SUMMARY
A long-held view is that stroke causes many distinct neurological syndromes due to damage of specialized cortical and subcortical centers. However, it is unknown if a syndrome-based description is helpful in characterizing behavioral deficits across a large number of patients. We studied a large prospective sample of first-time stroke patients with heterogeneous lesions at 1–2 weeks post-stroke. We measured behavior over multiple domains and lesion anatomy with structural MRI and a probabilistic atlas of white matter pathways. Multivariate methods estimated the percentage of behavioral variance explained by structural damage.
A few clusters of behavioral deficits spanning multiple functions explained neurological impairment. Stroke topography was predominantly subcortical, and disconnection of white matter tracts critically contributed to behavioral deficits and their correlation. The locus of damage explained more variance for motor and language than memory or attention deficits. Our findings highlight the need for better models of white matter damage on cognition.
Keywords: Neuropsychology, focal injury, thalamus, basal ganglia, white matter pathways, and connectivity
INTRODUCTION
A cornerstone of clinical neurology is that focal brain injury causes specific behavioral symptoms or syndromes that reflect the functional specialization of different brain modules. A Broca’s aphasia is characterized by speech output deficits and classically localizes to the inferior frontal cortex/anterior insula (plus underlying white matter), whereas alexia without agraphia is characterized by reading deficits and classically localizes to the left occipito-temporal cortex (plus or minus splenium of the corpus callosum). However, most of these syndromes have been described in a handful of patients selected based on their unique behavioral profile or lesion location. Hence it is unknown if a syndrome-based description is helpful in characterizing behavioral deficits across a large number of patients (for an historical perspective on the origin of syndromes, Doody, Brain & Language, 1993).
In contrast, studies that have examined patterns of neurological deficits in large samples with heterogeneous lesion locations have reported that a small number of behavioral factors explain well neurological impairment. For instance, studies on the factor structure of the NIH Stroke Scale (NIHSS)(Lyden et al., 2004; Lyden et al., 1999; Zandieh et al., 2012) indicate that two factors, one related to left hemisphere and one to right hemisphere damage, capture the great majority of variability in performance. However, this simplified factor structure may simply reflect a lack of sensitivity as cognitive functions are only cursorily assessed in the NIHSS.
The first goal of this study was then to determine patterns of behavioral impairments and their variability across subjects in a large (n=132) sample selected to be representative of the stroke population at large. A description of the main axes of behavioral impairment is not only important for neuropsychological correlation at the population level, but also to identify phenotypes for recovery or treatment studies in stroke, a disease that is the major cause of disability worldwide (Duncan et al., 2005).
The second goal was to describe the anatomy of stroke. Several large-scale prospective studies have shown that the majority of lesions are subcortical, and that cortical lesions account for less than 15% of the total number of strokes (Bogousslavsky et al., 1988; Kang et al., 2003; Wessels et al., 2006). This fact runs counter to the emphasis given to cortical strokes both in terms of behavioral correlates, mechanisms of recovery, and funding at the National Institutes of Health. In previous studies, however, lesions were classified by visual inspection in broad anatomical categories (cortical, subcortical, and cortical-subcortical). Here we perform single subject segmentation of lesions and atlas-based voxel-wise analyses to generate an average map of stroke damage.
The third goal was to understand the relationship between structural damage and behavioral impairment across multiple domains of function (e.g. language, motor, memory). Previous studies on the topography of stroke did not measure behavior. There have also been numerous neuropsychological studies that have considered the relationship between structural damage and specific behavioral deficits (e.g. aphasia) in so called “lesion-symptom” mapping studies (Barbey et al., 2013; Bates et al., 2003; Dronkers et al., 2004; Glascher et al., 2009; Hillis et al., 2002; Karnath et al., 2004). These studies have reported specific loci of cortical or subcortical damage for different syndromes, or for specific deficits within a syndrome (Barbey et al., 2013; Hillis et al., 2005; Verdon et al., 2010). To our knowledge no study has considered concurrently different kinds of deficits and their correlation in the same cohort, as well as the related variability both behavioral and structural. Furthermore, in previous studies patients were selected based on the presence of the deficit of interest (e.g. aphasia) or a lesion in the location of interest (e.g. left hemisphere lesions). Finally, these studies applied univariate regression models, which can produce lesion-symptom maps but cannot determine the amount of behavioral variance explained. Our goal was to use a multivariate approach to examine lesion-behavior relationships across multiple domains in a clinically relevant sample that was prospective and included patients with any neurological deficit and lesion location.
This design has several important features. First, it allows for the localization of regions of damage that are important across multiple cognitive domains. For instance, a recent paper argued that damage to specific cortical hubs defined by fMRI connectivity in healthy subjects causes widespread impairments of many cognitive functions (Warren et al., 2014). Second, it allows a comparison across different neurological impairments of the relative explanatory “weight” of structural damage, i.e. how much of the behavioral variance is explained by anatomical damage. For instance, motor deficits, which presumably are dependent on the corticospinal output, may have a strong association with structural damage (Carter et al., 2011; Schaechter et al., 2009). In contrast, attention and memory deficits, which rely on more distributed patterns of activity (Awh and Jonides, 2001; Corbetta and Shulman, 2002) may have a weaker relationship. Understanding the relative importance of structural damage on behavior is fundamental to our understanding of stroke pathogenesis, recovery, and outcome.
A final innovation is that clinical-anatomical correlations were carried out with a novel multivariate method based on machine learning (ridge regression). Multivariate methods control for hidden biases, such as the vascular distribution of damage, that consistently distort lesion-deficit maps computed using commonly used voxel-wise univariate methods (Mah et al., 2014; Phan et al., 2010). These biases can displace inferred critical regions from their true locations in a manner opaque to replication. Ridge regression models allow us to predict behavioral variance based on structural features including volume, location, white matter tracts affected, or functional features (e.g. patterns of functional connectivity).
RESULTS
Subjects (n=172) with a first symptomatic stroke, ischemic or hemorrhagic, anywhere in the brain and clinical evidence of any neurological impairment were prospectively recruited, with n=132 meeting post-enrollment inclusion criteria (see methods and supplementary information for details about subjects Supplementary Figure 1, Fig. S1, for enrollment flowchart). They were studied at 3 time points: 1–2 weeks; 3 months; and 12 months with a neurobehavioral battery and structural (T1/T2, Flair), functional (resting state), diffusion, and perfusion based magnetic resonance imaging (MRI). This report concentrates on the acute neurobehavioral assessment (mean 13 ± 4.9 days) and structural MRI studies (Fig. 1 for experimental design).
Figure 1. Experiments and time line.
The red outline indicates the data used in this report.
The behavioral battery was both broad, covering multiple domains (language, motor, memory, attention) and deep, as it assessed different processes within each domain (supplementary information for details about specific tests and references; Supplementary Table 1, Table S1, for list of tests and domain of function tested). For instance, the Motor battery measured strength, active range of motion, dexterity, and function in the upper extremity, distally and proximally, and strength, range of motion, and walking indexes in the lower extremity. The Language battery measured auditory comprehension, speech production, and reading both at the single word and sentence level; it also tested aspects of semantic and phonological processing. The Memory battery separately examined verbal and spatial memory, and in each domain immediate and delayed recall, recognition and familiarity. The battery also included measures of Executive Function like backward spatial span and animal fluency. The Attention battery included tests that are sensitive to neglect both acutely and chronically, and covered both goal-driven and stimulus-driven attention, two major axes in which attention deficits may manifest. It also separately measured general performance, associated with sustained attention, and visuospatial lateralized attention.
Clinical representativeness of study sample
To assess whether the study sample (n=132) was representative of the population of stroke patients at our medical center, we selected from the Cognitive Rehabilitation Research Group database (N=6260), which includes all the stroke patients admitted to Barnes-Jewish Hospital in the last five years, a source population control group selected based on the same inclusion/exclusion criteria of the study. This group includes n=1209 subjects who were compared in terms of demographics, risk factors, and neurological severity to the sample. We also ran a group of healthy control subjects (n=30), typically spouses or first-degree relatives of our patients, age- and education-matched to the stroke sample.
The study sample was well matched to the healthy controls on most variables including age distribution, gender frequency, race, education, and predisposing factors except incidence of hypertension (stroke 70%; controls 26%, p<0.001) and diabetes mellitus (stroke 31%; controls 16%, p<0.001). Coronary artery disease, atrial fibrillation, and depression were also well matched.
As compared to the source population, the study sample was younger (median age=54 vs. 62 years old; chi-square 39.1, p<0.001), and included a significantly greater proportion of African-Americans (64% vs. 35%; binomial test, p<0.001). Gender and education were matched. The sample had a higher proportion of smokers (p<0.001), a similar proportion of diabetes type II and hypertension, but a lower incidence of coronary artery disease (stroke sample 8%; source population 22%, p<0.001), atrial fibrillation (stroke sample 5%; source population 11%, p<0.01), and depression (stroke sample 5%; source population 11%, p<0.01)(Table S2).
In terms of stroke-related variables, the study sample and source population were equivalent for average NIHSS overall severity (mean=7.5 vs. 8.3), stroke side, and frequency of aphasia, motor symptoms, and neglect. The study sample had a significantly lower proportion of hemorrhagic strokes (17% vs. 24%, p=0.016), fewer patients with mild (53% vs. 58%) or severe (13% vs. 19%) strokes, and relatively more patients in the intermediate range (34% vs. 23%)(chi-square=8.843, p=0.012)(Figure 2S). Overall the sample is slightly skewed toward patients with moderate deficits who are admitted to an acute inpatient rehabilitation unit (~60% of all stroke patients at Barnes-Jewish Hospital).
Behavior: within domain factor analysis
The first major goal of the study was to describe the across-subject variability of neurological deficits. We used principal component analysis (PCA), a common data reduction strategy that identifies hidden variables or factors that capture the possible correlation of behavioral scores across subjects. A high number of factors within or across behavioral domains would be consistent with a large number of behavioral syndromes, while a small number of factors would be consistent with a few behavioral clusters common across many subjects.
A PCA with oblique rotation was run separately on the behavioral data for each domain (e.g. motor, language, attention, memory)(Table 1). The highest number of subjects tested in each domain was used. In the motor domain (N=117), two main factors were identified for the left and right sides of the body, respectively, which accounted for 77% of the variance across all motor tasks and subjects. There was a high degree of correlation between individual motor tests for both upper and lower extremity that ranged between r=0.73 and r=0.95 (Table 1, Figure 2A–B). Interestingly, tests of walking showed an intermediate correlation (r=0.56–0.68 with each factor (Figure 2A). The two motor factors were not correlated (Figure 2C).
Table 1.
Principal component analysis (PCA) within each domain of function.
| Motor battery | Variance explained: 77.2% | |
|---|---|---|
| Pattern Matrix | ||
| Component | ||
| 1 | 2 | |
| Left Shoulder Flexion | .947 | |
| Right Shoulder Flexion | .893 | |
| Left Wrist Extension | .874 | |
| Right Wrist Extension | .837 | |
| Left Grip Strength | .782 | |
| Right Grip Strength | .742 | |
| Left Hand 9-Hole Peg Test | .850 | |
| Right Hand 9-Hole Peg Test | .825 | |
| Left total ARA | .952 | |
| Right total ARA | .913 | |
| Timed Walk + FIM Walk Item | .681 | .558 |
| Left Lower Extremity TOTAL Motricity Index | .917 | |
| Right Lower Extremity TOTAL Motricity Index | .896 | |
| Left Ankle Motricity Index | .921 | |
| Right Ankle Motricity Index | .845 | |
Figure 2. Principal component analysis (PCA) within each domain of function.
A) Factor scores for left side (blue, right hemisphere) and right side (red, left hemisphere) motor tests. Note walking in between (green). B) Scatter plot of Arm Research Action (ARA) test scores vs. right motor factor scores. Healthy controls: green dots; Left hemisphere patients: red dots; right hemisphere patients: blue dots. Note that healthy controls were not included in PCA. C) Left vs. right motor factor scores. D) Oral command vs. language factor scores. E) Language factor scores vs. lesion hemisphere. F) Boston Naming vs. Word Comprehension scores. G) Spatial span vs. Spatial memory factor scores; H) Hopkins Verbal Memory (% retained) vs. Verbal memory factor scores; I) Verbal vs. Spatial memory factor scores; J) Posner visual field accuracy vs. Attention visual field factor scores; K) Posner overall reaction time vs. Attention general performance factor scores; L) Language vs. Attention visual field bias factor scores.
In the language domain (N=124), a single factor accounted for 76% of the variance across tasks and subjects (Table 1). This factor strongly correlated with tasks of auditory comprehension (Figure 2D), expression, and reading, with correlation coefficients ranging between r=0.82 and 0.91 and clearly separated subjects with lesions in the left vs. right hemisphere (Figure 2E). Interestingly, processes that are typically considered as separate in the aphasia literature were strongly correlated, e.g. speech output vs. comprehension (Figure 2F).
In light of the classic evidence for dissociations of different language disorders, we also performed a PCA only on the patients who scored 2 standard deviations below the healthy controls on the language factor (N=39). While we are aware of the small sample size, the robust outcomes are significant in descriptive terms. The analysis revealed two factors that accounted for 77% of the variance: Tests of verbal comprehension (complex ideational material, r=0.98; commands, r=0.80; reading comprehension, r=0.56) but also production (Boston naming, r=0.71; oral reading r=0.52) loaded on the first factor accounted for 66% of the variance, and tests of production/articulatory-phonetic processing (non-word reading r=0.95; stem completion r=0.6; oral reading=0.48) loaded on the second factor accounted for 11% of the variance (Table S3). Therefore, when the analysis of the language tests was restricted to aphasic patients, some evidence for separate comprehension and production deficits was obtained. However, when the entire stroke population was analyzed, patients who showed a comprehension deficit also showed some evidence of a production deficit relative to patients who did not show a comprehension deficit. A similar statement applies to patients who showed production deficits.
In the memory domain (N=98), two factors explained 66% of the variance: visuospatial memory loaded on one factor with correlations among tasks between r=0.68 and 0.80 (Figure 2G), and verbal memory loaded on a different factor with correlations among tasks between r=0.74 and 0.93 (Figure 2H). There was no clear separation between working, immediate, and delayed memory recall, and response criterion (discrimination index)(Table 1). The two memory factors were correlated and did not differentiate the side of the lesion (Figure 2I).
Finally, in the attention domain (N=101), the factor analysis identified three factors that accounted for 57% of the variance (Table 1). The first factor isolated a contralesional visual field bias, which included the Posner visual field effect (Figure 2J), and Mesulam and BIT center of cancellation scores. This deficit affected both left and right hemisphere patients, but more severely the latter group. The second factor isolated an overall performance (sustained attention) factor loading on low accuracy and slow reaction times, and was present in left and right hemisphere patients (Figure 2K). The third factor was related to deficits in shifting attention (Posner validity accuracy and disengagement RT)(Table 1).
To examine the relationship between within domain behavioral deficits and side of lesion, we ran t-tests on each domain score between left and right hemisphere lesion patients. As expected based on clinical experience, deficits of language were more severe in left hemisphere patients (p<0.0001), while deficits of spatial attention (visual field bias) were more severe in right hemisphere patients (p<0.03) and were not correlated with each other (Figure 2L). Motor deficits were lateralized as well (right motor, p<0.0001; left motor, p<0.0001). Verbal memory was weakly lateralized to the left hemisphere (p<0.051), while spatial memory, attention overall performance, and attention shifting were not lateralized.
In summary, the within domain factor analysis isolated one or a few robust factors that accounted for large amounts of behavioral variance indicating that deficits within a domain are correlated.
Behavior: across domain factor analysis
Next, we looked at the relationship among deficits in different behavioral domains, i.e. the factors isolated in the within domain PCA, using a higher-order PCA across domains. This is a well-accepted strategy to look at the hierarchical structure of factors (Turken and Dronkers, 2011). The data from 67 subjects included all of the test scores necessary for the analysis.
Three main uber-factors accounted for 69% of the variance. Language (0.88), verbal memory (0.89), and, at an intermediate level, spatial memory (0.59) loaded on a first factor. Left-side motor (0.77), visual field bias (0.80), general performance (−0.66), and spatial memory (0.53) loaded on a second factor. A third factor was associated with right-side motor (0.85), visual field bias (−0.35), and attention shifting (0.68). We examined the lateralization of these factors by comparing factor scores for left vs. right hemisphere lesions. Factor 1 (language, verbal and spatial memory) was not lateralized (p=n.s.) likely because of the bilateral representation of spatial memory; factor 2 (left motor, visual field bias, general performance, and spatial memory) was lateralized to the right hemisphere (p<0.01); and, factor 3 (right motor, visual field bias, and attention shifting) was lateralized to the left hemisphere (p<0.001). This factor structure is represented in Figure 3 where the size of each red circle indicates the percentage of variance across subjects accounted for by each uber-factor, while the size of the labels for each domain of function indicates the strength of loading on that uber-factor (see Table S4 for the loadings of each uber-factor).
Figure 3. Higher-order PCA.
The diameter of each red circle corresponds to the % variance accounted for by each factor. The font size corresponds to the loading of each function onto the factor.
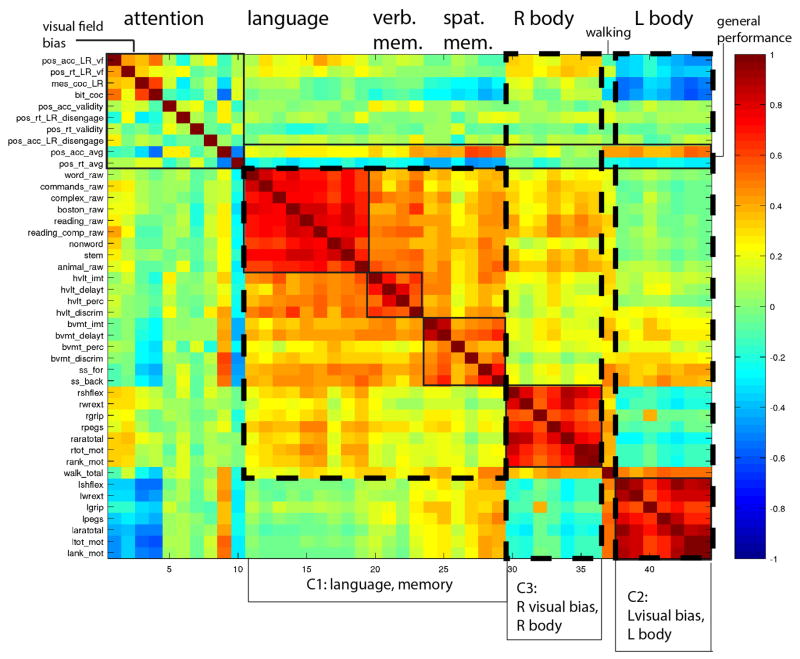
A different way to examine the correlation among behavioral scores is simply to compute a correlation matrix of raw scores for the different tests across subjects (Figure 4)(see Table S5 for PCA on raw scores). This approach allows for the visualization of strength of correlation within and between domains. The “block” structure along the diagonal indicates strong correlation among the different tests within each domain (language, verbal memory, spatial memory, left and right motor). Tests of spatial attention and exploration for left and right visual field correlate (Posner L/R visual field; BIT and Mesulam cancellation). Overall accuracy and reaction times on the Posner task (last two rows of the “attention” block in Figure 3) were correlated, consistent with a general performance factor. Note that general performance correlated with many tests in other domains, consistent with the clinical observation that sustained attention is necessary for performance of many cognitive functions. Walking scores correlated with both left and right motor scores as one might expect for a function that involves both sides of the body.
Figure 4. Correlation matrix of behavioral scores.
The color scale indicates Pearson r-values. C1–C3 refers to factors 1–3 as described in the text. Y-axis: individual tests. X-axis: functional domains.
Across domains there was a robust correlation between language, verbal memory, and spatial memory consistently with uber-factor 1 as detected in the higher order PCA. Each motor factor (left or right) correlates with congruent visual field biases consistent with uber-factors 2 and 3. These impressions were confirmed by a PCA on the raw scores for the individual tests. This analysis also identified three main factors that accounted for 48% of the variance and were similar to those identified by the higher-order factor analysis (regions defined by dashed lines in Fig. 3). Factor 1 loaded on language and verbal and spatial memory tests. Factor 2 loaded on left-side motor tests for upper and lower extremities, including tests for strength, range of motion, dexterity, and function; it also loaded on visual field bias as captured by accuracy and reaction time on the Posner task and Mesulam center of cancellation. Finally, factor 3 loaded mainly on right-side motor tests and modestly with visual field bias.
In summary, both the higher order PCA and the PCA on the raw scores identified three main axes of behavioral impairment: one related to language and memory, one related to left-body motor and left spatial attention, and one related to right-body motor deficits and right spatial attention.
Anatomy
Because previous studies have described the topography of stroke using visual inspection, the second major goal of the study was to characterize the topography of stroke using a voxel-wise analysis of structural lesions. Each lesion was manually segmented on structural MRI scans and checked by two board certified neurologists (MC, AC).
Figure 5 shows a voxel-wise average of the segmented lesions normalized to a standard brain atlas (see Methods, Table S6 for individual lesion information, and Table S7 for localization of lesions in this and other published studies). The majority of strokes were localized subcortically and concentrated in the basal ganglia, central white matter, and thalamus. Cortical lesions were less common, and predominantly occurred in the middle cerebral artery distribution. The location (cortico-subcortical, subcortical, white-matter only) of each individual lesion was assigned with an unsupervised K-means clustering on the percentage of total cortical/subcortical gray and white matter masks overlay, and was as follows: brainstem, 7%; cerebellum, 17%; cortical 13%; cortico-subcortical 23%; subcortical, 16%; and white matter only 23%. The majority of lesions (39%) were subcortical, including white matter.
Figure 5. Topography of stroke.

Lesion overlay map in atlas space. Color scale: number of subjects with lesions at each voxel.
The average lesion volume was 34,176 mm3 (4272 voxels of 2×2×2 mm). The periventricular white matter disease load was low (mean=1.2±1.5 out of 9 grades), as were the number of lacunes (mean=1.6±2.7). Low white matter disease and low number of lacunes were part of the inclusion/exclusion criteria to insure that the neurological impairments were related to first time stroke.
Behavior-to-Anatomy relationships: single domain prediction
To map lesion-behavior relationships, we applied ridge regression models separately for each factor. Ridge regression is a multivariate machine-learning method. In this application, the distribution of damaged voxels is used to generate leave-one-out predictions of continuous factor scores in each domain (language, left and right motor, attention (visual field bias), spatial and verbal memory)(see Figure S3, methods, and supplementary information for explanation of methods). The accuracy of the model can be assessed by the percentage of variance explained. The weights (ω) from the model can be back-projected to the brain to display the most predictive voxels. Importantly, multivariate methods such as ridge regression control for hidden biases such as the vascular distribution of damage (Mah et al., 2014).
Figure 6 shows the ridge regression ω maps for each factor score domain by domain. The color scale reflects z-scored predictive weights for damage to that voxel as compared to a random distribution. The bar graphs on the right show the percentage behavioral variance accounted for respectively by the lesion volume and spatial distribution. In other words, the % variance measure indicates the strength of the structural prediction for different deficits.
Figure 6. Ridge regression maps relating behavioral deficits to anatomical damage.
The color scale indicates weights (ω) determined by ridge regression for six behavioral factors, normalized to have a standard deviation of 1. Inset bar graphs show the percent of variance explained by lesion size alone (blue) or by lesion location (red). The dashed lines indicate significance thresholds determined by 10,000 random permutations of factor scores (5–6%).
Several results are notable. First, lesion volume accounts for only small amounts of variance (<20%) in all domains. Second, the location of structural damage explains different amounts of variance for different behavioral factors: high variance for left motor deficits (54% of variance) and language (44%); moderate variance for right motor deficits (27%) and attention/visual field biases (34%); and little variance for verbal (17%) and spatial memory (4%) deficits.
Third, each deficit is associated with damage to many different regions, cortical and subcortical, often in different vascular distributions. Motor deficits were associated with damage to the corona radiata, internal capsule, basal ganglia (caudate, putamen), and insula/frontal cortex. Language deficits were associated with damage to regions vascularized by both middle (MCA) and posterior cerebral arteries (PCA): classic language regions such as left (Broca) and right inferior frontal gyrus, left middle frontal gyrus, left anterior insula, left caudate, left posterior superior (Wernicke), middle temporal gyrus, left inferior parietal lobule, and basal ganglia in the MCA distribution, and left ventral occipito-temporal cortex near/at the word form area and thalamus in the PCA distribution. There was also extensive damage in ventral and dorsal white matter of left frontal, temporal, and parietal cortex. Visuospatial biases were stronger for damage to the right dorsal periventricular white matter, right inferior frontal/anterior insula, and basal ganglia (MCA), and thalamus and medial temporal cortex (PCA). Finally, verbal memory deficits were more strongly associated with damage of the frontal white matter, basal ganglia, especially caudate, and thalamus in the left hemisphere; whereas spatial memory was related to bilateral damage of the same structures, but also right posterior parietal and underlying white matter damage.
Behavior-to-Anatomy relationships: across domains
Next, we looked at the anatomical relationships between factors. To map regions of the brain whose damage corresponded to behavioral deficits in multiple domains, we created a conjunction of ridge regression maps, one per domain (L/R motor; language; verbal and spatial memory; L/R attention visual field). The maps were weighted for the amount of behavioral variance explained by multiplying the z-scored ω values (maps in Figure 5) by the % variance explained (bar graphs in Figure 5). Before conjunction, the maps were binarized using a threshold of 25 corresponding to a z score of 2.5 and 10% of behavioral variance explained. Interestingly, we found that the regions of damage most strongly associated with deficits in multiple domains (5 or 6 domains) localized bilaterally to the dorsal frontal white matter, thalami, and basal ganglia (Figure 7A). The contribution of cortical regions could not be sensitively evaluated given lesion overlap was lower in cortex.
Figure 7. Anatomy of correlated behavioral deficits.
A) Conjunction map of ridge regression maps for seven behavioral domains (L/R motor, language, verbal memory, attention visual field bias, general performance, attention shifting). Each single domain map was corrected for multiple comparisons correction and binarized. The color scale (1–8) indicates at each voxel the number of significant ridge regression maps. B) Correlated behavior and disconnection. Lesions affecting white matter regions of high tract overlap (SLF/AF/CST/etc.) cause deficits in multiple domains (motor, language, etc). The gray-to-white overlay on the atlas-registered subject average is a conjunction of 57 white matter tracts. White indicates 1 tract and black indicates 8+ tracts overlap (at 40% tract probability) in that voxel. The yellow-to-red overlay is the conjunction of eight domain specific Logistic Regresion maps. Yellow indicates 3 behavioral domains affected and red indicates 6 or more domains affected by lesion to that voxel. Graph: as the number of domains effected by lesion to a given voxel increases, the number of WM tracts contained in that voxels increases.
The white matter regions qualitatively map onto regions that contain multiple long-range white matter tracts such as the longitudinal fasciculus, arcuate fasciculus, and cortico-spinal tracts. To test this idea more closely we hypothesized that the number of fiber tracts damaged should relate to the number of impaired behavioral domains.
To examine this hypothesis we mapped the conjunction of ridge regression maps onto a probabilistic tractography atlas (Thiebaut de Schotten et al., 2011) generated in 40 healthy individuals. The 57 reconstructed tracts were generated at 20, 40, 60, 80, and 100% probability of across subject overlap and overlaid on a single image to measure tract overlap. Figure 7B shows the tract conjunction image created by summing 40% probability tracts with white regions corresponding to 1 tract and black regions corresponding to 8+ tracts. The red-orange color scale shows the regions of damage across multiple domains based on the conjunction of the single domain ridge regression maps. We found that regions of maximal damage across domains overlapped with regions of high white matter tract overlap. The tracts with most overlap across domains (>3) included anterior thalamic projection, cortico-spinal, frontal aslant, SLF II, and SLF III. Quantitatively, there was a significant regression between behavioral domains affected and number of white matter tracts damaged, and this relationship was independent of the percentage of overlap across healthy subjects used to define the tracts (p<0.0001 independently at each level of tract overlap: 20, 40, 60, and 80% of healthy subjects)(Graph Figure 7B). To ensure that any potential bias in the lesion sample distribution was removed, the statistical analysis was carried out on voxels that were affected by at least 8 lesions, and after regressing out the linear trend between behavioral domain overlap and number of lesions per voxel (Figure S4).
In summary, the behavior-to-anatomy analyses both within and across domains indicate that each behavioral domain is associated with a specific set of regions of damage. These regions are relatively distributed, especially for cognitive deficits, and involve multiple vascular distributions. That is, lesions in different parts of the brain cause a correlated, domain-wide set of impairments in language, memory, and attention. Notably, deficits across multiple domains correlate with damage to subcortical regions like thalamus and striatum, and specific regions of the white matter that contain multiple white matter tracts. Damage to these structures likely leads to disconnection of many cortical regions whose physiological dysfunction also likely contributes to the correlation among deficits.
Control for lesion volume and location
An important potential confound for the results noted above is lesion volume. Larger lesions may impact larger parts of gray or white matter and produce multiple deficits within a domain or across domains, hence inducing correlation. To examine this possibility we ran the correlation across all behavioral scores after removing lesion volume as a covariate of no interest. Figure S5 shows the correlation matrix of raw scores as total correlation and as partial correlation after regression of lesion volume. Note that the last row in the top matrix (total correlation) represents the correlation between lesion size and all other scores. A marginal effect is present only with attention scores (Posner, Mesulam, BIT). The correlation structure and clusters of deficits were unchanged. We conclude that lesion volume did not contribute to the behavioral clusters.
The results were the same after removing patients with brainstem and cerebellar lesions.
Finally, to examine if the pattern of behavioral correlation within domain or across domains was related to the predominantly subcortical distribution of lesions, a PCA was also run for a purely subcortical group (thalamus, basal ganglia, n=30) and a highly heterogeneous group of cortical lesions (n=35). The correlation structure both within and across domains was similar, with the same number of factors identified explaining the same amount of variance (cumulative variance explained for subcortical group=70%; for cortical group=72%)(Figure S6). The spatial correlation between the two matrices was r=0.6318. We tested the null hypothesis that this value is not different from a random distribution obtained by scrambling the lesion location assigned to each patient (i.e. cortical vs. subcortical) 10,000 times. The mean correlation value of the cortical and subcortical matrices computed over the 10,000 scrambled assignments was: r=0.5952±0.0455, with 95% confidence intervals of 0.5092 – 0.6577. Based on these findings the two correlation matrices are not different. However, given the small number of patients and uneven sampling of cortical space, we consider this analysis preliminary.
DISCUSSION
In this study, we examined whether behavioral variability following stroke is best described by a large number of mostly independent syndromes or by a relatively small number of factors comprising clusters of correlated deficits within and between domains of function (motor, language, attention, and memory). We also mapped the topography of stroke lesions and determined the association between lesion damage and our factor scores of behavioral impairment.
Subcortical stroke topography
The anatomy of stroke was predominantly subcortical with a relative paucity of cortical lesions (<20%) in line with other prospective clinical samples (Kang et al., 2003) (Wessels et al., 2006). Kang et al. (Kang et al., 2003) studied with diffusion imaging n=172 acute stroke cases, of which n=104 were single lesions: 50% of these strokes were classified based on visual inspection as subcortical including basal ganglia and thalamus; 33% were cortico-subcortical; and 16% were cortical. Wessels et al. (Wessels et al., 2006) studied a total of n=510 acute strokes, of which n=302 were single lesions also classified by inspection: 66% were subcortical; 16% were cortico-subcortical; and 14% were pure cortical lesions. Kang et al. classified 50% of their single lesions as subcortical (including white matter) while Wessels et al classified 66% of their single lesions as subcortical. The percentage of cortical only lesions was comparable across the three studies (this study=13%; Kang et al: 16%; Wessels et al=14%)(Kang et al., 2003; Wessels et al., 2006).
Demographic factors (e.g. greater proportion of African-Americans) or differences in risk factors (e.g. lower incidence of atrial fibrillation or coronary artery disease) in our sample as compared to the source population, or the inclusion requirements of the study (i.e. suitability for MRI scans and neuropsychological testing), did not likely bias the predominantly subcortical topography. Our study and Kang et al.’s (Kang et al., 2003) were drawn from metropolitan areas in the US (St. Louis, Washington DC) that include relatively large proportions of African-Americans, while patients in Wessels et al. (Wessels et al., 2006) came from a small city in central Germany (Giessen) with a population almost entirely Caucasian. Cardio-embolic strokes common after atrial fibrillation are associated with a cortico-subcortical topography (Kang et al., 2003; Wessels et al., 2006) whose frequency in our sample was well matched to others. Hypertension and diabetes type 2, two of the most important risk factors for subcortical strokes, and in African-Americans, were well matched to the source population. Finally, the percentage of hemorrhagic strokes, which are more likely to occur in subcortical structures (e.g. putamen), was actually lower in our sample.
In conclusion, the topography of damage as shown in Figure 5 is representative of the stroke population with single lesions.
Correlated behavioral deficits within and across domains of function
Behavioral deficits were strongly correlated within each domain (e.g. motor, language, attention, and memory) with one or a few factors accounting for the majority of variability.
In the motor domain, the neurological exam separates deficits of strength, coordination, and planning. Neuropsychological investigations have distinguished between sensory vs. memory-driven movements and reaching vs. grasping (Kalaska et al., 1997; Rizzolatti et al., 1997; Wise et al., 1997). In this study two factors (left body, right body) accounted for 76% of the variance with tests of strength, coordination, dexterity, and function all being highly correlated. This result has been shown on smaller samples of patients with motor deficits (Lang and Beebe, 2007). It is also known that acute impairment of shoulder function is strongly predictive of recovery of hand function (Beebe and Lang, 2009). Our study clearly shows that lesions in different parts of the motor system do not in general selectively impair one type of movement, e.g. reaching vs. grasping, or one type of process, e.g. strength vs. coordination, but disrupt movements concurrently across multiple joints. These findings are consistent with the emerging idea that natural movements can be described by a small number of correlated components, or muscle synergies, that involve both proximal and distal movements, and are represented in patterns of neural activity at multiple levels in the motor system (Cheung et al., 2009; Cheung et al., 2012; Howard et al., 2009; Ingram et al., 2008). Correspondingly, lesions in many regions including motor cortex, putamen, insula, frontal and parietal cortex, and descending motor pathways and brainstem resulted in the same motor factor deficits (Fig. 7).
In the language domain, a single factor accounted for 76% of impairment variability across tests of expression, auditory comprehension, and reading, both at the single word and sentence level. As a result, patients with a language dysfunction, e.g. in reading, will nevertheless show some impairment across all language functions, as compared to patients without any impairment in that domain. Therefore, when measured over a heterogeneous population, classic aphasia syndromes (e.g. Broca, Wernicke, or Alexia) account for more modest amounts of variance (20–30%) than overall impairments in language. Anatomically, this common language impairment mapped to multiple nodes of a distributed language network, which included not only classic perisylvian regions (left inferior frontal, superior temporal gyrus, anterior temporal, inferior parietal), but also left caudate, thalamus, fusiform gyrus, and right inferior frontal. Both dorsal and ventral white matter were also involved (Fig. 7).
In the attention domain, we obtained three factors related to deficits of lateralized spatial attention (both perceptual and motor), sustained attention/general performance, and attention shifting/re-orienting. Notably, these components were strongly correlated among subjects. While the literature on spatial neglect has been concerned with identifying “pure” neglect sub-types, and many dissociations have been proposed (e.g. perceptual vs. intentional; spatial attention vs. vigilance), our findings emphasize that most neglect patients have multiple concurrent deficits. Anatomically, attention biases were most strongly localized to the right dorsal periventricular white matter near/at the superior longitudinal fasciculus in line with a growing literature (Thiebaut de Schotten et al., 2014), but also right inferior frontal gyrus, right insula, thalamus, and parahippocampal gyrus, classic regions associated with neglect. A notable absence was the right temporo-parietal junction, which, however, was not well sampled (Fig. 5).
In the memory domain, we found a strong correlation among multiple processes (long-term, short-term, and working memory), and between spatial and verbal memory (Fig. 2I). Anatomically, verbal memory had a left hemisphere damage distribution similar to language, while spatial memory had a bilateral distribution of damage that included both cortical and subcortical tissue.
Behavioral deficits across domains were also well correlated. Three uber-factors explained about 70% of inter-individual variability in behavioral performance (Fig. 3), of which one loaded on language/memory and two loaded on motor/attention, one in each hemisphere. Overall, much of the complexity of a neurological evaluation was summarized with a few scores.
This conclusion is consistent with previous studies of the factor structure of the NIHSS (Lyden et al., 2004; Lyden et al., 1999). When we analyzed our patient sample using the NIHSS recorded at the same time as the behavioral battery, we obtained a similar factor structure to what previously reported (Table S8).
Therefore our findings indicate that even when behavioral domains are tested in detail, neurological impairment in a heterogeneous population is surprisingly well described by a few behavioral clusters. These clusters are ground knowledge, serving as behavioral phenotypes for large-scale future studies in genomics, recovery, outcome, and treatment.
Why are behavioral deficits correlated in stroke?
There are many possible reasons for the observed behavioral correlation. Our control analysis rules out lesion volume. This variable did not have any effect on the overall correlation structure of behavioral impairment and in each domain accounted for little variance (<20%).
Another explanation is the distribution of behavioral deficits in a group of patients with heterogeneous lesions. Consider four patients: patient A with a left hemisphere lesion producing severe speech output deficits, but only moderate auditory comprehension deficits; patient B with a different lesion who has the opposite pattern of impairment; and patients C and D with no language deficits. In an across-subject correlation analysis, different language impairments will correlate in a single factor. To isolate more specific language factors one has to restrict the analysis to patients with language deficits (i.e. A and B). For example, when we ran the PCA just on patients with aphasia two factors emerged, one more related to speech output and one to auditory comprehension. While “pure” syndromes are rarely seen in practice, future studies will need to examine finer behavioral clusters within each behavioral domain using more homogeneous groups of patients.
The statistical association of behavioral deficits partly reflects the relative frequency of strokes in different vascular distributions. Strokes are most common in the middle cerebral artery distribution, especially in the deep branches. Hence the association of visuospatial attention and motor deficits (uber-factor 2 and 3) is likely related to the proximity in the middle cerebral artery territory of cortical regions and white matter pathways (e.g. cortico-spinal and superior longitudinal fasciculus) whose damage causes these deficits (Schaechter et al., 2009; Thiebaut de Schotten et al., 2014). However, this argument does not work for factor 1, which does not show a clear hemispheric lateralization and combines domains with a relatively left dominant (language, verbal memory) vs. bilateral anatomy (spatial memory).
The correlation among deficits within and across domains reflects a complex interaction between multiple factors, including the type of domain impaired (e.g. sensory-motor vs. language vs. cognitive), the location and volume of the lesion, the white matter pathways affected, and the secondary physiological disruption caused either by cortical or subcortical/white matter damage on otherwise structurally normal regions of the brain (see below, ‘Physiological correlates of behavioral variability/correlation’). Hence a complete account of behavioral variability and correlation will require multivariate models that take into account all of the above variables.
In this study, we have made two important observations on the effect of structural damage on behavioral variability and correlation among deficits.
Anatomical correlates of behavioral variability/correlation
Symptom-lesion mapping studies in the last decade or so have identified a number of cortical loci whose damage cause specific deficits or syndromes (Barbey et al., 2013; Bates et al., 2003; Dronkers et al., 2004; Glascher et al., 2009; Hillis et al., 2002; Karnath et al., 2004) (Barbey et al., 2013; Hillis et al., 2005; Verdon et al., 2010). These studies, based on univariate analyses that consider each voxel in the brain independently, cannot estimate the amount of behavioral variance explained by lesion location or volume. Univariate analyses also do not control for hidden biases such as the vascular distribution of damage that may consistently distort lesion-deficit maps (Mah et al., 2014; Phan et al., 2010).
Here, we applied ridge regression, a multivariate machine learning method that uses a leave-one-out procedure to develop and test an optimal model relating the multi-voxel distribution of lesion damage, as well as lesion volume, to a measured behavioral impairment, in this case factor scores in each domain. This procedure allows an estimate of how well the model predicts behavioral variance in individual subjects.
Using this novel methodology, we show that lesion volume accounts for less than 20% of variance in any domain. Furthermore, structural damage explains a higher proportion of variance for motor (~40% on average between left and right side) and language deficits (44%) than other cognitive deficits: attention/visual field biases (34%), verbal memory (17%), and spatial memory deficits (4%). We believe that this distinction reflects the cortical organization of these functions, with motor behavior strongly related to the integrity of the motor outflow tract and language dependent on highly specialized cortical regions. In contrast, cognitive functions such as attention and memory depend on highly distributed patterns of activation and appear to be less sensitive to structural damage. An important prediction is that motor, and perhaps sensory deficits, will be more strongly correlated with structural damage of input/output pathways, whereas cognitive deficits should be more strongly correlated with widespread functional abnormalities of brain networks (see below, “Putative physiological correlates…”).
The second important observation is that we show that patients with deficits across multiple domains have damage to “cross-road” regions of the white matter containing multiple white matter tracts (as well as in the basal ganglia and/or thalamus)(Fig. 7). Notably, this multiple-domains/multiple-tracts relationship was independent of the greater frequency of subcortical lesions in our sample. The tracts whose damage was correlated with deficits in multiple domains (>3) included anterior thalamic projections, corticospinal, frontal aslant, and SLF II and III, which connect wide swaths of prefrontal cortex to thalamus, anterior to posterior cortex, and cortex with spinal cord. While the importance of white matter disconnection as a pathogenetic mechanism for specific neurological syndromes has been appreciated since Jules Dejerine (Catani and Mesulam, 2008; Geschwind, 1965; Henderson, 1984), these results indicate that white matter disconnection is generally important for post-stroke behavioral deficits, not just for uncommon deficits such as alien hand syndrome or alexia.
The disconnection of white matter leads to remote physiological effects in regions of cortex that are structurally intact. The cortical distribution of these abnormalities may partly account for the observed behavioral correlations.
Putative physiological correlates of behavioral variability/correlation
One possible mechanism of remote cortical dysfunction is metabolic diaschisis, i.e. a reduction of blood flow and/or metabolism caused by anatomical disconnection after subcortical stroke. Cortical diaschisis has been associated with cortical deficits like aphasia and spatial neglect, and their resolution (Hillis et al., 2002; Karnath et al., 2005; Perani et al., 1987). Diaschisis of specific cortical “hub” regions that connect multiple networks could explain the correlation of behavioral deficits (Warren et al., 2014).
However, there are several reasons why this explanation is not satisfactory. First, the same behavioral impairment can be obtained for lesions in widely separate regions that would produce different patterns of diaschisis. For instance, different patterns of diaschisis would be observed after left caudate and left fusiform gyrus lesions, yet both loci are associated with language impairment (Fig. 6). Similarly, across domains, language and spatial memory deficits are highly correlated (0.88 and 0.59, respectively) with the first uber-factor, yet localize to different anatomical loci, even in different hemispheres. Second, in preliminary work, we show that the factor structure of behavior correlation is independent of the subcortical vs. cortical location of damage. Therefore, cortical diaschisis alone cannot account for the observed results.
A second mechanism is the disruption of inter-regional signal coherence measured at rest with BOLD-fMRI. We originally identified this novel physiological abnormality in small groups of patients with attention and motor deficits post-stroke (Carter et al., 2010; He et al., 2007), and these findings have been replicated in various studies (Wang et al., 2010);(van Meer et al., 2010). In a recent study on a subset of the same cohort of first-time stroke examined here (Baldassarre et al., 2014), we found that visuospatial biases, as in uber-factor 2, were associated with multi-network and multi-regional coherence abnormalities. These abnormalities included both decreased inter-hemispheric correlation in attention and sensory-motor networks and increased intra-hemispheric correlation with fronto-parietal/default or basal ganglia regions in the damaged hemisphere. Critically, multi-network coherence abnormalities were widespread and were independent of structural damage, making them a plausible explanation of the behavioral correlations that are not well accounted for by lesion location.
Future work will apply multivariate methods to measures of lesion damage, white matter integrity, and functional connectivity to explore this issue in-depth.
Study limitations
There were two important limitations to this study. First, some important cognitive domains (emotion, social cognition, theory of mind, and, to some extent, executive functions) were either not tested or only coarsely evaluated. This limitation reflected a cost-benefit analysis of how much behavioral testing was feasible at the acute stage. Now that we have shown strong correlations among many different measures within important cognitive functions, many tests could be dropped in future studies in exchange for a more thorough investigation of the above functions.
The absence of measures in some domains may partly account for unexplained behavioral variance within and across the domains that were investigated, as may the imperfect sampling of processes within those latter domains. For example, more variance might have been explained in the attention domain if processes such as attention to the body or objects were assessed. In addition, some of the unexplained variability reflected noise in the measurements. Across domains, the three factors explained 69% of the variance even though fewer subjects (n=67) had measures in all domains. The variance explained would be higher if more subjects were available.
A second limitation was the relatively small number of lesions that overlapped the same cortical location, which limited the sensitivity of the multivariate ridge regression at those locations. Previous large-scale studies have used a threshold of n=4 subjects (Glascher et al., 2009), but more recent work indicates that as many as 10 subjects per voxel are necessary to avoid false positives in univariate analyses (Medina et al., 2010). The current distribution of lesions reflects the fact that strokes have a predominantly subcortical topography at the population level, and that white matter disconnection is an important contributor to behavioral correlation, but it renders the anatomo-clinical association in cortical regions less robust.
Conclusions
Neurologists have long concentrated on rare and interesting cases, leading to a proliferation of behavioral syndromes. Neuropsychologists have used double dissociations between deficits as the gold standard for the identification of mental operations. As a result we think of neurological deficits as highly specific and indicative of a highly modular functional organization of cortex. While we do not deny the importance of syndromes, our results show that syndromes represent the “tip of the iceberg.”
Here we focus on the great majority of patients who do not attract a lot of attention on rounds in stroke and rehabilitation units, the “bulk of the iceberg.” In these patients deficits are correlated and can be summarized with a few uber-factors divided between motor-attention and language-memory impairments. The behavioral correlation is due to the predominant subcortical/white matter topography of stroke and damage of white matter pathways, especially cross-road regions with high tract overlap.
Hence, in contrast to prevailing views and the majority of funding by agencies, stroke is better conceptualized as a subcortical than cortical disease. Novel models of subcortical damage and cognition are necessary to understand this disease, which is the most important cause of disability in the world. Finally, our results strongly argue that the symptoms and signs of stroke that are observed at the bedside do not reflect just the effect of structural lesions but also their remote physiological effect on connected regions. Therefore, a stronger correlation of brain measures with behavior will be obtained if structural information is integrated with physiological information.
METHODS (see Supplementary Information for more details)
Subjects
Study sample
Subjects (n=172) were prospectively recruited, of whom 132 met post-enrollment inclusion criteria. Figure S1 shows the diagram of enrollment (CONSORT) for this study.
Inclusion criteria
1. Age 18 or greater. No upper age limit. 2. First symptomatic stroke, ischemic or hemorrhagic. 3. Up to two lacunes, clinically silent, less than 15 mm in size on CT scan. 4. Clinical evidence of motor, language, attention, visual, or memory deficits based on neurological examination. 5. Time of enrollment: <2 weeks from stroke onset. 6. Awake, alert, and capable of participating in research.
Exclusion criteria
Previous stroke based on clinical imaging. 2. Multi-focal strokes. 3. Inability to maintain wakefulness in the course of testing. 4. Presence of other neurological, psychiatric or medical conditions that preclude active participation in research and/or may alter the interpretation of the behavioral/imaging studies (e.g. dementia, schizophrenia), or limit life expectancy to less than 1 year (e.g. cancer or congestive heart failure class IV). 5. Report of claustrophobia or metal object in body.
Healthy control group
A healthy control group (N=31) were matched with the study sample for age, gender, and years of education.
Stroke source population
A larger control group (N=1209) was selected from from a clinical database (N=6260) using the same inclusion/exclusion criteria. Stroke and controls provided informed consent according to procedures approved by the Washington University Institutional Review Board.
MRI and lesion analysis
Lesions were manually segmented on structural MRI images (T1,T2, Flair) using the Analyze biomedical imaging software system (Robb and Hanson, 1991), and automatically classified based on their overlap with three masks (gray matter, white matter, and subcortical regions including basal ganglia and thalamus). Each mask was computed as 50% conjunction of 38 single subject FreeSurfer (http://surfer.nmr.mgh.harvard.edu/)(Dale et al., 1999) gray and white matter segmentations obtained from an independent group of healthy volunteers (age range=18–35) on 1×1×1 mm MP-Rage T1-weighted images. A K-means clustering in MatLab (MatLab Works) was run using the percent of lesion volume that intersected with each mask (i.e. number voxels in the lesion overlapping with each mask/total number of voxels in the lesion) as input, to display the overlap of each lesion group with gray matter, white matter, and subcortical nuclei. The number of lacunes on the MRI was recorded and the periventricular white matter was rated according to (Longstreth et al., 1996).
Behavioral data reduction
A principle component analysis was used on the data within each of the domains (motor, language, attention, memory) to reduce the number of variables to a smaller number of composites. We expected the components to be correlated, so a oblique rotation was used. Components had to satisfy two criteria: (1) the eigenvalues had to be >1; (2) the percentage of variance accounted for had to be >10%. A higher-order principal component analysis was used on the components that resulted from the within-domain analyses. In this case the correlations between the resulting components were low, and the results were similar for oblique and orthogonal rotations, so we chose to use orthogonal rotations, since these are easier to interpret. The distributions of some of the variables are not normal and could not be made normal through transformation. To ensure this is not influencing our results we compared the correlations between the variables using a Pearson’s correlation and a rank order correlation (Spearman’s Rho). To make sure the structure was similar we replicated the within-domain principle component analyses using a rank-order correlation (Spearman’s Rho).
Lesion-behavior mapping
The voxel-wise relationship between lesion maps and behavioral scores for each domain was analyzed using a multivariate leave-one-out ridge regression machine learning algorithm (Fig. S3). First, the dimensionality of voxel-wise lesion maps was reduced using PCA. Second, model weights were optimized to predict a behavioral score based on lesion PC scores on all but one subject. Third, optimal model weights were applied to the lesion of the left-out subject to predict that subject’s behavioral score. A prediction was generated for all subjects in this way. We chose to use a linear ridge regression function to minimize bias but retain the ability to plot predictive weights back to brain anatomy (Mah et al., 2014; Phan et al., 2010). See supplemental methods for further details. Solving for behavioral scores in each domain produced two outputs: 1) accuracy – % variance explained (r2), and 2) a weight map – a vector (ω) containing relative predictive weights for every voxel in the brain (Fig. S3). The weight map was then weighted by amount of behavioral variance explained by multiplying ω*r2. A conjunction across behavioral domains was computed by masking at ω*r2>25 and adding the masks for L/R motor, language, verbal and spatial memory, attention visual field bias, general task performance, and attention shifting.
Quantification of white matter damage and relationship to behavior
We used a probabilistic tractography atlas (Thiebaut de Schotten et al., 2011) from 40 healthy right-handed adults. The atlas includes 57 tracts each at 20, 40, 60, 80, and 100% probabilities of overlap across subjects. The 57 tracts were thresholded at different probabilities and then overlaid in a single image to measure tract overlap.
To compute the impact of lesions on white matter tracts, we compared the conjunction of weighted ridge regression maps for the 8 deficits listed above and computed the relationship between functional domains and white matter tract overlap. Only voxels with at least 5 lesions were included in Figure 7 (only voxels with at least 8 lesions were included in the equivalent graph in the Figure S4).
Supplementary Material
Table 2.
| Language battery | Variance explained: 76.5% |
|---|---|
| Component Matrix | |
| Component | |
| 1 | |
| Word Comprehension | .858 |
| Commands | .885 |
| Complex Ideational Material | .855 |
| Boston Naming Test | .925 |
| Oral Reading of Sentences ( | .910 |
| Comprehension of Oral Reading of Sentences | .890 |
| Non-word Reading | .825 |
| Stem completion | .914 |
| Animal Naming | .804 |
Table 3.
| Memory Battery | Variance explained: 66.2% | |
|---|---|---|
| Pattern Matrix | ||
| Component | ||
| 1 | 2 | |
| BVMT Total Immediate Recall (T-score) | .687 | |
| BVMT Delayed Recall (T-Score) | .805 | |
| BVMT Percent Retained | .766 | |
| BVMT Recognition Discrimination Index | .699 | |
| HVLT Total Immediate Recall (T-score) | −.733 | |
| HVLT Delayed Recall (T-Score) | −.929 | |
| HVLT Percent Retained | −.897 | |
| HVLT Recognition Discrimination Index | −.710 | |
| Spatial Span Forward | .689 | |
| Spatial Span Backward | .808 | |
| Attention Battery | Variance explained: 57.1% | ||
|---|---|---|---|
| Pattern Matrix | |||
| Component | |||
| 1 | 2 | 3 | |
| Posner RT: Visual Field | .671 | .499 | |
| Posner accuracy: Visual Field | .828 | ||
| Posner RT: Validity effect | .539 | ||
| Posner accuracy: Validity effect | .779 | ||
| Posner RT: Disengagement | .584 | ||
| Posner accuracy: Disengagement | .361 | −.419 | |
| Posner RT: Average | .779 | ||
| Posner accuracy: Average | −.408 | −.605 | .484 |
| Mesulam Center of Cancellation | .749 | ||
| BIT Stars Center of Cancellation | .607 | −.335 | −.314 |
Acknowledgments
Grants: R01 HD061117-05 (MC); NIH 5T32GM007200-40, American Heart Association 14PRE19610010 (JS); and, R01 HD068290-03 (CEL). The authors acknowledge Kilian Weinberger, PhD, for providing code for the ridge regression.
Footnotes
They declare that they have no competing interests.
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Awh E, Jonides J. Overlapping mechanisms of attention and spatial working memory. Trends Cogn Sci. 2001;5:119–126. doi: 10.1016/s1364-6613(00)01593-x. [DOI] [PubMed] [Google Scholar]
- Baldassarre A, Ramsey L, Hacker CL, Callejas A, Astafiev SV, Metcalf NV, Zinn K, Rengachary J, Snyder AZ, Carter AR, et al. Large-scale changes in network interactions as a physiological signature of spatial neglect. Brain: a journal of neurology. 2014;137:3267–3283. doi: 10.1093/brain/awu297. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbey AK, Colom R, Grafman J. Architecture of cognitive flexibility revealed by lesion mapping. Neuroimage. 2013;82:547–554. doi: 10.1016/j.neuroimage.2013.05.087. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bates E, Wilson SM, Saygin AP, Dick F, Sereno MI, Knight RT, Dronkers NF. Voxel-based lesion-symptom mapping. Nature neuroscience. 2003;6:448–450. doi: 10.1038/nn1050. [DOI] [PubMed] [Google Scholar]
- Beebe JA, Lang CE. Active range of motion predicts upper extremity function 3 months after stroke. Stroke. 2009;40:1772–1779. doi: 10.1161/STROKEAHA.108.536763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bogousslavsky J, Van Melle G, Regli F. The Lausanne Stroke Registry: analysis of 1,000 consecutive patients with first stroke. Stroke. 1988;19:1083–1092. doi: 10.1161/01.str.19.9.1083. [DOI] [PubMed] [Google Scholar]
- Carter AR, Astafiev SV, Lang CE, Connor LT, Rengachary J, Strube MJ, Pope DL, Shulman GL, Corbetta M. Resting interhemispheric functional magnetic resonance imaging connectivity predicts performance after stroke. Ann Neurol. 2010;67:365–375. doi: 10.1002/ana.21905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carter AR, Patel KR, Astafiev SV, Snyder AZ, Rengachary J, Strube MJ, Pope A, Shimony JS, Lang CE, Shulman GL, Corbetta M. Upstream Dysfunction of Somatomotor Functional Connectivity After Corticospinal Damage in Stroke. Neurorehabilitation and neural repair. 2011 doi: 10.1177/1545968311411054. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Catani M, Mesulam M. What is a disconnection syndrome? Cortex. 2008;44:911–913. doi: 10.1016/j.cortex.2008.05.001. [DOI] [PubMed] [Google Scholar]
- Cheung VC, Piron L, Agostini M, Silvoni S, Turolla A, Bizzi E. Stability of muscle synergies for voluntary actions after cortical stroke in humans. Proc Natl Acad Sci U S A. 2009;106:19563–19568. doi: 10.1073/pnas.0910114106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cheung VC, Turolla A, Agostini M, Silvoni S, Bennis C, Kasi P, Paganoni S, Bonato P, Bizzi E. Muscle synergy patterns as physiological markers of motor cortical damage. Proc Natl Acad Sci U S A. 2012;109:14652–14656. doi: 10.1073/pnas.1212056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nature reviews Neuroscience. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I. Segmentation and surface reconstruction. Neuroimage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Dronkers NF, Wilkins DP, Van Valin RD, Jr, Redfern BB, Jaeger JJ. Lesion analysis of the brain areas involved in language comprehension. Cognition. 2004;92:145–177. doi: 10.1016/j.cognition.2003.11.002. [DOI] [PubMed] [Google Scholar]
- Duncan PW, Zorowitz R, Bates B, Choi JY, Glasberg JJ, Graham GD, Katz RC, Lamberty K, Reker D. Management of Adult Stroke Rehabilitation Care: a clinical practice guideline. Stroke. 2005;36:e100–143. doi: 10.1161/01.STR.0000180861.54180.FF. [DOI] [PubMed] [Google Scholar]
- Geschwind N. Disconnexion syndromes in animals and man, Part 1. Brain: a journal of neurology. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- Glascher J, Tranel D, Paul LK, Rudrauf D, Rorden C, Hornaday A, Grabowski T, Damasio H, Adolphs R. Lesion mapping of cognitive abilities linked to intelligence. Neuron. 2009;61:681–691. doi: 10.1016/j.neuron.2009.01.026. [DOI] [PMC free article] [PubMed] [Google Scholar]
- He BJ, Snyder AZ, Vincent JL, Epstein A, Shulman GL, Corbetta M. Breakdown of functional connectivity in frontoparietal networks underlies behavioral deficits in spatial neglect. Neuron. 2007;53:905–918. doi: 10.1016/j.neuron.2007.02.013. [DOI] [PubMed] [Google Scholar]
- Henderson VW. Jules Dejerine and the third alexia. Archives of neurology. 1984;41:430–432. doi: 10.1001/archneur.1984.04050160096022. [DOI] [PubMed] [Google Scholar]
- Hillis AE, Newhart M, Heidler J, Barker PB, Herskovits EH, Degaonkar M. Anatomy of spatial attention: insights from perfusion imaging and hemispatial neglect in acute stroke. J Neurosci. 2005;25:3161–3167. doi: 10.1523/JNEUROSCI.4468-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hillis AE, Wityk RJ, Barker PB, Beauchamp NJ, Gailloud P, Murphy K, Cooper O, Metter EJ. Subcortical aphasia and neglect in acute stroke: the role of cortical hypoperfusion. Brain: a journal of neurology. 2002;125:1094–1104. doi: 10.1093/brain/awf113. [DOI] [PubMed] [Google Scholar]
- Howard IS, Ingram JN, Kording KP, Wolpert DM. Statistics of natural movements are reflected in motor errors. J Neurophysiol. 2009;102:1902–1910. doi: 10.1152/jn.00013.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ingram JN, Kording KP, Howard IS, Wolpert DM. The statistics of natural hand movements. Exp Brain Res. 2008;188:223–236. doi: 10.1007/s00221-008-1355-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalaska JF, Scott SH, Cisek P, Sergio LE. Cortical control of reaching movements. Curr Opin Neurobiol. 1997;7:849–859. doi: 10.1016/s0959-4388(97)80146-8. [DOI] [PubMed] [Google Scholar]
- Kang DW, Chalela JA, Ezzeddine MA, Warach S. Association of ischemic lesion patterns on early diffusion-weighted imaging with TOAST stroke subtypes. Archives of neurology. 2003;60:1730–1734. doi: 10.1001/archneur.60.12.1730. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Fruhmann Berger M, Kuker W, Rorden C. The anatomy of spatial neglect based on voxelwise statistical analysis: a study of 140 patients. Cereb Cortex. 2004;14:1164–1172. doi: 10.1093/cercor/bhh076. [DOI] [PubMed] [Google Scholar]
- Karnath HO, Zopf R, Johannsen L, Fruhmann Berger M, Nagele T, Klose U. Normalized perfusion MRI to identify common areas of dysfunction: patients with basal ganglia neglect. Brain: a journal of neurology. 2005;128:2462–2469. doi: 10.1093/brain/awh629. [DOI] [PubMed] [Google Scholar]
- Lang CE, Beebe JA. Relating movement control at 9 upper extremity segments to loss of hand function in people with chronic hemiparesis. Neurorehabilitation and neural repair. 2007;21:279–291. doi: 10.1177/1545968306296964. [DOI] [PubMed] [Google Scholar]
- Longstreth WT, Jr, Manolio TA, Arnold A, Burke GL, Bryan N, Jungreis CA, Enright PL, O’Leary D, Fried L. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- Lyden P, Claesson L, Havstad S, Ashwood T, Lu M. Factor analysis of the National Institutes of Health Stroke Scale in patients with large strokes. Archives of neurology. 2004;61:1677–1680. doi: 10.1001/archneur.61.11.1677. [DOI] [PubMed] [Google Scholar]
- Lyden P, Lu M, Jackson C, Marler J, Kothari R, Brott T, Zivin J. Underlying structure of the National Institutes of Health Stroke Scale: results of a factor analysis. NINDS tPA Stroke Trial Investigators. Stroke. 1999;30:2347–2354. doi: 10.1161/01.str.30.11.2347. [DOI] [PubMed] [Google Scholar]
- Mah YH, Husain M, Rees G, Nachev P. Human brain lesion-deficit inference remapped. Brain: a journal of neurology. 2014;137:2522–2531. doi: 10.1093/brain/awu164. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medina J, Kimberg DY, Chatterjee A, Coslett HB. Inappropriate usage of the Brunner-Munzel test in recent voxel-based lesion-symptom mapping studies. Neuropsychologia. 2010;48:341–343. doi: 10.1016/j.neuropsychologia.2009.09.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perani D, Vallar G, Cappa S, Messa C, Fazio F. Aphasia and neglect after subcortical stroke: a clinical/cerebral perfusion correlation study) Brain: a journal of neurology. 1987;110:1211–1229. doi: 10.1093/brain/110.5.1211. [DOI] [PubMed] [Google Scholar]
- Phan TG, Chen J, Donnan G, Srikanth V, Wood A, Reutens DC. Development of a new tool to correlate stroke outcome with infarct topography: a proof-of-concept study. Neuroimage. 2010;49:127–133. doi: 10.1016/j.neuroimage.2009.07.067. [DOI] [PubMed] [Google Scholar]
- Rizzolatti G, Fogassi L, Gallese V. Parietal cortex: from sight to action. Curr Opin Neurobiol. 1997;7:562–567. doi: 10.1016/s0959-4388(97)80037-2. [DOI] [PubMed] [Google Scholar]
- Robb RA, Hanson DP. A software system for interactive and quantitative visualization of multidimensional biomedical images. Australas Phys Eng Sci Med. 1991;14:9–30. [PubMed] [Google Scholar]
- Schaechter JD, Fricker ZP, Perdue KL, Helmer KG, Vangel MG, Greve DN, Makris N. Microstructural status of ipsilesional and contralesional corticospinal tract correlates with motor skill in chronic stroke patients. Hum Brain Mapp. 2009;30:3461–3474. doi: 10.1002/hbm.20770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Ffytche DH, Bizzi A, Dell’Acqua F, Allin M, Walshe M, Murray R, Williams SC, Murphy DG, Catani M. Atlasing location, asymmetry and inter-subject variability of white matter tracts in the human brain with MR diffusion tractography. Neuroimage. 2011;54:49–59. doi: 10.1016/j.neuroimage.2010.07.055. [DOI] [PubMed] [Google Scholar]
- Thiebaut de Schotten M, Tomaiuolo F, Aiello M, Merola S, Silvetti M, Lecce F, Bartolomeo P, Doricchi F. Damage to white matter pathways in subacute and chronic spatial neglect: a group study and 2 single-case studies with complete virtual “in vivo” tractography dissection. Cereb Cortex. 2014;24:691–706. doi: 10.1093/cercor/bhs351. [DOI] [PubMed] [Google Scholar]
- Turken AU, Dronkers NF. The neural architecture of the language comprehension network: converging evidence from lesion and connectivity analyses. Frontiers in systems neuroscience. 2011;5:1. doi: 10.3389/fnsys.2011.00001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van Meer MP, van der Marel K, Wang K, Otte WM, El Bouazati S, Roeling TA, Viergever MA, Berkelbach van der Sprenkel JW, Dijkhuizen RM. Recovery of sensorimotor function after experimental stroke correlates with restoration of resting-state interhemispheric functional connectivity. J Neurosci. 2010;30:3964–3972. doi: 10.1523/JNEUROSCI.5709-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Verdon V, Schwartz S, Lovblad KO, Hauert CA, Vuilleumier P. Neuroanatomy of hemispatial neglect and its functional components: a study using voxel-based lesion-symptom mapping. Brain: a journal of neurology. 2010;133:880–894. doi: 10.1093/brain/awp305. [DOI] [PubMed] [Google Scholar]
- Wang L, Yu C, Chen H, Qin W, He Y, Fan F, Zhang Y, Wang M, Li K, Zang Y, et al. Dynamic functional reorganization of the motor execution network after stroke. Brain: a journal of neurology. 2010;133:1224–1238. doi: 10.1093/brain/awq043. [DOI] [PubMed] [Google Scholar]
- Warren DE, Power JD, Bruss J, Denburg NL, Waldron EJ, Sun H, Petersen SE, Tranel D. Network measures predict neuropsychological outcome after brain injury. Proc Natl Acad Sci U S A. 2014;111:14247–14252. doi: 10.1073/pnas.1322173111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wessels T, Wessels C, Ellsiepen A, Reuter I, Trittmacher S, Stolz E, Jauss M. Contribution of diffusion-weighted imaging in determination of stroke etiology. AJNR Am J Neuroradiol. 2006;27:35–39. [PMC free article] [PubMed] [Google Scholar]
- Wise SP, Boussaoud D, Johnson PB, Caminiti R. Premotor and parietal cortex: corticocortical connectivity and combinatorial computations. Annu Rev Neurosci. 1997;20:25–42. doi: 10.1146/annurev.neuro.20.1.25. [DOI] [PubMed] [Google Scholar]
- Zandieh A, Kahaki ZZ, Sadeghian H, Pourashraf M, Parviz S, Ghaffarpour M, Ghabaee M. The underlying factor structure of National Institutes of Health Stroke scale: an exploratory factor analysis. The International journal of neuroscience. 2012;122:140–144. doi: 10.3109/00207454.2011.633721. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.