Abstract
Best practice tariff (BPT) was introduced as a financial incentive model to improve compliance with evidence-based care, such as operation for hip fracture within 36 hours of admission. We previously evaluated the impact of warfarin on patients with hip fracture, revealing significant delay to operation and subsequent loss of revenue. As a result of this, an “early trigger” intravenous vitamin K (IVK) pathway was introduced and the service reaudited a year later. The first cycle was a retrospective audit of all cases with hip fracture against BPT standards over a 32-month period. Subsequent protocol change resulted in all warfarinised cases being given 2 mg IVK in the emergency department prior to blood testing. This protocol was reaudited against the same BPT standards 12 months later. An intention-to-treat approach was used, despite breaches of protocol and other reasons for patients not progressing to theater. The data were analyzed with parametric tools to establish true clinical and statistical impact of the introduction of the protocol. In the first cycle, 80 patients were admitted on warfarin with a mean time to theater of 53.71 hours. Of these patients, 79% breached BPT due to anticoagulation. Twelve months following protocol introduction, 42 patients had a mean time to theater of 37.61 hours. Of these patients, 34% breached BPT due to anticoagulation. These data are both clinically and statistically significant (P < .001). No adverse events occurred. We have shown for the first time that “early-trigger” IVK can reduce delay to theater and maximize tariff payments in warfarinised patients with hip fracture. This is in addition to other established benefits associated with early surgery such as decreasing risk of pressure lesions and pneumonia. It affords high-quality patient-centered care while ensuring trauma units achieve maximal financial reimbursement through pay for improved performance and supports a culture of change behavior.
Keywords: fragility fractures, geriatric trauma, geriatric medicine, osteoporosis, trauma surgery, systems of care
Introduction
The global population continues to age, presenting an indefatigable fragility fracture burden to health care systems struggling to balance quality of care and financial stability. A total of 75 000 new cases of hip fracture are seen annually in the United Kingdom,1 and as only half of these patients return to community dwelling,2 the ongoing cost implications are significant.3 Hip fractures are thus contributing to a major health economic problem.4
This injury pattern is not going to go away, and the financial consequences are considerable. It is predicted that 100 000 patients annually will require surgery for fractured neck of femur by 2033 in England, with a 30-day mortality of 9%, costing £3.6 to 5.6 billion per annum in total care.5
Improving the processes of hip fracture care benefits patients, clinicians, and managers. Reward for alignment with targets is increasingly a feature of modern patient care, particularly in the population with hip fracture. While never losing sight of the patient, it is important that opportunities for efficient ways of working with patients with hip fracture are explored.
Complicating this drive for progress, comorbidities in patients with hip fracture delay progression to surgery, negatively impacting on patient outcome, efficiency, and threatening target achievement.6 Time to surgery is a key aspect of the hip fracture best practice tariff (BPT): a payment by results process instigated by the UK Department of Health to reimburse and incentivize quality and cost-effective care.7 The BPT for hip fractures consists of 6 domains: surgery within 36 hours, admission under consultant-led joint orthopedic–geriatric care, use of a multidisciplinary assessment protocol, geriatrician review within 72 hours, geriatrician-directed multiprofessional rehabilitation, and falls and bone protection assessment. This incentivization, particularly the drive to reduce time to operation, occurs in order to avoid the main sequelae of protracted bed rest—pressure ulceration, thromboembolism, and pneumonia. In hip fracture units meeting BPT criteria, there have been demonstrated improvements in patient care.8 In addition to improved patient outcomes, there is also financial reward, but in order to realize this benefit (an extra £ 1,335 per patient), all domains of the BPT must be met.
With regard to these domains, anticoagulation reversal is a common cause of operative delay, loss of BPT, and greater overall mortality.9 This was confirmed by our previous study10 in which a 2 mg oral regime with no emphasis on early administration demonstrated significant delays to surgery. In this work, 80 patients given oral vitamin K at an uncontrolled time, once admitted to the ward, had a mean time to theater of 53.71 hours, a 79% breach of BPT. In contrast, the control group of 908 nonwarfarinised patients took on average 24.5 hours: a 28% breach of BPT. This equates to a £87 536 loss in BPT over the study period.10
With regard to the management of patients on warfarin, watching and waiting’—allowing anticoagulation to reverse without pharmaceutical intervention—has been used in this population. Ashouri et al however demonstrated the failing of such an approach in this patient group. In an analysis of 57 patients awaiting hip fracture surgery, those in which no reversal was employed waited almost 2 days longer for surgery.11 In order to safely hasten surgery, there is evidence for use of both oral and intravenous forms of vitamin K for prompt reversal, and intravenous vitamin K (IVK) has been shown more recently to have greater efficacy in shortening reversal time.12,13
This is clearly a patient population in which processes can be improved,14 and the literature supports pharmaceutical intervention to address anticoagulation.15 “Active management”16—intervening to reverse anticoagulation efficiently and safely—is key. Following the protracted time to surgery highlighted by our initial audit, a multidisciplinary meeting was held, a literature review was undertaken, and a new anticoagulation reversal protocol was devised for this population. This article explains the development of the protocol and the key feature of early trigger IVK administration to warfarinised patients with hip fracture once diagnosed in the emergency department (ED) and before knowledge of the International Normalised Ratio (INR). In closing the information loop, we present the results of the reaudit 12 months later. This is the first study in this population to reflect on improved ways of working in light of target-driven care and the reality of BPT uplift.
Methods
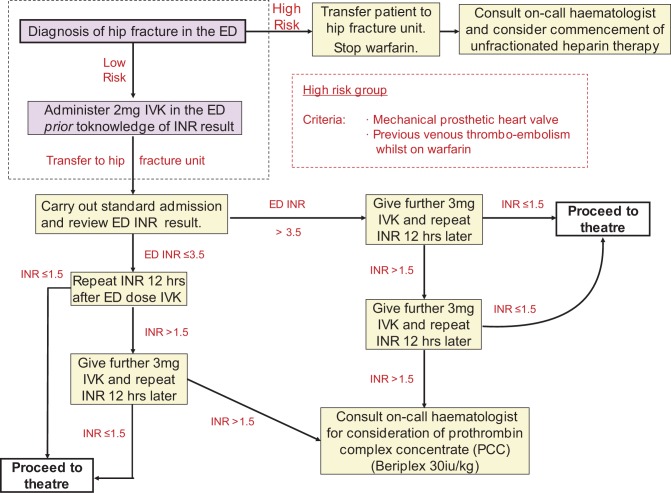
An existing nurse-led fast-track pathway for patients admitted to the ED with a confirmed hip fracture was augmented with an updated anticoagulation reversal protocol (Figure 1). Key to this is the administration of 2 mg IVK on admission to the ED prior to the INR result being known.
Figure 1.
Nurse-led early management protocol for all warfarinised patients with hip fracture.
Following introduction of the protocol, interim analysis was performed at 3 months to assess the impact of “blind” IVK administration. No complications were identified, so the new protocol remained in place and further review was carried out at 12 months.
Data were collected prospectively as part of the ongoing mandatory data collection for the National Hip Fracture Database. This involves collation of baseline demographics, injury characteristics, comorbidity scoring with the Nottingham Hip Fracture Score (NHFS), surgical procedure, timelines from admission to surgery, and discharge. In addition, readmission to hospital following discharge and complications such as venous thromboembolic events are recorded prospectively, continually as part of the trust quality assurance protocols (Figure 1).
Results
In the first 32-month audit loop, 83 eligible patients were admitted on warfarin, treated with an oral vitamin k protocol and with a mean time to theater of 53.71 hours (Table 1). Of these, 79% of patients breached BPT for the theater domain. In the subsequent 12-month audit loop, 42 patients (mean age 83 years; 25 female all, of which were warfarinised for underlying arrhythmia) were admitted through the ED (Table 1). In addition to the warfarinised patients, data were also collected on the nonwarfarinised (control) patients (n = 403) over the 12 months following protocol introduction (Table 2). Of the 42 patients, 21 had an extracapsular fracture and were treated with either an extramedullary sliding hip screw construct11 or cephalomedullary device.10 Twenty-one patients had an extracapsular fracture and were treated with either an extramedullary sliding hip screw construct11 or cephalomedullary device.10 Twenty-one patients had an intracapsular fracture and underwent cemented hemiarthroplasty. Mean The American Society of Anesthesiologists Physical Status classification (ASA) grade of 3.17 and mean NHFS of 4.83 serve as surrogate markers of significant comorbidity. Mean INR on admission was 2.5 (1.5-4.4) and following 1 dose early-trigger IVK administration in the ED, the mean INR after 12 hours was 1.9 (1.2-4.2).
Table 1.
Demographics and Comparison Data for the Pre- and Postprotocol Warfarinised Patient Groups With Hip Fracture.
| Total | Preprotocol | Postprotocol | ||
|---|---|---|---|---|
| 122 | 80 | 42 | P Value, ∼ | |
| Age, years | 82.08 (64-100) | 83.34 (72-95) | .34 | |
| ASA | 3.09 (2-4) | 3.17 (2-4) | .41 | |
| NHFS | 4.7 (2-9) | 4.83 (3-7) | .57 | |
| Time to theater, h | 53.71 (1.7-128) | 37.61 (14.73-71.75) | <.0001a | |
| LOS, days | 16.69 (2-65) | 15.79 (8-44) | .64 |
Abbreviations: ASA, American Society of Anesthesiologists Score; NHFS, Nottingham Hip Fracture Score; LOS, length of stay.
Figures within parentheses are the range of values.
a t test, 2-tailed.
Table 2.
Demographics and Comparison Data for Warfarinised and Control Patient Groups With Hip Fracture Following Protocol Introduction.
| Total | Postprotocol Warfarin | Postprotocol Control | ||
|---|---|---|---|---|
| 445 | 42 | 403 | P Value, ∼ | |
| Age, years | 83.34 (72-95) | 81.65 (61-100) | .11 | |
| ASA | 3.17 (2-4) | 2.79 (2-4) | .06 | |
| NHFS | 4.83 (3-7) | 4.63 (4-9) | .2 | |
| Time to theater, h | 37.61 (14.73-71.75) | 28.36 (5.02-286.38) | <.002a | |
| LOS, days | 15.79 (8-44) | 14.14 (2-67) | .88 |
Abbreviation: ASA, American Society of Anesthesiologists Score; NHFS, Nottingham Hip Fracture Score; LOS, length of stay.
Figures within parentheses are the range of values.
a t test, 2-tailed.
Seventeen patients had satisfactory reversal following the single admission 2 mg dose of IVK. The remainder required further IVK administration per protocol. There were no high-risk patients (Figure 1) and none required more advanced medication, for example, prothrombin complex concentrate (PCC) or fresh frozen plasma (FFP).
Following protocol introduction, 21 (50%) patients were operated on within the 36-hour target and 33 (79%) within 48 hours . The mean time to theater was 37.61 hours, a statistically significant (P < .0001) decrease in the preprotocol population (Figure 2). Of the 21 patients who exceeded the 36-hour target, 13 had additional causes for delays (pneumonia, cardiac event, or insufficient theater capacity) and of these, 11 (85%) patients had an INR < 1.5, thus had achieved satisfactory reversal. Ten patients were delayed purely due to failure of adequate anticoagulation reversal. The mean length of stay was 16 days. There have been no thromboembolic complications (deep vein thrombosis/pulmonary embolism) or mortalities attributed instigation of the new regime over the 2 years since its introduction (Figure 2).
Figure 2.
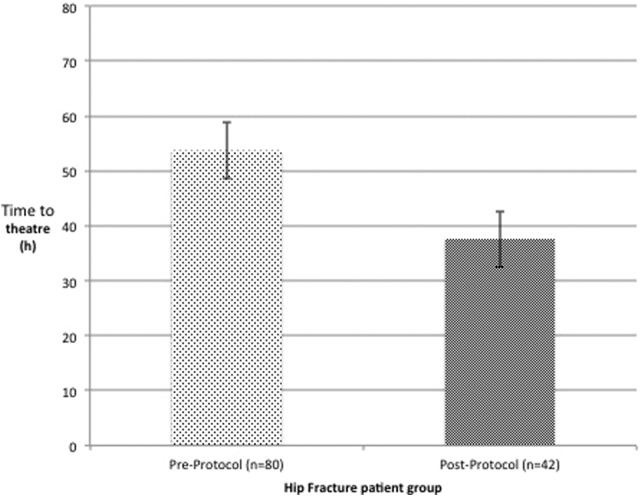
Comparison of time to theater for pre- and postprotocol warfarinised groups with hip fracture.
Discussion
Improving expediency in time to theater due to an anticoagulation reversal pathway is not new. Placing reduction in delays in the context of target driven care and delivering intravenous vitamin K, however, is a new approach. We have, through this work, simply introduced an element of kaizen to fragility fracture care: a process of small steps toward increased efficiency.17
These marginal but important changes that underpin process improvement are not unique in hip fracture care. They are also illustrated by Ahmed et al in their successful pathway for anticoagulation management in a small study comparing a preprotocol and postprotocol warfarinised hip fracture cohort.18 Finding a significant reduction in time to operation, Ahmed et al however do not account for reasons other than anticoagulation that may have affected time to theater and also do not comment on performance in terms of BPT. Of interest, the authors use a repeated 24-hour INR check policy. Leaving 24 hours between checking INR samples after intervention threatens the BPT operative domain, as, should the INR remain raised after 24 hours the patient will wait a further 24 hours before another check is done. This would entail a 48-hour mandatory delay for this population group, preventing operative intervention within the 36-hour window required for BPT.
Interestingly, Ahmed et al found that 27 of their 40 patients required a 48-hour window before an optimal reversal occurred. In addition, the authors report the mean number of patients proceeded to theater within 4 hour of the INR being known. While the logistics of their operating capacity are not mentioned, it is highly improbable that this ability to ensure patients are operated on so rapidly following a decision to operate is made could be replicated elsewhere. Ahmed et al reiterate the message that protocols reduce delays but their model will negate the ability to achieve BPT due to their delay to IVK administration and their choice of an excessively lengthy 24-hour INR window.
Options are numerous for the clinician attempting to optimize anticoagulation reversal. Key to making the most appropriate choice is the trade-off between the effect of delay such as in a watch and wait policy and the potential complications of more aggressive therapeutic intervention (prothrombin complex concentrate/fresh frozen plasma). Prompt IVK administration forms the balance of this scale. Reviewing options for reversal of anticoagulation, Hanley describes rapid with PCC, fast with FFP, prompt with intravenous vitamin K, slow with oral vitamin K, and ultraslow with warfarin cessation alone.19 The rapid and fast approaches may appear attractive as first-line agents although they are intended for reversal in the emergency setting, predominantly in head injury or active bleeding gastrointestinal20,21 and, in addition, carry the risks inherent with blood product infusion.22 These factors render them a second-line choice in our protocol, for individual cases only and only following consultation with a hematologist. At the slow end of this spectrum, our previously used oral vitamin K protocol has been shown to be less effective. The ultraslow option of cessation only has been demonstrated23 to be similarly ineffectual. It is for these reasons that we chose the intravenous route for our new protocol. We chose an initial 2 mg dose and subsequent 3 mg dose of vitamin K based on previous studies15 and remaining conscious of the impact of greater dosing regimes on postoperative time to therapeutic anticoagulation. Low-dose IVK similar to that used in our protocol and that previously published by Tharmarajah and Ahmed, among others has been shown to have negligible effects on reloading times.24
We can add to this evidence in showing that our new protocol did not cause an increase in length of stay, an established surrogate for protracted rewarfarinisation duration. Concordant with the wider literature, our first audit demonstrated significant delays to theater with an oral vitamin K policy, administered often many hours following admission. This impacts on care and BPT.10 Prior to the new protocol introduction, warfarinised patients, despite the fast-track pathway, faced protracted delays due to human and system factors: review by a doctor on the ward, prescription, and receipt of vitamin K for example.25
Warfarinised patients are thus a predictable challenge. This population should trigger early reversal with IVK on admission to the ED prior to the knowledge of the initial INR.
The literature regarding agent choice, dose, and mode of delivery is disparate. Clinicians are faced with choosing a protocol that balances efficacy and safety in addition to preventing prolonged reanticoagulation postoperatively.18,26 Established practice suggests IVK administration based on a known INR. We have challenged this premise with a new protocol characterized by IVK administration prior to knowledge of the INR. It is acknowledged that introduction of a “blind” IVK administration protocol must be done with caution. This is why we reaudited within the first 3 months and during this time (14 patients) there were no complications. In the original audit of over 80 patients on warfarin, all but 3 had “low risk indications” and it is unlikely that reversal would be harmful. Despite this, anecdotal concerns still exist regarding the broad use of IVK, therefore long-term prospective audit was carried out which has again showed no adverse events. Further reconciling anecdotal concerns over IVK use, in addition to our results, Leonidou et al (2007) in an assessment of reversal regimes found that IVK administration not only decreased delay to theater but also demonstrated the lowest complication rate.
Time to theater is the metric by which perioperative anti coagulation protocols are assessed. The mean time to theater following change in protocol was 37.6 hours compared to 53.7 hours for the same population in the first loop. Using an intention to treat analysis, this corresponds to 50% of patients gaining BPT for the theater domain compared to 21% prior to protocol refinement. If other delaying factors are excluded (theater constraints, comorbidities, etc.) for the postprotocol change group, the percentage of patients undergoing surgery within 36 hours increases to 66%. The hospital would have gained over £60 000, adjusting for other factors limiting achievement of BPT, had this been the case in the first loop.
The protocol was the first system change on the hip fracture unit for a number of years. It was well publicized and supported, and therefore there is a risk of performance or confirmation bias due to the effort put in by the staff to enforce the protocol. While this limitation is accepted, it is put into context by the fact that all interventions are nurse led, the first dose of IVK is given in the ED, remote from the hip fracture unit by inconsistent staff. In addition, the lead investigator was out of the region on Fellowship training or deployed on military duty for the second 6-month period. This represents a truly generalizable nurse-led protocol.
Further threatening the results of this study, no attempt to randomize patients was made, and the groups are not stratified. Outcome data other than length of stay are not included. There is no alternative treatment group and therefore no attempt to blind intervention made. This, like many health care change processes, is prone to bias at all stages. Carrying out robust randomized controlled trials in the trauma population is fraught with difficulty. In the hip fracture setting, these inherent issues of patient recruitment, matching, and stratification are compounded by the unique aspects of investigating this vulnerable population. Full informed consent in an octogenarian study group is a challenge. Threats to postoperative function are multiple, and recovery is heavily reliant on availability of physiotherapy services, which are not standardized across the community setting. The multiple comorbidities that characterize this group impact on their outcome as much or even more than their anticoagulation and are difficult to stratify. Long-term follow-up is threatened by the considerable mortality seen in this population.
These limitations notwithstanding and accepting that no such study can fully attribute an increase in performance to a protocol refinement, we have provided, to the best of our ability, evidence that beneficial change has occurred. Is the change optimized by 2 mg IVK or 3 mg? Is the effect size due to the rapidity of whichever route or dose chosen or is it purely due to a variant of the Hawthorne effect? These elements cannot be addressed in this work. We have shown however that refining a warfarin reversal protocol results in a decrease in delay to theater and optimizes financial gains without complications and this effect is sustained over 12 months.
Furthermore, the introduction to hip fracture care of the philosophy of kaizen has demonstrated what may be achieved through this approach of small changes yielding marginal but important gains in order to potentially improve population outcomes and drive staff engagement.
Early-trigger IVK is the first of several marginal areas of hip fracture care, which may be optimized to drive improvements in patient centered care. The time taken for the INR sample to reach the laboratory, to be interpreted, and acted upon is also now being addressed in our institution as these also compound the issues with BPT time constraints. We are introducing near-patient INR testing and IVK administration to enable and empower the nursing staff to obtain a bedside result and act on it to further reduce delays in reversal of anticoagulation.
Conclusions
With an aging population and care increasingly tied to financial incentives, hip fracture management will continue to dominate the orthopedic agenda. Within our unit, BPT loss in the warfarin group over the initial audit period equated to over £60 000. This article has described a protocol that has reduced delays to theater and maximized BPT. While it is acknowledged that there are cautions in the blind administration of IVK, we have shown that this protocol minimizes disruption to the patient journey with no recorded complications.
This work, highlighting that early IVK administration to warfarinised patients with hip fracture, reduces operative delay, improves care, and reduces financial loss and will be of interest to clinicians and managers. It enables high-quality, patient-centered care while ensuring trauma units achieve maximal financial reimbursement.
Footnotes
Authors’ Note: This project has been adjudged exempt from full ethics submission. It was approved however by our institution’s Clinical Audit Department. Mr Will Eardley was the project coordinator overseeing the data collection and edited the final article. Miss Marina Diament contributed to the data collection of the second audit cycle and writing of the paper. Dr Kirsty MacLeod contributed to the data collection of the first audit cycle and writing of the article. Dr Jon O’Hare contributed to the data collection of the second audit cycle and drafting of this article. Mrs Anne Tate was involved in the data collection in both audit cycles.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. British Orthopaedic Association (Blue Book). The Care of Patients with Fragility Fracture. First ed United Kingdom: British Orthopaedic Association; 2007. [Google Scholar]
- 2. Wiktorowicz ME, Goeree R, Papaioannou A, et al. Economic implications of hip fracture: health service use, institutional care and cost in Canada. Osteoporos Int. 2001;12(4):271–278. [DOI] [PubMed] [Google Scholar]
- 3. Braithwaite RS, Col NF, Wong JB. Estimating hip fracture morbidity, mortality and costs. J Am Geriatr Soc. 2003;51(3):364–370. [DOI] [PubMed] [Google Scholar]
- 4. Reginster JY, Gillet P, Ben Sedrine W, et al. Direct costs of hip fractures in patients over 60 years of age in Belgium. Pharmacoeconomics. 1999;15(5):507–514. [DOI] [PubMed] [Google Scholar]
- 5. White SM, Griffiths R. Projected incidence of proximal femoral fracture in England: a report from the NHS Hip Fracture Anaesthesia Network (HIPFAN). Injury. 2011;42(11):1230–1233. [DOI] [PubMed] [Google Scholar]
- 6. Shiga T, Wajima Z, Ohe Y. Is operative delay associated with increased mortality of hip fracture patients? Systematic review, meta-analysis, and meta-regression. Can J Anaesth. 2008;55(3):146–154. [DOI] [PubMed] [Google Scholar]
- 7. Payment by results and best practice tariff [Internet]. London, United Kingdom: British Orthopaedic Association; 2013. updated 29th December 2013; cited 30th December 2013. Web site http://www.boa.ac.uk/LIB/LIBPUB/Pages/PbR.aspx. Published December 29, 2013. Accessed October 23, 2014. [Google Scholar]
- 8. Khan SK, Weusten A, Bonczek S, et al. The Best Practice Tariff helps improve management of neck of femur fractures: a completed audit loop. Br J Hosp Med (Lond). 2013;74(11):644–647. [DOI] [PubMed] [Google Scholar]
- 9. Ranhoff AH, Martinsen MI, Holvik K, Solheim LF. Use of warfarin is associated with delay in surgery for hip fracture in older patients. Hosp Pract. 2011;39(1):37–40. [DOI] [PubMed] [Google Scholar]
- 10. Eardley WGP, Macleod KE, Freeman H, Tate A. “Tiers of delay”: warfarin, hip fractures, and target-driven care. Geriatr Orthop Surg Rehabil. 2014;5(3):103–108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Ashouri F, Al-Jundi W, Patel A, Mangwani J. Management of warfarin anticoagulation in patients with fractured neck of femur. ISRN Hematol. 2011;2011:294628. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Meehan R, Tavares M, Sweeney J. Clinical experience with oral versus intravenous vitamin K for warfarin reversal. Transfusion. 2013;53(3):491,8; quiz 490. [DOI] [PubMed] [Google Scholar]
- 13. Tsu LV, Dienes JE, Dager WE. Vitamin K dosing to reverse warfarin based on INR, route of administration, and home warfarin dose in the acute/critical care setting. Ann Pharmacother. 2012;46(12):1617–1626. [DOI] [PubMed] [Google Scholar]
- 14. Vitale MA, Vanbeek C, Spivack JH, et al. Pharmacologic reversal of warfarin-associated coagulopathy in geriatric patients with hip fractures: a retrospective study of thromboembolic events, postoperative complications, and time to surgery. Geriatr Orthop Surg Rehabil. 2011;2(4):128–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Tharmarajah P, Pusey J, Keeling D, et al. Efficacy of warfarin reversal in orthopedic trauma surgery patients. J Orthop Trauma. 2007;21(1):26–30. [DOI] [PubMed] [Google Scholar]
- 16. Gleason LJ, Mendelson DA, Kates SL, Friedman SM. Anticoagulation management in individuals with hip fracture. J Am Geriatr Soc. 2014;62(1):159–164. [DOI] [PubMed] [Google Scholar]
- 17. Tetteh HA. Kaizen: a process improvement model for the business of health care and perioperative nursing professionals. AORN J. 2012;95(1):104–108. [DOI] [PubMed] [Google Scholar]
- 18. Ahmed I, Khan MA, Nayak V, Mohsen A. An evidence-based warfarin management protocol reduces surgical delay in hip fracture patients. J Orthop Traumatol. 2014;15(1):21–27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Hanley JP. Warfarin reversal. J Clin Pathol. 2004;57(11):1132–1139. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Leiblich A, Mason S. Emergency management of minor head injury in anticoagulated patients. Emerg Med J. 2011;28(2):115–118. [DOI] [PubMed] [Google Scholar]
- 21. Wilson MD, Davis JE. Antithrombotic reversal agents. Emerg Med Clin North Am. 2014;32(3):715–725. [DOI] [PubMed] [Google Scholar]
- 22. Gilliss BM, Looney MR, Gropper MA. Reducing noninfectious risks of blood transfusion. Anesthesiology. 2011;115(3):635–649. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Douketis JD. Perioperative management of patients who are receiving warfarin therapy: an evidence-based and practical approach. Blood. 2011;117(19):5044–5049. [DOI] [PubMed] [Google Scholar]
- 24. Shetty HG, Backhouse G, Bentley DP, et al. Effective reversal of warfarin-induced excessive anticoagulation with low dose vitamin K1. Thromb Haemost. 1992;67(1):13–15. [PubMed] [Google Scholar]
- 25. Bhatia M, Talawadekar G, Parihar S, et al. An audit of the role of vitamin K in the reversal of International Normalised Ratio (INR) in patients undergoing surgery for hip fracture. Ann R Coll Surg Engl. 2010;92(6):473–476. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Leonidou A, Rallan R, Cox N, et al. Comparison of different warfarin reversal protocols on surgical delay and complication rate in hip fracture patients. J Orthop Surg (Hong Kong). 2013;21(2):142–145. [DOI] [PubMed] [Google Scholar]