteogenic media and mRNA expression was analyzed by quantitative real-time PCR at the indicated time points.
Values in all graphs are mean ± SEM from three independent experiments presented as percentage of WT. mRNA levels were normalized for 18 s rRNA expression, and results are presented as percentage of WT values. ∗p < 0.05, ∗∗p < 0.01; n.s., not significant compared to WT.
Previous studies have shown that the deubiquitinase enzyme CYLD plays an important negative regulatory role in NF-κB signaling in osteoclasts by interacting with p62 and TRAF6 (Jin et al., 2008). It has also been reported that optineurin is required for CYLD-dependent inhibition of NF-κB activation in immune cells (Nagabhushana et al., 2011). Since the D477N mutation is located in the region that binds to CYLD (Nagabhushana et al., 2011), we studied the effects of the mutant protein on CYLD binding by immunoprecipitation in osteoclasts. This confirmed that the D477N Optn variant had an impaired ability to bind CYLD compared with WT Optn (Figure 4C), indicating that the inhibitory effect of Optn on osteoclast is mediated, in part, by a CYLD-dependent pathway. It has previously been shown that the expression of mutant OptnD477N protein was higher than that of WT in multiple tissues including BMDMs (Gleason et al., 2011). In line with these findings, we also observed that the expression of the mutant OptnD477N protein during osteoclast differentiation was higher than that of the WT (Figures 4C and 4E). This difference was more noticeable from day 3 post-RANKL stimulation (Figure 4E), possibly due to increased NF-κB activity, since a putative NF-κB binding site has been reported in Optn promoter (Sudhakar et al., 2009). However, the increased levels of expression of the mutant OptnD477N protein were clearly not sufficient to compensate for its reduced ability to suppress NF-κB activity and osteoclast differentiation.
Studies have shown that while RANKL stimulates osteoclast activity, it also initiates a negative auto-regulatory effect on osteoclasts through induction of interferon beta (IFN-β) expression (Takayanagi et al., 2002, Hayashi et al., 2002), which has an inhibitory effect on osteoclast by interfering with RANKL-induced expression of c-Fos (Takayanagi et al., 2002). Since previous studies have shown that optineurin is involved in IFN-β signaling in immune cells (Gleason et al., 2011, Munitic et al., 2013), we investigated if Optn plays a role in this negative regulatory loop in osteoclast by studying the expression of IFN-β and c-Fos in both WT and OptnD477N/D477N mutant cells. We found that RANKL induced the expression of IFNβ in both WT and OptnD477N/D477N mutant cells, but the expression levels were significantly lower in mutant cells compared to those observed in WT (Figure 4D). Additionally, c-Fos expression during later stages of osteoclast differentiation (3 days post-RANKL stimulation onward) from OptnD477N/D477N mice was higher than that observed in WT (Figure 4E). These data suggest that in addition to its inhibitory effect on NF-κB signaling, Optn may also exert an inhibitory role on osteoclast differentiation by modulating the IFN-β signaling pathway.
Coupling between bone formation by osteoblasts and bone resorption by osteoclasts is a complex mechanism, but it is well known that osteoblasts produce many pro-osteoclastogenic cytokines to regulate osteoclast function such as TNFSF11 (RANKL) and IL-6. Expression of these two cytokines in osteoblast cultures derived from OptnD477N/D477N mice was significantly lower than that observed in WT cultures, which is consistent with the reduced ability of mutant osteoblast to support osteoclast differentiation (Figures 4F and 4G).
Conclusions
In conclusion, we have demonstrated that reduced expression or loss of Optn function in mice leads to enhanced osteoclast differentiation, identifying Optn as a negative regulator of osteoclast differentiation. The underlying mechanisms are complex but involve RANKL-induced NF-κB activation, an interaction with CYLD, and regulation of IFN-β signaling with regulatory effects that are cell-type specific, dependent on its expression level and on its ability to bind polyubiquitin. Our data suggest that the common genetic variant rs1561570 at the OPTN locus increases susceptibility to PDB by reducing levels of OPTN expression, probably leading to enhanced osteoclast differentiation.
Experimental Procedures
Reagents
Details of the materials and reagents can be found in Supplemental Experimental Procedures.
Mice
The generation of OptnD477N/D477N knockin mice was described previously (Gleason et al., 2011). To generate OptnΔEx12/ΔEx12, the OptnD477N/D477N mice were first crossed to Flpe−/− mice to remove the Flpe transgene, and these mice were then crossed to Bal-1 Cre mice to delete exon 12. All experiments were performed according to institutional, national, and European animal regulations.
Micro-Computed Tomography Analysis
Micro-CT analysis was performed using an in vivo Skyscan 1076 or an ex vivo Skyscan 1172 system. Live animals were scanned on the in vivo scanner looking for the development of PDB-like lesions as previously described (Daroszewska et al., 2011), and those showing evidence of lesions were further analyzed on the ex vivo scanner and then subjected to histological analysis as described below. Image reconstruction was performed using the Skyscan NRecon package and trabecular bone parameters measured using Skyscan CTAn software.
Histomorphometrical Analysis
Animals received two intraperitoneal injections of calcein 3 days apart before being culled. The hindlimbs were fixed for 24 hr in 4% formalin-buffered saline and stored in 70% ethanol prior to embedding in methyl methacrylate. The bones were processed for static and dynamic histomorphometry according to standard techniques. Sections were stained for tartrate-resistant acid phosphatase (TRAP) with aniline blue counterstain to visualize osteoclasts and osteoid analysis was done after Von Kossa staining with Van Gieson counterstaining according to standard protocols as previously described (Erben and Glösmann, 2012). Histomorphometry was performed using custom-built software and followed standardized nomenclature and recommendations (Dempster et al., 2013).
Osteoclast Culture
Bone marrow cells were isolated from the long bones of 4-month-old mice and cultured for 48 hr in the presence of M-CSF (100 ng/ml) to generate BMDMs. Adherent cells were reseeded and stimulated with M-CSF (25 ng/ml) and RANKL for 5 days until osteoclasts were formed. Cells were fixed with 4% (v/v) formaldehyde in PBS and stained for TRAP. TRAP-positive multinucleated osteoclasts (more than three nuclei) were counted, and numbers were compared to the WT control. For the NF-κB activation assay, BMDMs were generated as described above and incubated under standard conditions for 24 hr. Cells were serum starved for 1 hr followed by stimulation with 100 ng/ml RANKL. Cell lysates were collected at different time points and assayed by immunoblotting. For the NF-κB luciferase reporter assay, BMDMs were transduced with lentiviral NF-κB luciferase reporter for 2 days followed by selection for 48 hr using puromycin (5 μg/ml). The cells were then plated in 96-well plates and luciferase activity was measured with a SteadyGlo-luciferase reporter assay system at the indicated time points following M-CSF (25 ng/ml) and RANKL stimulation (100 ng/ml) using a Bio-Tek Synergy HT plate reader. The medium used was complete minimum essential medium alpha modification (αMEM), and all cultures were incubated under standard conditions of 5% CO2 and 37°C in a humidified atmosphere.
Osteoblast Culture and Bone Nodule Assay
Osteoblasts were isolated from the calvarial bones of 2-day-old mice by sequential collagenase/EDTA digestion and cultured in complete αMEM medium. On reaching confluence, cells were detached with trypsin, re-plated in 12-well plates at a density of 1 × 105 cells/well, and cultured in osteogenic medium (complete αMEM supplemented with 50 μg/ml vitamin C and 3 mM β-glycerophosphate). The medium was replaced three times per week, and cultures were continued for up to 21 days. Mineralized nodules were detected using alizarin red staining, and bone nodule formation was quantified by destaining the cultures in 10% (w/v) cetylpyridinium chloride and dissolving the stain in 10 mM sodium phosphate (pH 7.0). The absorbance of the extracted stain was then measured at 562 nm and compared to an alizarin red standard curve. Alizarin red values were corrected for viable cell number as determined by the alamar blue assay.
Optn Knockdown
Bone marrow cells from WT mice were isolated and cultured for 48 hr in complete αMEM supplemented with M-CSF (100 ng/ml). The adherent BMDMs were then transduced with either lentiviral particles containing shRNA targeted against the Optn gene or negative control particles (non-targeting lentiviral particles). Transduced cells were selected for 48 hr using puromycin (5 μg/ml). Optn-depleted BMDMs were then plated in 96-well plates and stimulated with M-CSF (25 ng/ml) and RANKL (25 or 50 ng/ml) until osteoclasts were formed. TRAP-positive multinucleated osteoclasts were counted and numbers were compared to the non-targeted negative control. Optn-depletion was confirmed at all stages of osteoclast differentiation by immunoblotting.
Statistical Analyses
Values in the graphs indicate group means ± SEM, as indicated in the legends. Comparisons between groups were performed by two-tailed t test. p < 0.05 was considered to indicate statistical significance. Experiments were performed as independent replicates as indicated in figure legends.
Author Contributions
R.O. and S.E.W. performed the majority of the experiments with participation from A.A. and R.J. T.H. contributed to Optn overexpression experiments. P.C. provided the OptnD477N and OptnΔEx12 mice and contributed to study design. S.H.R contributed to study design and paper writing. O.M.E.A. designed and supervised the whole project and wrote the paper.
Acknowledgments
We thank Rob van ’t Hof for help in bone histomorphometry and micro-CT, Antonia Sophocleous and Euphemie Landao-Basonga for assistance in histology, and Catherine Gleason for initial work related to this project. This study was supported mainly by a consolidator grant from the European Research Council to O.M.E.A. (311723-GENEPAD) and by grants from Arthritis Research UK to S.H.R. and O.M.E.A. (19799 and 19520).
Published: October 29, 2015
Footnotes
This is an open access article under the CC BY-NC-ND license (http://creativecommons.org/licenses/by-nc-nd/4.0/).
Supplemental information includes Supplemental Experimental Procedures, two figures, and one table and can be found with this article online at http://dx.doi.org/10.1016/j.celrep.2015.09.071.
Supplemental Information
References
- Albagha O.M., Visconti M.R., Alonso N., Langston A.L., Cundy T., Dargie R., Dunlop M.G., Fraser W.D., Hooper M.J., Isaia G. Genome-wide association study identifies variants at CSF1, OPTN and TNFRSF11A as genetic risk factors for Paget’s disease of bone. Nat. Genet. 2010;42:520–524. doi: 10.1038/ng.562. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Albagha O.M., Wani S.E., Visconti M.R., Alonso N., Goodman K., Brandi M.L., Cundy T., Chung P.Y., Dargie R., Devogelaer J.P., Genetic Determinants of Paget’s Disease (GDPD) Consortium Genome-wide association identifies three new susceptibility loci for Paget’s disease of bone. Nat. Genet. 2011;43:685–689. doi: 10.1038/ng.845. [DOI] [PubMed] [Google Scholar]
- Cavey J.R., Ralston S.H., Sheppard P.W., Ciani B., Gallagher T.R., Long J.E., Searle M.S., Layfield R. Loss of ubiquitin binding is a unifying mechanism by which mutations of SQSTM1 cause Paget’s disease of bone. Calcif. Tissue Int. 2006;78:271–277. doi: 10.1007/s00223-005-1299-6. [DOI] [PubMed] [Google Scholar]
- Daroszewska A., van ’t Hof R.J., Rojas J.A., Layfield R., Landao-Basonga E., Rose L., Rose K., Ralston S.H. A point mutation in the ubiquitin-associated domain of SQSMT1 is sufficient to cause a Paget’s disease-like disorder in mice. Hum. Mol. Genet. 2011;20:2734–2744. doi: 10.1093/hmg/ddr172. [DOI] [PubMed] [Google Scholar]
- Dempster D.W., Compston J.E., Drezner M.K., Glorieux F.H., Kanis J.A., Malluche H., Meunier P.J., Ott S.M., Recker R.R., Parfitt A.M. Standardized nomenclature, symbols, and units for bone histomorphometry: a 2012 update of the report of the ASBMR Histomorphometry Nomenclature Committee. J. Bone Miner. Res. 2013;28:2–17. doi: 10.1002/jbmr.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erben R.G., Glösmann M. Histomorphometry in rodents. Methods Mol. Biol. 2012;816:279–303. doi: 10.1007/978-1-61779-415-5_19. [DOI] [PubMed] [Google Scholar]
- Gleason C.E., Ordureau A., Gourlay R., Arthur J.S., Cohen P. Polyubiquitin binding to optineurin is required for optimal activation of TANK-binding kinase 1 and production of interferon β. J. Biol. Chem. 2011;286:35663–35674. doi: 10.1074/jbc.M111.267567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hayashi T., Kaneda T., Toyama Y., Kumegawa M., Hakeda Y. Regulation of receptor activator of NF-kappa B ligand-induced osteoclastogenesis by endogenous interferon-beta (INF-beta ) and suppressors of cytokine signaling (SOCS). The possible counteracting role of SOCSs- in IFN-beta-inhibited osteoclast formation. J. Biol. Chem. 2002;277:27880–27886. doi: 10.1074/jbc.M203836200. [DOI] [PubMed] [Google Scholar]
- Hiruma Y., Kurihara N., Subler M.A., Zhou H., Boykin C.S., Zhang H., Ishizuka S., Dempster D.W., Roodman G.D., Windle J.J. A SQSTM1/p62 mutation linked to Paget’s disease increases the osteoclastogenic potential of the bone microenvironment. Hum. Mol. Genet. 2008;17:3708–3719. doi: 10.1093/hmg/ddn266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin W., Chang M., Paul E.M., Babu G., Lee A.J., Reiley W., Wright A., Zhang M., You J., Sun S.C. Deubiquitinating enzyme CYLD negatively regulates RANK signaling and osteoclastogenesis in mice. J. Clin. Invest. 2008;118:1858–1866. doi: 10.1172/JCI34257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas G.J., Riches P.L., Hocking L.J., Cundy T., Nicholson G.C., Walsh J.P., Ralston S.H. Identification of a major locus for Paget’s disease on chromosome 10p13 in families of British descent. J. Bone Miner. Res. 2008;23:58–63. doi: 10.1359/jbmr.071004. [DOI] [PubMed] [Google Scholar]
- Maruyama H., Morino H., Ito H., Izumi Y., Kato H., Watanabe Y., Kinoshita Y., Kamada M., Nodera H., Suzuki H. Mutations of optineurin in amyotrophic lateral sclerosis. Nature. 2010;465:223–226. doi: 10.1038/nature08971. [DOI] [PubMed] [Google Scholar]
- Munitic I., Giardino Torchia M.L., Meena N.P., Zhu G., Li C.C., Ashwell J.D. Optineurin insufficiency impairs IRF3 but not NF-κB activation in immune cells. J. Immunol. 2013;191:6231–6240. doi: 10.4049/jimmunol.1301696. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nagabhushana A., Bansal M., Swarup G. Optineurin is required for CYLD-dependent inhibition of TNFα-induced NF-κB activation. PLoS ONE. 2011;6:e17477. doi: 10.1371/journal.pone.0017477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ralston S.H., Langston A.L., Reid I.R. Pathogenesis and management of Paget’s disease of bone. Lancet. 2008;372:155–163. doi: 10.1016/S0140-6736(08)61035-1. [DOI] [PubMed] [Google Scholar]
- Sudhakar C., Nagabhushana A., Jain N., Swarup G. NF-kappaB mediates tumor necrosis factor alpha-induced expression of optineurin, a negative regulator of NF-kappaB. PLoS ONE. 2009;4:e5114. doi: 10.1371/journal.pone.0005114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takayanagi H., Kim S., Matsuo K., Suzuki H., Suzuki T., Sato K., Yokochi T., Oda H., Nakamura K., Ida N. RANKL maintains bone homeostasis through c-Fos-dependent induction of interferon-beta. Nature. 2002;416:744–749. doi: 10.1038/416744a. [DOI] [PubMed] [Google Scholar]
- Westra H.J., Peters M.J., Esko T., Yaghootkar H., Schurmann C., Kettunen J., Christiansen M.W., Fairfax B.P., Schramm K., Powell J.E. Systematic identification of trans eQTLs as putative drivers of known disease associations. Nat. Genet. 2013;45:1238–1243. doi: 10.1038/ng.2756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wild P., Farhan H., McEwan D.G., Wagner S., Rogov V.V., Brady N.R., Richter B., Korac J., Waidmann O., Choudhary C. Phosphorylation of the autophagy receptor optineurin restricts Salmonella growth. Science. 2011;333:228–233. doi: 10.1126/science.1205405. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeller T., Wild P., Szymczak S., Rotival M., Schillert A., Castagne R., Maouche S., Germain M., Lackner K., Rossmann H. Genetics and beyond--the transcriptome of human monocytes and disease susceptibility. PLoS ONE. 2010;5:e10693. doi: 10.1371/journal.pone.0010693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu G., Wu C.J., Zhao Y., Ashwell J.D. Optineurin negatively regulates TNFalpha- induced NF-kappaB activation by competing with NEMO for ubiquitinated RIP. Curr. Biol. 2007;17:1438–1443. doi: 10.1016/j.cub.2007.07.041. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.