Abstract
Background:
The tumour-node-metastasis (TNM) classification is the most widely used tool for penile cancer. However, the current system is based on few studies and has been unchanged since 2009. We determined whether a modified pathological N staging system that incorporates the laterality and number of lymph node metastases (LNMs) increases the accuracy of the results in predicting survival compared with the 7th edition of the pathological N staging system of the American Joint Committee on Cancer (AJCC) for penile cancer.
Methods:
The clinical and histopathologic data from 111 patients with penile cancer with LNMs were analysed. Univariate and multivariate Cox proportional hazard regression analyses were used to determine the impact of the clinical and pathological factors on disease-specific survival of these patients. The predictive accuracy was further assessed using the concordance index.
Results:
According to the 7th edition of the pathological N classification, the 3-year disease-specific survival (DSS) rates for patients with pN1, pN2, and pN3 disease are 89.6%, 65.9%, and 33.6%, respectively (PN1–N2=0.030, PN2–N3<0.001, P<0.001). Under the modified pathological N category criteria, the 3-year DSS rates for pN1, pN2, and pN3 patients were 90.7%, 60.5%, and 31.4%, respectively (PN1–N2=0.005, PN2–N3=0.004, P<0.001). In separate multivariate Cox regression models, only modified N stages (hazard ratio: 4.877, 10.895; P=0.018, P<0.001) exhibited independent effects on the outcome. The accuracy of the modified pathological N category was significantly increased.
Conclusions:
The modified pathological N staging system is a better reflection of the prognosis of patients with penile cancer. Our study should contribute to the improvement of prognostic stratification and systemic treatment to avoid overtreatment of patients.
Keywords: penile neoplasms, lymph node metastasis, neoplasm staging, lymph node excision, prognosis
In 2010, the 7th edition of the American Joint Committee on Cancer (AJCC)- tumour-node-metastasis (TNM) staging system for penile cancer was published with two major changes in the N category: (1) the distinction between superficial and deep inguinal lymph node (LN) involvement was ignored and (2) extranodal extension (ENE) of regional lymph node metastasis (LNM) was classified as pN3 disease (Edge, 2009). Although the N staging system of the 7th edition more accurately reflects the prognosis of patients with penile squamous cell carcinoma compared with previous staging systems, there is still room for improvement (Graafland et al, 2010; Zhu et al, 2011; Zhu and Ye, 2012).
The status of inguinal LNM is the most important prognostic factor in patients with penile cancer (Clark et al, 2014; Hakenberg et al, 2014). The presence of bilateral metastasis (Leijte et al, 2008; Graafland et al, 2010; Zhu et al, 2011) and the number of LNMs (Ravi, 1993; Pandey et al, 2006; Svatek et al, 2009) have been reported to exert adverse effects on survival. The difference between the pN1 and pN2 categories in the 7th edition of the pN staging system is based on bilateral inguinal involvement and/or the number of involved nodes (1 vs 2 or more). However, the pN2 subgroup, which includes patients with multiple or bilateral inguinal nodal disease, is heterogeneous (Pandey et al, 2006; Zhu and Ye, 2012; Li et al, 2014). Because controversy exists with regard to this disease and the TNM staging system, the European Urology Association recommends adjuvant chemotherapy only for ⩾pN2 disease(OW Hakenberg and A Necchi, 2014), whereas the NCCN recommends such therapy for LN size ⩾4 cm (Clark et al, 2014). Therefore, the identification of patients in these subgroups not only helps determine the need for multimodal treatments but also helps avoid overtreatment.
The aim of our study is to discuss the predictive value and feasibility of our amended pathological N staging system, which included the laterality and the number of LNMs, compared with the 7th edition of the AJCC-TNM staging system.
Materials and Methods
Patients
The clinical and pathological information of 246 patients with penile carcinoma who were treated at Sun Yat-Sen University Cancer Center from March 1999 to January 2013 were reviewed. The eligibility criteria were histologically confirmed penile squamous cell carcinoma and bilateral inguinal lymph node dissection (ILND) with ⩾8 LNs harvested (Johnson et al, 2010; Zhu et al, 2013). None of the enroled patients had distant metastases or received neoadjuvant chemotherapy/radiotherapy. Only 111 patients met the criteria.
Treatment and study assessments
All of the ILNDs were performed by three surgeons. The criteria, boundaries and technologies of our ILND have previously been described in detail (Yao et al, 2010). Prior to January 2009, pelvic lymphadenectomy was not routinely performed except in cases with clear evidence of solitary pelvic metastasis and with the patient's consent. Patients with pN2 and pN3 disease were recommended to undergo adjuvant chemotherapy or radiotherapy after surgery. Since 2009, pelvic lymphadenectomy has been performed simultaneously when two or more positive inguinal LNs were found by examination of frozen sections; patients with pN2 and pN3 disease have also been recommended to undergo adjuvant chemotherapy after surgery. All of the patients were followed up every 3 months for the first 2 years after surgery, every 6 months in the 3rd and 4th years, and then once yearly thereafter. The deadline for follow-up was August 2014.
Statistical analyses
After pathological review, the LN status was determined again according to the AJCC's 7th edition TNM staging system and the modified pathological N staging system (Table 1). Disease-specific survival (DSS) was calculated using the Kaplan–Meier method, and group differences were assessed using log-rank tests. We did not evaluate the role of adjuvant therapies in a multivariate analysis because they were not routinely given to all of the enroled patients. Because ENE and pelvic LNM were already included in the 7th edition of the N staging system and because LNM laterality and number were already included in the modified N staging system, only the T staging system, the N staging system and the grade candidate predictors were included in the multivariate analysis. The likelihood ratio, Akaike information criterion and the concordance index (C-index) were investigated to evaluate the accuracy of the models. Bootstrap corrected C-indexes were used to internal validation for better gauge expected future predictive accuracy (500 times sampling). All of the statistical analyses were performed with R2.11.1 (http://www.r-project.org), and a P<0.05 indicated statistical significance.
Table 1. AJCC 7th edition N staging system and the modified N staging system.
| Stage | AJCC 7th edition N staging system | Modified N staging system |
|---|---|---|
| N0 | No regional lymph node metastasis | No regional lymph node metastasis |
| Nx | Regional lymph nodes cannot be assessed | Regional lymph nodes cannot be assessed |
| N1 | Metastasis in a single inguinal lymph node | Metastasis in one to two unilateral inguinal lymph nodes without ENE |
| N2 | Metastasis in multiple or bilateral inguinal lymph nodes | Metastasis in three unilateral or metastasis in two to three bilateral inguinal lymph nodes without ENE |
| N2 | Metastasis in multiple or bilateral inguinal lymph nodes | Unilateral or bilateral metastasis in four or more inguinal lymph nodes without ENE, ENE of LNM or pelvic lymph node(s) |
| N3 | Unilateral or bilateral ENE of LNM or pelvic lymph node(s) | Unilateral or bilateral metastasis in four or more inguinal lymph nodes without ENE, ENE of LNM or pelvic lymph node(s) |
Abbreviations: ENE=extranodal extension; LNM=lymph node metastasis.
Results
Patients' characteristics
After a median time of 36 months (range, 3–124 months), 36 patients died of penile cancer. The clinical and pathological characteristics of the patients are shown in Table 2.
Table 2. Clinical and pathological characteristics of the patients.
| Variable | No. (%) or variable unit | 3-year DSS (95% CI) | P-value |
|---|---|---|---|
| Age at surgery, year, median (range) | 52 (24–87) | 0.162 | |
| <52 | 55 (49.5) | 70.3 (57.7–83.0) | |
| ⩾52 | 56 (50.5) | 52.3 (35.8–68.8) | |
| BMI, kg m−2, median (range) | 22.6 (16–37) | 0.881 | |
| <22.6 | 55 (49.5) | 60.2 (45.3–75.1) | |
| ⩾22.6 | 56 (50.5) | 63.8 (48.9–78.7) | |
| Number of LNs, n, median (range) | 21 (8–55) | 0.01 | |
| <21 | 55 (49.5) | 48.1 (33.0–63.2) | |
| ⩾21 | 56 (50.5) | 78.6 (66.4–90.8) | |
| LNM laterality, no. (%) | <0.001 | ||
| Unilateral | 60 (54.1) | 86.7 (76.5–96.9) | |
| Bilateral | 51 (45.9) | 36.8 (21.5–52.1) | |
| ENE, no. (%) | <0.001 | ||
| Yes | 28 (25.2) | 30.7 (8.0–53.4) | |
| No | 83 (74.7) | 71.1 (59.9–82.3) | |
| Pelvic LNM, no. (%) | 0.113 | ||
| Yes | 17 (15.3) | 52.1 (22.7–81.5) | |
| No | 94 (84.7) | 64.0 (52.8–75.2) | |
| Adjuvant therapy, no. (%) | 0.031 | ||
| Yes | 37 (33.3) | 49.3 (29.1–69.5) | |
| Adjuvant chemotherapy | 28 (24.3) | ||
| Adjuvant radiotherapy alone | 6 (5.4) | ||
| Adjuvant chemotherapy+radiotherapy | 3 (2.7) | ||
| None | 74 (66.7) | 67.7 (55.4–80.0) | |
| Number of LNMs, no. (%) | <0.001 | ||
| 1 | 26 (23.4) | 89.6 (75.9–100.0) | |
| 2 | 22 (19.8) | 89.6 (75.9–100.0) | |
| 3 | 25 (22.5) | 48.8 (23.7–73.9) | |
| ⩾4 | 38 (34.2) | 31.1 (13.2–49.1) | |
| T, no. (%) | <0.001 | ||
| ⩽pT1 | 17 (15.3) | 80.8 (56.1–100.0) | |
| pT2 | 76 (68.4) | 68.2 (56.4–80.0) | |
| ⩾pT3 | 18 (16.2) | 19.6 (2.5–41.7) | |
| G, no. (%) | 0.144 | ||
| G1 | 55 (49.5) | 67.0 (52.9–81.1) | |
| G2 | 41 (36.9) | 62.3 (45.6–79.0) | |
| G3 | 15 (13.5) | 42.9 (9.6–76.2) | |
| 7th edition pN, no. (%) | <0.001 | ||
| pN1 | 26 (23.4) | 89.6 (75.9–100.0) | |
| pN2 | 48 (43.2) | 65.9 (51.0–80.8) | |
| pN3 | 37 (33.3) | 33.6 (13.4–53.8) | |
| Modified pN, no. (%) | <0.001 | ||
| pN1 | 40 (36.0) | 90.7 (80.5–100.0) | |
| pN2 | 28 (25.2) | 60.5 (39.3–81.7) | |
| pN3 | 43 (38.7) | 31.4 (13.4–49.4) |
Abbreviations: BMI=Body mass index; CI=confidence interval, ENE=extranodal extension; LN=lymph node; LNM=lymph node metastasis.
Disease-specific survival
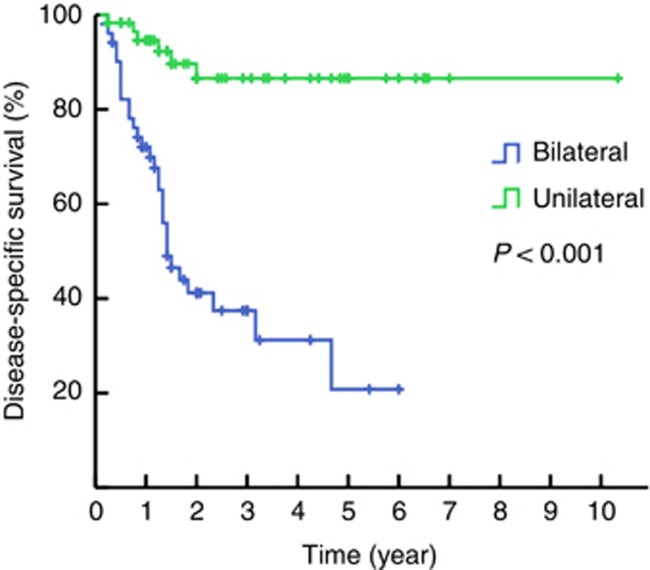
Patients with unilateral and bilateral LNMs showed a 3-year DSS of 86.6% and 37.4% (P<0.001, Figure 1), respectively. The 3-year DSS rates for patients with 1 (26) and 2 (14) unilateral inguinal LNMs without ENE or pelvic LNMs were 89.6% and 91.7%, respectively (P=0.983). The 3-year DSS rates for patients with 1–2 (40) and 3 (10) positive unilateral inguinal LNM without ENE or pelvic LNMs were 90.7% and 66.7%, respectively (P=0.023). No significant survival difference was observed between patients with 2 (7) and 3 (12) bilateral inguinal LNMs without ENE or pelvic LNMs (83.3% vs 36.5% P=0.094). The 3-year DSS was 51.5% for 2–3 (20) bilateral inguinal LNMs without ENE or pelvic LNMs; however, three of the four corresponding patients with ⩾4 involved LNs died from the disease.
Figure 1.
Kaplan–Meier estimates for DSS stratified by LNM laterality.
No significant difference in survival was observed between patients with pN2-3 disease regardless of whether they received adjuvant therapy (49.3% vs 56.1% P=0.405).
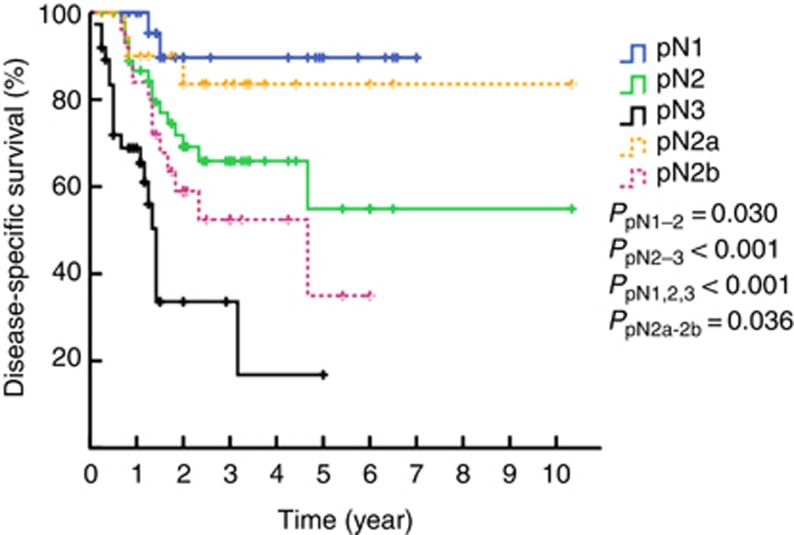
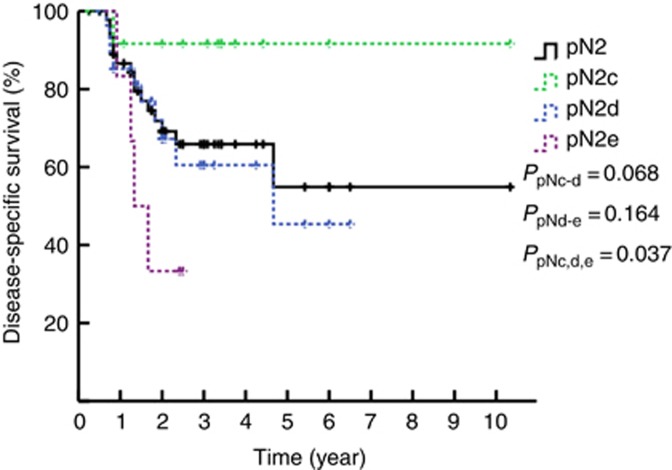
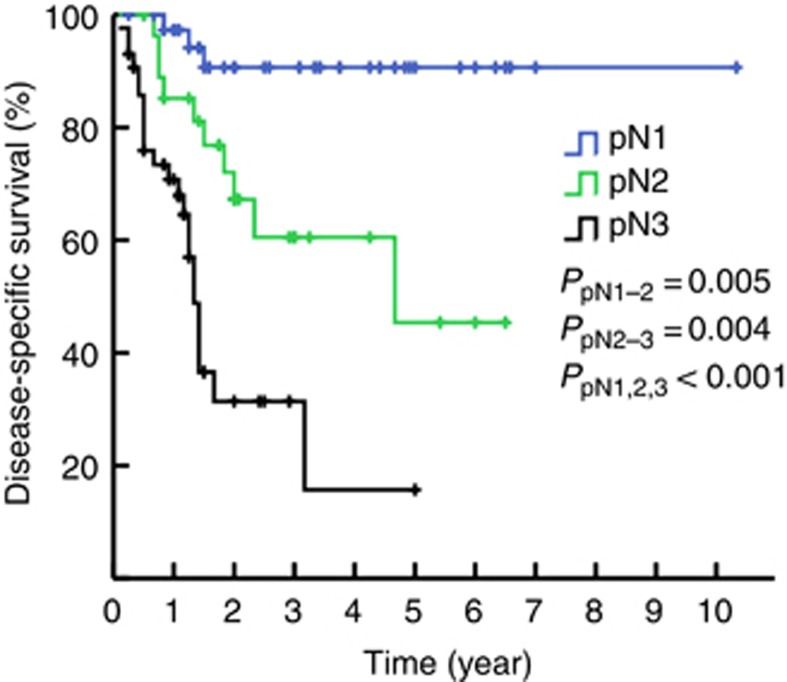
The 3-year DSS rates of patients with N1, N2, and N3 disease as determined by 7th edition of the N staging system were 89.6%, 65.9%, and 33.6%, respectively (PN1–N2=0.030, PN2–N3<0.001, P<0.001, Figure 2, solid line). However, when pN2 was divided into the N2a (23 patients with metastasis in unilateral inguinal LNs) and N2b groups (25 patients with metastasis in bilateral inguinal LNs), a difference in survival was noted (P=0.036, dashed line, Figure 2). Furthermore, had the more complicated stratified subdivision of the laterality and the number of inguinal LNMs been applied to the pN2 groups, metastasis in two unilateral inguinal lymph nodes (pN2c), metastasis in three unilateral or metastasis in 2–3 bilateral inguinal nodes (pN2d) and metastasis in four or more inguinal lymph nodes (pN2e) would have been considered heterogeneous groups (P=0.037, Figure 3). Therefore, the corresponding DSS rates of patients with N1, N2, and N3 disease according to the modified N staging system were 90.7%, 60.5%, and 31.4%, respectively (PN1–N2=0.005, PN2–N3=0.004, Figure 4).
Figure 2.
Kaplan–Meier estimates for DSS stratified by the 2009 pN staging system and pN2a, pN2b.
Figure 3.
Kaplan–Meier estimates for DSS stratified by pN2 and pN2c, pN2d, and pN2e.
Figure 4.
Kaplan–Meier estimates for DSS stratified by the modified pN staging system multivariate Cox regression analyses.
The proposed classification system provided improved predictive capacity with a higher hazard ratio (HR) and narrower confidence interval (CI) on the multivariate analysis (Table 3). Compared with stage pN1, stage pN2 (using the 7th edition of the pN staging system) was not significantly associated with an increased risk of death from this disease (P=0.074, Table 3). In contrast, the modified pathological N staging system exhibited an independent contribution to the Cox regression models (HR: reference, 4.302, 10.432; P: reference, 0.033, <0.001, Table 3).
Table 3. Multivariate Cox regression analyses for DSS.
| Variable |
AJCC 7th edition pN stage |
Modified pN stage |
||||
|---|---|---|---|---|---|---|
| HR | CI (95%) | P-value | HR | CI (95%) | P-value | |
| T | 0.047 | 0.15 | ||||
| ⩽T1 | Ref | – | – | Ref | – | – |
| T2 | 1.909 | 0.436–8.361 | 0.391 | 2.124 | 0491–9.196 | 0.314 |
| ⩾T3 | 4.277 | 0.921–19.871 | 0.064 | 3.711 | 0.802–17.169 | 0.093 |
| G | 0.783 | 0.506 | ||||
| G1 | Ref | – | – | Ref | – | – |
| G2 | 1.002 | 0.476–2.112 | 0.995 | 1.16 | 0.551–2.444 | 0.696 |
| G3 | 1.39 | 0.515–3.749 | 0.516 | 1.771 | 0.677–4.632 | 0.244 |
| pN stage | 0.002 | – | ||||
| pN1 | Ref | – | – | – | – | – |
| pN2 | 3.979 | 0.861–17.475 | 0.078 | – | – | – |
| pN3 | 10.561 | 2.317–48.127 | 0.002 | – | – | – |
| Modified pN stage | 0.001 | |||||
| pN1 | – | – | – | Ref | – | – |
| pN2 | – | – | – | 4.877 | 1.316–18.074 | 0.018 |
| pN3 | – | – | – | 10.895 | 3.104–38.244 | <0.001 |
Abbreviations: CI (95%)=95% confidence interval; HR=hazard ratio; Ref=reference.
Concordance index
In our study, we found that the addition of the pathological information significantly increased the predictive accuracy of the basic model (Table 4). We further evaluated discrimination and calibration. The bootstrap corrected C-index of the modified N stage categories was 0.742, which was inferior to that of the 7th pathological N staging system (P<0.001).
Table 4. Predictive accuracy of models including the AJCC 7th edition pN staging system and the modified N staging system.
| Stage | P-valuea | HR (95% CI)a | LRa | AIC | C-index | Bootstrap C-index |
|---|---|---|---|---|---|---|
| AJCC 7th edition of pN staging | 0.001 | 3.052 (1.626–5.726) | 14.148 | 130.14 | 0.698 | 0.699 |
| Modified pN staging | <0.001 | 3.335 (1.889–5.886) | 21.325 | 122.55 | 0.745 | 0.742 |
Abbreviations: AIC=Akaike information criterion, C-index=concordance index, LR=likelihood ratio.
Logistic.
Discussion
Modified N stage categories for penile cancer, which have been proposed in our study, include more of the above-mentioned parameters compared with the 7th pathological N staging system of the AJCC; our proposed system also provides validated prognostic value in terms of the DSS of patients with penile cancer.
The laterality of LNMs and the number of LNMs are important prognostic indicators for survival in patients with penile cancer (Ravi, 1993; Pandey et al, 2006; Leijte and Horenblas, 2009; Clark et al, 2014; OW Hakenberg and A Necchi, 2014). In our study, patients with bilateral metastases showed poorer survival than others (36.8% vs 86.7% P<0.001, Figure 1). We also found no significant difference in the survival rates in patients with one (pN1, 7th edition of the pN staging system) and two (pN2, 7th edition of the pN staging system) positive unilateral inguinal LNMs (P=0.983) as well as in patients with two and three bilateral positive LNMs (P=0.094) without ENE or pelvic LNM. In contrast, the survival rates between patients with one to two and three positive nodes were significantly different (P=0.023) under similar conditions. Moreover, three of four corresponding patients with more than equal to four involved LNs died from the disease. These studies show that an increased number of LNMs significantly increases the probability of a poor prognosis and a more strongly invasive penile cancer. Thus, identification of the number threshold and the laterality of LNMs in patients with penile cancer is necessary to facilitate clinical treatment decisions (Leijte and Horenblas, 2009; Pagliaro et al, 2010; Sonpavde et al, 2013).
The pN2 subgroup is heterogeneous (Graafland et al, 2010; Zhu et al, 2011; Li et al, 2014). According to our analysis, patients with pN2 disease (AJCC 7th edition of the pN staging system) in our study were divided into two subgroups (that is, pN2a and pN2b), showed 3-year DSS rates of 83.6% and 49.3%, respectively (P=0.044, Figure 1, dashed line). Interestingly, among the pN2 (48) cases, 14(29.2%) were down-staged to pN1, and 6 were (12.5%) up-staged to pN3 and were thus differentiated into new categories with improved clinical usefulness and prognostic value. Therefore, because of cryptomorphic heterogeneity, statistical significance was obtained by the Kaplan–Meier method and by a univariate analysis. According to our data, only the modified N categories retained an association with DSS in multivariate Cox regression analyses (Table 3) with a higher HR and narrower CI. In addition, the modified N categories are a significantly better prognostic factor for DSS (Table 4) with anticipated indexes. We presume that the addition of important information related to the status and extent of inguinal LNMs could increase the capability for predictive migration and have a positive predictive effect on survival for micro-metastasis or tumour load.
Based on this assumption, our modified N staging system prevented overtreatment in ∼30% (14/48) of patients with pN2 disease without missing any cases where treatment should have been given. We know that the value of adjuvant chemotherapy after ILND in node-positive penile cancer is heterogeneous (Pagliaro et al, 2010; Noronha et al, 2012; Nicholson et al, 2013; Sonpavde et al, 2013). Adjuvant therapy is not recommended for pN1 cases (Clark et al, 2014; OW Hakenberg and A Necchi, 2014). Multimodal treatment can improve patient outcomes in many tumour entities, and adjuvant chemotherapy is an option for patients with pN2–3 tumours (Noronha et al, 2012; Nicholson et al, 2013; Sonpavde et al, 2013). We admit that not all of the enroled patients with pN2 tumours were given adjuvant chemotherapy for various reasons, but according to our hypothesis, 14 of the patients who were down-staged to pN1 should not have been recommended to undergo further chemotherapy after ILND.
We acknowledge that our study has some limitations. First, the data collection was retrospective in design. However, our study included large sample sizes with few changes in treatment paradigms. Moreover, the median number of LNs removed was 21 (range, 8–55 nodes), which might better reflect the real status of LNMs (Lopes et al, 1996; de Carvalho et al, 2011; Li et al, 2014). Second, follow-up was brief. In our study, the median time was 36 months (range, 3–124 months), and this may cause follow-up duration time bias. So, future studies with longer follow-up have to define the role of modification of N staging systems would certainly be helpful. Third, adjuvant therapy (including adjuvant chemotherapy or/and radiotherapy) and pelvic lymphadenectomy may potentially affect other parameters. Pelvic lymphadenectomy was not routinely performed before 2009 because the unified standard was not recommended by the guidelines, whereas the adjuvant therapies were in varied forms and course of treatment is not unified. Unfortunately, someone who should have treated with adjuvant therapies also chooses nothing lead to lose the chance of treatment. We considered an adjuvant approach to lymphadenectomy, the results may still be subjected to selection bias that is inherent in this study design. We also know that the limited number of patients and consequent events in this study inhibited our ability to perform multivariate analysis. The predictive accuracy of each therapy should be tested in an external cohort population to determine its validity for clinical prediction. Thus, all analyses may be considered exploratory rather than hypothesis-tested. However, we believe that the present analysis will be important in future validation studies of larger and multicentre data sets.
Notwithstanding the above limitations, the modified pathological N staging system may increase the accuracy of survival prediction and help avoid overtreatment in patients with penile cancer.
Acknowledgments
The authors thank the staff of Department of Urology, Sun Yat-sen University Cancer Center. Ke-jian Gan and John N for editing the manuscript for English style from Chinese Journal of Cancer, Sun Yat-sen University Cancer Center, Guangzhou, P R China and Department of Urology, UT MD Anderson Cancer Center, TX, USA. The authors thank of Zhao Qi for the integrity of the statistical analysis from Sun Yat-sen University, School of Life Science.
Footnotes
This work is published under the standard license to publish agreement. After 12 months the work will become freely available and the license terms will switch to a Creative Commons Attribution-NonCommercial-Share Alike 4.0 Unported License.
The study is supported by grants from the Planned Science and Technology Project of Guangdong Province, China (2012B031800079), Sun Yat-sen University Cancer Center and State Key Laboratory of Oncology in Southern China, Guangzhou, China.
References
- Clark PE, Spiess PE, Agarwal N, Biagioli MC, Eisenberger MA, Greenberg RE, Herr HW, Inman BA, Kuban DA, Kuzel TM, Lele SM, Michalski J, Pagliaro L, Pal SK, Patterson A, Plimack ER, Pohar KS, Porter MP, Richie JP, Sexton WJ, Shipley WU, Small EJ, Trump DL, Wile G, Wilson TG, Dwyer M, Ho M National Comprehensive Cancer Network (2014) Penile cancer: NCCN Clinical Practice Guidelines in Oncology (Version 1. 2014). The National Comprehensive Cancer Network (NCCN).
- de Carvalho JP, Patricio BF, Medeiros J, Sampaio FJ, Favorito LA (2011) Anatomic aspects of inguinal lymph nodes applied to lymphadenectomy in penile cancer. Adv Urol 2011: 952532. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edge SB BDCM (2009) AJCC Cancer Staging Manual 7th edn. Springer: New York, NY, USA. [Google Scholar]
- Graafland NM, van Boven HH, van Werkhoven E, Moonen LM, Horenblas S (2010) Prognostic significance of extranodal extension in patients with pathological node positive penile carcinoma. J Urol 184: 1347–1353. [DOI] [PubMed] [Google Scholar]
- Hakenberg OW, Compeérat EM, Minhas S, Necchi A, Protzel C, Watkin N (2014) EAU guidelines on penile cancer: 2014 update. Eur Urol e-pub ahead of print 16 December 2014 doi:10.1016/j.eururo.2014.12.014. [DOI] [PubMed]
- Johnson TV, Hsiao W, Delman KA, Jani AB, Brawley OW, Master VA (2010) Extensive inguinal lymphadenectomy improves overall 5-year survival in penile cancer patients: results from the surveillance, epidemiology, and end results program. Cancer 116: 2960–2966. [DOI] [PubMed] [Google Scholar]
- Leijte JA, Gallee M, Antonini N, Horenblas S (2008) Evaluation of current TNM classification of penile carcinoma. J Urol 180: 933–938 discussion 938. [DOI] [PubMed] [Google Scholar]
- Leijte JA, Horenblas S (2009) Shortcomings of the current TNM classification for penile carcinoma: time for a change? World J Urol 27: 151–154. [DOI] [PubMed] [Google Scholar]
- Li ZS, Yao K, Chen P, Zou ZJ, Qin ZK, Liu ZW, Li YH, Zhou FJ, Han H (2014) Disease-specific survival after radical lymphadenectomy for penile cancer: prediction by lymph node count and density. Urol Oncol 32: 893–900. [DOI] [PubMed] [Google Scholar]
- Lopes A, Hidalgo GS, Kowalski LP, Torloni H, Rossi BM, Fonseca FP (1996) Prognostic factors in carcinoma of the penis: multivariate analysis of 145 patients treated with amputation and lymphadenectomy. J Urol 156: 1637–1642. [DOI] [PubMed] [Google Scholar]
- Nicholson S, Hall E, Harland SJ, Chester JD, Pickering L, Barber J, Elliott T, Thomson A, Burnett S, Cruickshank C, Carrington B, Waters R, Bahl A (2013) Phase II trial of docetaxel, cisplatin and 5FU chemotherapy in locally advanced and metastatic penis cancer (CRUK/09/001). Br J Cancer 109: 2554–2559. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noronha V, Patil V, Ostwal V, Tongaonkar H, Bakshi G, Prabhash K (2012) Role of paclitaxel and platinum-based adjuvant chemotherapy in high-risk penile cancer. Urol Ann 4: 150–153. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pagliaro LC, Williams DL, Daliani D, Williams MB, Osai W, Kincaid M, Wen S, Thall PF, Pettaway CA (2010) Neoadjuvant paclitaxel, ifosfamide, and cisplatin chemotherapy for metastatic penile cancer: a phase II study. J Clin Oncol 28: 3851–3857. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pandey D, Mahajan V, Kannan RR (2006) Prognostic factors in node-positive carcinoma of the penis. J Surg Oncol 93: 133–138. [DOI] [PubMed] [Google Scholar]
- Ravi R (1993) Correlation between the extent of nodal involvement and survival following groin dissection for carcinoma of the penis. Br J Urol 72: 817–819. [DOI] [PubMed] [Google Scholar]
- Sonpavde G, Pagliaro LC, Buonerba C, Dorff TB, Lee RJ, Di Lorenzo G (2013) Penile cancer: current therapy and future directions. Ann Oncol 24: 1179–1189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Svatek RS, Munsell M, Kincaid JM, Hegarty P, Slaton JW, Busby JE, Gaston KE, Spiess PE, Pagliaro LC, Tamboli P, Pettaway CA (2009) Association between lymph node density and disease specific survival in patients with penile cancer. J Urol 182: 2721–2727. [DOI] [PubMed] [Google Scholar]
- Yao K, Tu H, Li YH, Qin ZK, Liu ZW, Zhou FJ, Han H (2010) Modified technique of radical inguinal lymphadenectomy for penile carcinoma: morbidity and outcome. J Urol 184: 546–552. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Gu CY, Ye DW (2013) Validation of the prognostic value of lymph node ratio in patients with penile squamous cell carcinoma: a population-based study. Int Urol Nephrol 45: 1263–1271. [DOI] [PubMed] [Google Scholar]
- Zhu Y, Ye DW (2012) Lymph node metastases and prognosis in penile cancer. Chin J Cancer Res 24: 90–96. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Y, Ye DW, Yao XD, Zhang SL, Dai B, Zhang HL (2011) New N staging system of penile cancer provides a better reflection of prognosis. J Urol 186: 518–523. [DOI] [PubMed] [Google Scholar]