Abstract
Arabidopsis AUXIN INDUCED IN ROOTS (AIR 12) is a predicted to encode a glycosylphosphatidylinositol tail anchored protein. It has been associated with extracellular redox processes, but little is known about its physiological role. An air12 mutant line demonstrated increased germination rates in the presence of a range of abiotic stress factors and hormones, but not in the presence of ABA. Disruption of AIR12 also affected primary and lateral root development and was linked to changes in root catalase activity and superoxide production. We suggest AIR12 is an extracellular constituent linking both hormone and reactive oxygen signaling in plants.
Keywords: Arabidopsis, Auxin, Germination, Root development, Reactive oxygen
Introduction
Auxins are critical regulators of every stage of development including embryonic development, germination, cell differentiation, root and shoot development, vascular tissue patterning, meristem maintenance, and seed dispersal (Weijers et al. 2005; Benjamins and Scheres 2008; Péret et al. 2009; Sorefan et al. 2009; Vanneste and Friml 2009). Though these developmental processes require auxin, they are influenced by other regulators in key areas of development such as seed germination and lateral root development. For example, though seed germination is classically thought to involve antagonism between the gibberellins and ABA, auxin acts synergistically with ABA to repress germination, with both hormones acting through an unknown signaling pathway (Brady et al. 2003). Furthermore, though auxin itself drives root development and forms gradients without the action of other hormones (Grieneisen et al. 2007; Lucas et al. 2008) it requires some degree of ABA signaling for proper initiation of lateral roots, despite the antagonistic action of ABA on this developmental process (Brady et al. 2003).
When examined individually, auxin and ABA have distinct signaling pathways mediated by reactive oxygen species (ROS) (Joo et al. 2005; Li et al. 2006). Less obvious and not experimentally examined is if ABA-auxin signaling cross-talk involves ROS. The present study was completed to examine the effects of auxin, ABA, and ROS on seed germination and lateral root development. The experimental system utilized an uncharacterized gene thought to be involved in lateral root development, Arabidopsis AUXIN INDUCED IN ROOTS12 (AIR12, At3g07390) (Neuteboom et al. 1999). A unique aspect of the current study is the integration of hormonal and reactive oxygen molecules in what appears to be a novel pathway involving extracellular proteins and lateral root development.
Materials and methods
Seed sterilization and growth medium
Arabidopsis air12 (54–4309–1) (Ito et al. 2002) and the parent line (CS8518) (Fedoroff and Smith 1993) were obtained from RIKEN and ABRC, respectively. Seeds were surface sterilized and placed onto the media (A. thaliana), incubated for 3 days at 4 °C in the dark (A. thaliana) and placed in a Conviron 01,114 growth chamber at 23o/18o C with a 16/8 day night photoperiod, respectively. Seed germination and root growth was monitored in response to different abiotic stressors. Standard medium was composed of ½ Murashige and Skoog basal salts (Sigma-Aldrich, St. Louis, MO, USA) supplemented with 1 % sucrose (w/v; Sigma-Aldrich, St. Louis, MO, USA) and either 1.2 % (w/w) or 0.8 % (w/w) phytagar (Caisson Laboratories, North Logan UT, USA). The medium was then autoclaved, followed by the addition of abiotic stressor chemicals or hormones. Media containing abiotic stressors were composed of the control medium and were supplemented with 60 mM mannitol, 30 mM KCl, 40 mM nitrate (composed of 20 mM KNO3, 20 mM NH4NO3; Fisher Scientific, Fair Lawn, NJ, USA), 400 nM α-napthaleneacetic acid (α-NAA; Sigma-Aldrich, St. Louis, MO, USA), 100 nM 2,4-dichlorophenoxyacetic acid (2,4-D; Sigma-Aldrich, St. Louis, MO, USA), 1 mM H2O2 (EMD Chemicals, Gibbstown, NJ, USA), 3.5 % sucrose (w/v), 3.5 μM ± abscisic acid (ABA; Sigma-Aldrich, St. Louis, MO, USA), 5 μM ± ABA, or 3.5 μM ± ABA and 400 nM α-NAA.
Germination and root measurement
Germination and root assays all utilized biological replicates with 10 seeds per replicate per line. Germination of A. thaliana seeds was monitored for eight days beginning one day after placement in the growth chamber. One observation was made per day, 4 h into the “day” portion of the photoperiod. Seeds were examined under a dissecting microscope and were scored as “germinated” once radicle protrusion from the seed could be observed.
After 13 days of growth on media, primary root length, lateral root number, and mean lateral root length were determined. Primary root length was measured under a dissecting microscope with a ruler. Lateral root number was manually counted under a dissecting microscope. Similarly, lateral root length of all seedlings was manually measured under a dissecting microscope using a ruler. Seedlings grown on 400 nM α-NAA, 100 nM 2,4-D, or 3.5 μM ± ABA could not be scored for lateral root number and mean lateral root length because of the high density of roots (α-NAA and 2,4-D) or lack of roots (ABA). Because auxin often causes a loss of primary root identity the longest root was assigned as the primary root and measured.
Arabidopsis catalase activity
Catalase activity of air12, the wild type equivalent and control were analyzed using an Amplex Red Catalase Assay kit (Invitrogen, Carlsbad, CA, USA). Prior to analysis, seeds were sterilized as previously described and plated on control medium, or control medium supplemented with 400 nM a-NAA or 1 mM H2O2. After 13 days, total roots were harvested. Roots were placed in 1.5 mL tubes, weighed, and flash frozen in liquid nitrogen.
The frozen roots were manually ground in 1.5 mL microfuge tubes using a pestle for 30 s, 200 mL sodium phosphate buffer (20 mM, pH 7.4) was added and the roots were ground for a further 30 s and placed at room temperature while the remaining samples were processed. The samples were vortexed for 3 min using a IKA-VIBRAX-VXR automatic vortexer with a Typ VX2E adaptor (Janke and Kunkel, Germany) and centrifuged for 5 min at 14,000 g. The supernatant was placed in a clean tube and 50 μL was used for the catalase assay. The remaining sample was frozen.
The catalase assay was completed according to the product manual using a Victor3v 1420 Multilabel Counter with excitation and emission filters at 530 and 615 nm (Perkin Elmer, Waltham, MA, USA), respectively. For each assay replicate a new standard curve was constructed using the catalase concentrations, 0, 6.25, 250, 500, 1000, 2000 mU mL−1. In total, 3 biological replicates per line were completed. Within each biological replicate, 3 technical replicates were completed. Each replicate used pooled roots from at least 10 seedlings.
ROS staining and microscopy
To visualize ROS accumulation in A. thaliana roots, the fluorescent dyes H2DFFDA (5-(and −6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate; carboxy-H2DFFDA) and Oxyburst green H2HFF (dihydro-2′,4,5,6,7,7′ hexafluoroflurescein)-BSA; both Invitrogen, Carlsbad, CA, USA) were used at 20 μM (in DMSO) and 100 μg mL−1, respectively. Roots were incubated in one of the two dyes for 15 min in the dark, followed by immediate visualization on the previously mentioned confocal microscope for no more than 10 min. Fluorescence was detected using an argon laser with a 488 nm excitation filter and a long pass emission range of wavelengths greater than 505 nm.
Results
Germination and root development is affected by loss of AIR12 function
Homozygous control and air12 seed from were surface sterilized and placed on media containing ½ MS, 1 % sucrose (w/v), or on media composed of ½ MS, 1 % sucrose +60 mM mannitol, + 30 mM KCl, + 40 mM Nitrate (20 mM each of KNO3 and NH4NO3), + 400 nM μ-NAA, + 100 nM 2,4-D, 3.5 μ M ± ABA, 1 mM H2O2, or +3.5 % sucrose (w/v). After 3 days at 4 °C the plates were placed vertically in a growth chamber. Seeds were considered germinated once radicle protrusion was visible under a dissecting microscope. After 13 days the lateral root number was counted and lengths of the primary root and all lateral roots were measured manually using a ruler and dissecting microscope.
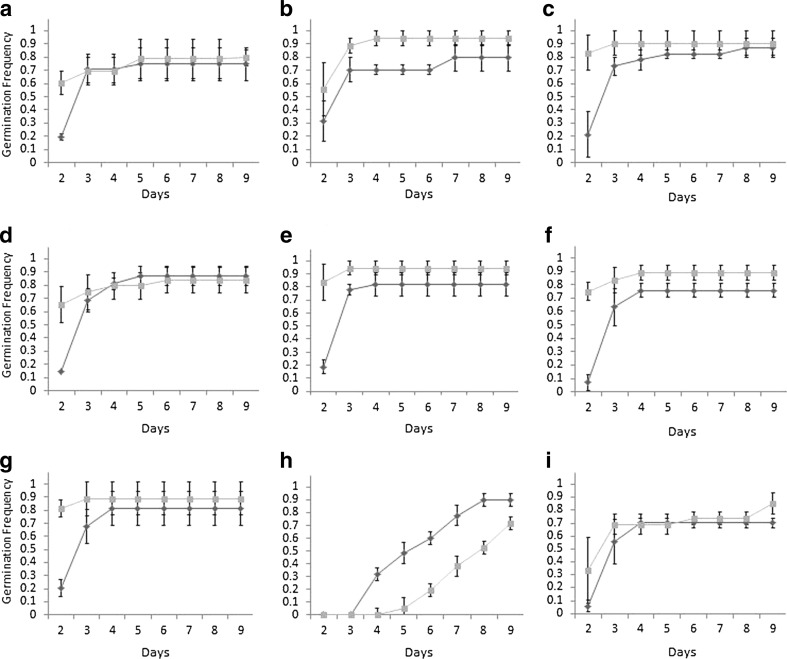
On all media except containing 3.5 μM ± ABA, air12 seeds germinated more rapidly than control. On ½ MS, 1 % sucrose 60 % of air12 seedlings germinated, compared to 20 % of control seedlings (Fig. 1a). By the third day, the majority of the air12 and control seedlings had germinated. On medium supplemented with 60 mM mannitol there was no initial germination difference, though by day 3 nearly 90 % of air12 seedlings were germinated compared to only 60 % of control (Fig. 1b). Germination rate of air12 seedlings on medium supplemented with 30 mM KCl was similar to that of control medium, but more rapid with 80 % germination by day 2 compared to 20 % of control (Fig. 1c). Like the germination rate on standard medium, the majority of control seedlings germinated one day later than air12 and by day 3 the number of germinated control seedlings was equivalent to air12. When medium was supplemented with nitrate, 65 % of air12 seedlings had germinated by day 2 compared with 15 % control seedlings (Fig. 1d). Similar to the trend on other media, by day 3 the number of germinated control seedlings was equivalent to air12. The greatest differences in seed germination occurred on media supplemented with H2O2 (Fig. 1e) or media supplemented with auxin (α-NAA or 2,4-D; Figs. 1f,g). By day 2 on α -NAA, 75 % of air12 seedlings had germinated compared with less than 10 % of control. By day 3, approximately 60 % of control seedlings had germinated, and by day 4, germination reached a maximum for both air12 and control at 90 % and 75 %, respectively. Germination of the majority of air12 seedlings on medium supplemented with 2,4-D occurred by day 2, whereas it took until day 3 for this to occur with the control seedlings (Fig. 1g). In addition, the total number of seedlings germinated on this synthetic auxin for both lines was reduced compared to α -NAA (Fig. 1f). When supplemented with hydrogen peroxide, the seedling germination rate resembled that of control medium with 80 % of air12 seedlings germinated by day 2 compared with 20 % control seedlings (Fig. 1e). Unlike standard medium, total germinated control seedlings did not reach air12 levels until day 4. The addition of a higher concentration of sucrose reduced germination rate of both air12 and control seedlings, but had a greater effect on air12, reducing initial germination rate by almost 50 % (Fig. 1i). On medium supplemented with +3.5 μM ± ABA, the germination frequency was inverted from all other media, with control seeds germinating more rapidly than air12 (Fig. 1h). The germinated control seedlings, however, were incapable of primary root elongation and arrested soon after germination (data not shown).
Fig. 1.
Seed germination on standard media (a) and media supplemented with mannitol (b), KCl (c), nitrate (d), H2O2 (e), α-NAA (f), 2,4-D (g), ABA (h) and 3.5 % sucrose (i). Gray symbols, air 12; black symbols, control. Data presented are the mean of three independent replicates consisting of at least 10 seedlings each. Error bars represent standard error
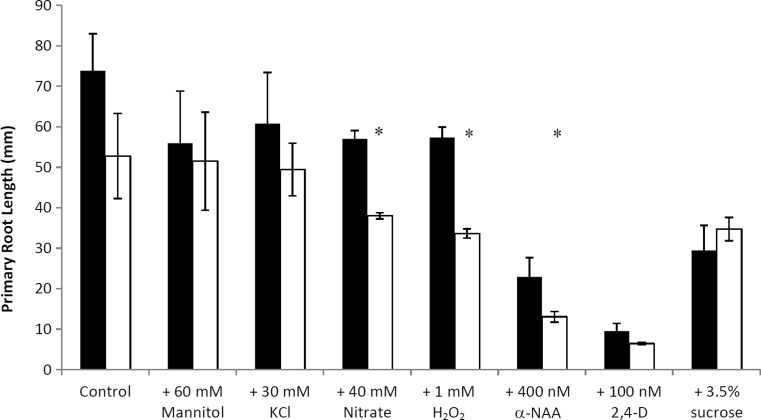
On standard medium no difference between air12 and control mean primary root length was observed (Fig. 2). The same trend was observed in seedlings grown on medium supplemented with mannitol, KCl, H2O2, 2,4-D and 3.5 % sucrose (Fig. 2). However, when supplemented with 40 mM nitrate, 1 mM H2O2 or 400 nM α-NAA air12 primary root length was significantly reduced when compared to that of control seedlings (Fig. 2). Among the treatments 100 nM 2,4-D had the greatest effect on reducing primary root length, but no significant difference was observed between genotypes.
Fig. 2.
Mean primary root length of Arabidopsis control and air12 seedlings grown 13 days on the indicated media. Black bars, control; white bars, air12. n = 3, bars represent standard error. Significant differences (Student’s t test, p < 0.05) between air12 and control plants are indicated with an asterisk
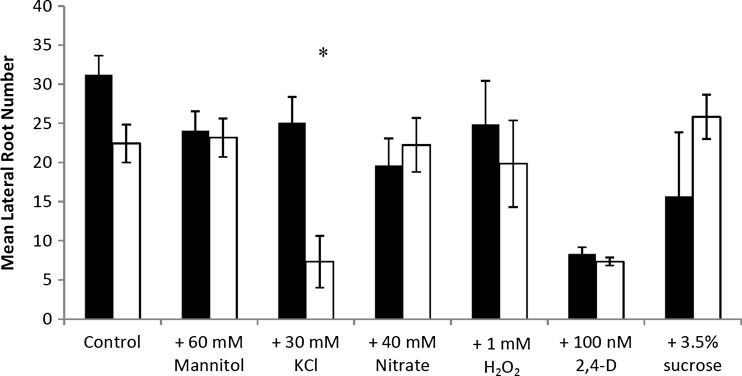
On standard medium a slight, but non-significant, difference in lateral root number decreases was observed in air12 seedlings compared to control. A much larger difference was evident on medium supplemented with KCl, where air12 lateral root number was diminished by 80 % that of control seedlings. No difference in lateral root number was observed between air12 and control seedlings grown on medium supplemented with mannitol, nitrate, H2O2, 2,4-D, or 3.5 % sucrose (Fig. 3).
Fig. 3.
Mean lateral root number of Arabidopsis control and air12 seedlings grown for 13 days on the indicated media. Black bars, control; white bars, air12. n = 3, bars represent standard error. Significant differences (Student’s t test, p < 0.05) between air12 and control plants are indicated with an asterisk
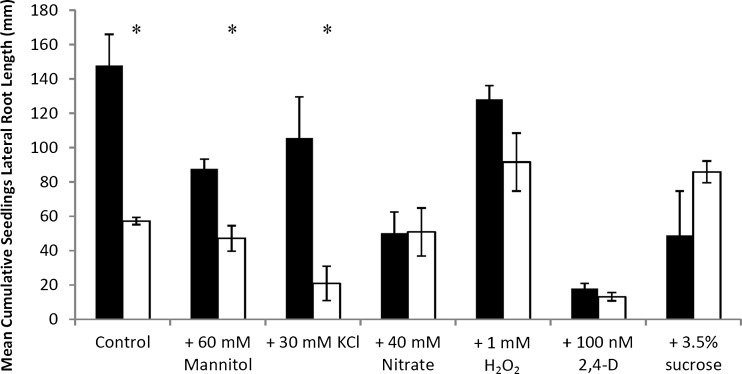
On standard medium, air12 cumulative lateral root length was 60 % shorter than control lateral roots (Fig. 4). Significant differences were also seen when air12 seedlings were grown on mannitol (55 % reduction) and on KCl, where air12 lateral roots were only 20 % the length of the control lateral roots (Fig. 4). Similar to the trend seen with lateral root development, no differences were present between air12 and control lateral root lengths when grown on nitrate, H2O2, 2,4-D, or 3.5 % sucrose (Fig. 4). Interestingly, 40 mM nitrate reduced control lateral root lengths to that of the air12 seedlings.
Fig. 4.
Mean cumulative seedling lateral root length of control and air12 seedlings grown for 13 days on the indicated media. Black bars, control; white bars, air12. n = 3, bars represent standard error. Significant differences (Student’s t test, p < 0.05) between air12 and control plants are indicated with an asterisk
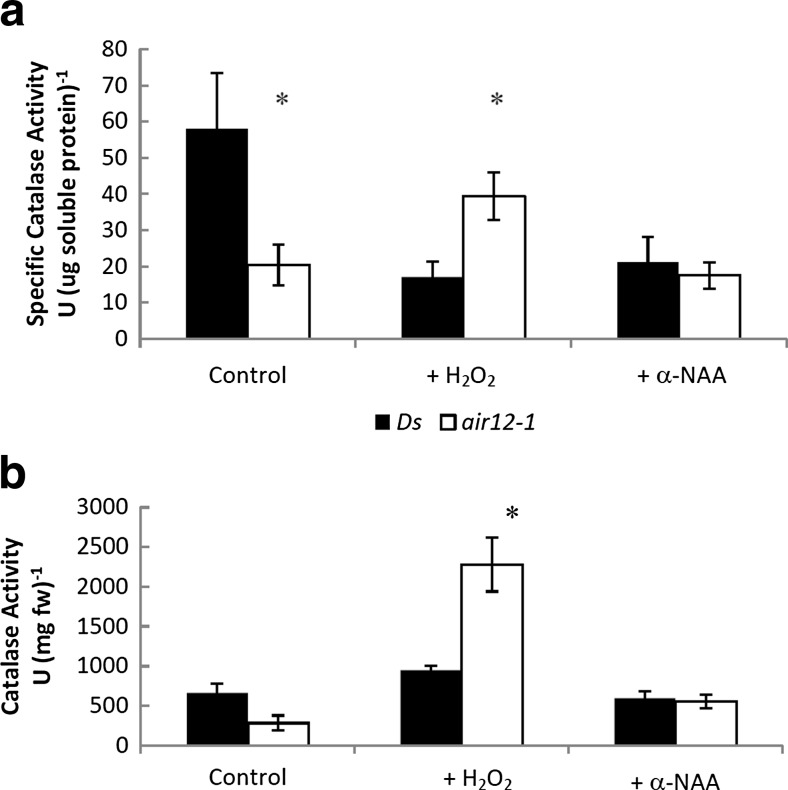
Catalase activity is increased in air12 plants
AIR12 is auxin-induced and is involved in lateral root development. There is a demonstrated role for ROS in this process (Joo et al. 2005) so we assayed catalase activity on standard media and in the presence of H2O2 and α -NAA. When grown on standard medium, catalase specific activity of air12 seedlings was significantly lower than the control line (Fig. 5). This trend was reversed when seedlings were grown on medium supplemented with 1 mM H2O2, with air12 seedlings having greater catalase specific activity. On a fresh weight basis the same pattern was observed. Supplementing medium with 400 nM α-NAA decreased control to air12 levels of catalase activity on both a fresh weight and specific activity basis. No significant differences in H2O2 concentration were observed (data not shown).
Fig. 5.
Catalase activity in control and air12 seedlings. a, Specific catalase activity; b, Catalase activity per mg fresh weight. Black bars, control; white bars, air12. n = 3, bars represent standard error. Significant differences (Student’s t test, p < 0.05) between air12 and control plants are indicated with an asterisk
air12 mutants exhibit differences in intracellular and extracellular superoxide
Because of the differences in catalase activity and previously discussed involvement of ROS in lateral root development we wanted to ascertain the amount of superoxide present in developing roots. We attempted to elucidate the redox status of air12 plants using the superoxide green fluorescent probes H2DFFDA (5-(and-6)-carboxy-2′,7′-difluorodihydrofluorescein diacetate) and Oxyburst (Invitrogen, Carlsbad CA, USA) as described by Monshausen et al. (2007). Oxyburst (dihydro-2′,4,5,6,7,7′-hexafluorofluorescein) and H2DFFDA are fluorinated derivatives of fluorescein. Oxyburst is also conjugated to bovine serum albumin (BSA) to prevent the probe from crossing membranes and thus can be used to determine extracellular superoxide content. H2DFFDA is capable of crossing membranes and can be used to assess intracellular superoxide. Using these probes, a picture of wild type and air12 superoxide content can be constructed.
Vertically grown, 8 day old seedlings were incubated in an Oxyburst or H2DFFDA solution for 15 min, followed by confocal laser-scanning microscopic analysis. To standardize lateral root imaging, all microscopic analysis began at the primary root apex. From the root apex, the root was scanned basipetally (toward root base) until an emerging lateral root was found. The first emerging lateral root was classified as a lateral root primordium that has grown at least one cell width, but not more than 5 cell widths beyond the epidermal cell file of the primary root.
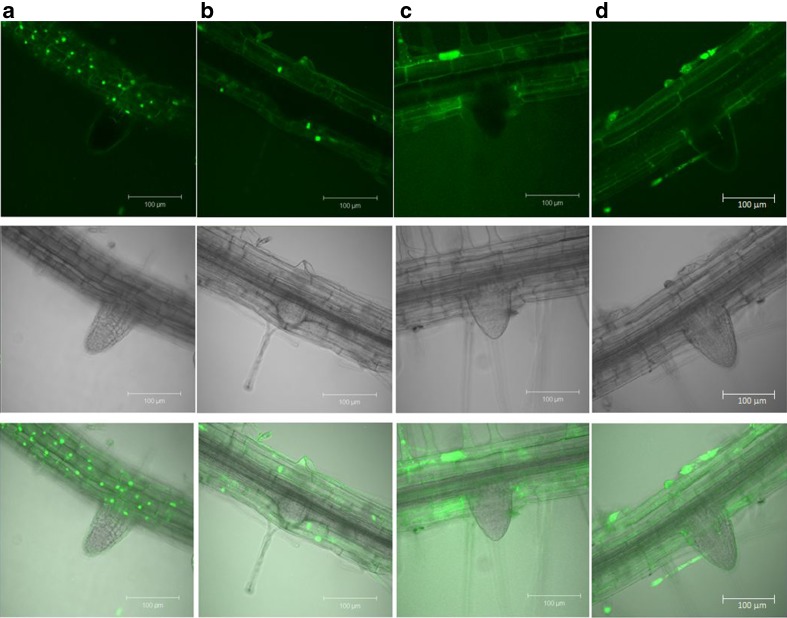
Comparisons of O2− accumulation were made between control and air12 seedlings grown for 8 days on standard medium, medium supplemented with 400 nM α-NAA, or medium supplemented with 1 mM H2O2. On the standard medium, reduced fluorescence was visible in the emerging lateral roots from air12stained with Oxyburst compared to controls (Fig. 6a, b). However no difference was observed in fluorescence intensity between air12 and controls using H2DFFDA (Fig. 6c, d). The punctate staining with Oxyburst was consistent and may be indicative of localized regions of superoxide production.
Fig. 6.
Qualitative analysis of extra- and intracellular superoxide in Arabidopsis using Oxyburst (a, b) and H2DFFDA (c, d) to stain 8-day old control (a, c) and air12 (b, d) seedlings grown on ½ MS, 1 % sucrose (w/v). Intensity of fluorescence is proportional to the amount of superoxide present
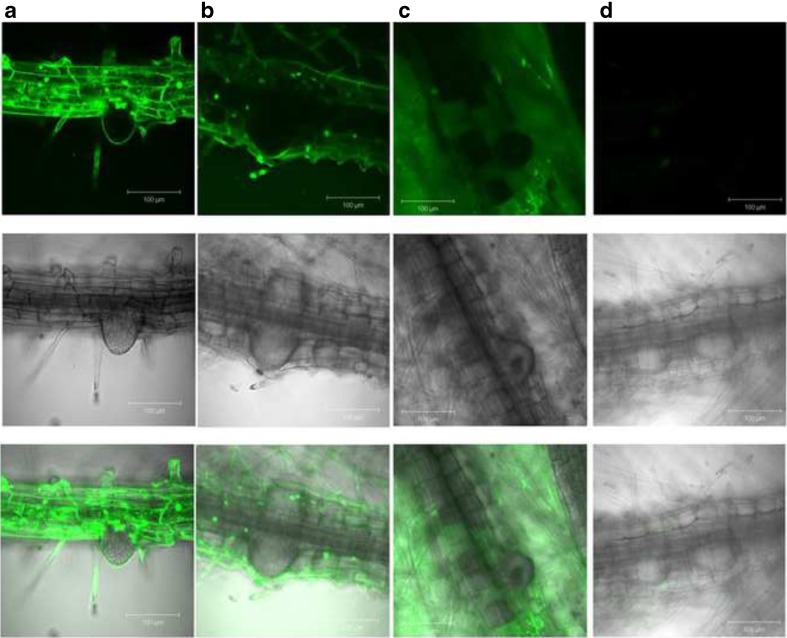
When stained with Oxyburst, fluorescence in air12 seedlings grown on medium supplemented with 400 nM α-NAA is markedly reduced compared to control seedlings (Fig. 7a, b). This pattern of fluorescence in Oxyburst-stained air12 seedlings is indicative of a decrease in extracellular ROS generation. Reduced fluorescence was also observed in air12 seedlings compared to controls after staining with H2DFFDA. (Fig. 7c, d). No differences in fluorescence could be detected when seeds were grown on 1 mM H2O2 using both Oxyburst and H2DFFDA. The intensity of the fluorescent signal was extremely weak in most cases (data not shown).
Fig. 7.
Analysis of extra- and intracellular superoxide in Arabidopsis using Oxyburst (a, b) and H2DFFDA (c, d) to stain 8-day old control (a, c) and air12 (b, d) seedlings grown on ½ MS, 1 % sucrose (w/v) supplemented with 400 nM α-NAA. Intensity of fluorescence is proportional to the amount of superoxide present
Discussion
Germination and root growth
Root development is a dynamic process, integrating plant-wide signals to determine growth in three dimensional space. Initiation and growth of lateral root primordia are stimulated by plant hormones. The present study examined the link between lateral root development Arabidopsis AIR12. Decreases in lateral root development were observed when expression of these genes was eliminated. Similar to the effects of reducing Brassica carinata CIL1, an orthologue of AIR12, root development in response to the hormones auxin and abscisic acid was altered in the mutants compared to control plants (Gibson et al. 2012). The hormone treatments used act through discrete and separate pathways; however, the response to both hormones was attenuated in mutants. A possible intersection point lies with reactive oxygen species, a class of molecules containing unpaired electrons capable of acting as membrane-transmissible signals (Schopfer et al. 2002; Foreman et al. 2003; Liszkay et al. 2004; Li et al. 2005; Lin et al. 2009).
AIR12 and BcCIL1 code for proteins containing a signal peptide and a glycosylphosphatidylinositol (GPI) modification signal. One of the defining features of GPI anchored proteins (GPI-APs) is the potential for activity in two positions: 1) anchored to the extracellular leaflet of the plasma membrane or 2) free extracellular protein (Sherrier et al. 1999; Borner et al. 2002). In addition, these proteins can be cleaved from the anchor by phospholipase C (Borner et al. 2003), a protein often produced following an ABA-mediated abiotic stress response (Hunt et al. 2003). BcCIL1 is released to the apoplast following osmotic shock (Gibson et al. 2012) as is AIR12 (data not shown). The air12 seeds germinated faster than controls on all abiotic stress treatments examined (Fig. 1). On all media except mannitol, control seeds were able to reach air12 germination levels by day 3 indicating sensitivity toward osmotic stress that was not shared by air12 seeds. This indicates that AIR12 is a negative regulator in response to osmotic stressors during seed germination and may act downstream of ABA-mediated GPI anchor cleavage by PLC. Germination and subsequent development under environmental stress reduces seed yield while increasing plant mortality (Wan et al. 2009). Inhibition of germination can allow a seed to remain dormant until stressful conditions have ceased, when it can then complete its lifecycle under more optimal conditions.
Though germination rate was not significantly altered by growth on media containing KCl (Fig. 1), lateral root production was severely reduced in air12 seedlings while control seedlings were largely unaffected (Figures 3 and 4). This suggests AIR12 is a positive regulator of the salt stress response during lateral root development. The response of Arabidopsis seedlings to the treatments examined all utilize signals mediated by ABA to adapt to abiotic stressors (Hodge 2009; Jiang et al. 2009). Furthermore, these stressors initially have a negative effect on lateral root development, indirectly diminishing the plant’s photosynthetic output and fecundity. An abiotic stress is perceived by plants after local accumulation of ABA occurs at the initial site of environmental challenge (Nambara and Marion-Poll 2005). Following accumulation, ABA is perceived by a receptor activating signaling pathways leading to stress response. ABI2, a protein phosphatase 2C involved in ABA signaling (Finkelstein and Somerville 1990), was shown to interact directly with Glutathione Peroxidase 3 (Miao et al. 2006), providing a direct link between ABA signaling and ROS detoxification. When challenged with an osmotic or nitrate stress, perception occurs by ABA followed by redox cascades mediated by ABI2, finally resulting in repression of post-initiation lateral root development. In air12 mutants, the response was attenuated, and no adverse effect on lateral root growth was observed. The decreased lateral root growth seen in control plants may represent different carbon usage strategies: instead of below-ground lateral branching to locate nitrogen and other micronutrients, the plant may invest solely in primary root growth until the high nitrogen region is exhausted relying on root hairs for absorption. Following exhaustion of local nitrogen and micronutrients, the plant would likely invest in lateral growth to find a new source of metabolic building blocks.
Response to salinity stress, unlike osmotic and nitrogen stress, utilize both ABA-dependent and –independent pathways acting through ROS intermediates (Guo et al. 2009; Lim et al. 2010; Mahajan and Tuteja 2005; Zhu 2002). The air12 seedling response to KCl differed than that of either nitrate or mannitol and may proceed through a pathway not mediated by ABA. Involvement of ABA in the salinity response is not a complete binary response; some responses show partial involvement of ABA while others show no response. The SALT OVERLY SENSITIVE (SOS) proteins are Na+/H+ antiporters in the plasma membrane that use NOX-generated extracellular ROS to convey salinity tolerance to plants (Kamei et al. 2005; Chung et al. 2008). Reduced extracellular O2− concentration correlated with increased KCl susceptibility in air12 seedlings.
In the present study, growth of air12 seedlings on standard medium was shown to result in a decrease in lateral root number while leaving the primary root length unaffected, resulting in a significantly lower total lateral root length (Fig. 4). While resembling a phenotype indicative of reduced auxin sensitivity or production superficially, the phenotype observed in air12 seedlings does not match any described auxin mutant. Root development is a process primarily controlled by the plant hormone auxin. The most physiologically active form of auxin in plants, indole-3-acetic acid (IAA), is a membrane impermeable compound requiring active transport to cross the plasma membrane. Normally, when Arabidopsis is grown on auxin, primary root development is greatly reduced while lateral proliferation increases (Casimiro et al. 2001; Christian et al. 2008). air12 plants exhibited reduced sensitivity to α-NAA, relative to wild type. On medium containing 2,4-D however, root growth matched that of wild type seedlings. Despite the appearance of an auxin deficiency, the response of air12 and cil1 seedlings (Gibson et al. 2012) to synthetic auxin did not match any described model, though it is worth noting that these studies utilized synthetic hormones, resulting in a plant response that may differ compared to the response to endogenous hormones.
Involvement of ROS during auxin and ABA-induced lateral growth
A major trade-off of all organisms that utilize oxygen is the potential for harmful radical formation. Although ROS can be detrimental to cells in high concentrations, causing formation of lipid radicals that can compromise the plasma membrane (Halliwell and Gutteridge 1984, 1992), in low concentrations they are used as signaling molecules in such processes as root hair development (Foreman et al. 2003), axillary branching of aerial tissue (Sagi et al. 2004; Sagi and Fluhr,2006)], and guard cell aperture control (Li et al. 2006). Because air12 seedlings displayed moderate insensitivity to both auxin and abscisic acid, signaling molecules downstream of perception were examined as a possible intersection point between the two hormones. In the current study, after growth on media containing 1 mM H2O2, air12 mean lateral root length appeared to elongate to control amounts. This suggests that H2O2 is able to compensate for a deficiency in the air12 mutant, though has little effect on seedlings with functional AIR12. Reactive oxygen species represent an important component of root development, and act as signals following auxin perception regulating processes such as gravitropism in Arabidopsis (Joo et al. 2001, 2005), adventitious root formation in cucumber (Xuan et al. 2008) and cell elongation in maize (Schopfer 2001; Liszkay et al., 2004). The longer lateral roots of air12 seedlings may be a direct effect of H2O2, stimulating an increased rate of cell elongation. Proliferation in lateral root production may stem from weakening the pericycle cell wall polymers, stimulating the development of LR primordia. The Glycine max orthologue of AIR12 and CIL1 was identified as a cytochrome b561 isoform that binds a single heme in vitro and was postulated to interact with members of the plasma membrane redox system (Preger et al. 2009). In mammals, cytochrome b561 is involved with ascorbate regeneration across membranes (Su et al. 2006). In plants, ascorbate plays a role in scavenging ROS generated through photosynthesis pathways (Bowler et al. 1991; Smith and Veitch 1998; Scandalios 2005). The extracellular localization of AIR12 and CIL1 may lead to association with complexes on the plasma membrane. To ascertain if the loss of AIR12 affected ROS levels in air12 mutants, catalase activity and H2O2 concentration were investigated.
In air12 seedlings, neither catalase activity nor H2O2 concentration was affected by growth on medium containing α -NAA. These results run counter to the increased catalase transcript correlated with IAA or 2,4-D treatment of maize coleoptiles found by Guan and Scandalios (2002). However, sensitivity of plant cells to hormones is dependent on their developmental stage and these results may be indicative of reduced sensitivity to auxin-induced catalase expression in 8-day old seedlings. Alternatively, expression of catalase in response to auxin does not guarantee translation of the transcript to a functional enzyme. A third, more likely possibility is that the α-NAA concentration used in this study may have been below a threshold required to enhance catalase transcription. Previous studies used 1 mM IAA or 2,4-D and demonstrated an effect on catalase expression (Guan and Scandalios 2002; Tyburski et al. 2009), however, in planta the concentration of IAA is magnitudes lower, and measured in nM (Bhalerao et al. 2002). It is compelling to note, however, that a large difference was observed between catalase activity of air12 and control seedlings grown on both control medium and medium supplemented with 1 mM H2O2 (Fig. 5). Despite the air12 seedlings' reduced catalase activity compared to control seedlings on standard medium and increased activity on 1 mM H2O2, concentration of in vivo H2O2 was unchanged between the two lines on either treatment (data not shown). This suggests that increased catalase activity is a consequence of elevated H2O2 and that despite exogenous H2O2 application steady state H2O2 levels were maintained.
Plant cells capitalize on the ability of hydrogen peroxide, a relatively stable ROS, to freely diffuse across the plasma membrane, acting as a membrane diffusible signaling molecule (Levine et al. 1994) to control root development (Joo et al. 2001; Guo et al. 2009). Evidence from this work provides additional insights regarding the role of ROS during lateral root patterning and emergence. The question remains, however; how does extracellular AIR12 interact with ROS, auxin, and ABA to affect lateral root development? This question may be answered by examining NADPH oxidase (NOX), a plasma membrane-spanning enzyme complex that utilizes intracellular NADPH to reduce extracellular O2, forming superoxide (O2−) (Sagi and Fluhr 2006). Generation of extracellular O2− signals is mediated by the NOX enzyme complex and represents a method of transmitting intracellular signals to the apoplast of plants. The punctate distribution of fluorescence raises the possibility of localized production, perhaps in lipid rafts. Altered catalase activity observed in air12 mutants may be the result of changes in the rate of O2− production from O2 via NOX. Following extracellular O2− production, it can be detoxified using SOD, resulting in H2O2 which can be used as a membrane-transmissible signal. The catalase enzyme acts on H2O2, converting it into water and oxygen, detoxifying it.
Auxin has a promotive effect on ROS production, stimulating cell elongation in maize coleoptiles (Rodriguez et al. 2002) and cell elongation requires an extracellular pool of ROS (Schopfer et al. 2002). As the major enzyme complex involved in the production of extracellular ROS, NOX may be the central component required for cell elongation. Indeed, elongation of root hairs specifically requires NOX activity, mediated by RHD2 (Foreman et al. 2003) and then spatially regulated by SCN1 (Carol and Dolan 2006). Interestingly, there appeared to be no difference in O2− accumulation on control medium or control medium supplemented with H2O2, suggesting that mutation of air12 affects catalase and NOX activities differently, depending on the environment of the seedling.
Extracellular O2− provided the most pertinent data with respect to the air12 phenotype, as O2− cannot readily cross the plasma membrane in significant amounts (Takahashi and Asada 1983). On control medium and on α -NAA there was greater fluorescence in control seedlings relative to air12 (Figs. 6 and 7). As above, these fluctuations in O2− may reflect regulation of the NOX enzyme complex by α-NAA, and in turn may reflect a reduction in activity due to the mutation of AIR12 or, may be the result of induced changes in ROS scavenging or turnover, though this was not examined. Curiously, supplementing medium with H2O2 did not affect O2− accumulation, suggesting that H2O2 concentration does not regulate NOX activity but instead may have an effect on the cell’s redox state, causing increases in ROS scavenger activity.
Leaves of cil1 plants were characterized by significant increases in NOX and SOD activity while simultaneously containing a reduced amount of O2− relative to control plants (Gibson et al. 2012). The quantified reduction in cil1 O2− concentration supports the qualitatively determined reduced extracellular O2− observed in the air12 mutants. In addition, the NOX and SOD activity data suggest a possible causative agent for the altered O2− patterns observed in mutant plants. Increased NOX activity leads to increased O2− production (Sagi 2006), which in turn, may lead to increased SOD activity. In response to an oxidative stress, conversion from O2− to H2O2 mediated by SOD also increases. In both air12 and cil1 mutants, little difference was observed in H2O2 concentration compared to control plants. However, catalase activity was significantly increased in the mutants. Though NOX and SOD activity were not monitored and O2− concentration not quantitatively determined, it seems reasonable based on phenotypic, localization, and sequence similarity to hypothesize that air12 mutants too exhibit increased NOX and SOD activity. Given the magnitude of increased catalase activity compared to SOD in mutant plants, the total pool of H2O2 may appear similar to wild type while actually indicating a more rapid turnover of H2O2 into H2O and O2. These data may indicate that AIR12 modifies, through an unknown mechanism, the first three enzymatic steps involving the reduction of extracellular O2. Given the involvement of AIR12 with ABA-specific and nonspecific stress responses, and that both ABA, auxin, and abiotic stress responses all utilize ROS, AIR12 may act at an intersection point of the three pathways; possibly at, or close to, the NOX enzyme complex.
Acknowledgments
This work was supported by the Natural Science and Engineering Research Council of Canada’s Discovery Grant Program.
References
- Benjamins R, Scheres B. Auxin: the looping star in plant development. Ann Rev Plant Bio. 2008;59:443–465. doi: 10.1146/annurev.arplant.58.032806.103805. [DOI] [PubMed] [Google Scholar]
- Bhalerao RP, Eklöf J, Ljung K, Marchant A, Bennett M, Sandberg G. Shoot-derived auxin is essential for early lateral root emergence in Arabidopsis seedlings. Plant J. 2002;29:325–332. doi: 10.1046/j.0960-7412.2001.01217.x. [DOI] [PubMed] [Google Scholar]
- Borner GH, Sherrier DJ, Stevens TJ, Arkin IT, Dupree P. Prediction of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A genomic analysis. Plant Physiol. 2002;129:486–499. doi: 10.1104/pp.010884. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Borner GH, Lilley KS, Stevens TJ, Dupree P. Identification of glycosylphosphatidylinositol-anchored proteins in Arabidopsis. A proteomic and genomic analysis. Plant Physiol. 2003;132:568–577. doi: 10.1104/pp.103.021170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bowler C, Slooten L, Vandenbranden S, De Rycke R, Botterman J, Sybesma C, et al. Manganese superoxide dismutase can reduce cellular damage mediated by oxygen radicals in transgenic plants. Embo J. 1991;10:1723–1732. doi: 10.1002/j.1460-2075.1991.tb07696.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brady SM, Sarkar SF, Bonetta D, McCourt P. The abscisic acid insensitive 3 (ABI3) gene is modulated by farnesylation and is involved in auxin signaling and lateral root development in Arabidopsis. Plant J. 2003;34:67–75. doi: 10.1046/j.1365-313X.2003.01707.x. [DOI] [PubMed] [Google Scholar]
- Carol RJ, Dolan L. The role of reactive oxygen species in cell growth: lessons from root hairs. J Exp Bot. 2006;57:1829–1834. doi: 10.1093/jxb/erj201. [DOI] [PubMed] [Google Scholar]
- Casimiro I, Marchant A, Bhalerao RP, Beeckman T, Dhooge S, Swarup R, et al. Auxin transport promotes Arabidopsis lateral root initiation. Plant Cell. 2001;13:843–852. doi: 10.1105/tpc.13.4.843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christian M. W.B. Hannah, H. Luthen, A.M. Jones, identification of auxins by a chemical genomics approach. J Exp Bot. 2008;59:2757–2767. doi: 10.1093/jxb/ern133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung JS, Zhu JK, Bressan RA, Hasegawa PM, Shi H. Reactive oxygen species mediate Na + −induced SOS1 mRNA stability in Arabidopsis. Plant J. 2008;53:554–565. doi: 10.1111/j.1365-313X.2007.03364.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fedoroff NV, Smith DL. A versatile system for detecting transposition in Arabidopsis. Plant J. 1993;3:273–289. doi: 10.1111/j.1365-313X.1993.tb00178.x. [DOI] [PubMed] [Google Scholar]
- Finkelstein R, Somerville C. Three classes of abscisic acid (ABA)–insensitive mutations of Arabidopsis define genes that control overlapping subsets of ABA responses. Plant Physiol. 1990;94:1172–1179. doi: 10.1104/pp.94.3.1172. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Foreman J, Demidchik V, Bothwell JH, Mylona P, Miedema H, Torres MA, et al. Reactive oxygen species produced by NADPH oxidase regulate plant cell growth. Nature. 2003;422:442–446. doi: 10.1038/nature01485. [DOI] [PubMed] [Google Scholar]
- Gibson SW, Conway AJ, Zheng Z, Uchacz TM, Taylor JL, Todd CD. Brassica carinata CIL1 mediates extracellular ROS production during auxin- and ABA-regulated lateral root development. J Plant Biol. 2012;55:361–372. doi: 10.1007/s12374-011-0328-4. [DOI] [Google Scholar]
- Grieneisen VA, Xu J, Maree AF, Hogeweg P, Scheres B. Auxin transport is sufficient to generate a maximum and gradient guiding root growth. Nature. 2007;449:1008–1013. doi: 10.1038/nature06215. [DOI] [PubMed] [Google Scholar]
- Guan LM, Scandalios JG. Catalase gene expression in response to auxin-mediated developmental signals. Physiol Plant. 2002;114:288–295. doi: 10.1034/j.1399-3054.2002.1140215.x. [DOI] [PubMed] [Google Scholar]
- Guo D, Liang J, Li L. Abscisic acid (ABA) inhibition of lateral root formation involves endogenous ABA biosynthesis in Arachis hypogaea L. Plant Growth Regul. 2009;58:173–179. doi: 10.1007/s10725-009-9365-0. [DOI] [Google Scholar]
- Halliwell B, Gutteridge JMC. Oxygen toxicity, oxygen radicals, transition metals and disease. Biochem J. 1984;219:1–14. doi: 10.1042/bj2190001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Halliwell B, Gutteridge JM. Biologically relevant metal ion-dependent hydroxyl radical generation. An Updat FEBS Lett. 1992;307:108–112. doi: 10.1016/0014-5793(92)80911-Y. [DOI] [PubMed] [Google Scholar]
- Hodge A. Root decisions. Plant Cell Environ. 2009;32:628–640. doi: 10.1111/j.1365-3040.2008.01891.x. [DOI] [PubMed] [Google Scholar]
- Hunt L, Mills LN, Pical C, Leckie CP, Aitken FL, Kopka J, et al. Phospholipase C is required for the control of stomatal aperture by ABA. Plant J. 2003;34:47–55. doi: 10.1046/j.1365-313X.2003.01698.x. [DOI] [PubMed] [Google Scholar]
- Ito T, Motohashi R, Kuromori T, Mizukado S, Sakurai T, Kanahara H, et al. A new resource of locally transposed dissociation elements for screening gene-knockout lines in silico on the Arabidopsis genome. Plant Physiol. 2002;129:1695–1699. doi: 10.1104/pp.002774. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jiang WS, Liu DH, Xu P. Cd-induced system of defence in the garlic root meristematic cells. Biol Plant. 2009;53:369–372. doi: 10.1007/s10535-009-0069-0. [DOI] [Google Scholar]
- Joo JH, Bae YS, Lee JS. Role of auxin-induced reactive oxygen species in root gravitropism. Plant Physiol. 2001;126:1055–1060. doi: 10.1104/pp.126.3.1055. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joo JH, Wang S, Chen JG, Jones AM, Fedoroff NV. Different signaling and cell death roles of heterotrimeric G protein alpha and beta subunits in the Arabidopsis oxidative stress response to ozone. Plant Cell. 2005;17:957–970. doi: 10.1105/tpc.104.029603. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kamei A, Seki M, Umezawa T, Ishida J, Satou M, Akiyama K, et al. Analysis of gene expression profiles in Arabidopsis salt overly sensitive mutants sos2-1 and sos3-1. Plant Cell Environ. 2005;28:1267–1275. doi: 10.1111/j.1365-3040.2005.01363.x. [DOI] [Google Scholar]
- Levine A, Tenhaken R, Dixon R, Lamb C. H2O2 from the oxidative burst orchestrates the plant hypersensitive disease resistance response. Cell. 1994;79:583–593. doi: 10.1016/0092-8674(94)90544-4. [DOI] [PubMed] [Google Scholar]
- Li J, Yang H, Ann Peer W, Richter G, Blakeslee J, Bandyopadhyay A, et al. Arabidopsis H + −PPase AVP1 regulates auxin-mediated organ development. Science. 2005;310:121–125. doi: 10.1126/science.1115711. [DOI] [PubMed] [Google Scholar]
- Li S, Assmann SM, Albert R. Predicting essential components of signal transduction networks: a dynamic model of guard cell abscisic acid signaling. PLoS Biol. 2006;4:e312. doi: 10.1371/journal.pbio.0040312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lim PO, Lee IC, Kim J, Kim HJ, Ryu JS, Woo HR, Nam HG. Auxin response factor 2 (ARF2) plays a major role in regulating auxin-mediated leaf longevity. J Exp Bot. 2010;61:1419–1430. doi: 10.1093/jxb/erq010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lin F, Ding H, Wang J, Zhang H, Zhang A, Zhang Y, et al. Positive feedback regulation of maize NADPH oxidase by mitogen-activated protein kinase cascade in abscisic acid signalling. J Exp Bot. 2009;60:3221–3238. doi: 10.1093/jxb/erp157. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liszkay A, van der Zalm E, Schopfer P. Production of reactive oxygen intermediates (O(2)(.-), H(2)O(2), and (.)OH) by maize roots and their role in wall loosening and elongation growth. Plant Physiol. 2004;136:3114–3123. doi: 10.1104/pp.104.044784. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lucas M, Guedon Y, Jay-Allemand C, Godin C, Laplaze L. An auxin transport-based model of root branching in Arabidopsis thaliana. PLoS ONE. 2008;3:e3673. doi: 10.1371/journal.pone.0003673. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mahajan S, Tuteja N. Cold, salinity and drought stresses: an overview. Arch Biochem Biophys. 2005;444:139–158. doi: 10.1016/j.abb.2005.10.018. [DOI] [PubMed] [Google Scholar]
- Miao Y, Lv D, Wang P, Wang X-C, Chen J, Miao C, Song C-P. An Arabidopsis glutathione peroxidase functions as both a redox transducer and a scavenger in abscisic acid and drought stress responses. Plant Cell. 2006;18:2749–2766. doi: 10.1105/tpc.106.044230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monshausen GB, Bibikova TN, Messerli MA, Shi C, Gilroy S. Oscillations in extracellular pH and reactive oxygen species modulate tip growth of Arabidopsis root hairs. Proc Natl Acad Sci U S A. 2007;104:20996–21001. doi: 10.1073/pnas.0708586104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nambara E, Marion-Poll M. Abscisic acid biosynthesis and catabolism. Ann. Rev. Plant Biol. 2005;56:165–185. doi: 10.1146/annurev.arplant.56.032604.144046. [DOI] [PubMed] [Google Scholar]
- Neuteboom LW, Ng JM, Kuyper M, Clijdesdale OR, Hooykaas PJ, van der Zaal BJ. Isolation and characterization of cDNA clones corresponding with mRNAs that accumulate during auxin-induced lateral root formation. Plant Mol Biol. 1999;39:273–287. doi: 10.1023/A:1006104205959. [DOI] [PubMed] [Google Scholar]
- Péret B, De Rybel B, Casimiro I, Benková E, Swarup R, Laplaze L, et al. Arabidopsis lateral root development: an emerging story. Trends Plant Sci. 2009;14:399–408. doi: 10.1016/j.tplants.2009.05.002. [DOI] [PubMed] [Google Scholar]
- Preger V, Tango N, Marchand C, Lemaire SD, Carbonera D. Di Valentin, et al. Auxin-responsive genes AIR12 code for a new family of plasma membrane b-type cytochromes specific to flowering plants. Plant Physiol. 2009;150:606–620. doi: 10.1104/pp.109.139170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodriguez AA, Grunberg KA, Taleisnik EL. Reactive oxygen species in the elongation zone of maize leaves are necessary for leaf extension. Plant Physiol. 2002;129:1627–1632. doi: 10.1104/pp.001222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M. Fluhr, R production of reactive oxygen species by plant NADPH oxidases. Plant Physiol. 2006;141:336–340. doi: 10.1104/pp.106.078089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sagi M, Davydov O, Orazova S, Yesbergenova Z, Ophir R, Stratmann JW, Fluhr R. Plant respiratory burst oxidase homologs impinge on wound responsiveness and development in Lycopersicon esculentum. Plant Cell. 2004;16:616–628. doi: 10.1105/tpc.019398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Scandalios JG. Oxidative stress: molecular perception and transduction of signals triggering antioxidant gene defenses. Braz J Med Biol Res. 2005;38:995–1014. doi: 10.1590/S0100-879X2005000700003. [DOI] [PubMed] [Google Scholar]
- Schopfer P. Hydroxyl radical-induced cell-wall loosening in vitro and in vivo: implications for the control of elongation growth. Plant J. 2001;28:679–688. doi: 10.1046/j.1365-313x.2001.01187.x. [DOI] [PubMed] [Google Scholar]
- Schopfer P, Liszkay A, Bechtold M, Frahry G, Wagner A. Evidence that hydroxyl radicals mediate auxin-induced extension growth. Planta. 2002;214:821–828. doi: 10.1007/s00425-001-0699-8. [DOI] [PubMed] [Google Scholar]
- Sherrier DJ, Prime TA, Dupree P. Glycosylphosphatidylinositol-anchored cell-surface proteins from Arabidopsis. Electrophoresis. 1999;20:2027–2035. doi: 10.1002/(SICI)1522-2683(19990701)20:10<2027::AID-ELPS2027>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Smith AT, Veitch NC. Substrate binding and catalysis in heme peroxidases. Curr Op Chem Biol. 1998;2:269–278. doi: 10.1016/S1367-5931(98)80069-0. [DOI] [PubMed] [Google Scholar]
- Sorefan K, Girin T, Liljegren SJ, Ljung K, Robles P, Galván-Ampudia CS, et al. A regulated auxin minimum is required for seed dispersal in Arabidopsis. Nature; 2009;459:583–586. doi: 10.1038/nature07875. [DOI] [PubMed] [Google Scholar]
- Su D, May JM, Koury MJ, Asard H. Human erythrocyte membranes contain a cytochrome b561 that may be involved in extracellular ascorbate recycling. J. Biol. Chem. 2006;281:39852–39859. doi: 10.1074/jbc.M606543200. [DOI] [PubMed] [Google Scholar]
- Takahashi MA, Asada K. Superoxide anion permeability of phospholipid membranes and chloroplast thylakoids. Arch. Bioch. Biophys. 1983;226:558–566. doi: 10.1016/0003-9861(83)90325-9. [DOI] [PubMed] [Google Scholar]
- Tyburski J, Dunajska K, Mazurek P, Piotrowska B, Tretyn A. Exogenous auxin regulated H2O2 metabolism in roots of tomato (Lycopersicon esculentum mill.) seedlings affecting the expression and activity of CuZn-superoxide dismutase, catalase, and peroxidase. Acta Physiol Plant. 2009;31:249–260. doi: 10.1007/s11738-008-0225-8. [DOI] [Google Scholar]
- Vanneste S, Friml J. Auxin: trigger of change in plant development. Cell. 2009;136:1005–1016. doi: 10.1016/j.cell.2009.03.001. [DOI] [PubMed] [Google Scholar]
- Wan J, Griffiths R, Ying J, McCourt P, Huang Y. Development of drought-tolerant canola (Brassica napus L.) through genetic modulation of ABA-mediated stomatal responses. Crop Sci. 2009;49:1539–1554. doi: 10.2135/cropsci2008.09.0568. [DOI] [Google Scholar]
- Weijers D, Benkova E, Jager KE, Schlereth A, Hamann T, Kientz M, et al. Developmental specificity of auxin response by pairs of ARF and Aux/IAA transcriptional regulators. Embo J. 2005;24:1874–1885. doi: 10.1038/sj.emboj.7600659. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xuan W, Zhu FY, Xu S, Huang BK, Ling TF, Qi JY, et al. Heme oxygenase/carbon monoxide system is involved in auxin-induced cucumber adventitious rooting process. Plant Physiol. 2008;148:881–893. doi: 10.1104/pp.108.125567. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu JK. Salt and drought stress signal transduction in plants. Ann. Rev. Plant Biol. 2002;53:247–273. doi: 10.1146/annurev.arplant.53.091401.143329. [DOI] [PMC free article] [PubMed] [Google Scholar]