ABSTRACT
Integrins play crucial roles in epithelial adhesion, proliferation, wound healing and cancer. In the epidermis, the roles of many integrin subunits are incompletely defined and mechanistic details regarding their functions are lacking. We performed a multiplexed small hairpin (sh)RNA screen to define roles for each subunit in human organotypic skin. We show that integrin-αv (also known as ITGAV) heterodimers are essential for epidermal generation, with integrin-αv loss driving a keratinocyte G1–S cell cycle block. Surprisingly, integrin αv is not localized within keratinocyte focal adhesions, and instead maintains proliferation by controlling cellular (c)-Myc translation through FAK, p38β and p90RSK1. These phenotypes depend only on the binding partners of integrin-αv – integrin β5 and integrin β6 (also known as ITGB5 and ITGB6, respectively). Through inducible depletion of integrin αv in both normal organotypic epidermis and Ras-driven invasive neoplasia, we show that integrin αv is required for de novo tissue generation and neoplastic invasion but that it is dispensable for epidermal maintenance. Heterodimers of integrin αv with integrin β5 (integrin αvβ5) or integrin β6 (integrin αvβ6) are required to similar extents for neoplastic invasion, thus identifying integrin αvβ5 and integrin αvβ6 heterodimers as potential therapeutic targets for epidermal squamous cell carcinoma.
KEY WORDS: c-Myc; Focal adhesion kinase, FAK; Integrin αv; Skin; Squamous cell carcinoma
Summary: Integrin-αv plays a crucial role in regulating skin tissue generation and tumor invasion through a focal-adhesion-independent FAK–p38–p90RSK–c-Myc signaling axis.
INTRODUCTION
Integrin-αβ heterodimers transduce mechanical and chemical cues from the extracellular matrix into intracellular signals that vary with cell type and tissue microenvironment (Watt and Fujiwara, 2011; Watt and Huck, 2013). In the classic paradigm of adhesion signaling, integrin engagement leads to recruitment of structural, adaptor and signaling proteins to form a large membrane-associated adhesion complex called a focal adhesion (Hynes, 2002). The integrin family comprises 18 α-subunits and eight β-subunits, which combine to form 24 α–β heterodimers with variable focal adhesion compositions and distinct non-redundant functions (Humphries et al., 2009; Schiller et al., 2013). Integrin expression is dynamic, and subunit requirements differ between tissue development, homeostasis, injury repair and tumorigenesis (Janes and Watt, 2006; López-Rovira et al., 2005; Sachs et al., 2012). Integrin signaling is important for the generation and maintenance of epidermis, where basal proliferative cells directly interact with extracellular-matrix-rich basement membranes (Duperret and Ridky, 2013; Margadant et al., 2010; Watt and Huck, 2013).
Transgenic mouse models have provided insight into the specific requirements of some integrins in epidermal maintenance, wound healing and squamous cell carcinoma (SCC) (Duperret and Ridky, 2013; Margadant et al., 2010). Integrin α3β1 is essential for proper basement membrane matrix organization, hair growth and wound healing in mice (Conti et al., 2003; DiPersio et al., 1997, 2000; Margadant et al., 2009). Integrin α9β1 is necessary for murine wound re-epithelialization (Singh et al., 2009) and integrin α6β4, a hemidesmosomal component, is essential for epidermal adhesion to the basement membrane (DiPersio et al., 2000; Dowling et al., 1996; Niculescu et al., 2011; Raymond et al., 2005; van der Neut et al., 1996). Additionally, ablation or inhibition of integrin β1 (also known as ITGB1) or integrin α6β4 blocks tumor formation in mouse and human skin (Dajee et al., 2003; Reuter et al., 2009).
However, the roles of many integrins in normal or neoplastic adult human epidermis are poorly understood, and the signaling events downstream of specific integrin heterodimers that mediate their function in skin is largely unknown (Duperret and Ridky, 2013). Defining integrin activities in a medically relevant human context is important, as many blocking antibodies, small molecule inhibitors and peptide antagonists are being explored as potential cancer therapeutics (Desgrosellier and Cheresh, 2010; Millard et al., 2011; Weis and Cheresh, 2011).
Here, we have used a multiplexed small hairpin (sh)RNA screen to target each integrin expressed in organotypic human skin and establish an essential role for specific integrin-αv (also known as ITGAV) heterodimers in maintaining progenitor cell proliferative capacity during epidermal generation. Organotypic tissues incorporate human stromal extracellular matrix and cellular elements, and have been previously used to establish essential, medically relevant roles for genes in epidermal proliferation, differentiation and tumorigenesis (Dumesic et al., 2009; Jameson et al., 2013; Monteleon et al., 2015; Sen et al., 2012). We demonstrate that integrin αv signals outside of classic focal adhesions in order to maintain keratinocyte proliferation. This requires binding partners integrin β5 and integrin β6 (also known as ITGB5 and ITGB6, respectively), which together regulate cellular (c)-Myc translation through a signaling axis comprising focal adhesion kinase (FAK), p38β (also known as MAPK11) and p90RSK1 (also known as RPS6KA1). We define a crucial role for integrin αv in epidermal generation. Further, we demonstrate that integrin αv is required for neoplastic epidermal tumor invasion but that it is dispensable for the maintenance of established normal epidermis.
RESULTS
Integrin αv is required for human keratinocyte proliferation
To determine the degree to which each integrin subunit is required for human epidermal tissue formation, we developed an shRNA-based screening approach in architecturally faithful organotypic skin. Primary human keratinocytes, melanocytes and fibroblasts were incorporated into the appropriate epidermal or stromal compartments of devitalized acellular human dermis and supported at the air–liquid interface, where tissue stratifies and differentiates into three-dimensional (3D) skin (Fig. 1A) (Duperret et al., 2014; Monteleon et al., 2015; Ridky et al., 2010).
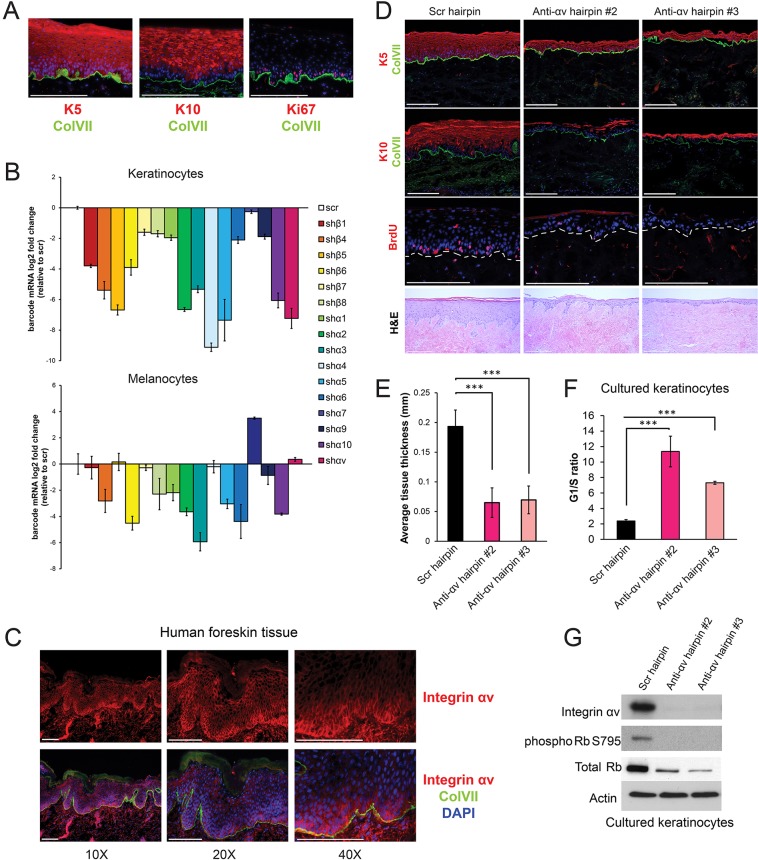
Fig. 1.
shRNA screen identifies integrin αv as a key regulator of keratinocyte cell cycle progression. (A) Representative images of regenerated human skin (K10, Keratin 10, early differentiation marker; K5, Keratin 5; Ki67, proliferation marker; ColVII, collagen VII basement membrane marker). (B) Quantification of cell populations remaining in the integrin knockdown competition screen performed in keratinocytes or melanocytes. Cell populations were measured by barcode mRNA expression compared to scrambled control cells. Values represent fold changes relative to that in cells infected with a scramble (scr) hairpin (n=3 independent organotypic culture tissues per experiment, mean±s.d.). sh, small hairpin against the indicated integrin. (C) Images of human neonatal foreskin tissue stained for integrin αv at the indicated magnifications. (D) Morphological analysis of organotypic tissue generated from keratinocytes infected with the indicated shRNAs (anti-ɑv hairpin #2 and #3, two independent shRNAs against integrin ɑv) showing BrdU, K5, K10 and ColVII staining. Bottom panel shows hematoxylin and eosin (H&E) stain. (E) Quantification of tissue thickness from D, measured in mm (mean±s.d.). Measurements taken from two experiments, each performed with n=3 organotypic culture tissues. P=3.55×10−6 using one-way ANOVA. (F) Propidium iodide staining and flow cytometry was performed on cultured keratinocytes infected with the indicated hairpins. The ratio of cells in the G1 phase to those in S phase was calculated from three biological replicates (mean±s.d.). P=2.0×10−4 using one-way ANOVA. (G) Western blot showing changes in Rb signaling upon integrin-ɑv knockdown with two independent hairpins . Scale bars: 200 μm (A,D); 100 µm (C). ***P<0.0005.
We first determined that primary keratinocytes transcribe 16 of the 26 integrin-encoding genes (Fig. S1A). We then screened shRNA libraries to identify individual hairpins with the ability to reduce transcript levels by more than 75% (Fig. S1C). Individual keratinocyte populations were transduced with a single shRNA, along with a second virus driving expression of a unique barcoded fluorescent reporter to allow for quantification of the relative representation of each cell population in a mixed group. Pooled integrin-knockdown cells and control cells were mixed in equal ratios and used to regenerate epidermis. The relative representation of each cell population in the starting mixture was compared to that in established day-14 tissue. We included all integrins expressed at the mRNA level in this screen, given the caveat that this might not reflect surface expression.
Many integrins appeared to be necessary for keratinocyte proliferation and survival (Fig. 1B). We chose to focus functional studies on integrin αv, because (1) it was strongly selected against in the screen, (2) it dimerizes with nearly all β subunits, (3) the role in human skin is poorly understood and (4) it has been shown to promote cancer in other tissues (Ricono et al., 2009; Weis and Cheresh, 2011). In contrast to the results in keratinocytes, we found that integrin-αv loss in melanocytes conferred no survival advantage or disadvantage in tissue (Fig. 1B). This helps to confirm that the fitness disadvantage in the integrin-αv-knockdown keratinocytes did not result from non-specific off-target toxicity.
Integrin αv is expressed in healing skin wounds (Cavani et al., 1993; Clark et al., 1996). We determined that integrin αv is also robustly expressed in both normal adult and neonatal skin, with highest expression in the plasma membrane of proliferative basal layer cells (Fig. 1C). To verify the crucial role of integrin αv in normal skin in a non-competitive context, we knocked down integrin αv in human keratinocytes using two independent hairpins and seeded these cells in organotypic culture (Fig. 1D). In each case, the resulting tissue was approximately one-third of the thickness of controls, and lacked proliferative BrdU+ basal cells (Fig. 1D,E). Proliferation arrest was not caused by premature differentiation, as skin tissue lacking integrin αv still lacks keratin 10 (K10) expression in the basal layer (Fig. 1D). Keratinocytes in two dimensional (2D) culture also displayed cell cycle arrest in response to integrin-αv depletion, with a 4–5 fold increase in the ratio of cells in the G1 phase to those in S phase and a near complete loss of Rb (also known as RB1) phosphorylation (Fig. 1F,G).
Integrin αv does not localize to keratinocyte focal adhesions
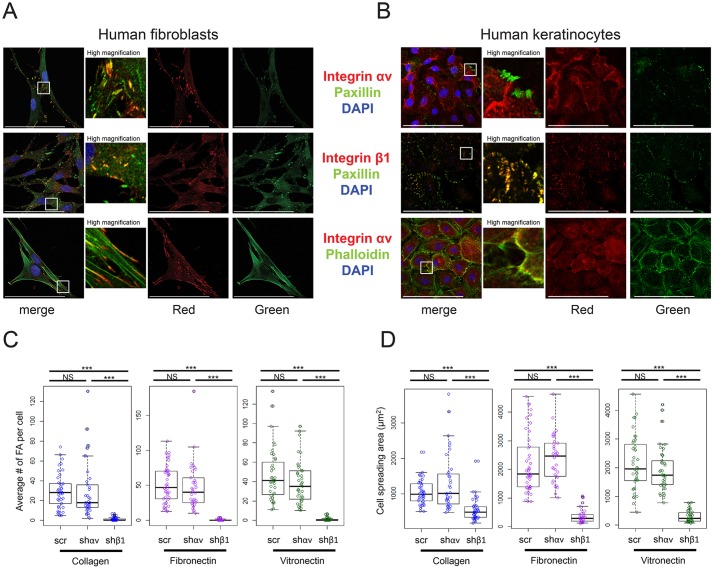
To identify crucial integrin-αv-activated signaling pathways, we first examined its spatial localization relative to focal adhesions in keratinocytes compared to that in dermal fibroblasts. Consistent with previous reports indicating a role for integrin αv in fibroblast adhesion maturation (Schiller et al., 2013), integrin αv localized to large paxillin-containing focal adhesions in fibroblasts, whereas integrin β1 localized to smaller, less mature, focal adhesions (Fig. 2A). In striking contrast, we found that integrin αv did not associate with keratinocyte focal adhesions, whereas integrin β1 was tightly co-localized with all keratinocyte focal adhesions (Fig. 2B). We confirmed this result on a variety of different substrates, including collagen, fibronectin and vitronectin (Fig. 2B; Fig. S2C). Keratinocytes on these substrates still secrete integrin β1 ligands and thus still form paxillin-containing adhesions (Alitalo et al., 1982; Chung et al., 2011). However, vitronectin, an integrin-αv-specific ligand, did not induce integrin αv focal adhesions (Fig. S2C). Further, although integrin β1 localized to the tips of actin filaments in keratinocytes, integrin αv did not, indicating that integrin αv is unlikely to connect the extracellular matrix to the actin cytoskeleton in keratinocytes (Fig. 2B; Fig. S2D). Consistent with this idea, integrin αv expression was not restricted to the cell-substrate basal adhesive surface in keratinocytes, and instead localized throughout the cell membrane (Fig. S2A,B). We confirmed integrin αv expression at the cell surface by using immunofluorescence without permeabilization (Fig. S2E). Integrin-αv knockdown did not alter the number or size distribution of focal adhesions within keratinocytes grown on collagen, fibronectin or vitronectin (Fig. 2C; Fig. S2F). In contrast, integrin-β1 knockdown abolished nearly all keratinocyte focal adhesions (Fig. 2C). Loss of integrin β1, but not integrin αv, also significantly decreased the area of cell spreading and the mechanical adhesion to the growth surface (Fig. 2D).
Fig. 2.
Integrin αv does not localize to focal adhesions in keratinocytes. (A,B) Representative images of human fibroblasts (A) or human keratinocytes (B) stained for integrin ɑv, integrin β1 and paxillin, and/or incubated with phalloidin, then imaged with confocal microscopy. (C) Box plots showing quantification of the number of focal adhesions per cell for keratinocytes infected with a scramble hairpin, an integrin αv hairpin (shαv) or an integrin β1 hairpin (shβ1), and seeded onto coverslips coated with collagen, fibronectin or vitronectin (n=30–40 cells per condition, box-plot whisker ends are at the 1.5 interquartile range, boxes are at the 1st and 3rd quartile, and the line is at the median). P=2.45×10−12 (collagen), 8.15×10−15 (fibronectin), 2×10−16 (vitronectin) using one-way ANOVA. (D) Box plots showing quantification of cell spreading area for the same cells shown in C. P=3.79×10−9 (collagen), 2×10−16 (fibronectin), 2×10−16 (vitronectin) using one-way ANOVA. Scale bars: 100 μm. ***P<0.0005; NS, not significant.
The mechanistic basis for the differential integrin αv localization between keratinocytes and fibroblasts is unclear. We considered the possibility that integrin β3, which is absent in human keratinocytes yet present in fibroblasts, directs integrin αv to focal adhesions. To test this possibility, we expressed integrin β3 in human keratinocytes and examined the localization of the integrin αvβ3 heterodimer (Fig. S2G,H). Although we achieved high levels of integrin β3 expression, which localized to the plasma membrane, it was insufficient to direct integrin αv to paxillin-containing focal adhesions (Fig. S2G,H). Consistent with this, depletion of integrin β3 in human fibroblasts decreased the number of integrin-αv-containing focal adhesions, but did not abolish them completely (Fig. S2I).
Integrin αv controls cell cycle progression in keratinocytes through a FAK–c-Myc signaling axis
Despite the lack of integrin αv at keratinocyte focal adhesions, we observed a near-complete loss of both phosphorylated and total FAK, a key regulator of focal adhesion signaling, upon integrin αv knock down (Fig. 3A). FAK localized to focal adhesions in both human fibroblasts and keratinocytes (Fig. S3C). Loss of FAK in murine keratinocytes leads to anoikis in in vitro culture (McLean et al., 2004); however, we did not observe anoikis in our human integrin-αv-depleted keratinocytes lacking FAK (Fig. S3A). In contrast, integrin-β1 depletion in keratinocytes led to significant anoikis (Fig. S3A). To determine whether this discrepancy might be due to differences between mouse and human keratinocytes, we examined anoikis upon FAK inhibition in both human and murine primary keratinocytes (Fig. S3A,B). Although FAK inhibition did not induce anoikis in human keratinocytes, FAK inhibition in murine keratinocytes led to a modest increase in terminal deoxynucleotidyl transferase dUTP nick end labeling (TUNEL) staining (Fig. S3A,B). These differences might be the result of in vitro culture conditions because mouse keratinocytes that lack FAK can proliferate under certain optimized conditions (Schober et al., 2007).
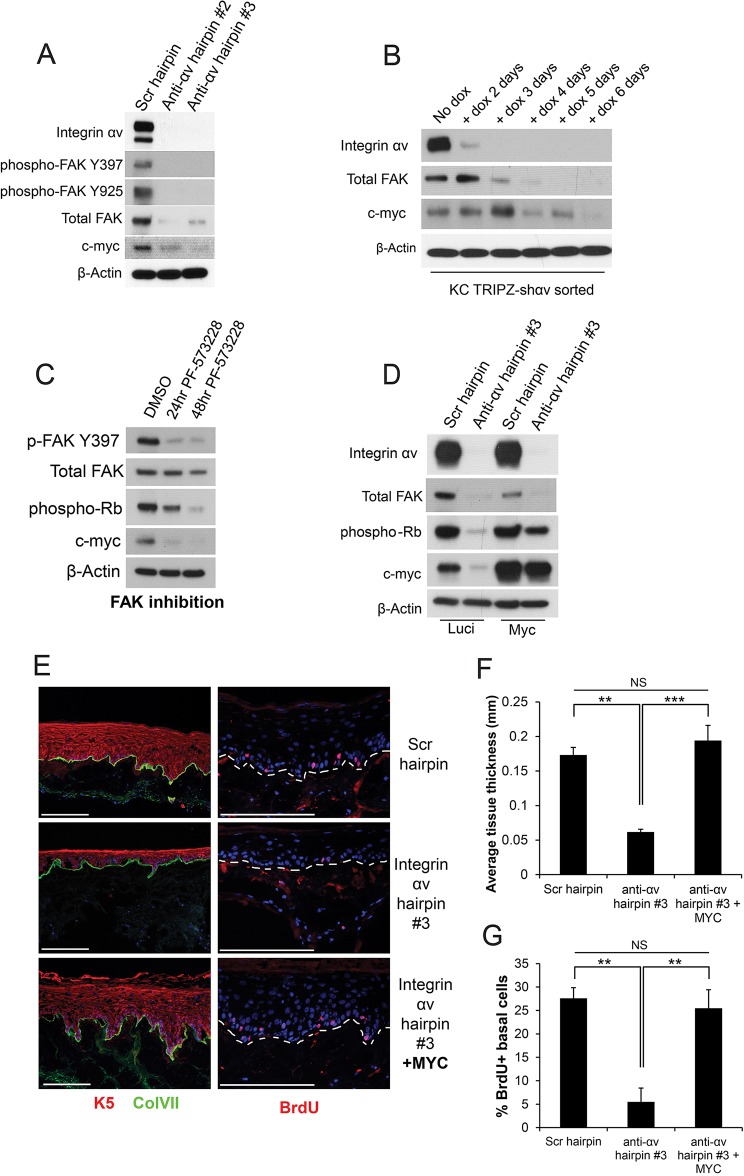
Fig. 3.
Integrin αv controls the cell cycle in keratinocytes through an integrin-αv–FAK–p38–c-Myc signaling axis. (A) Western blot showing signaling pathway changes in keratinocytes that had been infected with the indicated shRNAs. Scr, scrambled shRNA; anti-ɑv hairpin #2 and #3, two independent shRNAs against integrin ɑv. p-FAK Y397 and p-FAK Y925, FAK phosphorylated at residues Y397 and Y925, respectively. (B) Western blot showing temporal changes in signaling pathways upon integrin-αv loss using doxycycline (dox)-inducible integrin-αv knockdown. KC TRIPZ-shαv sorted, keratinocytes transduced with doxycycline-inducible shRNA targeting integrin αv, sorted for high RFP+ expression upon dox induction. (C) Western blot showing signaling pathway changes upon treatment of keratinocytes with DMSO or a FAK inhibitor (1 μM PF-573228) for 24 or 48 h. (D) Western blot showing signaling pathway changes upon expression of c-Myc in keratinocytes that had been infected with the indicated hairpins. Luci, luciferase. (E) Morphological analysis of organotypic tissue generated with keratinocytes infected with the indicated hairpins and constructs. ColVII, collagen VII; K5, keratin 5. (F) Quantification of tissue thickness of the samples shown in E, measured in mm (mean±s.d.). n=3 organotypic tissues. P=0.000253 using one-way ANOVA. (G) Quantification of BrdU uptake in the samples shown in E, measured as the percentage of BrdU+ basal epidermal cells (mean±s.d.). n=3 organotypic tissues. P=0.000952 using one-way ANOVA. **P<0.005; ***P<0.0005; NS, not significant. Scale bars: 200 μm.
We also observed a striking loss of c-Myc protein upon either integrin-αv knockdown, specific FAK inhibition or FAK knockdown (Fig. 3A,C; Fig. S4F). To gain further insight into mechanisms driving cell cycle arrest after integrin αv loss, we used a doxycycline-inducible shRNA against integrin αv to define the sequence and timing of the loss of downstream signaling pathways (Fig. 3B). Integrin αv loss was nearly complete at 3 days post doxycycline induction (dpi); FAK depletion followed at 4–5 dpi (consistent with the long half-life of FAK), and ultimately c-Myc loss at 6 dpi, immediately before cell cycle arrest (Fig. 3B). Re-expression of c-Myc was also sufficient to rescue the proliferation arrest without restoring upstream FAK (Fig. 3D). This phenotype was re-capitulated in organotypic culture, where re-expression of c-Myc in integrin-αv-null skin was sufficient to rescue tissue thickness and basal cell proliferation, while preserving normal stratification and differentiation (Fig. 3E–G). These data suggest that integrin αv controls cell cycle progression through a FAK–c-Myc signaling pathway.
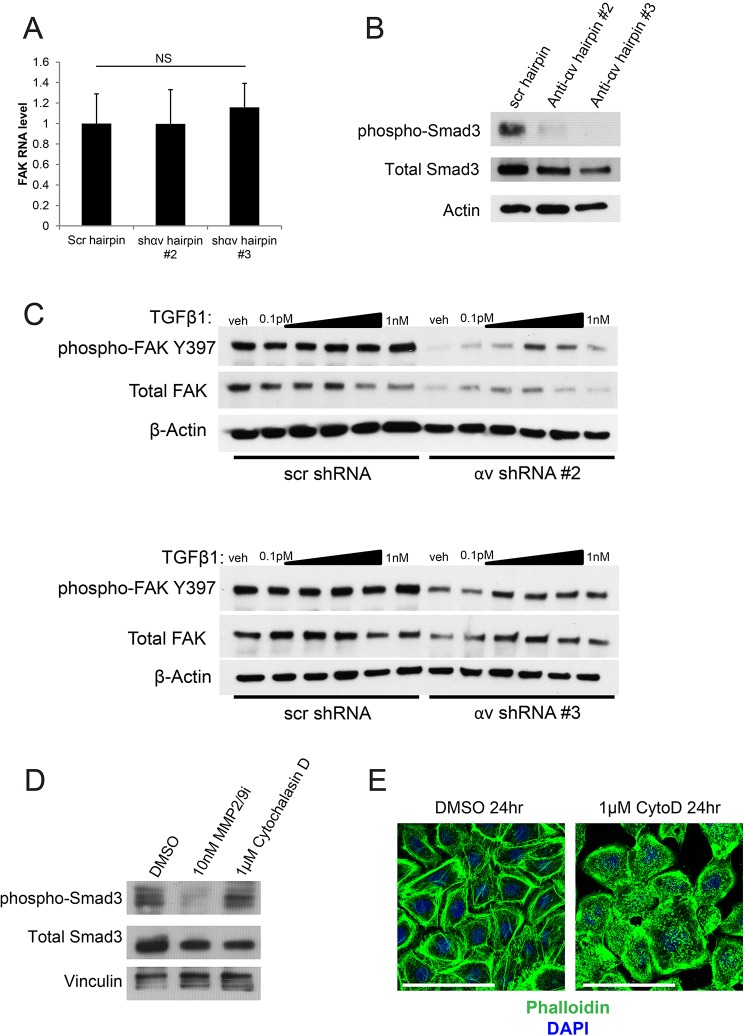
Integrin αv controls FAK expression and activation through TGFβ signaling
We next questioned how integrin αv controls FAK expression and activation. Integrin αv regulates FAK at the post-transcriptional level, because integrin-αv loss does not alter the levels of FAK mRNA (Fig. 4A). Previous efforts have linked TGFβ signaling to FAK activity and/or FAK expression through a variety of different mechanisms (Brooks et al., 1996; Cicchini et al., 2008; Kracklauer et al., 2003; Rolli et al., 2003; Thannickal et al., 2003; Wang et al., 2004; Wendt and Schiemann, 2009). Because of the role of integrin αv in activating latent TGFβ through force-dependent or matrix metalloproteinase (MMP)-dependent mechanisms, we questioned whether this could explain the loss of FAK observed upon depletion of integrin αv (Mamuya and Duncan, 2013). Consistent with the hypothesis that integrin αv loss inhibits TGFβ, we observed a loss of Smad3 phosphorylation upon integrin-αv knockdown (Fig. 4B). We treated control or integrin-αv-knockdown cells with exogenous TGFβ1 and observed a dose-dependent increase in both phosphorylated and total FAK protein in integrin-αv-null cells that peaked at treatment with 10 pM of TFGβ1 and tapered off at higher concentrations (Fig. 4C). The lack of dose-dependent increase in FAK phosphorylation in control cells is potentially due to saturation of FAK activation (Asthagiri et al., 1999). This suggests that integrin-αv-mediated control of TGFβ signaling is at least partially responsible for maintaining FAK expression and activity. It is possible that the fitness disadvantage observed upon loss of other integrins is likewise due to changes in TGFβ signaling, or to entirely distinct mechanisms (Margadant and Sonnenberg, 2010).
Fig. 4.
TGFβ signaling is partially responsible for the regulation of FAK signaling by integrin αv. (A) qPCR analysis of FAK transcript levels in keratinocytes that had been infected with the indicated shRNAs. NS, not statistically significant, measured with one-way ANOVA (P=0.906). Scr, scrambled shRNA; shɑv #2 and #3, two independent shRNAs against integrin ɑv. (B) Western blot showing signaling pathway changes in keratinocytes that had been infected with the indicated shRNAs. anti-ɑv hairpin #2 and #3, two independent shRNAs against integrin ɑv. (C) Western blot showing signaling pathway changes upon addition of varying doses of TGFβ1 in keratinocytes that had been infected with the indicated hairpins. TGFβ1 doses range from 0.1 pM to 1 nM, increasing by tenfold each time. Veh, vehicle. (D) Western blot showing signaling pathway changes in keratinocytes upon treatment with 10 nM of a MMP2 and MMP9 inhibitor (MMP2/9i), or 1 µM cytochalasin D treatment for 24 h. (E) Phalloidin staining of keratinocytes treated with control (DMSO) or 1 µM cytochalasin D (CytoD) for 24 h. Scale bars: 100 μm.
To determine whether TGFβ is regulated by MMPs or force-dependent mechanisms in keratinocytes, we treated keratinocytes with either a dual MMP2 and MMP9 inhibitor or the actin polymerization inhibitor cytochalasin D. We observed a decrease in phosphorylated Smad3 with MMP inhibition, but not with cytochalasin-D-mediated cytoskeleton disruption (Fig. 4D,E). We did not observe a difference in the expression of secreted MMP2 and MMP9 or the activity of these proteins upon integrin-αv knockdown. However, integrin αv is thought to control the local coordinated activities of MMPs at the membrane (Brooks et al., 1996; Mu et al., 2002; Wipff and Hinz, 2008; Yu and Stamenkovic, 2000). These results suggest that integrin αv does not control TGFβ signaling through adhesion-mediated forces, but rather through MMPs. It is also possible that MMPs and integrin αv are required in parallel for the regulation of TGFβ signaling.
To further explore the mechanism by which integrin αv controls the FAK protein activity in keratinocytes, we expressed wild-type FAK or hyperactive SuperFAK and then induced integrin-αv knockdown. Exogenously expressed FAK or SuperFAK was properly phosphorylated at the Tyr397 auto-phosphorylation site in control cells (Fig. S3D,F). Expression of exogenous FAK or SuperFAK was sufficient to rescue total FAK protein, but not FAK activity in integrin-αv-null keratinocytes (Fig. S3D,F). Furthermore, FAK localization at focal adhesions was lost upon integrin-αv knockdown, even in the presence of supplemental exogenous FAK (Fig. S3E). This indicates that integrin αv contributes to both FAK expression and the localization to focal adhesions in human keratinocytes. Although TGFβ has a well-established role in regulating FAK, the specific mechanisms are unclear. We did not observe consistent downregulation of Src, ERK or p70 S6K signaling pathways – which have been implicated in TGFβ-mediated regulation of FAK – upon integrin-αv knockdown (Fig. S3H; Fig. 5D; Fig. S4E) (Cicchini et al., 2008; Suer et al., 2009; Walsh et al., 2008; Wang et al., 2005). There is evidence for TGFβ-mediated regulation of talin proteins, which is known in certain situations to recruit FAK to focal adhesions (Chen et al., 1995; Frame et al., 2010; Lawson et al., 2012). In keratinocytes, integrin-αv knockdown led to a reduction in talin-1 protein levels, which was rescued upon addition of TGFβ1 (Fig. S3I). This integrin-αv- and TGFβ1-dependent control of talin-1 expression might be necessary to orchestrate proper localization of FAK to focal adhesions.
Fig. 5.
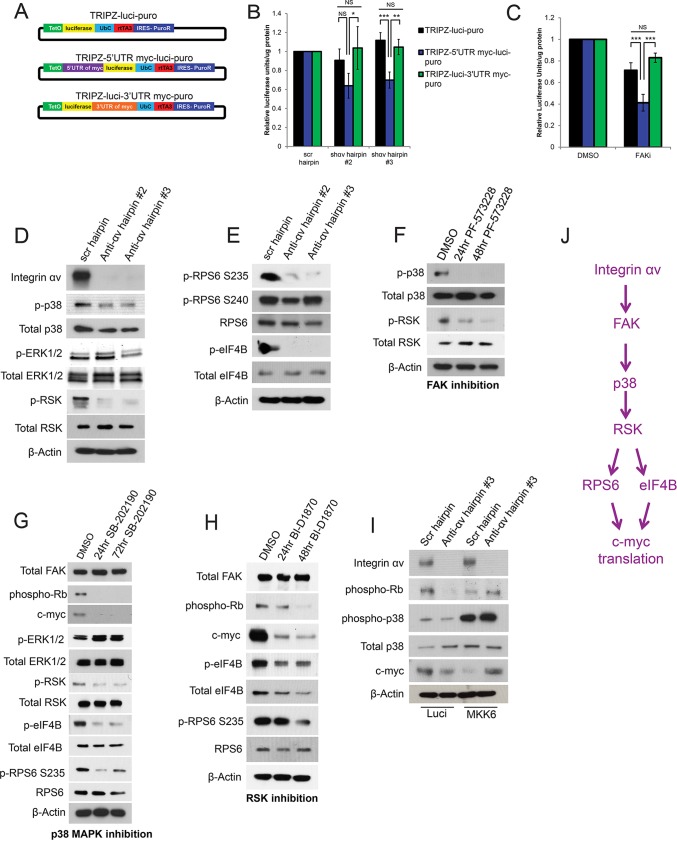
Integrin αv and FAK control 5′UTR-dependent c-Myc translation in keratinocytes through p38 and p90RSK. (A) Plasmid maps for chimeric luciferase constructs containing the 5′UTR or 3′UTR of c-Myc. (B,C) Luciferase activity measured after 6 h of doxycycline (dox) induction in keratinocytes that had been infected with the chimeric constructs shown in (A) and subsequently infected with the indicated hairpins (B) or treated with DMSO (vehicle) or 1 μM PF-573228 (FAK inhibitor) for 24 h (C). Scr, scrambled shRNA; shɑv #2 and #3, two independent shRNAs against integrin ɑv. n=3 biological replicates. P=0.0217 (hairpin #2), 0.000111 (hairpin #3) (B) and 2.33×10−5 (C) using one-way ANOVA. *P<0.05; **P<0.005; ***P<0.0005; NS, not significant. (D,E) Western blots showing p38–ERK–p90RSK (D) or RPS6–eIF4B (E) signaling pathway changes upon integrin-αv knockdown with two independent hairpins in keratinocytes (anti-ɑv hairpin #2 and #3). Pan-p38 MAPK and pan-90RSK antibodies were used. (F) Western blot showing signaling pathway changes upon treatment of keratinocytes with DMSO or FAK inhibitor (1 μM PF-573228) for 24 or 48 h. (G) Western blot showing changes in signaling pathways upon treatment of keratinocytes with DMSO or p38 MAPK inhibitor (10 μM SB-202190) for 24 or 72 h. (H) Western blot showing changes in the signaling pathways upon treatment of keratinocytes with DMSO or p90RSK inhibitor (1 µM BI-D1870) for 24 or 48 h. (I) Western blot showing signaling pathway changes upon expression of MKK6(glu) in keratinocytes that had been infected with the indicated hairpins. Luci, luciferase. (J) Schematic of signaling pathway. D–I, prefixes ‘p-’ and ‘phospho-’ indicate phosphorylated proteins. For RPS6, the phosphorylated residue is also given.
Integrin αv controls c-Myc translation through a FAK–p38–p90RSK signaling axis
Integrin αv and FAK regulation of the c-Myc protein occurs post-transcriptionally as c-Myc transcript levels are unaltered upon either integrin-αv knockdown or inhibition of FAK (Fig. S4A,B). We next examined the c-Myc half-life in integrin-αv-knockdown or FAK-inhibited cells and found no enhancement of the degradation of the c-Myc protein in either setting (Fig. S4C,D). This indicates that integrin αv and FAK are likely to influence c-Myc translation. To test the hypothesis that translation of the c-Myc protein is regulated by integrin αv and FAK through elements in the 5′UTR or 3′UTR, we generated chimeric reporter constructs containing doxycycline-inducible luciferase with (1) no UTRs, (2) the 5′UTR of MYC, or (3) the 3′UTR of MYC (Fig. 5A). We transduced keratinocytes with these constructs, antagonized integrin αv and FAK, and then induced luciferase expression. Integrin-αv loss or FAK inhibition led to decreased luciferase activity only when the luciferase transcript contained the MYC 5′UTR (Fig. 5B,C). These findings indicate that integrin αv and FAK control 5′UTR-dependent translation of c-Myc.
Cap-dependent c-Myc protein translation is partially regulated by Akt–mTOR signaling in some settings (Gera et al., 2004). However, we observed an increase in both Akt and p70 S6K family members phosphorylation upon loss of integrin αv, indicating that translation of c-Myc is likely to be controlled through alternative integrin-αv- and FAK-regulated pathways (Fig. S4E). In that regard, ERK proteins and p38 MAPK family members are also known to control 5′UTR-dependent c-Myc translation through cap-dependent and -independent mechanisms (Shi et al., 2005; Stoneley et al., 2000; Subkhankulova et al., 2001). Integrin-αv depletion did not alter phosphorylation of ERK1/2, but did decrease phosphorylation of p38 MAPK family members, indicating that this pathway is involved in c-Myc translation (Fig. 5D). Furthermore, we observed a decrease in the phosphorylation of p90RSK family members upon integrin-αv knockdown (Fig. 5D). Although ERK1/2 has a well-established role in phosphorylating p90RSK family members, p38 MAPK family members have also been shown to promote activation of p90RSK indirectly in some cell types (Roux et al., 2007; Zaru et al., 2015). Furthermore, we observed a decrease in phosphorylation of two ribosomal kinase (RSK) translation machinery targets – RPS6 at residue Ser235 (but not Ser240) and eIF4B, upon integrin-αv knockdown (Fig. 5E) (Degen et al., 2013; Roux et al., 2007). RPS6 phosphorylation at Ser240 is controlled by p70 S6K (Pende et al., 2004). FAK inhibition or FAK knockdown led to a similar decrease in phosphorylation of both p38 MAPK family members and p90RSK family members (Fig. 5F; Fig. S4F). This indicates that FAK activity is necessary for activation of these signaling pathways downstream of integrin αv. To test whether the lack of p38 activation was directly responsible for these signaling events, we next inhibited p38α and p38β (MAPK14 and MAPK11, respectively) (Fig. 5G). Inhibition of p38α and p38β led to an immediate decrease in p90RSK phosphorylation, eIF4B phosphorylation, RPS6 phosphorylation at Ser235, c-Myc protein expression and Rb phosphorylation without altering ERK1/2 activity or upstream FAK protein levels (Fig. 5G). We confirmed these results with small molecule inhibitors by using genetic approaches. The p38 inhibitor SB202190 targets both p38α (encoded by MAPK14) and p38β (encoded by MAPK11). At the RNA level, MAPK11 is expressed at levels tenfold higher than those of MAPK14, and we thus targeted this transcript with shRNA (Fig. S4G), which recapitulated the effects of the small molecule p38 inhibitor (Fig. S4I). Furthermore, restoration of p38 phosphorylation levels in integrin-αv-null keratinocytes through expression of a constitutively active MKK6 (also known as MAP2K6) mutant [MKK6(glu)] was sufficient to rescue cell growth and c-Myc protein levels (Fig. 5I). To test whether p90RSK activity was directly responsible for these signaling pathway changes, we inhibited p90RSK (Fig. 5H). Inhibition of p90RSK led to a decrease in eIF4B phosphorylation, phosphorylation of RPS6 at Ser235, c-Myc protein expression and subsequent growth arrest, without altering FAK levels (Fig. 5H). We also confirmed these signaling pathway changes using genetic approaches. P90RSK1 (encoded by RPS6KA1) is the predominant p90RSK isoform expressed in keratinocytes (Fig. S4G). Further, only hairpins targeting p90RSK1 (but not the other two isoforms) reduced levels of all p90RSK family members (p90RSK1/2/3) (Fig. S4H). Knockdown of p90RSK1 recapitulated the effects of the small molecule p90RSK inhibitor (Fig. S4J). These data, taken together, support the notion of a pathway in which integrin αv controls c-Myc protein translation through activation of FAK, p38 and p90RSK proteins (Fig. 5J).
Integrin αv controls the cell cycle in keratinocytes through binding partners integrin β5 and integrin β6
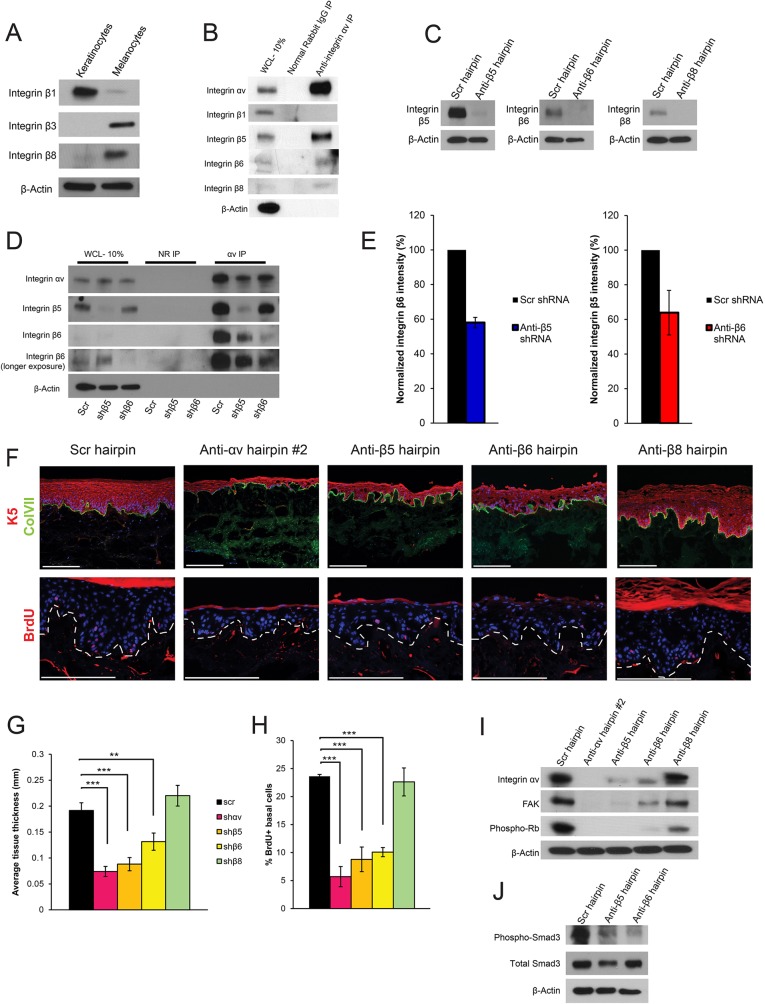
Integrin αv has several potential binding partners, including integrin β1, integrin β3, integrin β5, integrin β6 and integrin β8, all of which, except integrin β3, are expressed in cultured keratinocytes (Fig. 6A,B; Fig. S1A,B). In immunoprecipitation and western blot experiments, we found that integrin αv bound to integrin β5, integrin β6 and integrin β8, but not to integrin β1 (Fig. 6B). To determine which β subunits are most crucial in mediating the integrin-αv loss phenotype, we knocked down each β subunit individually in organotypic skin (Fig. 6C). Immunoprecipitation experiments showed that knock down of one β subunit did not lead to increased integrin-αv heterodimerization with the remaining β subunits (Fig. 6D). In contrast, knockdown of integrin β5 led to a slight reduction in integrin-αv immunoprecipitation with integrin β6 and vice versa (Fig. 6E). We found that loss of the integrin αvβ5 heterodimer or the integrin αvβ6 heterodimer individually largely phenocopied the loss of skin tissue proliferation seen upon depletion of all integrin αv heterodimers (Fig. 6F). The integrin αvβ5 requirement was slightly greater than that for integrin αvβ6, as determined by analyzing tissue thickness and BrdU incorporation (Fig. 6G,H). Loss of integrin β5 led to an absence of FAK protein, whereas depletion of integrin β6 partially reduced FAK levels (Fig. 6I). Loss of integrin β5 or integrin β6 also led to a similar reduction in phosphorylation of Smad3 (Fig. 6J). Thus, the cell cycle arrest in response to integrin-αv loss was due to the combined loss of both integrin αvβ5 and integrin αvβ6.
Fig. 6.
Integrin αv controls skin tissue generation through binding partners integrin β5 and integrin β6. (A) Western blot showing integrin expression in keratinocytes compared to that in melanocytes. (B) Immunoprecipitation (IP) of integrin αv from keratinocytes and subsequent western blot for β-subunits. WCL, whole cell lysate. (C) Western blots showing knockdown efficiency of integrin β5 (anti-β5 hairpin), integrin β6 (anti-β6 hairpin) and integrin β8 (anti-β8 hairpin) in keratinocytes. Scr, scrambled. (D) Immunoprecipitation of integrin αv from keratinocytes transduced with a control hairpin (scr), or hairpins against integrin β5 or integrin β6, and subsequent western blot of various integrin subunits. αv, integrin αv; NR, normal rabbit IgG. (E) Quantification of western blots shown in D. Means from three replicates are shown. (F) Morphologic analysis of organotypic tissue generated with keratinocytes that had been infected with a control hairpin, or hairpins targeting integrin αv, integrin β5, integrin β6 or integrin β8. Tissue was stained for K5 and ColVII, or BrdU. (G) Quantification of tissue thickness of the samples shown in E, measured in mm (mean±s.d.). n=3 independent organotypic tissues. P=1.07×10−6 using one-way ANOVA. (H) Quantification of BrdU uptake in the tissues shown in E, measured as the percentage of BrdU+ basal epidermal cells (mean±s.d.). n=3 independent organotypic tissues. P=3.01×10−7 using one-way ANOVA. (I) Western blot showing signaling pathway changes upon integrin-αv, integrin-β5, integrin-β6 or integrin-β8 knockdown in keratinocytes. (J) Western blot showing signaling pathway changes upon integrin-β5 or integrin-β6 knockdown in keratinocytes. **P<0.005; ***P<0.0005. Scale bars: 200 μm.
Integrin αv is required for organotypic skin tissue generation but not epidermal maintenance
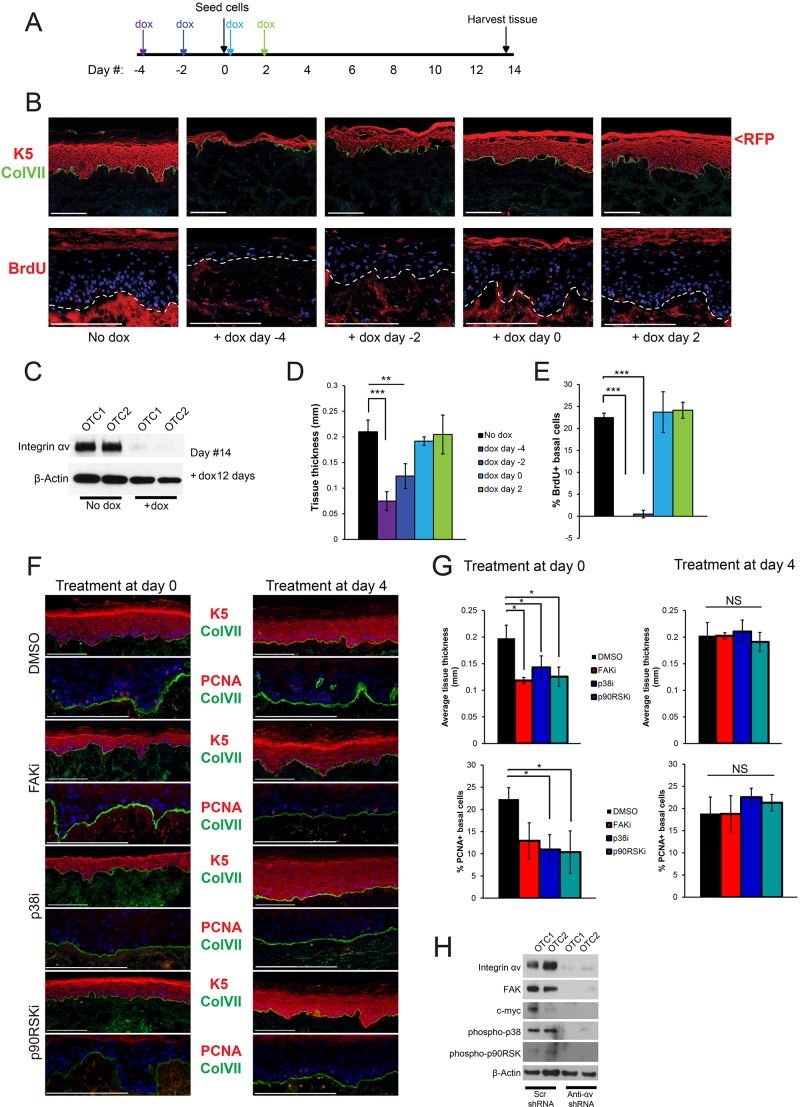
These initial analyses focused on the roles of integrins in tissue generation, and in cultured ‘activated’ keratinocytes. To determine the role of integrin αv in tissue maintenance, we used a doxycycline-inducible shRNA to knockdown integrin αv at successive time points during organotypic tissue regeneration (Fig. 7A). Loss of integrin αv occurred 3–4 dpi and persisted throughout the course of the experiment (Fig. 7C). The phenotypic tissue effects of integrin-αv loss were seen only at the earliest time points (Fig. 7B), correlating with thin tissue and a lack of BrdU incorporation (Fig. 7D,E). Tissue that had been treated with doxycycline 2 days before seeding (day −2) was slightly thicker than the tissue that had been treated with doxycycline 4 days before seeding (day −4). The day −2 tissue was able to proliferate for 1 to 2 days before integrin-αv knockdown occurred, whereas the day −4 tissue displayed loss of integrin αv before seeding. All other time points showed normal tissue thickness and normal basal epidermal proliferation (Fig. 7D,E) despite robust loss of integrin αv. These observations indicate that integrin αv is only necessary for tissue generation and is not required for the maintenance of normal epidermis.
Fig. 7.
Integrin-αv heterodimers are required for skin tissue generation but not tissue maintenance. (A) Experimental setup. Human keratinocytes that had been infected with TRIPZ-shintegrin-αv and sorted by using flow cytometry were induced with doxycycline (dox) at various time points in organotypic culture (indicated with arrows) – 4 days prior to seeding, 2 days prior to seeding, day of seeding, 2 days post seeding. (B) Morphological analysis of organotypic tissue in the doxycycline-inducible timecourse experiment described in A. Representative images were taken from two independent experiments, each performed in triplicate. (C) Western blot from tissue lysates that were not treated with dox, or treated with dox for 12 days. OTC, organotypic culture. (D) Quantification of tissue thickness of samples shown in B, measured in mm (mean±s.d.). Representative analysis from two independent experiments. Each experiment comprised three independent organotypic tissues. P=0.00015 using one-way ANOVA. (E) Quantification of BrdU+ basal epidermal cells from the samples shown in B (mean±s.d.). Representative analysis from two independent experiments. Each experiment comprised three independent organotypic tissues. P=7.52×10−8 using one-way ANOVA. (F) Morphological analysis of organotypic tissue treated with inhibitors (suffix ‘i’) of the indicated proteins at the indicated time points. (G) Quantification of tissue thickness measured in mm and PCNA+ basal cells from samples shown in F (mean±s.d.). n=3 independent organotypic tissues. P=0.00879 (upper left), P=0.423 (upper right), P=0.0157 (lower left), P=0.407 (lower right) using one-way ANOVA. (H) Western blot from tissue lysates generated from keratinocytes that had been infected with the indicated shRNAs. *P<0.05; **P<0.005; ***P<0.0005; NS, not significant. Scale bars: 200 μm.
Next, we sought to determine whether the FAK–p38–p90RSK pathway that mediates the effects of integrin-αv loss in cultured cells was required in a similar manner in 3D tissue. We inhibited each of these pathway elements at two different time points during epidermal tissue generation – day 0 and day 4. Inhibition of each pathway component led to a decrease in epidermal tissue thickness and S-phase basal cells when inhibitors were added at day 0, but not at day 4 (Fig. 7F,G). Furthermore, organotypic tissue that had been formed from integrin-αv-knockdown cells showed decreased levels of FAK and c-Myc, and of p38 and p90RSK phosphorylation, indicating that this pathway is also active in organotypic tissue (Fig. 7H). Taken together, these data indicate that the integrin-αv–FAK–p38–p90RSK signaling pathway plays a crucial role in organotypic epidermal tissue formation, but is relatively dispensable for epidermal maintenance.
Integrin αvβ5 and integrin αvβ6 are required for organotypic SCC invasion
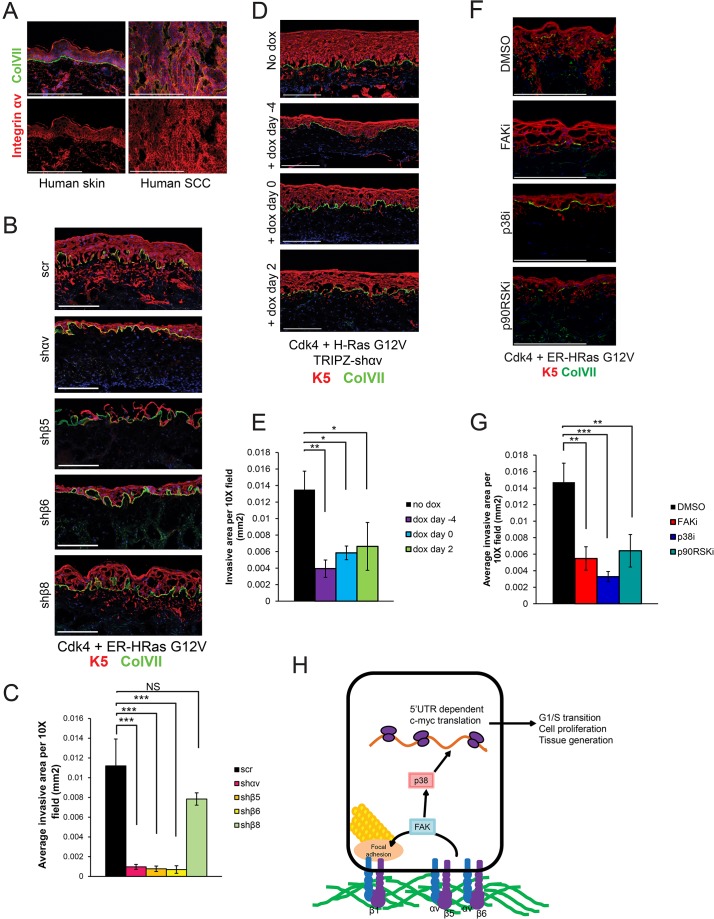
Because epidermal tissue generation and epidermal carcinoma are both associated with increased basal cell proliferation, we hypothesized that integrin αv is required for tumorigenesis. We examined integrin-αv expression and localization in human epidermal SCCs and found that nearly every tumor cell displayed intense membrane-localized staining of integrin-αv (Fig. 8A). To determine whether integrin αv or its binding partners, integrin β5 and integrin β6, are required for tumor initiation, we knocked down each of these subunits in keratinocytes that had been engineered to express a medically relevant oncogene pair, including active Cdk4 (mutant R24C), and H-Ras (mutant G12V), which are sufficient to convert normal organotypic epidermis into SCC that invades through the basement membrane (Lazarov et al., 2002; Ridky et al., 2010). We found that knockdown of integrin αv, integrin β5 or integrin β6 in tissue before oncogene activation blocked tumor invasion, whereas knockdown of integrin β8 had no effect (Fig. 8B,C). To determine whether integrin αv is also required for tumor invasion in established organotypic tumors, we performed a parallel doxycycline-inducible integrin-αv-knockdown time course in keratinocytes that expressed Cdk4 (mutant R24C) and H-Ras (mutant G12V). Doxycycline-induction was sufficient to inhibit neoplastic invasion at every time point, including 2 days post-seeding, indicating that integrin αv is required for both initiation of neoplastic invasion and subsequent progression (Fig. 8D,E). These results suggest that integrin αvβ5 and integrin αvβ6 might be useful therapeutic targets for the treatment of SCC.
Fig. 8.
Integrin αvβ5 and integrin αvβ6 are required for SCC tumor invasion. (A) Immunofluorescence staining of integrin αv in normal human skin and human SCC samples. ColVII, collagen VII. (B) Morphological analysis of organotypic tissue containing keratinocytes infected with Cdk4 (mutant R24C), ER-HRas G12V (an estrogen-receptor-H-Ras G12V fusion that is inducible with tamoxifen administration) and either a scramble (scr) hairpin or hairpins targeting integrin αv (shαv), integrin β5 (shβ5), integrin β6 (shβ6) or integrin β8 (shβ8), and that had been treated with 100 nM 4-O-HT. (C) Quantification of the invasive area of the samples shown in B, measured in mm2 (mean±s.d.). n=3 organotypic tissues. P=8.55×10−6 using one-way ANOVA. (D) The cells used in Fig. 6 were transduced with Cdk4 (mutant R24C) and ER-H-Ras (mutant G12V), and subjected to the same doxycycline (dox)-inducible time course. Representative images were taken from two independent experiments. (E) Quantification of the invasive area from samples shown in D, measured in mm2 (mean±s.d.). Representative of two independent experiments. n=3 organotypic tissues. P=0.00155 using one-way ANOVA. (F) Keratinocytes were transduced with Cdk4 (mutant R24C) and ER-H-Ras (mutant G12V) and treated with inhibitors (suffix ‘i’) of the indicated proteins. Representative images are shown. (G) Quantification of the invasive area of samples shown in F, measured in mm2 (mean±s.d.). n=3 organotypic tissues. P=0.000607 using one-way ANOVA. (H) Diagram of the signaling pathway described in this paper. Integrin αv dimerizes with integrin β5 or integrin β6 outside of focal adhesions to control FAK expression and localization to focal adhesions. Downstream, FAK controls phosphorylation of p38 proteins to induce 5′UTR-dependent c-Myc translation. *P<0.05; **P<0.005; ***P<0.0005; NS, not significant. Scale bars: 200 μm.
Next, we questioned whether the downstream pathway elements (FAK–p38–p90RSK) could also be therapeutic targets for the treatment of SCC. Inhibition of each of these elements with small molecule inhibitors, at doses that did not affect normal tissue, significantly attenuated Ras-driven neoplastic invasion (Fig. 8F,G).
DISCUSSION
Here, we have used both genetic and pharmacological approaches to define the functional roles of integrin-αv heterodimers in organotypic human skin and epidermal SCC. We show that integrin αv is required during the initial stages of skin tissue generation, but not for tissue maintenance. Integrin αv signals outside of focal adhesions though a FAK–p38–c-Myc signaling axis to promote keratinocyte proliferation. Further, we show that integrin αvβ5 and integrin αvβ6 are required for the invasive nature of human SCC, identifying these specific heterodimers as potential therapeutic targets (Fig. 8H).
Integrin-αv heterodimers and wound healing
Integrin-αv-null mice die during embryogenesis, or immediately after birth, whereas ablation of epidermal integrin αv during embryogenesis does not result in obvious morphological defects (Bader et al., 1998; Savar et al., 2014). Normal skin tissue proliferation in response to 7,12-Dimethylbenz[a]anthracene (DMBA) or 12-O-tetradecanoylphorbol-13-acetate (TPA) has not been examined in skin conditional integrin-αv-knockout mice (Savar et al., 2014). Ablation of integrin β5 or integrin β6 during embryogenesis does not lead to skin defects, indicating that these heterodimers are not necessary for epidermal development (Huang et al., 2000; Xie et al., 2012). However, the functional roles of integrin αvβ5 and integrin αvβ6 heterodimers in the wound-healing setting are unclear. In humans, integrin αv is upregulated during wound healing (Cavani et al., 1993; Clark et al., 1996). Young integrin-β6−/− mice do not display wound-healing defects; however, wound healing is delayed in aged integrin-β6-null mice compared to age-matched controls (AlDahlawi et al., 2006; Huang et al., 1996). In contrast, constitutive expression of integrin β6 in the epidermis leads to the formation of chronic wounds (Häkkinen et al., 2004). Integrin-β5−/− mice display normal cutaneous wound healing, although this has not been tested in aged mice (Huang et al., 2000). The contribution to these epidermal phenotypes is unclear because integrin β5 and integrin β6 have not been ablated specifically in mouse skin. It is clear that the role of integrin αvβ6 in wound healing is highly dependent on the expression level of this heterodimer and additional environmental or cellular factors. In human organotypic epidermis, keratinocytes adhere to the basement membrane, rapidly proliferate and stratify into full-thickness epidermis, processes that are similar to wound healing. Here, we have provided evidence that both integrin αvβ5 and integrin αvβ6 heterodimers are thus likely to play crucial roles in re-epithelialization after human skin wounding.
Integrin-αv heterodimers in tumorigenesis
Integrin-αv heterodimers are implicated in both tumor-promoting and tumor-suppressive roles in epithelial tissues. In mouse skin, integrin-αv deletion cooperates with p53 loss to transiently promote initial SCC formation, but ultimately results in decreased tumor growth (Savar et al., 2014). Knockout of integrin αv in mouse eyelids and conjunctiva also seems to promote SCC formation (McCarty et al., 2008). Integrin β6 has a growth-suppressive role in the mouse skin and hair follicles because skin and hair follicles lacking integrin β6 are thicker with more Ki67+ cells (Xie et al., 2012). These conflicting data could reflect inherent differences between mouse and human skin. Consistent with this, integrin αvβ6 is overexpressed in epidermal SCC; higher integrin αvβ6 expression correlates with decreased cell survival in human cervical SCC, and integrin αvβ6 promotes invasion in human oral SCC cell lines (Hazelbag et al., 2007; Nystrom et al., 2006; Reuter et al., 2009). Also potentially complicating direct comparisons between mouse and human systems is the fact that in most mouse models, integrin expression is depleted during embryogenesis, rather than in adult tissue. Acute loss of integrins in adult mouse skin has been shown to have markedly different phenotypic effects compared to loss during development (Brakebusch et al., 2000; López-Rovira et al., 2005; Raghavan et al., 2000).
Integrin-αv signaling in skin tissue
Here, we describe a focal-adhesion-independent role for integrin-αv heterodimers and show that the lack of localization of integrin αv to focal adhesions in keratinocytes is not due to a lack of keratinocyte integrin β3. Although keratinocyte integrin αv localizes to the plasma membrane, we cannot detect its concentration in a specific known subcellular compartment. It could be informative in future studies to characterize the specific protein component of focal-adhesion-independent integrin-αv adhesion complexes in keratinocytes, which could potentially provide insight into the mechanistic basis for its unique localization pattern. This might additionally provide insight regarding how integrin αv controls FAK expression levels without affecting the structural integrity of the integrin-β1-containing focal adhesions.
The phenotypes that we observe upon integrin-αv loss in skin are consistent with mouse models of epidermal c-Myc loss. c-Myc epidermal knockout during development leads to severe skin fragility, hypoproliferation and impaired wound healing (Zanet et al., 2005). However, inducible c-Myc deletion in adult mouse epidermis is well tolerated, with no obvious skin abnormalities (Oskarsson et al., 2006). These phenotypes are consistent with the intestinal epithelium, where c-Myc is necessary for crypt formation but dispensable for crypt homeostasis (Bettess et al., 2005). c-Myc is thus able to function differently based on various environmental stresses and physiological states.
Therapeutic utility of targeting integrin αvβ5 and integrin αvβ6 in SCC
Most cutaneous SCCs in immunocompetent individuals can be treated with local excisions or topical delivery of immunomodulatory and chemotherapy; however, there are frequently cases in which individuals are not good surgical candidates. Additionally, immunosuppressed SCC individuals often suffer from SCC metastasis and unfortunately have limited treatment options.
The idea of targeting specific integrin-αv heterodimers as an anti-cancer strategy is intriguing because these specific heterodimers appear to control both FAK and c-Myc. FAK is known to promote tumor formation in mouse SCC models, and several small molecule FAK inhibitors are in early-stage clinical trials (McLean et al., 2001, 2004; Sulzmaier et al., 2014). Targeting FAK indirectly through integrin αv might lead to greater specificity than small molecule kinase inhibitors, and as a cell surface protein, integrin αv could be vulnerable to blocking antibodies and peptide-based agents. It will also be interesting to determine whether integrin αv controls the FAK–p38–c-Myc pathway in other epithelial malignancies that are dependent on Myc signaling (Gabay et al., 2014).
Targeting each heterodimer individually might lead to fewer side-effects than antagonism of the entire integrin-αv group because the β subunits are not as ubiquitously expressed as integrin αv, and their corresponding knockout mice have minimal phenotypes (Huang et al., 1996, 2000). In summary, we have shown that acute loss of integrin αvβ5 and integrin αvβ6 leads to loss of de novo epidermal tissue generation and tumor invasion but not to loss of tissue maintenance; therefore, these heterodimers might be useful targets in the treatment of human epidermal cancers.
MATERIALS AND METHODS
Cell culture and reagents
Primary human keratinocytes, melanocytes and fibroblasts were isolated from neonatal foreskins obtained from the Hospital of the University of Pennsylvania. Foreskins were incubated in a 1:1 ratio of dispase (Fisher) to Dulbecco's modified Eagle's medium (DMEM; with high glucose, 4.5 g/l)+5% FBS (fetal bovine serum, Invitrogen) overnight at 4°C. The epidermis was carefully peeled from the underlying dermis and incubated in trypsin for 10 min at 37°C. The trypsin was neutralized with DMEM+5% FBS and 1% antibiotic-antimycotic (Gibco), and spun at 300 g for 5 min. The supernatant was removed, and the pellet was plated in keratinocyte medium containing 50% Gibco Keratinocyte-SFM+l-glutamine+EGF and bovine pituitary extract (BPE), 50% Gibco Cascade Biologics 154 medium for keratinocytes and 1% penicillin-streptomycin (100 U/ml, Gibco) for keratinocyte culture, or Melanocyte Medium 254 (Gibco) with Human Melanocyte Growth Supplement and 1% penicillin-streptomycin (100 U/ml, Gibco) for melanocyte culture. For fibroblast isolation, the dermis was chopped into small pieces and incubated in 1 ml of collagenase (10 mg/ml, Roche) at 37°C for 15 min. 1 ml of 0.05% trypsin (Gibco) was added and incubated for another 10 min at 37°C. 1 ml of DMEM+5% FBS was added to quench the trypsin, and the pieces of dermis were removed and discarded. The remaining solution was centrifuged at 300 g for 5 min. The supernatant was removed and the pellet plated in DMEM+5% FBS+1% antibiotic-antimycotic. HEK293T and Phoenix cells were purchased from American Type Culture Collection (ATCC) and also cultured in DMEM+5% FBS+1% antibiotic-antimycotic. All small molecules and recombinant proteins used are listed in Table S2.
Hybridoma culture and antibody purification
The mouse L230 hybridoma cell line was obtained from ATCC (HB-8448) and cultured according to ATCC guidelines. Supernatant was collected and filtered using a 0.22-µm filter. Antibody was isolated from the supernatant and concentrated using the Nab Protein G Spin Kit (Thermo). Antibody concentration was quantified by measuring the absorbance at 280 nm.
Lentiviral and retroviral constructs
A list of hairpins used in this study is included in Table S3. The following pRRL constructs were used in this study: pRRL-c-Myc, pRRL-luciferase and pRRL-MKK6(glu). The pRRL-MKK6(glu) construct was cloned from pcDNA3-Flag MKK6(glu), which was a gift from Roger Davis (Addgene plasmid #13518) (Raingeaud et al., 1996). The following LZRS retroviral constructs were used in this study: LZRS-ER-H-Ras G12V, LZRS-Cdk4 R24C and LZRS-luciferase. The TRIPZ-β3 construct was cloned using fibroblast cDNA.
Lentivirus and retrovirus production and transduction
Phoenix cells and HEK293T cells were used for retrovirus and lentivirus production, respectively. HEK293T cells were seeded at 70% confluence on 6-well plates and transfected with 1.22 µg lentiviral plasmid that had been mixed with packaging plasmids pCMVΔR8.91 (0.915 µg) and pUC-MDG (0.305 µg) per well using Fugene6 transfection reagent (Promega). Phoenix cells were transfected using the same protocol without the packaging plasmids. 10 mM sodium butyrate (Sigma) was added 16 h after transfection, and cell culture medium was replaced 24 h after transfection and virus-producing cells were moved to 32°C. Human keratinocytes, melanocytes and fibroblasts were transduced at 10–40% confluence with lentivirus harvested at 48 and 72 h post-transfection of packaging cells. Lentivirus was filtered through a 45-μm filter (Argos) and supplemented with 5 μg/ml of polybrene (hexadimethrine bromide, Sigma). Subsequently, cells were spun at 300 g for 1 h at room temperature. Complete growth medium was replaced after 15 min of incubation at 37°C.
Antibodies and immunoblot analysis
Adherent cells were washed with PBS and then lysed with RIPA Lite lysis buffer – 50 mM Tris pH 7.5, 150 mM NaCl, 1 mM EDTA, 1% NP-40 containing protease inhibitors (Roche) and phosphatase inhibitors (Roche). Lysates were quantified using Bradford assay, and reduced in Laemmli sample buffer containing β-mercaptoethanol (BioRad). Cell lysates were subjected to SDS gel electrophoresis on 4–15% Tris-Glycine precast polyacrylamide gels (BioRad) in running buffer (25 mM Tris, 192 mM glycine, 0.1% SDS, pH 8.3). Protein was transferred to PVDF membrane (Millipore) using a Trans-blot Semi-Dry Transfer Cell (BioRad) in semi-dry transfer buffer. Membranes were blocked with 5% milk in TBST or 5% BSA in TBST and incubated in primary antibodies at 4°C overnight. After incubation with horseradish peroxidase (HRP)-conjugated secondary antibody (Cell signaling) for 30 min–1 h at 4°C, proteins were detected using ECL western blotting detection reagents (GE-Amersham Biosciences) or Luminata Crescendo western HRP substrate (Millipore). All antibodies used are listed in Table S2.
RNA isolation and qPCR
RNA was isolated using an RNeasy Plus Mini Kit (Qiagen), and RNA was converted to cDNA using the Applied Biosystems High Capacity RNA to cDNA kit. Quantitative (q)PCR was performed using Power SYBR Green master mix using a ViiA 7 real-time PCR system (Life Technologies). Relative expression levels were calculated using the 2−ΔΔCt method. All primers used in the qPCR analyses are listed in Table S1.
Immunofluorescence
Skin tissues were embedded in optimal cutting temperature compound (OCT) and sectioned at 8-µm thickness using a cryostat. Tissue sections were fixed in cold methanol for 2 min. Cultured cells were fixed and permeabilized using microtubule stabilization buffer [MTSB; 0.1 M PIPES, pH 6.75, 1 mM EGTA, 1 mM MgSO4, 4% (w/v) poly(ethylene glycol), 1% Triton X-100, 2% paraformaldehyde]. Both tissue sections and cultured cells were blocked in 5% horse serum in PBS for 30 min and incubated in 1% horse serum in PBS for primary and secondary antibody incubation (30 min each). For FAK staining, cells were fixed in cold methanol for 10 min instead of paraformaldehyde. Tissue sections were mounted using Prolong Gold Antifade plus DAPI reagent. Images of tissues were taken using an Olympus BX-61 inverted microscope, and images of cultured cells were taken using a Zeiss LSM 710 confocal microscope. For BrdU staining, tissues were fixed in cold 70% EtOH for 5 min at room temperature. Tissue sections were rinsed with PBS, incubated in 1.5 M HCl for 30 min and then rinsed in PBS again. Tissues were blocked in 5% horse serum+0.3% Triton X-100 in PBS for 60 min and then incubated with primary antibody overnight in 1% BSA in PBS. Secondary antibody was incubated in 1% BSA for 1 h at room temperature. All antibodies used are listed in Table S2.
Immunoprecipitation
Keratinocytes were lysed in RIPA lysis buffer to extract membrane proteins (TBS, pH 7.5 supplemented with 2 mM CaCl2, 1 mM MgCl2, 1% NP-40 and 1% Triton X-100 plus protease and phosphatase inhibitors). The Pierce crosslink immunoprecipitation kit was used for immunoprecipitation according to the manufacturer's protocol (Thermo).
Fluorescence-activated cell sorting
For all doxycycline-inducible experiments, keratinocytes were sorted to achieve maximum hairpin induction. pTRIPZ-transduced human keratinocytes were induced with doxycycline for 24 h before cell sorting. Cells were trypsinized and resuspended in 1× PBS containing 1% BSA in a polypropylene tube (Falcon) at a density of 10×106 cells/ml. The top 20% of red fluorescent protein (RFP)+ cells was sorted onto 6-well plates containing keratinocyte growth medium using a BD FACSAria II cell sorter in the University of Pennsylvania flow cytometry and cell sorting facility. Cells were allowed to recover from the sorting process in doxycycline-free medium for 1 to 2 weeks before experimentation.
Propidium iodide staining and flow cytometry
Keratinocyte nuclei were isolated and stained with propidium iodide using the CycleTEST PLUS DNA reagent kit. Cells were analyzed using a BD FACSCalibur in the University of Pennsylvania flow cytometry and cell sorting facility. Data were analyzed and the percentages calculated using ModFit software.
Luciferase assay
Firefly luciferase activity was measured using the Dual-Glo luciferase assay system (Promega) and a BD Monolight 3096 microplate luminometer.
Organotypic culture
Split-thickness human skin was obtained and washed in PBS containing penicillin-streptomycin, and incubated at 37°C for 7 to 10 days. PBS was changed every 2 days. The epidermis was separated from the dermis and subsequently discarded. The dermis was washed and incubated in PBS at 4°C for 6 to 12 weeks. PBS was changed every 2–3 days. For assembly of organotypic tissue, the dermis was cut into 1 cm2 square pieces and placed into 12-well culture plates with the basement membrane side facing down. 100,000 fibroblasts were seeded into each well and incubated at 37°C with 5% humidified CO2 for 3 to 4 days. The dermis with fibroblasts was elevated onto a sterilized annular dermal support tissue culture insert device in a manner such that the basement membrane was oriented upwards. The growth medium was changed to keratinocyte growth medium (KGM) [3:1 mixture of DMEM:Ham's F12, supplemented with 10% FBS, adenine (1.8×10−4 M), hydrocortisone (0.4 μg/ml), insulin (5 μg/ml), cholera toxin (1×10−10 M), EGF (10 ng/ml), transferrin (5 μg/ml), and triido-l-thyronine (1.36 ng/ml)]. Epithelial cells were seeded onto the basement membrane side at a density of 1×106 per cm2, in a total volume of 80 µl. For organotypic skin, the upper chamber was kept dry and exposed only to air, and the KGM medium in the lower chamber was changed every day. Organotypic skin tissue was harvested at 14 days, and organotypic transformed tissue was harvested at 10 days. For BrdU labeling, organotypic tissue was incubated with BrdU-labeling reagent (Invitrogen) at a 1:100 dilution in KGM for 1 h.
Invasion assay
For invasion measurements, we established organotypic tissues (described in detail above) containing keratinocytes that had been transduced with mutant Cdk4 (mutant R24C) and oncogenic H-Ras (mutant G12V) in the epidermal compartment, and primary non-transduced human fibroblasts in the dermal compartment. These epidermal transformed keratinocytes spontaneously invade through the basement membrane of these organotypic tissues into the dermis. We quantified the invasion area (in mm2) per field by imaging these tissues across the length of the entire 1-cm2 tissue. We measured the area of keratin 5 (K5)+-labeled epidermal keratinocytes that invaded past the basement membrane (labeled with collagenVII) into the dermis using ImageJ. We then averaged the invasive area across the entire tissue, and then across biological replicates.
Quantification and statistical analysis
Tissue thickness and tumor invasion were quantified using ImageJ software. Focal adhesion size and number were also quantified using ImageJ software. For experiments with two groups, statistical significance was measured using a Student's t-test. For experiments with more than two groups, one-way ANOVA was used to measure statistical significance. For experiments in which ANOVA showed significance, Tukey's honest significance difference (HSD) test was performed. *P<0.05; **P<0.005; ***P<0.0005; NS, not statistically significant.
Acknowledgements
We would like to thank the Skin Disease Research Core (SDRC) at the University of Pennsylvania for tissue histology and primary cells. We would also like to thank Rick Assoian, Sarah Millar, John Seykora, Celeste Simon and Andrei Thomas-Tikhonenko for crucial presubmission review.
Footnotes
Competing interests
The authors declare no competing or financial interests.
Author contributions
E.K.D. and T.W.R. designed experiments and wrote the manuscript. E.K.D. and A.D. performed experiments. T.W.R. directed the project.
Funding
T.W.R. is supported by a grant from the National Institutes of Health – National Cancer Institute (NIH-NCI) [grant number RO1 CA163566]. E.K.D. is supported by an NIH – National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) training grant [grant number T32 AR0007465-30], an NIH-NCI F31 National Research Service Award (NRSA) individual fellowship [grant number F31 CA186446] and the Patel Family Scholar Award. Deposited in PMC for release after 12 months.
Supplementary information
Supplementary information available online at http://jcs.biologists.org/lookup/suppl/doi:10.1242/jcs.175539/-/DC1
References
- AlDahlawi S., Eslami A., Häkkinen L. and Larjava H. S. (2006). The alphavbeta6 integrin plays a role in compromised epidermal wound healing. Wound Repair Regen. 14, 289-297. 10.1111/j.1743-6109.2006.00123.x [DOI] [PubMed] [Google Scholar]
- Alitalo K., Kuismanen E., Myllylä R., Kiistala U., Asko-Seljavaara S. and Vaheri A. (1982). Extracellular matrix proteins of human epidermal keratinocytes and feeder 3T3 cells. J. Cell Biol. 94, 497-505. 10.1083/jcb.94.3.497 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Asthagiri A. R., Nelson C. M., Horwitz A. F. and Lauffenburger D. A. (1999). Quantitative relationship among integrin-ligand binding, adhesion, and signaling via focal adhesion kinase and extracellular signal-regulated kinase 2. J. Biol. Chem. 274, 27119-27127. 10.1074/jbc.274.38.27119 [DOI] [PubMed] [Google Scholar]
- Bader B. L., Rayburn H., Crowley D., Hynes R. O. and Hughes H. (1998). Extensive vasculogenesis, angiogenesis and organogenesis precede lethality in mice lacking all αv integrins. Cell 95, 507-519. 10.1016/S0092-8674(00)81618-9 [DOI] [PubMed] [Google Scholar]
- Bettess M. D., Dubois N., Murphy M. J., Dubey C., Roger C., Robine S. and Trumpp A. (2005). c-Myc is required for the formation of intestinal crypts but dispensable for homeostasis of the adult intestinal epithelium. Mol. Cell. Biol. 25, 7868-7878. 10.1128/MCB.25.17.7868-7878.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brakebusch C., Grose R., Quondamatteo F., Ramirez A., Jorcano J. L., Pirro A., Svensson M., Herken R., Sasaki T., Timpl R. et al. (2000). Skin and hair follicle integrity is crucially dependent on beta 1 integrin expression on keratinocytes. EMBO J. 19, 3990-4003. 10.1093/emboj/19.15.3990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brooks P. C., Strömblad S., Sanders L. C., von Schalscha T. L., Aimes R. T., Stetler-Stevenson W. G., Quigley J. P. and Cheresh D. A. (1996). Localization of matrix metalloproteinase MMP-2 to the surface of invasive cells by interaction with integrin alpha v beta 3. Cell 85, 683-693. 10.1016/S0092-8674(00)81235-0 [DOI] [PubMed] [Google Scholar]
- Cavani A., Zambruno G., Marconi A., Manca V., Marchetti M. and Giannetti A. (1993). Distinctive integrin expression in the newly forming epidermis during wound healing in humans. J. Invest. Dermatol. 101, 600-604. 10.1111/1523-1747.ep12366057 [DOI] [PubMed] [Google Scholar]
- Chen H.-C., Appeddu P. A., Parsons J. T., Hildebrand J. D., Schaller M. D. and Guan J.-L. (1995). Interaction of focal adhesion kinase with cytoskeletal protein talin. J. Biol. Chem. 270, 16995-16999. 10.1074/jbc.270.28.16995 [DOI] [PubMed] [Google Scholar]
- Chung H., Suh E.-K., Han I.-O. and Oh E.-S. (2011). Keratinocyte-derived laminin-332 promotes adhesion and migration in melanocytes and melanoma. J. Biol. Chem. 286, 13438-13447. 10.1074/jbc.M110.166751 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cicchini C., Laudadio I., Citarella F., Corazzari M., Steindler C., Conigliaro A., Fantoni A., Amicone L. and Tripodi M. (2008). TGFbeta-induced EMT requires focal adhesion kinase (FAK) signaling. Exp. Cell Res. 314, 143-152. 10.1016/j.yexcr.2007.09.005 [DOI] [PubMed] [Google Scholar]
- Clark R. A. F., Ashcroft G. S., Spencer M.-J., Larjava H. and Ferguson M. W. J. (1996). Re-epithelialization of normal human excisional wounds is associated with a switch from alpha v beta 5 to alpha v beta 6 integrins. Br. J. Dermatol. 135, 46-51. 10.1111/j.1365-2133.1996.tb03606.x [DOI] [PubMed] [Google Scholar]
- Conti F. J. A., Rudling R. J., Robson A. and Hodivala-Dilke K. M. (2003). Alpha3Beta1-integrin regulates hair follicle but not interfollicular morphogenesis in adult epidermis. J. Cell Sci. 116, 2737-2747. 10.1242/jcs.00475 [DOI] [PubMed] [Google Scholar]
- Dajee M., Lazarov M., Zhang J. Y., Cai T., Green C. L., Russell A. J., Marinkovich M. P., Tao S., Lin Q., Kubo Y. et al. (2003). NF-kappaB blockade and oncogenic Ras trigger invasive human epidermal neoplasia. Nature 421, 639-643. 10.1038/nature01283 [DOI] [PubMed] [Google Scholar]
- Degen M., Barron P., Natarajan E., Widlund H. R. and Rheinwald J. G. (2013). RSK activation of translation factor eIF4B drives abnormal increases of laminin γ2 and MYC protein during neoplastic progression to squamous cell carcinoma. PLoS ONE 8, e78979 10.1371/journal.pone.0078979 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desgrosellier J. S. and Cheresh D. A. (2010). Integrins in cancer: biological implications and therapeutic opportunities. Nat. Rev. Cancer 10, 9-22. 10.1038/nrc2748 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio C. M., Hodivala-Dilke K. M., Jaenisch R., Kreidberg J. A. and Hynes R. O. (1997). α3β1 Integrin is required for normal development of the epidermal basement membrane. J. Cell Biol. 137, 729-742. 10.1083/jcb.137.3.729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- DiPersio C. M., van der Neut R., Georges-Labouesse E., Kreidberg J. A., Sonnenberg A. and Hynes R. O. (2000). Alpha3Beta1 and Alpha6Beta4 integrin receptors for Laminin-5 are not essential for epidermal morphogenesis and homeostasis during skin development. J. Cell Sci. 113, 3051-3062. [DOI] [PubMed] [Google Scholar]
- Dowling J., Yu Q. C. and Fuchs E. (1996). β4 integrin is required for hemidesmosome formation, cell adhesion and cell survival. J. Cell Biol. 134, 559-572. 10.1083/jcb.134.2.559 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumesic P. A., Scholl F. A., Barragan D. I. and Khavari P. A. (2009). Erk1/2 MAP kinases are required for epidermal G2/M progression. J. Cell Biol. 185, 409-422. 10.1083/jcb.200804038 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duperret E. and Ridky T. W. (2013). Focal adhesion complex proteins in epidermis and squamous cell carcinoma. Cell Cycle 12, 3272-3285. 10.4161/cc.26385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Duperret E. K., Oh S. J., McNeal A., Prouty S. M. and Ridky T. W. (2014). Activating FGFR3 mutations cause mild hyperplasia in human skin, but are insufficient to drive benign or malignant skin tumors. Cell Cycle 13, 1551-1559. 10.4161/cc.28492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frame M. C., Patel H., Serrels B., Lietha D. and Eck M. J. (2010). The FERM domain: organizing the structure and function of FAK. Nat. Rev. Mol. Cell Biol. 11, 802-814. 10.1038/nrm2996 [DOI] [PubMed] [Google Scholar]
- Gabay M., Li Y. and Felsher D. W. (2014). MYC activation is a hallmark of cancer initiation and maintenance. Cold Spring Harb. Perspect. Med. 4, a014241 10.1101/cshperspect.a014241 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gera J. F., Mellinghoff I. K., Shi Y., Rettig M. B., Tran C., Hsu J.-h., Sawyers C. L. and Lichtenstein A. K. (2004). AKT activity determines sensitivity to mammalian target of rapamycin (mTOR) inhibitors by regulating cyclin D1 and c-myc expression. J. Biol. Chem. 279, 2737-2746. 10.1074/jbc.M309999200 [DOI] [PubMed] [Google Scholar]
- Häkkinen L., Koivisto L., Gardner H., Saarialho-Kere U., Carroll J. M., Lakso M., Rauvala H., Laato M., Heino J. and Larjava H. (2004). Increased expression of beta6-integrin in skin leads to spontaneous development of chronic wounds. Am. J. Pathol. 164, 229-242. 10.1016/S0002-9440(10)63113-6 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hazelbag S., Kenter G. G., Gorter A., Dreef E. J., Koopman L. A., Violette S. M., Weinreb P. H. and Fleuren G. J. (2007). Overexpression of the αvβ6 integrin in cervical squamous cell carcinoma is a prognostic factor for decreased survival. J. Pathol. 212, 316-324. 10.1002/path.2168 [DOI] [PubMed] [Google Scholar]
- Huang X. Z., Wu J. F., Cass D. and Erle D. J. (1996). Inactivation of the integrin beta 6 subunit gene reveals a role of epithelial integrins in regulating inflammation in the lung and skin. J. Cell Biol. 133, 921-928. 10.1083/jcb.133.4.921 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Huang X., Griffiths M., Wu J., Farese R. V. and Sheppard D. (2000). Normal development, wound healing, and adenovirus susceptibility in beta5-deficient mice. Mol. Cell. Biol. 20, 755-759. 10.1128/MCB.20.3.755-759.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Humphries J. D., Byron A., Bass M. D., Craig S. E., Pinney J. W., Knight D. and Humphries M. J. (2009). Proteomic analysis of integrin-associated complexes identifies RCC2 as a dual regulator of Rac1 and Arf6. Sci. Signal. 2, ra51 10.1126/scisignal.2000396 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hynes R. O. (2002). Integrins: bidirectional, allosteric signaling machines. Cell 110, 673-687. 10.1016/S0092-8674(02)00971-6 [DOI] [PubMed] [Google Scholar]
- Jameson K. L., Mazur P. K., Zehnder A. M., Zhang J., Zarnegar B., Sage J. and Khavari P. A. (2013). IQGAP1 scaffold-kinase interaction blockade selectively targets RAS-MAP kinase–driven tumors. Nat. Med. 19, 626-630. 10.1038/nm.3165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Janes S. M. and Watt F. M. (2006). New roles for integrins in squamous-cell carcinoma. Nat. Rev. Cancer 6, 175-183. 10.1038/nrc1817 [DOI] [PubMed] [Google Scholar]
- Kracklauer M. P., Schmidt C. and Sclabas G. M. (2003). TGFbeta1 signaling via alphaVbeta6 integrin. Mol. Cancer 2, 28 10.1186/1476-4598-2-28 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- Lawson C., Lim S.-T., Uryu S., Chen X. L., Calderwood D. A. and Schlaepfer D. D. (2012). FAK promotes recruitment of talin to nascent adhesions to control cell motility. J. Cell Biol. 196, 223-232. 10.1083/jcb.201108078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lazarov M., Kubo Y., Cai T., Dajee M., Tarutani M., Lin Q., Fang M., Tao S., Green C. L. and Khavari P. A. (2002). CDK4 coexpression with Ras generates malignant human epidermal tumorigenesis. Nat. Med. 8, 1105-1114. 10.1038/nm779 [DOI] [PubMed] [Google Scholar]
- López-Rovira T., Silva-Vargas V. and Watt F. M. (2005). Different consequences of beta1 integrin deletion in neonatal and adult mouse epidermis reveal a context-dependent role of integrins in regulating proliferation, differentiation, and intercellular communication. J. Invest. Dermatol. 125, 1215-1227. 10.1111/j.0022-202X.2005.23956.x [DOI] [PubMed] [Google Scholar]
- Mamuya F. A. and Duncan M. K. (2013). αV integrins and TGF-β-induced EMT: a circle of regulation. J. Cell. Mol. Med. 16, 445-455. 10.1111/j.1582-4934.2011.01419.x [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C. and Sonnenberg A. (2010). Integrin–TGF-beta crosstalk in fibrosis, cancer and wound healing. EMBO Rep. 11, 97-105. 10.1038/embor.2009.276 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margadant C., Raymond K., Kreft M., Sachs N., Janssen H. and Sonnenberg A. (2009). Integrin alpha3beta1 inhibits directional migration and wound re-epithelialization in the skin. J. Cell Sci. 122, 278-288. 10.1242/jcs.029108 [DOI] [PubMed] [Google Scholar]
- Margadant C., Charafeddine R. A. and Sonnenberg A. (2010). Unique and redundant functions of integrins in the epidermis. FASEB J. 24, 4133-4152. 10.1096/fj.09-151449 [DOI] [PubMed] [Google Scholar]
- McCarty J. H., Barry M., Crowley D., Bronson R. T., Lacy-Hulbert A. and Hynes R. O. (2008). Genetic ablation of alphav integrins in epithelial cells of the eyelid skin and conjunctiva leads to squamous cell carcinoma. Am. J. Pathol. 172, 1740-1747. 10.2353/ajpath.2008.070700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McLean G., Brown K., Arbuckle M., Wyke A., Pikkarainen T., Ruoslahti E. and Frame M. (2001). Decreased focal adhesion kinase suppresses papilloma formation during experimental mouse skin carcinogenesis. Cancer Res. 61, 8385-8389. [PubMed] [Google Scholar]
- McLean G. W., Komiyama N. H., Serrels B., Asano H., Reynolds L., Conti F., Hodivala-Dilke K., Metzger D., Chambon P., Grant S. G. N. et al. (2004). Specific deletion of focal adhesion kinase suppresses tumor formation and blocks malignant progression. Genes Dev. 18, 2998-3003. 10.1101/gad.316304 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Millard M., Odde S. and Neamati N. (2011). Integrin targeted therapeutics. Theranostics 1, 154-188. 10.7150/thno/v01p0154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Monteleon C. L., McNeal A., Duperret E. K., Oh S. J., Schapira E. and Ridky T. W. (2015). IQGAP1 and IQGAP3 serve individually essential roles in normal epidermal homeostasis and tumor progression. J. Invest. Dermatol. 135, 2258-2265. 10.1038/jid.2015.140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mu D., Cambier S., Fjellbirkeland L., Baron J. L., Munger J. S., Kawakatsu H., Sheppard D., Broaddus V. C. and Nishimura S. L. (2002). The integrin αvβ8 mediates epithelial homeostasis through MT1-MMP-dependent activation of TGF-β1. J. Cell Biol. 157, 493-507. 10.1083/jcb.200109100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niculescu C., Ganguli-Indra G., Pfister V., Dupé V., Messaddeq N., De Arcangelis A. and Georges-Labouesse E. (2011). Conditional ablation of integrin alpha-6 in mouse epidermis leads to skin fragility and inflammation. Eur. J. Cell Biol. 90, 270-277. 10.1016/j.ejcb.2010.09.003 [DOI] [PubMed] [Google Scholar]
- Nystrom M. L., McCulloch D., Weinreb P. H., Violette S. M., Speight P. M., Marshall J. F., Hart I. R. and Thomas G. J. (2006). Cyclooxygenase-2 inhibition suppresses alphavbeta6 integrin-dependent oral squamous carcinoma invasion. Cancer Res. 66, 10833-10842. 10.1158/0008-5472.CAN-06-1640 [DOI] [PubMed] [Google Scholar]
- Oskarsson T., Essers M. A. G., Dubois N., Offner S., Dubey C., Roger C., Metzger D., Chambon P., Hummler E., Beard P. et al. (2006). Skin epidermis lacking the c-Myc gene is resistant to Ras-driven tumorigenesis but can reacquire sensitivity upon additional loss of the p21Cip1 gene. Genes Dev. 20, 2024-2029. 10.1101/gad.381206 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pende M., Um S. H., Mieulet V., Sticker M., Goss V. L., Mestan J., Mueller M., Fumagalli S., Kozma S. C. and Thomas G. (2004). S6K1-/-/S6K2-/- mice exhibit perinatal lethality and rapamycin-sensitive 5′-terminal oligopyrimidine mRNA translation and reveal a mitogen-activated protein kinase-dependent S6 kinase pathway. Mol. Cell. Biol. 24, 3112-3124. 10.1128/MCB.24.8.3112-3124.2004 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raghavan S., Bauer C., Mundschau G., Li Q. and Fuchs E. (2000). Conditional ablation of β1 integrin in skin: severe defects in epidermal proliferation, basement membrane formation, and hair follicle invagination. J. Cell Biol. 150, 1149-1160. 10.1083/jcb.150.5.1149 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raingeaud J., Whitmarsh A. J., Barrett T., Dérijard B. and Davis R. J. (1996). MKK3- and MKK6-regulated gene expression is mediated by the p38 mitogen-activated protein kinase signal transduction pathway. Mol. Cell. Biol. 16, 1247-1255. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raymond K., Kreft M., Janssen H., Calafat J. and Sonnenberg A. (2005). Keratinocytes display normal proliferation, survival and differentiation in conditional beta4-integrin knockout mice. J. Cell Sci. 118, 1045-1060. 10.1242/jcs.01689 [DOI] [PubMed] [Google Scholar]
- Reuter J. A., Ortiz-Urda S., Kretz M., Garcia J., Scholl F. A., Pasmooij A. M. G., Cassarino D., Chang H. Y. and Khavari P. A. (2009). Modeling inducible human tissue neoplasia identifies an extracellular matrix interaction network involved in cancer progression. Cancer Cell 15, 477-488. 10.1016/j.ccr.2009.04.002 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ricono J. M., Huang M., Barnes L. A., Lau S. K., Weis S. M., Schlaepfer D. D., Hanks S. K. and Cheresh D. A. (2009). Specific cross-talk between epidermal growth factor receptor and integrin alphavbeta5 promotes carcinoma cell invasion and metastasis. Cancer Res. 69, 1383-1391. 10.1158/0008-5472.CAN-08-3612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ridky T. W., Chow J. M., Wong D. J. and Khavari P. A. (2010). Invasive three-dimensional organotypic neoplasia from multiple normal human epithelia. Nat. Med. 16, 1450-1455. 10.1038/nm.2265 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rolli M., Fransvea E., Pilch J., Saven A. and Felding-Habermann B. (2003). Activated integrin alphavbeta3 cooperates with metalloproteinase MMP-9 in regulating migration of metastatic breast cancer cells. Proc. Natl. Acad. Sci. USA 100, 9482-9487. 10.1073/pnas.1633689100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roux P. P., Shahbazian D., Vu H., Holz M. K., Cohen M. S., Taunton J., Sonenberg N. and Blenis J. (2007). RAS/ERK signaling promotes site-specific ribosomal protein S6 phosphorylation via RSK and stimulates cap-dependent translation. J. Biol. Chem. 282, 14056-14064. 10.1074/jbc.M700906200 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sachs N., Secades P., van Hulst L., Kreft M., Song J.-Y. and Sonnenberg A. (2012). Loss of integrin alpha3 prevents skin tumor formation by promoting epidermal turnover and depletion of slow-cycling cells. Proc. Natl. Acad. Sci. USA 109, 21468-21473. 10.1073/pnas.1204614110 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Savar A., Acin S., Gonzalez C. L., El-Sawy T., Mejia O., Li Z., Esmaeli B., Lacy-Hulbert A., El-Naggar A. K., McCarty J. H. et al. (2014). Loss of epithelial p53 and αv integrin cooperate through Akt to induce squamous cell carcinoma yet prevent remodeling of the tumor microenvironment. Oncogene 34, 516-524. 10.1038/onc.2013.585 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schiller H. B., Hermann M.-R., Polleux J., Vignaud T., Zanivan S., Friedel C. C., Sun Z., Raducanu A., Gottschalk K.-E., Théry M. et al. (2013). β1- and αv-class integrins cooperate to regulate myosin II during rigidity sensing of fibronectin-based microenvironments. Nat. Cell Biol. 15, 625-636. 10.1038/ncb2747 [DOI] [PubMed] [Google Scholar]
- Schober M., Raghavan S., Nikolova M., Polak L., Pasolli H. A., Beggs H. E., Reichardt L. F. and Fuchs E. (2007). Focal adhesion kinase modulates tension signaling to control actin and focal adhesion dynamics. J. Cell Biol. 176, 667-680. 10.1083/jcb.200608010 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sen G. L., Boxer L. D., Webster D. E., Bussat R. T., Qu K., Zarnegar B. J., Johnston D., Siprashvili Z. and Khavari P. A. (2012). ZNF750 is a p63 target gene that induces KLF4 to drive terminal epidermal differentiation. Dev. Cell 22, 669-677. 10.1016/j.devcel.2011.12.001 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi Y., Sharma A., Wu H., Lichtenstein A. and Gera J. (2005). Cyclin D1 and c-myc internal ribosome entry site (IRES)-dependent translation is regulated by AKT activity and enhanced by rapamycin through a p38 MAPK- and ERK-dependent pathway. J. Biol. Chem. 280, 10964-10973. 10.1074/jbc.M407874200 [DOI] [PubMed] [Google Scholar]
- Singh P., Chen C., Pal-Ghosh S., Stepp M. A., Sheppard D. and Van De Water L. (2009). Loss of integrin alpha9beta1 results in defects in proliferation, causing poor re-epithelialization during cutaneous wound healing. J. Invest. Dermatol. 129, 217-228. 10.1038/jid.2008.201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoneley M., Chappell S. A., Jopling C. L., Dickens M., MacFarlane M. and Willis A. E. (2000). c-Myc protein synthesis is initiated from the internal ribosome entry segment during apoptosis. Mol. Cell. Biol. 20, 1162-1169. 10.1128/MCB.20.4.1162-1169.2000 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Subkhankulova T., Mitchell S. A. and Willis A. E. (2001). Internal ribosome entry segment-mediated initiation of c-Myc protein synthesis following genotoxic stress. Biochem. J. 359, 183-192. 10.1042/bj3590183 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suer S., Ampasala D., Walsh M. F. and Basson M. D. (2009). Role of ERK/mTOR signaling in TGFbeta-modulated focal adhesion kinase mRNA stability and protein synthesis in cultured rat IEC-6 intestinal epithelial cells. Cell Tissue Res. 336, 213-223. 10.1007/s00441-009-0776-z [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sulzmaier F. J., Jean C. and Schlaepfer D. D. (2014). FAK in cancer: mechanistic findings and clinical applications. Nat. Rev. Cancer 14, 598-610. 10.1038/nrc3792 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thannickal V. J., Lee D. Y., White E. S., Cui Z., Larios J. M., Chacon R., Horowitz J. C., Day R. M. and Thomas P. E. (2003). Myofibroblast differentiation by transforming growth factor-beta1 is dependent on cell adhesion and integrin signaling via focal adhesion kinase. J. Biol. Chem. 278, 12384-12389. 10.1074/jbc.M208544200 [DOI] [PubMed] [Google Scholar]
- van der Neut R., Krimpenfort P., Calafat J., Niessen C. M. and Sonnenberg A. (1996). Epithelial detachment due to absence of hemidesmosomes in integrin beta 4 null mice. Nat. Genet. 13, 366-369. 10.1038/ng0796-366 [DOI] [PubMed] [Google Scholar]
- Walsh M. F., Ampasala D. R., Hatfield J., Vander Heide R., Suer S., Rishi A. K. and Basson M. D. (2008). Transforming growth factor-beta stimulates intestinal epithelial focal adhesion kinase synthesis via Smad- and p38-dependent mechanisms. Am. J. Pathol. 173, 385-399. 10.2353/ajpath.2008.070729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H., Radjendirane V., Wary K. K. and Chakrabarty S. (2004). Transforming growth factor beta regulates cell–cell adhesion through extracellular matrix remodeling and activation of focal adhesion kinase in human colon carcinoma Moser cells. Oncogene 23, 5558-5561. 10.1038/sj.onc.1207701 [DOI] [PubMed] [Google Scholar]
- Wang S. E., Wu F. Y., Shin I., Qu S. and Arteaga C. L. (2005). Transforming growth factor {beta} (TGF-{beta})-Smad target gene protein tyrosine phosphatase receptor type kappa is required for TGF-{beta} function. Mol. Cell. Biol. 25, 4703-4715. 10.1128/MCB.25.11.4703-4715.2005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M. and Fujiwara H. (2011). Cell-extracellular matrix interactions in normal and diseased skin. Cold Spring Harb. Perspect. Biol. 3, a005124 10.1101/cshperspect.a005124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Watt F. M. and Huck W. T. S. (2013). Role of the extracellular matrix in regulating stem cell fate. Nat. Rev. Mol. Cell Biol. 14, 467-473. 10.1038/nrm3620 [DOI] [PubMed] [Google Scholar]
- Weis S. M. and Cheresh D. A. (2011). αv integrins in angiogenesis and cancer. Cold Spring Harb. Perspect. Med. 1, a006478 10.1101/cshperspect.a006478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wendt M. K. and Schiemann W. P. (2009). Therapeutic targeting of the focal adhesion complex prevents oncogenic TGF-beta signaling and metastasis. Breast Cancer Res. 11, R68 10.1186/bcr2360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wipff P.-J. and Hinz B. (2008). Integrins and the activation of latent transforming growth factor beta1 – an intimate relationship. Eur. J. Cell Biol. 87, 601-615. 10.1016/j.ejcb.2008.01.012 [DOI] [PubMed] [Google Scholar]
- Xie Y., McElwee K. J., Owen G. R., Häkkinen L. and Larjava H. S. (2012). Integrin β6-deficient mice show enhanced keratinocyte proliferation and retarded hair follicle regression after depilation. J. Invest. Dermatol. 132, 547-555. 10.1038/jid.2011.381 [DOI] [PubMed] [Google Scholar]
- Yu Q. and Stamenkovic I. (2000). Cell surface-localized matrix metalloproteinase-9 proteolytically activates TGF-beta and promotes tumor invasion and angiogenesis. Genes Dev. 14, 163-176. [PMC free article] [PubMed] [Google Scholar]
- Zanet J., Pibre S., Jacquet C., Ramirez A., de Alborán I. M. and Gandarillas A. (2005). Endogenous Myc controls mammalian epidermal cell size, hyperproliferation, endoreplication and stem cell amplification. J. Cell Sci. 118, 1693-1704. 10.1242/jcs.02298 [DOI] [PubMed] [Google Scholar]
- Zaru R., Edgar A. J., Hanauer A. and Watts C. (2015). Structural and functional basis for p38-MK2-activated Rsk signaling in toll-like receptor-stimulated dendritic cells. Mol. Cell. Biol. 35, 132-140. 10.1128/MCB.00773-14 [DOI] [PMC free article] [PubMed] [Google Scholar]