Abstract
Abiotic stresses constitute a serious threats to the world food security as they cause significant economic losses in terms of reduction in crop productivity and also greatly limit the geographical locations where crops can be grown. Exposure to abiotic stress causes over-production of reactive oxygen species, leading to oxidative stress in plants. Induction of oxidative stress is primarily responsible for a variety of detrimental changes in the cellular physiology. However, plants have evolved intricate anti-oxidative defence machinery, for their survival under stress. Plant defence strategies for stress tolerance rely on the expression of anti-oxidative genes required for scavenging the toxic reactive oxygen species. Monodehydroascorbate reductase is one of the key anti-oxidant enzyme responsible for scavenging reactive oxygen species. In the present study, efforts have been made to understand the role of monodehydroascorbate reductase in finger millet under different abiotic stresses (drought, salt and UV radiation). The study establishes a differential link between mdar gene expression and enzyme activity under oxidative stress that is validated under different types of imposed stresses. Alteration in correlation between gene expression and enzyme activities under varying magnitude of oxidative stress is elucidated.
Keywords: Anti-oxidative defence, Environmental stress, Finger millet, Monodehydroascorbate reductase, Ascorbate-glutathione pathway
Introduction
Increasing demand for food, in order to feed the burgeoning population, is compelling the farming community to expand agriculture to hitherto fragile areas inundated with various environmental vagaries. Such areas generally have poor irrigation and low quality soils, which are considered unfit for optimal agricultural practices. Such stresses constitute a major barrier for agricultural production, due to the excessive production of reactive oxygen species (ROS) in plants. These ROS are also produced during the normal course of cellular metabolism, but their level is kept under control due to the presence of an effective anti-oxidative defence system. However, under stress, the production of ROS overrides the rate of their detoxification, and causes oxidative stress (Bhatt et al. 2011). Excess accumulation of ROS negatively effects the growth and productivity of crop plants by interfering with various metabolic activities, resulting in protein oxidation, lipid peroxidation and damage to the nucleic acids; which under severe conditions may also lead to cell death. Therefore, one of the prudent strategies to produce stress tolerant crop plants could be the strengthening of the anti-oxidative potential of susceptible plants, through over-production of key anti-oxidative enzymes.
Effectiveness of anti-oxidative defence system varies from species to species. Only select native plants possess strong inherent antioxidative properties, to survive under sub-optimal stressful environments. Finger millet (Eleusine coracana), an important millet which is cultivated extensively in Asia and Africa, possesses significant stress tolerance and has superior nutritional qualities as compared to rice and wheat. According to reports, the anticipated food demand for 2025 will require the yield of cereals, including millets, to rise from 2.5 to 4.5 t ha−1 (Borlaug 2002); and finger millet could be a useful crop for ensuring food and nutritional security and for providing a potential source of key stress tolerant isoforms of important antioxidant genes.
Plants contain several antioxidant enzymes for ROS detoxification. The primary components of this system include low molecular weight antioxidants (ascorbate, glutathione, carotenoids, flavonoids, and tocopherols), enzymes (superoxide dismutase, catalase, glutathione peroxidase, and other peroxidases), along with the enzymes involved in the ascorbate–glutathione cycle (MDAR: Monodehydroascorbate reductase, DHAR: Dehydroascorbate reductase, GR: Glutathione reductase and APX: Ascorbate peroxidase). Ascorbate-glutathione pathway is the central antioxidant defence pathway in plants, responsible for metabolizing toxic ROS (Shamsi et al. 2010). Ascorbate is one of the essential water-soluble antioxidant in organisms across all kingdoms. Ascorbate peroxidase catalyses the reduction of H2O2 with the simultaneous oxidation of ascorbate and generates monodehydroascorbate (MDA) radical as the primary product. Monodehydroascorbate reductase (MDAR) is critical in maintaining the optimal concentration of ascorbate in cells by reducing the MDA radical directly to ascorbate at the expense of NAD(P)H oxidation (Asada 1994).
Monodehydroascorbate reductase is ubiquitous in all the phylogenetic groups and its isoforms existing in chloroplasts (Hossain et al. 1984), cytosol (Dalton et al. 1993), peroxisomes and mitochondria (Jimenez et al. 1997) have been reported. The activity of MDAR has been shown to be up-regulated under several stress conditions, for instance in tomato by salinity stress (Mittova et al. 2003) and high light intensity (Gechev et al. 2003), in rice by low temperature (Oidaira et al. 2000), and in Arabidopsis by UV-C exposure (Kubo et al. 1999). However, among the antioxidant enzymes much of the research efforts have been focused on expression and activity of GR and APX, and only limited information is available concerning the expression and activation of MDAR upon exposure to oxidative stress in millets.
In the present investigations, attempts were made to validate the crucial role played by MDAR in regulating the levels of ascorbate, by monitoring the expression of mdar gene and activity of MDAR enzyme under different abiotic stress conditions (drought, salinity and UV-C stress). In addition to the expression profiling, the possible reasons for the differential expression under various stress conditions (drought, salt and UV) are also discussed.
Material and methods
Plant material and treatments
Seeds of E. coracana variety PR202 were obtained from G. B. Pant University of Agriculture and Technology, India. The seeds were washed with Tween-20 detergent and surface sterilization with 0.1 % HgCl2. Seeds were sown in pots, containing soil:sand:vermicompost in the ratio 2:1:1 and kept in a poly-house under controlled connditions. Another set of seeds was inoculated in culture bottles containing MS medium (Murashige and Skoog 1962), supplemented with different concentrations of NaCl and kept in tissue culture facility under controlled temperature (24/18 °C) and 16/8 h light/dark photoperiod.
Stress treatment
For stress treatment, 45-day-old seedlings were subjected to drought stress by restricting irrigation to 50 % of soil water withholding capacity. Control plants were irrigated on alternate days and maintained at 80–85 % of soil water holding capacity.
Salt stress was imposed by irrigating the 45-day-old seedlings with different concentrations of NaCl solution (0, 25, 50, 75 and 100 mM). The seedlings were irrigated with respective concentration of salt solution for 4 consecutive days followed by biochemical & molecular analysis on the 5th day. In another experiment, seedlings were raised in MS media supplemented without (control) and with 25, 50, 75 and 100 mM NaCl; in culture bottles.
In another set of experiments seedlings grown in MS media were exposed to UV-C radiations (252 nm, 50 kJ/m2) through 30 W germicide fluorescent lamps for increasing time intervals (15, 30, 120, 240 and 480 min).
Analysis of stress vigor
Electrolyte leakage
Electrolyte leakage (EL) was estimated according to the method of Dionisio-Sese and Tobita (1998). Fresh leaf samples (1 g) were washed with triple distilled water, and cut into small pieces (1 cm2 segments) and suspended in test tubes containing 15 ml of deionized water. Tubes were incubated in a water bath at 25 °C for half an hour. After incubation, electrical conductivity (EC1) of the bathing solution was recorded with an electrical conductivity meter (Eutech Instruments, Singapore). These samples were then kept at 100 °C for 30 min to completely disintegrate the membrane and release the electrolytes. Samples were then cooled and final electrical conductivity (EC2) was measured. The percent leakage of electrolytes was calculated using the equation .
Expression analysis
RNA isolation and cDNA preparation
Total RNA was isolated from the leaf samples of different treatments, using RNA Xpress reagent (Hi-Media India) according to the manufacturer’s protocol. Total RNA was used to make cDNA using RevertAid cDNA synthesis kit from Fermentas. A pair of gene specific primers MDAR-F1 and MDAR-R1 were designed by using Lasergene DNASTAR software and used for mdar gene expression. The sequence of the designated primers is as follows:
MDAR F1: 5’CTT GCG TTG GTG CTA ATG ATG AGT 3′.
MDAR R1: 5’CAA CGT GAA GAC CCT GGA GTA GAA 3′.
Semi-quantitative PCR (RT-PCR)
Semi-quantitative PCR was performed under standardised conditions with gene specific primers. The PCR cycle conditions were standardized at 94 °C for 3 min followed by 32 cycles at 94 °C for 50s (denaturation), 58 °C for 45s (annealing) and 72 °C for 50s (extension), and a final extension at 72 °C for 7 min. The housekeeping gene, actin was used as an internal control. The expression levels are represented as fold increase over control.
Antioxidative Enzyme Assay (Monodehydroascorbate reductase activity)
Specific activity of Monodehydroascorbate reductase was assayed as described by Hossain et al. (1984), with slight modification. Leaf samples (0.5 g fresh weight) were homogenized in a pre-chilled pestle mortar in 6 ml ice-cold extraction buffer containing 50 mM KH2PO4–KOH (pH 7.5), 0.1 mM ethylenediamine tetraacetate, 0.3 % (v/v) Triton X-100 and 2 % (w/v) insoluble polyvinylpolypyrrolidone-40. The homogenate was kept on ice for 10 min, and then centrifuged at 13,000 xg for 15 min at 4 °C. An aliquot of supernatant was used for determination of enzyme activity. The assay was performed at 340 nm (using absorbance coefficient of 6.2 mM−1cm−1) in a 2 ml reaction mixture containing 50 mmol L−1 Potassium phosphate buffer (pH 7.0), 0.2 mmol L−1 NADH, 2.5 mmol L−1 AsA, 0.25 U of ascorbate oxidase and 60 μl of enzyme extract. The decrease in absorbance due to ascorbate oxidase-induced oxidation of NADH was followed at 340 nm. The enzyme activity was calculated in terms of μmol of NADH oxidized per minute. Total protein was determined according to Bradford (1976).
Results
Abiotic stress induced generation of reactive oxygen species in cells, leads to structural and functional perturbations. Plants have developed specialized ROS scavenging mechanisms such as ascorbate-glutathione pathway (also known as Halliwell-Asada pathway) to reduce the ROS load. Monodehydroascorbate reductase is one of the crucial enzymes of ascorbate-glutathione pathway. Monodehydroascorbate reductase activity is induced by distinct regulatory mechanisms in response to various environmental stresses and plays a synergistic role in protecting cellular organelles, thus minimizing tissue injury (Hossain et al. 1984).
Electrolyte leakage
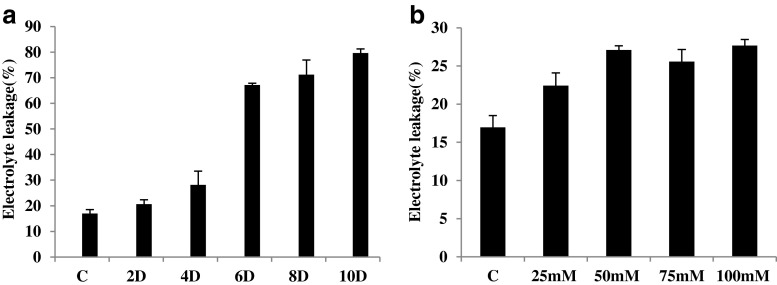
Seedlings exposed to salt and drought stress exhibited wilting and leaf rolling symptoms (Fig. 1), on exposure to different levels of stresses. Increase in electrolyte leakage of leaf tissues was recorded in seedlings exposed to different stresses. This decrease in membrane integrity on exposure to stress shows a direct relationship between stress induced reactive oxygen species production and the electrolyte leakage caused by membrane damage. In seedlings exposed to drought stress, no statistically significant enhancement was observed in electrolyte leakage till 4th day. However, on 6th day after exposure to drought stress, a fourfold increase in electrolyte leakage was recorded, over that of control seedlings (Fig. 2a). Further, no significant differences in electrolyte leakage were observed amongst drought stressed seedlings on 6th, 8th and 10th day after exposure to stress. Under salt stress, electrolyte leakage increased up to 50 mM NaCl concentration, with a 1.8 fold increase with respect to the control seedlings; and after that it remained steady till 100 mM (Fig. 2b).
Fig. 1.
Finger millet seedlings exposed to increasing concentration of NaCl
Fig. 2.
Electrolyte leakage in the leaves of finger millet seedlings at different levels of stress conditions a Drought and b salinity stress. Values represent Mean ± SE (n = 3)
Expression profiling of mdar under different abiotic stress conditions
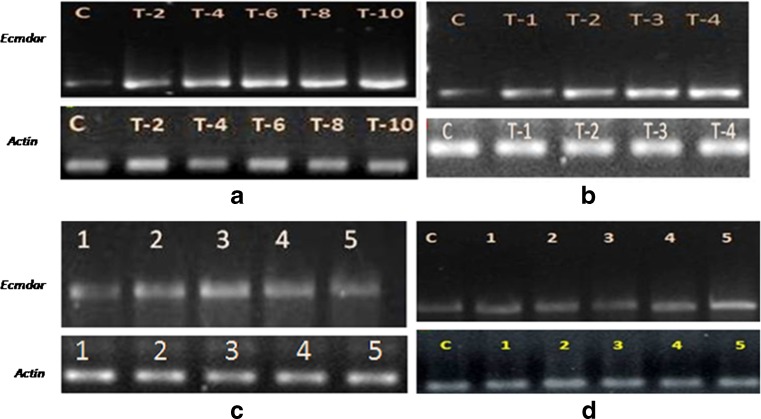
The expression pattern of mdar gene was recorded under different stress conditions. In all the experiments ‘actin’ was taken as the internal control, as its expression remained unaffected by changes in the imposed stress regimes. The mdar gene expression was monitored after 0, 2nd, 4th, 6th, 8th and 10th day of imposition of stress. The transcript level of mdar increased continuously with increasing duration of drought stress exposure. On 2, 4, and 6 days after drought stress imposition, a 2.2, 2.6 and 2.8 fold increase was recorded in mdar transcript levels, respectively, with respect to the control plants. A maximum of 4.1 fold increase was recorded in mdar gene expression on 10th day of drought stress (Fig. 3a).
Fig. 3.
Expression profiling of mdar under different stress conditions. a different days of drought stress treatment (C-control, T-2 = 2D, T-4 = 4D, T-6 = 6D, T-8 = 8D, T-10 = 10D), b different treatments of salt stress (C-control, T-1 = 25 mM, T-2 = 50 mM, T-3 = 75 mM, T-4 = 100 mM salt solutions), c seedlings raised in MS media supplemented with different concentrations of NaCl (1-control, 2 = 25 mM, 3 = 50 mM, 4 = 75 mM, 5 = 100 mM salt solutions), and d UV-C exposure for different time periods (C-control, 1 = 15 min, 2 = 30 min, 3 = 2 h, 4 = 4 h, 5 = 8 h of UV-B exposure)
In glasshouse experiments, a steady increase in mdar transcript was recorded in the seedlings irrigated with increasing strength of NaCl solution. At 25, 50 and 75 mM NaCl concentration a 1.3, 1.5 and 2.4 fold higher expression of mdar gene was recorded, respectively, over that of control levels (Fig. 3b). However, no statistically significant increase in mdar gene expression was detected between 75 and 100 mM NaCl treatments.
Seedlings raised in MS media supplemented with different concentrations of NaCl, illustrated a differential gene expression pattern when compared to the pot experiments. In MS media supplemented with NaCl, the transcript levels of mdar were up-regulated up to 50 mM NaCl concentration, and after that a down-regulation of mdar gene expression was recorded at 75 mM and 100 mM NaCl concentration (Fig. 3c). At 50 mM NaCl concentration, a 2.2 fold amplification was recorded in mdar transcript level whereas only 1.7 fold amplification was recorded at 100 mM NaCl concentration, relative to the control transcript levels.
In case of seedlings exposed to UV-C radiations, the mdar gene expression remained statistically unaltered up to 60 min of exposure time (Fig. 3d). At 240 min and higher exposure times a moderate increase in transcript level was recorded that reached a maximum value of 2.15 folds after 480 min of UV-C exposure.
Specific activity of MDAR under different abiotic stress condition
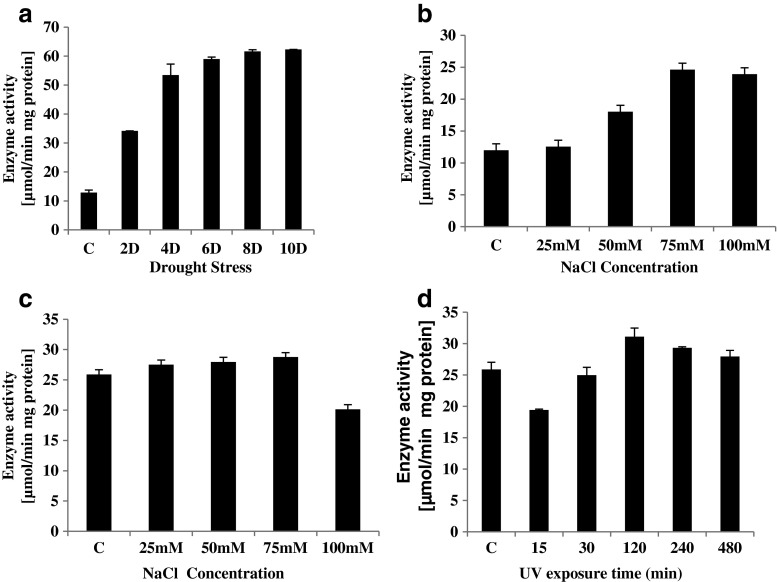
The enzymatic activity of MDAR was significantly affected by the duration and intensity of imposed stress. However, irrespective of the type of stress, MDAR specific activity increased under stress. In case of drought stress, the MDAR activity started increasing from 2nd day after exposure to stress and recorded a 6 fold increase in enzyme activity on 6th day after exposure to stress (Fig. 4a). After that the increase in MDAR activity was only minimalistic.
Fig. 4.
Specific activity of MDAR under different stress conditions, a drought, b plants treated with NaCl solution, c plants grown in MS media supplemnted with NaCl solution and d UV-C stress. Values represent Mean ± SE (n = 3)
In case of seedlings irrigated with different concentrations of NaCl, no significant change in specific activity of MDAR was observed at lower salt concentration (25 mM). However the enzymatic activity went up by 1.5 and 2 folds respectively, at 50 and 75 mM NaCl concentration (Fig. 4b). Beyond 75 mM NaCl concentration, no further increase in enzyme activity was recorded.
Interestingly, seedlings grown in MS media supplemented with different concentration of NaCl recorded no significant changes in MDAR enzyme activity with respect to the control seedlings. On the contrary a decrease in MDAR specific activity was recorded in seedlings grown in MS media supplemented with 100 mM NaCl concentration (Fig. 4c).
Under UV-C exposure a differential change in MDAR enzyme activity was recorded (Fig. 4d). The specific activity of MDAR decreased during the first 30 min of UV exposure time. A further increase in the UV exposure time lead to a continuous increase in the enzyme activity up to 120 min of exposure. Further increase in the exposure time led to a decrease in the MDAR specific activity at 480 min of exposure time. Thus, UV stress elicited a temporal expression of MDAR activity.
Discussion
Production of reactive oxygen species in plants exposed to various environmental stresses is a well-established phenomenon. Excessive production and accumulation of these ROS causes deleterious effects on the overall growth and development of the affected plants. Therefore, plants have developed a battery of antioxidant enzymes to combat these toxic species. However, it is also known that the extent of expression and activity of these antioxidant enzymes varies amongst different plant species. Therefore, one of the prudent strategies to produce stress tolerant plants could be the strengthening of the anti-oxidative potential of the affected plants, through overproduction of key anti-oxidative enzymes. As scavenging of ROS is a multi-enzyme process, therefore selection of the right anti-oxidative enzyme is a crucial step for successful development of stress tolerant varieties of crop plants. Monodehydroascorbate reductase (MDAR), is a regulatory enzyme of the ascorbate glutathione pathway, and helps the plant to cope up with increased ROS load, by catalyzing the reduction of monodehydroascorbate radical and generation of reduced ascorbate pool. Several researchers have reported a direct correlation between stress tolerance and MDAR activity (Kubo et al. 1999; Oidaira et al. 2000; Gechev et al. 2003; Mittova et al. 2003), thus underlining its vital role in the overall ROS detoxification process, under various abiotic stresses. However, few researchers have studied the relationship between gene expression and protein activity of mdar gene under stress. In the current study we have tried to elucidate the relationship between the role of monodehydroascorbate reductase under different in vivo and in vitro stress conditions, with respect to its expression profile and enzymatic activity.
Exposure of plants to different stresses led to an increase in the electrolyte leakage from the leaf tissues of affected plants. In case of drought induced ROS production, the signs of membrane damage appeared only on 4th day after exposure to stress, while in case of salt stress, the membrane damage was evident at 25 mM NaCl treatment. Membrane damage via ROS induced lipid peroxidation contributes to the loss of cellular integrity, inactivation of membrane enzymes and to some extent even of cytoplasmic proteins. All these changes adversely affect the functioning of the plant cell. During initial stages such an increase in ROS generation, acts as a signal for the induction of cellular defence machinery, including anti-oxidant enzymes. In case of monodehydroascorbate reductase, such an induction effect is visible at the transcript level as well as at the protein activity level also, indicating that the induction of stress induced MDAR activity occurs due to the induction of mdar gene.
It was found that mdar gene responds differentially to different external stresses. The mdar gene expression was found to be critical under drought stress. Up-regulation in the expression level of the mdar gene was recorded right from 2nd day after exposure to stress and this increase continued till the 10th day after exposure to stress. Although small incremental difference was recorded in the mdar gene expression between 8th and 10th of stress. The enzyme activity also co-related with the mdar gene expression, for successive days of drought stress treatment; but under severe stress conditions such as on 10th day of drought stress the enzyme activity did not increase in line with the gene expression. This could be because of various translational and post-transcriptional activities, adversely affected under stress, including an overall decrease in protein synthesis efficiency. Increased levels of mdar transcript and enzyme activity under drought stress attribute an important role to this gene for providing stress tolerance in Eleusine coracana. As exposure to drought induces the over-production of various ROS (O2− and H2O2), an increased pool of ascorbate is require for their detoxification which could be maintained by increasing mdar gene expression. An increase in the level of mdar transcript under cold stress has been reported in winter wheat by Baek and Skinner (2003), and in Arabidopsis by Vadassery et al. (2009).
In case of salt stress treatment, the seedlings irrigated with NaCl solution of different concentrations showed a differential response of the mdar gene with increasing salt concentration. There was little increase in mdar gene expression and enzyme activity at 25 mM NaCl concentration, with respect to controls, however, there was a 2 fold enhancement in gene expression and enzyme activity at 75 mM NaCl concentration. At 100 mM concentration, gene expression increased but enzyme activity was commensurate with the increased gene activity. These results showed that at lower salt concentration gene expression directly correlates with enzyme activity; but as the magnitude of stress (NaCl concentration) increases the protein activity is not able to keep pace with the increased level of gene expression.
Interestingly, in seedlings raised in MS media supplemented with different concentrations of NaCl, the mdar expression level first increased up to 50 mM NaCl concentration, followed by a decrease at 75 mM and 100 mM NaCl concentration respectively. The inconsistency in mdar gene expression in “in vitro” and “in vivo” salt stress could be due to changes in micro-environments of the seedlings. Under in vivo stress conditions, the seeds were germinated and the seedlings were grown in MS medium supplemented with NaCl, which enhanced the stress exposure of the seedlings, whereas in poly-house grown plants (in vitro stress treatment) the seeds were germinated and the seedlings were first grown under optimal conditions and only later when they have grown to a particular stage, they were subjected to stress. It is well-known that the plants are more prone to stress induced injury at the time of seed germination, as compared to the vegetative growth stages, wherein they develop the capability to combat the imposed stress (Cakmak et al. 1993). Therefore the level of mdar gene expression as well as the protein activity were subject to severe stress consequences in the in vivo stress conditions as compared to the in vitro stress conditions. This could be a prime reason behind the observed differential response of the seedlings to NaCl stress. Moreover, in case of seedlings raised in MS media supplemented with NaCl, the expression of mdar gene and the monodehydroascorbate reductase enzyme activity were observed to be higher when compared to seedlings irrigated with NaCl solution. This clearly indicates that the seedlings have a limited capacity to augment the mdar gene and protein activity under stress, and once the threshold limit is reached, there is limited scope to further augment this activity under continuously increasing external stress. Sultana (2010) have also reported that transgenic rice plants over-expressing mdar showed tolerance up to 100 mM NaCl at germination stage. Several scientists have reported an increase in MDAR activity in Oryza sativa (Vaidyanathan et al. 2003; Hossain et al. 2013) and Vigna radiata (Sumithra et al. 2006) under salt stress. However, Gomez et al. (2007) reported a reduction in the MDAR activity in pea plants grown on high salt concentration. These reports also indirectly support our observations that plants have a limited capacity to augment mdar activity.
In case of UV radiation exposed finger millet seedlings, only a moderate increase in mdar transcript level was recorded up to 120 min of UV-C exposure. However, at 480 min of UV-C exposure, there was a significant increase in mdar expression level. Gao and Zhang (2008) have reported an increased mdar gene expression in Arabidopsis thaliana under UV-B exposure. It was observed that mdar expression was not radically up-regulated by UV-C radiation exposure; rather a small lag period was there from signal perception to gene expression, hence mdar gene can be categorized as delayed-early stress responsive gene. Monodehydroascorbate reductase enzyme activity decreased at 15 min UV exposure and after that it increased up to 120 min followed by reduction in MDAR activity at 480 min. As time period of UV exposure increases, it induces production of ROS and subsequently expression and activity of antioxidant enzyme is up-regulated. However, it is again evident here that as in case of NaCl stress also, at higher magnitude of the imposed stress, there seems to be a lack of coordination between mdar gene expression and protein activity.
Thus the current study, establishes a differential link between mdar gene expression and enzyme activity under oxidative stress that is validated under different types of externally imposed stresses. Alteration in correlation between gene expression and enzyme activities under varying magnitude of oxidative stress has been established. Under mild stress, gene expression and enzyme activity are directly correlated. However, at higher magnitude of the imposed stress, there appears to be a clear lack of coordination between mdar gene expression and enzyme activity. This could have serious consequences for the experiments over-expressing mdar gene for stress tolerance. The pathways involved in cellular response to different abiotic stresses are controlled by a number of highly conserved signalling molecules and transcriptional regulators which eventually govern gene expression. After transcription, various regulatory mechanisms exist at post-transcription, translation and post-translation levels which ultimately influence the level and activity of MDAR enzyme. Under severe stress conditions, plants suffer acute disruption of metabolic activities and also denaturation of proteins leading to a loss in enzyme activity. Therefore, the current experiments clearly validate the differential correlation between mdar gene expression and enzyme activity under different types and magnitude of external stresses.
Acknowledgements
This work is supported by the University Grants Commission, Govt. of India under research award F.41-540/2012 (SR).
Footnotes
Jebi Sudan and Bhawana Negi have contributed equally and are joint first authors.
References
- Asada K. Mechanisms for scavenging reactive molecules generated in chloroplasts under light stress. In: Baker NR, Bower JR, editors. Photoinhibition of photosynthesis. Oxford: Bios Scientific Publishers; 1994. pp. 131–145. [Google Scholar]
- Baek KH, Skinner DZ. Alteration of antioxidant enzyme gene expression during cold acclimation of near-isogenic wheat lines. Plant Sci. 2003;165:1221–1227. doi: 10.1016/S0168-9452(03)00329-7. [DOI] [Google Scholar]
- Bhatt D, Negi M, Sharma P, Saxena SC, Dobriyal AK, Arora S. Responses to drought induced oxidative stress in five finger millet varieties differing in their geographical distribution. Physiol Mol Bio Plants. 2011;17(4):347–353. doi: 10.1007/s12298-011-0084-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borlaug NE. Feeding a world of 10 billion people: the miracle ahead. In Vitro Cell Dev Biol Plant. 2002;8:221–228. doi: 10.1079/IVP2001279. [DOI] [Google Scholar]
- Bradford MM. A rapid and sensitive method for the quantification of microgram quantities of protein utilizing the principle of protein-dye binding. Anal Biochem. 1976;72:248–254. doi: 10.1016/0003-2697(76)90527-3. [DOI] [PubMed] [Google Scholar]
- Cakmak I, Strbac D, Marschner H. Activities of hydrogen peroxide scavenging enzymes in germinated wheat seeds. J Exp Bot. 1993;44:127–132. doi: 10.1093/jxb/44.1.127. [DOI] [Google Scholar]
- Dalton DA, Baird LM, Langeberg L, Taugher CY, Anyan WR., Vance CP, Sarath G (1993) Subcellular localization of oxygen defense enzymes in soybean (Glycine max [L.] merr.) root nodules. Plant Physiol 102:481–489. [DOI] [PMC free article] [PubMed]
- Dionisio-Sese M, Tobita S. Antioxidant responses of rice seedlings to salinity stress. Plant Sci. 1998;135:1–9. doi: 10.1016/S0168-9452(98)00025-9. [DOI] [Google Scholar]
- Gao Q, Zhang L (2008) Ultraviolet-B-induced oxidative stress and antioxidant defense system responses in ascorbate-deficient vtc1 mutants of Arabidopsis thaliana. J Plant Physiol 165:138–148 [DOI] [PubMed]
- Gechev T, Willekens H, Van Montagu M, Inze D, Van Camp W, Toneva V, Minkov I. Different responses of tobacco antioxidant enzymes to light and chilling stress. J Plant Physiol. 2003;160:509–515. doi: 10.1078/0176-1617-00753. [DOI] [PubMed] [Google Scholar]
- Gomez M, Romero-Puertas MC, Corpas FJ, Rodrıguez-Serrano M, Del Rio LA, Sandalio LM. Differential expression and regulation of antioxidative enzymes by cadmium in pea plants. J Plant Physiol. 2007;164:1346–1357. doi: 10.1016/j.jplph.2006.06.018. [DOI] [PubMed] [Google Scholar]
- Hossain MA, Nakanoand Y, Asada K. Monodehydroascorbate reductase in spinach chloroplast and its participation in regeneration of ascorbate for scavenging hydrogen peroxide. Plant Cell Physiol. 1984;25:388–395. [Google Scholar]
- Hossain MA, Ismail MR, Uddin MK, Islam MZ, Ashrafuzzaman M (2013) Efficacy of ascorbate-glutathione cycle for scavenging H2O2 in two contrasting rice genotypes during salinity stress. Aust J Crop Sci 7(12):1801–1808
- Jimenez A, Hernandez JA, Del Rio LA, Sevilla F. Evidence for the presence of the ascorbate-glutathione cycle in mitochondria and peroxisomes of pea leaves. Plant Physiol. 1997;114:275–228. doi: 10.1104/pp.114.1.275. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kubo A, Aono M, Nakajima N, Saji H, Tanaka K, Kondo N (1999) Differential responses in activity of antioxidant enzymes to different environmental stresses in Arabidopsis thaliana. J Plant Res 112:279–290
- Mittova V, Tal M, Volokita M, Guy M (2003) Up-regulation of the leaf mitochondrial and peroxisomal antioxidative systems in response to salt-induced oxidative stress in the wild salt-tolerant tomato species Lycopersicon pennellii. Plant Cell Environ 6:845–856 [DOI] [PubMed]
- Murashige T, Skoog F. A revised medium for rapid growth and bioassays with tobacco tissue cultures. Physiol Plant. 1962;15:473–497. doi: 10.1111/j.1399-3054.1962.tb08052.x. [DOI] [Google Scholar]
- Oidaira H, Sano S, Koshiba T, Ushimaru T. Enhancement of oxidative enzyme activities in chilled rice seedlings. J Plant Physiol. 2000;156:811–913. doi: 10.1016/S0176-1617(00)80254-0. [DOI] [Google Scholar]
- Shamsi HI, Jiang S, Hussain N, Lin X, Jiang L. Coordinate role of ascorbate–glutathione in response to abiotic stresses. In: Anjum NA, Chan M-T, Umar S, editors. Ascorbate-glutathione pathway and stress tolerance in plants. Berlin: Springer; 2010. pp. 323–336. [Google Scholar]
- Sultana S. Towards the development of salt tolerant rice varieties by overexpressing cDNAs from a mangrove plant Acanthus ebracteatus vahl. Dissertation: Universiti Putra Malaysia; 2010. [Google Scholar]
- Sumithra K, Jutur PP, Carmel BD, Reddy AR. Salinity-induced changes in two cultivars of Vigna radiata: responses of antioxidative and proline metabolism. Plant Growth Regul. 2006;50:11–22. doi: 10.1007/s10725-006-9121-7. [DOI] [Google Scholar]
- Vadassery J, Tripathi S, Prasad R, Varma A, Oelmuller R (2009) Monodehydroascorbate reductase 2 and dehydroascorbate reductase 5 are crucial for a mutualistic interaction between Piriformospora indica and Arabidopsis. J Plant Physiol 166:1263–1274 [DOI] [PubMed]
- Vaidyanathan H, Sivakumar P, Chakrabarty R, Thomas G. Scavenging of reactive oxygen species in NaCl-stressed rice (Oryza sativa L.) – differential response in salt-tolerant and sensitive varieties. Plant Sci. 2003;165:1411–1418. doi: 10.1016/j.plantsci.2003.08.005. [DOI] [Google Scholar]