Abstract
The type 2 diabetes pandemic in recent decades is a huge global health threat. This pandemic is primarily attributed to the surplus of nutrients and the increased prevalence of obesity worldwide. In contrast, calorie restriction and weight reduction can drastically prevent type 2 diabetes, indicating a central role of nutrient excess in the development of diabetes. Recently, the molecular links between excessive nutrients, organelle stress, and development of metabolic disease have been extensively studied. Specifically, excessive nutrients trigger endoplasmic reticulum stress and increase the production of mitochondrial reactive oxygen species, leading to activation of stress signaling pathway, inflammatory response, lipogenesis, and pancreatic beta-cell death. Autophagy is required for clearance of hepatic lipid clearance, alleviation of pancreatic beta-cell stress, and white adipocyte differentiation. ROS scavengers, chemical chaperones, and autophagy activators have demonstrated promising effects for the treatment of insulin resistance and diabetes in preclinical models. Further results from clinical trials are eagerly awaited.
1. Introduction
Type 2 Diabetes Mellitus and Obesity: The Role of Nutrient Oversupply. Type 2 diabetes mellitus (T2DM) has become a global pandemic with huge health impact in recent decades. T2DM is a chronic progressive disorder characterized by peripheral insulin resistance in skeletal muscle, liver, and adipose tissue and the failure of pancreatic beta-cells to compensate for peripheral insulin resistance. Peripheral insulin resistance usually appears before the onset of hyperglycemia. Attenuated insulin action leads to reduced glucose uptake in skeletal muscle, reduced glucose uptake and increased lipolysis in adipose tissue, and decreased glycogen synthesis and increased glucose output of the liver, resulting in elevated plasma glucose and fatty acid levels [1]. To compensate for peripheral insulin resistance, pancreatic β-cells, which constitute only ~1% of pancreatic mass, have to dramatically increase proinsulin synthesis, imposing heavy biosynthesis burden on β-cells. Ultimately, pancreatic β-cells fail to overcome the resistance and frank hyperglycemia develops.
Obesity is the major driver of insulin resistance and T2DM. Obesity results from chronic imbalance of energy intake in excess of energy expenditure. Large prospective studies showed that lifestyle modification including diet restriction and exercise prevented the progression from prediabetes to diabetes by ~60% [2, 3]. In rhesus monkeys, long-term caloric-restricted diet drastically reduces incident diabetes or prediabetes [4]. These data clearly demonstrate excessive nutrient is critical for the development of obesity, leading to insulin resistance and T2DM.
Molecular Mechanism of Insulin Resistance. The molecular mechanism of insulin resistance is still not fully elucidated. Binding of insulin to insulin receptor triggers tyrosine autophosphorylation of the insulin receptor, which in turn phosphorylates the adaptor proteins insulin receptor substrate (IRS) proteins on tyrosine residues [5]. Tyrosine-phosphorylated IRS proteins recruit phosphoinositide-3-kinase (PI3K), a heterodimer consisting of a regulatory subunit p85 and a tightly associated catalytic subunit p110. Binding of the p85 regulatory subunit to phosphorylated IRS relieves catalytic subunit p110 and initiates a complex of signaling cascades that mediates downstream insulin action.
IRS proteins harbor several serine/threonine phosphorylation sites, which served as negative regulatory nodes that block insulin signaling triggered by tyrosine phosphorylation [6]. Several serine/threonine kinases including the cellular nutrient sensor mammalian target of rapamycin (mTOR) and ribosomal S6 kinase 1 (S6K1), the stress mediators c-Jun NH2-terminal kinases (JNK), and the proinflammatory IκB kinase β (IKKβ) and protein kinase θ (PKC-θ) block insulin signaling by serine-phosphorylation of IRS [6].
2. The Role of Endoplasmic Reticulum Stress and Unfolded Protein Response (UPR) in Diabetes and Obesity
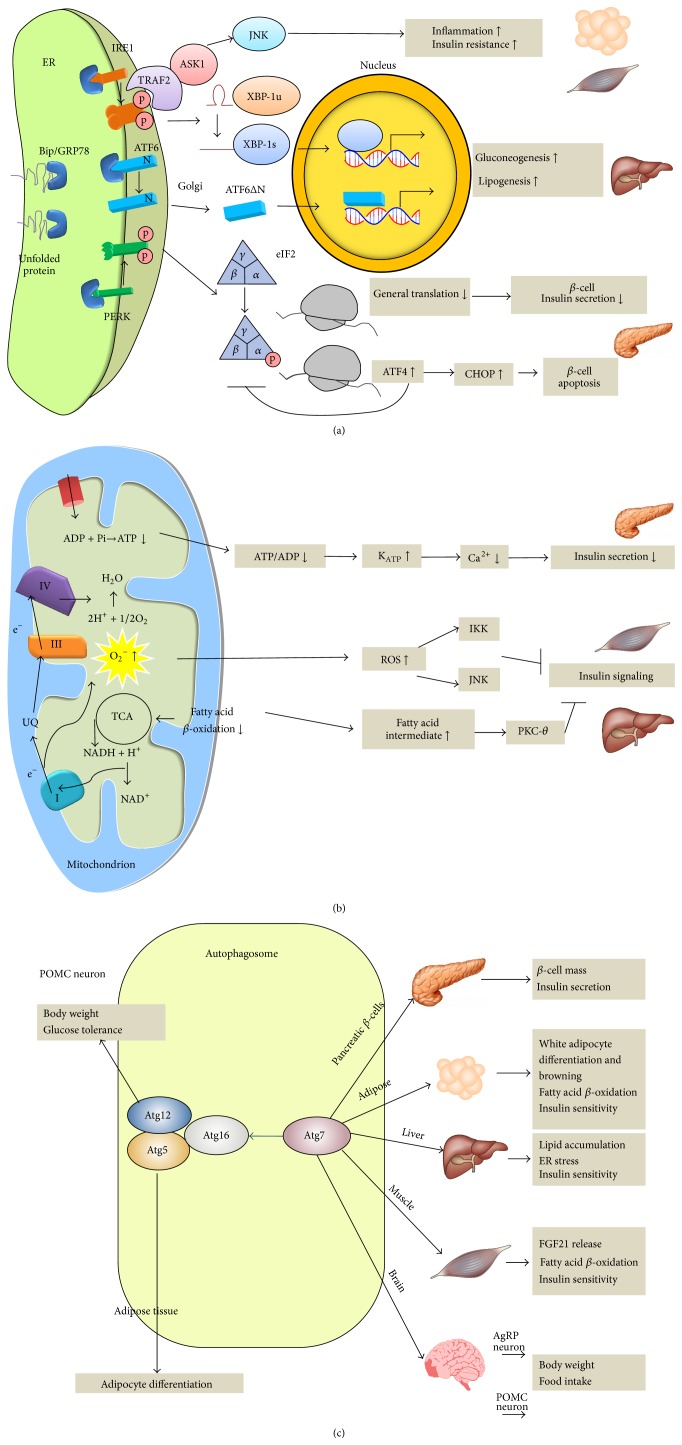
The ER is a specialized organelle essential for synthesis and folding of secreted and ER-resident proteins, maintenance of intracellular calcium homeostasis, and lipid synthesis. The protein concentration in ER lumen is very high. Therefore, increased demand for protein synthesis or accumulation of misfolded protein in the ER luminal causes “ER stress,” which triggers conserved transcriptional and translation programs, termed unfolded protein response (UPR), to cope with the ER stress [7]. The UPR are mediated by three ER membrane-bound mediators including inositol-requiring enzyme-1 (IRE-1), PKR-like endoplasmic reticulum kinase (PERK), and activating transcription factor 6 (ATF6), which are bound by the abundant ER chaperones glucose-regulated protein 78 (GRP78) in unstressed conditions. In stressful conditions when misfolded proteins accumulated, GRP78 chaperones are sequestered by misfolded proteins, releasing these UPR mediators. IRE1, an ancient ribonuclease and the oldest branch of UPR, cleaves 26-bp segment from the mRNA of x-box binding-1 (XBP-1) gene, creating an active/splice form of XBP-1 (XBP-1s). XBP-1s launches transcriptional programs to increase chaperone production, membrane biosynthesis, and gradation of misfolded proteins. Release of ATF6 from ER membrane unmasks its Golgi localization sequence. After processing by two proteases in Golgi, ATF6 is translocated to the nucleus to regulate the expression of genes encoding chaperones, enzymes for protein degradation, and ER membrane biogenesis. The release of PERK form membrane leads to its oligomerization and autophosphorylation. Activated PERK phosphorylates the eukaryotic initiation factor 2α (eIF2α), thereby suppressing general mRNA translation. However, specific mRNAs are preferentially translated when eIF2α is inhibited, including the transcriptional factor ATF4. Two downstream genes of ATF4 are the proapoptotic transcription factor C/EBP homologous protein (CHOP) and the growth arrest and DNA damage–inducible 34 (GADD34) which counteracts PERK's action by dephosphorylating eIF2α, thus promoting translational recovery. Collectively, the UPR relieves ER stress by decreased global protein synthesis, increased degradation of misfolded proteins, promoting chaperone synthesis, expansion of ER membrane volume, and triggering cell death [7].
2.1. Nutrient Excess, ER Stress, and Insulin Signaling
Several lines of evidence in human and mice indicate that chronic nutrient excess causes ER stress [8]. In contrast, ER stress is reduced by weight loss [9, 10]. Genetically manipulated mice models clearly demonstrate that ER stress and UPR influence insulin signaling and glucose homeostasis (Table 1, Figure 1(a)). Xbp1 haploinsufficient mice show abnormal glucose intolerance and impaired insulin signaling in adipose tissue and liver on high-fat diet (HFD) [11]. The increased insulin resistance is mediated, at least in part, through IRE1-dependent activation of JNK. Conversely, hepatic overexpression of Xbp1 lowers glucose in mice through interaction with FoxO1, a key transcriptional factor of gluconeogenesis [12], or uridine diphosphate (UDP) galactose-4-epimerase, an enzyme involved in galactose metabolism [13]. Mice with homozygous mutation at the eIF2α phosphorylation site (Ser51Ala) died at neonatal stage with defective gluconeogenesis [14]. Intriguingly, hepatic overexpression of Gadd34, which encodes an eIF2α-specific phosphatase that selectively counteracts PERK-eIF2α action, results in improved insulin sensitivity and diminished hepatic steatosis on HFD [15]. Hepatic overexpression of Atf6 reduces gluconeogenesis [16] while silencing of hepatic Atf6 increases gluconeogenesis [16]. The effect of ATF6 to suppress gluconeogenesis is mediated by disrupting the interaction between cAMP response element-binding protein (CREB) and transducer of regulated CREB protein 2 (TORC2), thereby decreasing the expression of gluconeogenic genes [16]. In addition, overexpression of chaperone GRP78 alleviates ER stress, restores insulin sensitivity, and resolves fatty liver in obese mice [17]. Similarly, deficiency of ER chaperone ORP150 results in impaired insulin signaling and impaired glucose tolerance, while overexpression of Orp150 improves glucose tolerance and insulin signaling in obese mice [18]. These pieces of evidence strongly support that UPR modulates glucose homeostasis.
Table 1.
Genetically modified mice model linking organelle stress to metabolic diseases.
| Model | Gene function | Tissue | Phenotypes |
|---|---|---|---|
| Xbp1 | UPR | Global haploinsufficiency | Weight gain, glucose intolerance, and insulin resistance on HFD [11] |
| Xbp1 | UPR | Liver-specific KO | Diminished hepatic cholesterol and triglyceride secretion and hepatic lipogenesis [22] |
| Xbp1 | UPR | Liver-specific OE | Reducing serum glucose concentrations and increasing glucose tolerance [12] Fasting and postprandial hypoglycemia; increased hepatic triglyceride content [13] |
| Xbp1 | UPR | β-cell-specific KO | Hyperglycemia and glucose intolerance resulting from decreased insulin secretion [14] |
| Perk | UPR | Mammary epithelium-specific KO | Reduced accumulation of lipid content and the milk produced [23] |
| Perk | UPR | β-cell-specific KO | Hyperglycemia associated with loss of islet and β-cell architecture [29, 30] |
| eIF2α | UPR | Phosphorylation site mutation | Defective gluconeogenesis and deficiency of pancreatic beta-cell [14] |
| Gadd34 | UPR | Liver-specific OE | Lower liver glycogen levels, fasting hypoglycemia, diminished hepatics steatosis [15] |
| Atf6 | UPR | Liver-specific OE/silencing | Increased hepatic glucose output/lowered hepatic glucose output [16] |
| Atf6 | UPR | Global KO | Hepatic steatosis [24] |
| Atf6, eIF2α, Ire1 | UPR | Global KO/phosphorylation site mutation | Hepatic steatosis [25] |
| Chop | UPR | Global KO | Delayed the onset of diabetes and beta-cell apoptosis [32] |
| Grp78 | Chaperone | Liver-specific OE | Reduced hepatic triglyceride and cholesterol contents and improved insulin sensitivity improved [17] |
| Orp150 | Chaperone | Liver-specific OE/Silencing | Improved insulin resistance and ameliorated glucose tolerance/increased insulin resistance [18] |
| Aif | Mitochondrion-localized flavoprotein | Muscle and liver-specific KO | Improved glucose tolerance, reduced fat mass, and increased insulin sensitivity [49] |
| Pgc-1α | Mitochondrial biogenesis | Global KO | Resistance to diet-induced obesity and insulin resistance [50, 51] |
| Tfam | Mitochondrial DNA transcription | Muscle-specific and adipose-specific KO | Improved glucose disposal [52, 53] |
| Tfam | Mitochondrial DNA transcription | β-cell-specific KO | Reduced β-cell mass and insulin secretion [61] |
| Cisd1 | Mitochondrial iron transport | Global and liver-specific OE | Massive expansion of adipose tissue but improved insulin sensitivity [54] |
| Fxn | Assembly of iron-sulfur cluster in mitochondria | β-cell-specific KO | Increased islet oxidative stress, reduced islet mass, and diabetes [62] |
| Atg5 | Autophagy | Adipose-specific KO | Impaired adipocyte differentiation [124] |
| Atg5 | Autophagy | Global OE | Lean, enhanced glucose tolerance, insulin sensitivity, and extended lifespan [125] |
| Atg7 | Autophagy | Global KO | Increased hepatic ER stress and impaired insulin sensitivity [69] |
| Atg7 | Autophagy | β-cell-specific KO | Reduction of β-cells mass, reduced insulin secretion, mitochondria swelling, and lower ATP production [74, 75] |
| Atg7 | Autophagy | Adipose-specific KO | Lean, browning of white adipose tissue, increased fatty acid oxidation, and improved insulin sensitivity [82, 83] |
| Atg7 | Autophagy | Muscle-specific KO | Reduced weight and body fat, enhanced glucose tolerance and insulin sensitivity, enhanced lipolysis and fatty acid oxidation, and increased FGF21 level [85] |
| Atg7 | Autophagy | AgRP neuron-specific KO | Lean with decreased food intake [126] |
| Atg7 | Autophagy | POMC neuron-specific KO | Increased body weight and food intake, impaired glucose tolerance [127, 128] |
| Atg7 | Autophagy | Myf5+ progenitors-specific KO | Impaired brown adipose tissue and skeletal muscle differentiation, browning of white adipose tissue, increased energy expenditure, increased body temperature, impaired glucose tolerance [129] |
| Atg7 | Autophagy | β-cell-specific KO in hIAPP transgenics | Decreased β-cell mass and diabetes [77–79] |
| Atg7 | Autophagy | Global haploinsufficiency in ob/ob mice | Reduces ER stress; improves insulin sensitivity and glucose tolerance ob/ob mice [84] |
| Atg7 | Autophagy | Liver-specific OE in ob/ob mice | Improved insulin sensitivity and glucose tolerance [69] |
| Atg12 | Autophagy | POMC neuron-specific KO | Weight gain, adiposity, and impaired glucose tolerance under HFD [130] |
KO: knockout; OE: overexpression; UPR: unfolded protein response; HFD: high-fat diet; AgRP: agouti-related peptide; POMC: proopiomelanocortin; hIAPP: human islet amyloid polypeptide.
Figure 1.
(a) Endoplasmic reticulum (ER) stress response and unfolded protein response (UPR) are linked to insulin resistance, inflammation lipogenesis, and pancreatic beta-cell survival. (b) Defective mitochondrial function leads to inflammation, insulin resistance, and reduced insulin secretion. (c) Autophagy regulates hepatic lipogenesis, adipocyte physiology, pancreatic beta-cell function, and appetite control. UPR: unfolded protein response; ROS: reactive oxygen species; NAD: nicotinamide adenine dinucleotide; NADH: reduced nicotinamide adenine dinucleotide; ADP: adenosine diphosphate; ATP: adenosine triphosphate; TCA: tricarboxylic acid cycle; KATP: ATP-dependent potassium channel; UQ: ubiquinol; FGF21: fibroblast growth factor-21; AgRP: agouti-related peptide; POMC: proopiomelanocortin.
Mechanistically, all three canonical branches of UPR have been shown to promote inflammatory pathways. The activated IRE-1 recruits the tumor necrosis factor receptor associated factor 2 (TRAF2) and the apoptosis signal-regulating kinase 1 (ASK1) to the ER membrane, thereby activating JNK [19]. The PERK signaling has been shown to inhibit the translation of IKKβ, the main negative regulator of NF-κB, through phosphorylation of eIF2α [20]. ATF6 has also been shown to activate the NF-κB pathway [21]. Both NF-κB and JNK pathways are critical mediators of inflammatory response that impairs insulin signaling by serine phosphorylation of IRS1.
2.2. ER Stress and Lipid Synthesis
In addition to glucose homeostasis, the three UPR branches also regulate lipid synthesis (Table 1, Figure 1(a)). Selective deletion of Xbp-1s in the liver resulted in marked diminished hepatic cholesterol and triglyceride secretion and hepatic lipogenesis by downregulating genes involved in fatty acid synthesis [22], whereas liver-specific overexpression of Xbp-1s increases hepatic triglycerides content [13]. Targeted deletion of Perk in mammary gland inhibits lipogenic enzymes expression, resulting in reduced lipid content and milk production [23]. Atf6 knockout mice developed hepatic steatosis upon ER stress through regulation of genes involved in lipogenesis [24]. Similar phenotypes were observed in liver-specific Ire1-knockout mice and eIF2α loss-of-function mutation [25].
2.3. ER Stress and Insulin Secretion
Pancreas is exocrine and endocrine organ with heavy protein synthesis load. A transgenic green fluorescent mouse model for dynamic monitoring of ER stress detects significant ER stress signal (Xbp1 mRNA splicing) in the pancreas 16 days after birth [26]. Several lines of evidence showed that UPR affect pancreatic islet survival and function (Table 1, Figure 1(a)). For example, mice with β-cell-specific deletion of Xbp-1 displayed hyperglycemia and glucose intolerance resulting from decreased insulin secretion [27]. Translation attenuation through eIF2α phosphorylation prevents the oxidative stress and maintains the differentiated state of β-cells [28]. Preventing eIF2α phosphorylation in β-cells also causes hyperglycemia, indicating a significant role in PERK-eIF2α for islet survival [14]. Perk-deficient mice develop severe hyperglycemia due to reduced islet mass [29, 30]. In human, a loss-of-function mutation in Perk causes a heritable form of juvenile diabetes (the Wolcott-Rallison syndrome) (Table 2), characterized by severe defects in pancreatic β-cells [31]. Furthermore, loss of CHOP, a downstream proapoptotic transcription factor of PERK-eIF2α arm, protects islets from apoptosis in the diabetic mice [32]. Hence, the two major pathological features of type 2 diabetes including peripheral insulin resistance and defective insulin secretion are both affected by ER stress and UPR.
Table 2.
Human hereditary syndrome linking organelle stress and diabetes mellitus.
| Disease | Gene | Function | Phenotypes |
|---|---|---|---|
| Wolcott-Rallison syndrome | PERK | UPR | Neonatal or early-infancy diabetes, epiphyseal dysplasia, osteoporosis, and growth retardation [31] |
| Wolfram syndrome | WFS1 | Negative regulator of UPR | Neurological dysfunctions and diabetes [131] |
| Friedreich's ataxia | FXN | Assembly of iron-sulfur cluster in mitochondria | Ataxia, cardiac dysfunction, and diabetes [63] |
| Kearns-Sayre syndrome | Large deletion of mitochondrial DNA | Respiratory chain | Ataxia, weakness, ptosis, pigmentary retinopathy, and diabetes [58] |
| MELAS (Mitochondrial encephalomyopathy, lactic acidosis, and stroke-like episodes) | Mitochondrial tRNA | tRNA | Seizure, ataxia, hemiparesis, cortical blindness, diabetes, and short stature [58] |
3. The Role of Mitochondrial Dysfunction in Diabetes and Obesity
3.1. Mitochondrial Dysfunction and Insulin Resistance
Mitochondrion is a specialized organelle where tricarboxylic acid cycle, oxidative phosphorylation, and fatty acid β-oxidation occur. Reduced mitochondrial phosphorylation and fatty acid β-oxidation are consistently observed in skeletal muscle and liver of insulin-resistant human [33–35]. Furthermore, expression of genes involved in mitochondrial oxidative phosphorylation is coordinately reduced in insulin-resistant or type 2 diabetic subjects [36, 37]. Therefore, it is long hypothesized that, in the presence of excessive nutrient flux, defective mitochondria lead to increased superoxide production and fatty acid accumulation in skeletal muscle and liver, leading to insulin resistance.
In support of these findings, HFD has been shown to increase mitochondrial reactive oxygen species (ROS) emission and shift the cellular environment to oxidized state in muscle in mice and human [38–40]. Mitochondrion-targeted overexpression of catalase reduces mitochondrial ROS emission and prevents diet-induced insulin resistance in mice [38]. ROS has been shown to activate the proinflammatory JNK and through modulation of cysteine residue or IKKβ [41–43], which in turn impairs insulin signaling via serine phosphorylation of IRS-1 (Figure 1(b)).
In addition to ROS, defective mitochondrial fatty acid β-oxidation leads to accumulation of triglycerides and fatty acids intermediates (e.g., diacylglycerol or ceramide) that activate PKC-θ, a serine/threonine kinase, thus attenuating insulin signaling [44, 45] (Figure 1(b)). Knockout of acetyl-CoA carboxylase 2 (Acc2), an enzyme generating malonyl-CoA which is a strong inhibitor of fatty acid oxidation, resulted in increased fatty acid oxidation, reduced adiposity, and improved insulin sensitivity [46]. Fat infusion increases fatty acids intermediates accumulation in muscle and induces insulin resistance in humans [47]. In contrast, pharmacological inhibition of ceramide (a fatty acid intermediate) production prevented fat-induced insulin resistance in mice and human [48] (Figure 1(b)).
However, whether the observed reduced mitochondrial function in insulin-resistant human is causative or compensatory for the development of insulin resistance is not certain in experimental mice model. Muscle- or liver-specific deletion of Aif, a mitochondrial protein essential for respiratory chain function, leads to decreased mitochondrial oxidative phosphorylation but improves insulin sensitivity [49]. Knockout of peroxisome proliferator-activated receptor-gamma coactivator 1-alpha (Pgc1α), a master regulator of mitochondrial biogenesis, resulted in decreased mitochondrial oxidative phosphorylation but protection from diet-induced obesity and insulin resistance in mice [50, 51]. Similarly, muscle- or adipose-specific knockout of the transcription factor A, mitochondria (Tfam), a key transcription factor for mitochondrial DNA transcription, causes abnormal mitochondrial morphology and function but improved glucose disposal [52, 53]. Furthermore, lower rate of fatty acid beta-oxidation and compromised mitochondrial oxidative phosphorylation caused by overexpression of the CDGSH iron sulfur domain 1 protein (Cisd1), which encodes an outer mitochondrial membrane protein blocking iron transport iron into the mitochondria, resulted in massive fat accumulation but improved insulin sensitivity [54] (Table 1). These data suggest that mitochondrial dysfunction does not cause insulin resistance.
From electrochemical point of view, mitochondrial superoxide (mostly from complex I) is generated when complex I is fully reduced with electrons but downstream electron transfer components are also fully reduced and thus cannot accept any more electrons (“electron jam”). In this situation, the saturated electrons in complex I leak and react prematurely with oxygen to form superoxide, a partially reduced form of molecular oxygen. This occurs when adenosine triphosphate (ATP) synthesis is not required or when the reduced nicotinamide adenine dinucleotide (NADH)/nicotinamide adenine dinucleotide (NAD+) ratio is high [55]. For mitochondria that are actively making ATP, the electrons are passed smoothly in the electron transfer train and hence the extent of superoxide production is low. When the ratio of NADH/NAD+ is low (such as diet restriction), complex I is not reduced so that electron leak is also low [55]. It is actually not certain whether reduced mitochondrial biogenesis or reduced oxidative phosphorylation rate by genetic manipulation would actually decrease or increase ROS production. This may explain the controversies between insulin resistance and various mitochondrial dysfunction models.
Another point of view, termed “mitohormesis” holds that increased ROS production from mitochondria may act as downstream effectors that trigger nuclear compensatory response including antioxidant defense and metabolic adaptation. An example comes from the observation that antioxidant treatment blocks the extension of life induced by nutrient deprivation in worm [56]. Mild mitochondrial stress appears to be beneficial for organism to adapt for subsequent metabolic perturbations [57].
3.2. Mitochondrial Dysfunction and Insulin Secretion
Mitochondrial ATP generation plays a pivotal role in insulin secretion of pancreatic β-cell. Increased mitochondrial ATP production in response to hyperglycemia closes the ATP-sensitive potassium channel, leading to membrane depolarization, opening of voltage-sensitive calcium channel, calcium ion influx, and insulin granule exocytosis (Figure 1(b)). Several forms of syndromic mitochondrial diseases are characterized with diabetes [58] (Table 2). Mutations in the mitochondrial DNA (mtDNA), especially those in tRNA genes such as A3243G mutation, cause approximately 0.5–1% of all types of diabetes [59, 60]. Consistently, β-cell-specific disruption of Tfam causes severe mtDNA depletion, deficient oxidative phosphorylation, abnormal appearing mitochondria in islets, and impaired insulin secretion [61]. Similarly, targeted disruption of frataxin, a mitochondrial iron-binding protein in pancreatic β-cell, causes increased islet ROS, decreased islet mass, and diabetes in mice [62]. Furthermore, patients with mutations in the frataxin gene develop diabetes in 23% of cases [63] (Table 2).
4. The Role of Autophagy in Diabetes and Obesity
Autophagy is a cellular housekeeping process which trafficked cytoplasmic misfolded protein and damaged organelles for lytic degradation and recycle, hence maintaining a normal cellular function [64]. During autophagy, part of the cytoplasm containing sequestered materials is bounded by a double membrane to form an autophagolysosome, which further fuses with lysosome for degradation. This process involves induction, cargo recognition, and nucleation that are tightly controlled by a group of over 30 autophagy-related (ATG) proteins [65].
Autophagy is originally considered as a protein turnover process to replenish amino acid pool during starvation. This signaling process is converged to the mammalian target of rapamycin complex 1 (mTORC1) pathway and is strongly affected by the nutrient level or growth factors such as insulin. During nutrient-rich condition, mTORC1 is activated to phosphorylate Atg1/UNC51-like kinase 1 (ULK-1) complex and inactivate the autophagy process. Conversely, during starvation, the adenosine monophosphate (AMP) to ATP increases. The energy depletion is sensed by AMP-activated protein kinase (AMPK) which activates autophagy by blocking mTORC1 activity and direct phosphorylation of Atg1/ULK1 [66]. A study using transgenic mouse model expressing a fluorescent marker of autophagy revealed that starvation activates autophagy in liver, heart, skeletal muscle, and kidney [67]. During starvation, autophagy provides amino acid for cellular fueling, protein synthesis, gluconeogenesis, and lipid mobilization.
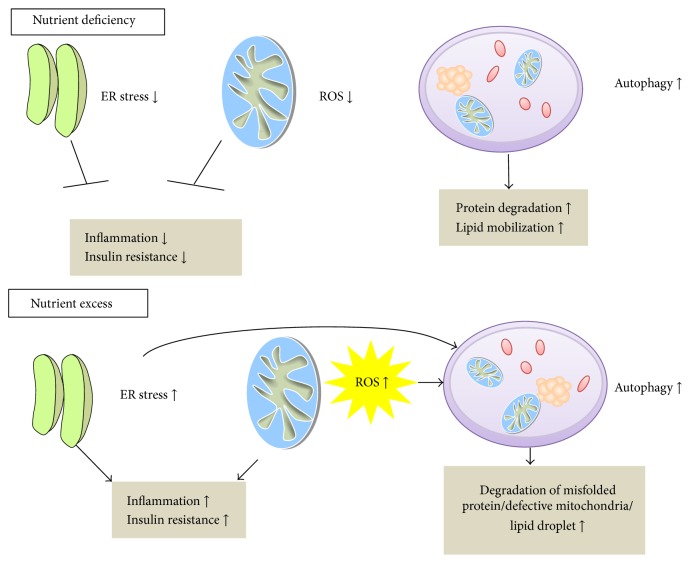
In stressful condition such as increased mitochondrial ROS, ER stress, or accumulation of excessive lipid droplet, autophagy is activated to degrade defective mitochondria (mitophagy), stressed ER (ER-phagy), or accumulated lipid (lipophagy) to remove excessive ROS, ER stress, or lipid [68] (Figure 2).
Figure 2.
Interactions between endoplasmic reticulum (ER) stress, mitochondrial reactive oxygen species (ROS), and autophagy during nutrient deficiency and excess.
4.1. Autophagy and Hepatic Lipid Metabolism
Obesity is associated with downregulation of autophagy in the liver [69]. Autophagy of lipid droplet (lipophagy) in hepatocyte facilitates the degradation of lipid in the liver and defective autophagy leads to massive accumulation of triglyceride, ER stress, and insulin resistance in the liver [69, 70]. In contrast, restoration of the Atg7 expression in liver resulted in alleviated ER stress and improved hepatic sensitivity in obese mice [69].
4.2. Autophagy and Insulin Secretion
Pancreatic β-cells keep on synthesizing large amount of insulin to maintain normoglycemia. When the protein folding cannot keep pace with the massive synthesis rate such as during hyperglycemia, UPR occurred to halt the process [71]. ER-phagy is the specific term for autophagic control to degrade excessive misfolded protein to the lysosome for degradation and prevent insulin secretory defects [72, 73]. Disruption of Atg7 in pancreatic β-cells causes ER stress, reduction of β-cells mass, and increase in β-cells apoptosis [74, 75]. IAPP is another peptide hormone released from β-cells, which normally are cosecreted with insulin [76]. Intracellular oligomer accumulation of human islet amyloid polypeptide (hIAPP) is toxic to β-cells, which is a typical morphological change in T2DM. Abnormal hIAPP aggregates are primary degraded by autophagy. Transgenic mice expressing hIAPP with β-cell-specific Atg7 deletion accumulate hIAPP oligomers and develop diabetes with increased oxidative damage and decreased β-cell mass [77–79] (Table 1, Figure 1(c)). Density volume of autophagic vacuoles and autophagosomes was significantly higher in β-cells of diabetic human [80].
Mitophagy also acts to prevent the accumulation of depolarized mitochondria and maintain optimal β-cells mitochondrial function [81]. β-cells-specific Atg7 knockout mice showed swollen mitochondria and reduced insulin secretion (Table 1, Figure 1(c)) [75].
4.3. Autophagy, Adipose Tissue, and Skeletal Muscle
In contrast to the role of autophagy in hepatic lipid clearance and alleviating stress of pancreatic β-cells, the function of autophagy in adipose tissue and skeletal muscle deserves separate mention. Mice targeted with Atg7 disruption in adipose tissue have reduced body fat, increased fatty acid β-oxidation, and improved insulin sensitivity [82, 83], indicating that autophagy is required for the production of large lipid droplets characteristic of white adipose tissue. However, Atg7+/−-ob/ob mice showed exacerbated insulin resistance with elevated lipid levels [84] (Table 1, Figure 1(c)). Muscle-specific Atg7 knockout mice exhibit lean phenotype with increased lipolysis and β-oxidation rate in adipose tissue, enhanced glucose tolerance, and improved insulin sensitivity [85]. This is due to the impairment of autophagy to degrade defective mitochondria, which leads to the fibroblast growth factor (FGF21) release, causing lipolysis and β-oxidation rate in adipose tissue [85]. These diverse results of the same gene exerting different function in different organs may be a result of noncell autonomous function.
4.4. Autophagy and Appetite Control
Furthermore, food intake in mice with agouti-related peptide (AgRP) neuron-specific Atg7 deletion was decreased while it increased in proopiomelanocortin (POMC) neuron-specific Atg7 deletion. The changes of the functional consequences converge on the controlling of a common neuropeptide, α-melanocyte-stimulating hormone (α-MSH), level (Table 1, Figure 1(c)).
5. Targeting Organelle Stress for Treating Metabolic Diseases
Chemical chaperones including tauroursodeoxycholate (TUDCA) and 4-phenylbutyrate (PBA) have been shown to reduce ER stress and improve insulin sensitivity in rodents and human [86, 87] (Table 3). These two drugs have been approved by the US Food and Drug Administration for the treatment of primary biliary cirrhosis. Numerous small molecules are identified to increase chaperone expression or to modulate specific arm of UPR in vitro using various screening strategies. For example, GSK2606414 has been shown to inhibit PERK kinase activity [88], azoramide to activate ATF6 [89], valproate to increase GRP78 expression [90], salubrinal and guanabenz to inhibit eIF2α dephosphorylation [91, 92], and 3-ethoxy-5,6-dibromosalicylaldehyde [93], STF-083010 [94], MKC-3946 [95], 4μ8C [96], and KIRA6 to inhibit IRE1 RNase activity [97]. Among them, valproate has been shown to attenuate atherosclerosis and alleviate hepatic steatosis [90] and azoramide has been shown to improve insulin sensitivity and pancreatic β-cell function in rodent models [89].
Table 3.
Treatment targeting organelle stress for diabetes mellitus and obesity.
| Agent | Specific mechanism | Highest level of studies | Result |
|---|---|---|---|
| Tauroursodeoxycholic acid | Chemical chaperone | Randomized controlled trials | Improved insulin sensitivity in muscle and liver in obese individuals [86] |
| Phenylbutyrate | Chemical chaperone | Randomized controlled trials | Improved insulin sensitivity and beta-cell function in lipid-infused individuals [87] |
| Azoramide | ATF6 activators | Rodents | Improves insulin sensitivity and beta-cell function in obese mice [89] |
| Valproate | Increasing GRPP78 | Rodents | Ameliorates atherosclerosis and hepatic steatosis in Apoe −/−mice [90] |
| L-Carnitine or carnitine-orotate | Fatty acid transfer for beta-oxidation | Randomized controlled trials | Twelve of 17 studies showing improved insulin sensitivity or glycemic control in type 2 diabetic patients or alleviation of hepatic steatosis [98, 99] |
| Co-enzyme Q10 | Electron carrier from complex I and II to complex III | Randomized controlled trials | No net effect on glycemic control in type 2 diabetic patients [100] |
| α-lipoic acid | Antioxidant | Randomized controlled trials; rodent | Weight-reducing, glucose-lowering, and insulin-sensitizing effect; prevention of hepatic steatosis [101–110] |
| Vitamin E | Antioxidant | Randomized controlled trials | Inconsistent results on glycemic control [111–115]; reduced hepatic steatosis [122] |
| N-acetylcysteine | Antioxidant | Rodents | Prevents diet-induced obesity [116–118] |
| Peptide SS31 | Mitochondria-targeted antioxidant peptide | Rodent | Improved glucose tolerance in diet-induced obese mice [38] |
| Resveratrol | SIRT1 agonist | Randomized controlled trials | Improved insulin sensitivity and glycemic control in diabetic patients; no effect in nondiabetic patients [120]; |
| GSK5182 | Estrogen-related receptor gamma inverse agonist | Rodents | Reduces hyperglycemia due to inhibition of hepatic gluconeogenesis [121] |
| Trehalose, imanitib | Enhance autophagy | Rodents |
Improved glucose tolerance and insulin sensitivity in obese mice [84] |
| Dh404 | Nrf2 activator | Rodents | Increased viability of islet by enhancing autophagy [123] |
Pharmacological approaches to alleviate mitochondrial stress include carnitine [98, 99], Coenzyme Q10 [100], ROS scavengers (peptide SS31 [38], α-lipoic acid [101–110], vitamin E, beta-carotene, vitamin C [111–114], N-acetylcysteine [115–118], and mitoQ [119]), stimulators of mitochondrial biogenesis (resveratrol and other sirtuin activators [120], and estrogen-related receptor modulators [121]). Specifically, carnitine or carnitine-orotate complex, which promotes fatty acid β-oxidation, improves insulin sensitivity or attenuates hepatic steatosis in most randomized clinical trials [98, 99]. Coenzyme Q1, however, showed no net effect on glycemic control in most type 2 diabetic patients [100]. Most evidence demonstrated that α-lipoic acid is a potent weight-reducing and insulin sensitizing agent in human clinical trials and rodent models [101–110]. Multiple small clinical trials investigating the effect of antioxidant vitamin E, vitamin C, and beta-carotene on glycemic control in diabetic patients yielded inconsistent results [111–115]. However, in a randomized clinical trial of 247 adults with nonalcoholic steatohepatitis, vitamin E use, as compared with placebo, was associated with a significantly higher rate of improvement in nonalcoholic steatohepatitis [122]. N-acetylcysteine, an approved drug for acetaminophen intoxication and mucolysis, has been demonstrated to prevent diet-induced obesity in rodent models [116–118]. Significant controversies remained regarding the metabolic action of resveratrol in human; a meta-analysis of 11 randomized controlled trials revealed that resveratrol significantly reduces glucose, insulin, and insulin resistance in diabetic patients but not in nondiabetics [120] (Table 3). Further results from clinical trials and more potent SIRT1-activating compounds (STAC) such as SRT1720 and SRT2104 are awaited.
Various therapeutic agents may be used to enhance autophagy. Trehalose is an autophagy enhancer which improves the glucose intolerance of hIAPP transgenic mice fed a HFD and further reduced hIAPP oligomer accumulation and improved β-cells function [77]. Both Imatinib and trehalose were reported to improve metabolic parameters of Atg7−/− -ob/ob mice by enhanced autophagic flux [84]. Dihydro-CDDO-trifluoroethyl amide (dh404) is an Nrf2 activator which can reduce oxidative stress in isolated rat islet by enhancing autophagy [123] (Table 3).
6. Future Perspectives
The interaction between ER stress, mitochondrial oxidative stress, and autophagy is complex. Most small molecules used to date do not have the required specificity. Furthermore, the multiple intrinsic feedback pathways, the cross-organ communication, and the interplay between autophagy and carcinogenesis make it difficult to target a single pathway to treat metabolic diseases without triggering unwanted side effects. Currently, the most efficient and safe way to reduce organelle stress and to treat metabolic disease is probably prevention of overnutrition.
Conflict of Interests
The authors declare that there is no conflict of interests regarding the publication of this paper.
Authors' Contribution
Yi-Cheng Chang and Siow-Wey Hee contributed equally to this work.
References
- 1.Saltiel A. R. Series introduction: the molecular and physiological basis of insulin resistance: emerging implications for metabolic and cardiovascular diseases. The Journal of Clinical Investigation. 2000;106(2):163–164. doi: 10.1172/jci10533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Knowler W. C., Barrett-Connor E., Fowler S. E., et al. Reduction in the incidence of type 2 diabetes with lifestyle intervention or metformin. The New England Journal of Medicine. 2002;346(6):393–403. doi: 10.1056/nejmoa012512. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Tuomilehto J., Lindström J., Eriksson J. G., et al. Prevention of type 2 diabetes mellitus by changes in lifestyle among subjects with impaired glucose tolerance. The New England Journal of Medicine. 2001;344(18):1343–1350. doi: 10.1056/nejm200105033441801. [DOI] [PubMed] [Google Scholar]
- 4.Colman R. J., Anderson R. M., Johnson S. C., et al. Caloric restriction delays disease onset and mortality in rhesus monkeys. Science. 2009;325(5937):201–204. doi: 10.1126/science.1173635. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Saltiel A. R., Kahn C. R. Insulin signalling and the regulation of glucose and lipid metabolism. Nature. 2001;414(6865):799–806. doi: 10.1038/414799a. [DOI] [PubMed] [Google Scholar]
- 6.Copps K. D., White M. F. Regulation of insulin sensitivity by serine/threonine phosphorylation of insulin receptor substrate proteins IRS1 and IRS2. Diabetologia. 2012;55(10):2565–2582. doi: 10.1007/s00125-012-2644-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ron D., Walter P. Signal integration in the endoplasmic reticulum unfolded protein response. Nature Reviews Molecular Cell Biology. 2007;8(7):519–529. doi: 10.1038/nrm2199. [DOI] [PubMed] [Google Scholar]
- 8.Sharma N. K., Das S. K., Mondal A. K., et al. Endoplasmic reticulum stress markers are associated with obesity in nondiabetic subjects. Journal of Clinical Endocrinology and Metabolism. 2008;93(11):4532–4541. doi: 10.1210/jc.2008-1001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gregor M. F., Yang L., Fabbrini E., et al. Endoplasmic reticulum stress is reduced in tissues of obese subjects after weight loss. Diabetes. 2009;58(3):693–700. doi: 10.2337/db08-1220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsutsumi A., Motoshima H., Kondo T., et al. Caloric restriction decreases ER stress in liver and adipose tissue in ob/ob mice. Biochemical and Biophysical Research Communications. 2011;404(1):339–344. doi: 10.1016/j.bbrc.2010.11.120. [DOI] [PubMed] [Google Scholar]
- 11.Özcan U., Cao Q., Yilmaz E., et al. Endoplasmic reticulum stress links obesity, insulin action, and type 2 diabetes. Science. 2004;306(5695):457–461. doi: 10.1126/science.1103160. [DOI] [PubMed] [Google Scholar]
- 12.Zhou Y., Lee J., Reno C. M., et al. Regulation of glucose homeostasis through a XBP-1-FoxO1 interaction. Nature Medicine. 2011;17(3):356–365. doi: 10.1038/nm.2293. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Deng Y., Wang Z. V., Tao C., et al. The Xbp1s/GalE axis links ER stress to postprandial hepatic metabolism. The Journal of Clinical Investigation. 2013;123(1):455–468. doi: 10.1172/jci62819. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Scheuner D., Song B., McEwen E., et al. Translational control is required for the unfolded protein response and in vivo glucose homeostasis. Molecular Cell. 2001;7(6):1165–1176. doi: 10.1016/S1097-2765(01)00265-9. [DOI] [PubMed] [Google Scholar]
- 15.Oyadomari S., Harding H. P., Zhang Y., Oyadomari M., Ron D. Dephosphorylation of translation initiation factor 2α enhances glucose tolerance and attenuates hepatosteatosis in mice. Cell Metabolism. 2008;7(6):520–532. doi: 10.1016/j.cmet.2008.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wang Y., Vera L., Fischer W. H., Montminy M. The CREB coactivator CRTC2 links hepatic ER stress and fasting gluconeogenesis. Nature. 2009;460(7254):534–537. doi: 10.1038/nature08111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Kammoun H. L., Chabanon H., Hainault I., et al. GRP78 expression inhibits insulin and ER stress-induced SREBP-1c activation and reduces hepatic steatosis in mice. The Journal of Clinical Investigation. 2009;119(5):1201–1215. doi: 10.1172/jci37007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Nakatani Y., Kaneto H., Kawamori D., et al. Involvement of endoplasmic reticulum stress in insulin resistance and diabetes. The Journal of Biological Chemistry. 2005;280(1):847–851. doi: 10.1074/jbc.m411860200. [DOI] [PubMed] [Google Scholar]
- 19.Kaneko M., Niinuma Y., Nomura Y. Activation signal of nuclear factor-κB in response to endoplasmic reticulum stress is transduced via IRE1 and tumor necrosis factor receptor-associated factor 2. Biological and Pharmaceutical Bulletin. 2003;26(7):931–935. doi: 10.1248/bpb.26.931. [DOI] [PubMed] [Google Scholar]
- 20.Deng J., Lu P. D., Zhang Y., et al. Translational repression mediates activation of nuclear factor kappa B by phosphorylated translation initiation factor 2. Molecular and Cellular Biology. 2004;24(23):10161–10168. doi: 10.1128/mcb.24.23.10161-10168.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Yamazaki H., Hiramatsu N., Hayakawa K., et al. Activation of the Akt-NF-κB pathway by subtilase cytotoxin through the ATF6 branch of the unfolded protein response. The Journal of Immunology. 2009;183(2):1480–1487. doi: 10.4049/jimmunol.0900017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lee A.-H., Scapa E. F., Cohen D. E., Glimcher L. H. Regulation of hepatic lipogenesis by the transcription factor XBP1. Science. 2008;320(5882):1492–1496. doi: 10.1126/science.1158042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Bobrovnikova-Marjon E., Hatzivassiliou G., Grigoriadou C., et al. PERK-dependent regulation of lipogenesis during mouse mammary gland development and adipocyte differentiation. Proceedings of the National Academy of Sciences of the United States of America. 2008;105(42):16314–16319. doi: 10.1073/pnas.0808517105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamamoto K., Takahara K., Oyadomari S., et al. Induction of liver steatosis and lipid droplet formation in ATF6α-knockout mice burdened with pharmacological endoplasmic reticulum stress. Molecular Biology of the Cell. 2010;21(17):2975–2986. doi: 10.1091/mbc.e09-02-0133. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Rutkowski D. T., Wu J., Back S.-H., et al. UPR pathways combine to prevent hepatic steatosis caused by ER stress-mediated suppression of transcriptional master regulators. Developmental Cell. 2008;15(6):829–840. doi: 10.1016/j.devcel.2008.10.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Iwawaki T., Akai R., Kohno K., Miura M. A transgenic mouse model for monitoring endoplasmic reticulum stress. Nature Medicine. 2004;10(1):98–102. doi: 10.1038/nm970. [DOI] [PubMed] [Google Scholar]
- 27.Lee A.-H., Heidtman K., Hotamisligil G. S., Glimcher L. H. Dual and opposing roles of the unfolded protein response regulated by IRE1α and XBP1 in proinsulin processing and insulin secretion. Proceedings of the National Academy of Sciences of the United States of America. 2011;108(21):8885–8890. doi: 10.1073/pnas.1105564108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Back S. H., Scheuner D., Han J., et al. Translation attenuation through eIF2α phosphorylation prevents oxidative stress and maintains the differentiated state in β cells. Cell Metabolism. 2009;10(1):13–26. doi: 10.1016/j.cmet.2009.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gao Y., Sartori D. J., Li C., et al. PERK is required in the adult pancreas and is essential for maintenance of glucose homeostasis. Molecular and Cellular Biology. 2012;32(24):5129–5139. doi: 10.1128/MCB.01009-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Zhang W., Feng D., Li Y., Iida K., McGrath B., Cavener D. R. PERK EIF2AK3 control of pancreatic beta cell differentiation and proliferation is required for postnatal glucose homeostasis. Cell Metabolism. 2006;4(6):491–497. doi: 10.1016/j.cmet.2006.11.002. [DOI] [PubMed] [Google Scholar]
- 31.Delépine M., Nicolino M., Barrett T., Golamaully M., Lathrop G. M., Julier C. EIF2AK3, encoding translation initiation factor 2-α kinase 3, is mutated in patients with Wolcott-Rallison syndrome. Nature Genetics. 2000;25(4):406–409. doi: 10.1038/78085. [DOI] [PubMed] [Google Scholar]
- 32.Oyadomari S., Koizumi A., Takeda K., et al. Targeted disruption of the Chop gene delays endoplasmic reticulum stress-mediated diabetes. Journal of Clinical Investigation. 2002;109(4):525–532. doi: 10.1172/jci200214550. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Petersen K. F., Dufour S., Befroy D., Garcia R., Shulman G. I. Impaired mitochondrial activity in the insulin-resistant offspring of patients with type 2 diabetes. TheNew England Journal of Medicine. 2004;350(7):664–671. doi: 10.1056/nejmoa031314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Petersen K. F., Befroy D., Dufour S., et al. Mitochondrial dysfunction in the elderly: possible role in insulin resistance. Science. 2003;300(5622):1140–1142. doi: 10.1126/science.1082889. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Befroy D. E., Petersen K. F., Dufour S., et al. Impaired mitochondrial substrate oxidation in muscle of insulin-resistant offspring of type 2 diabetic patients. Diabetes. 2007;56(5):1376–1381. doi: 10.2337/db06-0783. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mootha V. K., Lindgren C. M., Eriksson K.-F., et al. PGC-1α-responsive genes involved in oxidative phosphorylation are coordinately downregulated in human diabetes. Nature Genetics. 2003;34(3):267–273. doi: 10.1038/ng1180. [DOI] [PubMed] [Google Scholar]
- 37.Patti M. E., Butte A. J., Crunkhorn S., et al. Coordinated reduction of genes of oxidative metabolism in humans with insulin resistance and diabetes: potential role of PGC1 and NRF1. Proceedings of the National Academy of Sciences of the United States of America. 2003;100(14):8466–8471. doi: 10.1073/pnas.1032913100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Anderson E. J., Lustig M. E., Boyle K. E., et al. Mitochondrial H2O2 emission and cellular redox state link excess fat intake to insulin resistance in both rodents and humans. The Journal of Clinical Investigation. 2009;119(3):573–581. doi: 10.1172/jci37048. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Bonnard C., Durand A., Peyrol S., et al. Mitochondrial dysfunction results from oxidative stress in the skeletal muscle of diet-induced insulin-resistant mice. Journal of Clinical Investigation. 2008;118(2):789–800. doi: 10.1172/jci32601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Furukawa S., Fujita T., Shimabukuro M., et al. Increased oxidative stress in obesity and its impact on metabolic syndrome. The Journal of Clinical Investigation. 2004;114(12):1752–1761. doi: 10.1172/jci200421625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Ichijo H., Nishida E., Irie K., et al. Induction of apoptosis by ASK1, a mammalian MAPKKK that activates SAPK/JNK and p38 signaling pathways. Science. 1997;275(5296):90–94. doi: 10.1126/science.275.5296.90. [DOI] [PubMed] [Google Scholar]
- 42.Imoto K., Kukidome D., Nishikawa T., et al. Impact of mitochondrial reactive oxygen species and apoptosis signal-regulating kinase 1 on insulin signaling. Diabetes. 2006;55(5):1197–1204. doi: 10.2337/db05-1187. [DOI] [PubMed] [Google Scholar]
- 43.Asehnoune K., Strassheim D., Mitra S., Kim J. Y., Abraham E. Involvement of reactive oxygen species in Toll-like receptor 4-dependent activation of NF-κB. The Journal of Immunology. 2004;172(4):2522–2529. doi: 10.4049/jimmunol.172.4.2522. [DOI] [PubMed] [Google Scholar]
- 44.Griffin M. E., Marcucci M. J., Cline G. W., et al. Free fatty acid-induced insulin resistance is associated with activation of protein kinase C theta and alterations in the insulin signaling cascade. Diabetes. 1999;48(6):1270–1274. doi: 10.2337/diabetes.48.6.1270. [DOI] [PubMed] [Google Scholar]
- 45.Kim J. K., Fillmore J. J., Sunshine M. J., et al. PKC-θ knockout mice are protected from fat-induced insulin resistance. Journal of Clinical Investigation. 2004;114(6):823–827. doi: 10.1172/jci200422230. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Cheol S. C., Savage D. B., Abu-Elheiga L., et al. Continuous fat oxidation in acetyl-CoA carboxylase 2 knockout mice increases total energy expenditure, reduces fat mass, and improves insulin sensitivity. Proceedings of the National Academy of Sciences of the United States of America. 2007;104(42):16480–16485. doi: 10.1073/pnas.0706794104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Itani S. I., Ruderman N. B., Schmieder F., Boden G. Lipid-induced insulin resistance in human muscle is associated with changes in diacylglycerol, protein kinase C, and IkappaB-alpha. Diabetes. 2002;51(7):2005–2011. doi: 10.2337/diabetes.51.7.2005. [DOI] [PubMed] [Google Scholar]
- 48.Holland W. L., Brozinick J. T., Wang L.-P., et al. Inhibition of ceramide synthesis ameliorates glucocorticoid-, saturated-fat-, and obesity-induced insulin resistance. Cell Metabolism. 2007;5(3):167–179. doi: 10.1016/j.cmet.2007.01.002. [DOI] [PubMed] [Google Scholar]
- 49.Pospisilik J. A., Knauf C., Joza N., et al. Targeted deletion of AIF decreases mitochondrial oxidative phosphorylation and protects from obesity and diabetes. Cell. 2007;131(3):476–491. doi: 10.1016/j.cell.2007.08.047. [DOI] [PubMed] [Google Scholar]
- 50.Lin J., Wu P.-H., Tarr P. T., et al. Defects in adaptive energy metabolism with CNS-linked hyperactivity in PGC-1α null mice. Cell. 2004;119(1):121–135. doi: 10.1016/j.cell.2004.09.013. [DOI] [PubMed] [Google Scholar]
- 51.Leone T. C., Lehman J. J., Finck B. N., et al. PGC-1alpha deficiency causes multi-system energy metabolic derangements: muscle dysfunction, abnormal weight control and hepatic steatosis. PLoS Biology. 2005;3(4, article e101) doi: 10.1371/journal.pbio.0030101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Wredenberg A., Freyer C., Sandström M. E., et al. Respiratory chain dysfunction in skeletal muscle does not cause insulin resistance. Biochemical and Biophysical Research Communications. 2006;350(1):202–207. doi: 10.1016/j.bbrc.2006.09.029. [DOI] [PubMed] [Google Scholar]
- 53.Vernochet C., Mourier A., Bezy O., et al. Adipose-specific deletion of TFAM increases mitochondrial oxidation and protects mice against obesity and insulin resistance. Cell Metabolism. 2012;16(6):765–776. doi: 10.1016/j.cmet.2012.10.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Kusminski C. M., Holland W. L., Sun K., et al. MitoNEET-driven alterations in adipocyte mitochondrial activity reveal a crucial adaptive process that preserves insulin sensitivity in obesity. Nature Medicine. 2012;18(10):1539–1551. doi: 10.1038/nm.2899. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Murphy M. P. How mitochondria produce reactive oxygen species. Biochemical Journal. 2009;417(1):1–13. doi: 10.1042/bj20081386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Schulz T. J., Zarse K., Voigt A., Urban N., Birringer M., Ristow M. Glucose restriction extends Caenorhabditis elegans life span by inducing mitochondrial respiration and increasing oxidative stress. Cell Metabolism. 2007;6(4):280–293. doi: 10.1016/j.cmet.2007.08.011. [DOI] [PubMed] [Google Scholar]
- 57.Yun J., Finkel T. Mitohormesis. Cell Metabolism. 2014;19(5):757–766. doi: 10.1016/j.cmet.2014.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Maechler P., Wollheim C. B. Mitochondrial function in normal and diabetic β-cells. Nature. 2001;414(6865):807–812. doi: 10.1038/414807a. [DOI] [PubMed] [Google Scholar]
- 59.DiMauro S., Schon E. A. Mitochondrial respiratory-chain diseases. The New England Journal of Medicine. 2003;348(26):2656–2668. doi: 10.1056/nejmra022567. [DOI] [PubMed] [Google Scholar]
- 60.Kadowaki T., Kadowaki H., Mori Y., et al. A subtype of diabetes mellitus associated with a mutation of mitochondrial DNA. The New England Journal of Medicine. 1994;330(14):962–968. doi: 10.1056/nejm199404073301403. [DOI] [PubMed] [Google Scholar]
- 61.Silva J. P., Köhler M., Graff C., et al. Impaired insulin secretion and beta-cell loss in tissue-specific knockout mice with mitochondrial diabetes. Nature Genetics. 2000;26(3):336–340. doi: 10.1038/81649. [DOI] [PubMed] [Google Scholar]
- 62.Ristow M., Mulder H., Pomplun D., et al. Frataxin deficiency in pancreatic islets causes diabetes due to loss of β cell mass. Journal of Clinical Investigation. 2003;112(4):527–534. doi: 10.1172/jci200318107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Hewer R. L. Study of fatal cases of Friedreich's ataxia. British Medical Journal. 1968;3(619):649–652. doi: 10.1136/bmj.3.5619.649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Mizushima N., Komatsu M. Autophagy: renovation of cells and tissues. Cell. 2011;147(4):728–741. doi: 10.1016/j.cell.2011.10.026. [DOI] [PubMed] [Google Scholar]
- 65.Feng Y., He D., Yao Z., Klionsky D. J. The machinery of macroautophagy. Cell Research. 2014;24(1):24–41. doi: 10.1038/cr.2013.168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kim J., Kundu M., Viollet B., Guan K.-L. AMPK and mTOR regulate autophagy through direct phosphorylation of Ulk1. Nature Cell Biology. 2011;13(2):132–141. doi: 10.1038/ncb2152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Mizushima N., Yamamoto A., Matsui M., Yoshimori T., Ohsumi Y. In vivo analysis of autophagy in response to nutrient starvation using transgenic mice expressing a fluorescent autophagosome marker. Molecular Biology of the Cell. 2004;15(3):1101–1111. doi: 10.1091/mbc.e03-09-0704. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.He C., Klionsky D. J. Regulation mechanisms and signaling pathways of autophagy. Annual Review of Genetics. 2009;43:67–93. doi: 10.1146/annurev-genet-102808-114910. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 69.Yang L., Li P., Fu S., Calay E. S., Hotamisligil G. S. Defective hepatic autophagy in obesity promotes ER stress and causes insulin resistance. Cell Metabolism. 2010;11(6):467–478. doi: 10.1016/j.cmet.2010.04.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Singh R., Kaushik S., Wang Y., et al. Autophagy regulates lipid metabolism. Nature. 2009;458(7242):1131–1135. doi: 10.1038/nature07976. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lee J., Ozcan U. Unfolded protein response signaling and metabolic diseases. The Journal of Biological Chemistry. 2014;289(3):1203–1211. doi: 10.1074/jbc.r113.534743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Bernales S., McDonald K. L., Walter P. Autophagy counterbalances endoplasmic reticulum expansion during the unfolded protein response. PLoS Biology. 2006;4(12, article e423) doi: 10.1371/journal.pbio.0040423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Yorimitsu T., Nair U., Yang Z., Klionsky D. J. Endoplasmic reticulum stress triggers autophagy. Journal of Biological Chemistry. 2006;281(40):30299–30304. doi: 10.1074/jbc.m607007200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Ebato C., Uchida T., Arakawa M., et al. Autophagy is important in islet homeostasis and compensatory increase of beta cell mass in response to high-fat diet. Cell Metabolism. 2008;8(4):325–332. doi: 10.1016/j.cmet.2008.08.009. [DOI] [PubMed] [Google Scholar]
- 75.Jung H. S., Chung K. W., Kim J. W., et al. Loss of autophagy diminishes pancreatic β cell mass and function with resultant hyperglycemia. Cell Metabolism. 2008;8(4):318–324. doi: 10.1016/j.cmet.2008.08.013. [DOI] [PubMed] [Google Scholar]
- 76.Westermark P., Wernstedt C., Wilander E., Sletten K. A novel peptide in the calcitonin gene related peptide family as an amyloid fibril protein in the endocrine pancreas. Biochemical and Biophysical Research Communications. 1986;140(3):827–831. doi: 10.1016/0006-291x(86)90708-4. [DOI] [PubMed] [Google Scholar]
- 77.Kim J., Cheon H., Jeong Y. T., et al. Amyloidogenic peptide oligomer accumulation in autophagy-deficient β cells induces diabetes. Journal of Clinical Investigation. 2014;124(8):3311–3324. doi: 10.1172/jci69625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 78.Rivera J. F., Costes S., Gurlo T., Glabe C. G., Butler P. C. Autophagy defends pancreatic β cells from human islet amyloid polypeptide-induced toxicity. Journal of Clinical Investigation. 2014;124(8):3489–3500. doi: 10.1172/jci71981. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Shigihara N., Fukunaka A., Hara A., et al. Human IAPP-induced pancreatic β cell toxicity and its regulation by autophagy. Journal of Clinical Investigation. 2014;124(8):3634–3644. doi: 10.1172/jci69866. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Masini M., Bugliani M., Lupi R., et al. Autophagy in human type 2 diabetes pancreatic beta cells. Diabetologia. 2009;52(6):1083–1086. doi: 10.1007/s00125-009-1347-2. [DOI] [PubMed] [Google Scholar]
- 81.Ashrafi G., Schwarz T. L. The pathways of mitophagy for quality control and clearance of mitochondria. Cell Death & Differentiation. 2013;20(1):31–42. doi: 10.1038/cdd.2012.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Zhang Y., Goldman S., Baerga R., Zhao Y., Komatsu M., Jin S. Adipose-specific deletion of autophagy-related gene 7 (atg7) in mice reveals a role in adipogenesis. Proceedings of the National Academy of Sciences of the United States of America. 2009;106(47):19860–19865. doi: 10.1073/pnas.0906048106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Singh R., Xiang Y., Wang Y., et al. Autophagy regulates adipose mass and differentiation in mice. Journal of Clinical Investigation. 2009;119(11):3329–3339. doi: 10.1172/jci39228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Lim Y. M., Lim H., Hur K. Y., et al. Systemic autophagy insufficiency compromises adaptation to metabolic stress and facilitates progression from obesity to diabetes. Nature Communications. 2014;5, article 4934 doi: 10.1038/ncomms5934. [DOI] [PubMed] [Google Scholar]
- 85.Kim K. H., Jeong Y. T., Oh H., et al. Autophagy deficiency leads to protection from obesity and insulin resistance by inducing Fgf21 as a mitokine. Nature Medicine. 2013;19(1):83–92. doi: 10.1038/nm.3014. [DOI] [PubMed] [Google Scholar]
- 86.Kars M., Yang L., Gregor M. F., et al. Tauroursodeoxycholic Acid may improve liver and muscle but not adipose tissue insulin sensitivity in obese men and women. Diabetes. 2010;59(8):1899–1905. doi: 10.2337/db10-0308. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Xiao C., Giacca A., Lewis G. F. Sodium phenylbutyrate, a drug with known capacity to reduce endoplasmic reticulum stress, partially alleviates lipid-induced insulin resistance and β-cell dysfunction in humans. Diabetes. 2011;60(3):918–924. doi: 10.2337/db10-1433. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Axten J. M., Medina J. R., Feng Y., et al. Discovery of 7-methyl-5-(1-{[3-(trifluoromethyl)phenyl]acetyl}-2,3-dihydro-1H-indol-5-yl)-7H-pyrrolo[2,3-d]pyrimidin-4-amine (GSK2606414), a potent and selective first-in-class inhibitor of protein kinase R (PKR)-like endoplasmic reticulum kinase (PERK) Journal of Medicinal Chemistry. 2012;55(16):7193–7207. doi: 10.1021/jm300713s. [DOI] [PubMed] [Google Scholar]
- 89.Fu S., Yalcin A., Lee G. Y., et al. Phenotypic assays identify azoramide as a small-molecule modulator of the unfolded protein response with antidiabetic activity. Science Translational Medicine. 2015;7(292) doi: 10.1126/scitranslmed.aaa9134.292ra98 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.McAlpine C. S., Bowes A. J., Khan M. I., Shi Y., Werstuck G. H. Endoplasmic reticulum stress and glycogen synthase kinase-3β activation in apolipoprotein E-deficient mouse models of accelerated atherosclerosis. Arteriosclerosis, Thrombosis, and Vascular Biology. 2012;32(1):82–91. doi: 10.1161/atvbaha.111.237941. [DOI] [PubMed] [Google Scholar]
- 91.Boyce M., Bryant K. F., Jousse C., et al. A selective inhibitor of elF2α dephosphorylation protects cells from ER stress. Science. 2005;307(5711):935–939. doi: 10.1126/science.1101902. [DOI] [PubMed] [Google Scholar]
- 92.Tsaytler P., Harding H. P., Ron D., Bertolotti A. Selective inhibition of a regulatory subunit of protein phosphatase 1 restores proteostasis. Science. 2011;332(6025):91–94. doi: 10.1126/science.1201396. [DOI] [PubMed] [Google Scholar]
- 93.Volkmann K., Lucas J. L., Vuga D., et al. Potent and selective inhibitors of the inositol-requiring enzyme 1 endoribonuclease. The Journal of Biological Chemistry. 2011;286(14):12743–12755. doi: 10.1074/jbc.m110.199737. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 94.Papandreou I., Denko N. C., Olson M., et al. Identification of an Ire1alpha endonuclease specific inhibitor with cytotoxic activity against human multiple myeloma. Blood. 2011;117(4):1311–1314. doi: 10.1182/blood-2010-08-303099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 95.Mimura N., Fulciniti M., Gorgun G., et al. Blockade of XBP1 splicing by inhibition of IRE1α is a promising therapeutic option in multiple myeloma. Blood. 2012;119(24):5772–5781. doi: 10.1182/blood-2011-07-366633. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Cross B. C. S., Bond P. J., Sadowski P. G., et al. The molecular basis for selective inhibition of unconventional mRNA splicing by an IRE1-binding small molecule. Proceedings of the National Academy of Sciences of the United States of America. 2012;109(15):E869–E878. doi: 10.1073/pnas.1115623109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Ghosh R., Wang L., Wang E. S., et al. Allosteric inhibition of the IRE1α RNase preserves cell viability and function during endoplasmic reticulum stress. Cell. 2014;158(3):534–548. doi: 10.1016/j.cell.2014.07.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Ringseis R., Keller J., Eder K. Role of carnitine in the regulation of glucose homeostasis and insulin sensitivity: evidence from in vivo and in vitro studies with carnitine supplementation and carnitine deficiency. European Journal of Nutrition. 2012;51(1):1–18. doi: 10.1007/s00394-011-0284-2. [DOI] [PubMed] [Google Scholar]
- 99.Bae J. C., Lee W. Y., Yoon K. H., et al. Improvement of nonalcoholic fatty liver disease with carnitine-orotate complex in type 2 diabetes (CORONA): a randomized controlled trial. Diabetes Care. 2015;38(7):1245–1252. doi: 10.2337/dc14-2852. [DOI] [PubMed] [Google Scholar]
- 100.Suksomboon N., Poolsup N., Juanak N. Effects of coenzyme Q10 supplementation on metabolic profile in diabetes: a systematic review and meta-analysis. Journal of Clinical Pharmacy and Therapeutics. 2015;40(4):413–418. doi: 10.1111/jcpt.12280. [DOI] [PubMed] [Google Scholar]
- 101.Ratliff J. C., Palmese L. B., Reutenauer E. L., Tek C. An open-label pilot trial of alpha-lipoic acid for weight loss in patients with schizophrenia without diabetes. Clinical Schizophrenia and Related Psychoses. 2015;8(4):196–200. doi: 10.3371/CSRP.RAPA.030113. [DOI] [PubMed] [Google Scholar]
- 102.Porasuphatana S., Suddee S., Nartnampong A., Konsil J., B.Pharm B. H., Santaweesuk A. Glycemic and oxidative status of patients with type 2 diabetes mellitus following oral administration of alphalipoic acid: a randomized double-blinded placebocontrolled study. Asia Pacific Journal of Clinical Nutrition. 2012;21(1):12–21. [PubMed] [Google Scholar]
- 103.Huerta A. E., Navas-Carretero S., Prieto-Hontoria P. L., Martínez J. A., Moreno-Aliaga M. J. Effects of α-lipoic acid and eicosapentaenoic acid in overweight and obese women during weight loss. Obesity. 2015;23(2):313–321. doi: 10.1002/oby.20966. [DOI] [PubMed] [Google Scholar]
- 104.Koh E. H., Lee W. J., Lee S. A., et al. Effects of alpha-lipoic acid on body weight in obese subjects. The American Journal of Medicine. 2011;124(1):85.e1–85.e8. doi: 10.1016/j.amjmed.2010.08.005. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y., Han P., Wu N., et al. Amelioration of lipid abnormalities by α-lipoic acid through antioxidative and anti-inflammatory effects. Obesity. 2011;19(8):1647–1653. doi: 10.1038/oby.2011.121. [DOI] [PubMed] [Google Scholar]
- 106.Prieto-Hontoria P. L., Pérez-Matute P., Fernández-Galilea M., Alfredo Martinez J., Moreno-Aliaga M. J. Effects of lipoic acid on AMPK and adiponectin in adipose tissue of low- and high-fat-fed rats. European Journal of Nutrition. 2013;52(2):779–787. doi: 10.1007/s00394-012-0384-7. [DOI] [PubMed] [Google Scholar]
- 107.Chen W. L., Kang C. H., Wang S. G., Lee H. M. alpha-Lipoic acid regulates lipid metabolism through induction of sirtuin 1 (SIRT1) and activation of AMP-activated protein kinase. Diabetologia. 2012;55(6):1824–1835. doi: 10.1007/s00125-012-2530-4. [DOI] [PubMed] [Google Scholar]
- 108.Wang Y., Li X., Guo Y., Chan L., Guan X. Alpha-lipoic acid increases energy expenditure by enhancing adenosine monophosphate-activated protein kinase-peroxisome proliferator-activated receptor-gamma coactivator-1alpha signaling in the skeletal muscle of aged mice. Metabolism. 2010;59(7):967–976. doi: 10.1016/j.metabol.2009.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Kim M.-S., Park J.-Y., Namkoong C., et al. Anti-obesity effects of α-lipoic acid mediated by suppression of hypothalamic AMP-activated protein kinase. Nature Medicine. 2004;10(7):727–733. doi: 10.1038/nm1061. [DOI] [PubMed] [Google Scholar]
- 110.Castro M. C., Francini F., Gagliardino J. J., Massa M. L. Lipoic acid prevents fructose-induced changes in liver carbohydrate metabolism: role of oxidative stress. Biochimica et Biophysica Acta. 2014;1840(3):1145–1151. doi: 10.1016/j.bbagen.2013.12.005. [DOI] [PubMed] [Google Scholar]
- 111.Jain S. K., McVie R., Jaramillo J. J., Palmer M., Smith T. Effect of modest vitamin E supplementation on blood glycated hemoglobin and triglyceride levels and red cell indices in type I diabetic patients. Journal of the American College of Nutrition. 1996;15(5):458–461. doi: 10.1080/07315724.1996.10718624. [DOI] [PubMed] [Google Scholar]
- 112.Ceriello A., Giugliano D., Quatraro A., Donzella C., Dipalo G., Lefebvre P. J. Vitamin E reduction of protein glycosylation in diabetes: new prospect for prevention of diabetic complications? Diabetes Care. 1991;14(1):68–72. doi: 10.2337/diacare.14.1.68. [DOI] [PubMed] [Google Scholar]
- 113.Shab-Bidar S., Mazloum Z., Mousavi-Shirazifard Z. Daily vitamin E supplementation does not improve metabolic and glycemic control in type 2 diabetic patients: a double blinded randomized controlled trial. Journal of Diabetes. 2013;5(1):57–58. doi: 10.1111/j.1753-0407.2012.00206.x. [DOI] [PubMed] [Google Scholar]
- 114.Economides P. A., Khaodhiar L., Caselli A., et al. The effect of vitamin E on endothelial function of micro- and macrocirculation and left ventricular function in type 1 and type 2 diabetic patients. Diabetes. 2005;54(1):204–211. doi: 10.2337/diabetes.54.1.204. [DOI] [PubMed] [Google Scholar]
- 115.Gómez-Pérez F. J., Valles-Sánchez V. E., López-Alvarenga J. C., et al. Vitamin E modifies neither fructosamine nor HbA1c levels in poorly controlled diabetes. Revista de Investigación Clínica. 1996;48(6):421–424. [PubMed] [Google Scholar]
- 116.Chang Y.-C., Yu Y.-H., Shew J.-Y., et al. Deficiency of NPGPx, an oxidative stress sensor, leads to obesity in mice and human. The EMBO Molecular Medicine. 2013;5(8):1165–1179. doi: 10.1002/emmm.201302679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 117.Kim J.-R., Ryu H.-H., Chung H. J., et al. Association of anti-obesity activity of N-acetylcysteine with metallothionein-II down-regulation. Experimental and Molecular Medicine. 2006;38(2):162–172. doi: 10.1038/emm.2006.20. [DOI] [PubMed] [Google Scholar]
- 118.Novelli E. L. B., Santos P. P., Assalin H. B., et al. N-acetylcysteine in high-sucrose diet-induced obesity: energy expenditure and metabolic shifting for cardiac health. Pharmacological Research. 2009;59(1):74–79. doi: 10.1016/j.phrs.2008.10.004. [DOI] [PubMed] [Google Scholar]
- 119.Mercer J. R., Yu E., Figg N., et al. The mitochondria-targeted antioxidant MitoQ decreases features of the metabolic syndrome in ATM+/−/ApoE−/− mice. Free Radical Biology and Medicine. 2012;52(5):841–849. doi: 10.1016/j.freeradbiomed.2011.11.026. [DOI] [PubMed] [Google Scholar]
- 120.Liu K., Zhou R., Wang B., Mi M.-T. Effect of resveratrol on glucose control and insulin sensitivity: a meta-analysis of 11 randomized controlled trials. American Journal of Clinical Nutrition. 2014;99(6):1510–1519. doi: 10.3945/ajcn.113.082024. [DOI] [PubMed] [Google Scholar]
- 121.Kim D.-K., Ryu D., Koh M., et al. Orphan nuclear receptor estrogen-related receptor γ (ERRγ) is key regulator of hepatic gluconeogenesis. Journal of Biological Chemistry. 2012;287(26):21628–21639. doi: 10.1074/jbc.m111.315168. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Sanyal A. J., Chalasani N., Kowdley K. V., et al. Pioglitazone, vitamin E, or placebo for nonalcoholic steatohepatitis. The New England Journal of Medicine. 2010;362(18):1675–1685. doi: 10.1056/nejmoa0907929. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 123.Li W., Wu W., Song H., et al. Targeting Nrf2 by dihydro-CDDO-trifluoroethyl amide enhances autophagic clearance and viability of β-cells in a setting of oxidative stress. FEBS Letters. 2014;588(12):2115–2124. doi: 10.1016/j.febslet.2014.04.046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Baerga R., Zhang Y., Chen P.-H., Goldman S., Jin S. Targeted deletion of autophagy-related 5 (atg5) impairs adipogenesis in a cellular model and in mice. Autophagy. 2009;5(8):1118–1130. doi: 10.4161/auto.5.8.9991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 125.Pyo J.-O., Yoo S.-M., Ahn H.-H., et al. Overexpression of Atg5 in mice activates autophagy and extends lifespan. Nature Communications. 2013;4, article 2300 doi: 10.1038/ncomms3300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Kaushik S., Rodriguez-Navarro J. A., Arias E., et al. Autophagy in hypothalamic agrp neurons regulates food intake and energy balance. Cell Metabolism. 2011;14(2):173–183. doi: 10.1016/j.cmet.2011.06.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Kaushik S., Arias E., Kwon H., et al. Loss of autophagy in hypothalamic POMC neurons impairs lipolysis. EMBO Reports. 2012;13(3):258–265. doi: 10.1038/embor.2011.260. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 128.Coupé B., Ishii Y., Dietrich M. O., Komatsu M., Horvath T. L., Bouret S. G. Loss of autophagy in pro-opiomelanocortin neurons perturbs axon growth and causes metabolic dysregulation. Cell Metabolism. 2012;15(2):247–255. doi: 10.1016/j.cmet.2011.12.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 129.Martinez-Lopez N., Athonvarangkul D., Sahu S., et al. Autophagy in Myf5+ progenitors regulates energy and glucose homeostasis through control of brown fat and skeletal muscle development. EMBO Reports. 2013;14(9):795–803. doi: 10.1038/embor.2013.111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 130.Malhotra R., Warne J. P., Salas E., Xu A. W., Debnath J. Loss of Atg12, but not Atg5, in pro-opiomelanocortin neurons exacerbates diet-induced obesity. Autophagy. 2015;11(1):145–154. doi: 10.1080/15548627.2014.998917. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 131.Fonseca S. G., Ishigaki S., Oslowski C. M., et al. Wolfram syndrome 1 gene negatively regulates ER stress signaling in rodent and human cells. The Journal of Clinical Investigation. 2010;120(3):744–755. doi: 10.1172/jci39678. [DOI] [PMC free article] [PubMed] [Google Scholar]