Abstract
In the Fenofibric Acid (FA) Intervention and Event Lowering in Diabetes (FIELD) study, FA, a lipid-lowering drug, has been shown to significantly reduce macular edema in diabetic patients. In the present study, we investigated whether FA reduces vascular permeability by inhibiting cyclooxygenase-2 (COX-2), a critical mediator of inflammation, and reducing overexpression of fibronectin (FN) and collagen IV (Coll IV), two basement membrane (BM) components upregulated in diabetic retinopathy. Rat retinal endothelial cells (RRECs) were grown in normal (N:5 mM glucose) or high (HG:30 mM glucose) medium with or without FA for 7 days. Total protein isolated from these cells was assessed for FN, Coll IV, COX-2, and zonula occludens-1 (ZO-1), a tight junction protein, using Western blot analysis. In addition, the distribution and localization of ZO-1 was determined by immunofluorescence microscopy, and cell monolayer permeability was studied by in vitro permeability (IVP) assay. RRECs grown in HG medium showed significant increase in FN, Coll IV, and COX-2 expression (179%, 144%, 139% of N respectively), and a decrease in ZO-1 expression (48% of N) compared to those of N cells. Cells grown in HG medium supplemented with FA significantly reduced FN, Coll IV, and COX-2 expression by 47%, 32%, and 34% respectively, with concomitant increase in ZO-1 expression by 42%. In parallel studies, IVP assays showed a significant increase (139% of N) in cell monolayer permeability in RRECs grown in HG medium, which was significantly reduced with FA treatment. Additionally, immunostaining results indicated FA prevents HG-induced downregulation of ZO-1. The findings indicate that the beneficial effect of FA in reducing excess permeability is mediated, at least in part, by downregulating abnormal overexpression of BM components and inflammatory factors and preventing compromised tight junctions associated with diabetic retinopathy.
Keywords: Fenofibric acid, fibronectin, collagen type IV, retinal endothelial cells
1. Background
FA is a lipid lowering drug that has been shown to be effective in reducing the risk of developing cardiovascular disease events (Keech et al., 2005). There is now consistent evidence from two major trials, the FA Intervention and Event Lowering in Diabetes (FIELD) study (Keech et al., 2007) and the Action to Control Cardiovascular Risk in Diabetes Eye (ACCORD-Eye) study (Chew et al., 2010) that FA reduces the risk of progression of diabetic retinopathy. In the FIELD study, which involved an average follow-up of 5 years, FA reduced the frequency of first laser treatment for macular edema by 31% and proliferative diabetic retinopathy by 30%. In addition, in the ACCORD-Eye study FA treatment was associated with a 40% decrease in diabetic retinopathy progression over 4 years [3]. However, the mechanism by which FA prevents retinal vascular permeability in diabetic retinopathy remains unclear.
Diabetic retinopathy is the leading cause of blindness in the working age population (Cheung et al., 2010; Yau et al., 2012). Basement membrane (BM) thickening and increased vascular permeability are two major retinal vascular changes associated with the early stages in the pathogenesis of this disease (Cherian et al., 2009; Oshitari et al., 2006; Roy and Lorenzi, 1996). Studies indicate that HG or hyperhexosemia results in overexpression of BM components, which in turn, contributes to excess retinal vascular permeability (Oshitari et al., 2006; Roy and Lorenzi, 1996). It has been shown that normalization of diabetes-induced overexpression of BM components may lead to beneficial effects in preventing excess permeability, the development of acellular capillaries, and pericyte loss in diabetic retinopathy (Evans et al., 2000; Oshitari et al., 2006; Robison et al., 1998; Roy and Lorenzi, 1996). In addition, we have recently reported that downregulation of basement membrane components by FA (FA), the active metabolite of FA, may have a protective effect against the outer blood retinal barrier (BRB) leakage associated with diabetic retinopathy (Trudeau et al., 2011). However, it is unknown whether this beneficial effect of FA may also occur at the level of the inner BRB in the context of diabetic retinopathy.
FA has been shown to improve endothelial cell function in resistance arteries of aged rats by enhancing anti-oxidant capacity of the vessel wall (Alvarez de Sotomayor et al., 2007), improve GFR in the diabetic kidney by reducing nitrosative stress (Chen et al., 2004), and prevent HG-induced apoptosis in human endothelial cells (Zanetti et al., 2008). Each of these studies show FA imparts its beneficial effects, at least in part, by modulating cyclooxygenase-2 (COX-2) levels. More recently, we have observed that FA reduces overexpression of BM components in retinal pigment epithelial cells, and thereby reduces cell monolayer permeability (Trudeau et al., 2011). However, the effect of FA on COX-2 production in microvascular retinal cells in the context of diabetic retinopathy is currently unknown.
In the present study we have investigated whether FA prevents retinal vascular breakdown under conditions that mimic the diabetic milieu. Results from this study indicate that FA may impart beneficial effects by ameliorating abnormal overexpression of vascular basement membrane components and COX-2 expression. Findings from this study provide mechanistic insight into FA’s beneficial effects in preventing breakdown of inner BRB associated with diabetic retinopathy.
2. Materials and methods
2.1. Cell Culture – Rat Retinal Endothelial Cells
Rat retinal endothelial cells (RRECs) ascertained positive for von Willebrand factor were grown in Dulbecco’s modified Eagle’s medium (DMEM) with 10% FBS (Hyclon, Thermo Scientific, Waltham, MA), antibiotics, and antimycotics. Second to fourth passage cells were used in this study. All experiments were repeated at least four times. To examine the effect of FA on retinal vascular BM components, FN and Coll IV expression, and ZO-1 and COX-2 expression, RRECs were grown in normal medium (5 mmol/l glucose) or HG medium (30 mmol/l) for 7 days followed by exposure to 100 µM FA for the last 3 days of the experiment. In parallel, RRECs were grown in HG medium for 7 days followed by exposure to 100 µM FA and 1 µM MK886, a PPAR-α antagonist, for the last 3 days of the experiment to investigate whether the effect of FA on these proteins is PPAR-α dependent. The FA concentration used in this study was based on our previous work examining the effect of different FA doses 25 or 100 µM (Trudeau et al., 2011). Total protein was isolated and subjected to Western blot analysis as described in the next section. In parallel, immunostaining for BM components and COX-2, and in vitro permeability assay were performed. The cells were subjected to serum starvation (1% FBS) during the treatments. In order to rule out a potential bias by an osmotic effect the experiment was also performed using mannitol (5.5 mM D-glucose + 24.5 mM mannitol vs. 30 mM D-glucose) as an osmotic control agent.
2.2. Western Blot
Western Blot analysis was performed to determine FN, Coll IV, COX-2, and ZO-1 protein expression. Protein was isolated from RRECs and bicinchoninic acid assay (Pierce Chemical, Rockford, IL) was used to determine total protein concentration. Western blots were performed with 25µg protein/lane and after electrophoresis, the gels were transferred onto PVDF membranes (Millipore, Billerica, MA) using a semi-dry apparatus according to Towbin’s procedure (Towbin et al., 1979). The membranes were blocked with 5% nonfat dry milk for 2 hours and then exposed to rat FN (Millipore, Billerica, MA; 1:1000), Coll IV (Fitzgerald, Acton, MA; 1:2000), COX-2 (Cayman Chemical, San Diego, CA; 1:500), or rabbit ZO-1 (Invitrogen, Carlsbad, CA; 1:500) antibody solution overnight at 4°C. Blots were washed with TTBS and then incubated with rabbit anti-mouse IgG secondary antibody (Sigma, St. Louis, MO) solution (1:15,000) or rat anti-rabbit IgG secondary antibody (Cell Signaling, Danvers, MA) solution (1:3000) for 1 hour. The membranes were again washed and exposed to Immun- Star Chemiluminescent Protein Detection System (Bio-Rad, Hercules, CA) to detect protein signals on X-ray film. Protein loading in the gels was confirmed by beta-actin antibody binding. Densitometric analysis of the protein signals was performed at non-saturating exposures and analyzed using the NIH ImageJ analysis program.
2.3. In Vitro Permeability
The permeability of RREC monolayer was determined at 7 days in culture by measuring the movement of fluorescein isothiocyanate (FITC) dextran (40 kDa) (Sigma; Saint Louis, MO) from the upper chamber to the lower chamber. RRECs were plated on transwell inserts, and the FITC-dextran was added to the upper chamber of the transwell apparatus at 100 µg/ml. 200 µl samples were collected from the lower chamber 1 hour after adding the FITC-dextran. The medium in the lower chamber was replaced by fresh medium after the collection of every sample. A minimum of four wells was used for each time point measurement. Absorbance was measured at 485 nm of excitation and 528 nm of emission using a microplate reader (SpectraMax Gemini; Molecular Devices, Sunnyvale, CA, USA).
2.4. Statistical analysis
Data are presented as mean ± standard deviation. Comparisons between groups were performed using ANOVA followed by the Student’s t test, and p < 0.05 was considered statistically significant.
3. Results
3.1. Fenofibric Acid Abrogates Fibronectin and Collagen IV Overexpression Induced by HG Condition
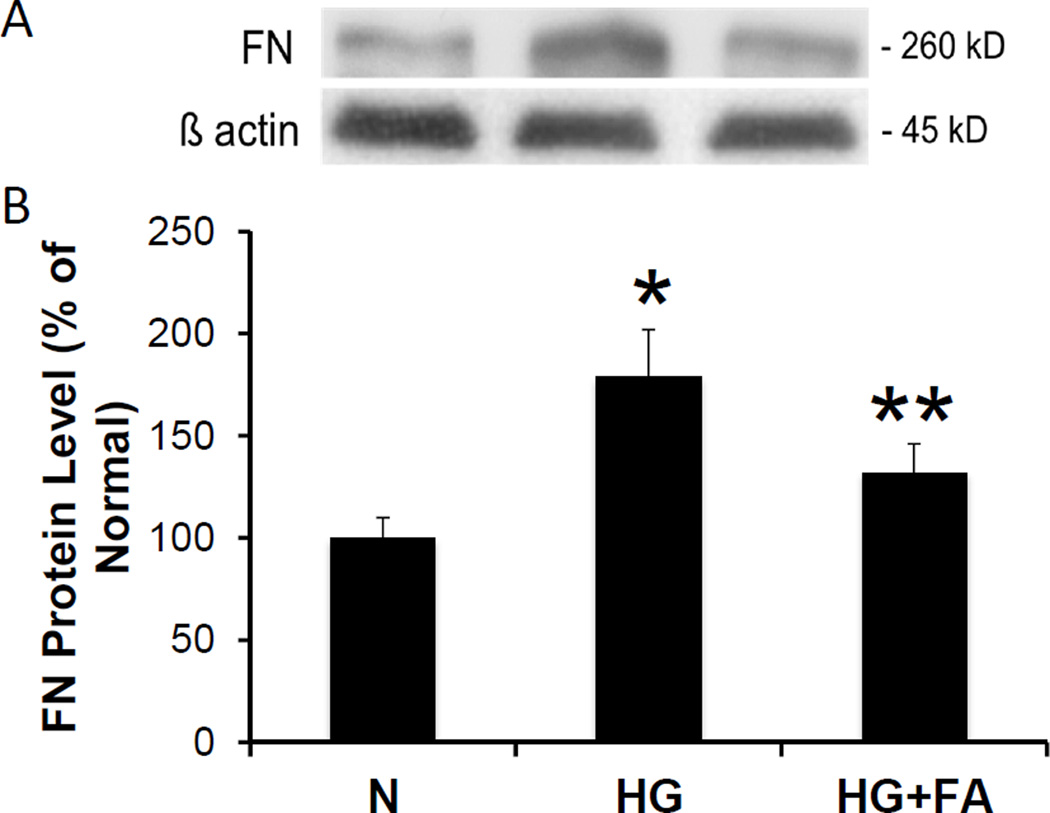
Western blot analysis showed significantly increased FN protein expression in RRECs grown in HG medium when compared to those grown in normal medium (179 ± 23% of normal, p < 0.001, n = 6). When RRECs grown in HG medium were treated with FA, a significant reduction in FN protein level was observed compared to untreated RRECs grown in HG medium (132 ± 14% of normal vs. 179 ± 23% of normal, p = 0.001, n = 6) (Figure 1A, 1B).
Figure 1. Effect of FA on FN Protein Level.
(1A) Representative WB image shows FA significantly reduces HG-induced FN overexpression. (1B) Graphical representations of cumulative WB data. FN protein level was significantly increased in RRECs grown in HG medium compared to those grown in N medium. When treated with FA, cells grown in HG medium showed a significant reduction in FN expression (* = N vs. HG, p < 0.05; ** = HG vs. HG + FA, p < 0.05).
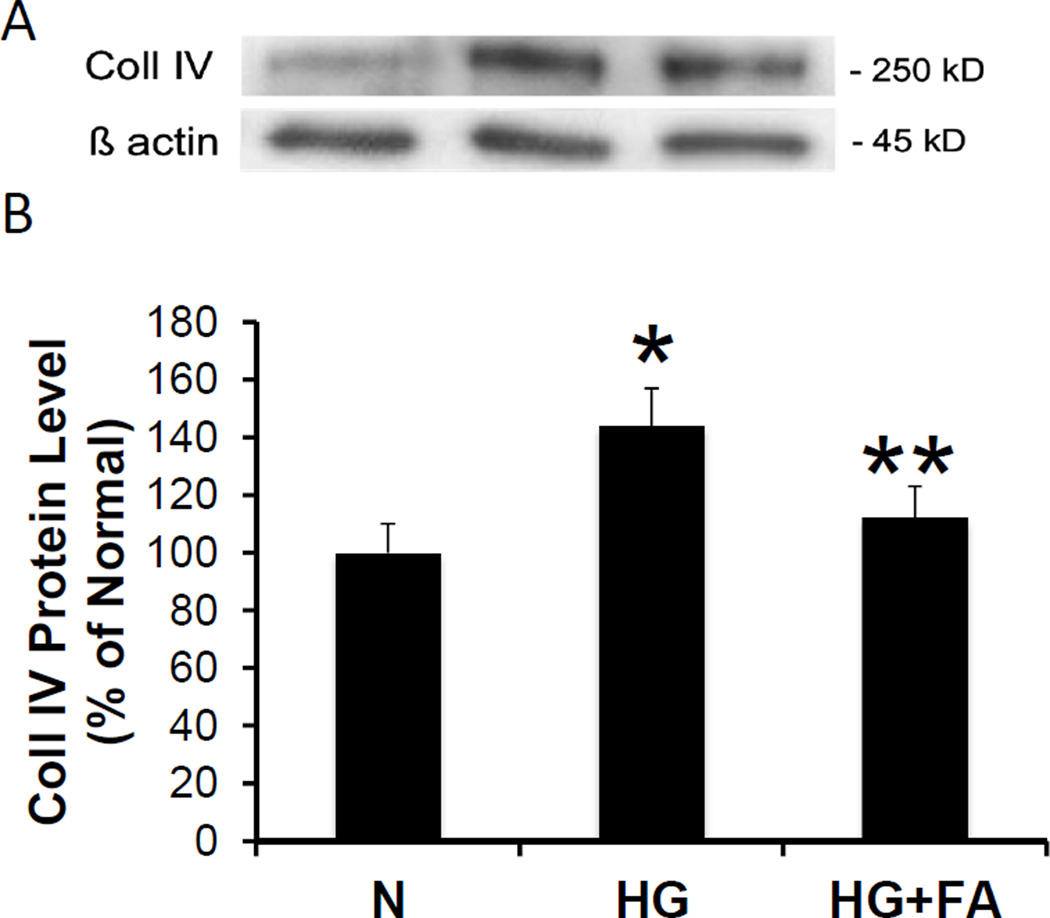
In parallel experiments, Western blot analysis showed significantly increased Coll IV protein expression in RRECs grown in HG medium compared to those grown in normal medium (144 ± 13% of normal, p < 0.001, n = 5). RRECs grown in HG medium treated with FA showed significantly decreased Coll IV protein expression when compared to untreated RRECs grown in HG medium (112 ± 11% of normal vs. 144 ± 13% of normal, p = 0.003, n = 5) (Figure 2A, 2B).
Figure 2. Effect of FA on Coll IV Protein Level.
(2A) Representative WB image shows FA significantly reduces HG-induced Coll IV overexpression in RRECs compared to those grown in N medium. (2B) Graphical representations of cumulative WB data. Coll IV protein level was significantly increased in RRECs grown in HG medium compared to those grown in N medium. When treated with FA, cells grown in HG medium showed a significant reduction in Coll IV expression (* = N vs. HG, p < 0.05; ** = HG vs. HG + FA, p < 0.05).
3.2. Fenofibric Acid Downregulates COX-2 Synthesis in RRECs Under HG Condition
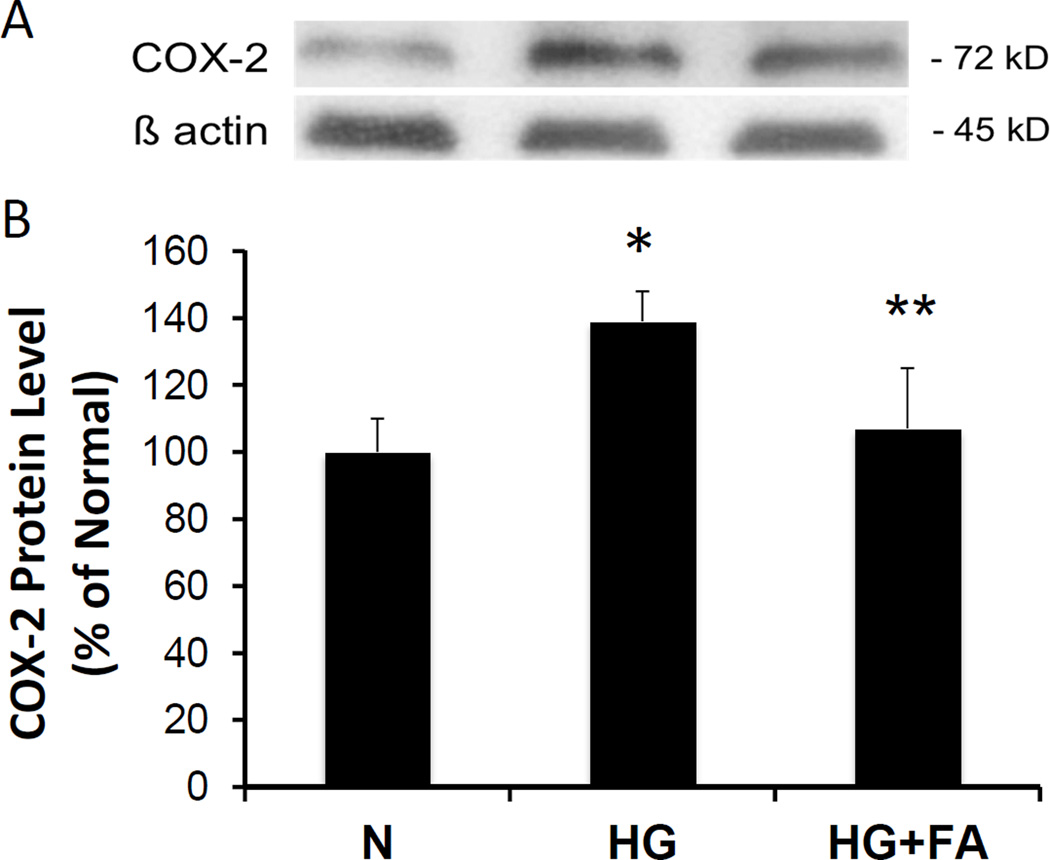
COX-2 protein expression was significantly upregulated in RRECs grown in HG medium compared to those grown in normal medium (139 ± 9% of normal, p = 0.001, n = 3). When RRECs grown in HG medium were treated with FA, a significant reduction in COX-2 protein level was observed compared to untreated RRECs grown in HG medium (107 ± 18% of normal vs. 139 ± 9% of normal, p < 0.05, n = 3) (Figure 3A, 3B).
Figure 3. Effect of FA on COX-2 Protein Level.
(3A) Representative WB image shows FA significantly reduces HG-induced COX-2 overexpression. (3B) Graphical representations of WB data. Coll IV protein level was significantly increased in RRECs grown in HG medium compared to those grown in N medium. When treated with FA, cells grown in HG medium showed a significant reduction in COX-2 (* = N vs. HG, p < 0.05; ** = HG vs. HG + FA, p < 0.05).
3.3. Effect of Fenofibric Acid on ZO-1 Expression, Localization and Distribution in RRECs Under HG Condition
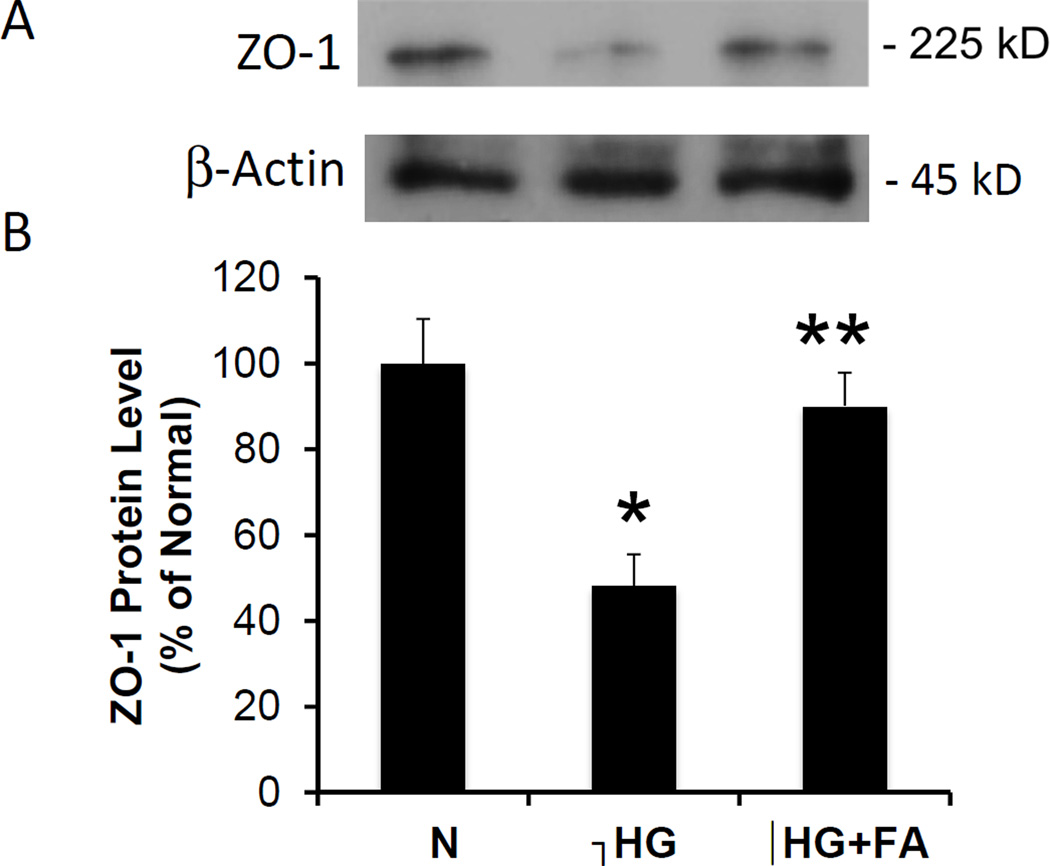
Western blot analysis showed significantly decreased ZO-1 protein expression in RRECs grown in HG medium when compared to those grown in normal medium (48 ± 7% of normal, p < 0.05, n = 4). When RRECs grown in HG medium were treated with FA, a significant increase in ZO-1 protein level was observed compared to untreated RRECs grown in HG medium (89 ± 8% of normal vs. 48 ± 7% of normal, p < 0.05, n = 4) (Figure 4A, 4B).
Figure 4. Effect of FA on ZO-1 Protein Level.
(4A) Representative WB image shows FA significantly increases ZO-1 expression. (4B) Graphical representation of ZO-1 WB data. ZO-1 protein levels were significantly decreased in RRECs grown in HG medium compared to those grown in N medium. When treated with FA, cells grown in HG medium showed a significant increase in ZO-1 expression compared to untreated HG cells (** = N vs. HG, HG vs. HG + FA, p < 0.01).
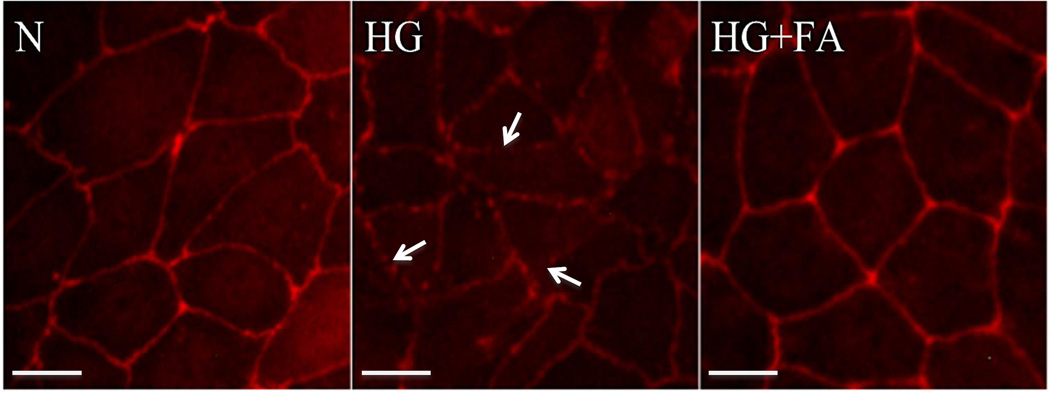
ZO-1 immunostaining showed reduced intensity in areas of discontinuity reflective of disruption of the cell monolayer induced by HG. RRECs grown in HG and treated with FA prevented the disorganization of the tight junction and restored the integrity of the cell monolayer (Figure 5).
Figure 5. Effect of FA on ZO-1 Immunostaining.
Representative images of ZO-1 localization shows reduced fluorescence intensity and areas of discontinuity (arrow) of ZO-1 immunostaining in RRECs grown in HG condition as compared to those grown in N medium. When treated with FA, cells grown in HG medium showed near normalization of ZO-1 immunostaining. Scale bar: 10 um.
3.4. Fenofibric Acid Reduces Retinal Endothelial Cell Monolayer Permeability Induced by HG Condition
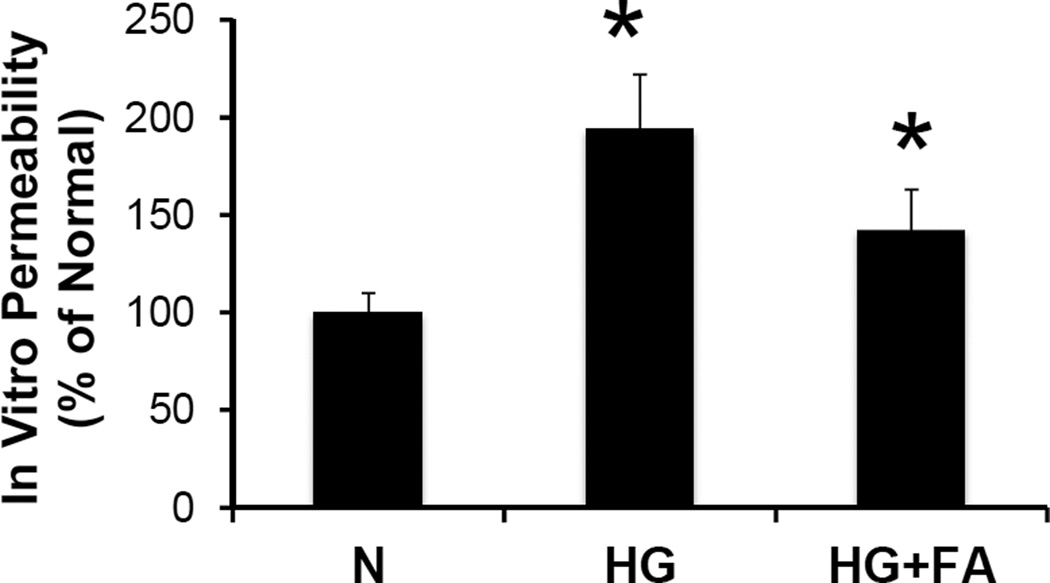
The effect of different conditions tested on the permeability of RREC monolayers is displayed in Figure 6. HG alone significantly increases excess permeability (175 ± 13% of normal, p < 0.001, n = 8). Data related to osmotic control experiments using mannitol indicate that the excess permeability, and the effect of HG is independent of hyperosmotic effects (data not shown). When cells grown in HG medium were treated with FA, a significant reduction in permeability was observed (139 ± 13% of normal vs. 175 ± 13% of normal, p < 0.001, n = 8).
Figure 6. Effect of FA on In Vitro Permeability.
Graphical representation of cumulative data from in vitro permeability assay. RRECs grown in HG medium showed significantly increased cell monolayer permeability compared to those grown in N medium. When treated with FA, cells grown in HG medium showed a significant reduction in cell monolayer permeability compared to untreated HG cell monolayer (* = N vs. HG, p < 0.05; ** = HG vs. HG + FA, p < 0.01).
3.5. The Effect of Fenofibric Acid on FN, Coll IV, ZO-1, and COX-2 Proteins Are PPAR-α Independent
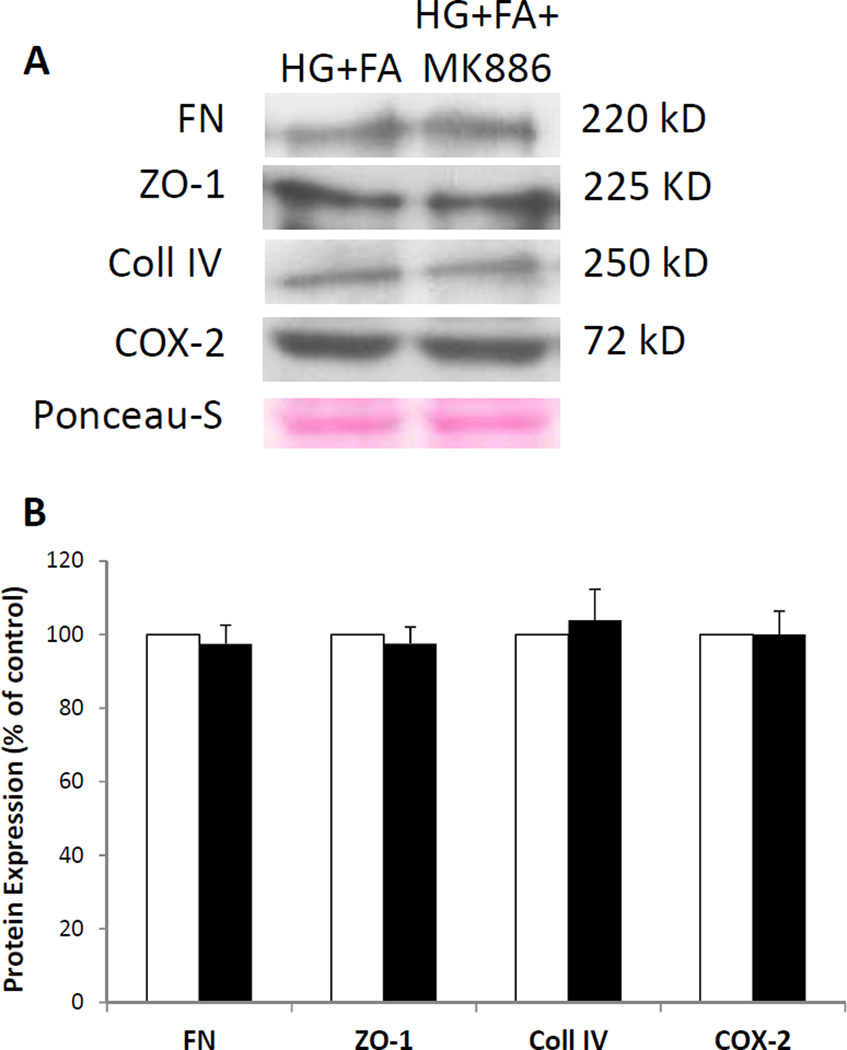
The role of PPAR-α inhibition on FA’s effect on FN, Coll IV, ZO-1, and COX-2 protein expression is displayed in Figure 7. When cells grown in HG medium were treated with FA and with MK886, a PPAR-α inhibitor, no significant changes in FN, Coll IV, ZO-1, and COX-2 protein levels were observed (p > 0.1, n = 4) compared to cells grown in HG medium and treated with FA alone.
Figure 7. Effect of FA on FN, Coll IV, ZO-1, and COX-2 Protein Levels with Inhibition of PPAR-α activity.
(7A) Representative WB image shows there is no significant difference in protein levels of FN, Coll IV, ZO-1, and COX-2 between cells grown in HG treated with FA alone (HG+FA) and cells grown in HG and treated with FA with MK886 (HG+FA+MK886). (7B) Graphical representation of cumulative WB data. Protein levels of FN, Coll IV, ZO-1, and COX-2 are not significantly altered when FA is treated with a PPAR-α inhibitor, demonstrating that the effects of FA on these proteins are PPAR-α independent in RRECs (HG+FA vs. HG+FA+MK886, p > 0.10).
4. Discussion
While substantial clinical benefits with FA on diabetic retinopathy were observed in the FIELD (Keech et al., 2007) and ACCORD-Eye studies (Chew et al., 2010), the underlying therapeutic mechanisms are not yet clear. In this study, we investigated the effect of FA on the inner BRB and how it may contribute to the maintenance of vascular integrity under HG condition. Our findings indicate that FA treatment prevents increased retinal endothelial cell monolayer permeability induced by the HG condition, and that this beneficial effect of FA is associated with downregulation of HG-induced FN and Coll IV overexpression, and possibly also through downregulation of COX-2 level. Our previous studies from in vitro cell culture experiments and animal models of diabetes have shown that HG-induced excess permeability in the diabetic retina is linked to extracellular matrix (ECM) overproduction and basement membrane thickening of the retinal capillaries (Oshitari et al., 2006). Other studies have shown detrimental effects of ECM produced by endothelial cells on viability of pericytes and impaired pericyte adhesion on these matrices under HG condition (Beltramo et al., 2003; Beltramo et al., 2002). Our findings suggest that FA can prevent breakdown of BRB permeability, at least in part, by reducing excess ECM protein synthesis. In addition, we confirmed that preventing ECM upregulation facilitates the maintenance of tight junction protein expression and their function, and that the discontinuity of the tight junction proteins as observed in the ZO-1 immunostaining of RRECs in HG may be indicative of compromised endothelial cell barrier permeability. Our current findings indicate that the protective effect of FA on RREC’s barrier functionality is at least in part mediated by its ability to prevent the accumulation of ECM components and the aberrant distribution of tight junction proteins.
The mechanisms by which FA exerts its beneficial effects in diabetic retinopathy have been intensively investigated in recent years and it is generally accepted that the non-lipidic mechanisms are more important than those attributed to its effect on circulating lipids (Simo et al., 2013). In fact, in the FIELD and ACCORD-Eye studies (Chew et al., 2010; Keech et al., 2007) no relationship was observed between FA -induced changes in plasma lipids and the incidence or progression of diabetic retinopathy. However, a possible involvement of the lipid-modifying effects of FA on the development of diabetic retinopathy cannot be completely discounted. It remains uncertain whether the effects of FA in modulating the qualitative properties of lipoproteins (i.e., reducing remnants and sLDL) contribute to observed benefits in diabetic retinopathy. In addition, the fact that in the present study we provide evidence that the improvement of sealing function of the inner BRB induced by FA is not PPAR-α mediated does not preclude that FA could have potential additive beneficial effects due to its capacity in modulating the qualitative properties of lipoproteins. In this regard, it has been recently reported that increased levels of circulating oxidized LDL immune complexes (ox-LDL-IC) predict risk for severe non-proliferative diabetic retinopathy and proliferative diabetic retinopathy (Simo et al., 2013).
In addition, it is well-known that FA upregulates apoA1 production in the liver, and in a recent report circulating apoA1 was shown to be an independent protective factor for the development of diabetic retinopathy (Simo et al., 2009). To ascertain whether the beneficial effects of FA in the FIELD and ACCORD-Eye studies were related to elevation in apoA1 or to a reduction in oxidised LDL/ox-LDL-IC levels requires further investigation. Finally, it should be noted that the mechanisms involved in regulating intraretinal lipid transport may play a robust role than plasma lipid levels in the pathogenesis of diabetic retinopathy (Simo and Hernandez, 2007). ApoA1 is expressed in the human retina and is a key factor involved in the intraretinal reverse transport of lipids, thus preventing lipid deposition and lipotoxicity (Simo et al., 2009). Therefore, it could be envisaged that FA -induced apoA1 production in the retina could be an additional beneficial effect of FA in diabetic retinopathy. However, specific studies to test this hypothesis are needed. Nevertheless, there is robust evidence that the effects of FA on diabetic retinopathy are mainly mediated by non-lipidic mechanisms which include: 1) endothelial function improvement and anti-apoptotic activity (Kim et al., 2007; Zanetti et al., 2008); 2) preventive effects on BRB breakdown (Chen et al., 2013; Villarroel et al., 2011); 3) antiangiogenic activity (Chen et al., 2013) and 4) antioxidant and anti-inflammatory effects (Simo et al., 2013). In the present study we provide evidence that the reduction of permeability of the inner BRB is associated with the downregulation of ECM components induced by FA in the retinal microvascular cells. A similar mechanism by which FA prevented outer BRB breakdown was previously observed in RPE cells (Trudeau et al., 2011). These findings support and extend a previous report by Mysiorek et al (Mysiorek et al., 2009) demonstrating that FA protects brain capillary endothelial cells from oxygen-glucose deprivation-induced hyperpermeability in the blood-brain barrier of mice, and the recent study by Chen et al (Chen et al., 2013) who showed that both oral and intravitreal administration of FA ameliorated retinal vascular leakage in murine models.
Our finding that COX-2 is downregulated by FA deserves further comments. COX-2, which is regulated at least in part by NF-kβ, has been intensely studied for many years as inflammatory mediator of DR (Kern, 2007). In this regard, it has been recently reported that fenofibrate prevents the disruption of the outer BRB through downregulation of NF-kβ activity (Garcia-Ramírez et al., 2015). Given that inflammation plays an essential role in the pathogenesis of the blood-retinal barrier disruption (Joussen et al., 2001; Kern, 2007; Tang and Kern, 2011), the downregulation of COX-2 can be included in the mechanisms by which FA exerts beneficial effects in reducing the excess permeability associated with DR. In addition, COX-2 and COX-2-induced prostaglandin E2 (PGE2) regulate the expression of VEGF in experimental models of DR (Ayalasomayajula et al., 2004; Li et al., 2014). Since VEGF is a potent factor involved in the induction of retinal permeability (Simo et al., 2014) it is reasonable to postulate that FA reduces VEGF expression through the inhibition of COX-2/PGE2. Therefore, this could be one of the mechanisms accounting for the beneficial effects of FA in preventing vascular leakage. Additionally, we have shown that HG-induced FN and Coll IV upregulation decreases expression of connexin 43 (Cx43), a gap junction protein (Moon et al., 2011). We have recently shown that decreased Cx43 reduces ZO-1 and occludin expression in retinal endothelial cells, which contributes to increased cell monolayer permeability (Tien et al., 2013). Taken together, these findings suggest increased levels of ECM components and COX-2 may disrupt tight junctional integrity, leading to breakdown of the inner BRB.
However, further studies are necessary to elucidate the mechanism by which FA protects the integrity of the inner BRB. The precise mechanism by which FA regulates the ECM components also remains to be elucidated. Previous studies demonstrated that FA reduced ECM accumulation in the renal cortex of streptozotocin-induced diabetic rats (Chen et al., 2006) and in the kidney of spontaneously hypertensive rats by inhibiting oxidative stress and MAPK activity (Hou et al., 2010). In a model of diabetic nephropathy, there is evidence suggesting that FA attenuates HG-induced upregulation of Coll IV by reducing phosphorylation and activation of ERK ½ and PI3K/Akt signaling pathways (Zeng et al., 2013). Additionally, a study using a model of hypertension has found that FA significantly reduced collagen production in the myocardium, which may be due to FA-induced reduction in the formation of activator protein-1 involved in increased production of collagen (Li et al., 2009). Furthermore, we previously reported that FA was able to prevent the overexpression of FN and Coll IV in human retinal pigment epithelial cells induced by IL-1β in the presence of HG (Trudeau et al., 2011). In the present study, we provide evidence that these beneficial effects of FA also occurs in endothelial cells but without the presence of IL-1β. In addition, these effects were maintained when PPAR-α receptor was inhibited. Therefore, other PPAR-α independent mechanisms unrelated to anti-inflammatory action remain to be investigated.
The present study complements our previous study showing beneficial effect of FA on the outer BRB (Trudeau et al.). Taken together, findings from the current study on FA’s beneficial effect on the inner BRB and those from the previous study clearly show that at least one of the mechanisms by which FA imparts its beneficial effect is by preventing the abnormally high ECM production induced by diabetes. In this context, FA’s anti-inflammatory effect may even be facilitated by the reduced ECM production as RAGE-mediated inflammatory cascades may be compromised (Goldin et al., 2006; Zong et al., 2011). Overall, these findings contribute to a better understanding of the mode of action of FA and shed light on its significant clinical translational value as it could be useful for direct application and treatment of diabetic retinopathy, in particular DME.
Highlights.
Mechanistic insight into fenofibric acid’s beneficial effect in DR
Fenofibric acid prevents high glucose-induced ECM overexpression in endothelial cells.
Fenofibric acid reduces retinal inflammation associated with DR.
Acknowledgements
Research was supported by NEI, NIH EY014702, NEI, NIH EY018218, and in part by a departmental grant from the Massachusetts Lions Organization (SR). In addition, this study was supported by grants from Ministerio de Ciencia y Tecnología (SAF2012-35562) and CIBER for Diabetes and Associated Metabolic Diseases (CIBERDEM). CIBERDEM is an initiative of the Instituto de Salud Carlos III. We acknowledge the assistance of Solvay Pharma S.A. in providing FA.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alvarez de Sotomayor M, Mingorance C, Andriantsitohaina R. Fenofibrate improves age-related endothelial dysfunction in rat resistance arteries. Atherosclerosis. 2007;193:112–120. doi: 10.1016/j.atherosclerosis.2006.08.041. [DOI] [PubMed] [Google Scholar]
- Ayalasomayajula SP, Amrite AC, Kompella UB. Inhibition of cyclooxygenase-2, but not cyclooxygenase-1, reduces prostaglandin E2 secretion from diabetic rat retinas. Eur J Pharmacol. 2004;498:275–278. doi: 10.1016/j.ejphar.2004.07.046. [DOI] [PubMed] [Google Scholar]
- Beltramo E, Buttiglieri S, Pomero F, Allione A, D'Alu F, Ponte E, Porta M. A study of capillary pericyte viability on extracellular matrix produced by endothelial cells in high glucose. Diabetologia. 2003;46:409–415. doi: 10.1007/s00125-003-1043-6. [DOI] [PubMed] [Google Scholar]
- Beltramo E, Pomero F, Allione A, D'Alu F, Ponte E, Porta M. Pericyte adhesion is impaired on extracellular matrix produced by endothelial cells in high hexose concentrations. Diabetologia. 2002;45:416–419. doi: 10.1007/s00125-001-0761-x. [DOI] [PubMed] [Google Scholar]
- Chen LL, Zhang JY, Wang BP. Renoprotective effects of fenofibrate in diabetic rats are achieved by suppressing kidney plasminogen activator inhibitor-1. Vascular pharmacology. 2006;44:309–315. doi: 10.1016/j.vph.2006.01.004. [DOI] [PubMed] [Google Scholar]
- Chen Q, Shinohara N, Abe T, Watanabe T, Nonomura K, Koyanagi T. Significance of COX-2 expression in human renal cell carcinoma cell lines. Int J Cancer. 2004;108:825–832. doi: 10.1002/ijc.11646. [DOI] [PubMed] [Google Scholar]
- Chen Y, Hu Y, Lin M, Jenkins AJ, Keech AC, Mott R, Lyons TJ, Ma JX. Therapeutic effects of PPARalpha agonists on diabetic retinopathy in type 1 diabetes models. Diabetes. 2013;62:261–272. doi: 10.2337/db11-0413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cherian S, Roy S, Pinheiro A. Tight glycemic control regulates fibronectin expression and basement membrane thickening in retinal and glomerular capillaries of diabetic rats. Invest Ophthalmol Vis Sci. 2009;50:943–949. doi: 10.1167/iovs.08-2377. [DOI] [PubMed] [Google Scholar]
- Cheung N, Mitchell P, Wong TY. Diabetic retinopathy. Lancet. 2010;376:124–136. doi: 10.1016/S0140-6736(09)62124-3. [DOI] [PubMed] [Google Scholar]
- Chew EY, Ambrosius WT, Davis MD, Danis RP, Gangaputra S, Greven CM, Hubbard L, Esser BA, Lovato JF, Perdue LH, Goff DC, Jr, Cushman WC, Ginsberg HN, Elam MB, Genuth S, Gerstein HC, Schubart U, Fine LJ. Effects of medical therapies on retinopathy progression in type 2 diabetes. N Engl J Med. 2010;363:233–244. doi: 10.1056/NEJMoa1001288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans T, Deng DX, Chen S, Chakrabarti S. Endothelin receptor blockade prevents augmented extracellular matrix component mRNA expression and capillary basement membrane thickening in the retina of diabetic and galactose-fed rats. Diabetes. 2000;49:662–666. doi: 10.2337/diabetes.49.4.662. [DOI] [PubMed] [Google Scholar]
- Garcia-Ramírez M, Hernández C, Palomer X, Vázquez-Carrera M, Simó R. Fenofibrate prevents the disruption of the outer blood retinal barrier through downregulation of NF-κB activity. Acta Diabetologica. 2015 doi: 10.1007/s00592-015-0759-3. May 5th. [Epub ahead of print]. PMID: 25936740. [DOI] [PubMed] [Google Scholar]
- Goldin A, Beckman JA, Schmidt AM, Creager MA. Advanced glycation end products: sparking the development of diabetic vascular injury. Circulation. 2006;114:597–605. doi: 10.1161/CIRCULATIONAHA.106.621854. [DOI] [PubMed] [Google Scholar]
- Hou X, Shen YH, Li C, Wang F, Zhang C, Bu P, Zhang Y. PPARalpha agonist fenofibrate protects the kidney from hypertensive injury in spontaneously hypertensive rats via inhibition of oxidative stress and MAPK activity. Biochemical and biophysical research communications. 2010;394:653–659. doi: 10.1016/j.bbrc.2010.03.043. [DOI] [PubMed] [Google Scholar]
- Joussen AM, Murata T, Tsujikawa A, Kirchhof B, Bursell SE, Adamis AP. Leukocyte-mediated endothelial cell injury and death in the diabetic retina. The American journal of pathology. 2001;158:147–152. doi: 10.1016/S0002-9440(10)63952-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Keech A, Simes RJ, Barter P, Best J, Scott R, Taskinen MR, Forder P, Pillai A, Davis T, Glasziou P, Drury P, Kesaniemi YA, Sullivan D, Hunt D, Colman P, d'Emden M, Whiting M, Ehnholm C, Laakso M. Effects of long-term fenofibrate therapy on cardiovascular events in 9795 people with type 2 diabetes mellitus (the FIELD study): randomised controlled trial. Lancet. 2005;366:1849–1861. doi: 10.1016/S0140-6736(05)67667-2. [DOI] [PubMed] [Google Scholar]
- Keech AC, Mitchell P, Summanen PA, O'Day J, Davis TM, Moffitt MS, Taskinen MR, Simes RJ, Tse D, Williamson E, Merrifield A, Laatikainen LT, d'Emden MC, Crimet DC, O'Connell RL, Colman PG. Effect of fenofibrate on the need for laser treatment for diabetic retinopathy (FIELD study): a randomised controlled trial. Lancet. 2007;370:1687–1697. doi: 10.1016/S0140-6736(07)61607-9. [DOI] [PubMed] [Google Scholar]
- Kern TS. Contributions of inflammatory processes to the development of the early stages of diabetic retinopathy. Experimental diabetes research. 2007;2007:95103. doi: 10.1155/2007/95103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim J, Ahn JH, Kim JH, Yu YS, Kim HS, Ha J, Shinn SH, Oh YS. Fenofibrate regulates retinal endothelial cell survival through the AMPK signal transduction pathway. Experimental eye research. 2007;84:886–893. doi: 10.1016/j.exer.2007.01.009. [DOI] [PubMed] [Google Scholar]
- Li CB, Li XX, Chen YG, Zhang C, Zhang MX, Zhao XQ, Hao MX, Hou XY, Gong ML, Zhao YX, Bu PL, Zhang Y. Effects and mechanisms of PPARalpha activator fenofibrate on myocardial remodelling in hypertension. Journal of cellular and molecular medicine. 2009;13:4444–4452. doi: 10.1111/j.1582-4934.2008.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li T, Hu J, Du S, Chen Y, Wang S, Wu Q. ERK1/2/COX-2/PGE2 signaling pathway mediates GPR91-dependent VEGF release in streptozotocin-induced diabetes. Molecular vision. 2014;20:1109–1121. [PMC free article] [PubMed] [Google Scholar]
- Moon J, Knack HP, Roy S. Effect of Inhibiting High Glucose-induced Upregulation of ECM on Connexin 43 (Cx43) Expression in Retinal Endothelial Cells, Investigative ophthalmology & visual science; ARVO 2011 Conference; 2011. E-Abstract 3554. [Google Scholar]
- Mysiorek C, Culot M, Dehouck L, Derudas B, Staels B, Bordet R, Cecchelli R, Fenart L, Berezowski V. Peroxisome-proliferator-activated receptor-alpha activation protects brain capillary endothelial cells from oxygen-glucose deprivation-induced hyperpermeability in the blood-brain barrier. Current neurovascular research. 2009;6:181–193. doi: 10.2174/156720209788970081. [DOI] [PubMed] [Google Scholar]
- Oshitari T, Polewski P, Chadda M, Li AF, Sato T, Roy S. Effect of combined antisense oligonucleotides against high-glucose- and diabetes-induced overexpression of extracellular matrix components and increased vascular permeability. Diabetes. 2006;55:86–92. [PubMed] [Google Scholar]
- Robison WG, Jr, Jacot JL, Glover JP, Basso MD, Hohman TC. Diabetic-like retinopathy: early and late intervention therapies in galactose-fed rats. Investigative ophthalmology & visual science. 1998;39:1933–1941. [PubMed] [Google Scholar]
- Roy S, Lorenzi M. Early biosynthetic changes in the diabetic-like retinopathy of galactose-fed rats. Diabetologia. 1996;39:735–738. doi: 10.1007/BF00418547. [DOI] [PubMed] [Google Scholar]
- Simo R, Garcia-Ramirez M, Higuera M, Hernandez C. Apolipoprotein A1 is overexpressed in the retina of diabetic patients. American journal of ophthalmology. 2009;147:319–325. e311. doi: 10.1016/j.ajo.2008.08.009. [DOI] [PubMed] [Google Scholar]
- Simo R, Hernandez C. Fenofibrate for diabetic retinopathy. Lancet. 2007;370:1667–1668. doi: 10.1016/S0140-6736(07)61608-0. [DOI] [PubMed] [Google Scholar]
- Simo R, Roy S, Behar-Cohen F, Keech A, Mitchell P, Wong TY. Fenofibrate: a new treatment for diabetic retinopathy. Molecular mechanisms and future perspectives. Current medicinal chemistry. 2013;20:3258–3266. doi: 10.2174/0929867311320260009. [DOI] [PubMed] [Google Scholar]
- Simo R, Sundstrom JM, Antonetti DA. Ocular Anti-VEGF therapy for diabetic retinopathy: the role of VEGF in the pathogenesis of diabetic retinopathy. Diabetes care. 2014;37:893–899. doi: 10.2337/dc13-2002. [DOI] [PubMed] [Google Scholar]
- Tang J, Kern TS. Inflammation in diabetic retinopathy. Progress in retinal and eye research. 2011;30:343–358. doi: 10.1016/j.preteyeres.2011.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tien T, Barrette KF, Chronopoulos A, Roy S. Effects of high glucose-induced Cx43 downregulation on occludin and ZO-1 expression and tight junction barrier function in retinal endothelial cells. Investigative ophthalmology & visual science. 2013;54:6518–6525. doi: 10.1167/iovs.13-11763. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Towbin H, Staehelin T, Gordon J. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc Natl Acad Sci USA. 1979;76:4350–4354. doi: 10.1073/pnas.76.9.4350. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Trudeau K, Roy S, Guo W, Hernandez C, Villarroel M, Simo R, Roy S. Fenofibric acid reduces fibronectin and collagen type IV overexpression in human retinal pigment epithelial cells grown in conditions mimicking the diabetic milieu: functional implications in retinal permeability. Investigative ophthalmology & visual science. 2011;52:6348–6354. doi: 10.1167/iovs.11-7282. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Villarroel M, Garcia-Ramirez M, Corraliza L, Hernandez C, Simo R. Fenofibric acid prevents retinal pigment epithelium disruption induced by interleukin-1beta by suppressing AMP-activated protein kinase (AMPK) activation. Diabetologia. 2011;54:1543–1553. doi: 10.1007/s00125-011-2089-5. [DOI] [PubMed] [Google Scholar]
- Yau JW, Rogers SL, Kawasaki R, Lamoureux EL, Kowalski JW, Bek T, Chen SJ, Dekker JM, Fletcher A, Grauslund J, Haffner S, Hamman RF, Ikram MK, Kayama T, Klein BE, Klein R, Krishnaiah S, Mayurasakorn K, O'Hare JP, Orchard TJ, Porta M, Rema M, Roy MS, Sharma T, Shaw J, Taylor H, Tielsch JM, Varma R, Wang JJ, Wang N, West S, Xu L, Yasuda M, Zhang X, Mitchell P, Wong TY. Global prevalence and major risk factors of diabetic retinopathy. Diabetes care. 2012;35:556–564. doi: 10.2337/dc11-1909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zanetti M, Stocca A, Dapas B, Farra R, Uxa L, Bosutti A, Barazzoni R, Bossi F, Giansante C, Tedesco F, Cattin L, Guarnieri G, Grassi G. Inhibitory effects of fenofibrate on apoptosis and cell proliferation in human endothelial cells in high glucose. J Mol Med (Berl) 2008;86:185–195. doi: 10.1007/s00109-007-0257-3. [DOI] [PubMed] [Google Scholar]
- Zeng R, Xiong Y, Zhu F, Ma Z, Liao W, He Y, He J, Li W, Yang J, Lu Q, Xu G, Yao Y. Fenofibrate attenuated glucose-induced mesangial cells proliferation and extracellular matrix synthesis via PI3K/AKT and ERK1/2. PloS one. 2013;8:e76836. doi: 10.1371/journal.pone.0076836. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zong H, Ward M, Stitt AW. AGEs, RAGE, and diabetic retinopathy. Current diabetes reports. 2011;11:244–252. doi: 10.1007/s11892-011-0198-7. [DOI] [PubMed] [Google Scholar]