Abstract
Testosterone plays a central role in male development and health. Likewise, androgen deficiency, or hypogonadism, is associated with a variety of symptoms including decreased energy, diminished libido and erectile dysfunction, among others. Male androgen levels steadily decline with age, and, in a subset of symptomatic older men, can result in late-onset hypogonadism (LOH). Over the last decade, increased awareness of hypogonadism among patients and providers has led to a significant rise in the use of testosterone replacement therapy (TRT) for hypogonadism, and especially in LOH. Accompanying the rise in TRT are concerns of potential adverse effects, including cardiovascular risks and the promotion of prostate cancer. The ‘androgen hypothesis’ asserts that prostate cancer development and progression is driven by androgens, and thus TRT has the theoretical potential to drive prostate cancer development and progression. In this review, we examine existing data surrounding testosterone and prostate cancer. There is significant evidence that androgens promote prostate cancer in experimental systems. However, there is no clear evidence that elevations in endogenous testosterone levels promote the development of prostate cancer in humans. As a result of experimental and historical data on the progression of prostate cancer following TRT, there has been widespread belief that TRT will promote disease progression in prostate cancer patients. Despite these fears, there are a growing number of studies demonstrating no increase in prostate cancer incidence among men on TRT. Furthermore, in studies involving a small number of patients, there has been no discernable increase in disease progression in prostate cancer patients on TRT. While data from large, prospective, randomized, controlled trials are absent, TRT in select prostate cancer patients is likely safe. In the end, the use of TRT in prostate cancer patients is still considered experimental and should only be offered after well-informed shared decision making and with close monitoring.
Keywords: prostate cancer, testosterone, hypogonadism
Introduction
Androgen deficiency, or hypogonadism, is characterized by decreased serum testosterone and variable symptoms including decreased muscle mass, decreased energy, depressed mood, decreased libido and erectile dysfunction [Wang et al. 2008; Basaria, 2014]. As male androgen levels decline with age, a subset of symptomatic hypogonadal men develop so-called late-onset hypogonadism (LOH). LOH is associated with a variety of other disease states including hypertension, diabetes, hyperlipidemia and obesity [Mulligan et al. 2006; Basaria, 2014]. Although estimates vary, LOH is a common condition and affects an estimated 2.4 million US men over 40 years of age [Araujo et al. 2004].
Testosterone replacement therapy (TRT) encompasses the administration of exogenous testosterone and other agents aimed at raising androgen levels in hypogonadal men. While TRT has been used for decades by endocrinologists and urologists to treat men with hypogonadism, the last decade has seen a dramatic increase in the use of TRT. The percentage of men in the United States over 40 years of age prescribed TRT increased from less than 1% in 2001 to nearly 3% in 2011 [Baillargeon et al. 2013]. In 2011, global testosterone sales reached an estimated $1.8 billion [Handelsman, 2013]. With continued population growth of men over 65 years old, the number of men with LOH who are candidates for TRT can be expected grow by over 400,000 per year [Howden and Meyer, 2011; Handelsman, 2013].
The increase in TRT and lack of data from large, long-term, randomized controlled trials (RCTs) has raised concern for unrecognized adverse health risks, including potential increases in cardiovascular disease and prostate cancer (PrCa). In this review we examine the effects of testosterone on PrCa pathogenesis and implications for TRT and risk of progression in PrCa patients. We attempt to make important distinctions between patient populations and sources of testosterone. As such, we examine the role of endogenous testosterone in patients without PrCa, the role of endogenous testosterone in PrCa patients, and the potential oncologic risks of exogenous testosterone from TRT in PrCa patients. Other aspects of TRT, including the potential benefits to hypogonadal men and risk of adverse cardiovascular effects are beyond the scope of our review and have been expertly reviewed elsewhere [Swerdloff and Wang, 2011; Spitzer et al. 2013; Miner et al. 2014; Al-Khazaali et al. 2015; Morgentaler and Conners, 2015].
Androgens and prostate physiology
Androgens play a critical role in male sexual development and prostate physiology. The two principal androgens in men are testosterone, produced by testicular Leydig cells, and dihydrotestosterone (DHT), produced from testosterone in peripheral tissues by 5-α reductase. In circulation, testosterone is bound primarily to sex hormone-binding globulin (SHBG) while the unbound, or free testosterone, is the most bioavailable and active form. In the second trimester, fetal testosterone induces development of the epididymis, vas deferens and seminal vesicles, while DHT mediates development of the prostate, urethra and external genitalia [Siiteri and Wilson, 1974]. From birth through puberty, the prostate remains small and immature, while in postpubertal males the surge in androgens drives gland development and an increase in prostate volume up to 10 times its prepubertal size [Swyer, 1944]. DHT also plays a well-established role in promoting continued growth of the adult prostate, leading to benign prostatic hypertrophy (BPH) [Huggins, 1947; Andriole et al. 2004].
LOH
Serum androgen levels in men steadily decline with age, beginning in the fourth decade of life [Harman et al. 2001; Mulligan et al. 2006; Basaria, 2014]. Accordingly, in the Baltimore Longitudinal Study on Aging (BLSA), roughly 10% of men in their 40s and 25% of men in their 70s were hypogonadal, based on serum testosterone levels [Harman et al. 2001]. While age-related decline in testosterone is common among US populationsit is not universal. For instance, Ellison and colleagues demonstrated young adult elevations in testosterone and subsequent age-related declines in US and Congo populations, but not in Nepal or Paraguay [Ellison et al. 2002]. In a subset of men, the age-related decline in androgens will lead to signs and symptoms of hypogonadism, termed LOH or androgen deficiency in the aging male (ADAM).
Male hypogonadism may be caused by testicular (primary) or hypothalamic–pituitary (secondary) dysfunction. LOH is typically characterized by mixed testicular and hypothalamic–pituitary dysfunction [Vermeulen and Kaufman, 1995; Wang et al. 2008; Basaria, 2014]. Testicular changes with aging include loss of Leydig cells, decreased testosterone production, and decreased responsiveness of the testes to luteinizing hormone (LH) [Rubens et al. 1974; Neaves et al. 1984]. The resulting amplitude of peak morning testosterone is decreased in older men, making morning testosterone measurement a useful laboratory marker in the diagnosis of LOH [Bremner et al. 1983; Tenover et al. 1988; Wang et al. 2008]. Older men also demonstrate decreased amplitude and slowing of LH pulses and this hypothalamic dysfunction in LOH is characterized by low-normal LH levels, even in the presence of low testosterone [Bremner et al. 1983; Tenover et al. 1988]. The decline in total testosterone is further influenced by an increase in SHBG that occurs with aging, which may lower bioavailable testosterone [Vermeulen and Kaufman, 1995; Muller et al. 2003].
An international consensus, including the International Society of Andrology (ISA) and the European Association of Urology (EAU), defines LOH as a syndrome characterized by symptoms of hypogonadism and testosterone below the young adult reference range [Wang et al. 2008]. While common symptoms of hypogonadism in postpubertal men include decreased muscle mass, decreased energy, depressed mood, decreased libido, decreased spontaneous erections and erectile dysfunction, these symptoms can be considered subjective [Wang et al. 2008; Basaria, 2014]. In a large, multi-institutional study of 3369 men aged 40 to 79 years, the European Male Aging Study (EMAS) attempted to better define the symptom complex of LOH. Compared with constitutional symptoms, sexual symptoms of decreased morning erections, decreased libido and erectile dysfunction were most closely associated with low testosterone levels [Wu et al. 2010].
Prevalence estimates of LOH vary widely depending on study methods, populations and diagnostic criteria used. The laboratory diagnosis of LOH, including guideline recommendations, has been reviewed elsewhere [Matsumoto, 2003; Wang et al. 2008; Basaria, 2014]. Importantly, the inclusion of symptoms in the diagnosis of LOH helps to separate the pathologic condition of LOH from normal, and expected, age-dependent declines in testosterone (Figure 1). For instance, in the Hypogonadism in Males (HIM) study, 38.7% of men over 45 years met criteria for androgen deficiency, defined as a morning total serum testosterone of <10.4 nmol/l (300 ng/dl) [Mulligan et al. 2006]. In contrast, in the European Male Aging Study (EMAS), using criteria of testosterone <11.1 nmol/l (320 ng/dl) combined with the presence of symptoms, an estimated 2.1% of men aged 40–79 years met these criteria [Wu et al. 2010]. As expected, the prevalence of LOH in EMAS increased with age, from 0.1% for men in their 40s to 5.1% for men in their 70s [Wu et al. 2010]. Using both laboratory and symptoms for diagnosis, the Massachusetts Male Aging Study estimated that 2.4 million US men met criteria for LOH [Araujo et al. 2004].
Figure 1.
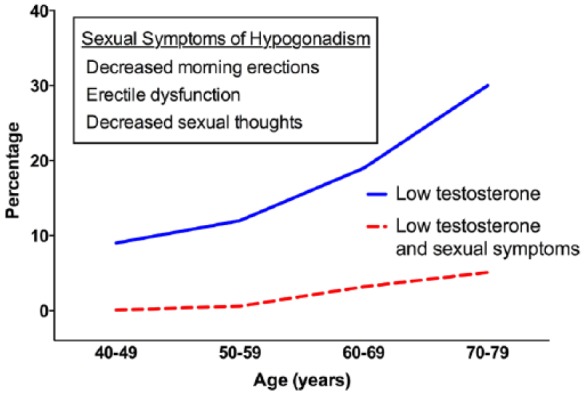
Prevalence of symptomatic hypogonadism. Percentage of hypogonadal men, with total serum testosterone of <11.3 nmol/l (325 ng/dl) (solid), or with both low testosterone and the listed sexual symptoms.
Data from Wu et al. [2010] and Harmon et al. [2001].
Endogenous testosterone and PrCa risk
There is large body of both historic and modern data supporting a role for androgens in PrCa pathogenesis and progression, also known as the ‘androgen hypothesis’. In 1941, Huggins and Hodges proposed that PrCa growth was driven by androgens, after observing the benefits of castration in PrCa patients [Huggins and Hodges, 1941]. Current laboratory data demonstrate that many well-differentiated PrCa cell lines are androgen responsive and undergo programmed cell death upon androgen withdrawal [Kyprianou et al. 1990; Webber et al. 1996; Schwab et al. 2000]. Likewise, androgens promote tumorigenesis and xenograft growth in animal models, and tumor regression is seen upon androgen deprivation [Bladou et al. 1996; Ahmad et al. 2008]. Clinically, androgen deprivation therapy (ADT) remains a mainstay in PrCa treatment, especially in advanced disease [Rove and Crawford, 2014]. Yet, despite basic science data supporting a role for androgens in PrCa pathogenesis, there are conflicting clinical data on the role of endogenous testosterone in human PrCa pathogenesis de novo. In reviewing the literature involving PrCa development in PrCa naive patients, there are studies implicating elevated testosterone, studies implicating lower testosterone, and studies with no association of testosterone and PrCa risk (Table 1).
Table 1.
Endogenous testosterone and prostate cancer (PrCa) risk.
| Study | Number of patients (Number of PrCa) | Study design | Results (95% CI) | Comments |
|---|---|---|---|---|
| Studies correlating elevated testosterone and PrCa risk associate | ||||
| Gann et al. [1996] | 621 (222) | Prospective, nested case-control | For men with highest quartile of T, PrCa OR 2.6 (1.34–5.02) | Patients from Physicians’ Health Study |
| Non-AM serum T | ||||
| Pierorazio et al. [2010] | 781 (145) | Prospective, population-based | Calculated free T and high-risk PrCa HR 1.61 (1.18–2.20) | Long-term serum storage, from BLSA Non-AM serum T |
| Shaneyfelt et al. [2000] | 817 (320) | Meta-analysis | OR 2.34 (1.3–4.2) | Includes data form Gann et al. [1996].No association with serum DHT |
| Yano et al. [2007] | 420 (216) | Retrospective cohort | PrCa HR 1.31 (CI 1.03–1.65) in men with PSA < 10 ug/l | Cohort referred for suspected PrCa |
| Studies correlating lower testosterone and PrCa | ||||
| Morgentaler and Conners [2015] | 345 (52) | Retrospective cohort | OR 2.02 (1.10–3.72) | Cohort with PSA < 4 ug/l |
| Shin et al. [2010] | 565 (194) | Prospective cohort | OR 1.99 (1.25–3.16) | Cohort with PSA > 4 ug/l or abnormal DRE |
| Mearini et al. [2013] | 206 (103) | Retrospective cohort | OR 0.70 (0.55–0.89) | PrCa cohort compared with BPH cohort |
| Garcia-Cruz et al. [2012] | 45 (10) | Prospective cohort | Calculated free T of 6.9 versus 9.3 in men with versus men without PrCa | Cohort re-biopsied for HGPIN |
| Studies with no correlation between testosterone and PrCa | ||||
| Roddam et al. [2008] | 10324 (3886) | Meta-analysis of prospective studies | For men with highest tertile of T, PrCa RR 0.94 (0.82–1.07) | Pooled data from 18 prospective studies |
| Muller et al. [2012] | 3242 (819) | Prospective cohort | PrCa rate of 25.5% versus 25.1% in men with low versus high T | Placebo arm of the REDUCE trial |
AM, morning; BLSA, Baltimore Longitudinal Study on Aging; BPH, benign prostatic hypertrophy; CL, confidence interval; DHT, dihydrotestosterone; DRE, digital rectal exam; HGPIN, high grade prostatic intraepithelial neoplasia; HR, hazard ratio; OR, odds ratio; RR, relative risk; PSA, prostate-specific antigen; REDUCE, Reduction by Dutasteride of Prostate Cancer Events; T, testosterone.
Elevated testosterone and PrCa risk
Several longitudinal studies have established a relationship between elevated testosterone and subsequent development of PrCa. Using serum samples and PrCa incidence data from the Physicians’ Health Study, Gann and colleagues identified 222 men with PrCa and 399 controls, matched for age, smoking status and follow up. Compared with controls, men in the highest quartile of serum testosterone were more likely to develop PrCa [odds ratio (OR) 2.6, 95% confidence interval (CI) 1.34–5.02, p = 0.004] [Gann et al. 1996]. In another longitudinal analysis of a cohort of 781 patients enrolled in the BLSA, Pierorazio and colleagues examined the relationship between PrCa, serum testosterone, SHBG and free testosterone index (FTI), calculated as total serum testosterone divided by SHBG [Pierorazio et al. 2010]. Of 145 cases of PrCa, 36 were categorized as high risk, based on modified D’Amico criteria [D’Amico et al. 2003]. They found that high risk PrCa was associated with both FTI [hazard ratio (HR) 1.91, 95% CI 1.10–3.32, p = 0.02] and calculated free testosterone (HR 1.61, 95% CI 1.18–2.20, p = 0.003) [Pierorazio et al. 2010]. It should be noted that longitudinal studies of this kind are limited in that serum testosterone levels were not routinely drawn in the morning, and thus may not reflect the true peak circulating androgen levels. Lastly, Shaneyfelt and colleagues performed a meta-analysis of three prospective nested case-control studies, including the study by Gann and colleagues. Controlling for testosterone, estradiol, SHBG, age and body mass index (BMI), they demonstrated an increase in PrCa for men in the highest quartile of serum testosterone (OR 2.34, 95% CI 1.3–4.2), but no association of PrCa with DHT or estradiol [Shaneyfelt et al. 2000].
Lower testosterone and PrCa risk
While some longitudinal studies have demonstrated increased risk of PrCa with elevations in testosterone, smaller, well-designed studies have demonstrated the opposite, that is, increased PrCa risk in patients with lower testosterone levels. A large study of untreated hypogonadal men with prostate-specific antigen (PSA) <4.0 ng/ml revealed a relationship between low testosterone and PrCa (OR 2.02, 95% CI 1.10–3.72) [Morgentaler and Rhoden, 2006]. A prospective cohort study of Korean men undergoing prostate biopsy for suspected PrCa compared biopsy results among men with low testosterone, defined as total testosterone levels below the median of 13.3 nmol/l (385 ng/dl). On multivariate analysis, low testosterone was associated with PrCa risk (OR 1.99, 95% CI 1.25–3.16, p = 0.003), but not PrCa grade [Shin et al. 2010].
No association of testosterone and PrCa
Arguably the most robust data on the role of endogenous testosterone and PrCa risk comes from the placebo arm of the Reduction by Dutasteride of Prostate Cancer Events (REDUCE) trial which prospectively collected data on androgens and PrCa while comparing men treated with dutasteride or placebo [Muller et al. 2012]. The placebo arm included 3242 patients between the ages of 50 and 75 years who all had at least one prior negative prostate biopsy. Men had baseline androgen levels drawn and underwent prostate biopsy at 2 and 4 years, or for increases in PSA or abnormal digital rectal exam (DRE). In analyzing the placebo arm, researchers identified no association of testosterone or DHT with PrCa incidence or Gleason grade [Muller et al. 2012]. Although information on the time of day for serum measurements was unknown and the study only included men with prior negative biopsy, the large number of patients and routine prostate biopsies make this population-based data in PrCa naive patients highly informative. Similarly, in a large meta-analysis Roddam and colleagues examined the relationship between testosterone and PrCa in patients pooled from the Endogenous Hormones and Prostate Cancer Collaborative Group, which included data from 3886 PrCa patients and 6438 controls. They identified an inverse association of PrCa with SHBG, but not serum testosterone, with a relative risk (RR) of 0.86 [Roddam et al. 2008].
Testosterone and PrCa risk at biopsy
There are a number of studies examining testosterone levels prior to prostate biopsy in men with suspected PrCa. Similar to studies on the general population, data are mixed on the relationship between testosterone and PrCa risk at biopsy. Yano and colleagues performed a prospective study in 420 men referred for prostate biopsy. They measured morning serum testosterone and examined the relationship to PrCa detection. There was no overall association of total testosterone with PrCa. However, in men with PSA levels of <10 ug/l, there was a small but significant increase in PrCa risk among men with increased testosterone (HR 1.31, 95% CI 1.03–1.65, p = 0.02) [Yano et al. 2007].
Conversely, Mearini and colleagues examined the relationship between PrCa and testosterone among 206 men referred for evaluation of suspected PrCa or lower urinary tract symptoms (LUTS). They measured morning testosterone levels and compared 103 men diagnosed with BPH with 103 men diagnosed with PrCa. In multivariate analysis, lower testosterone levels were associated with PrCa (OR 0.70, 95% CI 0.55–0.89, p = 0.004), especially in patients with total testosterone <8.3 nmol/l (240 ng/dl) (OR 0.134, 95% CI 0.039–0.453, p = 0.001) [Mearini et al. 2013]. An association of low testosterone and PrCa was also found in men with high grade prostatic intraepithelial neoplasia (HGPIN), where lower free testosterone was associated with PrCa diagnosis on re-biopsy (p = 0.04) [Garcia-Cruz et al. 2012].
There are several negative studies with mixed data on testosterone levels and associated PrCa risk at prostate biopsy. In a prospective study of 478 patients undergoing prostate biopsy for elevated PSA or abnormal DRE, testosterone levels were compared with biopsy results including cancer detection rates, PSA and Gleason grade. Neither total testosterone nor free testosterone levels were associated with PrCa diagnosis or Gleason grade [Morote et al. 2009]. Likewise, Botelho and colleagues examined total testosterone and biopsy results in a large study of 1570 patients referred for prostate biopsy for abnormal DRE or elevated PSA, and found no association between PrCa on biopsy and total testosterone [Botelho et al. 2012].
Endogenous testosterone in PrCa patients
Despite the large amount of basic science data implicating androgens in PrCa pathogenesis and progression, there is a lack of direct evidence that endogenous testosterone promotes PrCa progression in clinically localized disease. In fact, clinical studies have mainly associated lower testosterone levels with PrCa disease severity.
Several studies have implicated lower testosterone with higher Gleason grade at the time of prostatectomy. Lane and colleagues prospectively measured total testosterone levels of 455 patients with clinically localized PrCa undergoing radical prostatectomy. On multivariate analysis, low testosterone, defined as <7.6 nmol/l (220 ng/dl) was associated with the predominance of Gleason grades 4 and 5 (OR 2.4, 95% CI 1.01–5.7, p = 0.048) [Lane et al. 2008]. Likewise, Dai and colleagues reported on preoperative testosterone levels in 110 Chinese men undergoing prostatectomy for clinically localized disease. Men with Gleason 8 or greater had significantly lower serum testosterone, 14.2 nmol/l (410 ng/dl) versus 11.1 nmol/l (320 ng/dl) (p = 0.028). Furthermore, hypogonadal men, with total morning testosterone <8.6 nmol/l (250 ng/dl), were more likely to have Gleason 8 or greater disease (p = 0.005) [Dai et al. 2012].
In addition to Gleason grade, low preoperative testosterone has been is associated with higher stage at prostatectomy. Xylinas and colleagues found that men with preoperative total testosterone <10.4 nmol/l (300 ng/dl) were more likely to have high-risk disease including Gleason grade >7 and stage pT3–4 on final pathology (p = 0.01 and p = 0.04) [Xylinas et al. 2011]. In a study of 673 men undergoing prostatectomy, Salonia and colleagues examined the association of morning testosterone with surgical pathology outcomes. They identified no association between total testosterone and Gleason grade, but identified an increased risk of seminal vesicle invasion in severely hypogonadal men, defined as testosterone <3.4 nmol/l (100 ng/dl) (OR 3.11, p = 0.006) [Salonia et al. 2010]. In another study of men undergoing prostatectomy, lower pretreatment testosterone was associated with extraprostatic disease (p = 0.046), but not biochemical recurrence [Massengill et al. 2003]. Finally, in examining total testosterone levels in patients undergoing prostatectomy, Imamoto and colleagues found that lower total testosterone was associated with non-organ confined disease (HR 2.16, 95% CI 1.29–3.63, p = 0.003) [Imamoto et al. 2005] .
Testosterone therapy and PrCa
TRT in patients without PrCa
Androgens, including exogenous testosterone, have been shown to play a role in PrCa pathogenesis in cell lines and animal models [Bladou et al. 1996; Ahmad et al. 2008]. Despite this evidence, currently available data do not suggest an increased risk of PrCa in men undergoing TRT.
Haider and colleagues reported on three prospective cohort studies comprising a total of 1023 hypogonadal men with PSA <4 ug/l who were treated with TRT at three centers [Haider et al. 2014]. With a median follow up of 5 years, the incidence of PrCa was lower in TRT-treated populations than accepted incidence rates from large population-based studies with long-term follow up [Andriole et al. 2009; Schroder et al. 2012; Haider et al. 2014]. Likewise, in a smaller study of men at increased risk of PrCa, TRT appeared to be safe with respect to PrCa risk. Rhoden and Morgentaler treated 20 men with HGPIN and 55 men with negative biopsies with 12 months of TRT, and observed no changes in PSA or DRE [Rhoden and Morgentaler, 2003]. This is in agreement with a separate study demonstrating higher incidence of PrCa on re-biopsy in men with HGPIN and lower serum testosterone levels [Garcia-Cruz et al. 2012].
The majority of studies on TRT and PrCa are small and there have been no prospective studies on TRT with sufficient power to determine increased PrCa risk. By one estimate, 6000 patients receiving 5 years of TRT would be needed to detect a 30% increase in PrCa incidence [Bhasin et al. 2003]. In a systematic review of 40 prospective studies of TRT in men without PrCa, no study demonstrated an association between TRT and PrCa risk. Furthermore, in a meta-analysis of 19 placebo-controlled studies, comprising 651 men in the TRT group and 433 men in the placebo group, there was no significant increase in PrCa, PSA >4 ng/dl, or need for prostate biopsy [Calof et al. 2005].
TRT in patients with PrCa
The androgen hypothesis and robust clinical data supporting ADT in advanced PrCa have helped foster the dogma that TRT in PrCa patients is ‘like feeding the fire’. Historically, there are data supporting this concept. In 1982, Fowler and Whitemore reported on 52 men with metastatic PrCa patients with bone metastasis, with 65% having had a prior orchiectomy. With testosterone treatment there was elevation in prostatic acid phosphatase in 38% of men, two cases of measurable metastatic progression, and four deaths [Fowler and Whitemore, 1982]. Importantly, these data are from the pre-PSA era in a mixture of patients with advanced disease that were either untreated, in remission or relapse, and many of whom had prior androgen deprivation [Fowler and Whitemore, 1982]. Thus, it would not be appropriate to apply these observations to men with clinically localized disease in the PSA era who commonly receive early primary treatment and PSA monitoring.
In contrast to historical data, a number of studies suggest that TRT in selected PrCa patients does not lead to disease progression. Using Surveillance, Epidemiology, and End Results (SEER)-Medicare data, Kaplan and colleagues reported on 149,354 men including 1181 men who received TRT after a diagnosis with PrCa. Overall, TRT was not associated with overall or cancer-specific mortality [Kaplan et al. 2014]. Similarly, Pastuszak and colleagues reported on 103 men treated with TRT after prostatectomy. There was an overall increase in serum PSA, but no evidence of increased cancer recurrence over 36 months [Pastuszak et al. 2013]. Similarly, there was no evidence of disease progression following brachytherapy for clinically localized disease among 31 men who underwent TRT for a median duration of 5 years [Sarosdy, 2007].
It should be noted that there have been reports of PrCa patients who have progressed on TRT. Leibowitz and colleagues reported on 96 men with PrCa who began high-dose TRT after undergoing primary PrCa treatment. After a median of 15 months on TRT, men achieved a mean serum testosterone of 48 nmol/l (1391 ng/dl); 43% had evidence of PSA progression, with seven patients showing radiographic progression and PSA decreasing in 59% of men after discontinuation [Leibowitz et al. 2010]. Whether the disease progression in this small number of patients was due to TRT will remain unknown, but it should serve to highlight the need for close monitoring of PrCa patients who are offered TRT.
Studies on TRT in patients with PrCa enrolled in active surveillance (AS) programs provide valuable information on possible tumor progression in PrCa patients, given the frequency of exams, biopsies and close follow up in this population. In a review of 154 men enrolled in AS for very low risk PrCa, free testosterone was found to be associated with reclassification. Compared with men who remained on AS, men with free testosterone levels <1.56 nmol/l (45 ng/dl) were more likely to be reclassified (HR 2.40, 95% CI 1.13–5.10, p = 0.02) [San Francisco et al. 2014]. Finally, in a smaller study, Morgentaler and colleagues examined 13 AS patients receiving TRT. After a median follow up of 2.5 years, two men had upgrading on subsequent biopsy, but no cases of disease or PSA progression were seen [Morgentaler et al. 2011].
There is an apparent disconnect between data supporting androgen-driven PrCa growth and the clinical data, although limited, demonstrating no increase in PrCa growth or progression in men on TRT. In an effort to reconcile these differences, a saturation model was proposed which integrates data on androgen receptor responsiveness with clinical data on the effects of testosterone [Morgentaler and Traish, 2009]. The saturation model proposes a biologic saturation point for the maximal stimulation of prostate tissues by androgens, which falls in the lower range of serum testosterone levels, approximately 8.7 nmol/l (250 ng/dl). Androgen levels above the saturation point will have no further stimulatory effect, thus raising serum testosterone concentrations above the saturation point, as often seen with TRT, fails to induce growth of prostate tissues. Although the merits of the saturation model are beyond the scope of this review, they recently been reviewed in detail elsewhere [Morgentaler and Conners, 2015]. Importantly, the model provides a framework for understanding the effects of testosterone in the context of androgen receptor responsiveness, and highlights the differences between TRT in hypogonadal and eugonadal men.
Conclusion
Overall, there remains no clear answer to the question ‘Does testosterone promote PrCa pathogenesis in humans?’. There is good evidence that androgens can promote PrCa in animal models and that ADT is beneficial in PrCa patients. However, after a large number of mostly retrospective studies, there remains no clear association with higher endogenous testosterone and the development or severity of PrCa. To the contrary, there are several studies associating lower testosterone levels with increased PrCa severity. Whether hypogonadism promotes high-risk disease or is rather a symptom of high-risk disease remains unknown.
With concerns that testosterone can stimulate cancer growth, TRT in men with PrCa remains controversial. Currently, there is a growing amount of evidence that TRT is safe in well-selected men with clinically localized PrCa. However, these results are based on TRT in a small number of patients. Furthermore, the heterogeneity seen clinically in PrCa progression and aggressiveness may give rise to heterogeneity in the responsiveness of tumors to TRT. As patients and providers continue to weigh the potential risks and benefits of TRT, better defining the influence of testosterone on PrCa disease progression is of paramount importance. Thus, until the results of future RCTs are available, TRT should only be offered to select patients who are carefully monitored and well-informed about the potential risks and benefits.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The authors declare no conflicts of interest in preparing this article.
Contributor Information
Jason E. Michaud, The James Buchanan Brady Urological Institute, The Johns Hopkins Medical Institutions, 600 N, Wolfe Street, Baltimore, MD 21287, USA.
Kevin L. Billups, The James Buchanan Brady Urological Institute, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
Alan W. Partin, The James Buchanan Brady Urological Institute, The Johns Hopkins University School of Medicine, Baltimore, MD, USA
References
- Ahmad I., Sansom O., Leung H. (2008) Advances in mouse models of prostate cancer. Expert Rev Mol Med 10: e16. [DOI] [PubMed] [Google Scholar]
- Al-Khazaali A., Arora R., Muttar S. (2015) Controversial effects of exogenous testosterone on cardiovascular diseases. Am J Ther. 13 Febriary 2015. [Epub ahead of print]. [DOI] [PubMed] [Google Scholar]
- Andriole G., Bruchovsky N., Chung L., Matsumoto A., Rittmaster R., Roehrborn C., et al. (2004) Dihydrotestosterone and the prostate: the scientific rationale for 5alpha-reductase inhibitors in the treatment of benign prostatic hyperplasia. J Urol 172: 1399–1403. [DOI] [PubMed] [Google Scholar]
- Andriole G., Crawford E., Grubb R., Buys S., Chia D., Church T., et al. (2009) Mortality results from a randomized prostate-cancer screening trial. New Engl J Med 360: 1310–1319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Araujo A., O’Donnell A., Brambilla D., Simpson W., Longcope C., Matsumoto A., et al. (2004) Prevalence and incidence of androgen deficiency in middle-aged and older men: estimates from the Massachusetts male aging study. J Clin Endocrinol Metab 89: 5920–5926. [DOI] [PubMed] [Google Scholar]
- Baillargeon J., Urban R., Ottenbacher K., Pierson K., Goodwin J. (2013) Trends in androgen prescribing in the United States, 2001 to 2011. JAMA Intern Med 173: 1465–1466. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basaria S. (2014) Male hypogonadism. Lancet 383: 1250–1263. [DOI] [PubMed] [Google Scholar]
- Bhasin S., Singh A., Mac R., Carter B., Lee M., Cunningham G. (2003) Managing the risks of prostate disease during testosterone replacement therapy in older men: recommendations for a standardized monitoring plan. J Androl 24: 299–311. [DOI] [PubMed] [Google Scholar]
- Bladou F., Vessella R., Buhler K., Ellis W., True L., Lange P. (1996) Cell proliferation and apoptosis during prostatic tumor xenograft involution and regrowth after castration. Int J Cancer 67: 785–790. [DOI] [PubMed] [Google Scholar]
- Botelho F., Pina F., Figueiredo L., Cruz F., Lunet N. (2012) Does baseline total testosterone improve the yielding of prostate cancer screening? Eur J Cancer 48: 1657–1663. [DOI] [PubMed] [Google Scholar]
- Bremner W., Vitiello M., Prinz P. (1983) Loss of circadian rhythmicity in blood testosterone levels with aging in normal men. J Clin Endocrinol Metab 56: 1278–1281. [DOI] [PubMed] [Google Scholar]
- Calof O., Singh A., Lee M., Kenny A., Urban R., Tenover J., et al. (2005) Adverse events associated with testosterone replacement in middle-aged and older men: a meta-analysis of randomized, placebo-controlled trials. J Gerontol A Biol Sci Med Sci 60: 1451–1457. [DOI] [PubMed] [Google Scholar]
- D’Amico A., Moul J., Carroll P., Sun L., Lubeck D., Chen M. (2003) Surrogate end point for prostate cancer-specific mortality after radical prostatectomy or radiation therapy. J Natl Cancer Inst 95: 1376–1383. [DOI] [PubMed] [Google Scholar]
- Dai B., Qu Y., Kong Y., Ye D., Yao X., Zhang S., et al. (2012) Low pretreatment serum total testosterone is associated with a high incidence of Gleason score 8–10 disease in prostatectomy specimens: data from ethnic Chinese patients with localized prostate cancer. BJU Int 110: E667–E672. [DOI] [PubMed] [Google Scholar]
- Ellison P., Bribiescas R., Bentley G., Campbell B., Lipson S., Panter-Brick C., et al. (2002) Population variation in age-related decline in male salivary testosterone. Hum Reprod 17: 3251–3253. [DOI] [PubMed] [Google Scholar]
- Fowler J., Whitemore W. (1982) Considerations for the use of testosterone with systemic chemotherapy in prostate cancer. Cancer 49: 1373–1377. [DOI] [PubMed] [Google Scholar]
- Gann P., Hennekens C., Ma J., Longcope C., Stampfer M. (1996) Prospective study of sex hormone levels and risk of prostate cancer. J Natl Cancer Inst 88: 1118–1126. [DOI] [PubMed] [Google Scholar]
- Garcia-Cruz E., Piqueras M., Ribal M., Huguet J., Serapiao R., Peri L., et al. (2012) Low testosterone level predicts prostate cancer in re-biopsy in patients with high grade prostatic intraepithelial neoplasia. BJU Int 110: E199–E202. [DOI] [PubMed] [Google Scholar]
- Haider A., Zitzmann M., Doros G., Isbarn H., Hammerer P., Yassin A. (2014) Incidence of prostate cancer in hypogonadal men receiving testosterone therapy: observations from 5-year median followup of 3 registries. J Urol 193: 80–86. [DOI] [PubMed] [Google Scholar]
- Handelsman D. (2013) Global trends in testosterone prescribing, 2000–2011: expanding the spectrum of prescription drug misuse. Med J Aust 199: 548–551. [DOI] [PubMed] [Google Scholar]
- Harman S., Metter E., Tobin J., Pearson J., Blackman M. and Baltimore Longitudinal Study of Aging. (2001) Longitudinal effects of aging on serum total and free testosterone levels in healthy men. Baltimore longitudinal study of aging. J Clin Endocrinol Metab 86: 724–731. [DOI] [PubMed] [Google Scholar]
- Howden L., Meyer J. (2011) Age and sex composition: 2010, 2010 Census briefs. Vol. 2015 Washington, DC: United States Census Bureau. [Google Scholar]
- Huggins C. (1947) The etiology of benign prostatic hypertrophy. Bull NY Acad Med 23: 696–704. [PMC free article] [PubMed] [Google Scholar]
- Huggins C., Hodges C. (1941) Studies on prostatic cancer: I. The effect of castration, of estrogen and of androgen injection on serum phosphatases in metastatic carcinoma of the prostate. Cancer Res 1: 293–297. [DOI] [PubMed] [Google Scholar]
- Imamoto T., Suzuki H., Fukasawa S., Shimbo M., Inahara M., Komiya A., et al. (2005) Pretreatment serum testosterone level as a predictive factor of pathological stage in localized prostate cancer patients treated with radical prostatectomy. Eur Urol 47: 308–312. [DOI] [PubMed] [Google Scholar]
- Kaplan A., Trinh Q., Sun M., Carter S., Nguyen P., Shih Y., et al. (2014) Testosterone replacement therapy following the diagnosis of prostate cancer: outcomes and utilization trends. J Sex Med 11: 1063–1070. [DOI] [PubMed] [Google Scholar]
- Kyprianou N., English H., Isaacs J. (1990) Programmed cell death during regression of Pc-82 human prostate cancer following androgen ablation. Cancer Res 50: 3748–3753. [PubMed] [Google Scholar]
- Lane B., Stephenson A., Magi-Galluzzi C., Lakin M., Klein E. (2008) Low testosterone and risk of biochemical recurrence and poorly differentiated prostate cancer at radical prostatectomy. Urology 72: 1240–1245. [DOI] [PubMed] [Google Scholar]
- Leibowitz R., Dorff T., Tucker S., Symanowski J., Vogelzang N. (2010) Testosterone replacement in prostate cancer survivors with hypogonadal symptoms. BJU Int 105: 1397–1401. [DOI] [PubMed] [Google Scholar]
- Massengill J., Sun L., Moul J., Wu H., Mcleod D., Amling C., et al. (2003) Pretreatment total testosterone level predicts pathological stage in patients with localized prostate cancer treated with radical prostatectomy. J Urol 169: 1670–1675. [DOI] [PubMed] [Google Scholar]
- Matsumoto A. (2003) Fundamental aspects of hypogonadism in the aging male. Rev Urol 5: S3–S10. [PMC free article] [PubMed] [Google Scholar]
- Mearini L., Zucchi A., Nunzi E., Villirillo T., Bini V., Porena M. (2013) Low serum testosterone levels are predictive of prostate cancer. World J Urol 31: 247–252. [DOI] [PubMed] [Google Scholar]
- Miner M., Barkin J., Rosenberg M. (2014) Testosterone deficiency: myth, facts, and controversy. Can J Urol 21(Suppl. 2): 39–54. [PubMed] [Google Scholar]
- Morgentaler A., Conners W. (2015) Testosterone therapy in men with prostate cancer: literature review, clinical experience, and recommendations. Asian J Androl 17: 206–211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgentaler A., Lipshultz L., Bennett R., Sweeney M., Avila D., Jr., Khera M. (2011) Testosterone therapy in men with untreated prostate cancer. J Urol 185: 1256–1260. [DOI] [PubMed] [Google Scholar]
- Morgentaler A., Miner M., Caliber M., Guay A., Khera M., Traish A. (2015) Testosterone therapy and cardiovascular risk: advances and controversies. Mayo Clin Proc 90: 224–251. [DOI] [PubMed] [Google Scholar]
- Morgentaler A., Rhoden E. (2006) Prevalence of prostate cancer among hypogonadal men with prostate-specific antigen levels of 4.0 Ng/Ml or less. Urology 68: 1263–1267. [DOI] [PubMed] [Google Scholar]
- Morgentaler A., Traish A. (2009) Shifting the paradigm of testosterone and prostate cancer: the saturation model and the limits of androgen-dependent growth. Eur Urol 55: 310–320. [DOI] [PubMed] [Google Scholar]
- Morote J., Ramirez C., Gomez E., Planas J., Raventos C., De Torres I., et al. (2009) The relationship between total and free serum testosterone and the risk of prostate cancer and tumour aggressiveness. BJU Int 104: 486–489. [DOI] [PubMed] [Google Scholar]
- Muller M., Den Tonkelaar I., Thijssen J., Grobbee D., Van Der Schouw Y. (2003) Endogenous sex hormones in men aged 40–80 years. Eur J Endocrinol 149: 583–589. [DOI] [PubMed] [Google Scholar]
- Muller R., Gerber L., Moreira D., Andriole G., Castro-Santamaria R., Freedland S. (2012) Serum testosterone and dihydrotestosterone and prostate cancer risk in the placebo arm of the reduction by dutasteride of prostate cancer events trial. Eur Urol 62: 757–764. [DOI] [PubMed] [Google Scholar]
- Mulligan T., Frick M., Zuraw Q., Stemhagen A., McWhirter C. (2006) Prevalence of hypogonadism in males aged at least 45 years: the HIM study. Int J Clin Pract 60: 762–769. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Neaves W., Johnson L., Porter J., Parker C., Jr., Petty C. (1984) Leydig cell numbers, daily sperm production, and serum gonadotropin levels in aging men. J Clin Endocrinol Metab 59: 756–763. [DOI] [PubMed] [Google Scholar]
- Pastuszak A., Pearlman A., Lai W., Godoy G., Sathyamoorthy K., Liu J., et al. (2013) Testosterone replacement therapy in patients with prostate cancer after radical prostatectomy. J Urol 190: 639–644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pierorazio P., Ferrucci L., Kettermann A., Longo D., Metter E., Carter H. (2010) Serum testosterone is associated with aggressive prostate cancer in older men: results from the Baltimore longitudinal study of aging. BJU Int 105: 824–829. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rhoden E., Morgentaler A. (2003) Testosterone replacement therapy in hypogonadal men at high risk for prostate cancer: results of 1 year of treatment in men with prostatic intraepithelial neoplasia. J Urol 170: 2348–2351. [DOI] [PubMed] [Google Scholar]
- Roddam A., Allen N., Appleby P., Key T. (2008) Endogenous sex hormones and prostate cancer: a collaborative analysis of 18 prospective studies. J Natl Cancer Inst 100: 170–183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rove K., Crawford E. (2014) Traditional androgen ablation approaches to advanced prostate cancer: new insights. Can J Urol 21: 14–21. [PubMed] [Google Scholar]
- Rubens R., Dhont M., Vermeulen A. (1974) Further studies on Leydig cell function in old age. J Clin Endocrinol Metab 39: 40–45. [DOI] [PubMed] [Google Scholar]
- Salonia A., Gallina A., Briganti A., Abdollah F., Suardi N., Capitanio U., et al. (2010) Preoperative hypogonadism is not an independent predictor of high-risk disease in patients undergoing radical prostatectomy. Cancer 117: 3953–3962. [DOI] [PubMed] [Google Scholar]
- San Francisco I., Rojas P., Dewolf W., Morgentaler A. (2014) Low free testosterone levels predict disease reclassification in men with prostate cancer undergoing active surveillance. BJU Int 114: 229–235. [DOI] [PubMed] [Google Scholar]
- Sarosdy M. (2007) Testosterone replacement for hypogonadism after treatment of early prostate cancer with brachytherapy. Cancer 109: 536–541. [DOI] [PubMed] [Google Scholar]
- Schroder F., Hugosson J., Roobol M., Tammela T., Ciatto S., Nelen V., et al. (2012) Prostate-cancer mortality at 11 years of follow-up. New Engl J Med 366: 981–990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schwab T., Stewart T., Lehr J., Pienta K., Rhim J., Macoska J. (2000) Phenotypic characterization of immortalized normal and primary tumor-derived human prostate epithelial cell cultures. Prostate 44: 164–171. [DOI] [PubMed] [Google Scholar]
- Shaneyfelt T., Husein R., Bubley G., Mantzoros C. (2000) Hormonal predictors of prostate cancer: a meta-analysis. J Clin Oncol 18: 847. [DOI] [PubMed] [Google Scholar]
- Shin B., Hwang E., Im C., Kim S., Jung S., Kang T., et al. (2010) Is a decreased serum testosterone level a risk factor for prostate cancer? A cohort study of Korean men. Korean J Urol 51: 819–823. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siiteri P., Wilson J. (1974) Testosterone formation and metabolism during male sexual differentiation in the human embryo. J Clin Endocrinol Metab 38: 113–125. [DOI] [PubMed] [Google Scholar]
- Spitzer M., Huang G., Basaria S., Travison T., Bhasin S. (2013) Risks and benefits of testosterone therapy in older men. Nat Rev Endocrinol 9: 414–424. [DOI] [PubMed] [Google Scholar]
- Swerdloff R., Wang C. (2011) Testosterone treatment of older men: why are controversies created? J Clin Endocrinol Metab 96: 62–65. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Swyer G. (1944) Post-natal growth changes in the human prostate. J Anat 78: 130–145. [PMC free article] [PubMed] [Google Scholar]
- Tenover J., Matsumoto A., Clifton D., Bremner W. (1988) Age-related alterations in the circadian rhythms of pulsatile luteinizing hormone and testosterone secretion in healthy men. J Gerontol 43: M163–M169. [DOI] [PubMed] [Google Scholar]
- Vermeulen A., Kaufman J. (1995) Ageing of the hypothalamo-pituitary-testicular axis in men. Horm Res 43: 25–28. [DOI] [PubMed] [Google Scholar]
- Wang C., Nieschlag E., Swerdloff R., Behre H., Hellstrom W., Gooren L., et al. (2008) Investigation, treatment and monitoring of late-onset hypogonadism in males: ISA, ISSAM, EAU, EAA and ASA recommendations. Eur J Endocrinol 159: 507–514. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Webber M., Bello D., Quader S. (1996) Immortalized and tumorigenic adult human prostatic epithelial cell lines: characteristics and applications. Part I. Cell markers and immortalized nontumorigenic cell lines. Prostate 29: 386–394. [DOI] [PubMed] [Google Scholar]
- Wu F., Tajar A., Beynon J., Pye S., Silman A., Finn J., et al. (2010) Identification of late-onset hypogonadism in middle-aged and elderly men. N Engl J Med 363: 123–135. [DOI] [PubMed] [Google Scholar]
- Xylinas E., Ploussard G., Durand X., Fabre A., Salomon L., Allory Y., et al. (2011) Low pretreatment total testosterone (<3 ng/ml) predicts extraprostatic disease in prostatectomy specimens from patients with preoperative localized prostate cancer. BJU Int 107: 1400–1403. [DOI] [PubMed] [Google Scholar]
- Yano M., Imamoto T., Suzuki H., Fukasawa S., Kojima S., Komiya A., et al. (2007) The clinical potential of pretreatment serum testosterone level to improve the efficiency of prostate cancer screening. Eur Urol 51: 375–380. [DOI] [PubMed] [Google Scholar]


