Abstract
Urothelial carcinoma is a common malignancy that carries a poor prognosis when the disease includes muscle invasion. Metastatic urothelial carcinoma is almost uniformly fatal. The evidence behind treatment options in the neoadjuvant, adjuvant and metastatic settings are discussed in this manuscript, with a focused review of standard and investigational cytotoxic, targeted, and immunotherapy approaches. We have focused especially on neoadjuvant cisplatin-based therapy (supported by level one evidence) and on novel immunotherapy agents such as checkpoint inhibitors, which have shown great promise in early clinical studies.
Keywords: bladder cancer, chemotherapy, immunotherapy, targeted therapy, urothelial carcinoma
Introduction
Bladder cancer is the most common urinary system malignancy, with urothelial carcinoma (UC; formerly transitional cell carcinoma) being the most prevalent histologic subtype in the United States and Europe. An estimated 74,000 cases of UC will be diagnosed in the United States in 2015 with almost 16,000 expected deaths [Siegel et al. 2015]. Progress in systemic therapy for muscle-invasive bladder carcinoma (MIBC) has been stagnant for decades, with few new systemic therapies being evaluated until recently [Hussain et al. 2009]. Simultaneously, therapeutic interventions proven to effect survival outcomes have not been widely adopted [Pal et al. 2013]. Recently, intense interest has developed in the molecular profiling of UC, both to understand the biology of these tumors and to develop novel therapies [Cancer Genome Atlas Research, 2014]. This review will outline some of the pivotal studies which led to the current use of chemotherapy in the neoadjuvant, adjuvant, and metastatic settings for pure or predominant UC, as well as future directions in immunotherapies and targeted therapies in these various disease settings.
Neoadjuvant chemotherapy
Prior to 2003, cystectomy alone was the standard of care treatment for MIBC. The disease often recurred at distant sites, suggesting the presence of micro-metastases at the time of surgery. As a result, studies began to examine neoadjuvant chemotherapy (NAC) as a means to target these micro-metastases, with the hope of improving survival in this population [Hussain et al. 2009]. Although several initial randomized trials did not show a survival advantage of NAC in patients with localized MIBC [Wallace et al. 1991; Martinez-Pineiro et al. 1995; Coppin et al. 1996; Sherif et al. 2002], these trials have been critiqued as being small, having design limitations, using only single-agent chemotherapy, and lacking standardized local therapy. More recent data clearly supports the utility of multidrug NAC in UC patients with MIBC prior to cystectomy and is discussed below.
A randomized controlled trial published in 1999 looked at a neoadjuvant three-drug regimen (cisplatin, methotrexate, vinblastine) followed by surgery or radiation or a combination of radiation and surgery (definitive treatment), versus definitive treatment alone [International Collaboration of Trialists, 1999]. After 8 years, the survival advantage in the group receiving NAC became statistically significant with a 16% risk reduction in death [hazard ratio (HR) 0.84, 95% confidence interval (CI) 0.72–0.99, p = 0.037] and a 6% increase in 10-year survival [Griffiths et al. 2011].
A second prospective randomized controlled trial supporting the use of NAC came from the Southwest Oncology Group (SWOG); this trial evaluated NAC in 317 patients with locally advanced MIBC (pT2-pT4a) [Grossman et al. 2003]. Half were treated with NAC, a regimen of traditional methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) followed by cystectomy, which resulted in a 31-month increase in median survival (p = 0.06) as compared with the group receiving cystectomy alone. The patients who received neoadjuvant MVAC also had a significant improvement in pathologic complete response (38% versus 15%, p < 0.001).
A smaller Japanese phase III study of 130 patients with MIBC (T2-T4) randomized patients to either two cycles of MVAC followed by cystectomy or cystectomy alone; this trial showed an overall survival (OS) of 102 months in the NAC arm versus 82 months in the control, although the difference was not statistically significant (p = 0.07) [Kitamura et al. 2014]. There was, however, a significant increase in the pathologic complete response in the chemotherapy arm (34% versus 9%, p < 0.01).
Finally, a meta-analysis published in 2005 reviewed studies of 3005 patients from 11 trials who received platinum-based combination chemotherapy in the neoadjuvant setting [Advanced Bladder Cancer Meta-Analysis, 2005]. There was a 5% statistically significant increase in OS at 5 years (HR = 0.86, 95% CI 0.77–0.95, p = 0.003), and a 9% improvement in disease-free survival (DFS) at 5 years (HR = 0.78, 95% CI 0.71–0.86, p < 0.0001).
Based on these data, NAC is routinely recommended for treatment of localized MIBC in the United States [Clark et al. 2013]. However, despite this level one evidence, several studies utilizing the National Cancer Data Base (NCDB) showed suboptimal use of NAC, with perioperative chemotherapy being administered in only 11.6% of stage III bladder cancer patients in one study [David et al. 2007] and 34.5% of any-stage patients undergoing surgery in another study [Fedeli et al. 2011]. Historically, there have been concerns that NAC causes significant toxicities, surgical delays, and ultimately worsens surgical morbidity and mortality [Donat, 2009; Bajorin and Herr, 2011]. Importantly, the SWOG group study regimen using standard MVAC was developed decades ago, prior to the use of growth factors, antiemetics, and other supportive medications that are routinely used today. Therefore, there were high rates of toxicities, most frequently cytopenias, stomatitis, nausea and vomiting [Grossman et al. 2003]. A study published in 2000 found that gemcitabine and cisplatin (GC) had similar efficacy and improved tolerability compared to standard MVAC in the metastatic setting [Von Der Maase et al. 2000]. This was then extrapolated to the neoadjuvant setting when a retrospective study published in 2008 showed that four cycles (12 weeks) of GC given prior to cystectomy had similar rates of pathologic response compared with standard MVAC [Dash et al. 2008].
To address some of the toxicity concerns, studies looking at variations to the delivery of MVAC have been published, including a high dose-intensity regimen known as accelerated MVAC (AMVAC) in which each of the four drugs are given every two weeks along with granulocyte colony-stimulating factor (G-CSF). A phase III study of AMVAC in the metastatic setting showed improvements in disease progression and OS, with improved tolerability compared with standard MVAC [Sternberg et al. 2006]. AMVAC was then tested in the neoadjuvant setting in two single arm phase II trials published in 2014 by Plimack and colleagues [Plimack et al. 2014] and Choueiri and coworkers [Choueiri et al. 2014]. The trial by Plimack and colleagues enrolled 44 patients with cT2-cT4a MIBC who were treated with three cycles of AMVAC followed by cystectomy. This study showed that 6 weeks of NAC led to a 38% (95% CI 23–53%) complete pathologic response rate with an additional 14% being downstaged to non-muscle-invasive cancer. This trial published similar pathologic response rates compared with the standard MVAC regimen, and it had good tolerability with most patients (82%) having only grade 1 and 2 toxicities. Choueiri and colleagues reported that of 39 patients with cT2-cT4 MIBC who received four cycles of AMVAC followed by cystectomy, 49% (80% CI 38–61) were downstaged to non-muscle-invasive disease, and that the regimen was similarly well tolerated with only 10% of patients having grade 3 or higher toxicity. Current guidelines in the United States list AMVAC as well as GC as optimal choices for NAC in advanced UC [National Comprehensive Cancer Network, 2015]. Neoadjuvant trials [e.g. ClinicalTrials.gov identifier: NCT02365766] exploring the use of checkpoint inhibitors are in early phases of development, and may increase treatment options in the future. Table 1 summarizes these findings.
Table 1.
Key trials supporting the current neoadjuvant, adjuvant and metastatic chemotherapy regimens.
| Chemotherapy | Trial characteristics |
Outcomes | Reference | ||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | N | R | Line | ||||||||
| Neoadjuvant | Response rates | Survival outcomes (months) | |||||||||
| MVAC versus cystectomy or RT alone (T2 to T4aN0) |
III | 317 | Y | 1st | MVAC
cystectomy alone |
↓pT0 = 38%
↓pT0 = 15% p<0.001 |
MVAC
Cystectomy/RT alone (months) |
MS
MS |
77
46 p=0.06 |
Grossman et al. (2003) | |
| CMV versus cystectomy alone (T2 to T4aN0) |
III | 976 | Y | 1st | CMV | ↓pT0 = 32.5% | CMV
cystectomy alone (10-year f/u) |
OS
OS |
36%
30% |
International Collaboration of Trialists (1999) | |
| AMVAC
(cT2-T4a and N0-1) |
II | 44 | N | 1st | AMVAC | ↓pT0 = 38%
↓pT1 = 14% |
Plimack et al. 2014 | ||||
| AMVAC
(cT2-cT4, N0-1) |
II | 39 | N | 1st | AMVAC | PaR ≤ pT1 in 49% | with PaR
without PaR |
1 yr DFS | 89%
67% |
Choueiri et al. (2014) | |
| Adjuvant | 5-year recurrence outcomes | 5-year survival outcomes | |||||||||
| GC versus chemo at relapse (T2G3, T3 to T4, N0-2) |
III | 194 | Y | 1st | GC
chemo at relapse |
DFS
DFS |
42.3%
37.2% p=0.70 |
GC
Chemo at Relapse |
OS
OS |
53.7%
43.4% p=0.24 |
Cognetti et al. (2012) |
| MVAC versus observation (T1 and T2N0) |
III | 114 | Y | 1st | MVAC
observation |
TTR | 0.20; p=0.62; HR=0.78 | MVAC
observation |
OS
OS |
85%
85% |
Stadler et al. (2011) |
| AMVAC/GC/MVAC versus chemo at relapse (T3 to T4 and/or N+M0) |
III | 284 | Y | 1st | adjuvant chemo
chemo at relapse |
PFS
PFS |
31.8%
47.6% p<0.05 |
adjuvant chemo
chemo at relapse |
OS
OS |
47.7%
53.6% p=0.13 |
Sternberg et al. (2015) |
| Metastatic | Response rates | Survival outcomes (months) | |||||||||
| MVAC versus cisplatin | III | 269 | Y | 1st | MVAC
Cisplatin |
ORR
ORR |
39%
12% |
MVAC
Cisplatin |
PFS
OS PFS OS |
10.0
12.5 4.3 8.2 |
Loehrer et al. (1992) |
| GC versus MVAC | III | 405 | Y | 1st | GC
MVAC |
CR PR ORR CR PR ORR |
12.2%
37.2% 49.4% 11.9% 33.8% 45.7% |
GC
MVAC |
PFS
MS 5-yr OS PFS MS 5-yr OS |
7.7
14.0 13.0% 8.3 15.2 15.3% |
Von der Maase et al. (2005) |
| Classical MVAC versus AMVAC | III | 263 | Y | 1st | MVAC | CR
PR ORR |
9%
41% 50% |
MVAC | PFS
MS 5-yr OS |
8.1
14.9 13.5% |
Sternberg et al. (2006) |
| AMVAC | CR
PR ORR |
21%
41% 64% |
AMVAC | PFS
MS 5-yr OS |
9.5
15.1 21.8% |
||||||
Abbreviations: N, number randomized; R, randomized; Y, yes; N, no; MVAC, methotrexate, vinblastine, doxorubicin, cisplatin; MS, median survival; CMV, cisplatin, methotrexate, vinblastine; OS, overall survival; MRC, Medical Research Council; EORTC, European Organization for Research and Treatment of Cancer; AMVAC, accelerated MVAC; PaR, pathologic response; GC, gemcitabine, cisplatin; DFS, disease free survival; TTR, time to recurrence; PFS, progression-free survival; ORR, overall response rate; CR, complete response; PR, partial response; RT, radiation therapy.
Adjuvant chemotherapy
Despite the phase II and III data supporting the use of NAC for MIBC, many of these patients go directly to radical cystectomy (RC) without receiving NAC. Until recently perioperative chemotherapy has most commonly been administered in the adjuvant setting where supporting evidence for this approach is weaker [Schrag et al. 2005; David et al. 2007]. One significant limitation to the timely initiation of adjuvant chemotherapy (AC) after RC is the high surgical complication rate following RC. For example, of 1142 patients evaluated at Memorial Sloan Kettering Cancer Center, 64% experienced one or more postoperative complications, and it was estimated that 30% of these patients were unable to receive AC after RC due to these complications [Donat et al. 2009].
For patients who remain candidates for chemotherapy after RC, AC should be considered, especially in patients with known high-risk features such as non-organ-confined (pT3 or T4) disease and pathologic node positivity. A recent meta-analysis reviewed outcomes after cisplatin-based AC from 945 patients enrolled in nine randomized trials [Leow et al. 2014]. Of these trials, some were unpublished, and most were prematurely terminated for a variety of reasons including poor accrual. While all patients included in these trials received cisplatin, there was heterogeneity in regards to dosing regimens, duration of therapy, and drug combinations. Individually, some of the older studies included in the analysis had shown improvements in DFS favoring AC without OS data [Skinner et al. 1990; Stockle et al. 1995; Freiha et al. 1996]. More contemporary randomized trials included in the analysis looked at AC with either gemcitabine plus cisplatin versus observation or gemcitabine plus cisplatin and paclitaxel versus observation and had conflicting results, with only the three-drug regimen showing an improvement in outcomes including OS [Paz-Ares et al. 2010; Cognetti et al. 2012]. With less than 200 patients in each of these contemporary studies, the ability to draw conclusions based on the findings was limited. Nonetheless, the pooled analysis showed a statistically significant improvement in OS (HR 0.77, 95% CI 0.59–0.99; p = 0.049) and DFS (HR 0.66, 95% CI 0.45–0.91; p = 0.014). These findings are consistent with an international retrospective analysis of off-protocol AC in UC [Svatek et al. 2010]. This cohort included 3947 patients, of which 932 received AC and showed an improvement in OS (HR 0.83, 95% CI 0.72–0.97, p = 0.017). Risk stratification analysis based on primary tumor (T) and nodal (N) status revealed that the relative benefit of AC was contingent on severity of disease, with the higher-risk subgroups receiving the majority of the benefit [Svatek et al. 2010].
Alternatively, some trials have evaluated the differences in outcomes of immediate AC versus deferred chemotherapy at the time of relapse. The recently published phase III EORTC 30994 trial randomized 284 patients across Europe and Canada to immediate versus deferred cisplatin based chemotherapy with either GC, MVAC, or AMVAC [Sternberg et al. 2015]. Eligible patients had pT3-pT4 or pN1-3 disease. After a median follow-up of 7 years, there was no significant improvement in OS with immediate versus deferred therapy (HR 0.78, 95% CI 0.56–1.08; p = 0.13). However, 5-year progression-free survival (PFS) was significantly prolonged with immediate treatment (47.6% versus 31.8%). Like its predecessors, this study was underpowered, and hopefully larger studies can identify which subgroups will benefit most from AC in the future. Table 1 summarizes the findings of the key AC trials in UC.
While newer targeted drugs and immunotherapies are being studied in the metastatic setting, their usefulness in the neoadjuvant and adjuvant settings is also an intriguing area of ongoing research. A fully accrued, but ongoing study uses DN24-02, an autologous cellular immunotherapy targeting HER2/neu, as an adjuvant therapy in patients with high-risk HER2+ UC [ClinicalTrials.gov identifier: NCT01353222; Bajorin et al. 2014]. Of the 226 specimens screened, 75% of patients had HER2 expression of ⩾1+, and preliminary data suggests an immunologic humoral antibody response to the autologous cellular immunotherapy. As in the neoadjuvant setting, trials utilizing checkpoint inhibitors in the adjuvant setting are currently accruing [e.g. ClinicalTrials.gov identifier: NCT02450331].
Similar to the neoadjuvant recommendations, the NCCN currently suggests using AMVAC, GC, or CMV (cisplatin, methotrexate, and vinblastine) as AC in high-risk MIBC patients with T3, T4, or node-positive disease at cystectomy (category 2A) [National Comprehensive Cancer Network, 2015]. It is important to determine patient eligibility for cisplatin therapy including factors such as performance status (PS), creatinine clearance, and comorbidities [Galsky et al. 2011]. Advanced age alone should not be a deterrent for eligibility in good PS patients. A retrospective study demonstrated that while elderly patients (age ⩾ 70) had increased rates of toxicity with cisplatin-based chemotherapy, median survival was not inferior to those of younger age [Bamias et al. 2005]. However, elderly patients with a PS ⩾ 2 did have a significantly inferior survival, emphasizing the importance of this surrogate marker as a predictor of benefit from AC. Based on previous data, the substitution of carboplatin for cisplatin, cisplatin monotherapy, and non-cisplatin regimens are not recommended in the adjuvant or neoadjuvant settings [Studer et al. 1994; Gallagher et al. 2009; Lehmann et al. 2013, National Comprehensive Cancer Network, 2015].
Chemotherapy for metastatic disease
Most cases of bladder cancer are diagnosed before the disease has spread to distant sites, however, roughly 4% of all patients with new diagnoses of bladder cancer are found to have distant metastatic disease at the time of diagnosis [American Cancer Society, 2015]. However, for patients diagnosed with MIBC, the likelihood of micro-metastatic disease or overt clinical metastatic disease is much higher; an autopsy study of 367 patients with MIBC found 68% to have metastatic disease [Wallmeroth et al. 1999]. The standard of care remains systemic chemotherapy, using cisplatin-based combination regimens similar to those described above in the perioperative setting (e.g. AMVAC and GC).
Single-agent cisplatin was compared with standard MVAC in a randomized international intergroup trial for patients with advanced UC not curable by surgery or radiation therapy (RT) (n = 246), and MVAC was found to have superior response rates (39% versus 12%), PFS (10.0 versus 4.3 months), and OS (12.5 versus 8.2 months) [Loehrer et al. 1992]. As expected, the MVAC regimen had increased rates of toxicity compared with cisplatin, including mucositis and neutropenic fever, as well as drug-related mortality. Importantly, as mentioned above, this trial was conducted prior to the currently available supportive care modalities. MVAC was later compared with GC in a large (n = 405), randomized international phase III trial for patients with untreated locally advanced (T4b, or N2-N3) or metastatic UC [Von Der Maase et al. 2000]. The toxicity profiles differed between the two regimens, with GC having higher rate of grades 3/4 thrombocytopenia (57% in GC versus 21% in MVAC) and grades 3/4 anemia (27% in GC versus 18% in MVAC), and MVAC having higher rates of grades 3/4 neutropenia (82% in MVAC versus 71% in GC), neutropenic fever (14% in MVAC versus 2% in GC) and grades 3/4 mucositis (22% in MVAC versus 1% in GC). GC was found to have comparable efficacy with a difference in median OS which was not statistically significant (15.2 months in MVAC versus 14.0 months in GC, p = 0.66), as were the survival rates at 5 years (15.3% in MVAC versus 13% in GC, p = 0.53) [Von Der Maase et al. 2005]. Therefore, many clinicians have opted for GC as the standard first-line treatment for metastatic bladder cancer, especially at that time. Later studies, however, showed a statistically significant difference in response rate favoring AMVAC with G-CSF support compared with standard MVAC (64% versus 50%) in patients with advanced urothelial cancer; a difference in survival after a median follow-up of 7.3 years also favored AMVAC (24.6% in AMVAC versus 13.2% in standard MVAC), and thus if MVAC is to be used in the metastatic setting, the accelerated regimen is recommended over the traditional MVAC schedule [Sternberg et al. 2001, 2006].
Given the relative tolerability of the GC regimen, a third regimen which added paclitaxel to gemcitabine and cisplatin (PCG) was also evaluated in the metastatic UC setting. A phase III study performed by the EORTC randomized 626 patients to either PCG or GC alone. There was no statistical benefit for median OS (15.8 months in PCG versus 12.7 months in GC, p = 0.075) [Bellmunt et al. 2012]. More severe acute toxicities were noted in the PCG group (20.2%, versus 14.8% in the GC group). Based on the questionable benefit and risk for higher toxicity, PCG has not become widely adopted.
Finally, there is no established standard of care for second-line treatment and beyond. Many trials, most relatively small in size, have been conducted with a multitude of agents. Vinflunine is approved in Europe on the basis of a phase III trial of this drug versus best supportive care, which showed a PFS benefit favoring vinflunine, but no significant difference in OS. Given the lack of survival benefit, this drug is not approved in the US [Bellmunt et al. 2009]. In the US, single-agent second-line options with activity in UC include the taxanes (paclitaxel, docetaxel), pemetrexed, 5-FU, ifosfamide, as well as the drugs comprising the MVAC and GC regimens [Clark et al. 2013]. Recently, eribulin has been shown to be an active drug in the metastatic setting in treatment-naïve and pretreated patients, with an overall response rate (ORR) of 34.7% and a PFS of 4.1 months; this will likely be tested in the phase III setting [Quinn et al. 2015]. The treatment choice for second-line therapy depends on what was given in the first-line setting, as well as the patient’s PS, goals of care, and renal, hepatic and hematologic function. Regardless of what is offered, overall responses to second-line and beyond therapy are modest and enrollment in clinical trials is strongly encouraged.
Cisplatin-ineligible patients
Many patients diagnosed with metastatic bladder cancer may not be eligible for cisplatin-based therapies due to impaired renal function or other comorbidities. There have been trials evaluating carboplatin in place of cisplatin, including a randomized phase II/III trial which compared gemcitabine plus carboplatin (GCarbo) versus methotrexate, carboplatin and vinblastine (MCAVI) in patients with newly diagnosed UC and either impaired renal function [glomerular filtration rate (GFR) between 30 and 60 ml/min] or poor PS (WHO PS of 2) which rendered them unfit for cisplatin [De Santis et al. 2012]. This trial enrolled 238 patients (119 patients in each group) and found that there was not a statistically significant difference in median OS (9.3 months in GCarbo versus 8.1 months in MCAVI regimen, p = 0.65). Severe acute toxicities as defined by the authors occurred at rates of 21.2% in the MCAVI arm and only 9.3% in the GC arm. Due to its more favorable toxicity profile, gemcitabine plus carboplatin is thus a frequent regimen for patients who are able to receive chemotherapy but unable to tolerate cisplatin. However, carboplatin carries with it significant hematologic toxicity, and the doublet has never been compared with single-agent gemcitabine which can lead by itself to an ORR of 28% and a median PFS and OS of 20 and 54 weeks, respectively [Stadler et al. 1997], making single-agent gemcitabine a reasonable alternative for cisplatin-ineligible patients or for those who have progressed on cisplatin-based therapy.
New directions
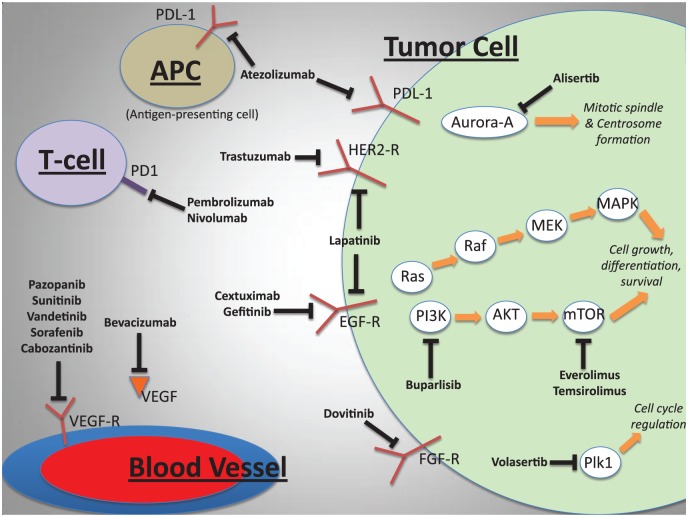
Given the limited success of the previously discussed treatment options in the neoadjuvant, adjuvant, and metastatic settings for UC, innovation is required. There is intense interest in developing molecularly targeted therapies using tumor genomic profiling and driver mutation identification, although no new therapies have been approved to date [Carneiro et al. 2015]. Below we discuss the relevant molecular pathways in UC and summarize the efforts undertaken thus far to exploit them in the treatment of metastatic UC (Figure 1). Table 2 summarizes the key targeted therapy trials to date and Table 3 lists the major ongoing and completed immunotherapy trials.
Figure 1.
Key molecular pathways, therapeutic targets and drugs under investigation in urothelial carcinoma. HER2, human epidermal growth factor receptor 2; EGFR, epidermal growth factor receptor; FGFR, fibroblast growth factor receptor; PDL1, programmed cell death ligand 1; PD1, programmed cell death protein 1; VEGFR, vascular endothelial growth factor receptor; VEGF, vascular endothelial growth factor.
Table 2.
Select targeted therapy trials in metastatic urothelial carcinoma.
| Drug | Trial characteristics |
Target | Response Rate (%): |
Clinical Outcomes (months) |
Reference | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| Phase | N | R | Line | Arm | CR | PR | SD | PFS/TTP | OS | |||
| Everolimus | II | 45 | N | 2nd–nth | mTOR | NA | 2 | 2 | 26.7 | 2.6 | 8.3 | Milowsky et al. [2013b] |
| Everolimus | II | 37 | N | 2nd–nth | mTOR | NA | 0 | 5.4 | 21.6 | 2 | 3.3 | Seront et al. [2012] |
| Temsirolimus | II | 15 | N | 2nd–nth | mTOR | NA | 0 | 0 | 26.7 | 2.5 | 3.5 | Gerullis et al. [2012] |
| Dovitinib | II | 44 | N | 2nd–4th | FGFR3 |
FGFR3 mutant FGFR3 non-mutant |
0 0 |
0 3 |
0 0 |
3 1.8 |
Milowsky et al. [2013a] | |
| Gemcitabine and cisplatin ± cetuximab | II | 88 | Y | 1st | EGFR |
GC GC + cetuximab |
10.7 3.5 |
46.4 57.9 |
32.1 19.3 |
8.5 7.6 |
17.4 14.3 |
Hussain et al. [2014] |
| Cetuximab ± paclitaxel | II | 39 | Y | 2nd | EGFR |
Cetuximab Paclitaxel + cetuximab |
0 10.7 |
0 14 |
0 39 |
1.7 3.8 |
3.9 9.6 |
Wong et al. [2012] |
| Trastuzumab, paclitaxel, carboplatin, gemcitabine | II | 44 | N | 1st | HER2 | NA | 11 | 59 | 11 | 9.3 | 14.1 | Hussain et al. [2007] |
| Gemcitabine and either cisplatin or carboplatin ± trastuzumab | II | 61 | Y | 1st | HER2 |
GC or GCarb GC+Trastuzumab |
20.7 21.9 |
44.8 31.3 |
20.7 21.9 |
10.2 8.2 |
15.7 14.1 |
Oudard et al. [2015] |
| Lapatinib | II | 59 | N | 2nd | HER1/2 | NA | 0 | 2 | 31 | 2 | 4 | Wulfing et al. [2009] |
| Lapatinib | II/III | 232 | Y | 2nd | HER1/2 |
Lapatinib Placebo |
14 8 |
4.6 5.1 |
12.6 12.0 |
Powles et al. [2015] | ||
| Cisplatin/gemcitabine/ bevacizumab | II | 43 | N | 1st | VEGF | NA | 19 | 53 | 9 | 8.2 | 19.1 | Hahn et al. [2011] |
| Carboplatin/gemcitabine/bevacizumab | II | 51 | N | 1st | VEGF | NA | 6 | 43 | 23 | 6.5 | 13.9 | Balar et al. [2013] |
| Volasertib | II | 50 | N | 2nd | Plk | NA | 0 | 14 | 26 | 1.4 | 8.5 | Stadler et al. [2014] |
Abbreviations: mTOR, mammalian target of rapamycin; FGFR3, fibroblast growth factor receptor 3; HER2, human epidermal growth receptor 2; EGFR, epidermal growth factor receptor; VEGF, vascular endothelial growth factor; GC gemcitabine cisplatin; GCarb, gemcitabine carboplatin; Plk, Polo-like kinase; PD-L1, programmed death ligand 1; PD-1, programmed cell death 1; IHC, immunohistochemistry; N, total number of patients in trial that were evaluable; R, randomized trial or not; OS, overall survival; PFS, progression-free survival; TTP, time to progression; NA, not applicable.
Unless otherwise specified numbers refer to months.
Table 3.
Key checkpoint inhibitor trials (ClinicalTrials.gov identifiers) in urothelial carcinoma.
| Setting | Drug | Phase |
|---|---|---|
| Metastatic | ||
| NCT02335424 | Pembrolizumab in cisplatin ineligible | II |
| NCT02256436 | Pembrolizumab (versus paclitaxel, docetaxel, or vinflunine) | III |
| NCT02108652 | Atezolizumab | II |
| NCT02302807 | Atezolizumab versus chemotherapy | III |
| NCT01848834 | Pembrolizumab | Ib |
| NCT02387996 | Nivolumab post cisplatin | II |
| NCT01524991 | Ipilimumab (plus gemcitabine and cisplatin) | II |
| Neoadjuvant | ||
| NCT02365766 | Pembrolizumab | Ib/II |
| Adjuvant | ||
| NCT02450331 | Atezolizumab versus observation | III |
PI3K/AKT/mTOR pathway
The Cancer Genome Atlas published genetic analysis of 131 cases of UC with the goal of identifying common molecular alterations [Cancer Genome Atlas Research, 2014]. Recurring mutations in 32 genes reached statistical significance including genes involved in regulation of chromatin and the cell cycle, as well as kinase signaling pathways. For example, in 69% of the tumors, potential therapeutic targets were identified, including the phophatidylinositol-3-OH kinase/AKT/mTOR (PI3K/AKT/mTOR) and RTK/MAPK pathways. Up to 40% of UC demonstrates mutations in the PI3K/AKT/mTOR pathway and this has been heavily explored [Yap et al. 2008; Gust and So, 2009; Platt et al. 2009; Askham et al. 2010; Hansel et al. 2010; Sjodahl et al. 2011; Carneiro et al. 2015; Houede and Pourquier, 2015]. Activation of the PI3K/AKT/mTOR pathway has been shown to contribute to chemotherapy resistance, and is generally associated with poorer prognosis [Moon Du et al. 2014]. As an example, Wagle and colleagues described a patient with chemotherapy-refractory metastatic UC who had a durable response (26 months) to everolimus (an mTOR inhibitor) and pazopanib on a phase I clinical trial [Wagle et al. 2014]. Pazopanib, a VEGF-R tyrosine kinase inhibitor which affects tumor cell proliferation and angiogenesis, was hypothesized to improve the anti-angiogenesis and antitumor effect of both drugs [O’Reilly et al. 2005], and the patient was found to harbor two activating mutations in the mTOR pathway, making his tumor sensitive to mTOR inhibition [Iyer et al. 2012, Wagle et al. 2014]. A phase II trial studied everolimus alone in 45 patients with metastatic UC, with only one patient having an objective response at 2 months [Milowsky et al. 2013b]. Another phase II study of single-agent everolimus evaluated 37 patients, with 10 patients having disease control at 8 weeks, but there were no objective responses [Seront et al. 2012]. Similarly, there was limited activity seen with single-agent temsirolimus, another mTOR inhibitor, in a small phase II study of 15 patients with advanced UC [Gerullis et al. 2012]. Although these trials collectively have not shown great success, there may be clinical benefit in a select group of patients with driver mutations in this pathway. Two trials studying this pathway are actively recruiting patients including a phase I study with gemcitabine, cisplatin and everolimus [ClinicalTrials.gov identifier: NCT01182168] and a phase II trial with everolimus and paclitaxel in cisplatin-ineligible patients [ClinicalTrials.gov identifier: NCT01215136]. Finally, another phase II study of a pan-PI3K-inhibitor buparlisib, which has been shown to have activity in some solid tumors [Rodon et al. 2014], is ongoing for patients with metastatic, chemotherapy-refractory UC [ClinicalTrials.gov identifier: NCT01551030].
Fibroblast growth factor family inhibition
Fibroblast growth factor receptor (FGFR) and its ligand fibroblast growth factor (FGF) lead to downstream activation of multiple signaling molecules and pathways including Ras/Raf/Mek/Erk, PI3K/Akt, Jnk-p38, MAPK, STAT3 and NF-κB, that control a diverse variety of developmental processes including angiogenesis, cell metabolism, proliferation, and survival and have also been identified as possible therapeutic targets for treatment of UC [Raju et al. 2014]. Dysregulation and mutations of FGF/FGFR-related signaling has been shown to contribute to carcinogenesis, tumor cell invasion, and metastasis in multiple malignancies, including UC [Allen and Maher, 1993; Cappellen et al. 1999; Sibley et al. 2001; Beenken and Mohammadi, 2009; Itoh and Ornitz, 2011]. FGFR3 in particular has been shown to have activating mutations in UC, as well as over-expression and alternative splicing of the receptor [Carneiro et al. 2015]. Preclinical studies have been performed on UC cell lines and xenograft models demonstrating the ability of FGFR inhibitors to block signaling pathways and ultimately cell proliferation [Zhao et al. 2011; Gozgit et al. 2012; Chell et al. 2013]. This formed the basis for clinical trials involving FGFR inhibitors [Wolf et al. 2012; Milowsky et al. 2013a; Bahleda et al. 2014; Tie et al. 2014]. Milowsky and colleagues reported the data from a phase II trial of dovitinib, an oral FGFR3 inhibitor in patients with advanced UC [Milowsky et al. 2013a]. In this trial, patients were stratified by whether their tumors harbored a FGFR3 mutation. A total of 44 patients were treated, 31 of them without mutations. In the nonmutated group, one patient had a partial response (ORR 3%) and median PFS was 1.8 months. In the mutated group, no patients responded (ORR 0%) and median PFS was 3 months. A phase I study treating 37 patients with advanced solid tumors with the pan-FGFR inhibitor, JNJ-42756493, had more promising results; two patients with advanced UC and mutations in the FGFR pathway demonstrated a response (one near-complete, one partial) [Bahleda et al. 2014]. This study is continuing to enroll patients with advanced solid tumors [ClinicalTrials.gov identifier: NCT01703481]. As a whole, these studies suggest that more research is needed to define the exact role of FGF inhibition in UC. Several phase I–II studies with FGF-R inhibitors in UC and other advanced malignancies are ongoing [ClinicalTrials.gov identifiers: NCT01004224, NCT01976741], and others are planned but not yet recruiting participants [ClinicalTrials.gov identifiers: NCT02278978, NCT02401542].
Epidermal growth factor family inhibition
The epidermal growth factor receptor (EGFR) family, which includes HER2, is a group of receptor tyrosine kinases involved in cancer pathogenesis and UC; several studies have linked EGFR expression to advanced disease and worse prognosis [Lonn et al. 1995; Sriplakich et al. 1999; Fleischmann et al. 2011; Tsai et al. 2012; Ross et al. 2014]. A meta-analysis of EGFR expression in UC showed that it was significantly associated with disease progression and mortality [Tsai et al. 2012]. Amplification or mutation of HER2 was seen in 9% of UC tumors based on TCGA data and was significantly associated with lymph node metastasis compared with matched primary tumors (15.3% versus 8.7%, p = 0.003) [Fleischmann et al. 2011]. HER2 mutations were also frequently seen in micropapillary UC specimens, which is an aggressive variety of UC [Ross et al. 2014].
Several trials have evaluated agents interfering with EGFR family pathways. Cetuximab, a monoclonal antibody inhibiting EGFR, was studied in patients with advanced UC in both the first- and second-line settings. A phase II trial enrolled 88 previously untreated patients, randomizing them to receive gemcitabine and cisplatin with or without cetuximab. The group receiving cetuximab demonstrated a worse PFS (7.6 versus 8.5 months) and OS (14.3 versus 17.4 months) and had more adverse events [Hussain et al. 2014]. Another phase II trial randomized 39 previously treated patients to cetuximab with or without paclitaxel [Wong et al. 2012]. The monotherapy arm had poor results with most patients progressing at the first evaluation, but the dual therapy arm showed an ORR of 25%, median PFS of 16.4 weeks, and median OS of 42 weeks, suggesting that cetuximab may improve the efficacy of paclitaxel in this patient population. Two earlier phase II studies with gefitinib, an EGFR TKI, in both the first- and second-line setting for patients with advanced UC did not yield any improvements in PFS or OS [Philips et al. 2009; Petrylak et al. 2010].
Trastuzumab, a monoclonal antibody against HER2, has also been studied in advanced UC. A nonrandomized phase II trial published by Hussain and colleagues in 2007 studied 44 untreated patients with HER2/neu-positive tumors treated with gemcitabine, carboplatin, paclitaxel, and trastuzumab showing an ORR of 70%, median PFS of 9.3 months, and OS 14.1 months [Hussain et al. 2007]. A phase II trial randomized 61 patients with advanced UC with over-expression of HER2 to receive gemcitabine with cisplatin or carboplatin with (arm A) or without (arm B) trastuzumab [Oudard et al. 2015]. Median PFS for arms A and B was 10.2 versus 8.2 months (p = 0.689), ORR 65% versus 53.2% (p = 0.39), and median OS 15.7 versus 14.1 months (p = 0.684), respectively. A major limitation with this study was that few patients had HER2 overexpression (75 of 563), limiting the power of the study. Lapatinib, a TKI targeting both EGFR and HER, has been studied in the first- and second-line setting in UC. In a phase I study, lapatinib was added to gemcitabine and cisplatin in untreated patients with advanced UC. This trial is completed, but the results have not yet been published [ClinicalTrials.gov identifier: NCT00623064]. A phase II study of lapatinib in the second-line setting reported limited responses in these pre-treated patients, but there was a significantly prolonged OS in those patients with tumors harboring EGFR and/or HER2 expression [Wulfing et al. 2009]. In the metastatic setting, Powles and colleagues recently reported on a phase II/III randomized trial comparing maintenance lapatinib versus placebo after first-line chemotherapy in HER1 or 2-positive UC. The ORR was 14% for lapatinib and 8% for placebo, which was not a significant result (p = 0.14). The authors concluded that lapatinib does not have clinical benefit in the second-line setting of metastatic UC [Powles et al. 2015]. Although these trials have not established a definitive role for EGFR and HER2 blockade in treatment of UC, they suggest there may be clinical benefit for select patients.
Anti-angiogenesis agents
Angiogenesis is significantly increased in UC specimens in comparison to normal urothelial tissue, which makes molecules propagating angiogenesis possible therapeutic targets in UC [O’Brien et al. 1995, 1996; Campbell et al. 1998; Miyake et al. 1999]. Vascular endothelial growth factor (VEGF) overexpression in particular has been associated with more invasive tumors and worse prognosis in UC [Crew et al. 1997; Crew et al. 1999; Miyake et al. 1999; Nakanishi et al. 2009]. Therefore, agents targeting the tumor vasculature, such as bevacizumab, sunitinib, vandetanib, pazopanib, sorafenib, and cabozantinib have been studied in UC [Sonpavde et al. 2015]. Bevacizumab, a monoclonal antibody against VEGF, has been utilized in several nonrandomized, phase II trials [Chaudhary et al. 2011; Hahn et al. 2011; Siefker-Radtke et al. 2012; Balar et al. 2013]. In the neoadjuvant setting, it was paired with GC and AMVAC, and although these trials were relatively small and nonrandomized, they concluded that the addition of bevacizumab did not contribute to down-staging of the primary tumor and that there were higher rates of surgical complications [Chaudhary et al. 2011; Siefker-Radtke et al. 2012]. Both Hahn and colleagues [Hahn et al. 2011] and Balar and coworkers [Balar et al. 2013] published studies of previously untreated patients with metastatic UC receiving a platinum agent, gemcitabine, and bevacizumab in the first-line setting. The median PFS was 8.2 and 6.5 months, with median OS of 19.1 months and 13.9 months, respectively. This formed the basis for a phase III randomized trial of cisplatin, gemcitabine with or without bevacizumab [ClinicalTrials.gov identifier: NCT00942331], which has completed accrual with results pending. The other anti-angiogenic agents listed above have been evaluated in multiple settings in UC, with some trials still ongoing (sunitinib [ClinicalTrials.gov identifier: NCT01118351], pazopanib [ClinicalTrials.gov identifier: NCT01622660], sorafenib [ClinicalTrials.gov identifier: NCT00461851], and cabozantinib [ClinicalTrials.gov identifier: NCT01688999]): these have not yet shown a clear clinical benefit.
Cell cycle inhibitors
Drugs interrupting the cell cycle are another area of interest in UC treatment. For example, the aurora kinase family is crucial to cell cycle regulation, and interruptions in aurora kinase signaling contribute to carcinogenesis via chromosomal instability and aneuploidy [Bufo et al. 2010; Dar et al. 2010]. Aurora-A gene expression has been shown to be increasingly dysregulated in more invasive forms of UC [Comperat et al. 2008]. An oral aurora kinase inhibitor known as MLN8237 (alisertib) was studied in vitro and in vivo and found to induce apoptosis in UC cell lines in vitro via cell cycle arrest, and aneuploidy, mitotic spindle failure as well as halting tumor growth in a mouse UC xenograft model [Zhou et al. 2013]. A phase II trial with alisertib in patients with relapsed or refractory UC is underway [ClinicalTrials.gov identifier: NCT02109328]. Polo-like kinases (Plks), similar to aurora kinases, are associated with cell cycle regulation. Plks have an important role in the centrosome cycle, and are also known to cause aneuploidy and chromosomal instability when inhibited [Takai et al. 2005]. In UC, Plk over-expression is associated with aneuploidy, higher pathologic grade, and growth of multiple tumors [Yamamoto et al. 2006]. A phase I study of volasertib (BI 6727), a potent and selective Plk inhibitor, was undertaken in 65 patients with advanced solid tumors and did show antitumor activity. One of three patients with partial responses had advanced UC, and maintained a PFS of 403 days with this drug [Schoffski et al. 2012]. Volasertib was then studied in phase II trial involving patients with advanced UC. A total of 50 patients previously treated with a platinum agent were enrolled. The ORR was 14%, with a median PFS of 1.4 months and median OS of 8.5 months. The most common grade 3 and 4 adverse events were cytopenias. The authors concluded that volasertib was inefficient as monotherapy in the second-line setting in patients with UC [Stadler et al. 2014].
Immunotherapy
Immunotherapy has long been viewed as a potential therapeutic option for UC, given that immune dysregulation is known to play a vital role in this disease [Herr et al. 1976; Mukamel et al. 1982; Loskog et al. 2007]. In the 1980s, dysregulation of T cells in patients with this disease was shown to correlate with tumor aggressiveness, and the immunologic profile of these patients improved after cystectomy [Mukamel et al. 1982]. Carcinogenesis was thought to be due to immune impairment particularly influenced by T-regulatory cells and Th1 inhibitory cytokines [Loskog et al. 2007]. For example, Sharma and colleagues showed in 2007 that higher numbers of tumor-infiltrating cytotoxic T lymphocytes (TILs) in 69 patients with MIBC was associated with improved DFS (p < 0.001) and OS (p = 0.018) and concluded that intratumoral CD8 T-cell concentrations can be used as a prognostic tool for patients with MIBC [Sharma et al. 2007].
Among the most promising classes of immunotherapy drugs developed in recent years are the checkpoint blockade agents [Nguyen and Ohashi, 2015; Postow et al. 2015; Topalian et al. 2015]. These drugs inactivate inhibitory pathways (‘checkpoints’) which regulate T-cell activity, thus allowing for increased immune surveillance and antitumor efficacy. Examples include drugs targeting cytotoxic T-lymphocyte associated antigen 4 (CTLA-4) and drugs targeting the interaction between programmed cell death receptor 1 protein (PD-1) and its ligands PD-L1 and PD-L2. PD-1 is a negative co-stimulatory receptor on T cells and when bound to its ligands exerts an inhibitory effect on the T cells. As a result, expression of PD-L1 and PD-L2 on tumor cells and mononuclear cells leads to immune suppression and tumor evasion from the host immune surveillance, and this pathway has become an active target for anti-cancer therapies [Chen et al. 2012].
In a 2008 study of over 300 patients with urothelial bladder cancer, Boorjian and colleagues showed that expression of PD-L1 and PD-1 was associated with more advanced pathologic stages (p < 0.001 and p = 0.012, respectively) [Boorjian et al. 2008]. In addition, PD-L1 expression independently predicted all-cause mortality postoperatively for patients with localized disease. In another study of 280 UCs, PD-L1 was expressed significantly more often in high-grade tumors (p < 0.001) [Inman et al. 2007]. Finally, a recent study by Bellmunt and colleagues showed that PD-L1 expression in tumor-infiltrating mononuclear cells was associated with longer survival in patients with metastatic UC (median OS of 12 months versus 23 months, p = 0.04 on univariate analysis; HR 3.19, p = 0.0007 on multivariable analysis adjusted for ECOG status and visceral disease) [Bellmunt et al. 2015].
These and other studies formed the basis for multiple ongoing and completed trials of checkpoint inhibitors in UC. Powles and colleagues reported on an expansion cohort of patients with metastatic UC in a phase I clinical trial of atezolizumab, a monoclonal antibody against PD-L1, which blocks interaction of PD-L1 with PD-1 and B7.1 (CD80) [Powles et al. 2014]. In this trial, 68 patients were treated with atezolizumab, with the majority of patients having been pretreated with platinum-based chemotherapy, and having had two or more chemotherapy regimens in the past. Most patients had poor prognostic factors including visceral metastases, anemia, renal dysfunction with creatinine clearance less than 60 ml/min, and PS of ECOG 1. Patients were treated for a median of 65 days, with the majority of adverse events being mild (grades 1 and 2) and transient. Objective responses were seen in 17 of 65 patients (all continued on treatment at the time of data cutoff), and two patients had a complete response. The degree of PD-L1 staining on tumor-infiltrating immune cells in the tumor sample correlated to the response to atezolizumab. For example, the ORR was 50% for the patients whose tumors stained strongly for tumor-infiltrating immune cells (IHC 3) versus 8.3% for those with tumors that stained poorly (IHC 0). Overall, these study results showed a clinically significant response rate, with some sustained responses and acceptable tolerability to the drug. Petrylak and colleagues recently reported on the updated response and survival data for the 92 patients with UC enrolled in the ongoing dose-expansion phase. He reported similar drug tolerability and ORR in this patient population (50% with IHC 2/3, 17% in IHC 0/1). A total of 20 of 30 patients who responded had ongoing responses at the time of data cutoff in December 2014 and the 1-year OS was 57% for those with IHC 2/3 and 38% for those with IHC 0/1 [Petrylak et al. 2015].
Further encouraging immunotherapy data was presented by Plimack and colleagues in an updated analysis of a phase Ib study of pembrolizumab (MK-3475), an anti-PD1 monoclonal antibody, in patients with advanced UC [Plimack et al. 2015]. Expression of PD-L1 in stroma or tumor cells was required for entry into the study. A total of 33 patients were enrolled to receive pembrolizumab monotherapy every 2 weeks. The most common adverse events were fatigue, peripheral edema, and nausea. ORR was 27.6% (95% CI 12.7–47.2%) with three patients (10%) having complete responses. Median PFS was 2 months (95% CI 1.7–4.0), median OS was 12.7 months (95% CI 5.0– not reached) and over 30% of patients were alive at 18 months. The response duration was 8.1 to 64.1+ weeks, with 3 patients on treatment for over 60 weeks.
The results of the trials with both atezolizumab and pembrolizumab are promising, especially given that this population is heavily pretreated, and demonstrate that both of these agents will likely have a role in the treatment of UC. A number of other trials targeting the PD-1/PD-L1 axis in the neoadjuvant, adjuvant and metastatic setting are recently closed to accrual, ongoing, soon to be open or in rapid development with a subset of trials focusing on patients who are cisplatin ineligible.
Conclusion
Traditionally, muscle-invasive and metastatic bladder cancer has been associated with poor survival and limited treatment options. Although treatments and outcomes for bladder cancer have been largely unchanged over the last few decades, the landscape is evolving. Clear evidence now exists supporting the use of neoadjuvant cisplatin-based chemotherapy and should be the standard-of-care in all eligible patients. The evidence for cisplatin-based adjuvant therapy is weaker compared with the neoadjuvant setting, but should be offered in the appropriate patient. Given that overall only a subset of patients will benefit from neoadjuvant or adjuvant cisplatin-based therapy, further studies of predictive molecular profiles to help identify those likely to respond favorably are necessary and ongoing. Targeted therapies for metastatic bladder cancer are continuing to be explored with no approved drugs to date and success will likely depend on appropriately selecting patients who stand to benefit from a particular drug. The dawn of immunotherapy is upon us and early promising results have led to a renewed sense of excitement and hope, with multiple trials actively accruing or completed. How to appropriately sequence, combine and administer these therapies will be the work of the next decade.
Footnotes
Funding: This research received no specific grant from any funding agency in the public, commercial, or not-for-profit sectors.
Conflict of interest statement: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Contributor Information
Hayley Knollman, Department of Medical Oncology, Fox Chase Cancer Center-Temple University Health System, Philadelphia, PA, USA.
J. Luke Godwin, Department of Medical Oncology, Fox Chase Cancer Center-Temple University Health System, Philadelphia, PA, USA.
Rishi Jain, Department of Medical Oncology, Fox Chase Cancer Center-Temple University Health System, Philadelphia, PA, USA.
Yu-Ning Wong, Department of Medical Oncology, Fox Chase Cancer Center-Temple University Health System, Philadelphia, PA, USA.
Elizabeth R. Plimack, Department of Medical Oncology, Fox Chase Cancer Center-Temple University Health System, Philadelphia, PA, USA
Daniel M. Geynisman, Assistant Professor of Medical Oncology, Fox Chase Cancer Center-Temple University Health System, 333 Cottman Avenue, Philadelphia, PA 19111, USA.
References
- Advanced Bladder Cancer Meta-Analysis (2005) Neoadjuvant chemotherapy in invasive bladder cancer: update of a systematic review and meta-analysis of individual patient data advanced bladder cancer (ABC) meta-analysis collaboration. Eur Urol 48: 202–205. [DOI] [PubMed] [Google Scholar]
- Allen L., Maher P. (1993) Expression of basic fibroblast growth factor and its receptor in an invasive bladder carcinoma cell line. J Cell Physiol 155: 368–375. [DOI] [PubMed] [Google Scholar]
- American Cancer Society. (2015). What are the key statistics about bladder cancer? Available at: http://www.cancer.org/cancer/bladdercancer/detailedguide/bladder-cancer-key-statistics (accessed 17 April 2015).
- Askham J., Platt F., Chambers P., Snowden H., Taylor C., Knowles M. (2010) AKT1 mutations in bladder cancer: identification of a novel oncogenic mutation that can co-operate with E17K. Oncogene 29: 150–155. [DOI] [PubMed] [Google Scholar]
- Bahleda R., Dienstmann R., Adamo B., Gazzah A., Infante J., Zhong B., et al. (2014) Phase 1 Study of JNJ-42756493, a pan-fibroblast growth factor receptor (FGFR) inhibitor, in patients with advanced solid tumors. J Clin Oncol 32(Suppl.): abstract 2501. [DOI] [PubMed] [Google Scholar]
- Bajorin D., Herr H. (2011) Kuhn’s paradigms: are those closest to treating bladder cancer the last to appreciate the paradigm shift? J Clin Oncol 29: 2135–2137. [DOI] [PubMed] [Google Scholar]
- Bajorin D., Sharma P., Gomella L., Plimack E., O’donnell P., Hoffman-Censits J., et al. (2014) Neuact, a phase II, randomized, open-label trial of DN24-02: updated analysis of HER2 expression, immune responses, product parameters, and safety in patients with surgically resected HER2+ urothelial cancer. J Clin Oncol 32(Suppl.): abstract 296. [Google Scholar]
- Balar A., Apolo A., Ostrovnaya I., Mironov S., Iasonos A., Trout A., et al. (2013) Phase II study of gemcitabine, carboplatin, and bevacizumab in patients with advanced unresectable or metastatic urothelial cancer. J Clin Oncol 31: 724–730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bamias A., Efstathiou E., Moulopoulos L., Gika D., Hamilos G., Zorzou M.P., et al. (2005) The outcome of elderly patients with advanced urothelial carcinoma after platinum-based combination chemotherapy. Ann Oncol 16: 307–313. [DOI] [PubMed] [Google Scholar]
- Beenken A., Mohammadi M. (2009) The FGF family: Biology, pathophysiology and therapy. Nat Rev Drug Discov 8: 235–253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bellmunt J., Mullane S., Werner L., Fay A., Callea M., Leow J., et al. (2015) Association of PD-L1 expression on tumor-infiltrating mononuclear cells and overall survival in patients with urothelial carcinoma. Ann Oncol 26: 812–817. [DOI] [PubMed] [Google Scholar]
- Bellmunt J., Theodore C., Demkov T., Komyakov B., Sengelov L., Daugaard G., et al. (2009) Phase III trial of vinflunine plus best supportive care compared with best supportive care alone after a platinum-containing regimen in patients with advanced transitional cell carcinoma of the urothelial tract. J Clin Oncol 27: 4454–4461. [DOI] [PubMed] [Google Scholar]
- Bellmunt J., Von Der Maase H., Mead G., Skoneczna I., De Santis M., Daugaard G., et al. (2012) Randomized Phase III study comparing paclitaxel/cisplatin/gemcitabine and gemcitabine/cisplatin in patients with locally advanced or metastatic urothelial cancer without prior systemic therapy: EORTC intergroup study 30987. J Clin Oncol 30: 1107–1113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorjian S., Sheinin Y., Crispen P., Farmer S., Lohse C., Kuntz S., et al. (2008) T-cell coregulatory molecule expression in urothelial cell carcinoma: clinicopathologic correlations and association with survival. Clin Cancer Res 14: 4800–4808. [DOI] [PubMed] [Google Scholar]
- Bufo P., Sanguedolce F., Tortorella S., Cormio L., Carrieri G., Pannone G. (2010) Expression of mitotic kinases phospho-aurora A and aurora B correlates with clinical and pathological parameters in bladder neoplasms. Histol Histopathol 25: 1371–1377. [DOI] [PubMed] [Google Scholar]
- Campbell S., Volpert O., Ivanovich M., Bouck N. (1998) Molecular mediators of angiogenesis in bladder cancer. Cancer Res 58: 1298–1304. [PubMed] [Google Scholar]
- Cancer Genome Atlas Research. (2014) Comprehensive molecular characterization of urothelial bladder carcinoma. Nature 507: 315–322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cappellen D., De Oliveira C., Ricol D., De Medina S., Bourdin J., Sastre-Garau X., et al. (1999) Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet 23: 18–20. [DOI] [PubMed] [Google Scholar]
- Carneiro B., Meeks J., Kuzel T., Scaranti M., Abdulkadir S., Giles F. (2015) Emerging therapeutic targets in bladder cancer. Cancer Treat Rev 41: 170–178. [DOI] [PubMed] [Google Scholar]
- Chaudhary U., Golshayan A., Brisendine A. (2011) Phase II trial of neoadjuvant GC and bevacizumab followed by radical cystectomy (RC) in patients with muscle-invasive transitional cell carcinoma (TCC) of the bladder. J Clin Oncol 29(Suppl. 7): abstract 276. [Google Scholar]
- Chell V., Balmanno K., Little A., Wilson M., Andrews S., Blockley L., et al. (2013) Tumour cell responses to new fibroblast growth factor receptor tyrosine kinase inhibitors and identification of a gatekeeper mutation in FGFR3 as a mechanism of acquired resistance. Oncogene 32: 3059–3070. [DOI] [PubMed] [Google Scholar]
- Chen D., Irving B., Hodi F. (2012) Molecular pathways: next-generation immunotherapy–inhibiting programmed death-ligand 1 and programmed death-1. Clin Cancer Res 18: 6580–6587. [DOI] [PubMed] [Google Scholar]
- Choueiri T., Jacobus S., Bellmunt J., Qu A., Appleman L., Tretter C., et al. (2014) Neoadjuvant dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin with pegfilgrastim support in muscle-invasive urothelial cancer: pathologic, radiologic, and biomarker correlates. J Clin Oncol 32: 1889–1894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark P., Agarwal N., Biagioli M., Eisenberger M., Greenberg R., Herr H., et al. (2013) Bladder cancer. J Natl Compr Canc Netw 11: 446–475. [DOI] [PubMed] [Google Scholar]
- Cognetti F., Ruggeri E., Felici A., Gallucci M., Muto G., Pollera C., et al. (2012) Adjuvant chemotherapy with cisplatin and gemcitabine versus chemotherapy at relapse in patients with muscle-invasive bladder cancer submitted to radical cystectomy: an Italian, multicenter, randomized phase III trial. Ann Oncol 23: 695–700. [DOI] [PubMed] [Google Scholar]
- Comperat E., Bieche I., Dargere D., Laurendeau I., Vieillefond A., Benoit G., et al. (2008) Gene expression study of Aurora-A reveals implication during bladder carcinogenesis and increasing values in invasive urothelial cancer. Urology 72: 873–877. [DOI] [PubMed] [Google Scholar]
- Coppin C., Gospodarowicz M., James K., Tannock I., Zee B., Carson J., et al. (1996) Improved local control of invasive bladder cancer by concurrent cisplatin and preoperative or definitive radiation. The National Cancer Institute of Canada Clinical Trials Group. J Clin Oncol 14: 2901–2907. [DOI] [PubMed] [Google Scholar]
- Crew J., O’Brien T., Bicknell R., Fuggle S., Cranston D., Harris A. (1999) Urinary vascular endothelial growth factor and its correlation with bladder cancer recurrence rates. J Urol 161: 799–804. [PubMed] [Google Scholar]
- Crew J., O’Brien T., Bradburn M., Fuggle S., Bicknell R., Cranston D., et al. (1997) Vascular endothelial growth factor is a predictor of relapse and stage progression in superficial bladder cancer. Cancer Res 57: 5281–5285. [PubMed] [Google Scholar]
- Dar A., Goff L., Majid S., Berlin J., El-Rifai W. (2010) Aurora kinase inhibitors–rising stars in cancer therapeutics? Mol Cancer Ther 9: 268–278. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dash A., Pettus J., Herr H., Bochner B., Dalbagni G., Donat S., et al. (2008) A role for neoadjuvant gemcitabine plus cisplatin in muscle-invasive urothelial carcinoma of the bladder: a retrospective experience. Cancer 113: 2471–2477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- David K., Milowsky M., Ritchey J., Carroll P., Nanus D. (2007) Low incidence of perioperative chemotherapy for stage III bladder cancer 1998 to 2003: a report from the National Cancer Data Base. J Urol 178: 451–454. [DOI] [PubMed] [Google Scholar]
- De Santis M., Bellmunt J., Mead G., Kerst J., Leahy M., Maroto P., et al. (2012) Randomized phase II/III trial assessing gemcitabine/carboplatin and methotrexate/carboplatin/vinblastine in patients with advanced urothelial cancer who are unfit for cisplatin-based chemotherapy: EORTC study 30986. J Clin Oncol 30: 191–199. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Donat S. (2009) Integrating perioperative chemotherapy into the treatment of muscle-invasive bladder cancer: strategy versus reality. J Natl Compr Canc Netw 7: 40–47. [DOI] [PubMed] [Google Scholar]
- Donat S., Shabsigh A., Savage C., Cronin A., Bochner B., Dalbagni G., et al. (2009) Potential impact of postoperative early complications on the timing of adjuvant chemotherapy in patients undergoing radical cystectomy: a high-volume tertiary cancer center experience. Eur Urol 55: 177–185. [DOI] [PubMed] [Google Scholar]
- Fedeli U., Fedewa S., Ward E. (2011) Treatment of muscle invasive bladder cancer: evidence from the National Cancer Database, 2003 to 2007. J Urol 185: 72–78. [DOI] [PubMed] [Google Scholar]
- Fleischmann A., Rotzer D., Seiler R., Studer U., Thalmann G. (2011) HER2 amplification is significantly more frequent in lymph node metastases from urothelial bladder cancer than in the primary tumours. Eur Urol 60: 350–357. [DOI] [PubMed] [Google Scholar]
- Freiha F., Reese J., Torti F. (1996) A randomized trial of radical cystectomy versus radical cystectomy plus cisplatin, vinblastine and methotrexate chemotherapy for muscle invasive bladder cancer. J Urol 155: 495–499; discussion 499–500. [PubMed] [Google Scholar]
- Gallagher D., Milowsky M., Iasonos A., Maluf F., Russo P., Dalbagni G., et al. (2009) Sequential adjuvant chemotherapy after surgical resection of high-risk urothelial carcinoma. Cancer 115: 5193–5201. [DOI] [PubMed] [Google Scholar]
- Galsky M., Hahn N., Rosenberg J., Sonpavde G., Hutson T., Oh W., et al. (2011) A consensus definition of patients with metastatic urothelial carcinoma who are unfit for cisplatin-based chemotherapy. Lancet Oncol 12: 211–214. [DOI] [PubMed] [Google Scholar]
- Gerullis H., Eimer C., Ecke T., Georgas E., Freitas C., Kastenholz S., et al. (2012) A phase II trial of temsirolimus in second-line metastatic urothelial cancer. Med Oncol 29: 2870–2876. [DOI] [PubMed] [Google Scholar]
- Gozgit J., Wong M., Moran L., Wardwell S., Mohemmad Q., Narasimhan N., et al. (2012) Ponatinib (AP24534), a multitargeted pan-FGFR inhibitor with activity in multiple FGFR-amplified or mutated cancer models. Mol Cancer Ther 11: 690–699. [DOI] [PubMed] [Google Scholar]
- Griffiths G., Hall R., Sylvester R., Raghavan D., Parmar M. (2011) International phase III trial assessing neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: long-term results of the BA06 30894 trial. J Clin Oncol 29: 2171–2177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grossman H., Natale R., Tangen C., Speights V., Vogelzang N., Trump D., et al. (2003) Neoadjuvant chemotherapy plus cystectomy compared with cystectomy alone for locally advanced bladder cancer. N Engl J Med 349: 859–866. [DOI] [PubMed] [Google Scholar]
- Gust K., So A. (2009) The role of mTOR in bladder cancer. Cancer Biol Ther 8: 2348–2350. [DOI] [PubMed] [Google Scholar]
- Hahn N., Stadler W., Zon R., Waterhouse D., Picus J., Nattam S., et al. (2011) Phase II trial of cisplatin, gemcitabine, and bevacizumab as first-line therapy for metastatic urothelial carcinoma: Hoosier Oncology Group GU 04-75. J Clin Oncol 29: 1525–1530. [DOI] [PubMed] [Google Scholar]
- Hansel D., Platt E., Orloff M., Harwalker J., Sethu S., Hicks J., et al. (2010) Mammalian target of rapamycin (mTOR) regulates cellular proliferation and tumor growth in urothelial carcinoma. Am J Pathol 176: 3062–3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herr H., Bean M., Whitmore W., Jr. (1976) Decreased ability of blood leukocytes from patients with tumors of the urinary bladder to act as stimulator cells in mixed leukocyte culture. Cancer Res 36: 2754–2760. [PubMed] [Google Scholar]
- Houede N., Pourquier P. (2015) Targeting the genetic alterations of the PI3K-AKT-mTOR pathway: its potential use in the treatment of bladder cancers. Pharmacol Ther 145: 1–18. [DOI] [PubMed] [Google Scholar]
- Hussain M., Daignault S., Agarwal N., Grivas P., Siefker-Radtke A., Puzanov I., et al. (2014) A randomized phase 2 trial of gemcitabine/cisplatin with or without cetuximab in patients with advanced urothelial carcinoma. Cancer 120: 2684–2693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hussain M., Macvicar G., Petrylak D., Dunn R., Vaishampayan U., Lara P., Jr, et al. (2007) Trastuzumab, paclitaxel, carboplatin, and gemcitabine in advanced human epidermal growth factor receptor-2/neu-positive urothelial carcinoma: results of a multicenter phase II National Cancer Institute Trial. J Clin Oncol 25: 2218–2224. [DOI] [PubMed] [Google Scholar]
- Hussain M., Wood D., Bajorin D., Bochner B., Dreicer R., Lamm D., et al. (2009) Bladder cancer: narrowing the gap between evidence and practice. J Clin Oncol 27: 5680–5684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Inman B., Sebo T., Frigola X., Dong H., Bergstralh E., Frank I., et al. (2007) PD-L1 (B7-H1) expression by urothelial carcinoma of the bladder and BCG-induced granulomata: associations with localized stage progression. Cancer 109: 1499–1505. [DOI] [PubMed] [Google Scholar]
- International Collaboration of Trialists (1999) Neoadjuvant cisplatin, methotrexate, and vinblastine chemotherapy for muscle-invasive bladder cancer: a randomised controlled trial. Lancet 354: 533–540. [PubMed] [Google Scholar]
- Itoh N., Ornitz D. (2011) Fibroblast growth factors: from molecular evolution to roles in development, metabolism and disease. J Biochem 149: 121–130. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iyer G., Hanrahan A., Milowsky M., Al-Ahmadie H., Scott S., Janakiraman M., et al. (2012) Genome sequencing identifies a basis for everolimus sensitivity. Science 338: 221. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kitamura H., Tsukamoto T., Shibata T., Masumori N., Fujimoto H., Hirao Y., et al. (2014) Randomised phase III study of neoadjuvant chemotherapy with methotrexate, doxorubicin, vinblastine and cisplatin followed by radical cystectomy compared with radical cystectomy alone for muscle-invasive bladder cancer: Japan Clinical Oncology Group Study JCOG0209. Ann Oncol 25: 1192–1198. [DOI] [PubMed] [Google Scholar]
- Lehmann J., Kuehn M., Fischer C. (2013) Randomized phase III study of adjuvant versus progression-triggered treatment with gemcitabine (G) after radical cystectomy (RC) for locally advanced bladder cancer (LABC) in patients not suitable for cisplatin-based chemotherapy (CBC) (AUO-trial AB22/00).J Clin Oncol 31(Suppl. 6): abstract 250. [Google Scholar]
- Leow J., Martin-Doyle W., Rajagopal P., Patel C., Anderson E., Rothman A., et al. (2014) Adjuvant chemotherapy for invasive bladder cancer: a 2013 updated systematic review and meta-analysis of randomized trials. Eur Urol 66: 42–54. [DOI] [PubMed] [Google Scholar]
- Loehrer P., Sr., Einhorn L., Elson P., Crawford E., Kuebler P., Tannock I., et al. (1992) A randomized comparison of cisplatin alone or in combination with methotrexate, vinblastine, and doxorubicin in patients with metastatic urothelial carcinoma: a cooperative group study. J Clin Oncol 10: 1066–1073. [DOI] [PubMed] [Google Scholar]
- Lonn U., Lonn S., Friberg S., Nilsson B., Silfversward C., Stenkvist B. (1995) Prognostic value of amplification of C-ERB-B2 in bladder carcinoma. Clin Cancer Res 1: 1189–1194. [PubMed] [Google Scholar]
- Loskog A., Ninalga C., Paul-Wetterberg G., De La, Torre M., Malmstrom P., Totterman T. (2007) Human bladder carcinoma is dominated by T-regulatory cells and Th1 inhibitory cytokines. J Urol 177: 353–358. [DOI] [PubMed] [Google Scholar]
- Martinez-Pineiro J., Gonzalez Martin M., Arocena F., Flores N., Roncero C., Portillo J., et al. (1995) Neoadjuvant cisplatin chemotherapy before radical cystectomy in invasive transitional cell carcinoma of the bladder: a prospective randomized phase III study. J Urol 153: 964–973. [PubMed] [Google Scholar]
- Milowsky M., Dittrich C., Martinez I., Jagdev S., Millard F., Sweeney C., et al. (2013a) Final results of a multicenter, open-label phase II trial of dovitinib (TKI258) in patients with advanced urothelial carcinoma with either mutated or nonmutated FGFR3. J Clin Oncol 31(Suppl.): abstract 255. [Google Scholar]
- Milowsky M., Iyer G., Regazzi A., Al-Ahmadie H., Gerst S., Ostrovnaya I., et al. (2013b) Phase II study of everolimus in metastatic urothelial cancer. BJU Int 112: 462–470. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyake H., Hara I., Yamanaka K., Gohji K., Arakawa S., Kamidono S. (1999) Increased angiogenin expression in the tumor tissue and serum of urothelial carcinoma patients is related to disease progression and recurrence. Cancer 86: 316–324. [PubMed] [Google Scholar]
- Moon Du G., Lee S., Oh M., Lee S., Jeong S., Hong S., et al. (2014) NVP-BEZ235, a dual PI3K/mTOR inhibitor synergistically potentiates the antitumor effects of cisplatin in bladder cancer cells. Int J Oncol 45: 1027–1035. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mukamel E., Shohat B., Servadio C. (1982) Immunological profile of patients with transitional cell carcinoma of the bladder. Br J Urol 54: 11–15. [DOI] [PubMed] [Google Scholar]
- Nakanishi R., Oka N., Nakatsuji H., Koizumi T., Sakaki M., Takahashi M., et al. (2009) Effect of vascular endothelial growth factor and its receptor inhibitor on proliferation and invasion in bladder cancer. Urol Int 83: 98–106. [DOI] [PubMed] [Google Scholar]
- National Comprehensive Cancer Network (NCCN). (2015) NCCN guidelines for treatment of cancer site Available at: http://www.nccn.org/professionals/physician_gls/f_guidelines.asp (accessed 14 May 2015).
- Nguyen L., Ohashi P. (2015) Clinical blockade of PD1 and LAG3–potential mechanisms of action. Nat Rev Immunol 15: 45–56. [DOI] [PubMed] [Google Scholar]
- O’Brien T., Cranston D., Fuggle S., Bicknell R., Harris A. (1995) Different angiogenic pathways characterize superficial and invasive bladder cancer. Cancer Res 55: 510–513. [PubMed] [Google Scholar]
- O’Brien T., Cranston D., Fuggle S., Bicknell R., Harris A. (1996) The angiogenic factor midkine is expressed in bladder cancer, and overexpression correlates with a poor outcome in patients with invasive cancers. Cancer Res 56: 2515–2518. [PubMed] [Google Scholar]
- O’Reilly T., Wood J., Littlewood-Evans A., Boulay A., Schnell C., Patrizia S. (2005) Differential anti-vascular effects of mTOR or VEGFR pathway inhibition: a rational basis for combining RAD001 and PTK787/ZK222584. Proc Amer Assoc Cancer Res 46: 3038 [Google Scholar]
- Oudard S., Culine S., Vano Y., Goldwasser F., Theodore C., Nguyen T., et al. (2015) Multicentre randomised phase II trial of gemcitabine+platinum, with or without trastuzumab, in advanced or metastatic urothelial carcinoma overexpressing HER2. Eur J Cancer 51: 45–54. [DOI] [PubMed] [Google Scholar]
- Pal S., Milowsky M., Plimack E. (2013) Optimizing systemic therapy for bladder cancer. J Natl Compr Canc Netw 11: 793–804. [DOI] [PubMed] [Google Scholar]
- Paz-Ares L., Solsona E., Esteban E., Saez A., Gonzalez-Larriba J., Anton A., et al. (2010) Randomized phase III trial comparing adjuvant paclitaxel/gemcitabine/cisplatin (PGC) to observation in patients with resected invasive bladder cancer: results of the Spanish Oncology Genitourinary Group (SOGUG) 99/01 study. J Clin Oncol 28(Suppl): abstract LBA4518. [Google Scholar]
- Petrylak D., Powles T., Bellmunt J., Braiteh F., Loriot Y., Zambrano C., et al. (2015) A Phase Ia study of MPDL3280a (anti-PDL1): updated response and survival data in urothelial bladder cancer (UBC). J Clin Oncol 33(Suppl.): abstract 4501. [Google Scholar]
- Petrylak D., Tangen C., Van Veldhuizen P., Jr, Goodwin J., Twardowski P., Atkins J., et al. (2010) Results of the Southwest Oncology Group phase II evaluation (study S0031) of ZD1839 for advanced transitional cell carcinoma of the urothelium. BJU Int 105: 317–321. [DOI] [PubMed] [Google Scholar]
- Philips G., Halabi S., Sanford B., Bajorin D., Small E. and Cancer and Leukemia Group B (2009) A phase II trial of cisplatin (C), gemcitabine (G) and gefitinib for advanced urothelial tract carcinoma: results of Cancer and Leukemia Group B (CALGB) 90102. Ann Oncol 20: 1074–1079. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Platt F., Hurst C., Taylor C., Gregory W., Harnden P., Knowles M. (2009) Spectrum of phosphatidylinositol 3-kinase pathway gene alterations in bladder cancer. Clin Cancer Res 15: 6008–6017. [DOI] [PubMed] [Google Scholar]
- Plimack E., Bellmunt J., Gupta S., Berger R., Montgomery R., Heath K., et al. (2015) Pembrolizumab (MK-3475) for advanced urothelial cancer: updated results and biomarker analysis from keynote-012. J Clin Oncol 33(Suppl.): abstract 4502. [Google Scholar]
- Plimack E., Hoffman-Censits J., Viterbo R., Trabulsi E., Ross E., Greenberg R., et al. (2014) Accelerated methotrexate, vinblastine, doxorubicin, and cisplatin is safe, effective, and efficient neoadjuvant treatment for muscle-invasive bladder cancer: results of a multicenter phase II study with molecular correlates of response and toxicity. J Clin Oncol 32: 1895–1901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Postow M., Callahan M., Wolchok J. (2015) Immune checkpoint blockade in cancer therapy. J Clin Oncol 33: 1974–1982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Powles T., Eder J., Fine G., Braiteh F., Loriot Y., Cruz C., et al. (2014) MPDL3280a (Anti-PD-L1) treatment leads to clinical activity in metastatic bladder cancer. Nature 515: 558–562. [DOI] [PubMed] [Google Scholar]
- Powles T., Huddart R., Elliott T., Jones R., Hussain S., Crabb S., et al. (2015) A phase II/III, double-blind, randomized trial comparing maintenance lapatinib versus placebo after first line chemotherapy in HER1/2 positive metastatic bladder cancer patients. J Clin Oncol 33(Suppl.): abstract 4505. [DOI] [PubMed] [Google Scholar]
- Quinn D., Ruel N., Twardowski P., Groshen S., Dorff T., Pal S., et al. (2015) Eribulin in advanced urothelial cancer (AUC) patients (Pts): a California Cancer Consortium trial—NCI/CTEP 7435. J Clin Oncol 33(Suppl.): abstract 4504. [Google Scholar]
- Raju R., Palapetta S., Sandhya V., Sahu A., Alipoor A., Balakrishnan L., et al. (2014) A network map of FGF-1/FGFR signaling system. J Signal Transduct 2014: 1–16 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rodon J., Brana I., Siu L., De Jonge M., Homji N., Mills D., et al. (2014) Phase I dose-escalation and -expansion study of Buparlisib (BKM120), an oral pan-class I PI3k inhibitor, in patients with advanced solid tumors. Invest New Drugs 32: 670–681. [DOI] [PubMed] [Google Scholar]
- Ross J., Wang K., Gay L., Al-Rohil R., Nazeer T., Sheehan C., et al. (2014) A high frequency of activating extracellular domain ERBB2 (HER2) mutation in micropapillary urothelial carcinoma. Clin Cancer Res 20: 68–75. [DOI] [PubMed] [Google Scholar]
- Schoffski P., Awada A., Dumez H., Gil T., Bartholomeus S., Wolter P., et al. (2012) A phase I, dose-escalation study of the novel polo-like kinase inhibitor volasertib (BI 6727) in patients with advanced solid tumours. Eur J Cancer 48: 179–186. [DOI] [PubMed] [Google Scholar]
- Schrag D., Mitra N., Xu F., Rabbani F., Bach P., Herr H., et al. (2005) Cystectomy for muscle-invasive bladder cancer: patterns and outcomes of care in the Medicare population. Urology 65: 1118–1125. [DOI] [PubMed] [Google Scholar]
- Seront E., Rottey S., Sautois B., Kerger J., D’hondt L., Verschaeve V., et al. (2012) Phase II study of everolimus in patients with locally advanced or metastatic transitional cell carcinoma of the urothelial tract: clinical activity, molecular response, and biomarkers. Ann Oncol 23: 2663–2670. [DOI] [PubMed] [Google Scholar]
- Sharma P., Shen Y., Wen S., Yamada S., Jungbluth A., Gnjatic S., et al. (2007) CD8 tumor-infiltrating lymphocytes are predictive of survival in muscle-invasive urothelial carcinoma. Proc Natl Acad Sci U S A 104: 3967–3972. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sherif A., Rintala E., Mestad O., Nilsson J., Holmberg L., Nilsson S., et al. (2002) Neoadjuvant cisplatin-methotrexate chemotherapy for invasive bladder cancer–Nordic cystectomy trial 2. Scand J Urol Nephrol 36: 419–425. [DOI] [PubMed] [Google Scholar]
- Sibley K., Cuthbert-Heavens D., Knowles M. (2001) Loss of heterozygosity at 4p16.3 and mutation of FGFR3 in transitional cell carcinoma. Oncogene 20: 686–691. [DOI] [PubMed] [Google Scholar]
- Siefker-Radtke A., Kamat A., Corn P. (2012) Neoadjuvant chemotherapy with DD-MVAC and bevacizumab in high-risk urothelial cancer: results from a phase II trial at the M.D. Anderson Cancer Center. J Clin Oncol 30(Suppl. 5): 261. [Google Scholar]
- Siegel R., Miller K., Jemal A. (2015) Cancer statistics, 2015. CA Cancer J Clin 65: 5–29. [DOI] [PubMed] [Google Scholar]
- Sjodahl G., Lauss M., Gudjonsson S., Liedberg F., Hallden C., Chebil G., et al. (2011) A systematic study of gene mutations in urothelial carcinoma; inactivating mutations in TSC2 and PIK3R1. PLoS One 6: e18583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skinner D., Daniels J., Russell C., Lieskovsky G., Boyd S., Krailo M., et al. (1990) Adjuvant chemotherapy following cystectomy benefits patients with deeply invasive bladder cancer. Semin Urol 8: 279–284. [PubMed] [Google Scholar]
- Sonpavde G., Jones B., Bellmunt J., Choueiri T., Sternberg C. (2015) Future directions and targeted therapies in bladder cancer. Hematol Oncol Clin North Am 29: 361–376. [DOI] [PubMed] [Google Scholar]
- Sriplakich S., Jahnson S., Karlsson M. (1999) Epidermal growth factor receptor expression: predictive value for the outcome after cystectomy for bladder cancer? BJU Int 83: 498–503. [DOI] [PubMed] [Google Scholar]
- Stadler W., Kuzel T., Roth B., Raghavan D., Dorr F. (1997) Phase II study of single-agent gemcitabine in previously untreated patients with metastatic urothelial cancer. J Clin Oncol 15: 3394–3398. [DOI] [PubMed] [Google Scholar]
- Stadler W., Lerner S., Groshen S., Stein J., Shi S., Raghavan D., et al. (2011) Phase III study of molecularly targeted adjuvant therapy in locally advanced urothelial cancer of the bladder based on p53 status. J Clin Oncol 29: 3443–3449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stadler W., Vaughn D., Sonpavde G., Vogelzang N., Tagawa S., Petrylak D., et al. (2014) An open-label, single-arm, phase 2 trial of the polo-like kinase inhibitor volasertib (BI 6727) in patients with locally advanced or metastatic urothelial cancer. Cancer 120: 976–982. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sternberg C., De Mulder P., Schornagel J., Theodore C., Fossa S., Van Oosterom A., et al. (2001) Randomized phase III trial of high-dose-intensity methotrexate, vinblastine, doxorubicin, and cisplatin (MVAC) chemotherapy and recombinant human granulocyte colony-stimulating factor versus classic MVAC in advanced urothelial tract tumors: European Organization for Research and Treatment of Cancer Protocol no. 30924. J Clin Oncol 19: 2638–2646. [DOI] [PubMed] [Google Scholar]
- Sternberg C., De Mulder P., Schornagel J., Theodore C., Fossa S., Van Oosterom A., et al. (2006) Seven year update of an EORTC phase III trial of high-dose intensity M-VAC chemotherapy and G-CSF versus classic M-VAC in advanced urothelial tract tumours. Eur J Cancer 42: 50–54. [DOI] [PubMed] [Google Scholar]
- Sternberg C., Skoneczna I., Kerst J., Albers P., Fossa S., Agerbaek M., et al. (2015) Immediate versus deferred chemotherapy after radical cystectomy in patients with pT3-pT4 or N+ M0 urothelial carcinoma of the bladder (EORTC 30994): an intergroup, open-label, randomised phase 3 trial. Lancet Oncol 16: 76–86. [DOI] [PubMed] [Google Scholar]
- Stockle M., Meyenburg W., Wellek S., Voges G., Rossmann M., Gertenbach U., et al. (1995) Adjuvant polychemotherapy of nonorgan-confined bladder cancer after radical cystectomy revisited: long-term results of a controlled prospective study and further clinical experience. J Urol 153: 47–52. [DOI] [PubMed] [Google Scholar]
- Studer U., Bacchi M., Biedermann C., Jaeger P., Kraft R., Mazzucchelli L., et al. (1994) Adjuvant cisplatin chemotherapy following cystectomy for bladder cancer: results of a prospective randomized trial. J Urol 152: 81–84. [DOI] [PubMed] [Google Scholar]
- Svatek R., Shariat S., Lasky R., Skinner E., Novara G., Lerner S., et al. (2010) The effectiveness of off-protocol adjuvant chemotherapy for patients with urothelial carcinoma of the urinary bladder. Clin Cancer Res 16: 4461–4467. [DOI] [PubMed] [Google Scholar]
- Takai N., Hamanaka R., Yoshimatsu J., Miyakawa I. (2005) Polo-like kinases (Plks) and Cancer. Oncogene 24: 287–291. [DOI] [PubMed] [Google Scholar]
- Tie J., Bang Y., Park Y., Kang Y., Monteith D., Hartsock K., et al. (2014) A phase I trial of LY2874455, a fibroblast growth factor receptor inhibitor, in patients with advanced cancer. Cancer Res 74: CT215. [Google Scholar]
- Topalian S., Drake C., Pardoll D. (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27: 450–461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tsai Y., Cheng H., Tzai T., Chow N. (2012) Clinical significance of ERBB receptor family in urothelial carcinoma of the bladder: a systematic review and meta-analysis. Adv Urol 2012: 1–11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Von Der Maase H., Hansen S., Roberts J., Dogliotti L., Oliver T., Moore M., et al. (2000) Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, phase III study. J Clin Oncol 18: 3068–3077. [DOI] [PubMed] [Google Scholar]
- Von Der Maase H., Sengelov L., Roberts J., Ricci S., Dogliotti L., Oliver T., et al. (2005) Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol 23: 4602–4608. [DOI] [PubMed] [Google Scholar]
- Wagle N., Grabiner B., Van Allen E., Hodis E., Jacobus S., Supko J., et al. (2014) Activating mTOR mutations in a patient with an extraordinary response on a phase I trial of everolimus and pazopanib. Cancer Discov 4: 546–553. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wallace D., Raghavan D., Kelly K., Sandeman T., Conn I., Teriana N., et al. (1991) Neo-adjuvant (pre-emptive) cisplatin therapy in invasive transitional cell carcinoma of the bladder. Br J Urol 67: 608–615. [DOI] [PubMed] [Google Scholar]
- Wallmeroth A., Wagner U., Moch H., Gasser T., Sauter G., Mihatsch M. (1999) Patterns of metastasis in muscle-invasive bladder cancer (pT2–4): an autopsy study on 367 patients. Urol Int 62: 69–75. [DOI] [PubMed] [Google Scholar]
- Wolf J., Lorusso P., Camidge R., Perez J., Tabernero J., Hidalgo M., et al. (2012) A phase I dose escalation study of NVP-BGJ398, a selective pan FGFR inhibitor in genetically preselected advanced solid tumors. Cancer Res 72(Suppl. 8): abstract LB-122. [Google Scholar]
- Wong Y., Litwin S., Vaughn D., Cohen S., Plimack E., Lee J., et al. (2012) Phase II trial of cetuximab with or without paclitaxel in patients with advanced urothelial tract carcinoma. J Clin Oncol 30: 3545–3551. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wulfing C., Machiels J., Richel D., Grimm M., Treiber U., De Groot M., et al. (2009) A single-arm, multicenter, open-label phase 2 study of lapatinib as the second-line treatment of patients with locally advanced or metastatic transitional cell carcinoma. Cancer 115: 2881–2890. [DOI] [PubMed] [Google Scholar]
- Yamamoto Y., Matsuyama H., Kawauchi S., Matsumoto H., Nagao K., Ohmi C., et al. (2006) Overexpression of polo-like kinase 1 (PLK1) and chromosomal instability in bladder cancer. Oncology 70: 231–237. [DOI] [PubMed] [Google Scholar]
- Yap T., Garrett M., Walton M., Raynaud F., De Bono J., Workman P. (2008) Targeting the PI3k-AKT-mTOR pathway: progress, pitfalls, and promises. Curr Opin Pharmacol 8: 393–412. [DOI] [PubMed] [Google Scholar]
- Zhao G., Li W., Chen D., Henry J., Li H., Chen Z., et al. (2011) A novel, selective inhibitor of fibroblast growth factor receptors that shows a potent broad spectrum of antitumor activity in several tumor xenograft models. Mol Cancer Ther 10: 2200–2210. [DOI] [PubMed] [Google Scholar]
- Zhou N., Singh K., Mir M., Parker Y., Lindner D., Dreicer R., et al. (2013) The investigational aurora kinase A inhibitor MLN8237 induces defects in cell viability and cell-cycle progression in malignant bladder cancer cells in vitro and in vivo. Clin Cancer Res 19: 1717–1728. [DOI] [PMC free article] [PubMed] [Google Scholar]