Abstract
Jasmonic acid (JA) is a very young candidate of plant growth regulators which is being explored for various antistress properties. Present study deals with the hypothesis that JA can modulate antioxidant mechanism of higher plants with tight regulation of biomembrane peroxidation, making plants tolerant to toxic Ni2+. 2 mM NiCl2 as a source of Ni2+ appeared as sub lethal dose for the growth of 15 days old Glycine max seedlings. Exogenous application of 1 μM and 1 nM JA prior to NiCl2 exposure, made seedlings of Glycine max more tolerant to Ni2+stress as compared to control untreated seedlings. Regulatory inhibition of MDA and H2O2 production by JA with or without Ni2+ treatment made plants more resistant to Ni2+ stress which may be associated with ameliorative activity of antioxidant enzymes system composed of SOD, POD, CAT and APOX. Ascorbate, a secondary metabolite synthesized from D-glucose act as an antioxidant in plant cells. Many fold enhancements in AsA content of Ni2+ treated seedlings supplemented with different concentrations of JA was observed. Significant improvement in AsA levels by JA with or without Ni2+ stress may involve two aspects, either denovo synthesis level regulation of AsA or recycling of AsA from an oxidized form. Improvement in total protein content showed the uplift modulation of transcriptional machinery by JA which was also maintained under Ni2+ stress. Photosynthetic pigments as total chl, chl a and b showed inhibition in presence of Ni2+ stress which was not found much effective under JA supplementation as compared to control. Present findings revealed that although JA was not helpful for protection of photosynthetic pigments but it modulates the other machinery of plants significantly including various antioxidants positively, while tightly inhibiting stress related processes responsible for lipid peroxidation to make plants tolerant to Ni2+ stress.
Keywords: Oxidative stress, SOD, POD, CAT, Chlorophyll
Introduction
Transition heavy metals are non-biodegradable pollutants which persist in the environment. Heavy metals cause toxicity in plants when they accumulate at a higher concentration than their threshold value and are also responsible for deterioration in morphology and physiology to significant levels (Dhankar and Solanki 2011). Ni is one of the heavy metal which is required by the plants in micro concentrations to regulate the activity of various enzymes such as glyoxalases, peptide deformylases, methyl-CoM reductase, ureases, hydrogenases and isoforms of superoxide dismutase (Kupper and Kroneck 2007). Being a micronutrient, Ni is responsible to regulate various metabolic processes by acting as cofactor of the enzymes involved in processes such as ureolysis where ureases enzymes use Ni as a cofactor (Mulrooney and Hausinger 2003). It can stimulate or inhibit enzyme activities in plant tissues depending on accumulated concentration. It has been reported that 50 μM NiCl2 increased the activities of ascorbate oxidase, CAT, glutathione reductase and peroxidase in Oryza sativa. Higher accumulation of Ni in photosynthetic plants cause degradation of photosynthetic apparatus and replace Mg ions present in the porphyrin ring of chlorophyll pigments. This replacement of Mg ions from the porphyrin ring of chlorophyll pigments, diminish chlorophyll content which ultimately decrease the photosynthetic efficiency of the plant (Ahmad et al. 2007; Gajewska et al. 2006). Ni is reported to inhibit the activities of Calvin cycle enzymes including rubisco, glyceraldehyde-3-phosphate dehydrogenase, 3-phsophoglycerate kinase, aldolase, fructose-1,6-bisphosphatase, and NADP- and NAD-dependent phosphoglyceraldehyde dehydrogenase in leaves collected from 30 day old plants of Cajanus cajan (Sheoran et al. 1990), nitrate metabolism by inhibiting nitrate reductase and glutamine synthease in Beta vulgaris (Kevresan et al. 1998) whereas low concentration of 0.2 mM Ni inhibit alanine aminotransferase activity which is responsible for transformation of alanine into pyruvate in Glycine max (El-Shintinawy and El-Ansary 2000).
Most widespread reaction resulted from heavy metal stress is the production of excessive reactive oxygen species (ROS) (Gill and Tuteja 2010). ROS causes oxidative stress in plants to which plants respond by enhancing the defense related enzymes particularly of antioxidant defense machinery. H2O2 is an active oxygen species which perform dual function in plant growth and development. Catalase and ascorbate peroxidase are the enzymes which oxidize H2O2 into H2O and O2. APOX showed higher affinity to H2O2 than catalase and is responsible for tight regulation of H2O2 for balanced growth and development, and protects plants from oxidative damage (Noctor and Foyer 1998). Therefore, it is crucial that plants should maintain the activities of these enzymes in order to accommodate metal induced oxidative damage.
Jasmonates (JAs) belongs to class of polyunsaturated fatty acid derived phytohormones and are available ubiquitously in plants. JAs are the signaling molecules, which activate number of signaling pathways responsible for diverse functions in plants, such as regulation of plant growth and development, induction of biotic and abiotic resistances, responses to various environmental signals and crosstalk with other phytohormones (Cheong and Choi 2003; Creelman and Mullet 1995). Methyl jasmonate is the esterific derivative of JA which mimic as stressor in the plant resulting in induction of senescence related genes (Westermarck and Hause 2002). The first evidence for direct interaction between the Me-JA induced ROS and peroxidases in vivo activity in the roots of sunflower seedlings has been reported by Garrido et al. 2003. Exogenous application of Me-JA ameliorates chilling and water stress in rice seedlings (Lee et al. 1996). However, modulation of enzymatic antioxidant defence system comprising of SOD, GPOX, APOX and CAT have not been studied yet in relation to exogenous application of JA in Glycine max for making crop adaptive to stress. However, Keramat et al. (2009) studied antioxidant defence in Glycine max under Me-JA treatment and found ameliorative effect of Me-JA under Cd stress. Present study was conducted to investigate the role of free JA on oxidative stress management in soybean plants to Ni stress by modulation of antioxidant defense system, if any.
Material and methods
Collection of seeds and experimental setup
Certified seeds of Glycine max L. cv. SL-525 were procured from PAU, Ludhiana, India. Healthy seeds were treated with 0.1 % hypochlorite (v/v) for 5 min and then washed in free flowing tap water. Surface sterilized seeds were primed with two concentrations of JA (1 μM and 1 nM) and DW as control, for 6 h and sowed in plastic cups with/or without NiCl2. 10 ml of 2 mM nickel chloride was given on zero and fourth day in thermocol cup and taking distilled water as control and grown for 15 days under controlled conditions in a plant growth chamber, having 24 °C temperature, 14/12 h dark and light period and a light intensity of 200 PAR with humidity set at 80 %. Present study was conducted to observe the effects of JA in modulating the photosynthetic pigments and regulating the antioxidant enzymes by mitigating the nickel toxicity in soybean plants.
Morphological parameters
The fresh-weight (fw) was measured by blot-drying roots, on an electronic balance (Mettler-Toledo) in milligrams (mg), shoot length and roots in centimeters (cm). All the growth parameters were measured on 15th day after treatments, whereas, the biochemical parameters were measures only after 15 days.
Biochemical analysis
Chlorophyll and carotenoids content was estimated by Lichtenthaler (1987). TBARS content (mmol g−1 fw) was estimated according to Heath and Packer (1968) using ε = 0.155 M g−1 fw for MDA-TBA adduct. The H2O2 (nmol g−1 fw) level was measured as described by Jena and Choudhuri (1981). For estimation of antioxidant enzyme activities, fresh samples of leaves (1 g each) were used following Kumar et al. (2013). Superoxide dismutase (SOD) activity was measured spectrophotometrically at 560 nm following Kono (1978) and presented as U mg−1 protein, where 1 U of SOD activity is the amount of protein required to inhibit 50 % of initial reduction of nitroblue tetrazolium (NBT) under light. Ascorbate peroxidase activity was measured following Nakano and Asada (1981) using ε = 2.8 mM−1cm−1 and the enzyme activity were expressed as μ moles of ascorbate oxidized min −1 mg −1 protein. Catalase activity was measured by method of Aebi (1983) and was expressed as mM min−1 mg−1 protein. Guaiacol peroxidase activity was assayed at 290 nm following Reddy et al. (1995) using ε = 2.8 mM−1cm−1 and expressed as μ moles of guaiacol oxidized min−1 mg−1protein. Protein content was determined according to Lowry et al. (1951) using bovine serum albumin as a standard. The amount of ascorbic acid in the soybean leaves was determined following Mukherjee and Choudhri (1983).
Statistical analysis
All analysis was done on a completely randomized design and the data obtained was subjected to Tukeys multiple comparisons test. Each data was the mean of three replicates (n = 3) and comparisons of p-values < 0.05 were considered significant and different from control.
Results
Effects of JA and Ni on growth parameters
JA treated seeds showed 10 % decrease in rate of seed germination as compared to control while in presence of 2 mM NiCl2 22 % decrease in germination rate was observed. Interestingly seeds grown in presence of 2 mM NiCl2 after treated with 1 μM and 1 nM JA showed 18 and 30 % decrease in germination rate as compared to control. JA treated seedlings showed increased in both root length (3.3 cm, 4.6 cm with 1 μM and 1 nM respectively) and increased shoot length (1.66 cm, 7.33 cm with 1 μM and 1 nM respectively) as compared to control as well as in 2 mM metal treated seedlings (Table 1).
Table 1.
Effect of different levels of JA on growth of Glycine max alone and in combination with 2 mM Ni
| Treatments | Without Ni | With Ni (2 mM) | ||||
|---|---|---|---|---|---|---|
| Germination (%) | RL (cm) | SL (cm) | Germination (%) | RL (cm) | SL (cm) | |
| 0 | 98a ± 0.061 | 7.96a ± 0.19 | 14.02a ± 0.17 | 78a ± 0.060 | 4.9a ± 0.34 | 8.80a ± 0.32 |
| 1 μM | 94a ± 0.080 | 11.33b ± 0.09 | 15.67a ± 0.06 | 91b ± 0.080 | 9.0b ± 0.64 | 15.50b ± 0.76 |
| 1ŋM | 96a ± 0.062 | 12.50b ± 1. | 21.33b ± 0.51 | 90b ± 0.015 | 8.3b ± 0.08 | 18.33b ± 0.08 |
Data are means ± SE of three replicates. Different letters indicate the significance of difference at P ≤ 0.05 levels by Tukeys multiple comparisons test
Photosynthetic pigments
Present results showed detrimental effects of exogenous application of JA on total chlorophyll as well as Chl a and Chl b. Total carotenoids are the accessory pigments of photosynthetic apparatus showed significant accumulation in 15 days old seedlings of G. max (Table 2).
Table 2.
Effect of different level of jasmonic acid and nickel on chlorophyll content (total chl, chl a and chl b), chl a/chl b ratio in Glycine max alone and in combination with 2 mM nickel
| Treatments | Without Ni | With Ni (2 mM) | ||||||
|---|---|---|---|---|---|---|---|---|
| Total chl | chla | chlb | a/b ratio | Total chl | chla | chlb | a/b ratio | |
| 0 | 0.51a ± 0.13 | 0.27a ± 0.10 | 0.25a ± 0.10 | 1.08 | 0.29b ± 0.09 | 0.18b ± 0.07 | 0.11a ± 0.05 | 1.63 |
| 1 μM | 0.32a ± 0.12 | 0.15a ± 0.07 | 0.17a ± 0.06 | 0.88 | 0.38b ± 0.13 | 0.21b ± 0.08 | 0.17b ± 0.09 | 1.23 |
| 1ŋM | 0.33a ± 0.10 | 0.19a ± 0.08 | 0.14a ± 0.07 | 1.35 | 0.21a ± 0.10 | 0.11a ± 0.08 | 0.15b ± 0.07 | 0.73 |
Data are means ± SE of three replicates. Different letters indicate the significance of difference at P ≤ 0.05 levels by Tukeys multiple comparisons test
JA ameliorates the antioxidant enzymes
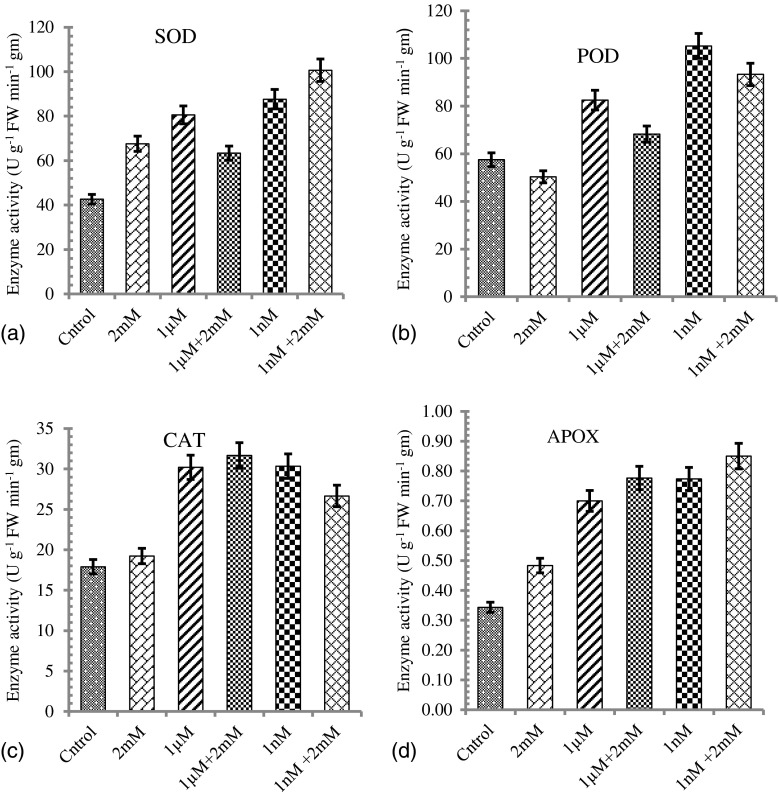
The plants raised under 2 mM Ni stress showed the induction of SOD enzymes to higher level which further ameliorated by exogenous application of JA in presents study. These results revealed that JA application enhanced the activity of SOD and thus scavenge higher amount of free radicals to protect plants from oxidative damage caused due to Ni. JA treated seedlings showed two times higher SOD activity which was 1.5 times in only Ni treated seedlings as compared to control distilled water (Fig. 1a). APOX belongs to peroxidases enzyme family responsible for oxidation of H2O2 and O2 and thus regulating normal growth and developmental functions as well as stress management of plant (Matamoros et al. 2003). APOX activity increases 3 folds as compared to control and metal treated seedlings (Fig. 1d). The seedlings treated with 1 nM JA showed higher activity of APOX which was further enhanced when concentration of JA increased to 1 μM with or without Ni stress. These results confirmed that the JA induced APOX involvement in plant growth and developmental regulation with or without Ni stress. The exogenous JA application increases CAT activity which helps to scavenge H2O2. Under NiCl2 alone CAT activity does not effected significantly as compared to control seedlings but JA increased CAT activity more than 2 folds (Fig. 1c).
Fig. 1.
Effects of Jasmonic acid young Glycine max seedlings cultivated on a control and a metal-polluted soil. Defined young seedlings were collected. Three biological replicates of each line and treatment were performed for measurements of antioxidant enzyme activities. Significant differences of antioxidant enzyme activity were found at *P < 0.05
The activity of POD decreased with Ni metal stress. Jasmonic acid treatments to plants further enhanced the POD activity that was maximum at both 1 μM and 1 nM alone as well as in combination with metal (Fig. 1b). In this study, a significant increase was found in SOD and APOX activities after application of JA in Ni-treated plants.
Protein and AsA contents
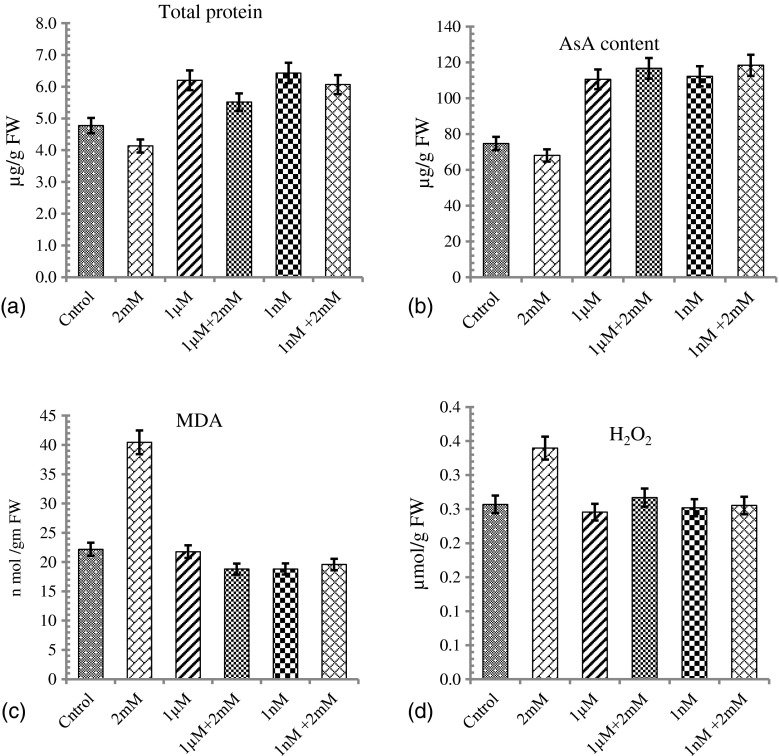
Nickel stress decreased soluble protein content in the seedlings of Glycine max compared with control. Application of Jasmonic acid significantly increased the soluble protein in non-stressed as well as in metal stressed plants (Fig. 2a). Maximum protein and ascorbate contents were observed in plants treated with 1 nM supplemented with JA as compared to control and 2 mM Ni treated plants (Fig. 2b).
Fig. 2.
Effects of Jasmonic acid on (a) total protein content (b) total ascorbate (c) lipid peroxidation (MDA) and (d) H2O2 content in young G.max seedlings cultivated on a control and a metal-polluted soil. Defined young seedlings were collected. Three biological replicates of each line and treatment were performed for measurements of total protein, total ascorbate, MDA and H2O2 content Significant differences were found at *P < 0.05
JA reduces the MDA and H2O2 content
The data regarding lipid peroxidation showed the enhancement of oxidative stress in presence of Ni (Fig. 2c) as compared to control seedlings. These findings suggested that for G. max 2 mM Ni proved to be sub lethal and responsible for degradation of plasma membrane and ultimately growth of plant. Thirty six percent (36 %) decreased in MDA content in presence of 1 nM JA in seedlings confirmed the role of JA in protecting plants due to oxidative damage. In seedlings treated with 1 μM JA decrease in the MDA content was noticed but not to the significant level. H2O2 content was enhanced in Ni treated seedlings to significant level as compared to control untreated seedlings. Presence of JA prior to Ni stress caused deterioration in H2O2 to significant level as compared to only Ni treated plants. Similar reduction in H2O2 content was calculated in JA treated plants as compared to control untreated seedlings (Fig. 2d).
Discussion
Ni is recognized as a trace element and its metabolism is very decisive for certain activities in plants. Present study indicated that 2 mM concentration of NiCl2 as source of Ni found to be toxic for photosynthetic pigments and enhancement of stress markers such as, MDA and H2O2 contents in cell. stress markers such as MDA and H2O2 contents in cell. Toxic level of Ni induced membrane degeneration due to peroxidation of lipids by H2O2 which was noted in terms of MDA content. Disintegration of membranes also includes thylakoid membranes ensued collapse of antenna complexes of photosynthetic machinery which causes decrease in level of photosynthetic pigments. In present study toxic concentration of Ni initiated reduction in photosynthetic pigments 1 in G. max. Our results are in coherence with Alam et al. (2007) in Cajanus with 1 mM and (Gajewska and Sklodowska 2007) in Triticum aestivum with 100 μM Ni. Latif (2010) reported similar results in Raphanus sativus where 200 ppm of NiSO4 as a source of Ni to inhibit photosynthetic pigments. From these results it has been found that Ni toxicity in plants is very much depended on plant species as well as source and concentration of Ni used.
Exogenous application of JA to Ni toxic plants enhanced the Ni tolerance level further but in very much dose dependent manner and best JA concentration for all above said photosynthetic parameters was 1 nM JA. However, the studies on in vitro spinach leaf showed that Ni affects the photosynthetic machinery at various levels and decrease in photosynthetic efficiency of plant under Ni toxicity cannot be attributed to single factor (Keramat et al. 2009). Supplementation of JA prior to Ni treatment in Glycine max plants ameliorate the chlorophyll contents as compared to plants without JA treatment illustrated that it may be JA which inhibit the interference of Ni with other metal ions required by the photosynthetic machinery. However, this confirmation needs more experiments to be done to prove. There is information that shows inhibitory effect of exogenous JA on germination rate of seeds of different plant species. Our results showed that JA ameliorate inhibitory effect of Ni on germination rate while, alone JA found itself to have inhibitory factor for rate of germination (Nojavan-Asghari and Ishizava 1998).
Ni toxicity in plants is associated with oxidative stress (Choudhury and Panda 2004). Our results supported the work of Hao et al. (2006) who reported the increased accumulation of H2O2 induced by plasma membrane based enzyme NADPH oxidase in presence of high Ni concentration. Ni at 2 mM concentration enhanced the production of cellular H2O2 to 36 % as compared to control distilled water raised seedlings in our case. This increase in H2O2 lead to higher disintegration of plasma membrane which was noticed as 87 % higher MDA content was calculated in Ni treated seedlings compared to control distilled water raised seedlings. Presence of JA in plants may be exogenous application or endogenous biosynthesis make plants ore protective from any oxidative damage. There is information that showed that methyl ester of JA (Me-JA) affects the activity and/or pools of stress enzymes to alleviate oxidative stress (Jung 2004). Me-JA mitigated the toxic effects of ROS in soybean under Cd stress by modulating activities of various enzymes involved in antioxidative metabolism (Keramat et al. 2009). Presence of Ni in growth medium of G. max seedlings raised with or without JA enhanced the activity of antioxidant enzymes in our results. These results showed partial coherence with those of Norastehnia and Nojavan-Asghari (2006) who reported mitigation of ROS effect in strawberry under water stress and maize seedlings by paraquat by exogenous application of JA.
In the present work sub-lethal dose of Ni promoted the activities of SOD, CAT and APOX but on the other hand diminish GPOD activity. SOD is the primary enzyme of Asada-Halliwell pathway responsible for dismutation of superoxide radicals produced under elevated level of stress into H2O2 which further hydrolyzed into H2O and O2 by CAT and APOX or various other POD enzymes present in various organelles of the cell. These findings are antagonize to those of Gajewska and Sklodowska (2007) who reported significant decrease in activities of SOD and CAT in presence of 100 μM Ni in wheat leaves.
Different forms of peroxidase enzymes have been identified as APOX, GPOD and POD in different organelles of the cell including cell cytoplasm, chloroplast and mitochondria (Foyer et al., 1997; Asada 1999). In contrast to APOX, POD activity was significantly lowered in Glycine max seedlings grown on metal polluted soil in our experiments. The decline of POD activity in present work after exposure to Ni stress indicates that the scavenging activity of this enzyme is more impaired to Ni toxicity as compared to other scavengers as SOD, CAT and APOX. Supplementation of different concentrations of JA in growing medium considerably enhanced activities of all scavenging enzymes signified the ameliorative properties of JA to decrease the damaging effect of metal stress on growing seedlings. Keramat et al. (2010) reported similar results of ameliorative effect of one form of JA as Me-JA on antioxidant mechanism to repair the damages initiated by ROS under metal stress.
Conclusion
In conclusion our results explored the potential of free form of JA in mitigating Ni induced oxidative stress by triggering the antioxidant defense mechanism of Glycine max consist of SOD, POD, CAT, APOX and AsA, concurrently decreasing MDA and H2O2 content to significant levels. Enhancement of total proteins to significant high levels in presence of different concentrations of JA in seedlings raised with or without Ni stress suggested the role of JA towards Ni tolerance in G. max. Present study revealed the protective role of JA from Ni toxicity in G. max seedlings, which has been achieved by managing the antioxidant machinery and protecting the DNA synthesis of total proteins.
Acknowledgments
This work is financially supported by DST New Delhi, F. No. SR/SO/PS-158/2012 and WOS-A of DST grant No. SR/WOS-A/LS-528/2012. Laboratory facility provided by Head Department of Botany, Punjabi University Patiala. The certified seeds provided by the Head Plant Breeding and Genetics, PAU, Ludhiana are also appreciated.
References
- Aebi H. Catalase. In: Bergmeyer HU, editor. Methods of enzymatic analysis. Weinhan: Verlag Chmie; 1983. pp. 673–684. [Google Scholar]
- Ahmad MSA, Hussain M, Saddiq R, Alvi AK. Mungbean: a nickel indicator, accumulator or excluder. Bull Environ Contam Toxicol. 2007;78:319–324. doi: 10.1007/s00128-007-9182-y. [DOI] [PubMed] [Google Scholar]
- Alam MM, Hayat S, Ali B, Ahmad A (2007) Effect of 28-homobrassinolide treatment on nickel toxicity in Brassica juncea. Photosynthetica 45:139--142
- Asada K. The water-water cycle in chloroplasts: scavenging of active oxygen and dissipation of excess photons. Annu Rev Plant Physiol Plant Mol Biol. 1999;50:601–639. doi: 10.1146/annurev.arplant.50.1.601. [DOI] [PubMed] [Google Scholar]
- Cheong JJ, Choi YD. Methyl jasmonate as a vital substance in plants. Trends Genet. 2003;19:409–413. doi: 10.1016/S0168-9525(03)00138-0. [DOI] [PubMed] [Google Scholar]
- Choudhury S, Panda SK. Role of salicylic acid in regulating cadmium induced oxidative stress in Oryza sativa L. roots. Bulg J Plant Physiol. 2004;30(3–4):95–110. [Google Scholar]
- Creelman RA, Mullet JE. Jasmonic acid distribution and action in plants, regulation during development and response to biotic and abiotic stresses. Proc Natl Acad Sci. 1995;92:4114–4119. doi: 10.1073/pnas.92.10.4114. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dhankar R, Solanki R. Effect of nickel and zinc toxicity on physiological and biochemical parameters in Vigna Mungo (L.) hepper. Int J Pharm Biol Sci. 2011;2:553. [Google Scholar]
- El-Shintinawy F, El-Ansary A. Differential effect of Cd2+ and Ni2+ on amino acid metabolism in soybean seedlings. Biol Plant. 2000;43:79–84. doi: 10.1023/A:1026507114354. [DOI] [Google Scholar]
- Foyer CH, Lopez-Delgado H, Dat JF, Scott IM (1997) Hydrogen peroxide and glutathione-associated mechanisms of acclamatory stress tolerance and signaling. Plant Physiol 100:241--254
- Gajewska E, Sklodowska M. Differential biochemical responces of wheat shoots and roots to nickel stress: antioxidative reactions and proline accumulation. Plant Growth Regul. 2007;54:179–188. doi: 10.1007/s10725-007-9240-9. [DOI] [Google Scholar]
- Gajewska E, Sklodowska M, Slaba M, Mazur J (2006) Effect of nickel on antioxidative enzyme activities and chlorophyll contents in wheat shoot. Biol Plant 50: 653--659
- Garrido I, Espinosa F, Cordoba-Pedregosa MC, Gonzalez-Reyes JA, Alvarez-Tinaut MC. Redox related peroxidative responses evoked by methyl jasmonate in axenically cultured aeroponic sunflower (Helianthus annus L.) seedling roots. Protoplasma. 2003;221:79–91. doi: 10.1007/s00709-002-0073-0. [DOI] [PubMed] [Google Scholar]
- Gill SS, Tuteja N (2010) Reactive oxygen species and antioxidant machinery in abiotic stress tolerance in crop plants. Plant Physiol Biochem 48:909--930 [DOI] [PubMed]
- Hao F, Wang X, Chen J. Involvement of plasma-membrane NADPH oxidase in nickel-induced oxidative stress in roots of wheat seed- lings. Plant Sci. 2006;170:151–158. doi: 10.1016/j.plantsci.2005.08.014. [DOI] [Google Scholar]
- Heath RL, Packer L. Photoperoxidation in isolated chloroplast 1 kinetics and stoichiometry of fatty acid peroxidation. Arch Biochem Biophys. 1968;125:189–198. doi: 10.1016/0003-9861(68)90654-1. [DOI] [PubMed] [Google Scholar]
- Jena S, Choudhuri MA (1981) Glycolate metabolism of three submerged aquatic angiosperms during aging. Aquatic Bot 12:345--354
- Jung S. Effect of chlorophyll reduction in Arabidopsis thaliana by methyl jasmonate or norflurazon on antioxidant systems. J Plant Physiol Biochem. 2004;42:231–255. doi: 10.1016/j.plaphy.2004.01.001. [DOI] [PubMed] [Google Scholar]
- Keramat B, Kalantari KM, Arvin MJ (2010) Effects of methyl jasmonate treatment on alleviation of cadmium damages in soyabean. J Plant Nutr 33:1016--1025
- Keramat B, Manouchehri KK, Java AM. Effects of methyl jasmonate in regulating cadmium induced oxidative stress in soybean plant Glycine max. Afr J Microbiol Res. 2009;3:240–244. [Google Scholar]
- Kevresan S, Petrovi N, Popovi M, Kandrac J. Effect of heavy metals on nitrate and protein metabolism in sugar beet. Biol Plant. 1998;41:235–240. doi: 10.1023/A:1001818714922. [DOI] [Google Scholar]
- Kono Y. Generation of superoxide radical during autoxidation of hydroxylamine and an assay for superoxide dismutase. Arch Biochem Biophys. 1978;186:189–195. doi: 10.1016/0003-9861(78)90479-4. [DOI] [PubMed] [Google Scholar]
- Kumar N, Mallick S, Yadava RN, Singh AP, Sinha S. Coapplication of selenite and phosphate reduces arsenite uptake in hydroponically grown rice seedlings: toxicity and defence mechanism. Ecotoxicol Environ Saf. 2013;91:171–179. doi: 10.1016/j.ecoenv.2013.01.027. [DOI] [PubMed] [Google Scholar]
- Kupper H and Kroneck PMH (2007) Nickel in the Environment and Its Role in the Metabolism of Plants and Cyanobacteria. In: A. Sigel, H. Sigel, R. K. O. Sigel (eds) Metal Ions in Life Sciences, vol 2. John Wiley & Sons, Chichester, UK, p 31--62
- Latif HH (2010) The influence of nickel sulphate on some physiological aspects of two cultivars of Raphanus sativus L. Arch Biol Sci Belgrade 62:683--691
- Lee J, Reeves RD, Brooks RR, Jaffre T. Isolation and identification of a citro-omplex of nickel from nickel-accumulating plants. Phytochemistry. 1996;16:1503–1505. doi: 10.1016/0031-9422(77)84010-7. [DOI] [Google Scholar]
- Lichtenthaler HK. Chlorophylls and carotenoids: pigments of photosynthetic biomembranes. Methods Enzymol. 1987;148:350–382. doi: 10.1016/0076-6879(87)48036-1. [DOI] [Google Scholar]
- Lowry H, Rosebrough NJ, Farr AL, Randall RJ. Protein estimation with folin-phenol reagent. J Biol Chem. 1951;193:265. [PubMed] [Google Scholar]
- Matamoros MA, Dalton DA, Ramos J, Clemente MR, Rubio MC, Becana M (2003) Biochemistry and molecular biology of antioxidants in the rhizobia-legume symbiosis. Plant Physiol 133:499--509 [DOI] [PMC free article] [PubMed]
- Mukherjee SP, Choudhuri MA (1983) Implications of water stree-induced changes in the leaves of indigenous ascorbic acid and hydrogen peroxide in Vigna seedlings. Physiol Plant 58:166--170
- Mulrooney SB, Hausinger RP. Nickel uptake and utilization by microorganisms. FEMS Microbiol Rev. 2003;27:239–261. doi: 10.1016/S0168-6445(03)00042-1. [DOI] [PubMed] [Google Scholar]
- Nakano Y, Asada K. Hydrogen peroxide is scavenged by ascorbate specific peroxidase in spinach chloroplast. Plant Cell Physiol. 1981;22:860–867. [Google Scholar]
- Noctor G, Foyer CH. Ascorbate and glutathione: keeping active oxygen under control. Annu Rev Plant Physiol Plant Mol Biol. 1998;49:249–279. doi: 10.1146/annurev.arplant.49.1.249. [DOI] [PubMed] [Google Scholar]
- Nojavan-Asghari, Ishizava MK (1998) Inhibitory effects of methyl jasmonate on the germination and ethylene production in cocklebur seeds. J Plant Growth Regul 17:13--18
- Norastehnia A, Nojavan-Asghari M. Effect of methyl jasmonate on the enzymatic antioxidant defense system in maize seedling subjected to paraquat. Asian J Plant Sci. 2006;5(1):17–23. doi: 10.3923/ajps.2006.17.23. [DOI] [Google Scholar]
- Reddy KP, Subhani SM, Khan PA, Kumar KB (1995) Effect of light and benzyl adenine and dark treated graving rice (Oryza sativa) leaves changes in peroxidases activity. Plant Cell Physiol 26:987--994
- Sheoran IS, Singal HR, Singh R. Effect of cadmium and nickel on photosynthesis and the enzymes of the photosynthetic carbon reduction cycle in pigeonpea (Cajanus cajan) Photosynth Res. 1990;23:345–351. doi: 10.1007/BF00034865. [DOI] [PubMed] [Google Scholar]
- Westermarck C, Hause B. Jasmonates and octadecanoids: signals in plant stress responses and development. Prog Nucleic Acid Res Mol Biol. 2002;72:165–221. doi: 10.1016/S0079-6603(02)72070-9. [DOI] [PubMed] [Google Scholar]