Abstract
Zinc (Zn) is an important micronutrient for the physiology of plants. It is poorly available to the plants in soil solution. A pot experiment was conducted to evaluate effectiveness of various Zn application methods on key enzyme activities and protein content of two contrasting rice genotypes viz., PD16 (Zn efficient) and NDR359 (Zn inefficient). The treatments were, control (0 mg Zn kg−1 soil), soil application (5 mg Zn kg−1 soil), foliar application (0.5 % ZnSO4 + 0.25 % lime at 30, 60 and 90 days after transplanting), soil (5 mg Zn kg−1 soil) + foliar application of 0.5 % ZnSO4 + 0.25 % lime at 30, 60 and 90 days after transplanting. Among all the methods tested soil+foliar application of Zn fertilizers was found most effective in increasing superoxide dismutase (SOD) and carbonic anhydrase (CA) activities as well as chlorophyll and protein content in both the rice varieties. NDR359, showed higher enzyme activities and more chlorophyll content in leaves than PD16, when Zn was applied either through foliar spray alone or in soil along with foliar application. Regarding the protein content in grains, PD16 showed higher protein content than NDR359, thus showed better translocation of Zn from leaves to grains.
Keywords: Oryza sativa L, Superoxide dismutase, Carbonic anhydrase, Protein content and chlorophyll content
Introduction
Rice (Oryza sativa L.) is an important staple food crops among all the cereals. About 90 % of rice grown and consumed in south and southeast Asia. In some parts of the world consumption of rice is as high as 990 g per person per day (Sharma et al. 2015). Due to polishing, rice grains are deficient for most of the micronutrients especially Zinc (Zn) and iron (Fe). Irrespective of the rice grain, nearly half of the cereal-growing areas of the world are Zn deficient (Cakmak 2002). The most critical conditions are in arid and semiarid regions, where soil organic matter content is low while pH and CaCO3 content in soils are high (Mirzapour and Khoshgoftar 2006). Zinc deficiency affects one third of the world’s population. In developing countries, this problem is more serious, where as much as 50 to 70 % of the daily calorie intake is derived from cereal grains (Cakmak 2008). In India, 47 % of the soils are Zn deficient. Critical limit of a nutrient in soils refers to a level below which the crops will readily respond to its application. This critical limit varies with soil, crops and varieties. Critical limit of Zn for rice was 0.74 ± 0.18 ppm across the soils and in different agro-ecological regions of India (Muthukumararaja and Sriramachandrasekharan 2012).
Regarding the importance of Zn, it is an essential element for all organisms. In its oxidized Zn (II) form, it acts as a catalytic or structural co-factor in a large number of enzymes and regulatory proteins (Maret 2009). Well-known examples of the enzymes in plants that contain Zn as a cofactor include SOD, CA, alcohol dehydrogenase and the structural Zn-finger domains mediating DNA-binding of transcription factors and protein-protein interactions (Sinclair and Kraemer 2012). Zinc also plays an important role in seed development and Zn-deficient plants show delayed maturity (Hansch and Mendel 2009). In higher organisms, it is reported that approximately 3000 proteins contain Zn as a prosthetic group. Zinc ions also act as a neurotransmitter in humans and cells of the salivary gland, prostate gland, immune system and intestine use Zn for signaling (Sharma et al. 2015). Many plant processes are regulated by Zn containing enzymes such as CO2 fixation, maintenance of biological membranes, protein synthesis, auxin synthesis and formation of pollen grains. Micronutrient Zn deficiency can also adversely affect the quality of harvested products, susceptibility of plants against high light, high temperature and fungal infection (Herschfinkel et al. 2007).
Considering the deficiency status of Zn in soil and its importance for both plants and humans, an attempt has been made to evaluate the effects of various methods of Zn application on key enzymes and quality of rice grains.
Materials and methods
The experimental soil was collected from E1 plot of Norman E. Borlaug Crop Research Center, G. B. Pant University of Agriculture and Technology, Pantnagar, India. The soil had sandy loam texture, 7.4 pH and 0.266 dSm−1 E.C., 10.5 g organic C, 0.47 mg DTPA extractable Zn kg−1 soil and 25.3 mg DTPA extractable Fe kg−1 soil. In order to study the translocation pattern of Zn, two contrasting genotypes of rice were used for the experiment viz. Pant Dhan16 (Zn efficient) and NDR359 (Zn inefficient). The efficient and non efficient genotypes were decided by 2 years consecutive field experiments with 20 more genotypes. Seedlings of both rice varieties were raised in plastic trays filled with this soil.
The soil was filled in plastic pots of 4 kg capacity. A basal dose of 22.3 mg N, 11.6 mg P and 18.5 mg K kg−1 soil, using stock solutions of urea, KH2PO4 and KCl, respectively was applied to all the pots. The treatments tested in triplicate were: control (0 mg Zn kg−1 soil), soil application (5 mg Zn kg−1 soil), foliar spray (0.5 % ZnSO4 + 0.25 % lime at 30 (tillering), 60 (panicle initiation) and 90 (grain filling) days after transplanting), soil application (5 mg Zn kg−1 soil) + foliar spray 0.5 % ZnSO4 + 0.25 % lime at 30 (tillering), 60 (panicle initiation) and 90 (grain filling) days after transplanting. After soil application of Zn in the specified treatments, the soil of each pot was thoroughly mixed, flood irrigated and left for 3 days for equilibration. Five seedlings (21 days old) of both rice varieties PD16 and NDR359 were planted in each pot. The solution of fertilizer applied in each foliar spray was 10 ml pot−1.
The statistical design was two factorial completely randomized design and the data analysis was done by analysis of variance (ANOVA) using a software developed by the university. The means were tested for significance at P ≤ 0.05.
Super oxide dismutase (SOD) activity
The SOD activity was estimated in vitro in the freshly harvested leaves as described by Giannopolitis and Ries 1977. The composition of reaction mixture (3.0 ml) was 50 mM phosphate buffer (pH 7.8), 0.1 μM EDTA, 13 mM methionine, 75 μM NBT, 2 μM riboflavin and 100 μl of enzyme extract. Riboflavin was added in the end and the tubes were illuminated with fluorescent tubes of 20 W after proper shaking.. After an incubation period of 15 min the light was switched off and the tubes were kept in dark. Absorbance of the samples was recorded at 560 nm. One unit of SOD activity (U) is defined as the amount of enzyme required to inhibit photoreduction of NBT by 50 % and is expressed as U mg−1 of fresh weight.
Estimation of carbonic anhydrase (CA) activity
The carbonic anhydrase activity (CA activity) was estimated in vitro in the freshly harvested leaves at flowering by using the method described by Rickli et al. 1964. Extraction of the carbonic anhydrase from rice leaf samples was done according to the method described by Everson and Slack 1968. Sufficiently diluted enzyme extract (1 ml) was added to the 0.0025 M veronal buffer (pH 8.2) containing bromothymol blue and then 2 ml of cold saturated CO2 solution was injected by means of a syringe into the veronal buffer. The time duration of the CO2 solution injection to the color change of indicator from blue to yellow was recorded.
Chlorophyll quantification
Chlorophyll was quantified at flowering using the protocol given by Hiscox and Israelstam 1979. Fifty milligrams of fresh leaf sample was taken and chopped into small pieces. Ten milliliters DMSO (Dimethyl sulphoxide) was added in each test tube and thereafter incubated for 3 h at 65 °C in an oven allowing chlorophyll extraction from the leaves. The clear supernatant was taken and absorbance was recorded at the wavelength 663 and 645 nm, using DMSO as a blank.
Quantification of proteins in grains
Extraction of total proteins from rice seeds was carried out according to the method followed by Reza et al. 2005. Rice grains (1 g) were finely powdered and stirred with 5 ml of cold extraction buffer. Add 2 mM of phenylmethanesulfonyl fluoride (PMSF) to it. The mixture was centrifuged at 10,000g for 20 min at 4 °C. Supernatant was collected and stored at −20 °C for further use. Proteins in each plant samples were quantified by Bradford’s method. A standard curve was prepared using stock solutions (1 mg/ml) of bovine serum albumin (BSA). The absorbance of all known standards and unknown samples was recorded at 595 nm in spectrophotometer. Amount of protein in the unknown sample was calculated with the help of standard curve.
Results and discussion
Superoxide dismutase (SOD) activity
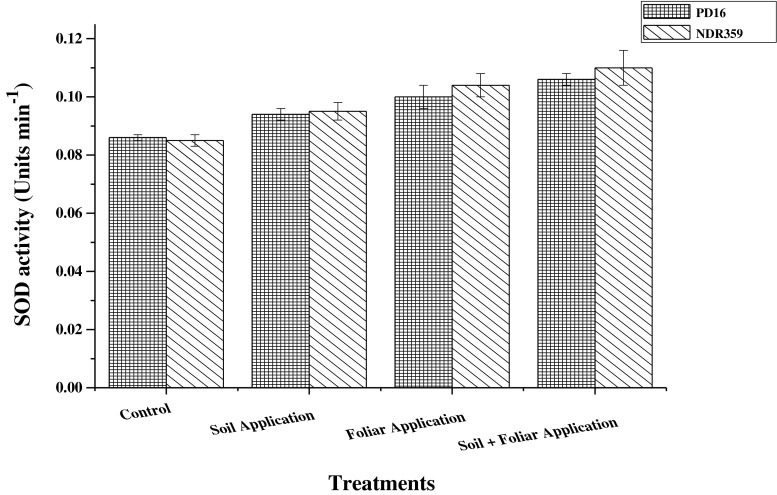
The effect of different methods of zinc application on SOD activity in leaves of both the rice varieties is shown in Fig. 1. Main effect of treatments on SOD activity was found to be statistically significant. Soil application of 5 mg Zn kg−1 soil along with foliar application of 0.5 % zinc sulphate solution was reported to be most effective among all the methods of Zn application and increased the SOD activity by 27.0 % as compared to no application of Zn. Foliar spray of 0.5 % zinc sulphate solution and soil application of 5 mg Zn/kg of soil, individually increased the SOD activity by 20.0 and 10.5 %, respectively, as compared to control. Main effect of varieties was found to be statistically significant and variety NDR359 showed higher enzyme activities as compared to PD16. Influence of interaction effect of variety and treatments on the SOD activity was found statistically nonsignificant.
Fig. 1.
Effect of different methods of zinc application on super oxide dismutase (SOD) activity (Units min−1) in leaves of two contrasting rice genotypes at flowering. Vertical bars indicate ± standard deviation
Superoxide dismutase uses Zn as a cofactor and its activity depend on it. It is most important enzyme in neutralizing reactive oxygen species in chloroplast and protects photosynthetic apparatus from photooxidation. Results of the present investigation clearly showed that soil application of 5 mg Zn kg−1 soil+foliar spray of 0.5 % zinc sulphate solution was most effective for increasing SOD activity in both the rice varieties. It might be due to that Zn efficient genotypes contain higher concentration of physiologically active Zn in their leaves at soil+foliar application on Zn and thus showed higher Cu/Zn-SOD activity. Under Zn deficiency, a reduction in the Cu/Zn SOD activity was recorded and the reduction was more in Zn inefficient wheat genotypes (Cakmak et al. 1999). Total SOD activity in leaf extract of wild type Medicago truncatula increased from 6.7 to 9.2 units SOD mg protein−1 (1.4 fold) when the Zn application was increased from 0 to 3 μM (Millan et al. 2005). In an another study, SOD activity increased with increasing Zn levels in different wheat genotypes and the maximum was found with soil application of Zn along with its foliar spray (Bharti et al. 2013).
Carbonic anhydrase (CA) activity
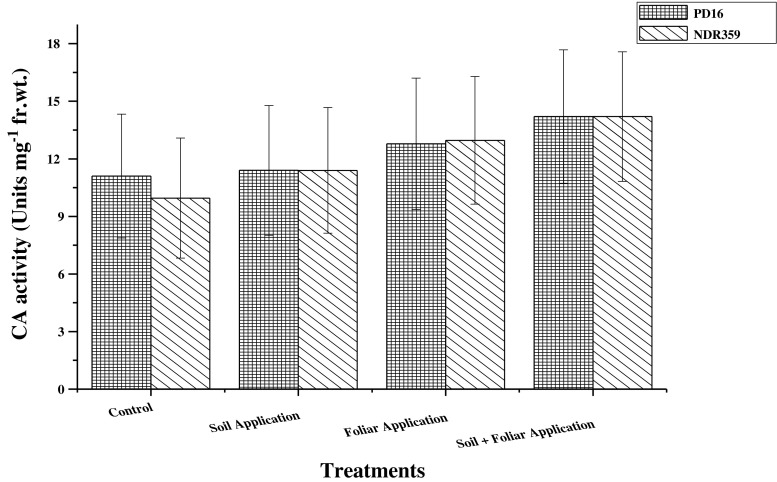
Carbonic anhydrase activity in leaves of both the rice varieties under different methods of Zn application is presented in Fig. 2. It was found to be increased with different Zn regimes; however the effect of treatments was statistically not significant. Among the various methods of Zn applications soil application of 5 mg Zn/kg soil along with foliar application of 0.5 % zinc sulphate solution was found to be most effective and increased the enzyme activity by 34.8 % over control. Application of 0.5 % zinc sulphate solution foliarly and soil application of 5 mg Zn/kg soil increased the activity of CA by 22.2 and 8.2 %, respectively. Main effect of varieties significantly influenced the CA activity and PD16 showed higher activity in comparison to NDR359. Regarding the main effect of interaction between treatments and varieties was found statistically nonsignificant.
Fig. 2.
Effect of different methods of zinc application on carbonic anhydrase (CA) activity (Units mg−1 fr. wt.) in leaves of two contrasting rice genotypes at flowering. Vertical bars indicate ± standard deviation
Another potential Zn containing enzyme is carbonic anhydrase that may be used to estimate physiological availability of Zn. Carbon-di-oxide hydration reaction in C4 plants is catalyzed by this enzyme and Zn is an integral part of this enzyme. Enzyme activity occurs in chloroplasts and cytoplasm which depends on Zn concentration in the plant cell. CA activity increased with increasing Zn levels and the maximum activity was recorded with soil application of 5 mg Zn kg−1 soil along with foliar application of 0.5 % zinc sulphate solution. As combined soil+foliar application of Zn increased the concentration of physiologically active Zn in the leaves, it enhanced the CA activity in leaves. It was earlier reported that activity of CA decreased in many plant species as a consequence of Zn deficiency (Gibson and Leece 1981). A Zn inefficient wheat genotype when grown under Zn deficient conditions showed reduced activity of CA (Hacisalihoglu et al. 2003). Carbonic anhydrase activity in leaf extract of wild type plants of Medicago trancatula increased from 5.2 to 14.7 units CA mg protein−1 s−1 when Zn supply in the nutrient solution was increased from 0 to 5 μM (Millan et al. 2005).
Total chlorophyll content
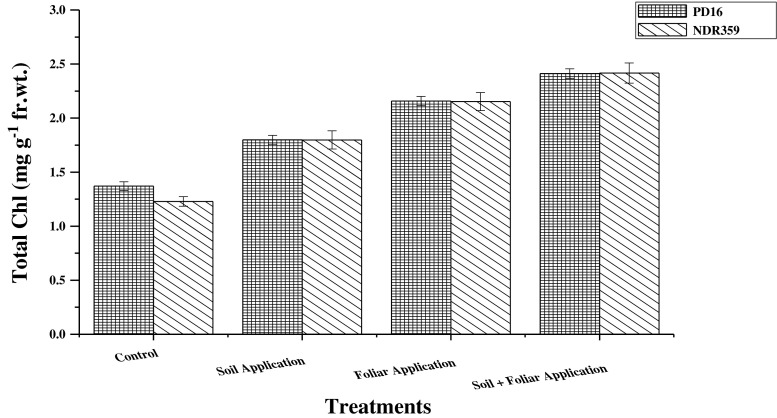
The total chlorophyll content in leaves of both contrasting rice varieties is shown in Fig. 3. Regarding the methods of Zn application the main effect of different treatments on total chlorophyll content was found to be statistically significant. Maximum chlorophyll content was found to be at combined soil application of 5 mg Zn kg−1 soil and foliar spray of 0.5 % zinc sulphate and the increment was recorded 85.3 % in comparison to control (0 Zn application). An increment of 65.3 % in total chlorophyll content was reported by the foliar application of 0.5 % zinc sulphate solution whereas least increment (37.6 %) was found by the soil application of 5 mg Zn/kg soil as compared to without application of Zn. Irrespective of the Zn application methods the main effect of varieties on total chlorophyll content was found to be statistically nonsignificant. However, PD16, a zinc efficient variety contains higher chlorophyll content than NDR359, a zinc inefficient variety. The effect of interaction between variety and treatments on total chlorophyll content in leaves of both the rice varieties was found to be statistically non significant.
Fig. 3.
Effect of different methods of zinc application on total chlorophyll content (mg g−1 fr. wt.) in leaves of two contrasting rice genotypes at flowering. Vertical bars indicate ± standard deviation
Zinc is found to be involved in chlorophyll biosynthesis via its important role in protein expression and carbohydrate metabolism. Photosynthetic rate in maize leaves was reduced under Zn deficiency. Chen et al. 2008, reported a decrease in photosynthetic rate by 42 % in IR8192 (Zn efficient genotype) and by 88 % in Erjiufeng (Zn inefficient variety) under Zn deficient conditions. This reduced photosynthetic rate was due to reduced chlorophyll biosynthesis under Zn deficiency. Bal et al. 2012 also reported more than double chlorophyll content in rice with the application of PGPR as compared to control which increased the availability of Zn in the soil. In rice leaves chlorophyll ‘a’ and chlorophyll ‘b’ content was found to be increased by a factor of 43 and 41 % respectively in response to Zn fertilization as compared to control (Arif et al. 2012).
Protein content
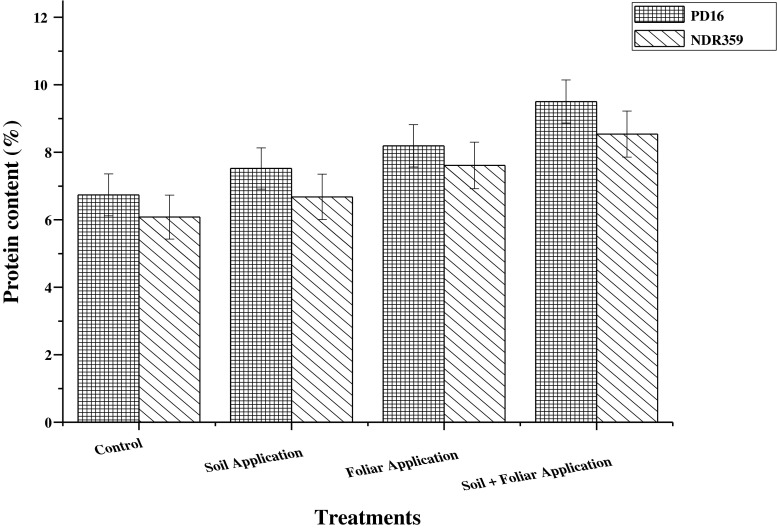
The effect of different methods of Zn application on protein content in grains of both contrasting rice varieties viz. PD16 and NDR359 is shown in Fig. 4. Different methods of Zn application significantly affect the protein content in grains of both the varieties. Among the various methods of Zn application, soil application of 5 mg Zn/kg soil along with foliar spray of 0.5 % zinc sulphate solution was reported most effective in increasing protein content in grains of both the rice varieties and the percent increment was 40.6 as compared to no application of Zn. Foliar application of 0.5 % zinc sulphate solution and soil application of 5 mg Zn/kg soil individually increased the protein content by 23.4 and 10.7 % respectively, in comparison to control. The main effect of varieties significantly influenced the protein content in grains of both rice varieties and the variety PD16, contain higher protein content than NDR359. The main effect of interaction between variety and treatments affects the protein content nonsignificantly.
Fig. 4.
Effect of different methods of zinc application on protein content (%) in grains of two contrasting rice genotypes. Vertical bars indicate ± standard deviation
Results of the study showed that increasing levels of Zn enhanced the protein content in grains of both the varieties and the Zn efficient variety (PD16) showed greater increment in protein content as compared to NDR359, Zn inefficient variety. It might be due to that Zn is a part of many regulatory proteins such as Zn finger motifs that regulate gene expression and hence enhance the synthesis of proteins (Roy et al. 2012). Similar results have been also reported by Morshedi and Farahbakhshb 2010, who showed maximum grain protein content in wheat under 40 kg Zn ha−1 as compared to without application of Zn. Similarly, Morgounov et al. 2007 and Roy et al. 2014 also reported a positive and close relationship between Zn levels and protein concentration in wheat and gram seed respectively.
Conclusion
Such a poor availability of Zn in soil gives an idea to supply Zn fertilizers through foliar spray as well as through both soil+foliar application. Among various methods tested in the study soil along with foliar application of Zn was found most effective in increasing SOD, CA activities and improving nutritional quality of grains. In future the results of present study can be useful in rice improvement programme for the development of stress tolerant genotypes and varieties with high protein content in the grains.
Acknowledgments
The financial assistance provided by NAIP, ICAR, New Delhi, during the period of this study is duly acknowledged.
References
- Arif M, Shehzad MA, Bashir F, Tasneem M, Yasin G, Iqbal M. Boron, zinc and microtone effects on growth, chlorophyll contents and yield attributes in rice (Oryza sativa L.) cultivar. Afr J Biotechnol. 2012;11:10851–10858. [Google Scholar]
- Bal HB, Nayak L, Das S, Adhya TK. Isolation of ACC deaminase producing PGPR from rice rhizosphere and evaluating their plant growth promoting activity under salt stress. Plant Soil. 2012 [Google Scholar]
- Bharti K, Pandey N, Shankhdhar D, Srivastava PC, Shankhdhar SC. Effect of different zinc levels on activity of superoxide dismutases & acid phosphatases and organic acid exudation on wheat genotypes. Physiol Mol Biol Plants. 2013 doi: 10.1007/s12298-013-0201-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cakmak I. Plant nutrition research: priorities to meet human needs for food in sustainable ways. Plant Soil. 2002;247:3–24. doi: 10.1023/A:1021194511492. [DOI] [Google Scholar]
- Cakmak I. Enrichment of cereal grains with zinc: agronomic or genetic biofortification? Plant Soil. 2008;302:1–17. doi: 10.1007/s11104-007-9466-3. [DOI] [Google Scholar]
- Cakmak I, Kalayci M, Ekizc H, Braund HJ, Kilinc Y, Yilmazc A, Lisbeth B, Meyer AS, Rasmussen SK. Zinc deficiency as a practical problem in plants and human nutrition in Turkey: a NATO-science for stability project. Field Crops Res. 1999;60:175–188. doi: 10.1016/S0378-4290(98)00139-7. [DOI] [Google Scholar]
- Chen W, Yang X, He Z, Feng Y, Hu F. Differential changes in photosynthetic capacity, chlorophyll fluorescence and chloroplast ultrastructure between Zn-efficient and Zn-inefficient rice (Oryza sativa L.) genotypes under low zinc stress. Physiol Plant. 2008;132:89–101. doi: 10.1111/j.1399-3054.2007.01013.x. [DOI] [PubMed] [Google Scholar]
- Everson RG, Slack CR. Distribution of carbonic anhydrase in relation to the C4 pathway of photosynthesis. Phytochemistry. 1968;7:581–584. doi: 10.1016/S0031-9422(00)88230-8. [DOI] [Google Scholar]
- Giannopolitis CN, Ries SK. Superoxide dismutases. I. Occurrence in higher plants. Plant Physiol. 1977;59:309–314. doi: 10.1104/pp.59.2.309. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gibson TS, Leece DR. Estimation of physiologically active zinc in maize by biochemical assay. Plant Sci. 1981;146:241–250. [Google Scholar]
- Hacisalihoglu G, Hart JJ, Wang YH, Cakmak I, Kochian LV. Zinc efficiency is correlated with enhanced expression and activity of zinc-requiring enzymes in wheat. Plant Physiol. 2003;131:595–602. doi: 10.1104/pp.011825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hansch R, Mendel RR. Physiological functions of mineral micronutrients (Cu, Zn, Mn, Fe, Ni, Mo, B, Cl) Curr Opin Plant Biol. 2009;12:259–266. doi: 10.1016/j.pbi.2009.05.006. [DOI] [PubMed] [Google Scholar]
- Herschfinkel M, Silverman WF, Sekler I. The zinc sensing receptor, a link between zinc and cell signaling. Mol Med. 2007;13:331–336. doi: 10.2119/2006-00038.Hershfinkel. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiscox JD, Israelstam GF. A method for extraction of chlorophyll from leaf tissues without maceration. Can J Bot. 1979;57:1332–1334. doi: 10.1139/b79-163. [DOI] [Google Scholar]
- Maret W. Molecular aspects of human cellular zinc homeostasis: redox control of zinc potentials and zinc signals. Biometals. 2009;22:149–157. doi: 10.1007/s10534-008-9186-z. [DOI] [PubMed] [Google Scholar]
- Millan AFL, Ellis DR, Grusak MA. Effect of zinc and manganese supply on the activities of superoxide dismutase and carbonic anhydrase in Medicago truncatula wild type and raz mutant plants. Plant Sci. 2005;168:1015–1022. doi: 10.1016/j.plantsci.2004.11.018. [DOI] [Google Scholar]
- Mirzapour MH, Khoshgoftar AH. Zinc application effects on yield and seed oil content of sunflower grown on soil. J Plant Nutr. 2006;29:1719–1727. doi: 10.1080/01904160600897430. [DOI] [Google Scholar]
- Morgounov A, Gomez-Becerra HF, Abugalieva A, Dzhunusova M, Yessimbekova M, Muminzanov H, Zelensky Y, Ozturk L, Cakmak I. Iron and zinc grain density in common wheat grown in Central Asia. Euphytica. 2007;155:193–203. doi: 10.1007/s10681-006-9321-2. [DOI] [Google Scholar]
- Morshedi A, Farahbakhshb H. Effects of potassium and zinc on grain protein contents and yield of two wheat genotypes under soil and water salinity and alkalinity stresses. Plant Ecophysiol. 2010;2:67–72. [Google Scholar]
- Muthukumararaja T, Sriramachandrasekharan MV. Critical limit of zinc for rice soils of veeranam command area, Tamilnadu, India. ARPN J Agric Biol Sci. 2012;7(1):23–34. [Google Scholar]
- Reza MIH, Chowdhury AQ, Pasha MK. Characterization of proteins of brown, bran and endosperm of raw and parboiled rice. Res J Agric Biol Sci. 2005;1(2):184–189. [Google Scholar]
- Rickli EE, Ghazanfar SAS, Gibbson BH, Edsall JT. Carbonic anhydrases from human erythrocytes. J Biol Chem. 1964;239:1065–1078. [PubMed] [Google Scholar]
- Roy S, Duttay S, Khanna K, Singla S, Sundar D. Prediction of DNA-binding specificity in zinc finger proteins. J Biosci. 2012;37(3):483–491. doi: 10.1007/s12038-012-9213-7. [DOI] [PubMed] [Google Scholar]
- Roy PD, Narwal RP, Malik RS, Saha BN, Kumar S. Impact of zinc application methods on green gram (Vigna radiata L.) productivity and grain zinc fertilization. J Environ Biol. 2014;35:851–854. [PubMed] [Google Scholar]
- Sharma A, Babita P, Shankhdhar D, Shankhdhar SC. Evaluation of different PGPR strains for yield enhancement and higher Zn content in different genotypes of rice (Oryza sativa L.) J Plant Nutr. 2015;38(3):456–472. doi: 10.1080/01904167.2014.934475. [DOI] [Google Scholar]
- Sinclair SA, Kraemer U. The zinc homeostasis network of land plants. Biochim Biophys Acta Mol Cell Res. 2012;1823(9):1553–1567. doi: 10.1016/j.bbamcr.2012.05.016. [DOI] [PubMed] [Google Scholar]