Abstract
To compare a novel cooling product, Physicool (P, Physicool Ltd, London, England, UK) with a well-established cryotherapy system, Cryocuff (C, Aircast, DJO Global, Vista, California, USA) using pain scores, range of movement (ROM), and cost as outcome measures in the early phase following total knee arthroplasty. We prospectively studied 90 consecutive patients undergoing unilateral total knee arthroplasty by a single surgeon. Following exclusions, 40 patients were recruited to each group. Visual analogue scale (VAS) for pain and ROM before and after application of cooling device was recorded at 24 and 48 hours after surgery. The cost of treatment per patient was also calculated. The VAS were significantly reduced in P on day 1 postsurgery (p = 0.013) and day 2 (p = 0.001) compared to C. A significant increase in ROM was recorded in P at 24 hours (p = 0.004) and at 48 hours (p = 0.009) postsurgery compared to C. The cost benefit of using P over C was approximately £25 per patient. The Physicool system is a safe and effective cooling method for improving pain and ROM in the early postoperative phase following total knee arthroplasty. Furthermore, it offers substantial cost savings.
Keywords: total knee arthroplasty, rehabilitation, cryotherapy, pain, range of movement
Introduction
Total knee arthroplasty (TKA) is one of the most successful operations in terms of patient-reported, quality-of-life outcomes, and as a result, 88, 257 procedures were performed in England and Wales in 2014.1 However, despite significant long-term benefits in mobility, pain, function, and health-related quality of life2, the initial rehabilitation following surgery remains challenging.3,4
Enhanced recovery programs have been used successfully to improve early pain management, range of movement (ROM), blood loss, and hospital stay.5–9 Cooling devices used as part of enhanced recovery programs have been shown to reduce pain, hasten discharge, and promote greater movement in the early postoperative phase following total knee arthroplasty.10,11
Cooling devices deliver localized cryotherapy which works by reducing intra-articular temperature and thus slowing the neuronal conduction of both C- and A-Delta pain fibres.12,13 Small decreases in temperature have also been shown to reduce enzyme activity in inflammation and as a result reduce the inflammatory response.13 Due to these molecular and cellular level actions, cryotherapy reduces localized swelling and perceived pain. In addition, a decrease in the measured blood loss has been shown following application of cryotherapy, presumably due to vasoconstriction in response to the reduced temperature.14
There are a number of commercially available cryotherapy systems used following TKA. The most recent Cochrane review supported their safety and efficacy.16 However, concerns have been raised due to the potential inconvenience to patients and the cost-effectiveness of these devices.
The aim of this study is to compare a novel cooling product, Physicool (P), with a well-established cooling system, Cryocuff (C). The outcome measures were postoperative pain, improvement in ROM, and cost savings. Both devices are used in our institution as part of our knee arthroplasty Enhanced Recovery Program (ERP).
Background
The Cryocuff device consists of four elements: A cooling reservoir that is filled with water and ice, a compression cuff that wraps around the knee and is secured by straps with an aperture anteriorly for the patella, a connecting tube that exchanges water between the cuff and the reservoir, and an insulation disk, which helps keep the water and ice cold. Once the cuff has been applied and connected to the filled reservoir, the air vent on the reservoir is opened and raised to no more than 15 inches above the knee for 30 seconds to fill the cuff. The air vent is then closed and the reservoir can be disconnected from the cuff. In order to recirculate the water, the reservoir is reattached, lowered, and warmed fluid is free to pass into the cooler, where it can mix with the ice to be cooled. After a minute or two, the filling process can be repeated. It is recommended that an initial fluid change be performed after 15 minutes and then hourly for up to 6 hours, without refilling the reservoir.
The Physicool system safely employs the cooling effects of latent heat evaporation rather than traditional direct external cooling. It utilizes a cotton bandage soaked in an ethanol-based solution. A presoaked bandage, stored in resealable foil pouch, is wrapped around the knee on top of a waterproof dressing and secured with the preattached self-grip strap. The cooling effect lasts for approximately 2 hours, by which time the bandage would have become dry. It can either be recharged by spraying the cooling fluid directly to the bandage in situ or it can be rerolled and placed in the resealable bag with additional cooling solution.
Methods
Outline
We performed a prospective study to evaluate P, a novel cryotherapy device, against C in patients undergoing primary total knee arthroplasty by a single surgeon (SG) using their default surgical technique. All patients were operated on in 1 hospital between March 2011 and February 2012.
During this period, all patients were managed as part of a protocol-driven ERP. Preoperative information leaflets including expected length of stay were routinely used. Patients were preassessed prior to surgery and attended on the day of their operation to a dedicated admission unit. Patients received either spinal or general anesthesia depending on their preference and clinical indications. Thigh tourniquets were in place for the entire procedure in all patients. The Triathlon Knee (Stryker, Kalamazoo, Michigan) system was used in all patients through a medial parapatellar approach. Local anesthetic infiltration using standardized volume, concentration, and technique was routinely used. A retransfusion drain was inserted prior to closure and removed on the first postoperative day. An integrated care pathway was used to record a patient’s progress throughout the admission, and postoperative rehabilitation was standardized.
Power Calculation
Following the results of a pilot study, a priori power calculation (G*Power, version 3.1.9.2; Universitat Kiel, Germany) was completed with several assumptions; our final study would have a standard power of 80% and that a p value <0.05 is significant. It revealed at least 32 patients in each group would be sufficient to show a significant difference in outcomes.
Patient Selection
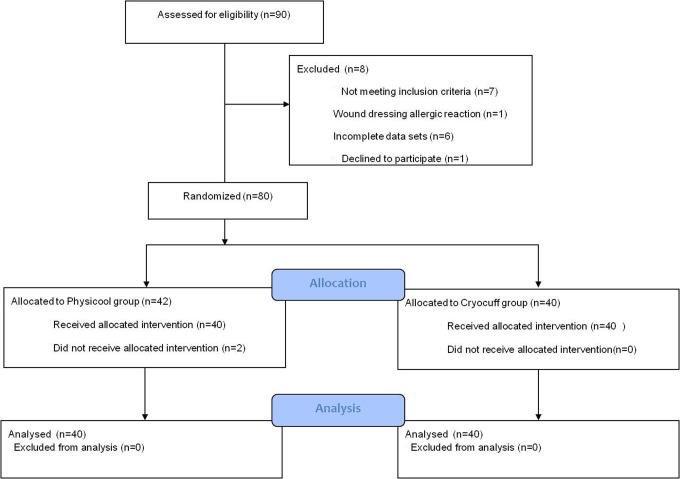
Ninety consecutive patients were entered into the study. Allocation into each group was based on their hospital numbers which are randomly designated. Those with even numbers were entered into the P group, and those with odd numbers were placed in the C group. Consent was obtained from all patients, and their rights were protected at all times. Those patients with incomplete data sets or with surgical complications not as a result of the cooling product were excluded (Figure 1)
Figure 1.
CONSORT 2010 flow diagram for patient enrollment and group allocation.
Eight patients were excluded from the study. Seven did not meet the inclusion criteria of which 1 patient in C group experienced an allergic reaction to the wound dressing (not the cryotherapy system) and 6 had incomplete data sets (4 in C vs 2 in P). One patient declined to take part in the trial who would have otherwise been allocated to the P group and therefore received the local standard intervention (C). Two patients in the P group did not receive the allocated intervention.
Outcome Measures
Primary outcome measures were pain and ROM. Pain was assessed using a visual analogue scale (VAS) and ROM using a long-arm goniometer. The VAS scores and ROM were recorded before and 30 minutes after application of the cooling device on the first and second postoperative day. The patients were often discharged home on the third postoperative day.
Data Collection
Data collection was performed by physiotherapists. All data for each patient was recorded on a custom designed data collection form. The patient hospital number was recorded on the cover sheet with subsequent pages for recording pain and ROM for each postoperative day. Although all physiotherapists were appropriately trained and briefed on the methodologies of the study, these were also detailed within this tool. Data was kept in a secure location throughout the study period.
The devices were assigned on the first postoperative day. Both devices were applied in the first instance by a physiotherapist. Following this, the patient was responsible for reapplication of the device, unless they were unable to do so.
In the C group, ice and water changes were performed by physiotherapists or nursing staff. The P group patients resoaked the bandage themselves via a spray bottle when they perceived the cooling benefit to have diminished. All patients in the group were given one extra fluid bottle for this.
Ethics
All methodology and data collection were approved and conformed to the hospital trust protocols.
Statistics
The age of the groups was compared using a Student t test, whereas the ROM and VAS were compared using a Mann-Whitney U test. Normality of the data was tested using a Shapiro-Wilk test. This statistical analysis was performed using SPSS (IBM, Chicago, Illinois). A p value <0.05 was considered statistically significant.
Results
Demographics
There was no significant difference in age or sex in either group (Table 1).
Table 1.
Comparable Age and Sex of Both Cryocuff and Physicool Groups.
| Cryocuff | Physicool | |
|---|---|---|
| Age, mean, years | 74.9 | 75.1 |
| Female, n | 19 | 18 |
| Male, n | 21 | 22 |
Range of Movement
Improvement in ROM (range) after intervention on day 1 C versus P was 12.34° (−10° to 60°) versus 20.38° (−5° to 55°), respectively. Improvement in ROM on day 2 C versus P was 8.88° (−20° to 45°) versus 16.25° (−15° to 50°), respectively. The difference in ROM between P and C was statistically significant on both day 1 (p = 0.004) and day 2 (p = 0.009) postoperatively (Figure 2).
Figure 2.
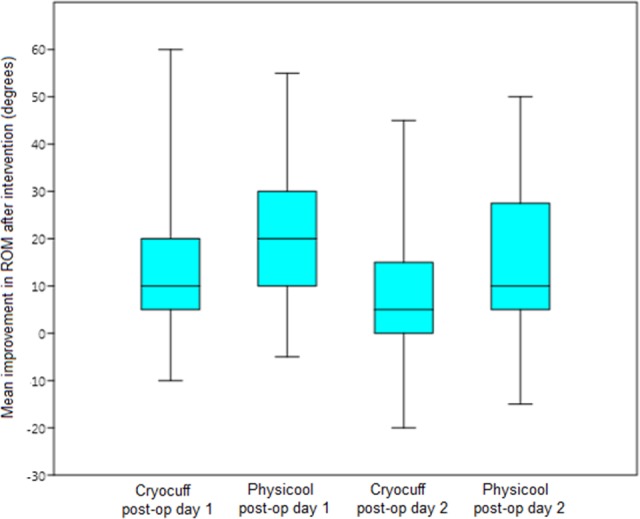
Box-plot displaying distributions of mean improvement in range of movement (ROM).
Pain
The difference in VAS was recorded pre- and postintervention. We report our results in terms of a difference in VAS. Therefore, the greater the decrease between the pre- and postintervention VAS, the better the improvement in pain. The difference in VAS after intervention on day 1 C versus P was −1.2 (−4 to 0) versus −1.73 (−5 to 1), respectively. The difference in VAS on day 2 C versus P was −0.8 (−4 to 1) versus −1.73 (−4 to 1), respectively. The greater improvement in VAS in the P group compared to the C group was statistically significant on both day 1 (p = 0.013) and day 2 (p = 0.001) postoperatively (Figure 3).
Figure 3.
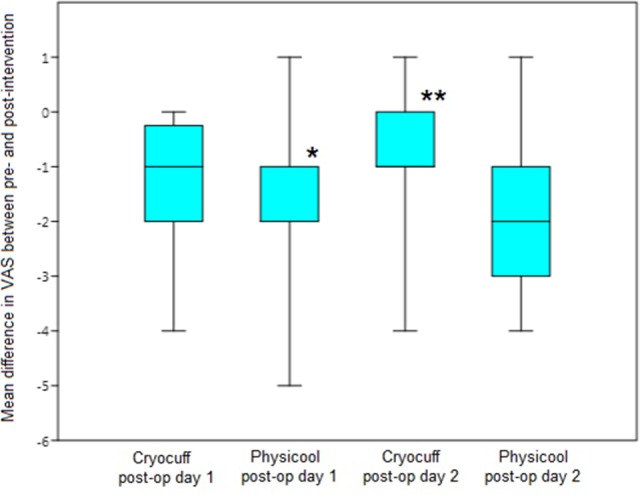
Box-plot displaying distributions of the mean visual analogue scale (VAS) improvement after interventions on postoperative day 1 and 2. *Median and 25th percentile values were both −2. **Median and 25th percentile values were both −1.
Cost
The cost of a Physicool presoaked bandage and sufficient additional cooling fluid is £15. The cost of a Cryocuff is £37, excluding the cost of water and ice production, which is difficult to quantify. The cooling reservoirs cost £80 each and are replaced as required. Patients are not able to take them home with them due to the cost. Cost savings of approximately £25 per patient were estimated accounting for the cost of disposables, replacement cooling reservoirs, and ice. This equates to a potential saving of £8750 per year based on a unit which performs 350 total knee replacements annually.
Discussion
A recent Cochrane meta-analysis concluded that potential benefits of cryotherapy on postoperative pain and ROM may be too small to justify its use when balanced against potential inconveniences and expense of using cryotherapy.15 With increasing constraints on resources and pressure on hospital beds, our department has successfully introduced enhanced recovery to reduce the length of inpatient stay without an increase in readmission rate.
The Cryocuff system of cryotherapy, while used successfully in our department’s ERP, is not without its limitations. The bulkiness of the cuff limits full knee flexion. The lack of constant flow compared to more technologically advanced but expensive cooling devices may limit its effectiveness of cooling, and regular ice and water changes are labor intensive. The ice reservoirs are expensive and designed to be reusable, it is therefore impractical for patients to take them home. Currently, we do not have an option for patients to purchase the reservoirs.
In view of these factors, we wanted to find a cooling product, which addressed the shortfalls of the Cryocuff system, without increasing cost. The Physicool system is easy to apply and recharge. Owing to its lack of bulk, it does not limit knee flexion while in situ on the limb. Furthermore, it remains effective once taken home by the patient for continued use. No complications were associated with the use of Physicool either in our study or in the literature.
Our results show that the P system was significantly better at providing pain relief and improving ROM compared to the Cryocuff system on both the first and second postoperative days. In addition, using the Physicool system provides a modest cost saving of £25 per patient, which multiplied by 350 knee replacements over the course of a year would save £8750 in an averaged size department.
Limitations
We accept that this was not a randomized blinded study, and therefore results are susceptible to bias. In view of the nature of the cryotherapy devices, patient blinding is not feasible. It may, however, have been possible to blind assessors, although due to resource and time limitations this was not included in our methodology.
While there was no control group, it was felt that sufficient evidence exists to support the efficacy of cryotherapy versus placebo for the outcome measures we examined. Previous work has shown cryotherapy to be superior to compression bandages and external ice at reducing pain and improving early ROM.16–18
Furthermore, we did not examine analgesic requirements between the 2 groups, although previous meta-analysis has shown a reduction in analgesia requirement with cryotherapy.19
The groups were matched on age and sex alone. Our pilot study suggested no difference in ROM and VAS on day 1 prior to administration of any form of cryotherapy, as this would aid in forming homogenous groups. Consequently, we did not record preoperative ROM and VAS. Our study showed no statistical difference in day 1 ROM (p = 0.79) and VAS (p = 0.102) between the patients in either group before cryotherapy was applied, which was in keeping with our findings in our pilot study. This helps to justify our decision, as we felt both groups had a similar point of reference. Patients were also not matched regarding anaesthetic type (general vs spinal). Although several studies have shown that spinal anesthesia provides improved analgesia post TKA,20 studies have also confirmed that local anesthetic periarticular injections provide pain relief for up to 48 hours postoperatively,21 which all of our patients had performed. We do appreciate that matching using preoperative ROM, VAS, and anesthetic type would have increased the strength of our cohorts, and therefore we will do so in further studies of a similar nature.
Pain scores and ROM data were only recorded for the first 2 postoperative days. The mean length of stay of total knee arthroplasty patients in our department is 4.2 days, with a significant number of patients being discharged on the third postoperative day. As no provision for measurement following patient discharge was made, we felt a shorter follow-up was preferable to multiple incomplete data sets.
We used VAS, as it provides a simple and validated scoring system.22 Intraobserver variability was controlled by having the same physiotherapist recording pre- and postcryotherapy measurements with a goniometer; however, no mechanisms were put in place to control interobserver variability.
For those who had been excluded from the study, 1 individual in the C group experienced an allergic reaction to the Tegaderm dressing (3M, St Paul, Minnesota). Seven patients did not undergo postoperative measurements of either ROM or VAS due to limitations on the ward, and these incomplete data sets were excluded. The 2 patients who had not received their allocated intervention complained of the cooling fluid-soaked bandage causing dampness on bed sheets and clothing, and some found the odor of the cooling fluid unpleasant. They were therefore given the standard C system and excluded from data analysis. It was noted that application of the P device can be challenging for those with poor manual dexterity.
Implications
This study clearly demonstrates the superiority of the P cryotherapy system over that of the C method for patients undergoing total knee arthroplasty when considering pain scores, ROM, and cost in the early postoperative period.
In addition to the acute postoperative phase of knee arthroplasty, cryotherapy has been used successfully following several arthroscopic procedures, particularly cruciate ligament reconstruction.23 It has been used to reduce both the recovery time from traumatic injuries and in the treatment of chronic sporting injuries. It would seem likely that the advantage of Physicool over Cryocuff would be similar in these situations, so this study could prompt the exploration of the use of the Physicool system in a wide range of operative and nonoperative scenarios.
Conclusion
This study shows that the P system is a viable alternative to other available cooling methods. It is relatively inexpensive and easy for the majority of patients to apply. It allows patients to mobilize without removing the device and can be used following discharge. This is the first study demonstrating that the Physicool system improves pain and ROM in the acute postoperative phase following total knee arthroplasty. We have shown it to be more effective in reducing pain and in increasing ROM of the knee and also more cost effective compared to the Cryocuff system.
Footnotes
Authors’ Note: This study was classified as a “Service Improvement Audit” with Dorset County Hospital audit department, as a result ethical approval was not required.
Declaration of Conflicting Interests: The author(s) declared no potential conflicts of interest with respect to the research, authorship, and/or publication of this article.
Funding: The author(s) received no financial support for the research, authorship, and/or publication of this article.
References
- 1. National Joint Registry. NJR Reports: Types of knee replacements undertaken. Website http://www.njrreports.org.uk. Accessed October 6, 2015.
- 2. Hawker G, Wright J, Coyte P, et al. Health-related quality of life after knee replacement. J Bone Joint Surgery Am. 1998;80(2):163–173. [DOI] [PubMed] [Google Scholar]
- 3. Larsen K, Hvass KE, Hansen TB, Thomsen PB, Søballe K. Effectiveness of accelerated perioperative care and rehabilitation intervention compared to current intervention after hip and knee arthroplasty. A before-after trial of 247 patients with a 3-month follow-up. BMC Musculoskelet Disord. 2008;9:59. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Webb JM, Williams D, Ivory JP, Day S, Williamson DM. The use of cold compression dressings after total knee replacement: a randomized controlled trial. Orthopedics. 1998;21(1):59–61. [DOI] [PubMed] [Google Scholar]
- 5. Khan F, Ng L, Gonzalez S, Hale T, Turner-Stokes L. Multidisciplinary rehabilitation programmes following joint replacement at the hip and knee in chronic arthropathy. Cochrane Database Syst Rev. 2008;(2):CD004957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Sorrells RB, Voorhorst PE, Murphy JA, Bauschka MP, Greenwald AS. Uncemented rotating-platform total knee replacement: a five to twelve-year follow-up study. J Bone Joint Surg Am. 2004;86-A(10):2156–2162. [PubMed] [Google Scholar]
- 7. Larsen K, Hansen TB, Thomsen PB, Christiansen T, Søballe K. Cost-effectiveness of accelerated perioperative care and rehabilitation after total hip and knee arthroplasty. J Bone Joint Surg Am. 2009;91(4):761–772. [DOI] [PubMed] [Google Scholar]
- 8. Abramson DI, Chu LS, Tuck S, Jr, Lee SW, Richardson G, Levin M. Effect of tissue temperatures and blood flow on motor nerve conduction velocity. JAMA. 1966;198(10):1082–1088. [PubMed] [Google Scholar]
- 9. Martin SS, Spindler KP, Tarter JW, et al. Does cryotherapy affect intraarticular temperature after knee arthroscopy? Clin Orthop. 2002;(400):184–189. [DOI] [PubMed] [Google Scholar]
- 10. Malviya A, Martin K, Harper I, et al. Enhanced recovery program for hip and knee replacement reduces death rate. Acta Orthop. 2011;82(5):577–581. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Kullenberg B, Ylipää S, Söderlund K, Resch S. Postoperative cryotherapy after total knee arthroplasty: a prospective study of 86 patients. J Arthroplasty. 2006;21(8):1175–1179. [DOI] [PubMed] [Google Scholar]
- 12. Matsen FA, III, Questad K, Matsen AL. The effect of local cooling on postfracture swelling. A controlled study. Clin Orthop Relat Res. 1975;(109):201–206. [DOI] [PubMed] [Google Scholar]
- 13. Ohkoshi Y, Ohkoshi M, Nagasaki S, Ono A, Hashimoto T, Yamane S. The effect of cryotherapy on intraarticular temperature and postoperative care after anterior cruciate ligament reconstruction. Am J Sports Med May. 1999;27(3):357–362. [DOI] [PubMed] [Google Scholar]
- 14. Gibbons CE, Solan MC, Ricketts DM, Patterson M. Cryotherapy compared with Robert Jones bandage after total knee replacement: a prospective randomized trial. Int Orthop. 2001;25(4):250–252. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Adie S, Kwan A, Naylor JM, Harris IA, Mittal R. Cryotherapy following total knee replacement. Cochrane Database Syst Rev. 2012;9:CD007911. [DOI] [PubMed] [Google Scholar]
- 16. Barbieri A, Vanhaecht K, Van Herck P, et al. Effects of clinical pathways in the joint replacement: a meta-analysis. BMC Med. 2009;7:32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Fischer HB, Simanski CJP, Sharp C, et al. A procedure-specific systematic review and consensus recommendations for postoperative analgesia following total knee arthroplasty. Anaesthesia. 2008;63(10):1105–1123. [DOI] [PubMed] [Google Scholar]
- 18. Price DD, McGrath PA, Rafii A, Buckingham B. The validation of visual analogue scales as ratio scale measures for chronic and experimental pain. Pain. 1983;17(1):45–56. [DOI] [PubMed] [Google Scholar]
- 19. Ayalon O, Liu S, Flics S, Cahill J, Juliano K, Cornell CN. A multimodal clinical pathway can reduce length of stay after total knee arthroplasty. HSS J. 2011;7(1):9–15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Macfarlane AJ, Prasad GA, Chan VW, Brull R. Does regional anesthesia improve outcome after total knee arthroplasty? Clin Orthop Relat Res. 2009;467(9):2379–2402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Anderson LO, Kehlet H. Analgesic efficacy of local infiltration analgesia in hip and knee arthroplasty: a systematic review. Br J Anaesth. 2014;113(3):360–374. [DOI] [PubMed] [Google Scholar]
- 22. Smith J, Stevens J, Taylor M, Tibbey J. A randomized, controlled trial comparing compression bandaging and cold therapy in postoperative total knee replacement surgery. Orthop Nurs. 2002;21(2):61–66. [DOI] [PubMed] [Google Scholar]
- 23. Whitelaw GP, DeMuth KA, Demos HA, Schepsis A, Jacques E. The use of the Cryo/Cuff versus ice and elastic wrap in the postoperative care of knee arthroscopy patients. Am J Knee Surg. 1995;8(1):28–30. [PubMed] [Google Scholar]