Abstract
Background:
The duration of the cancer diagnostic process has considerable influence on patients' psychosocial well-being. Breast diagnostic assessment units (DAUs) in Ontario, Canada are designed to improve the quality and timeliness of care during a breast cancer diagnosis. We compared the diagnostic duration of patients diagnosed through a DAU vs usual care (UC).
Methods:
Retrospective population-based cohort study of 2499 screen-detected breast cancers (2011) using administrative health-care databases linked to the Ontario Cancer Registry. The diagnostic interval was measured from the initial screen to cancer diagnosis. Diagnostic assessment unit use was based on the biopsy and/or surgery hospital. We compared the length of the diagnostic interval between the DAU groups using multivariable quantile regression.
Results:
Diagnostic assessment units had a higher proportion of patients diagnosed within the 7-week target compared with UC (79.1% vs 70.2%, P<0.001). The median time to diagnosis at DAUs was 26 days, which was 9 days shorter compared with UC (95% CI: 6.4–11.6). This effect was reduced to 8.3 days after adjusting for all study covariates. Adjusted DAU differences were similar at the 75th and 90th percentiles of the diagnostic interval distribution.
Conclusions:
Diagnosis through an Ontario DAU was associated with a reduced time to diagnosis for screen-detected breast cancer patients, which likely reduces the anxiety and distress associated with waiting for a diagnosis.
Keywords: breast cancer/neoplasms diagnosis, timeliness, health services research, cohort studies
Despite the recent controversy over its efficacy (Miller et al, 2014), breast cancer screening remains one of the most important strategies for reducing breast cancer mortality (Andersson et al, 1988; Nystrom et al, 1993). The benefits of breast cancer screening depend on the timely follow-up of an abnormal mammogram and timely treatment initiation (Ganry et al, 2004). Delayed diagnosis can lead to increased patient anxiety, the need for more aggressive treatment, and adverse clinical outcomes (Arndt et al, 2003; Brett et al, 1998; Poole and Lyne, 2000; Richards et al, 1999; Thorne et al, 1999).
The Ontario Breast Screening Program (OBSP) is an organised provincial screening programme established in 1990 that provides biennial breast screening services accessible to all women aged 50 and older (Cancer Care Ontario, 2013b). In 2011, there were 155 OBSP screening sites across the province (Cancer Care Ontario, 2011). The OBSP screened 43.2% of Ontario women aged 50–74 during 2010–2011, and a further 17.6% of women in this age group received screening mammograms ordered by a medical doctor outside of the OBSP, also known as opportunistic screening (Cancer Care Ontario, 2013b).
In the usual care route (UC), positive screening mammogram results (either through the OBSP or opportunistically) are sent back to the primary care physician, who must then order diagnostic tests and specialist consultation (Olivotto et al, 2000, 2001a; Quan et al, 2012). This UC route creates a disconnection between screening and assessment that can prolong the diagnostic interval (Olivotto et al, 2001b).
The OBSP introduced Breast Assessment Affiliates (BAA) in 2004 to improve the transition between an abnormal screen (or symptomatic presentation) and diagnosis and to ensure access to high-quality diagnostic services (Ontario Breast Screening Program, 2004; Cancer Care Ontario, 2009; Quan et al, 2012). Access to a BAA is through the OBSP or a primary care provider referral (for those who are symptomatic or screened opportunistically). BAAs are based in hospitals or independent facilities and include a multidisciplinary health-care team with a nurse navigator who coordinates diagnostic tests following a detailed pathway and who also provides psychosocial and information support (Ontario Breast Screening Program, 2004; Cancer Care Ontario, 2013a). At a BAA, the performance of diagnostic tests and specialist consultation to investigate a positive screening mammogram does not require referral recommendations from the patient's primary care physician. Diagnostic tests and specialist consultation are directly arranged on the advice of the reading radiologist. BAAs provide care according to the Canadian Association of Radiologist standards and are required to meet OBSP quality thresholds to maintain BAA status (Ontario Breast Screening Program, 2004; Cancer Care Ontario, 2009). Each BAA must ensure the availability of diagnostic specialists (Ontario Breast Screening Program, 2004) and have sufficient imaging, surgical biopsy and pathological assessment capacity (Brouwers et al, 2009; Quan et al, 2012). BAA structure varies. Some provide complete assessment in a single location, whereas others are virtual entities that coordinate diagnostic services across multiple institutions.
During the study period, there were 47 BAAs with at least one in each of the province's 14 regional cancer programmes (Cancer Care Ontario, 2012; Cancer Quality Council of Ontario, 2014). These included one rapid diagnostic clinic where assessments were completed within 24 h (Cancer Care Ontario, 2009, 2013a). At the time of this study, Ontario had two additional assessment centres designed to expedite the diagnostic process. Although they were not as well regulated as the BAAs, they shared the same goal of expediting the diagnostic process and were likely to have similar organisational components. Both BAAs and these two additional programmes were referred to as diagnostic assessment units (DAUs) in this study.
There is little evidence on how DAUs affect the timeliness of breast cancer diagnosis. One retrospective study suggested BAAs are more successful in achieving timeliness targets than UC for resolving abnormal screening results within the OBSP (Quan et al, 2012). We do not know the size of the DAU effect in days and we do not know how effective DAUs are at the population level. The purpose of this study was to examine the length of the diagnostic interval among all Ontario screen-detected breast cancer patients who were diagnosed through DAUs vs those diagnosed through UC.
Materials and methods
Patient population
We conducted a population-based retrospective cohort study of breast cancer patients diagnosed between 1 January 2011 and 31 December 2011 in Ontario, Canada. This report is on the subset whose cancer was detected through screening, which is defined as a cancer diagnosed within 12 months of an abnormal OBSP screening mammogram or within 6 months of an opportunistic screening.
We included patients who had histologically confirmed breast cancer and had no previous cancer diagnosis. At the time of this study, the Ontario Cancer Registry (OCR) did not collect information on in situ cancer cases so the study is restricted to those with invasive disease. Patients were excluded if they met the following criteria: (1) males (2) no data linkage (3) non-Ontario residents at the time of diagnosis (4) not having Ontario Health Insurance Plan (OHIP) coverage for at least 3 years before the diagnosis or (5) cancer not detected by screening.
Data sources
We used the Ontario Cancer Registry and population-based administrative health databases from the Institute for Clinical Evaluative Sciences (ICES) and Cancer Care Ontario. These administrative health-care databases are anonymously linked using an individual-level encrypted identifier. The Ontario Cancer Registry was used to identify breast cancer patients and determine the date of diagnosis, with its accuracy and completeness previously demonstrated (Walter et al, 1994; Hall et al, 2006). Screen-detected patients were identified using the OBSP Database combined with the OHIP Claims Database (OHIP), which contains billing codes that specify the patient's symptom status (asymptomatic vs symptomatic) when a mammogram was ordered. The National Ambulatory Care Reporting System, the Canadian Institute for Health Information/Discharge Abstract Database and the Same-day Surgery Database provided information on use of acute care services, associated dates and physicians, facilities and comorbid disease diagnoses. Patient demographics and OHIP coverage status were obtained from the Registered Persons Database. Collaborative Stage Data provided information on cancer stage and histology. A list of BAA hospitals was provided to us by the OBSP and we found two regional breast DAUs located in Cobourg and Brockville through an email survey to Cancer Care Ontario Regional Primary Care Leads and OBSP Regional Program Managers.
Study variables
The study outcome was the diagnostic interval, defined as the time from the initial screen to the date of diagnosis. The initial screen was defined as the earliest abnormal OBSP screening test within 12 months before diagnosis or the earliest opportunistic screening mammogram within 6 months before diagnosis. We chose 6 months for opportunistic screens based on evidence from the Canadian Partnership Against Cancer (Canadian Partnership Against Cancer, 2013) and our observation that fewer than 5% of abnormal OBSP screens occurred in 6–12 months before diagnosis (Jiang, 2013). We presumed that a similar pattern was present in the opportunistic group. As we had tests results for OBSP screens, we extended that window to 12 months for those with abnormal OBSP screening tests. Date of diagnosis was ascertained from the Ontario Cancer Registry, which uses the date of first positive histology/cytology or the date of the first breast cancer-related hospital admission/outpatient consultation, whichever is earliest. We analysed the diagnostic interval both as a continuous variable and dichotomized at 7 weeks, which reflects the Canadian breast screening timeliness targets during the study period (Public Health Agency of Canada, 2007).
DAU use was determined separately for patients whose initial screen was within the OBSP from those outside of the OBSP because the OBSP has a database that tracked the use of BAAs. OBSP patients were diagnosed through a DAU if (1) the OBSP database indicated a BAA payment record or (2) they had a biopsy/surgery performed at a regional breast DAU. Otherwise, they were diagnosed through UC. For patients whose initial screen was opportunistic, we considered them diagnosed through a DAU only if they had a biopsy/surgery performed at a DAU hospital (BAAs or regional breast DAUs). We tested this strategy using the OBSP group's BAA assignment as the criterion standard and determined that assigning DAU based on the biopsy/surgery hospital has a sensitivity of 90.1% and a specificity of 84.6%.
Study covariates included (1) patient characteristics: age, recent immigration status (yes/no), socio-economic status based on an area-level material deprivation index (Matheson et al, 2012), urban/rural residence based on the Rurality Index for Ontario 2008 (Kralj, 2009) (yes/no), comorbidity based on the Johns Hopkins Aggregated Diagnosis Groups (Austin et al, 2011), and benign breast disease history (yes/no) (2) disease characteristics: Nottingham/Bloom-Richardson histological grade (Elston et al, 1982; Elston and Ellis, 1998) (low/medium/high) and Tumour-Node-Metastasis (7th edition) cancer stage (Edge et al, 2009; Sobin et al, 2009) (3) usual health-care utilisation characteristics were assessed between 36 months and 12 months prior to the date of diagnosis, as evidence suggests that a 2-year look-back period provides stable estimates (Leung, 2012). Factors examined included: frequency of doctor visits, primary care provider (yes/no), continuity of care based on Usual Provider Continuity index (Jee and Cabana, 2006) (high/low/non-user) and a preventive services index (Leung, 2012), which was the proportion of preventive services used out of the total number of preventive services for which an individual was eligible. The component preventive services involved in calculating this index were annual health examination, influenza vaccination, breast cancer screening, colorectal cancer screening and cervical cancer screening.
Statistical analysis
We summarized the characteristics of the study subjects and statistically compared the distribution of study covariates between DAU patients and UC patients. The diagnostic interval distribution was positively skewed so all regression analyses on the continuous form of this variable used the quantile regression approach (Hao and Naiman, 2007). We constructed multivariable quantile regression models to assess the association between DAU use and the length of the diagnostic interval at the 50th, 75th and 90th percentiles of the diagnostic interval distribution. We included all study covariates in these models. A logistic regression model was also constructed to assess the success of DAUs in meeting recommended timeliness targets. We performed a sensitivity analysis that excluded regional breast DAUs from the DAU assignment to check for possible timeliness differences between BAAs and those other centres. All the analyses were performed at the ICES-Queen's Health Services Research Facility using SAS (Version 9.3, SAS Institute Inc., Cary, NC, USA). This study was approved by the Health Sciences Research Ethics Board at Queen's University at Kingston, Canada.
Results
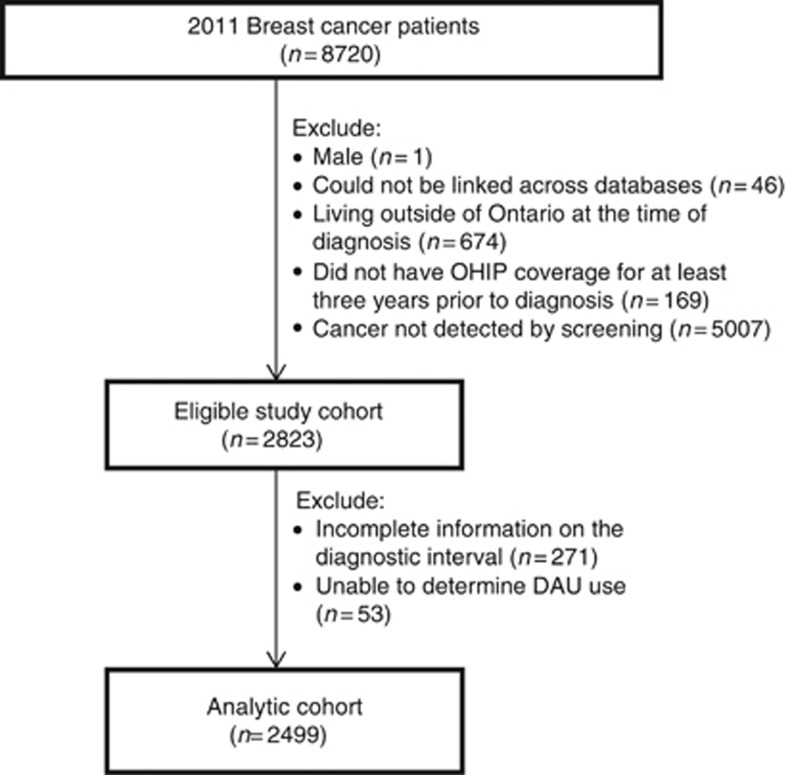
The final cohort consisted of 2499 study subjects (Figure 1). Of these, 1986 (79.5%) patients had an initial screen within the OBSP, and 513 (20.5%) were detected through opportunistic breast screening.
Figure 1.
Flow diagram of study subjects. OHIP, Ontario Health Insurance Plan; DAU, diagnostic assessment units.
Demographic and disease characteristics of the study population are shown in Table 1. The mean age was 63 and 69.5% were within the 50–69 year age group targeted by the OBSP. Fifty-one percent of the breast cancer patients attended a DAU for diagnostic assessment. Patients diagnosed through DAUs were slightly older than those in UC, although the difference was marginally statistically significant (P=0.08). Patients diagnosed through DAUs were more likely to live in a rural area (11.7% vs 8.2%, P=0.004) than UC patients. Over 90% were diagnosed at an early stage (stage 0–II). The proportion of stage III–IV cancers was 3% higher in the UC group (P=0.03). Table 2 displays the usual health-care utilisation characteristics of study subjects. The proportion of patients with a primary care provider was 2% lower in the DAU group (P=0.02). The preventive service index median values were the same in the two groups although their overall distributions were marginally statistically significantly different (P=0.06). Of the components that make up that index, DAU patients were less likely to get annual physical exams (33.9% vs 37.9%, P=0.04).
Table 1. Demographic and disease characteristics of screen-detected breast cancer patients in Ontario, Canada by diagnostic route (%).
| UC (n=1215) | DAU (n=1284) | P-value | |
|---|---|---|---|
|
Age | |||
| <50 | 6.5 | 6.2 | 0.08 |
| 50–59 | 33.4 | 29.4 | |
| 60–69 | 36.9 | 39.3 | |
| 70–79 | 18.4 | 21.3 | |
| 80+ | 4.8 | 3.8 | |
|
Deprivation index quintile | |||
| 1 (lowest) | 29.6 | 29.9 | 0.52 |
| 2 | 24.6 | 22.3 | |
| 3 | 18.8 | 21.5 | |
| 4 | 16.2 | 15.4 | |
| 5 (highest) | 10.8 | 10.9 | |
| Missing (n=297) | |||
| Benign breast disease history | 11.5 | 10.2 | 0.29 |
| Recent immigrant | 3.9 | 3.7 | 0.78 |
|
Co-morbidity | |||
| 0–3 ADGs | 20.8 | 22.9 | 0.14 |
| 4–5 ADGs | 21.2 | 22.3 | |
| 6–7 ADGs | 21.5 | 18.2 | |
| 8–9 ADGs | 16.6 | 18.5 | |
| 10+ ADGs | 19.8 | 18.2 | |
| Rural residence | 8.2 | 11.7 | 0.004 |
|
Histological grade | |||
| Low | 28.9 | 30.8 | 0.36 |
| Medium | 48.9 | 46.0 | |
| High | 22.2 | 23.2 | |
| Missing (n=207) | |||
|
Stage | |||
| Stage 0–I | 61.0 | 62.5 | 0.03 |
| Stage II | 28.8 | 30.3 | |
| Stage III–IV | 10.2 | 7.3 | |
| Stage UNK/missing (n=38) | |||
Abbreviations: ADG= the Johns Hopkins Aggregated Diagnosis Groups; DAU=diagnostic assessment units; UC=usual care; UNK=unknown.
Table 2. Usual health-care utilisation characteristics of screen-detected breast cancer patients in Ontario, Canada by diagnostic route (%).
| UC (n=1215) | DAU (n=1284) | P-value | |
|---|---|---|---|
|
Frequency of doctor visits | |||
| <10 | 36.9 | 41.4 | 0.12 |
| 10–19 | 34.5 | 32.6 | |
| 20–29 | 16.2 | 15.1 | |
| 30+ | 12.4 | 10.8 | |
| Had a primary care provider | 95.9 | 93.9 | 0.02 |
|
Continuity of care | |||
| Non-users | 6.4 | 7.8 | 0.22 |
| Low | 25.0 | 26.6 | |
| High | 68.6 | 65.6 | |
|
Preventive services indexa | |||
| Median | 0.40 | 0.40 | 0.06 |
| Interquartile range | 0.22–0.70 | 0.20–0.68 | |
Abbreviations: DAU=diagnostic assessment units; IQR=interquartile range; UC=usual care.
Preventive service index score is the proportion of preventive services used out of the total number of preventive services for which an individual was eligible.
Table 3 summarizes results of univariable and multivariable regression analyses. Overall, the median time to diagnosis was 29 days (IQR: 17–50). Ten percent of patients waited >79 days to be diagnosed. The median time to diagnostic resolution was 9 days shorter for patients in DAUs than those in UC (P<0.001). This effect estimate was slightly reduced to 8.3 days (P<0.001) after adjustment. Other factors associated with the median diagnostic interval included stage, with those in the Stage 0–I category waiting 9.9 days longer (P<0.001) and those in the Stage II category waiting 3.7 days longer compared with the Stage III/IV group (P=0.05). Those age 70–79 waited 4 days longer than the reference group (age 60–69, P=0.004), whereas being over 80 was marginally statistically significantly associated with a 3.8 day shorter interval (P=0.08). Having a history of benign breast disease was marginally associated with a 3 day longer diagnostic interval (P=0.05). A sensitivity analysis found similar results when we excluded the two non-BAA DAUs, with an unadjusted effect estimate of 9 days shorter than UC and an adjusted effect estimate of 8.2 days (results not shown). Regression at the 75th and 90th percentile of the diagnostic interval produced adjusted DAU effects that were similar to the median regression at 9.5 days (P<0.001) and 8.3 days (P<0.001), respectively. Breast cancer patients diagnosed at DAUs were more likely to have an abnormal screening resolved within 7 weeks than those in UC (79.1% vs 70.2%, P<0.001). The logistic regression for meeting the 7-week target in the DAU group vs UC produced an unadjusted odds ratio of 1.6 (95% CI: 1.3–1.9), which increased slightly to 1.7 (95% CI: 1.4–2.0) after adjusting for all covariates.
Table 3. Univariable and multivariable regression analyses results (N=2499).
| UC (n=1215) | DAU (n=1284) | Unadjusted difference (95% CI) | Adjusteda difference (95% CI) | |
|---|---|---|---|---|
|
Diagnostic Interval (days) | ||||
| Median | 35 | 26 (ref) | 9 (6.4, 11.6) | 8.3 (6.5, 10.2) |
| 75th Percentile | 55 | 43 (ref) | 12 (8.1, 15.9) | 9.5 (5.8, 13.3) |
| 90th Percentile | 82 | 77 (ref) | 5 (3.1, 13.1) | 8.3 (1.2, 15.3) |
| Meeting targets (%) | Unadjusted OR | Adjusteda OR | ||
| Diagnosed within 7 weeks | 70.2 (ref) | 79.1 | 1.6 (1.3, 1.9) | 1.7 (1.4, 2.0) |
Discussion
To the best of our knowledge, this is the first population-based study describing the effect of a specialized DAU on the cancer diagnostic process. We found that almost half of screen-detected breast cancer patients were diagnosed at DAUs and that those patients were diagnosed faster than patients diagnosed through UC. The median diagnostic interval was 4.1 weeks over all patients in our study, which is shorter than the 5.6 week estimate reported from seven Canadian provincial breast screening programmes in 1996 (Olivotto et al, 2001a) and the 5.9 week estimate reported from the province of Quebec in 2002/2003 (Bairati et al, 2007). This improvement was largely confined to the DAU group (at 3.7 weeks), as the UC route continued to take 5 weeks to diagnosis. The adjusted DAU's effect was 8.3 days, which was not affected by our inclusion of two regional DAUs that were not part of the OBSP BAA system. We studied three points in the upper end of the diagnostic interval distribution and found a consistent DAU effect throughout, suggesting that modifiable delays at an organisational level are similar for persons throughout that upper range.
Our DAU finding differs from two previous reports from single DAUs within Ontario. Those studies used a before–after design with one observing a 22-day median reduction between cancer suspicion and diagnosis (from 42 days to 20 days) (Gaskin and Fine, 2007) and the other observing a 4-day mean decrease between abnormal screen and biopsy (Arnaout et al, 2013). The discrepancy between these two previous studies could be due to variation in DAU structure, as this can differ as long as the DAU meets certain organisational criteria (Brouwers et al, 2009; Quan et al, 2012). Discrepancies with our current study may also be due to previous work's restriction to one DAU, the absence of contemporaneous comparison groups, and no statistical adjustment for covariates (Gaskin and Fine, 2007; Arnaout et al, 2013). Also, small sample sizes (n=76 and n=211, respectively) may have decreased their accuracy.
The Canadian breast screening timeliness targets, which are regularly updated based on evidence, recommend that 90% of abnormal screens should be resolved within 5 weeks (if no tissue biopsy is required), or within 7 weeks (if a tissue biopsy is required) (Public Health Agency of Canada, 2007; Canadian Partnership Against Cancer, 2013). So the 7-week target we used applies to open and/or core biopsy-diagnosed cancer cases but not those diagnosed clinically or through fine needle aspiration. Despite this liberal choice, only 79% and 70% met the target in the DAU and UC groups, respectively. A previous study that included all patients being investigated for possible breast cancer found that 59.9% in the DAU group compared 50.6% in UC met the 7-week target (Quan et al, 2012). Our higher rates might be explained by our restriction to breast cancer patients as women with invasive breast cancer get a quicker diagnosis compared to those with benign diseases (Chiarelli et al, 2005; Borugian et al, 2008).
We also observed some differences in the diagnostic interval among our covariate subgroups. Patients between 70 and 79 years of age waited four days longer (vs 60–69), which is inconsistent with the literature where most studies report no association. Some studies analysed age as continuous (Ferrante et al, 2007), whereas others dichotomised age at 50 years (Chang et al, 1996). In our study, age did not have a linear association with the diagnostic interval. We kept the age range of our categories relatively small to enhance model fit. The oldest (>80 years) patients may wait less time for their diagnoses (P=0.08), which agreed with the findings of Gorin et al (Gorin et al, 2006). Other covariates affecting timely diagnosis included a history of benign breast disease and an early-stage (Stage 0–II) cancer. The benign breast disease association was marginally significant. If true, it might be explained by false assurance from past false-positive screenings or the difficulty in reaching a diagnosis owing to multiple lesions in dense breasts. Further study of this question is warranted. Our observation that early staged patients take longer to be diagnosed has been repeatedly seen in the literature (Williams et al, 2010). This is likely explained by a lower sense of urgency with the symptoms of early-stage cancers.
The DAU effect could be due to any or all of their organisational components. Multidisciplinary care models have been consistently associated with shorter diagnostic wait times (Brouwers et al, 2007) with one study reporting a reduction of 4–10 days (Castellanos et al, 2008). Having a patient navigator who is responsible for coordinating diagnostic care and providing patient support shortens the wait times by 6–8 days (Psooy et al, 2004; Lobb et al, 2010), and a direct referral intervention reduces the median time to diagnostic resolution by 7–35 days (Olivotto et al, 2001b; Decker et al, 2004; Borugian et al, 2008). DAUs contain all of these components in the organisational structure, but the size of the effect we observed indicates that these effects are not additive. There is a need to understand the inter-relationship between these components and/or identify the component that explains most of the DAU effect as this could simplify the strategy used to achieve a timely diagnosis. Previous evidence also suggests that certain subintervals are more sensitive to interventions than others (Borugian et al, 2008). We are currently conducting a follow-up study to this one that will calculate these subintervals and how they related to DAU use.
It is unlikely that an 8-day quicker diagnosis will affect clinical outcomes. In the literature, the only conclusive evidence on the delay-survival association involves an interval longer than 3 months (Richards et al, 1999). Some studies have demonstrated that abnormal screening resolution of 6 or more months is associated with a larger tumour and more positive lymph nodes (Olivotto et al, 2002; Ganry et al, 2004; Elmore et al, 2005), but very few patients in this study waited that long. We do think that shortening the time to diagnosis by 8 days improves the patient experience as they often suffer from stress, anxiety and daily disruptions waiting for a diagnosis (Harcourt et al, 1998; Olivotto et al, 2000, 2001a, 2002; Quan et al, 2012). The patient perspective is also reflected in the 7-week diagnostic target, which considered evidence from patient quality of care research (Canadian Partnership Against Cancer, 2013) and we observed that the DAUs achieved a 9% improvement towards meeting that goal.
Most Canadian provincial screening programmes differ from those in the United Kingdom and Australia where the diagnostic assessment falls within the screening programme mandate (Olivotto et al, 2000). It would be useful to compare the diagnostic timeliness of DAUs to those integrated screening programmes. However, we were not able to make direct comparisons with published indicators from those countries (Australian Government: Department of Health and Aging, 2009; NHS Breast Screening Program and Association of Breast Surgery, 2013) because of our focus on the length of the whole diagnostic interval. Our follow-up study to this one will calculate and make comparisons with the indicators used in the United Kingdom and Australia.
It is important to emphasise that DAUs are not only designed to provide a rapid diagnosis, but also to improve the quality of care and patient experience. DAUs are required to ensure access to high-quality diagnostic equipment and clinical expertise. The use of a multidisciplinary care delivery model likely improves the diagnostic quality and efficiency. The navigation function is designed not only to streamline the process but also to provide supportive care, which has been shown to decrease patient anxiety and increase satisfaction (Ferrante et al, 2008). These features and associated outcomes need further study.
Conversely, there is a concern about the cost-effectiveness of DAUs (Gagliardi et al, 2004). Previous evidence suggests the direct referral component alone can greatly improve the timeliness with a modest cost increase (Olivotto et al, 2001b). Further evidence about the cost-effectiveness of Ontario DAUs would provide a more complete assessment of their impact. Our study quantified DAU's timeliness effect in days, which might be a useful part of a future economic evaluation.
This study had two key strengths. First, it was population-based, thus mitigating any concerns about selection bias. Second, it took advantage of a natural experiment in which DAU use is primarily determined by where a woman lives (Jiang, 2013). This natural experiment assertion is supported by the balanced covariate distribution we observed between the DAU and UC groups and by the fact that our DAU effect did not meaningfully change with adjustment.
This study has several limitations. We were restricted to the study of breast cancer patients rather than all women being investigated for a possible cancer due to the complexity of identifying this latter group in administrative data. Patients with benign diagnosis have been shown to wait longer for a final resolution compared with cancer patients (Borugian et al, 2008). Our observed DAU's effect is not generalisable to that group. We had to assume that an opportunistic screening mammogram performed within 6 months before diagnosis was abnormal because we did not have the test results. This may have increased the length of the diagnostic interval but errors were unlikely to be associated with DAU status. New physician billing codes specifying the purpose for the mammogram (screening vs diagnostic) as being for screening were introduced in 1 October 2010 and might not have been completely adopted in clinical practice during the study period. So, some opportunistically screened patients may have been left out of our study through use of the old, non-specific mammography code. We think the magnitude of this influence is likely to be small as frequency of use of the new code increased from October through December 2010 and had levelled off by 2011 (Institute for Clinical Evaluative Sciences, 2011). DAU use determination was subject to misclassification with sensitivity estimated at 90.1% and specificity at 84.6%. This misclassification only applies to the smaller (20.5%) group of patients detected through opportunistic screening. It would have resulted in an underestimate of the difference in time to diagnosis between DAU and UC. In addition, the facilities assigned to the UC route varied in the amount of diagnostic coordination they conducted with some of these facilities containing partial diagnostic assessment services. This variation would have decreased the magnitude of DAU's effect that we were able to observe. Some invasive cancer cases that were included in the study based on histology in the OCR had stage 0 disease in the collaborative staging data. We decided to use histology instead of stage to identify invasive cases, as the OCR's experience with this variable is much longer than its experience with collecting collaborative stage data. We excluded recent immigrants who did not have OHIP coverage in 3 years prior to the diagnosis because of our interest in capturing usual health utilisation patterns. Our study results cannot be generalised to very recent immigrants. We used an area-level proxy measure of socio-economic status based on the smallest census geographic unit available. Differences in individual-level socio-economic status between the DAU and UC groups may have persisted in our adjusted results. Lastly we were not able to measure some potential confounders and others were not measured optimally because of our use of administrative databases. Unmeasured confounding and residual confounding effects might have influenced the study results.
In conclusion, DAU use was associated with an 8-day improvement towards timely diagnosis for screen-detected breast cancer patients. This study provides evidence about one aspect of the effectiveness of specialised breast cancer DAUs. Future research examining the clinical, psychological and cost implications associated with DAUs is needed. Further documentation about which component(s) of a DAU is most effective in reducing the time to diagnosis is also warranted to inform breast cancer programme planning.
Acknowledgments
We thank Ms Marlo Whitehead for data linkage and data preparation, Dr Marcy Winget for methodologic advice and Ms Melissa Enmore for assistance in circulating the survey. This research is funded by a grant from the Canadian Institute for Health Research. Li Jiang was supported by an Ontario Graduate Scholarship and a Queen's University Graduate Award. This study was supported by the Institute for Clinical Evaluative Sciences (ICES), which is funded by an annual grant from the Ontario Ministry of Health and Long-Term Care (MOHLTC). The opinions, results and conclusions reported in this paper are those of the authors and are independent from the funding sources. No endorsement by ICES or the Ontario MOHLTC is intended or should be inferred.
The authors declare no conflict of interest.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
References
- Andersson I, Aspegren K, Janzon L, Landberg T, Lindholm K, Linell F, Ljungberg O, Ranstam J, Sigfusson B (1988) Mammographic screening and mortality from breast cancer: the Malmo mammographic screening trial. BMJ 297: 943–948. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnaout A, Smylie J, Seely J, Robertson S, Knight K, Shin S, Ramsey T, Mallick R, Watters J (2013) Improving breast diagnostic services with a Rapid Access Diagnostic and Support (RADS) program. Ann Surg Oncol 20: 3335–3340. [DOI] [PubMed] [Google Scholar]
- Arndt V, Sturmer T, Stegmaier C, Ziegler H, Becker A, Brenner H (2003) Provider delay among patients with breast cancer in Germany: a population-based study. J Clin Oncol 21: 1440–1446. [DOI] [PubMed] [Google Scholar]
- Austin PC, Van WC, Wodchis WP, Newman A, Anderson GM (2011) Using the Johns Hopkins Aggregated Diagnosis Groups (ADGs) to predict mortality in a general adult population cohort in Ontario, Canada. Med Care 49: 932–939. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Australian Government: Department of Health and Aging (2009) Screening Monograph No.1/2009: BreastScreen Australia Evaluation Evaluation Final Report. Available at http://www.cancerscreening.gov.au/internet/screening/publishing.nsf/Content/8463830B90E5BDF5CA25762A000193C6/$File/Breastscreen%20Aust_REPORT.pdf accessed 22 January 2015.
- Bairati I, Jobin E, Fillion L, Larochelle M, Vincent L (2007) Determinants of delay for breast cancer diagnosis. Cancer Detect Prev 31: 323–331. [DOI] [PubMed] [Google Scholar]
- Borugian MJ, Kan L, Chu CC, Ceballos K, Gelmon KA, Gordon PB, Poole B, Tyldesley S, Olivotto IA (2008) Facilitated "fast track" referral reduces time from abnormal screening mammogram to diagnosis. Can J Public Health 99: 252–256. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brett J, Austoker J, Ong G (1998) Do women who undergo further investigation for breast screening suffer adverse psychological consequences? A multi-centre follow-up study comparing different breast screening result groups five months after their last breast screening appointment. J Public Health Med 20: 396–403. [DOI] [PubMed] [Google Scholar]
- Brouwers M, Crawford J, Ellison P, Evans WK, Gagliardi A, Holmes D (2007) Organizational standards for diagnostic assessment programs. Available at https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=36872 accessed 22 January 2015.
- Brouwers M, Oliver TK, Crawford J, Ellison P, Evans WK, Gagliardi A, Lacourciere J, Lo D, Mai V, McNair S, Minuk T, Rabeneck L, Rand C, Ross J, Smylie J, Srigley J, Stern H, Trudeau M (2009) Cancer diagnostic assessment programs: standards for the organization of care in Ontario. Curr Oncol 16: 29–41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Canadian Partnership Against Cancer (2013) Report from the Evaluation Indicators Working Group: Guidelines for Monitoring Breast Cancer Screening Program Performance (Third Edition). Available at http://www.cancerview.ca/idc/groups/public/documents/webcontent/guideline_monitoring_breast.pdf accessed 22 January 2015.
- Cancer Care Ontario (2013. a) Current States of Diagnostic Assessment Programs. ESRS Report Prepared for the Diagnostic Assessment Program at Cancer Care Ontario.
- Cancer Care Ontario (2013. b) Ontario Breast Screening Program 2011 Report. Available at https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=288834 accessed 22 January 2015.
- Cancer Care Ontario (2012) Ontario Breast Cancer Screening Program (OBSP) Backgrounder. Available at https://www.cancercare.on.ca/common/pages/userfile.aspx?fileId=13032 accessed 22 January 2015.
- Cancer Care Ontario (2011) Ontario Breast Cancer Screening Program (OBSP) Backgrounder. Available at https://www.cancercare.on.ca/common/pages accessed 17 September 2013.
- Cancer Care Ontario (2009) Diagnostic Assessment Programs: An Environment Scan. Available at https://www.cancercare.on.ca/common/pages/UserFile.aspx?fileId=64068 accessed 22 January 2015.
- Cancer Quality Council of Ontario (2014) Patient Experience with the Diagnostic Assessment Program. Available at http://www.csqi.on.ca/ptjourney/diagnosis/dap_patient_exp/ accessed 22 January 2015.
- Castellanos MR, Conte J, Fadel DA, Raia C, Forte F, Ahern K, Smith M, Elsayeh D, Buchbinder S (2008) Improving access to breast health services with an interdisciplinary model of care. Breast J 14: 353–356. [DOI] [PubMed] [Google Scholar]
- Chang SW, Kerlikowske K, Napoles-Springer A, Posner SF, Sickles EA, Perez-Stable EJ (1996) Racial differences in timeliness of follow-up after abnormal screening mammography. Cancer 78: 1395–1402. [DOI] [PubMed] [Google Scholar]
- Chiarelli AM, Mai V, Halapy EE, Shumak RS, O'Malley FP, Klar NS (2005) Effect of screening result on waiting times to assessment and breast cancer diagnosis: results from the Ontario Breast Screening Program. Can J Public Health 96: 259–263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker KM, Harrison M, Chateau D (2004) Influence of direct referrals on time to diagnosis after an abnormal breast screening result. Cancer Detect Prev 28: 361–367. [DOI] [PubMed] [Google Scholar]
- Edge S, Byrd DR, Compton CC, Fritz AG, Greene FL, Trotti A eds (2009) AJCC Cancer Staging Manual 7th edn Springer New York: New York, NY, USA. [Google Scholar]
- Elmore JG, Nakano CY, Linden HM, Reisch LM, Ayanian JZ, Larson EB (2005) Racial inequities in the timing of breast cancer detection, diagnosis, and initiation of treatment. Med Care 43: 141–148. [DOI] [PubMed] [Google Scholar]
- Elston CW, Gresham GA, Rao GS, Zebro T, Haybittle JL, Houghton J, Kearney G (1982) The cancer research campaign (King's/Cambridge) trial for early breast cancer: clinico-pathological aspects. Br J Cancer 45: 655–669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elston C, Ellis I (1998) Assessment of histological grade. In Systemic Pathology, The Breast, CW Elston, IO Ellis, (eds) pp 365–384. Churchill Livingstone: London, UK. [Google Scholar]
- Ferrante JM, Chen P, Kim S (2008) The effect of patient navigation on time to diagnosis, anxiety, and satisfaction in urban minority women with abnormal mammograms: a randomized controlled trial. J Urban Health 85: 114–124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ferrante JM, Rovi S, Das K, Kim S (2007) Family physicians expedite diagnosis of breast disease in urban minority women. J Am Board Fam Med 20: 52–59. [DOI] [PubMed] [Google Scholar]
- Gagliardi A, Grunfeld E, Evans WK (2004) Evaluation of diagnostic assessment units in oncology: a systematic review. J Clin Oncol 22: 1126–1135. [DOI] [PubMed] [Google Scholar]
- Ganry O, Peng J, Dubreuil A (2004) Influence of abnormal screens on delays and prognostic indicators of screen-detected breast carcinoma. J Med Screen 11: 28–31. [DOI] [PubMed] [Google Scholar]
- Gaskin M, Fine S (2007) The Credit Valley Hospital Diagnostic Assessment Unit (DAU). Available at https://fr.cancercare.on.ca/common/pages/UserFile.aspx?fileId=47303 accessed 22 January 2015.
- Gorin SS, Heck JE, Cheng B, Smith SJ (2006) Delays in breast cancer diagnosis and treatment by racial/ethnic group. Arch Intern Med 166: 2244–2252. [DOI] [PubMed] [Google Scholar]
- Hall S, Schulze K, Groome P, Mackillop W, Holowaty E (2006) Using cancer registry data for survival studies: the example of the Ontario Cancer Registry. J Clin Epidemiol 59: 67–76. [DOI] [PubMed] [Google Scholar]
- Hao L, Naiman DQ (2007) Quantile regression. Sage Publications: Thousand Oaks, California, USA. [Google Scholar]
- Harcourt D, Ambler N, Rumsey N, Cawthorn SJ (1998) Evaluation of a one-stop breast lump clinic: a randomized controlled trial. Breast 7: 314–319. [Google Scholar]
- Institute for Clinical Evaluative Sciences (2011) Fee codes descriptions and counts by year. Available at https://outside.ices.on.ca/Data%20Holdings/Health%20Services/ohip/Variables/feecode.html accessed 11 November 2013.
- Jee SH, Cabana MD (2006) Indices for continuity of care: a systematic review of the literature. Med Care Res Rev 63: 158–188. [DOI] [PubMed] [Google Scholar]
- Jiang L (2013) Association between Use of A Specialized Diagnostic Assessment Unit and the Diagnostic Interval in Ontario Breast Cancer Patients. Master Thesis. Available at https://qspace.library.queensu.ca/jspui/bitstream/1974/8466/1/Jiang_Li_201311_MSc.pdf accessed 22 January 2015.
- Kralj B (2009) Measuring Rurality-RIO2008_BASIC: Methodology and Results. Available at https://www.oma.org/Resources/Documents/2008RIO-FullTechnicalPaper.pdf accessed 22 January 2015.
- Leung F (2012) The Association between Usual Health Care Utilization and Stage at Diagnosis in Laryngeal Cancer. Master Thesis. Available at https://qspace.library.queensu.ca/bitstream/1974/7121/1/Leung_Felicia_G_201204_MSc.pdf accessed 22 January 2015.
- Lobb R, Allen JD, Emmons KM, Ayanian JZ (2010) Timely care after an abnormal mammogram among low-income women in a public breast cancer screening program. Arch Intern Med 170: 521–528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matheson F, Dunn J, Smith K, Moineddin R, Glazier R (2012) ON-Marg: Ontario Marginalization Index-User Guide Version 1.0. Available at http://www.torontohealthprofiles.ca/onmarg/userguide_data/ON-Marg_user_guide_1.0_FINAL_MAY2012.pdf accessed 22 January 2015.
- Miller AB, Wall C, Baines CJ, Sun P, To T, Narod SA (2014) Twenty five year follow-up for breast cancer incidence and mortality of the Canadian National Breast Screening Study: randomised screening trial. BMJ 348: g366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- NHS Breast Screening Program and Association of Breast Surgery (2013) An audit of screen detected breast cancer for the year of screening April 2011 to March 2012. Available at http://www.cancerscreening.nhs.uk/breastscreen/publications/baso2011-2012.pdf accessed: 22 January 2015.
- Nystrom L, Rutqvist LE, Wall S, Lindgren A, Lindqvist M, Ryden S, Andersson I, Bjurstam N, Fagerberg G, Frisell J (1993) Breast cancer screening with mammography: overview of Swedish randomised trials. Lancet 341: 973–978. [DOI] [PubMed] [Google Scholar]
- Olivotto IA, Bancej C, Goel V, Snider J, McAuley RG, Irvine B, Kan L, Mirsky D, Sabine MJ, McGilly R, Caines JS (2001. a) Waiting times from abnormal breast screen to diagnosis in 7 Canadian provinces. CMAJ 165: 277–283. [PMC free article] [PubMed] [Google Scholar]
- Olivotto IA, Borugian MJ, Kan L, Harris SR, Rousseau EJ, Thorne SE, Vestrup JA, Wright CJ, Coldman AJ, Hislop TG (2001. b) Improving the time to diagnosis after an abnormal screening mammogram. Can J Public Health 92: 366–371. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Olivotto IA, Gomi A, Bancej C, Brisson J, Tonita J, Kan L, Mah Z, Harrison M, Shumak R (2002) Influence of delay to diagnosis on prognostic indicators of screen-detected breast carcinoma. Cancer 94: 2143–2150. [DOI] [PubMed] [Google Scholar]
- Olivotto IA, Kan L, King S (2000) Waiting for a diagnosis after an abnormal screening mammogram. SMPBC diagnostic process workgroup. Screening Mammography Program of British Columbia. Can J Public Health 91: 113–117. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ontario Breast Screening Program (2004) Ontario Breast Screening Program: Breast Assessment Initiative Orientation. Available at http://www.cancercare.on.ca accessed 17 September 2013.
- Poole K, Lyne PA (2000) The 'cues' to diagnosis: describing the monitoring activities of women undergoing diagnostic investigations for breast disease. J Adv Nurs 31: 752–758. [DOI] [PubMed] [Google Scholar]
- Psooy BJ, Schreuer D, Borgaonkar J, Caines JS (2004) Patient navigation: improving timeliness in the diagnosis of breast abnormalities. Can Assoc Radiol J 55: 145–150. [PubMed] [Google Scholar]
- Public Health Agency of Canada (2007) Report from the Evaluation Indicators Working Group: Guidelines for Monitoring Breast Screening Program Performance (Second Edition). Available at http://www.phac-aspc.gc.ca/publicat/2007/gmbspp-ldsppdcs/pdf/gmbspp-ldsppdcs_e.pdf accessed 22 January 2015.
- Quan ML, Shumak RS, Majpruz V (2012) Improving work-up of abnormal mammogram through organized assessment: results from the Ontario Breast Screening Program. J Oncol Prac 8: 107–112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richards MA, Westcombe AM, Love SB, Littlejohns P, Ramirez AJ (1999) Influence of delay on survival in patients with breast cancer: a systematic review. Lancet 353: 1119–1126. [DOI] [PubMed] [Google Scholar]
- Sobin LH, Gospodarowicz MK, Wittekind C eds (2009) The TNM Classification of Malignant Tumours. Wiley-Blackwell: Hoboken, NJ, USA. [Google Scholar]
- Thorne SE, Harris SR, Hislop TG, Vestrup JA (1999) The experience of waiting for diagnosis after an abnormal mammogram. Breast J 5: 42–51. [DOI] [PubMed] [Google Scholar]
- Walter SD, Birnie SE, Marrett LD, Taylor SM, Reynolds D, Davies J, Drake JJ, Hayes M (1994) The geographic variation of cancer incidence in Ontario. Am J Public Health 84: 367–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Williams DL, Tortu S, Thomson J (2010) Factors associated with delays to diagnosis and treatment of breast cancer in women in a Louisiana urban safety net hospital. Women Health 50: 705–718. [DOI] [PubMed] [Google Scholar]